Investigating the Causal Effects of Exercise-Induced Genes on Sarcopenia
Abstract
1. Introduction
2. Results
2.1. Exercise-Induced Histological and Transcriptome Differences in Aging Skeletal Muscle
2.2. Exploring the Potential miRNA–Target Network Response to Exercise
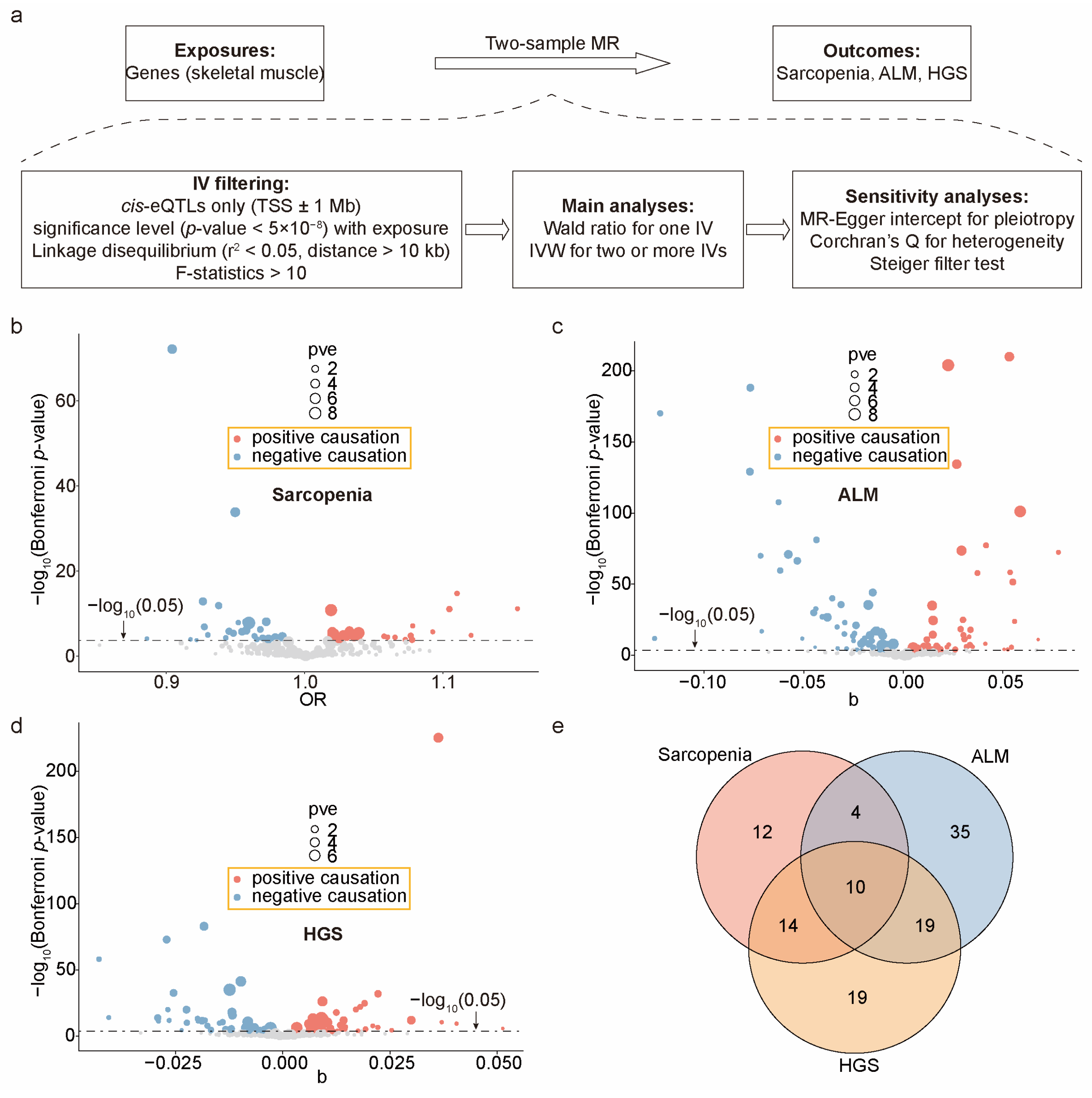
2.3. Calculating the Causal Effects of Exercise-Induced Genes on Sarcopenia Outcomes
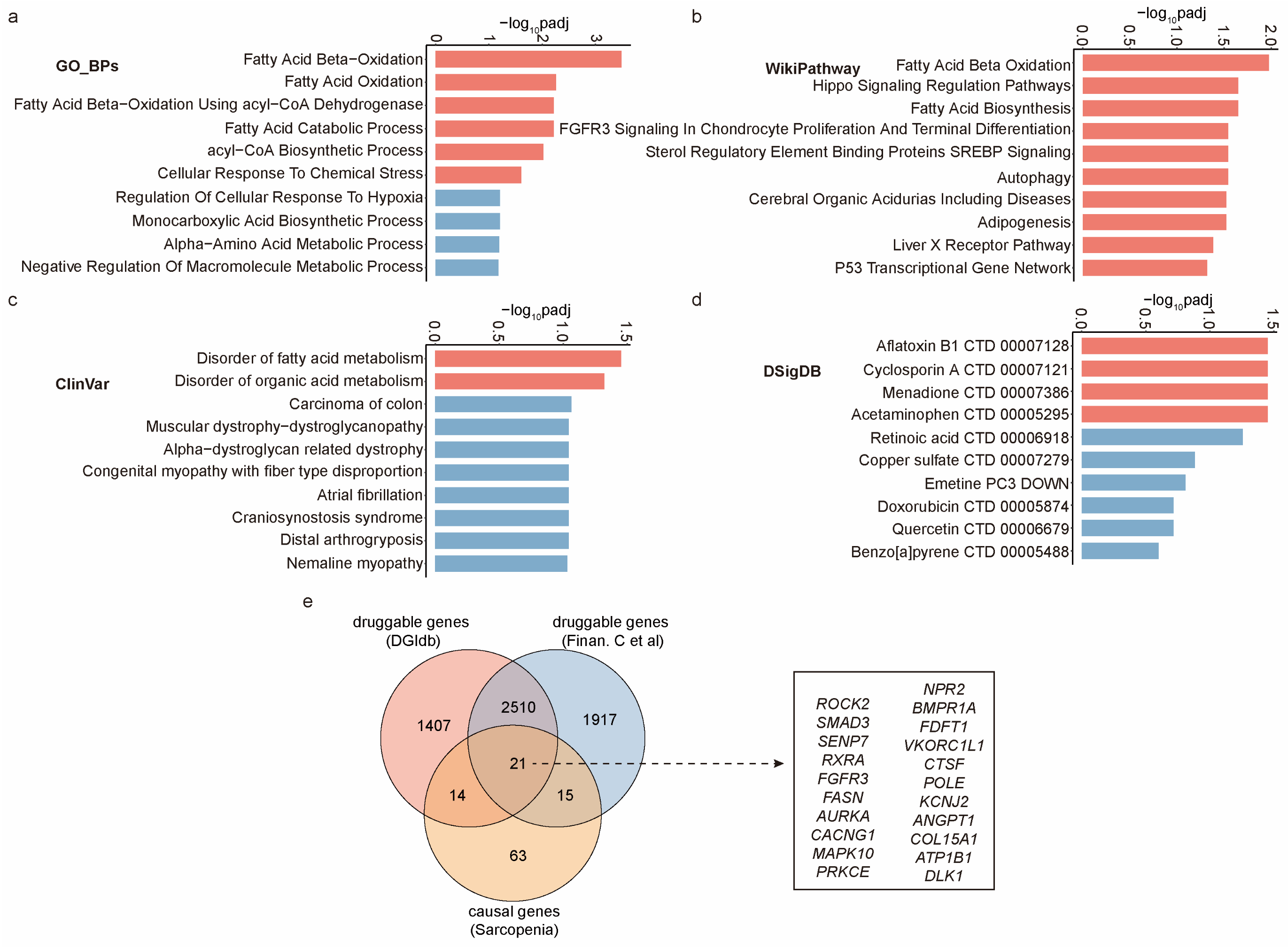
2.4. Unveiling Biological Mechanisms and Drug Interactions of These Causal Genes
3. Discussion
4. Materials and Methods
4.1. Animal Care and Use
4.2. Histological Investigation
4.3. Sequencing Library Construction
4.4. RNA-Seq Analysis
4.5. miRNA-Seq Analysis
4.6. Enrichment Analysis of TFs and GWAS Traits
4.7. Exposure Data and Outcome Data
4.8. Mendelian Randomization Analysis
4.9. Biological Mechanisms and Druggable Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guescini, M.; Tiano, L.; Genova, M.L.; Polidori, E.; Silvestri, S.; Orlando, P.; Fimognari, C.; Calcabrini, C.; Stocchi, V.; Sestili, P. The Combination of Physical Exercise with Muscle-Directed Antioxidants to Counteract Sarcopenia: A Biomedical Rationale for Pleiotropic Treatment with Creatine and Coenzyme Q10. Oxid. Med. Cell. Longev. 2017, 2017, 7083049. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, S.; Travison, T.G.; Manini, T.M.; Patel, S.; Pencina, K.M.; Fielding, R.A.; Magaziner, J.M.; Newman, A.B.; Kiel, D.P.; Cooper, C. Sarcopenia Definition: The Position Statements of the Sarcopenia Definition and Outcomes Consortium. J. Am. Geriatr. Soc. 2020, 68, 1410–1418. [Google Scholar] [CrossRef]
- Distefano, G.; Goodpaster, B.H. Effects of Exercise and Aging on Skeletal Muscle. Cold Spring Harb. Perspect. Med. 2018, 8, a029785. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Zhang, Y.; Olumi, S.; Karvar, M.; Argawal, S.; Neppl, R.L.; Sinha, I. Exercise-Induced Gene Expression Changes in Skeletal Muscle of Old Mice. Genomics 2021, 113, 2965–2976. [Google Scholar] [CrossRef] [PubMed]
- Petermann-Rocha, F.; Balntzi, V.; Gray, S.R.; Lara, J.; Ho, F.K.; Pell, J.P.; Celis-Morales, C. Global Prevalence of Sarcopenia and Severe Sarcopenia: A Systematic Review and Meta-analysis. J. Cachexia. Sarcopenia Muscle 2022, 13, 86–99. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M. Sarcopenia: European Consensus on Definition and Diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Brown, J.C.; Harhay, M.O.; Harhay, M.N. Appendicular Lean Mass and Mortality among Prefrail and Frail Older Adults. J. Nutr. Health Aging 2017, 21, 342–345. [Google Scholar] [CrossRef]
- Dennison, E.M.; Sayer, A.A.; Cooper, C. Epidemiology of Sarcopenia and Insight into Possible Therapeutic Targets. Nat. Rev. Rheumatol. 2017, 13, 340–347. [Google Scholar] [CrossRef]
- Martone, A.M.; Marzetti, E.; Calvani, R.; Picca, A.; Tosato, M.; Santoro, L.; Di Giorgio, A.; Nesci, A.; Sisto, A.; Santoliquido, A. Exercise and Protein Intake: A Synergistic Approach against Sarcopenia. BioMed Res. Int. 2017, 2017, 2672435. [Google Scholar] [CrossRef]
