Macrophages: Key Players in the Battle against Triple-Negative Breast Cancer
Abstract
:1. Introduction
2. Concept of Breast Cancer
2.1. Pathological and Molecular Characterization of TNBC
2.2. Prevalence, Epidemiology, and Risk Factors
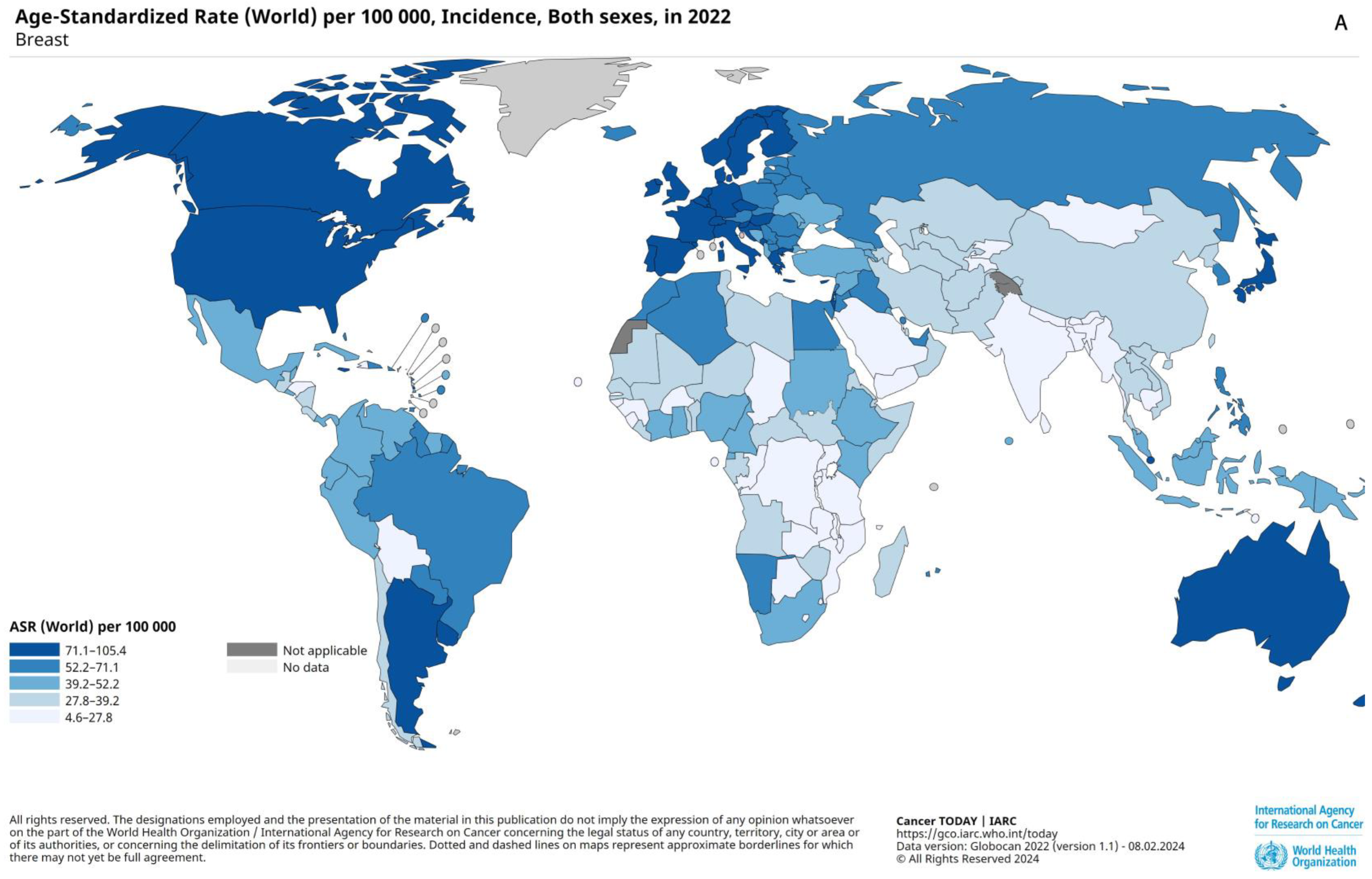
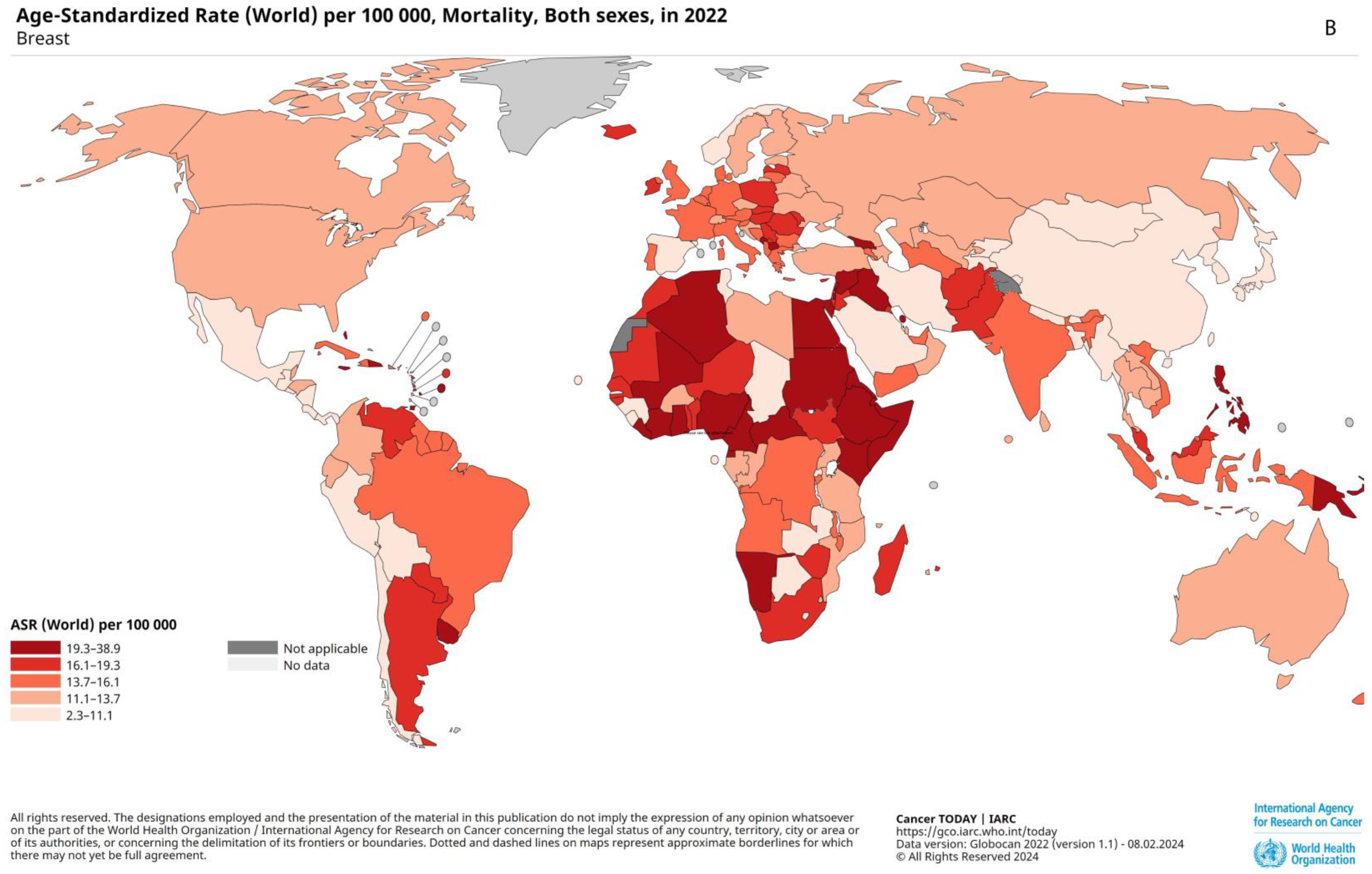
2.3. Prognostic Factors
2.4. Conventional Approaches in Diagnosis and Treatment
2.5. Modern Approach to TNBC Treatment
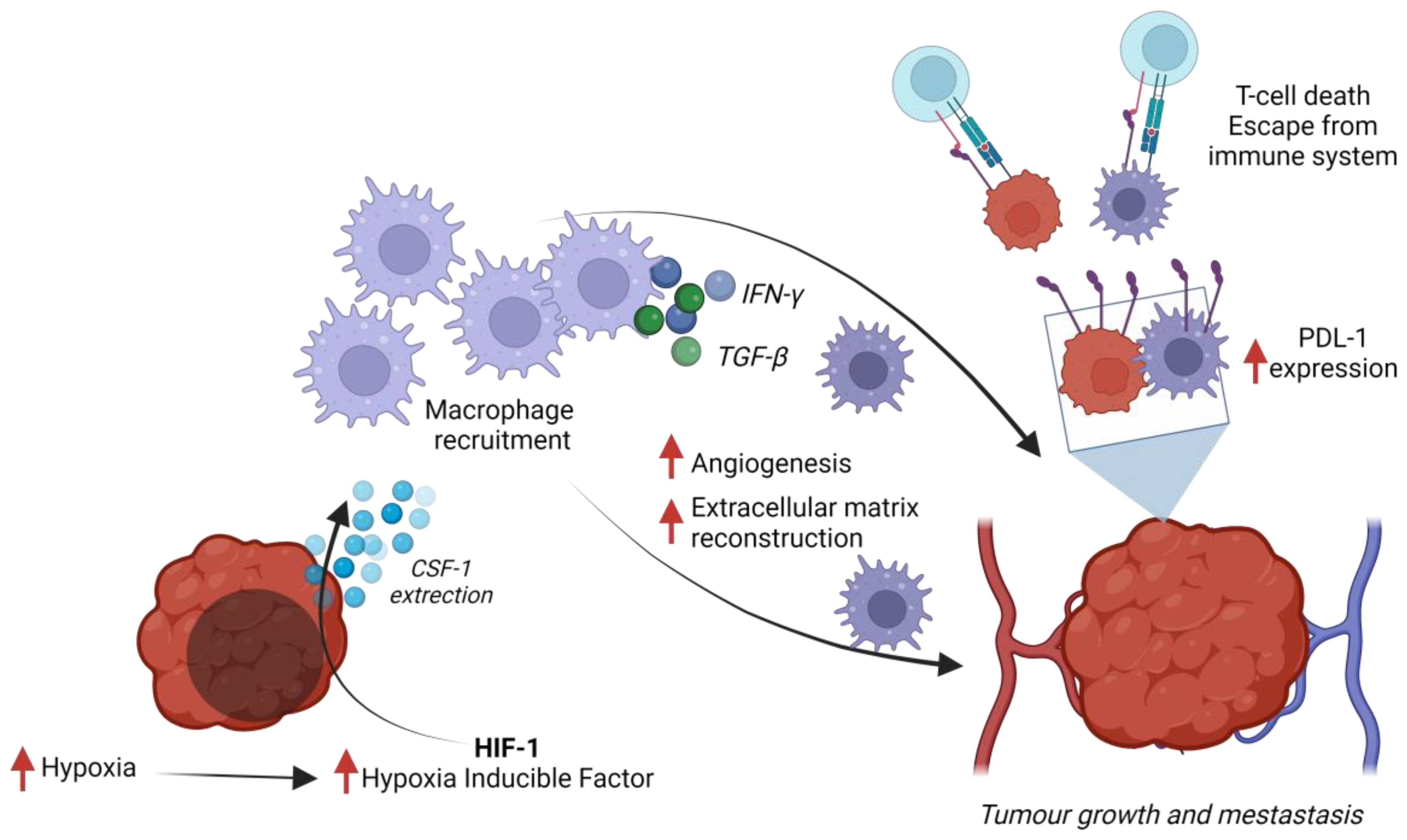
3. Tumor-Associated Macrophages in TNBC
3.1. Crosstalk between Tumor-Associated Macrophages and Triple-Negative Breast Cancer
3.2. Regulation of PD-1 Expression by TAMs
3.3. Targeting Tumor-Associated Macrophages in Triple-Negative Breast Cancer
3.3.1. Inhibition of Macrophage Recruitment to the Tumor
3.3.2. Direct Elimination of TAMs
3.3.3. Reprogramming TAMs into Anti-Tumor Macrophages
3.3.4. Activation of Macrophage Phagocytosis
3.4. Potential Challenges of TAM-Targeted Therapies
3.5. Future Perspectives: Clinical Trials Targeting Immune Checkpoints and Other Strategies in TNBC
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Obidiro, O.; Battogtokh, G.; Akala, E.O. Triple Negative Breast Cancer Treatment Options and Limitations: Future Outlook. Pharmaceutics 2023, 15, 1796. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.K.; Kadel, S.; Townsend, M.G.; Oliwa, M.; Guerriero, J.L. Macrophage Biology and Mechanisms of Immune Suppression in Breast Cancer. Front. Immunol. 2021, 12, 643771. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and Future Burden of Breast Cancer: Global Statistics for 2020 and 2040. Breast Off. J. Eur. Soc. Mastology 2022, 66, 15. [Google Scholar] [CrossRef] [PubMed]
- Ghoncheh, M.; Pournamdar, Z.; Salehiniya, H. Incidence and Mortality and Epidemiology of Breast Cancer in the World. Asian Pac. J. Cancer Prev. 2016, 17, 43–46. [Google Scholar] [CrossRef]
- Anders, C.K.; Carey, L.A. Biology, Metastatic Patterns, and Treatment of Patients with Triple-Negative Breast Cancer. Clin. Breast Cancer 2009, 9, S73. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Anderson, B.O.; Ilbawi, A.M.; Fidarova, E.; Weiderpass, E.; Stevens, L.; Abdel-Wahab, M.; Mikkelsen, B. The Global Breast Cancer Initiative: A Strategic Collaboration to Strengthen Health Care for Non-Communicable Diseases. Lancet Oncol. 2021, 22, 578–581. [Google Scholar] [CrossRef]
- Miao, H.; Verkooijen, H.M.; Chia, K.S.; Bouchardy, C.; Pukkala, E.; Larønningen, S.; Mellemkjær, L.; Czene, K.; Hartman, M. Incidence and Outcome of Male Breast Cancer: An International Population-Based Study. J. Clin. Oncol. 2011, 29, 4381–4386. [Google Scholar] [CrossRef]
