Unveiling the Hidden Responses: Metagenomic Insights into Dwarf Bamboo (Fargesia denudata) Rhizosphere under Drought and Nitrogen Challenges
Abstract
:1. Introduction
2. Results
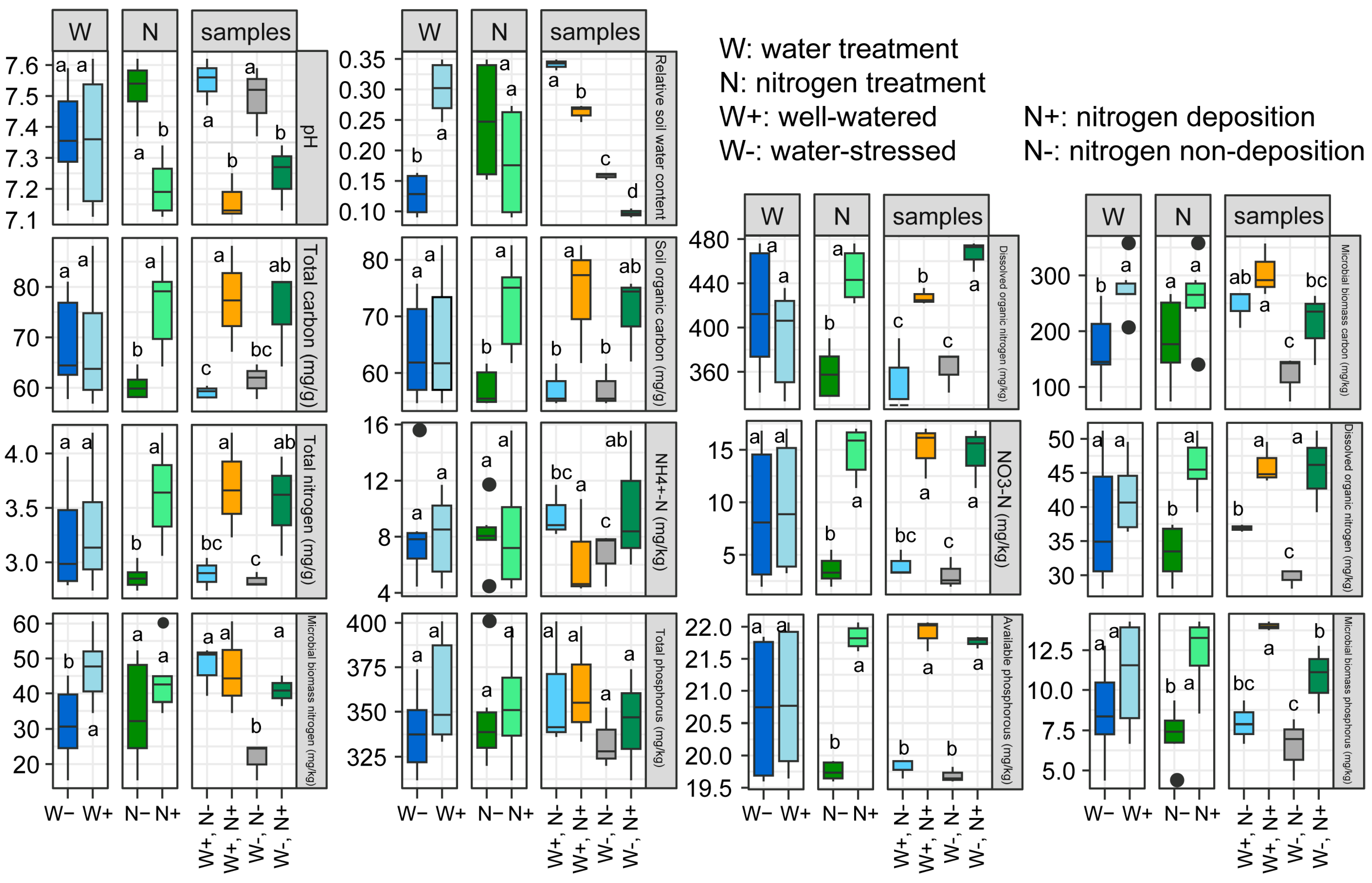
2.1. Effects of Nitrogen (N) Deposition and Drought Stress on Soil Physicochemical Index
2.2. Effects of Nitrogen (N) Deposition and Drought Stress on Microbial Communities and Functions
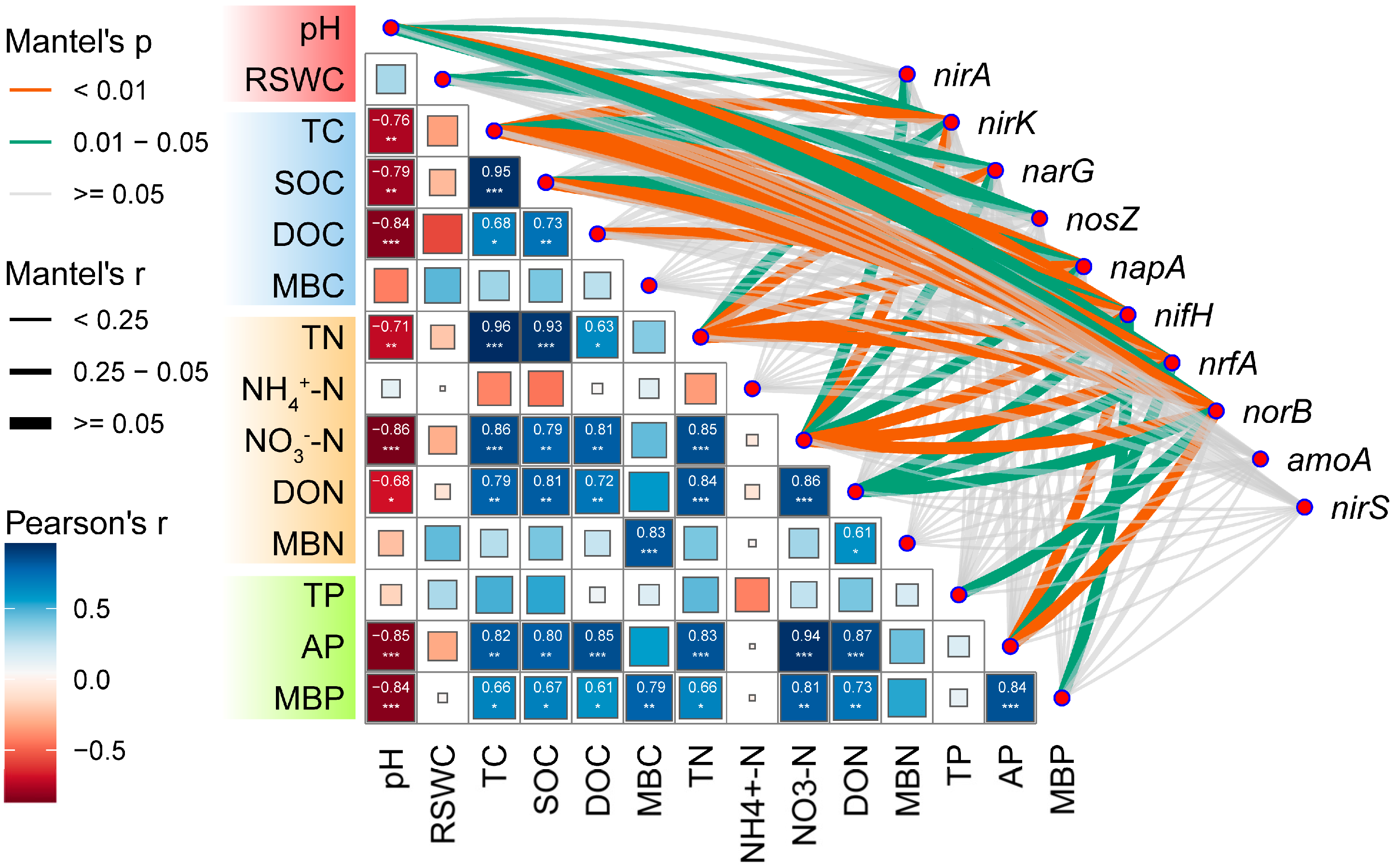
2.3. Correlation between N Cycling-Related Microorganisms and Soil Physicochemical Index
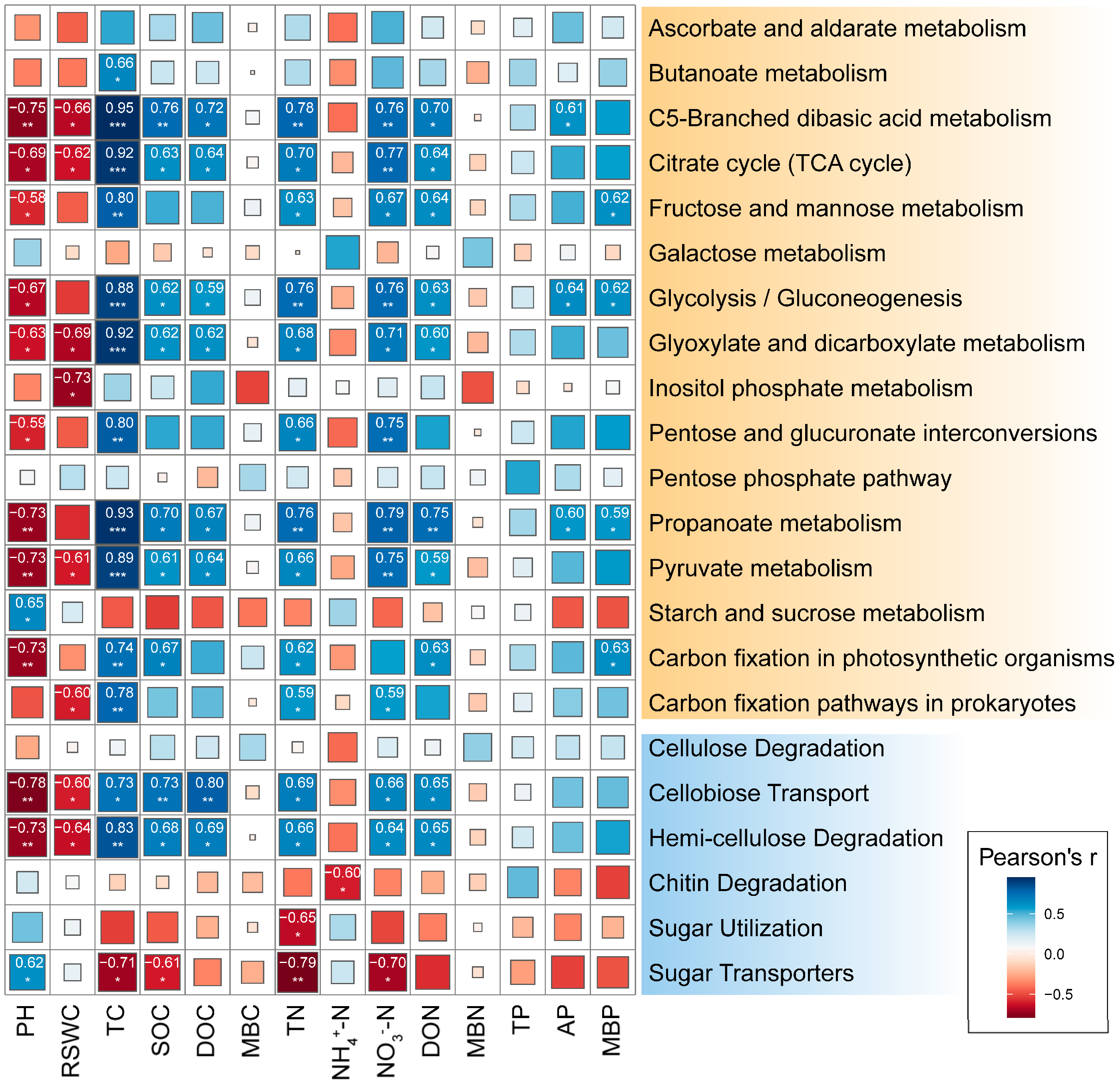
2.4. Correlation between C Cycling-Related Microorganisms and Soil Physicochemical Index