- Barbieri, E.; Agostini, D.; Polidori, E.; Potenza, L.; Guescini, M.; Lucertini, F.; Annibalini, G.; Stocchi, L.; De Santi, M.; Stocchi, V. The Pleiotropic Effect of Physical Exercise on Mitochondrial Dynamics in Aging Skeletal Muscle. Oxid. Med. Cell. Longev. 2015, 2015, 917085. [Google Scholar] [CrossRef]
- Meng, S.-J.; Yu, L.-J. Oxidative Stress, Molecular Inflammation and Sarcopenia. Int. J. Mol. Sci. 2010, 11, 1509–1526. [Google Scholar] [CrossRef] [PubMed]
- Dobrowolny, G.; Aucello, M.; Rizzuto, E.; Beccafico, S.; Mammucari, C.; Bonconpagni, S.; Belia, S.; Wannenes, F.; Nicoletti, C.; Del Prete, Z. Skeletal Muscle Is a Primary Target of SOD1G93A-Mediated Toxicity. Cell Metab. 2008, 8, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Hawley, J.A.; Hargreaves, M.; Joyner, M.J.; Zierath, J.R. Integrative Biology of Exercise. Cell 2014, 159, 738–749. [Google Scholar] [CrossRef] [PubMed]
- Tonevitsky, A.G.; Maltseva, D.V.; Abbasi, A.; Samatov, T.R.; Sakharov, D.A.; Shkurnikov, M.U.; Lebedev, A.E.; Galatenko, V.V.; Grigoriev, A.I.; Northoff, H. Dynamically Regulated MiRNA-MRNA Networks Revealed by Exercise. BMC Physiol. 2013, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Safdar, A.; Abadi, A.; Akhtar, M.; Hettinga, B.P.; Tarnopolsky, M.A. MiRNA in the Regulation of Skeletal Muscle Adaptation to Acute Endurance Exercise in C57Bl/6J Male Mice. PLoS ONE 2009, 4, e5610. [Google Scholar] [CrossRef]
- Nielsen, S.; Åkerström, T.; Rinnov, A.; Yfanti, C.; Scheele, C.; Pedersen, B.K.; Laye, M.J. The MiRNA Plasma Signature in Response to Acute Aerobic Exercise and Endurance Training. PLoS ONE 2014, 9, e87308. [Google Scholar] [CrossRef]
- Su, W.-M.; Gu, X.-J.; Dou, M.; Duan, Q.-Q.; Jiang, Z.; Yin, K.-F.; Cai, W.-C.; Cao, B.; Wang, Y.; Chen, Y.-P. Systematic Druggable Genome-Wide Mendelian Randomisation Identifies Therapeutic Targets for Alzheimer’s Disease. J. Neurol. Neurosurg. Psychiatry 2023, 94, 954–961. [Google Scholar] [CrossRef]
- Yazdanpanah, N.; Jumentier, B.; Yazdanpanah, M.; Ong, K.K.; Perry, J.R.B.; Manousaki, D. Mendelian Randomization Identifies Circulating Proteins as Biomarkers for Age at Menarche and Age at Natural Menopause. Commun. Biol. 2024, 7, 47. [Google Scholar] [CrossRef]
- Davey Smith, G.; Hemani, G. Mendelian Randomization: Genetic Anchors for Causal Inference in Epidemiological Studies. Hum. Mol. Genet. 2014, 23, R89–R98. [Google Scholar] [CrossRef]
- Burgess, S.; Butterworth, A.; Thompson, S.G. Mendelian Randomization Analysis with Multiple Genetic Variants Using Summarized Data. Genet. Epidemiol. 2013, 37, 658–665. [Google Scholar] [CrossRef]
- Davies, N.M.; Holmes, M.V.; Smith, G.D. Reading Mendelian Randomisation Studies: A Guide, Glossary, and Checklist for Clinicians. BMJ 2018, 362, k601. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, D.A. Commentary: Two-Sample Mendelian Randomization: Opportunities and Challenges. Int. J. Epidemiol. 2016, 45, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-B.; Wang, J.-Q.; Meng, X.-H.; Luo, Z.; Liu, X.-W.; Shen, H.; Xiao, H.-M.; Deng, H.-W. Putative Candidate Drug Targets for Sarcopenia-Related Traits Identified through Mendelian Randomization Analysis of the Blood Proteome. Front. Genet. 2022, 13, 923429. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Chen, T.; Gu, X.; Su, W.; Jiang, Z.; Lu, S.; Cao, B.; Chi, L.; Gao, X.; Chen, Y. Systematic Druggable Genome-wide Mendelian Randomization Identifies Therapeutic Targets for Sarcopenia. J. Cachexia. Sarcopenia Muscle 2024, 15, 1324–1334. [Google Scholar] [CrossRef]