- Yu, X.F.; Yang, H.J.; Yu, Y.; Zou, D.H.; Miao, L.L.; Coleman, W.B. A Prognostic Analysis of Male Breast Cancer (MBC) Compared with Post-Menopausal Female Breast Cancer (FBC). PLoS ONE 2015, 10, e0136670. [Google Scholar] [CrossRef]
- Perou, C.M. Molecular Stratification of Triple-Negative Breast Cancers. Oncologist 2011, 16 (Suppl. S1), 61–70. [Google Scholar] [CrossRef]
- Sørlie, T.; Perou, C.M.; Tibshirani, R.; Aas, T.; Geisler, S.; Johnsen, H.; Hastie, T.; Eisen, M.B.; Van De Rijn, M.; Jeffrey, S.S.; et al. Gene Expression Patterns of Breast Carcinomas Distinguish Tumor Subclasses with Clinical Implications. Proc. Natl. Acad. Sci. USA 2001, 98, 10869–10874. [Google Scholar] [CrossRef] [PubMed]
- Rakha, E.A.; Reis-Filho, J.S.; Ellis, I.O. Basal-like Breast Cancer: A Critical Review. J. Clin. Oncol. 2008, 26, 2568–2581. [Google Scholar] [CrossRef] [PubMed]
- Troester, M.A.; Herschkowitz, J.I.; Oh, D.S.; He, X.; Hoadley, K.A.; Barbier, C.S.; Perou, C.M. Gene Expression Patterns Associated with P53 Status in Breast Cancer. BMC Cancer 2006, 6, 276. [Google Scholar] [CrossRef]
- Davis, N.M.; Sokolosky, M.; Stadelman, K.; Abrams, S.L.; Libra, M.; Candido, S.; Nicoletti, F.; Polesel, J.; Maestro, R.; D’Assoro, A.; et al. Deregulation of the EGFR/PI3K/PTEN/Akt/MTORC1 Pathway in Breast Cancer: Possibilities for Therapeutic Intervention. Oncotarget 2014, 5, 4603–4650. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, B.D.; Pietenpol, J.A.; Tan, A.R. Triple-Negative Breast Cancer: Molecular Subtypes and New Targets for Therapy. Am. Soc. Clin. Oncol. Educ. B 2015, 35, e31–e39. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, B.D.; Jovanović, B.; Chen, X.; Estrada, M.V.; Johnson, K.N.; Shyr, Y.; Moses, H.L.; Sanders, M.E.; Pietenpol, J.A. Refinement of Triple-Negative Breast Cancer Molecular Subtypes: Implications for Neoadjuvant Chemotherapy Selection. PLoS ONE 2016, 11, e0157368. [Google Scholar] [CrossRef]
- Santonja, A.; Sánchez-Muñoz, A.; Lluch, A.; Chica-Parrado, M.R.; Albanell, J.; Chacón, J.I.; Antolín, S.; Jerez, J.M.; de la Haba, J.; de Luque, V.; et al. Triple Negative Breast Cancer Subtypes and Pathologic Complete Response Rate to Neoadjuvant Chemotherapy. Oncotarget 2018, 9, 26406. [Google Scholar] [CrossRef]
- Almansour, N.M. Triple-Negative Breast Cancer: A Brief Review About Epidemiology, Risk Factors, Signaling Pathways, Treatment and Role of Artificial Intelligence. Front. Mol. Biosci. 2022, 9, 836417. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer Global Cancer Observatory: Cancer Today. Age-Standardized Incidence Rates, Breast Cancer, Both Sexes, All Ages. Available online: https://gco.iarc.fr/today/en/dataviz/maps-heatmap?mode=population&cancers=20&types=1 (accessed on 19 August 2024).