- Jiang, W.; Zhan, W.; Zhou, L.; Dong, M.; Liu, L.; Xu, X.; Cao, Z. Potential Therapeutic Targets for Sarcopenia Identified by Mendelian Randomisation. Age Ageing 2023, 52, afad024. [Google Scholar] [CrossRef]
- Sun, S.; Ma, S.; Cai, Y.; Wang, S.; Ren, J.; Yang, Y.; Ping, J.; Wang, X.; Zhang, Y.; Yan, H. A Single-Cell Transcriptomic Atlas of Exercise-Induced Anti-Inflammatory and Geroprotective Effects across the Body. Innovation 2023, 4, 100380. [Google Scholar] [CrossRef]
- GTEx Consortium. The GTEx Consortium Atlas of Genetic Regulatory Effects across Human Tissues. Science 2020, 369, 1318–1330. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, H.; Loenneke, J.P.; Buckner, S.L.; Abe, T. Muscle Growth across a Variety of Exercise Modalities and Intensities: Contributions of Mechanical and Metabolic Stimuli. Med. Hypotheses 2016, 88, 22–26. [Google Scholar] [CrossRef]
- Mengeste, A.M.; Rustan, A.C.; Lund, J. Skeletal Muscle Energy Metabolism in Obesity. Obesity 2021, 29, 1582–1595. [Google Scholar] [CrossRef]
- Agarwal, M.; Sharma, A.; Kumar, P.; Kumar, A.; Bharadwaj, A.; Saini, M.; Kardon, G.; Mathew, S.J. Myosin Heavy Chain-Embryonic Regulates Skeletal Muscle Differentiation during Mammalian Development. Development 2020, 147, dev184507. [Google Scholar] [CrossRef]
- Schiaffino, S.; Rossi, A.C.; Smerdu, V.; Leinwand, L.A.; Reggiani, C. Developmental Myosins: Expression Patterns and Functional Significance. Skelet. Muscle 2015, 5, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.D.; Liu, G.; Luo, L.; Xiao, J.; Gerrein, J.; Juan-Guardela, B.; Tedrow, J.; Alekseyev, Y.O.; Yang, I.V.; Correll, M. Assessment of MicroRNA Differential Expression and Detection in Multiplexed Small RNA Sequencing Data. RNA 2015, 21, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.W.; Zhang, S.; Etheridge, A.; Ma, L.; Martin, D.; Galas, D.; Wang, K. Complexity of the MicroRNA Repertoire Revealed by Next-Generation Sequencing. RNA 2010, 16, 2170–2180. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Wang, Y.; Li, Y.; Cui, L.; Zhao, Y.; Zhao, B.; Li, K. MiR-206, a Key Modulator of Skeletal Muscle Development and Disease. Int. J. Biol. Sci. 2015, 11, 345. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Lee, Y.S.; Sivaprasad, U.; Malhotra, A.; Dutta, A. Muscle-Specific MicroRNA MiR-206 Promotes Muscle Differentiation. J. Cell Biol. 2006, 174, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Mytidou, C.; Koutsoulidou, A.; Zachariou, M.; Prokopi, M.; Kapnisis, K.; Spyrou, G.M.; Anayiotos, A.; Phylactou, L.A. Age-Related Exosomal and Endogenous Expression Patterns of MiR-1, MiR-133a, MiR-133b, and MiR-206 in Skeletal Muscles. Front. Physiol. 2021, 12, 708278. [Google Scholar] [CrossRef]
- Wosczyna, M.N.; Carbajal, E.E.P.; Wagner, M.W.; Paredes, S.; Konishi, C.T.; Liu, L.; Wang, T.T.; Walsh, R.A.; Gan, Q.; Morrissey, C.S. Targeting MicroRNA-Mediated Gene Repression Limits Adipogenic Conversion of Skeletal Muscle Mesenchymal Stromal Cells. Cell Stem Cell 2021, 28, 1323–1334. [Google Scholar] [CrossRef]
- Horak, M.; Novak, J.; Bienertova-Vasku, J. Muscle-Specific MicroRNAs in Skeletal Muscle Development. Dev. Biol. 2016, 410, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, C.; Zhang, A.; Cai, H.; Price, S.R.; Wang, X.H. MicroRNA-23a and MicroRNA-27a Mimic Exercise by Ameliorating CKD-Induced Muscle Atrophy. J. Am. Soc. Nephrol. JASN 2017, 28, 2631. [Google Scholar] [CrossRef]
- Lee, M.; Wada, S.; Oikawa, S.; Suzuki, K.; Ushida, T.; Akimoto, T. Loss of MicroRNA-23–27–24 Clusters in Skeletal Muscle Is Not Influential in Skeletal Muscle Development and Exercise-Induced Muscle Adaptation. Sci. Rep. 2019, 9, 1092. [Google Scholar] [CrossRef]
- Brzeszczyńska, J.; Brzeszczyński, F.; Hamilton, D.F.; McGregor, R.; Simpson, A.H.R.W. Role of MicroRNA in Muscle Regeneration and Diseases Related to Muscle Dysfunction in Atrophy, Cachexia, Osteoporosis, and Osteoarthritis. Bone Joint Res. 2020, 9, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Nie, M.; Liu, J.; Yang, Q.; Seok, H.Y.; Hu, X.; Deng, Z.L.; Wang, D.Z. MicroRNA-155 Facilitates Skeletal Muscle Regeneration by Balancing pro-and Anti-Inflammatory Macrophages. Cell Death Dis. 2016, 7, e2261. [Google Scholar] [CrossRef] [PubMed]
- Okamura, T.; Hashimoto, Y.; Osaka, T.; Senmaru, T.; Fukuda, T.; Hamaguchi, M.; Fukui, M. MiR-23b-3p Acts as a Counter-Response against Skeletal Muscle Atrophy. J. Endocrinol. 2020, 244, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-H.; Liu, S.; Zhou, H.; Qu, L.-H.; Yang, J.-H. StarBase v2. 0: Decoding MiRNA-CeRNA, MiRNA-NcRNA and Protein–RNA Interaction Networks from Large-Scale CLIP-Seq Data. Nucleic Acids Res. 2014, 42, D92–D97. [Google Scholar] [CrossRef]
- Tong, Z.; Cui, Q.; Wang, J.; Zhou, Y. TransmiR v2. 0: An Updated Transcription Factor-MicroRNA Regulation Database. Nucleic Acids Res. 2019, 47, D253–D258. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, C.; Sun, Y.; Li, Y.; Kang, L.; Jiang, Y. Dynamic Transcriptome and DNA Methylome Analyses on Longissimus Dorsi to Identify Genes Underlying Intramuscular Fat Content in Pigs. BMC Genom. 2017, 18, 1–18. [Google Scholar] [CrossRef]
- Vasyutina, E.; Lenhard, D.C.; Wende, H.; Erdmann, B.; Epstein, J.A.; Birchmeier, C. RBP-J (Rbpsuh) Is Essential to Maintain Muscle Progenitor Cells and to Generate Satellite Cells. Proc. Natl. Acad. Sci. USA 2007, 104, 4443–4448. [Google Scholar] [CrossRef]
- Paul, R.G.; Hennebry, A.S.; Elston, M.S.; Conaglen, J.V.; McMahon, C.D. Regulation of Murine Skeletal Muscle Growth by STAT5B Is Age-and Sex-Specific. Skelet. Muscle 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Jia, X.; Ouyang, H.; Abdalla, B.A.; Xu, H.; Nie, Q.; Zhang, X. MiR-16 Controls Myoblast Proliferation and Apoptosis through Directly Suppressing Bcl2 and FOXO1 Activities. Biochim. Biophys. Acta (BBA)-Gene Regul. Mech. 2017, 1860, 674–684. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, X.; Zhou, K.; Ling, X.; Zhang, J.; Wu, P.; Zhang, T.; Xie, K.; Dai, G. MiRNA-10a-5p Targeting the BCL6 Gene Regulates Proliferation, Differentiation and Apoptosis of Chicken Myoblasts. Int. J. Mol. Sci. 2022, 23, 9545. [Google Scholar] [CrossRef]