- Newman, L.A.; Kaljee, L.M. Health Disparities and Triple-Negative Breast Cancer in African American Women: A Review. JAMA Surg. 2017, 152, 485–493. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics of American 2019. CA. Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Xu, K.; Shi, Y.; Wang, X.; Chen, Y.; Tang, L.; Guan, X. A Novel BRCA1 Germline Mutation Promotes Triple-Negative Breast Cancer Cells Progression and Enhances Sensitivity to DNA Damage Agents. Cancer Genet. 2019, 239, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Churpek, J.E.; Walsh, T.; Zheng, Y.; Moton, Z.; Thornton, A.M.; Lee, M.K.; Casadei, S.; Watts, A.; Neistadt, B.; Churpek, M.M.; et al. Inherited Predisposition to Breast Cancer among African American Women. Breast Cancer Res. Treat. 2015, 149, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Couch, F.J.; Hart, S.N.; Sharma, P.; Toland, A.E.; Wang, X.; Miron, P.; Olson, J.E.; Godwin, A.K.; Pankratz, V.S.; Olswold, C.; et al. Inherited Mutations in 17 Breast Cancer Susceptibility Genes among a Large Triple-Negative Breast Cancer Cohort Unselected for Family History of Breast Cancer. J. Clin. Oncol. 2015, 33, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Dietze, E.C.; Chavez, T.A.; Seewaldt, V.L. Obesity and Triple-Negative Breast Cancer: Disparities, Controversies, and Biology. Am. J. Pathol. 2018, 188, 280–290. [Google Scholar] [CrossRef]
- Bandera, E.V.; Chandran, U.; Hong, C.C.; Troester, M.A.; Bethea, T.N.; Adams-Campbell, L.L.; Haiman, C.A.; Park, S.Y.; Olshan, A.F.; Ambrosone, C.B.; et al. Obesity, Body Fat Distribution, and Risk of Breast Cancer Subtypes in African American Women Participating in the AMBER Consortium. Breast Cancer Res. Treat. 2015, 150, 655–666. [Google Scholar] [CrossRef]
- Strober, J.W.; Brady, M.J. Dietary Fructose Consumption and Triple-Negative Breast Cancer Incidence. Front. Endocrinol. 2019, 10, 367. [Google Scholar] [CrossRef]
- Abdel-Maksoud, M.F.; Risendal, B.C.; Slattery, M.L.; Giuliano, A.R.; Baumgartner, K.B.; Byers, T.E. Behavioral Risk Factors and Their Relationship to Tumor Characteristics in Hispanic and Non-Hispanic White Long-Term Breast Cancer Survivors. Breast Cancer Res. Treat. 2012, 131, 169–176. [Google Scholar] [CrossRef]
- Kruk, J.; Czerniak, U. Physical Activity and Its Relation to Cancer Risk: Updating the Evidence. Asian Pac. J. Cancer Prev. 2013, 14, 3993–4003. [Google Scholar] [CrossRef]
- Opdahl, S.; Alsaker, M.D.K.; Janszky, I.; Romundstad, P.R.; Vatten, L.J. Joint Effects of Nulliparity and Other Breast Cancer Risk Factors. Br. J. Cancer 2011, 105, 731–736. [Google Scholar] [CrossRef]
- Russo, J.; Moral, R.; Balogh, G.A.; Mailo, D.; Russo, I.H. The Protective Role of Pregnancy in Breast Cancer. Breast Cancer Res. 2005, 7, 131–142. [Google Scholar] [CrossRef]
- Ma, H.; Ursin, G.; Xu, X.; Lee, E.; Togawa, K.; Duan, L.; Lu, Y.; Malone, K.E.; Marchbanks, P.A.; McDonald, J.A.; et al. Reproductive Factors and the Risk of Triple-Negative Breast Cancer in White Women and African-American Women: A Pooled Analysis. Breast Cancer Res. 2017, 19, 6. [Google Scholar] [CrossRef]
- ElShamy, W.M. The Protective Effect of Longer Duration of Breastfeeding against Pregnancy-Associated Triple Negative Breast Cancer. Oncotarget 2016, 7, 53941–53950. [Google Scholar] [CrossRef] [PubMed]
- Dolle, J.M.; Daling, J.R.; White, E.; Brinton, L.A.; Doody, D.R.; Porter, P.L.; Malone, K.E. Risk Factors for Triple-Negative Breast Cancer in Women under the Age of 45 Years. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1157–1166. [Google Scholar] [CrossRef]
- Beaber, E.F.; Malone, K.E.; Tang, M.T.C.; Barlow, W.E.; Porter, P.L.; Daling, J.R.; Li, C.I. Oral Contraceptives and Breast Cancer Risk Overall and by Molecular Subtype among Young Women. Cancer Epidemiol. Biomark. Prev. 2014, 23, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, V.; Bareket-Samish, A.; Chodick, G.; Siegelmann-Danieli, N. Hormone-Replacement Therapy and Its Association with Breast Cancer Subtypes: A Large Retrospective Cohort Study. Int. J. Womens Health 2021, 13, 1207. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Deming-Halverson, S.L.; Beeghly-Fadiel, A.; Lipworth, L.; Shrubsole, M.J.; Fair, A.M.; Shu, X.O.; Zheng, W. Interactions of Hormone Replacement Therapy, Body Weight, and Bilateral Oophorectomy in Breast Cancer Risk. Clin. Cancer Res. 2014, 20, 1169–1178. [Google Scholar] [CrossRef]
- Phipps, A.I.; Malone, K.E.; Porter, P.L.; Daling, J.R.; Li, C.I. Reproductive and Hormonal Risk Factors for Postmenopausal Luminal, HER-2-Overexpressing, and Triple-Negative Breast Cancer. Cancer 2008, 113, 1521–1526. [Google Scholar] [CrossRef]
- Dietel, M.; Lewis, M.A.; Shapiro, S. Hormone Replacement Therapy: Pathobiological Aspects of Hormone-Sensitive Cancers in Women Relevant to Epidemiological Studies on HRT: A Mini-Review. Hum. Reprod. 2005, 20, 2052–2060. [Google Scholar] [CrossRef]
- da Costa, R.E.A.R.; de Oliveira, F.T.R.; Araújo, A.L.N.; Vieira, S.C. Prognostic Factors in Triple-Negative Breast Cancer: A Retrospective Cohort. Rev. Assoc. Med. Bras. 2021, 67, 950–957. [Google Scholar] [CrossRef]
- Koca, B.; Yildirim, M.; Kuru, B. Prognostic Factors Affecting Disease-Free Survival in Triple-Negative Breast Cancer and Impact of Ki-67. Indian J. Surg. 2022, 84, 708–713. [Google Scholar] [CrossRef]
- Li, Z.; Han, Y.; Wang, J.; Xu, B. Prognostic Factors for Triple-Negative Breast Cancer with Residual Disease after Neoadjuvant Chemotherapy. J. Pers. Med. 2023, 13, 190. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, B.B.; Aktan, M.; Kanyilmaz, G. Prognostic Factors in Patients with Triple Negative Breast Cancer Undergoing Adjuvant Radiotherapy: A 10-Year Single Center Experience: Triple Negative Breast Cancer. Arch. Breast Cancer 2022, 9, 377–385. [Google Scholar] [CrossRef]
- Aebi, S.; Davidson, T.; Gruber, G.; Cardoso, F. Primary Breast Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2011, 22 (Suppl. S6), vi12–vi24. [Google Scholar] [CrossRef] [PubMed]
- Cserni, G.; Chmielik, E.; Cserni, B.; Tot, T. The New TNM-Based Staging of Breast Cancer. Virchows Arch. 2018, 472, 697–703. [Google Scholar] [CrossRef]
- Silber, J.H.; Rosenbaum, P.R.; Clark, A.S.; Giantonio, B.J.; Ross, R.N.; Teng, Y.; Wang, M.; Niknam, B.A.; Ludwig, J.M.; Wang, W.; et al. Characteristics Associated with Differences in Survival among Black and White Women with Breast Cancer. JAMA 2013, 310, 389–397. [Google Scholar] [CrossRef]
- Amirikia, K.C.; Mills, P.; Bush, J.; Newman, L.A. Higher Population-Based Incidence Rates of Triple-Negative Breast Cancer Among Young African American Women: Implications for Breast Cancer Screening Recommendations. Cancer 2011, 117, 2747. [Google Scholar] [CrossRef]
- Byrski, T.; Huzarski, T.; Dent, R.; Marczyk, E.; Jasiowka, M.; Gronwald, J.; Jakubowicz, J.; Cybulski, C.; Wisniowski, R.; Godlewski, D.; et al. Pathologic Complete Response to Neoadjuvant Cisplatin in BRCA1-Positive Breast Cancer Patients. Breast Cancer Res. Treat. 2014, 147, 401–405. [Google Scholar] [CrossRef]
- Carey, L.A.; Dees, E.C.; Sawyer, L.; Gatti, L.; Moore, D.T.; Collichio, F.; Ollila, D.W.; Sartor, C.I.; Graham, M.L.; Perou, C.M. The Triple Negative Paradox: Primary Tumor Chemosensitivity of Breast Cancer Subtypes. Clin. Cancer Res. 2007, 13, 2329–2334. [Google Scholar] [CrossRef]