- Jin, S.; Kim, J.; Willert, T.; Klein-Rodewald, T.; Garcia-Dominguez, M.; Mosqueira, M.; Fink, R.; Esposito, I.; Hofbauer, L.C.; Charnay, P. Ebf Factors and MyoD Cooperate to Regulate Muscle Relaxation via Atp2a1. Nat. Commun. 2014, 5, 3793. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Stephens, M. Large-Scale Genome-Wide Enrichment Analyses Identify New Trait-Associated Genes and Pathways across 31 Human Phenotypes. Nat. Commun. 2018, 9, 4361. [Google Scholar] [CrossRef] [PubMed]
- Barrenäs, F.; Chavali, S.; Alves, A.C.; Coin, L.; Jarvelin, M.-R.; Jörnsten, R.; Langston, M.A.; Ramasamy, A.; Rogers, G.; Wang, H. Highly Interconnected Genes in Disease-Specific Networks Are Enriched for Disease-Associated Polymorphisms. Genome Biol. 2012, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fu, Y.; Yang, Y.; Yi, G.; Lian, J.; Xie, B.; Yao, Y.; Chen, M.; Niu, Y.; Liu, L. Integration of Multi-Omics Data Reveals Cis-Regulatory Variants That Are Associated with Phenotypic Differentiation of Eastern from Western Pigs. Genet. Sel. Evol. 2022, 54, 1–22. [Google Scholar] [CrossRef]
- Sollis, E.; Mosaku, A.; Abid, A.; Buniello, A.; Cerezo, M.; Gil, L.; Groza, T.; Güneş, O.; Hall, P.; Hayhurst, J. The NHGRI-EBI GWAS Catalog: Knowledgebase and Deposition Resource. Nucleic Acids Res. 2023, 51, D977–D985. [Google Scholar] [CrossRef]
- Wang, C.; Chen, C.; Lei, B.; Qin, S.; Zhang, Y.; Li, K.; Zhang, S.; Liu, Y. Constructing ERNA-Mediated Gene Regulatory Networks to Explore the Genetic Basis of Muscle and Fat-Relevant Traits in Pigs. Genet. Sel. Evol. 2024, 56, 1–21. [Google Scholar] [CrossRef]
- Wang, M.; Hancock, T.P.; MacLeod, I.M.; Pryce, J.E.; Cocks, B.G.; Hayes, B.J. Putative Enhancer Sites in the Bovine Genome Are Enriched with Variants Affecting Complex Traits. Genet. Sel. Evol. 2017, 49, 1–16. [Google Scholar] [CrossRef]
- Lee, J.S.W.; Auyeung, T.-W.; Leung, J.; Kwok, T.; Leung, P.-C.; Woo, J. Physical Frailty in Older Adults Is Associated with Metabolic and Atherosclerotic Risk Factors and Cognitive Impairment Independent of Muscle Mass. J. Nutr. Health Aging 2011, 15, 857–862. [Google Scholar] [CrossRef]
- Schaap, L.A.; Koster, A.; Visser, M. Adiposity, Muscle Mass, and Muscle Strength in Relation to Functional Decline in Older Persons. Epidemiol. Rev. 2013, 35, 51–65. [Google Scholar] [CrossRef]
- Kunz, H.E.; Lanza, I.R. Age-Associated Inflammation and Implications for Skeletal Muscle Responses to Exercise. Exp. Gerontol. 2023, 177, 112177. [Google Scholar] [CrossRef]
- Cui, C.-Y.; Ferrucci, L. Macrophages in Skeletal Muscle Aging. Aging 2020, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- He, N.; Zhang, Y.; Zhang, L.; Zhang, S.; Ye, H. Relationship between Sarcopenia and Cardiovascular Diseases in the Elderly: An Overview. Front. Cardiovasc. Med. 2021, 8, 743710. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Trajanoska, K.; Santanasto, A.J.; Stringa, N.; Kuo, C.-L.; Atkins, J.L.; Lewis, J.R.; Duong, T.; Hong, S.; Biggs, M.L. Genome-Wide Meta-Analysis of Muscle Weakness Identifies 15 Susceptibility Loci in Older Men and Women. Nat. Commun. 2021, 12, 654. [Google Scholar] [CrossRef]
- Pei, Y.-F.; Liu, Y.-Z.; Yang, X.-L.; Zhang, H.; Feng, G.-J.; Wei, X.-T.; Zhang, L. The Genetic Architecture of Appendicular Lean Mass Characterized by Association Analysis in the UK Biobank Study. Commun. Biol. 2020, 3, 608. [Google Scholar] [CrossRef]
- Lyon, M.S.; Andrews, S.J.; Elsworth, B.; Gaunt, T.R.; Hemani, G.; Marcora, E. The Variant Call Format Provides Efficient and Robust Storage of GWAS Summary Statistics. Genome Biol. 2021, 22, 32. [Google Scholar] [CrossRef]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A. Enrichr: A Comprehensive Gene Set Enrichment Analysis Web Server 2016 Update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef]
- Yoo, M.; Shin, J.; Kim, J.; Ryall, K.A.; Lee, K.; Lee, S.; Jeon, M.; Kang, J.; Tan, A.C. DSigDB: Drug Signatures Database for Gene Set Analysis. Bioinformatics 2015, 31, 3069–3071. [Google Scholar] [CrossRef]
- Rushing, B.R.; Selim, M.I. Aflatoxin B1: A Review on Metabolism, Toxicity, Occurrence in Food, Occupational Exposure, and Detoxification Methods. Food Chem. Toxicol. 2019, 124, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, A.; Lopes, P.C.; Sereno, J.; Pedro, J.; Espinoza, D.O.; Pereira, M.J.; Reis, F.; Eriksson, J.W.; Carvalho, E. Molecular Mechanisms Underlying the Effects of Cyclosporin A and Sirolimus on Glucose and Lipid Metabolism in Liver, Skeletal Muscle and Adipose Tissue in an in Vivo Rat Model. Biochem. Pharmacol. 2014, 88, 216–228. [Google Scholar] [CrossRef]
- Ren, X.; Santhosh, S.M.; Coppo, L.; Ogata, F.T.; Lu, J.; Holmgren, A. The Combination of Ascorbate and Menadione Causes Cancer Cell Death by Oxidative Stress and Replicative Stress. Free Radic. Biol. Med. 2019, 134, 350–358. [Google Scholar] [CrossRef]
- Mast, C.; Dardevet, D.; Papet, I. Impact of Medication on Protein and Amino Acid Metabolism in the Elderly: The Sulfur Amino Acid and Paracetamol Case. Nutr. Res. Rev. 2018, 31, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Freshour, S.L.; Kiwala, S.; Cotto, K.C.; Coffman, A.C.; McMichael, J.F.; Song, J.J.; Griffith, M.; Griffith, O.L.; Wagner, A.H. Integration of the Drug–Gene Interaction Database (DGIdb 4.0) with Open Crowdsource Efforts. Nucleic Acids Res. 2021, 49, D1144–D1151. [Google Scholar] [CrossRef]
- Finan, C.; Gaulton, A.; Kruger, F.A.; Lumbers, R.T.; Shah, T.; Engmann, J.; Galver, L.; Kelley, R.; Karlsson, A.; Santos, R. The Druggable Genome and Support for Target Identification and Validation in Drug Development. Sci. Transl. Med. 2017, 9, eaag1166. [Google Scholar] [CrossRef] [PubMed]
- Durham, W.J.; Casperson, S.L.; Dillon, E.L.; Keske, M.A.; Paddon-Jones, D.; Sanford, A.P.; Hickner, R.C.; Grady, J.J.; Sheffield-Moore, M. Age-Related Anabolic Resistance after Endurance-Type Exercise in Healthy Humans. FASEB J. 2010, 24, 4117. [Google Scholar] [CrossRef] [PubMed]
- Raue, U.; Trappe, T.A.; Estrem, S.T.; Qian, H.-R.; Helvering, L.M.; Smith, R.C.; Trappe, S. Transcriptome Signature of Resistance Exercise Adaptations: Mixed Muscle and Fiber Type Specific Profiles in Young and Old Adults. J. Appl. Physiol. 2012, 112, 1625–1636. [Google Scholar] [CrossRef]
- Tuñón-Suárez, M.; Reyes-Ponce, A.; Godoy-Órdenes, R.; Quezada, N.; Flores-Opazo, M. Exercise Training to Decrease Ectopic Intermuscular Adipose Tissue in Individuals with Chronic Diseases: A Systematic Review and Meta-Analysis. Phys. Ther. 2021, 101, pzab162. [Google Scholar] [CrossRef]