- Sikov, W.M.; Berry, D.A.; Perou, C.M.; Singh, B.; Cirrincione, C.T.; Tolaney, S.M.; Kuzma, C.S.; Pluard, T.J.; Somlo, G.; Port, E.R.; et al. Impact of the Addition of Carboplatin and/or Bevacizumab to Neoadjuvant Once-per-Week Paclitaxel Followed by Dose-Dense Doxorubicin and Cyclophosphamide on Pathologic Complete Response Rates in Stage II to III Triple-Negative Breast Cancer: CALGB 40603 (Alliance). J. Clin. Oncol. 2015, 33, 13–21. [Google Scholar] [CrossRef]
- Martín, M.; Ruiz, A.; Borrego, M.R.; Barnadas, A.; González, S.; Calvo, L.; Vila, M.M.; Antón, A.; Rodríguez-Lescure, A.; Seguí-Palmer, M.A.; et al. Fluorouracil, Doxorubicin, and Cyclophosphamide (FAC) versus FAC Followed by Weekly Paclitaxel as Adjuvant Therapy for High-Risk, Node-Negative Breast Cancer: Results from the GEICAM/2003-02 Study. J. Clin. Oncol. 2013, 31, 2593–2599. [Google Scholar] [CrossRef]
- Alagizy, H.A.; Shehata, M.A.; Hashem, T.A.; Abdelaziz, K.K.; Swiha, M.M. Metronomic Capecitabine as Extended Adjuvant Chemotherapy in Women with Triple Negative Breast Cancer. Hematol. Oncol. Stem Cell Ther. 2015, 8, 22–27. [Google Scholar] [CrossRef] [PubMed]
- de Boo, L.W.; Jóźwiak, K.; Joensuu, H.; Lindman, H.; Lauttia, S.; Opdam, M.; van Steenis, C.; Brugman, W.; Kluin, R.J.C.; Schouten, P.C.; et al. Adjuvant Capecitabine-Containing Chemotherapy Benefit and Homologous Recombination Deficiency in Early-Stage Triple-Negative Breast Cancer Patients. Br. J. Cancer 2022, 126, 1401–1409. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.K. Ixabepilone: Overview of Effectiveness, Safety, and Tolerability in Metastatic Breast Cancer. Front. Oncol. 2021, 11, 617874. [Google Scholar] [CrossRef] [PubMed]
- Mylavarapu, S.; Das, A.; Roy, M. Role of BRCA Mutations in the Modulation of Response to Platinum Therapy. Front. Oncol. 2018, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Beniey, M.; Hubert, A.; Haque, T.; Cotte, A.K.; Béchir, N.; Zhang, X.; Tran-Thanh, D.; Hassan, S. Sequential Targeting of PARP with Carboplatin Inhibits Primary Tumour Growth and Distant Metastasis in Triple-Negative Breast Cancer. Br. J. Cancer 2023, 128, 1964–1975. [Google Scholar] [CrossRef]
- Kwa, M.J.; Adams, S. Checkpoint Inhibitors in Triple-Negative Breast Cancer (TNBC): Where to Go from Here. Cancer 2018, 124, 2086–2103. [Google Scholar] [CrossRef]
- Howard, F.M.; Pearson, A.T.; Nanda, R. Clinical Trials of Immunotherapy in Triple-Negative Breast Cancer. Breast Cancer Res. Treat. 2022, 195, 1–15. [Google Scholar] [CrossRef]
- Winer, E.P.; Lipatov, O.; Im, S.-A.; Goncalves, A.; Muñoz-Couselo, E.; Lee, K.S.; Schmid, P.; Testa, L.; Witzel, I.; Ohtani, S.; et al. Association of Tumor Mutational Burden (TMB) and Clinical Outcomes with Pembrolizumab (Pembro) versus Chemotherapy (Chemo) in Patients with Metastatic Triple-Negative Breast Cancer (MTNBC) from KEYNOTE-119. J. Clin. Oncol. 2020, 38, 1013. [Google Scholar] [CrossRef]
- Hattori, M.; Honma, N.; Nagai, S.; Narui, K.; Shigechi, T.; Ozaki, Y.; Yoshida, M.; Sakatani, T.; Sasaki, E.; Tanabe, Y.; et al. Trastuzumab Deruxtecan for Human Epidermal Growth Factor Receptor 2-Low Advanced or Metastatic Breast Cancer: Recommendations from the Japanese Breast Cancer Society Clinical Practice Guidelines. Breast Cancer 2024, 31, 335. [Google Scholar] [CrossRef]
- Fenn, K.M.; Kalinsky, K. Sacituzumab Govitecan: Antibody-Drug Conjugate in Triple Negative Breast Cancer and Other Solid Tumors. Drugs Today 2019, 55, 575. [Google Scholar] [CrossRef]
- Pascual, J.; Turner, N.C. Targeting the PI3-Kinase Pathway in Triple-Negative Breast Cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 1051–1060. [Google Scholar] [CrossRef] [PubMed]
- Batalini, F.; Xiong, N.; Tayob, N.; Polak, M.; Eismann, J.; Cantley, L.C.; Shapiro, G.I.; Adalsteinsson, V.; Winer, E.P.; Konstantinopoulos, P.A.; et al. Phase 1b Clinical Trial with Alpelisib plus Olaparib for Patients with Advanced Triple-Negative Breast Cancer. Clin. Cancer Res. 2022, 28, 1493–1499. [Google Scholar] [CrossRef] [PubMed]
- Jemal, M.; Shibabaw, T.; Dejenie, M.T.A. Ketogenic Diets and Their Therapeutic Potential on Breast Cancer: A Systemic Review. Cancer Manag. Res. 2021, 13, 9147. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, M.U.; Clear, K.Y.J.; Cornelius, Z.; Bawaneh, A.; Feliz-Mosquea, Y.R.; Wilson, A.S.; Ruggiero, A.D.; Cruz-Diaz, N.; Shi, L.; Kerr, B.A.; et al. Diet Impacts Triple-Negative Breast Cancer Growth, Metastatic Potential, Chemotherapy Responsiveness, and Doxorubicin-Mediated Cardiac Dysfunction. Physiol. Rep. 2022, 10, e15192. [Google Scholar] [CrossRef] [PubMed]
- Salvadori, G.; Zanardi, F.; Iannelli, F.; Lobefaro, R.; Vernieri, C.; Longo, V.D. Fasting-Mimicking Diet Blocks Triple-Negative Breast Cancer and Cancer Stem Cell Escape. Cell Metab. 2021, 33, 2247–2259.e6. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Jin, Z.; Yuan, Y.; Liu, R.; Xu, T.; Wei, H.; Xu, X.; He, S.; Chen, S.; Shi, Z.; et al. New Mechanisms of Tumor-Associated Macrophages on Promoting Tumor Progression: Recent Research Advances and Potential Targets for Tumor Immunotherapy. J. Immunol. Res. 2016, 2016, 9720912. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, J.; Li, Q.; Cao, B. The Effect of Omega-3 Polyunsaturated Fatty Acid Supplementations on Anti-Tumor Drugs in Triple Negative Breast Cancer. Nutr. Cancer 2021, 73, 196–205. [Google Scholar] [CrossRef]
- Wiggs, A.; Molina, S.; Sumner, S.J.; Rushing, B.R. A Review of Metabolic Targets of Anticancer Nutrients and Nutraceuticals in Pre-Clinical Models of Triple-Negative Breast Cancer. Nutrients 2022, 14, 1990. [Google Scholar] [CrossRef]
- Cassetta, L.; Pollard, J.W. Tumor-Associated Macrophages. Curr. Biol. 2020, 30, R246–R248. [Google Scholar] [CrossRef]
- Italiani, P.; Boraschi, D. From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation. Front. Immunol. 2014, 5, 514. [Google Scholar] [CrossRef]
- Szebeni, G.J.; Vizler, C.; Kitajka, K.; Puskas, L.G. Inflammation and Cancer: Extra- and Intracellular Determinants of Tumor-Associated Macrophages as Tumor Promoters. Mediat. Inflamm. 2017, 2017, 9294018. [Google Scholar] [CrossRef]
- Xue, J.; Schmidt, S.V.; Sander, J.; Draffehn, A.; Krebs, W.; Quester, I.; DeNardo, D.; Gohel, T.D.; Emde, M.; Schmidleithner, L.; et al. Transcriptome-Based Network Analysis Reveals a Spectrum Model of Human Macrophage Activation. Immunity 2014, 40, 274–288. [Google Scholar] [CrossRef] [PubMed]
- Cassetta, L.; Fragkogianni, S.; Sims, A.H.; Swierczak, A.; Forrester, L.M.; Zhang, H.; Soong, D.Y.H.; Cotechini, T.; Anur, P.; Lin, E.Y.; et al. Human Tumor-Associated Macrophage and Monocyte Transcriptional Landscapes Reveal Cancer-Specific Reprogramming, Biomarkers, and Therapeutic Targets. Cancer Cell 2019, 35, 588–602.e10. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Z.; Al-Eryani, G.; Roden, D.L.; Junankar, S.; Harvey, K.; Andersson, A.; Thennavan, A.; Wang, C.; Torpy, J.R.; Bartonicek, N.; et al. A Single-Cell and Spatially Resolved Atlas of Human Breast Cancers. Nat. Genet. 2021, 53, 1334–1347. [Google Scholar] [CrossRef]
- Aras, S.; Raza Zaidi, M. TAMeless Traitors: Macrophages in Cancer Progression and Metastasis. Br. J. Cancer 2017, 117, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Tang, Z.; Gao, S.; Li, C.; Feng, Y.; Zhou, X. Tumor-Associated Macrophages: Recent Insights and Therapies. Front. Oncol. 2020, 10, 188. [Google Scholar] [CrossRef]
- Nahrendorf, M.; Swirski, F.K. Abandoning M1/M2 for a Network Model of Macrophage Function. Circ. Res. 2016, 119, 414–417. [Google Scholar] [CrossRef]