- Kallinen, M.; Markku, A. Aging, Physical Activity and Sports Injuries: An Overview of Common Sports Injuries in the Elderly. Sport. Med. 1995, 20, 41–52. [Google Scholar] [CrossRef]
- Pan, Z.; Chen, X.; Wu, D.; Li, X.; Gao, W.; Li, G.; Du, G.; Zhang, C.; Jin, S.; Geng, Z. A Novel in Duck Myoblasts: The Transcription Factor Retinoid X Receptor Alpha (RXRA) Inhibits Lipid Accumulation by Promoting CD36 Expression. Int. J. Mol. Sci. 2023, 24, 1180. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Q.; Wu, Y.; Zhang, Y.; Zhang, B.; Zhang, H. Identification of Candidate Genes That Specifically Regulate Subcutaneous and Intramuscular Fat Deposition Using Transcriptomic and Proteomic Profiles in Dingyuan Pigs. Sci. Rep. 2022, 12, 2844. [Google Scholar] [CrossRef]
- Shi, Y.; Cheng, D. Beyond Triglyceride Synthesis: The Dynamic Functional Roles of MGAT and DGAT Enzymes in Energy Metabolism. Am. J. Physiol. Metab. 2009, 297, E10–E18. [Google Scholar] [CrossRef]
- He, J.; Yi, J.; Ji, L.; Dai, L.; Chen, Y.; Xue, W. ECHDC2 Inhibits the Proliferation of Gastric Cancer Cells by Binding with NEDD4 to Degrade MCCC2 and Reduce Aerobic Glycolysis. Mol. Med. 2024, 30, 69. [Google Scholar] [CrossRef] [PubMed]
- Van de Mark, D.; Kong, D.; Loncarek, J.; Stearns, T. MDM1 Is a Microtubule-Binding Protein That Negatively Regulates Centriole Duplication. Mol. Biol. Cell 2015, 26, 3788–3802. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Gu, J.; Li, Y.; Peng, C.; Shi, M.; Wang, X.; Wei, G.; Ge, O.; Wang, D.; Zhang, B. MiR-17-5p Enhances Pancreatic Cancer Proliferation by Altering Cell Cycle Profiles via Disruption of RBL2/E2F4-Repressing Complexes. Cancer Lett. 2018, 412, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.S.; Uzel, S.G.M.; Akagi, J.; Wlodkowic, D.; Andreeva, V.; Yelick, P.C.; Devitt-Lee, A.; Pare, J.; Levin, M. Bioelectric Signalling via Potassium Channels: A Mechanism for Craniofacial Dysmorphogenesis in KCNJ2-associated Andersen–Tawil Syndrome. J. Physiol. 2016, 594, 3245–3270. [Google Scholar] [CrossRef]
- Hargreaves, M.; Spriet, L.L. Skeletal Muscle Energy Metabolism during Exercise. Nat. Metab. 2020, 2, 817–828. [Google Scholar] [CrossRef]
- Li, N.; Shi, H.; Guo, Q.; Gan, Y.; Zhang, Y.; Jia, J.; Zhang, L.; Zhou, Y. Aerobic Exercise Prevents Chronic Inflammation and Insulin Resistance in Skeletal Muscle of High-Fat Diet Mice. Nutrients 2022, 14, 3730. [Google Scholar] [CrossRef]
- Silva, L.A.; Pinho, C.A.; Scarabelot, K.S.; Fraga, D.B.; Volpato, A.M.J.; Boeck, C.R.; De Souza, C.T.; Streck, E.L.; Pinho, R.A. Physical Exercise Increases Mitochondrial Function and Reduces Oxidative Damage in Skeletal Muscle. Eur. J. Appl. Physiol. 2009, 105, 861–867. [Google Scholar] [CrossRef]
- Yılmaz, D.; Mathavan, N.; Wehrle, E.; Kuhn, G.A.; Müller, R. Mouse Models of Accelerated Aging in Musculoskeletal Research for Assessing Frailty, Sarcopenia, and Osteoporosis—A Review. Ageing Res. Rev. 2024, 93, 102118. [Google Scholar] [CrossRef] [PubMed]
- Brett, J.O.; Arjona, M.; Ikeda, M.; Quarta, M.; de Morrée, A.; Egner, I.M.; Perandini, L.A.; Ishak, H.D.; Goshayeshi, A.; Benjamin, D.I. Exercise Rejuvenates Quiescent Skeletal Muscle Stem Cells in Old Mice through Restoration of Cyclin D1. Nat. Metab. 2020, 2, 307–317. [Google Scholar] [CrossRef]