- Azizi, E.; Carr, A.J.; Plitas, G.; Cornish, A.E.; Konopacki, C.; Prabhakaran, S.; Nainys, J.; Wu, K.; Kiseliovas, V.; Setty, M.; et al. Single-Cell Map of Diverse Immune Phenotypes in the Breast Tumor Microenvironment. Cell 2018, 174, 1293–1308.e36. [Google Scholar] [CrossRef]
- Locati, M.; Curtale, G.; Mantovani, A. Diversity, Mechanisms, and Significance of Macrophage Plasticity. Annu. Rev. Pathol. 2020, 15, 123–147. [Google Scholar] [CrossRef]
- Yu, T.; Di, G. Role of Tumor Microenvironment in Triple-Negative Breast Cancer and Its Prognostic Significance. Chin. J. Cancer Res. 2017, 29, 237–252. [Google Scholar] [CrossRef]
- Qiu, S.Q.; Waaijer, S.J.H.; Zwager, M.C.; de Vries, E.G.E.; van der Vegt, B.; Schröder, C.P. Tumor-Associated Macrophages in Breast Cancer: Innocent Bystander or Important Player? Cancer Treat. Rev. 2018, 70, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yang, L.; Yue, D.; Cao, L.; Li, L.; Wang, D.; Ping, Y.; Shen, Z.; Zheng, Y.; Wang, L.; et al. Macrophage-Derived CCL22 Promotes an Immunosuppressive Tumor Microenvironment via IL-8 in Malignant Pleural Effusion. Cancer Lett. 2019, 452, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Ruffell, B.; Chang-Strachan, D.; Chan, V.; Rosenbusch, A.; Ho, C.M.T.; Pryer, N.; Daniel, D.; Hwang, E.S.; Rugo, H.S.; Coussens, L.M. Macrophage IL-10 Blocks CD8+ T Cell-Dependent Responses to Chemotherapy by Suppressing IL-12 Expression in Intratumoral Dendritic Cells. Cancer Cell 2014, 26, 623–637. [Google Scholar] [CrossRef] [PubMed]
- Di Conza, G.; Tsai, C.H.; Gallart-Ayala, H.; Yu, Y.R.; Franco, F.; Zaffalon, L.; Xie, X.; Li, X.; Xiao, Z.; Raines, L.N.; et al. Tumor-Induced Reshuffling of Lipid Composition on the Endoplasmic Reticulum Membrane Sustains Macrophage Survival and pro-Tumorigenic Activity. Nat. Immunol. 2021, 22, 1403–1415. [Google Scholar] [CrossRef]
- Shang, L.; Zhong, Y.; Yao, Y.; Liu, C.; Wang, L.; Zhang, W.; Liu, J.; Wang, X.; Sun, C. Subverted Macrophages in the Triple-Negative Breast Cancer Ecosystem. Biomed. Pharmacother. 2023, 166, 115414. [Google Scholar] [CrossRef]
- Xiao, M.; He, J.; Yin, L.; Chen, X.; Zu, X.; Shen, Y. Tumor-Associated Macrophages: Critical Players in Drug Resistance of Breast Cancer. Front. Immunol. 2021, 12, 799428. [Google Scholar] [CrossRef]
- Wang, C.; Lin, Y.; Zhu, H.; Zhou, Y.; Mao, F.; Huang, X.; Sun, Q.; Li, C. The Prognostic and Clinical Value of Tumor-Associated Macrophages in Patients With Breast Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 2022, 12, 905846. [Google Scholar] [CrossRef]
- Zhang, W.J.; Wang, X.H.; Gao, S.T.; Chen, C.; Xu, X.Y.; Sun, Q.; Zhou, Z.H.; Wu, G.Z.; Yu, Q.; Xu, G.; et al. Tumor-Associated Macrophages Correlate with Phenomenon of Epithelial-Mesenchymal Transition and Contribute to Poor Prognosis in Triple-Negative Breast Cancer Patients. J. Surg. Res. 2018, 222, 93–101. [Google Scholar] [CrossRef]
- Kuroda, H.; Jamiyan, T.; Yamaguchi, R.; Kakumoto, A.; Abe, A.; Harada, O.; Masunaga, A. Tumor Microenvironment in Triple-Negative Breast Cancer: The Correlation of Tumor-Associated Macrophages and Tumor-Infiltrating Lymphocytes. Clin. Transl. Oncol. 2021, 23, 2513–2525. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.Y.; Luo, R.Z.; Peng, R.J.; Wang, S.S.; Xue, C. High Infiltration of Tumor-Associated Macrophages in Triple-Negative Breast Cancer Is Associated with a Higher Risk of Distant Metastasis. Onco. Targets Ther. 2014, 7, 1475–1480. [Google Scholar] [CrossRef]
- Hao, M.; Huang, B.; Wu, R.; Peng, Z.; Luo, K.Q. The Interaction between Macrophages and Triple-Negative Breast Cancer Cells Induces ROS-Mediated Interleukin 1α Expression to Enhance Tumorigenesis and Metastasis. Adv. Sci. 2023, 10, 2302857. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Thakor, P.; Vesey, D.A.; Gobe, G.C.; Morais, C. Conditioned Medium from Stimulated Macrophages Inhibits Growth but Induces an Inflammatory Phenotype in Breast Cancer Cells. Biomed. Pharmacother. 2018, 106, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Pe, K.C.S.; Saetung, R.; Yodsurang, V.; Chaotham, C.; Suppipat, K.; Chanvorachote, P.; Tawinwung, S. Triple-Negative Breast Cancer Influences a Mixed M1/M2 Macrophage Phenotype Associated with Tumor Aggressiveness. PLoS ONE 2022, 17, e0273044. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Yang, R.; Zhang, L.; Xi, Y.; Zhao, J.; Wang, F.; Zhang, H.; Li, Z. Macrophage Colony-Stimulating Factor Mediates the Recruitment of Macrophages in Triple Negative Breast Cancer. Int. J. Biol. Sci. 2019, 15, 2859–2871. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, P.; Gilkes, D.M.; Takano, N.; Semenza, G.L. Hypoxia-Inducible Factor-Dependent Signaling between Triple-Negative Breast Cancer Cells and Mesenchymal Stem Cells Promotes Macrophage Recruitment. Proc. Natl. Acad. Sci. USA 2014, 111, E2120–E2129. [Google Scholar] [CrossRef]
- Jiang, M.; Li, X.; Zhang, J.; Lu, Y.; Shi, Y.; Zhu, C.; Liu, Y.; Qin, B.; Luo, Z.; Du, Y.; et al. Dual Inhibition of Endoplasmic Reticulum Stress and Oxidation Stress Manipulates the Polarization of Macrophages under Hypoxia to Sensitize Immunotherapy. ACS Nano 2021, 15, 14522–14534. [Google Scholar] [CrossRef]
- Sami, E.; Paul, B.T.; Koziol, J.A.; El Shamy, W.M. The Immunosuppressive Microenvironment in BRCA1-IRIS-Overexpressing TNBC Tumors Is Induced by Bidirectional Interaction with Tumor-Associated Macrophages. Cancer Res. 2020, 80, 1102–1117. [Google Scholar] [CrossRef]
- Fu, L.Q.; Du, W.L.; Cai, M.H.; Yao, J.Y.; Zhao, Y.Y.; Mou, X.Z. The Roles of Tumor-Associated Macrophages in Tumor Angiogenesis and Metastasis. Cell. Immunol. 2020, 353, 104119. [Google Scholar] [CrossRef]
- Evans, R.; Flores-Borja, F.; Nassiri, S.; Miranda, E.; Lawler, K.; Grigoriadis, A.; Monypenny, J.; Gillet, C.; Owen, J.; Gordon, P.; et al. Integrin-Mediated Macrophage Adhesion Promotes Lymphovascular Dissemination in Breast Cancer. Cell Rep. 2019, 27, 1967–1978.e4. [Google Scholar] [CrossRef]
- Matejuk, A.; Dwyer, J.; Hopke, C.; Vandenbark, A.A.; Offner, H. Opposing Roles for TGF-Β1 and TGFβ3 Isoforms in Experimental Autoimmune Encephalomyelitis. Cytokine 2004, 25, 45–51. [Google Scholar] [CrossRef]
- Santoni, M.; Romagnoli, E.; Saladino, T.; Foghini, L.; Guarino, S.; Capponi, M.; Giannini, M.; Cognigni, P.D.; Ferrara, G.; Battelli, N. Triple Negative Breast Cancer: Key Role of Tumor-Associated Macrophages in Regulating the Activity of Anti-PD-1/PD-L1 Agents. Biochim. Biophys. Rev. Cancer 2018, 1869, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Bräutigam, K.; Kabore-Wolff, E.; Hussain, A.F.; Polack, S.; Rody, A.; Hanker, L.; Köster, F. Inhibitors of PD-1/PD-L1 and ERK1/2 Impede the Proliferation of Receptor Positive and Triple-Negative Breast Cancer Cell Lines. J. Cancer Res. Clin. Oncol. 2021, 147, 2923–2933. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zeng, Y.; Qu, Q.; Zhu, J.; Liu, Z.; Ning, W.; Zeng, H.; Zhang, N.; Du, W.; Chen, C.; et al. PD-L1 Induced by IFN-γ from Tumor-Associated Macrophages via the JAK/STAT3 and PI3K/AKT Signaling Pathways Promoted Progression of Lung Cancer. Int. J. Clin. Oncol. 2017, 22, 1026–1033. [Google Scholar] [CrossRef]
- Ramos Solis, N.; Cannon, A.; Dilday, T.; Abt, M.; Oblak, A.L.; Soloff, A.C.; Kaplan, M.H.; Yeh, E.S. HUNK as a Key Regulator of Tumor-Associated Macrophages in Triple Negative Breast Cancer. Oncoimmunology 2024, 13, 2364382. [Google Scholar] [CrossRef]
- Sonnessa, M.; Cioffi, A.; Brunetti, O.; Silvestris, N.; Zito, F.A.; Saponaro, C.; Mangia, A. NLRP3 Inflammasome From Bench to Bedside: New Perspectives for Triple Negative Breast Cancer. Front. Oncol. 2020, 10, 1587. [Google Scholar] [CrossRef]
- Liu, L.; Li, H.; Wang, J.; Zhang, J.; Liang, X.J.; Guo, W.; Gu, Z. Leveraging Macrophages for Cancer Theranostics. Adv. Drug Deliv. Rev. 2022, 183, 114136. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, S.; Wang, X.; Zheng, Y.; Yang, B.; Zhang, J.; Pan, B.; Gao, J.; Wang, Z. Research Trends in Pharmacological Modulation of Tumor-Associated Macrophages. Clin. Transl. Med. 2021, 11, e288. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, L.; Liu, J.; Dang, P.; Hu, S.; Yuan, W.; Sun, Z.; Liu, Y.; Wang, C. Roles of Tumor-Associated Macrophages in Anti-PD-1/PD-L1 Immunotherapy for Solid Cancers. Mol. Cancer 2023, 22, 58. [Google Scholar] [CrossRef]
- Cho, H.Y.; Choi, E.K.; Lee, S.W.; Jung, K.O.; Seo, S.K.; Choi, I.W.; Park, S.G.; Choi, I.; Lee, S.W. Programmed Death-1 Receptor Negatively Regulates LPS-Mediated IL-12 Production and Differentiation of Murine Macrophage RAW264.7 Cells. Immunol. Lett. 2009, 127, 39–47. [Google Scholar] [CrossRef]
- Roy, S.; Gupta, P.; Palit, S.; Basu, M.; Ukil, A.; Das, P.K. The Role of PD-1 in Regulation of Macrophage Apoptosis and Its Subversion by Leishmania Donovani. Clin. Transl. Immunol. 2017, 6, e137. [Google Scholar] [CrossRef]
- Allison, E.; Edirimanne, S.; Matthews, J.; Fuller, S.J. Breast Cancer Survival Outcomes and Tumor-Associated Macrophage Markers: A Systematic Review and Meta-Analysis. Oncol. Ther. 2023, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Zhao, T.; Luo, R.; Qiu, R.; Li, Z. Tumor-Associated Macrophages: Key Players in Triple-Negative Breast Cancer. Front. Oncol. 2022, 12, 772615. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.Z.; Li, J.; Zhang, H.; Kitamura, T.; Zhang, J.; Campion, L.R.; Kaiser, E.A.; Snyder, L.A.; Pollard, J.W. CCL2 Recruits Inflammatory Monocytes to Facilitate Breast-Tumour Metastasis. Nature 2011, 475, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Kaplanov, I.; Carmi, Y.; Kornetsky, R.; Shemesh, A.; Shurin, G.V.; Shurin, M.R.; Dinarello, C.A.; Voronov, E.; Apte, R.N. Blocking IL-1β Reverses the Immunosuppression in Mouse Breast Cancer and Synergizes with Anti-PD-1 for Tumor Abrogation. Proc. Natl. Acad. Sci. USA 2019, 116, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.B.; Yao, M.; Brummer, G.; Acevedo, D.; Alhakamy, N.; Berkland, C.; Cheng, N. Targeted Gene Silencing of CCL2 Inhibits Triple Negative Breast Cancer Progression by Blocking Cancer Stem Cell Renewal and M2 Macrophage Recruitment. Oncotarget 2016, 7, 49349–49367. [Google Scholar] [CrossRef]
- Brana, I.; Calles, A.; LoRusso, P.M.; Yee, L.K.; Puchalski, T.A.; Seetharam, S.; Zhong, B.; de Boer, C.J.; Tabernero, J.; Calvo, E. Carlumab, an Anti-C-C Chemokine Ligand 2 Monoclonal Antibody, in Combination with Four Chemotherapy Regimens for the Treatment of Patients with Solid Tumors: An Open-Label, Multicenter Phase 1b Study. Target. Oncol. 2015, 10, 111–123. [Google Scholar] [CrossRef]
- Steiner, J.L.; Davis, J.M.; McClellan, J.L.; Guglielmotti, A.; Murphy, E.A. Effects of the MCP-1 Synthesis Inhibitor Bindarit on Tumorigenesis and Inflammatory Markers in the C3(1)/SV40Tag Mouse Model of Breast Cancer. Cytokine 2014, 66, 60–68. [Google Scholar] [CrossRef]
- Zollo, M.; Di Dato, V.; Spano, D.; De Martino, D.; Liguori, L.; Marino, N.; Vastolo, V.; Navas, L.; Garrone, B.; Mangano, G.; et al. Targeting Monocyte Chemotactic Protein-1 Synthesis with Bindarit Induces Tumor Regression in Prostate and Breast Cancer Animal Models. Clin. Exp. Metastasis 2012, 29, 585–601. [Google Scholar] [CrossRef]
- Cioli, V.; Ciarniello, M.G.; Guglielmotti, A.; Luparini, M.R.; Durando, L.; Martinelli, B.; Catanese, B.; Fava, L.; Silverstrini, B. A New Protein Antidenaturant Agent, Bindarit, Reduces Secondary Phase of Adjuvant Arthritis in Rats. J. Rheumatol. 1992, 19, 1735–1742. [Google Scholar]
- D’Incalci, M.; Badri, N.; Galmarini, C.M.; Allavena, P. Trabectedin, a Drug Acting on Both Cancer Cells and the Tumour Microenvironment. Br. J. Cancer 2014, 111, 646. [Google Scholar] [CrossRef]
- Blum, J.L.; Gonçalves, A.; Efrat, N.; Debled, M.; Conte, P.; Richards, P.D.; Richards, D.; Lardelli, P.; Nieto, A.; Cullell-Young, M.; et al. A Phase II Trial of Trabectedin in Triple-Negative and HER2-Overexpressing Metastatic Breast Cancer. Breast Cancer Res. Treat. 2016, 155, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Riaz, N.; Burugu, S.; Cheng, A.S.; Leung, S.C.Y.; Gao, D.; Nielsen, T.O. Prognostic Significance of Csf-1r Expression in Early Invasive Breast Cancer. Cancers 2021, 13, 5769. [Google Scholar] [CrossRef] [PubMed]
- Calvo, A.; Joensuu, H.; Sebastian, M.; Naing, A.; Bang, Y.-J.; Martin, M.; Roda, D.; Hodi, F.S.; Veloso, A.; Mataraza, J.; et al. Phase Ib/II Study of Lacnotuzumab (MCS110) Combined with Spartalizumab (PDR001) in Patients (Pts) with Advanced Tumors. J. Clin. Oncol. 2018, 36, 3014. [Google Scholar] [CrossRef]
- Kuemmel, S.; Campone, M.; Loirat, D.; Lopez, R.L.; Thaddeus Beck, J.; de Laurentiis, M.; Im, S.A.; Kim, S.B.; Kwong, A.; Steger, G.G.; et al. A Randomized Phase II Study of Anti-CSF1 Monoclonal Antibody Lacnotuzumab (MCS110) Combined with Gemcitabine and Carboplatin in Advanced Triple-Negative Breast Cancer. Clin. Cancer Res. 2022, 28, 106–115. [Google Scholar] [CrossRef]
- Cannarile, M.A.; Weisser, M.; Jacob, W.; Jegg, A.M.; Ries, C.H.; Rüttinger, D. Colony-Stimulating Factor 1 Receptor (CSF1R) Inhibitors in Cancer Therapy. J. Immunother. Cancer 2017, 5, 53. [Google Scholar] [CrossRef]
- Dammeijer, F.; Lievense, L.A.; Kaijen-Lambers, M.E.; Van Nimwegen, M.; Bezemer, K.; Hegmans, J.P.; Van Hall, T.; Hendriks, R.W.; Aerts, J.G. Depletion of Tumor-Associated Macrophages with a CSF-1R Kinase Inhibitor Enhances Antitumor Immunity and Survival Induced by DC Immunotherapy. Cancer Immunol. Res. 2017, 5, 535–546. [Google Scholar] [CrossRef]
- Hartman, Z.C.; Poage, G.M.; Den Hollander, P.; Tsimelzon, A.; Hill, J.; Panupinthu, N.; Zhang, Y.; Mazumdar, A.; Hilsenbeck, S.G.; Mills, G.B.; et al. Growth of Triple-Negative Breast Cancer Cells Relies upon Coordinate Autocrine Expression of the Proinflammatory Cytokines IL-6 and IL-8. Cancer Res. 2013, 73, 3470–3480. [Google Scholar] [CrossRef]
- Heo, T.H.; Wahler, J.; Suh, N. Potential Therapeutic Implications of IL-6/IL-6R/Gp130-Targeting Agents in Breast Cancer. Oncotarget 2016, 7, 15460–15473. [Google Scholar] [CrossRef]
- Coward, J.; Kulbe, H.; Chakravarty, P.; Leader, D.; Vassileva, V.; Leinster, D.A.; Thompson, R.; Schioppa, T.; Nemeth, J.; Vermeulen, J.; et al. Interleukin-6 as a Therapeutic Target in Human Ovarian Cancer. Clin. Cancer Res. 2011, 17, 6083–6096. [Google Scholar] [CrossRef]
- Van Rooijen, N.; Sanders, A.; Van Den Berg, T.K. Apoptosis of Macrophages Induced by Liposome-Mediated Intracellular Delivery of Clodronate and Propamidine. J. Immunol. Methods 1996, 193, 93–99. [Google Scholar] [CrossRef]
- Hirano, R.; Okamoto, K.; Shinke, M.; Sato, M.; Watanabe, S.; Watanabe, H.; Kondoh, G.; Kadonosono, T.; Kizaka-Kondoh, S. Tissue-Resident Macrophages Are Major Tumor-Associated Macrophage Resources, Contributing to Early TNBC Development, Recurrence, and Metastases. Commun. Biol. 2023, 6, 144. [Google Scholar] [CrossRef] [PubMed]