- Xie, W.; He, M.; Yu, D.; Wu, Y.; Wang, X.; Lv, S.; Xiao, W.; Li, Y. Mouse Models of Sarcopenia: Classification and Evaluation. J. Cachexia. Sarcopenia Muscle 2021, 12, 538–554. [Google Scholar] [CrossRef]
- Li, Z.; Votava, J.A.; Zajac, G.J.M.; Nguyen, J.N.; Leyva Jaimes, F.B.; Ly, S.M.; Brinkman, J.A.; De Giorgi, M.; Kaul, S.; Green, C.L.; et al. Integrating Mouse and Human Genetic Data to Move beyond GWAS and Identify Causal Genes in Cholesterol Metabolism. Cell Metab. 2020, 31, 741–754. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A Fast Spliced Aligner with Low Memory Requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. FeatureCounts: An Efficient General Purpose Program for Assigning Sequence Reads to Genomic Features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. EdgeR: A Bioconductor Package for Differential Expression Analysis of Digital Gene Expression Data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 1–21. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. clusterProfiler: An R Package for Comparing Biological Themes among Gene Clusters. Omi. J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L. ClusterProfiler 4.0: A Universal Enrichment Tool for Interpreting Omics Data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]
- Friedländer, M.R.; Mackowiak, S.D.; Li, N.; Chen, W.; Rajewsky, N. MiRDeep2 Accurately Identifies Known and Hundreds of Novel MicroRNA Genes in Seven Animal Clades. Nucleic Acids Res. 2012, 40, 37–52. [Google Scholar] [CrossRef]
- Hemani, G.; Zheng, J.; Elsworth, B.; Wade, K.H.; Haberland, V.; Baird, D.; Laurin, C.; Burgess, S.; Bowden, J.; Langdon, R. The MR-Base Platform Supports Systematic Causal Inference across the Human Phenome. Elife 2018, 7, e34408. [Google Scholar] [CrossRef]
- Chen, L.; Peters, J.E.; Prins, B.; Persyn, E.; Traylor, M.; Surendran, P.; Karthikeyan, S.; Yonova-Doing, E.; Di Angelantonio, E.; Roberts, D.J. Systematic Mendelian Randomization Using the Human Plasma Proteome to Discover Potential Therapeutic Targets for Stroke. Nat. Commun. 2022, 13, 6143. [Google Scholar] [CrossRef]
- 1000 Genomes Project Consortium. A Map of Human Genome Variation from Population Scale Sequencing. Nature 2010, 467, 1061. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Zhang, S. Investigating the Causal Effects of Exercise-Induced Genes on Sarcopenia. Int. J. Mol. Sci. 2024, 25, 10773. https://doi.org/10.3390/ijms251910773
Wang L, Zhang S. Investigating the Causal Effects of Exercise-Induced Genes on Sarcopenia. International Journal of Molecular Sciences. 2024; 25(19):10773. https://doi.org/10.3390/ijms251910773
Chicago/Turabian StyleWang, Li, and Song Zhang. 2024. "Investigating the Causal Effects of Exercise-Induced Genes on Sarcopenia" International Journal of Molecular Sciences 25, no. 19: 10773. https://doi.org/10.3390/ijms251910773
APA StyleWang, L., & Zhang, S. (2024). Investigating the Causal Effects of Exercise-Induced Genes on Sarcopenia. International Journal of Molecular Sciences, 25(19), 10773. https://doi.org/10.3390/ijms251910773