- Paterson, A.H.G.; Anderson, S.J.; Lembersky, B.C.; Fehrenbacher, L.; Falkson, C.I.; King, K.M.; Weir, L.M.; Brufsky, A.M.; Dakhil, S.; Lad, T.; et al. Oral Clodronate for Adjuvant Treatment of Operable Breast Cancer (National Surgical Adjuvant Breast and Bowel Project Protocol B-34): A Multicentre, Placebo-Controlled, Randomised Trial. Lancet. Oncol. 2012, 13, 734. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Paterson, S.; Kanis, J.A.; McCloskey, E.; Ashley, S.; Tidy, A.; Rosenqvist, K.; Smith, I.; Ottestad, L.; Legault, S.; et al. Randomized, Placebo-Controlled Trial of Clodronate in Patients With Primary Operable Breast Cancer. J. Clin. Oncol. 2016, 20, 3219–3224. [Google Scholar] [CrossRef]
- Cai, X.J.; Wang, Z.; Cao, J.W.; Ni, J.J.; Xu, Y.Y.; Yao, J.; Xu, H.; Liu, F.; Yang, G.Y. Anti-Angiogenic and Anti-Tumor Effects of Metronomic Use of Novel Liposomal Zoledronic Acid Depletes Tumor-Associated Macrophages in Triple Negative Breast Cancer. Oncotarget 2017, 8, 84248–84257. [Google Scholar] [CrossRef]
- Gnant, M. Zoledronic Acid in Breast Cancer: Latest Findings and Interpretations. Ther. Adv. Med. Oncol. 2011, 3, 293. [Google Scholar] [CrossRef]
- Green, J.R.; Guenther, A. The Backbone of Progress--Preclinical Studies and Innovations with Zoledronic Acid. Crit. Rev. Oncol. Hematol. 2011, 77 (Suppl. S1), S3–S12. [Google Scholar] [CrossRef]
- Giraudo, E.; Inoue, M.; Hanahan, D. An Amino-Bisphosphonate Targets MMP-9-Expressing Macrophages and Angiogenesis to Impair Cervical Carcinogenesis. J. Clin. Investig. 2004, 114, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Moradi, S.Z.; Yarmohammadi, A.; Narimani, F.; Wallace, C.E.; Bishayee, A. Modulation of TLR/NF-ΚB/NLRP Signaling by Bioactive Phytocompounds: A Promising Strategy to Augment Cancer Chemotherapy and Immunotherapy. Front. Oncol. 2022, 12, 834072. [Google Scholar] [CrossRef]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-ΚB in Biology and Targeted Therapy: New Insights and Translational Implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef]
- Nguyen, B.L.; Phung, C.D.; Pham, D.V.; Le, N.D.; Jeong, J.H.; Kim, J.; Kim, J.H.; Chang, J.H.; Jin, S.G.; Choi, H.G.; et al. Liposomal Co-Delivery of Toll-like Receptors 3 and 7 Agonists Induce a Hot Triple-Negative Breast Cancer Immune Environment. J. Control. Release 2023, 361, 443–454. [Google Scholar] [CrossRef]
- Reddy, S.M.; Carter, M.; Chan, I.; Hullings, M.; Unni, N.; Medina, J.; Shakeel, S.; Armstrong, S.; Cade, L.; Fattah, F.J.; et al. Phase 1 Pilot Study with Dose Expansion of Chemotherapy in Combination with CD40 Agonist and Flt3 Ligand in Metastatic Triple-Negative Breast Cancer. J. Clin. Oncol. 2022, 40, TPS1126. [Google Scholar] [CrossRef]
- Olsson, A.; Nakhlé, J.; Sundstedt, A.; Plas, P.; Bauchet, A.L.; Pierron, V.; Bruetschy, L.; Deronic, A.; Törngren, M.; Liberg, D.; et al. Tasquinimod Triggers an Early Change in the Polarization of Tumor Associated Macrophages in the Tumor Microenvironment. J. Immunother. Cancer 2015, 3, 53. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shu, X.; Xu, J.; Su, S.M.; Chan, U.I.; Mo, L.; Liu, J.; Zhang, X.; Adhav, R.; Chen, Q.; et al. S100A9-CXCL12 Activation in BRCA1-Mutant Breast Cancer Promotes an Immunosuppressive Microenvironment Associated with Resistance to Immunotherapy. Nat. Commun. 2022, 13, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Vergadi, E.; Ieronymaki, E.; Lyroni, K.; Vaporidi, K.; Tsatsanis, C. Akt Signaling Pathway in Macrophage Activation and M1/M2 Polarization. J. Immunol. 2017, 198, 1006–1014. [Google Scholar] [CrossRef]
- Savas, P.; Lo, L.L.; Luen, S.J.; Blackley, E.F.; Callahan, J.; Moodie, K.; van Geelen, C.T.; Ko, Y.A.; Weng, C.F.; Wein, L.; et al. Alpelisib Monotherapy for PI3K-Altered, Pretreated Advanced Breast Cancer: A Phase II Study. Cancer Discov. 2022, 12, 2058–2073. [Google Scholar] [CrossRef]
- Long, Y.; Yu, X.; Chen, R.; Tong, Y.; Gong, L. Noncanonical PD-1/PD-L1 Axis in Relation to the Efficacy of Anti-PD Therapy. Front. Immunol. 2022, 13, 910704. [Google Scholar] [CrossRef]
- Meng, Z.; Zhang, R.; Wu, X.; Zhang, M.; Jin, T. PD-L1 Mediates Triple-Negative Breast Cancer Evolution via the Regulation of TAM/M2 Polarization. Int. J. Oncol. 2022, 61, 150. [Google Scholar] [CrossRef]
- Ali, M.A.; Aiman, W.; Shah, S.S.; Hussain, M.; Kashyap, R. Efficacy and Safety of Pembrolizumab Based Therapies in Triple-Negative Breast Cancer: A Systematic Review of Clinical Trials. Crit. Rev. Oncol. Hematol. 2021, 157, 103197. [Google Scholar] [CrossRef]
- Loibl, S.; Untch, M.; Burchardi, N.; Huober, J.; Sinn, B.V.; Blohmer, J.U.; Grischke, E.M.; Furlanetto, J.; Tesch, H.; Hanusch, C.; et al. A Randomised Phase II Study Investigating Durvalumab in Addition to an Anthracycline Taxane-Based Neoadjuvant Therapy in Early Triple-Negative Breast Cancer: Clinical Results and Biomarker Analysis of GeparNuevo Study. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 1279–1288. [Google Scholar] [CrossRef]
- Schmid, P.; Salgado, R.; Park, Y.H.; Muñoz-Couselo, E.; Kim, S.B.; Sohn, J.; Im, S.A.; Foukakis, T.; Kuemmel, S.; Dent, R.; et al. Pembrolizumab plus Chemotherapy as Neoadjuvant Treatment of High-Risk, Early-Stage Triple-Negative Breast Cancer: Results from the Phase 1b Open-Label, Multicohort KEYNOTE-173 Study. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2020, 31, 569–581. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Zhang, H.; Barrios, C.H.; Saji, S.; Jung, K.H.; Hegg, R.; Koehler, A.; Sohn, J.; Iwata, H.; Telli, M.L.; et al. Neoadjuvant Atezolizumab in Combination with Sequential Nab-Paclitaxel and Anthracycline-Based Chemotherapy versus Placebo and Chemotherapy in Patients with Early-Stage Triple-Negative Breast Cancer (IMpassion031): A Randomised, Double-Blind, Phase 3 Trial. Lancet 2020, 396, 1090–1100. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Cortes, J.; Dent, R.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; et al. Event-Free Survival with Pembrolizumab in Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2022, 386, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Cortés, J.; Dent, R.A.; Pusztai, L.; McArthur, H.L.; Kummel, S.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. LBA18 Pembrolizumab or Placebo plus Chemotherapy Followed by Pembrolizumab or Placebo for Early-Stage TNBC: Updated EFS Results from the Phase III KEYNOTE-522 Study. Ann. Oncol. 2023, 34, S1257. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Chen, Z.; Luo, J.; Guo, W.; Sun, L.; Lin, L. Targeting M2-like Tumor-Associated Macrophages Is a Potential Therapeutic Approach to Overcome Antitumor Drug Resistance. NPJ Precis. Oncol. 2024, 8, 31. [Google Scholar] [CrossRef]
- Wahdan-Alaswad, R.; Fan, Z.; Edgerton, S.M.; Liu, B.; Deng, X.S.; Arnadottir, S.S.; Richer, J.K.; Anderson, S.M.; Thor, A.D. Glucose Promotes Breast Cancer Aggression and Reduces Metformin Efficacy. Cell Cycle 2013, 12, 3759–3769. [Google Scholar] [CrossRef] [PubMed]
- Varghese, S.; Samuel, S.M.; Varghese, E.; Kubatka, P.; Büsselberg, D. High Glucose Represses the Anti-Proliferative and Pro-Apoptotic Effect of Metformin in Triple Negative Breast Cancer Cells. Biomolecules 2019, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Jiang, W.; Thompson, M.; McGinley, J.; Thompson, H. Metformin as an Energy Restriction Mimetic Agent for Breast Cancer Prevention. J. Carcinog. 2011, 10, 17. [Google Scholar] [CrossRef]
- Zimel, M.N.; Horowitz, C.B.; Rajasekhar, V.K.; Christ, A.B.; Wei, X.; Wu, J.; Wojnarowicz, P.M.; Wang, D.; Goldring, S.R.; Purdue, P.E.; et al. HPMA-Copolymer Nanocarrier Targets Tumor-Associated Macrophages in Primary and Metastatic Breast Cancer. Mol. Cancer Ther. 2017, 16, 2701. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, C.; Santa-Maria, C.A.; Emens, L.A.; Popel, A.S. Dynamics of Tumor-Associated Macrophages in a Quantitative Systems Pharmacology Model of Immunotherapy in Triple-Negative Breast Cancer. iScience 2022, 25, 104702. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Yang, Y.; Weng, L.; Wu, Q.; Zhang, J.; Zhao, P.; Fang, L.; Shi, Y.; Wang, P. Emerging Phagocytosis Checkpoints in Cancer Immunotherapy. Signal Transduct. Target. Ther. 2023, 8, 104. [Google Scholar] [CrossRef]
- Chen, C.; Wang, R.; Chen, X.; Hou, Y.; Jiang, J. Targeting CD47 as a Novel Immunotherapy for Breast Cancer. Front. Oncol. 2022, 12, 924740. [Google Scholar] [CrossRef] [PubMed]
- Kielbassa, K.; Vegna, S.; Ramirez, C.; Akkari, L. Understanding the Origin and Diversity of Macrophages to Tailor Their Targeting in Solid Cancers. Front. Immunol. 2019, 10, 488249. [Google Scholar] [CrossRef] [PubMed]
- Kloosterman, D.J.; Akkari, L. Macrophages at the Interface of the Co-Evolving Cancer Ecosystem. Cell 2023, 186, 1627–1651. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Yrigoyen, M.; Cassetta, L.; Pollard, J.W. Macrophage Targeting in Cancer. Ann. N. Y. Acad. Sci. 2021, 1499, 18–41. [Google Scholar] [CrossRef] [PubMed]
- Bonapace, L.; Coissieux, M.M.; Wyckoff, J.; Mertz, K.D.; Varga, Z.; Junt, T.; Bentires-Alj, M. Cessation of CCL2 Inhibition Accelerates Breast Cancer Metastasis by Promoting Angiogenesis. Nature 2014, 515, 130–133. [Google Scholar] [CrossRef]
- Varol, C.; Mildner, A.; Jung, S. Macrophages: Development and Tissue Specialization. Annu. Rev. Immunol. 2015, 33, 643–675. [Google Scholar] [CrossRef]
- Quail, D.F.; Joyce, J.A. Microenvironmental Regulation of Tumor Progression and Metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef]
- Kumar, V.; Donthireddy, L.; Marvel, D.; Condamine, T.; Wang, F.; Lavilla-Alonso, S.; Hashimoto, A.; Vonteddu, P.; Behera, R.; Goins, M.A.; et al. Cancer-Associated Fibroblasts Neutralize the Anti-Tumor Effect of CSF1 Receptor Blockade by Inducing PMN-MDSC Infiltration of Tumors. Cancer Cell 2017, 32, 654–668.e5. [Google Scholar] [CrossRef]
- Liu, J.; Geng, X.; Hou, J.; Wu, G. New Insights into M1/M2 Macrophages: Key Modulators in Cancer Progression. Cancer Cell Int. 2021, 21, 389. [Google Scholar] [CrossRef]
- Yates, L.R.; Seoane, J.; Le Tourneau, C.; Siu, L.L.; Marais, R.; Michiels, S.; Soria, J.C.; Campbell, P.; Normanno, N.; Scarpa, A.; et al. The European Society for Medical Oncology (ESMO) Precision Medicine Glossary. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2018, 29, 30–35. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Pietenpol, J.A. Identification and Use of Biomarkers in Treatment Strategies for Triple-Negative Breast Cancer Subtypes. J. Pathol. 2014, 232, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yu, D.; Kwon, Y.; Lee, K.S.; Sim, S.H.; Kong, S.Y.; Lee, E.S.; Park, I.H.; Park, C. Genomic Characteristics of Triple-Negative Breast Cancer Nominate Molecular Subtypes That Predict Chemotherapy Response. Mol. Cancer Res. 2020, 18, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Syrnioti, A.; Petousis, S.; Newman, L.A.; Margioula-Siarkou, C.; Papamitsou, T.; Dinas, K.; Koletsa, T. Triple Negative Breast Cancer: Molecular Subtype-Specific Immune Landscapes with Therapeutic Implications. Cancers 2024, 16, 2094. [Google Scholar] [CrossRef] [PubMed]
| Drugs for Inhibition of Macrophage Recruitment to the Tumor | ||||
|---|---|---|---|---|
| Drug | Mechanism of Action | Other Drugs/Interventions Used in Trial | ClinicalTrials.gov ID | Phase |
| Trabectedin | Alkylating agent suppressing monocyte recruitment | Dexamethasone | NCT00580112 | Phase II; completed |
| Dexamethasone | NCT00050427 | Phase II; completed | ||
| Lacnotuzumab (MSC110) | Monoclonal antibody against CSF-1 | PDR001 | NCT02807844 | Phase Ib/II; completed |
| Carboplatin, gemcitabine | NCT02435680 | Phase II; completed | ||
| PLX3397 | CSF-1/CSF-1R inhibitor | Eribulin | NCT01596751 | Phase Ib/II; completed |
| Multiple drugs | NCT01042379 | Phase II; recruiting | ||
| LY3022855 | CSF-1/CSF-1R inhibitor | - | NCT02265536 | Phase I; completed |
| Cabiralizumab | CSF-1/CSF-1R inhibitor | Nivolumab, carboplatin, paclitaxel | NCT04331067 | Phase Ib/II; active, not recruiting |
| Drugs for direct elimination of TAMs | ||||
| Drug | Mechanism of action | Other drugs/interventions used in trial | ClinicalTrials.gov ID | Phase |
| Clodronate | Bisphosphonate selectively killing macrophages. | Placebo | NCT00009945 | Phase III; completed |
| Ibandronate, zoledronic acid | NCT00127205 | Phase III; completed | ||
| Zoledronic acid | Bisphosphonate depleting TAMs and inhibiting angiogenesis | Atorvastatin, standard neoadjuvant chemotherapy | NCT03358017 | Phase II; completed |
| - | NCT04045522 | Unknown status | ||
| - | NCT02595138 | Phase III; unknown status | ||
| Drugs for reprogramming TAMs into anti-tumor macrophages | ||||
| Drug | Mechanism of action | Other drugs/interventions used in trial | ClinicalTrials.gov ID | Phase |
| Alpelisib | PI3Kα-specific inhibitor promoting M1-like phenotype | Nab-paclitaxel | NCT04251533 | Phase III; active, not recruiting |
| Nab-paclitaxel, iNOS inhibitor | NCT05660083 | Phase II; recruiting | ||
| Enzalutamide | NCT03207529 | Phase I; completed | ||
| Pembrolizumab | PD-1 inhibitor used in combination therapy | Lenvatinib | NCT04427293 | Phase I; recruiting |
| Intraoperative radiation therapy | NCT02977468 | Phase I; recruiting | ||
| Olinvacimab | NCT04986852 | Phase II; recruiting | ||
| Capivasertib (AZD5153) | BRD4 inhibitor promoting M1 polarization | Fulvestrant | NCT01226316 | Phase I; active, not recruiting |
| Paclitaxel | NCT02423603 | Phase II; active, not recruiting | ||
| Enzalutamide, Fulvestrant | NCT03310541 | Phase I; completed | ||
| Metformin | Agent used to inhibit M2 polarization and decrease tumor size | Night fasting | NCT05023967 | Phase IIb; recruiting |
| Doxycycline | NCT02874430 | Phase II; active, not recruiting | ||
| Calorie restriction | NCT04248998 | Phase II; active, not recruiting | ||
| Drugs for activation of macrophage phagocytosis | ||||
| Drug | Mechanism of action | Other drugs/interventions used in trial | ClinicalTrials.gov ID | Phase and stage |
| IMM2520 | Anti-CD47 and PD-L1 bispecific antibody | - | NCT05780307 | Phase I; recruiting |
| Evorpacept (ALX148) | CD47-SIRPα inhibitor improving macrophage phagocytosis | Fam-Trastuzumab Deruxtecan-Nxki | NCT05868226 | Phase I/Ib; recruiting |
| Hu5F9-G4 | Anti-CD47 antibody for targeting cancer cells | Olaparib | NCT05807126 | Phase I; withdrawn |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padzińska-Pruszyńska, I.; Kucharzewska, P.; Matejuk, A.; Górczak, M.; Kubiak, M.; Taciak, B.; Król, M. Macrophages: Key Players in the Battle against Triple-Negative Breast Cancer. Int. J. Mol. Sci. 2024, 25, 10781. https://doi.org/10.3390/ijms251910781
Padzińska-Pruszyńska I, Kucharzewska P, Matejuk A, Górczak M, Kubiak M, Taciak B, Król M. Macrophages: Key Players in the Battle against Triple-Negative Breast Cancer. International Journal of Molecular Sciences. 2024; 25(19):10781. https://doi.org/10.3390/ijms251910781
Chicago/Turabian StylePadzińska-Pruszyńska, Irena, Paulina Kucharzewska, Agata Matejuk, Małgorzata Górczak, Małgorzata Kubiak, Bartłomiej Taciak, and Magdalena Król. 2024. "Macrophages: Key Players in the Battle against Triple-Negative Breast Cancer" International Journal of Molecular Sciences 25, no. 19: 10781. https://doi.org/10.3390/ijms251910781