3. Discussion
3.1. Response of Soil Physicochemical Index to Drought Stress and Nitrogen Deposition
3.2. Response of Soil Microbial Community Structure and Diversity to Drought Stress and Nitrogen Deposition
3.3. The Effect of N Deposition on Microbial Functional Genes for Soil C-N Cycling
3.4. The Effect of Soil Physicochemical Index on Microbial Communities
4. Materials and Methods
4.1. Research Area and Experiment Design
4.2. Soil Physiochemistry Properties
4.3. Metagenome Sequencing
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, S.; Zheng, Q.; Yuan, M.; Shi, Z.; Chiariello, N.R.; Docherty, K.M.; Dong, S.; Field, C.B.; Gu, Y.; Gutknecht, J. Long-term elevated CO2 shifts composition of soil microbial communities in a Californian annual grassland, reducing growth and N utilization potentials. Sci. Total Environ. 2019, 652, 1474–1481. [Google Scholar] [CrossRef]
- Rillig, M.C.; Ryo, M.; Lehmann, A.; Aguilar-Trigueros, C.A.; Buchert, S.; Wulf, A.; Iwasaki, A.; Roy, J.; Yang, G. The role of multiple global change factors in driving soil functions and microbial biodiversity. Science 2019, 366, 886–890. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Dong, S.; Li, S.; Xiao, J.; Han, Y.; Yang, M.; Zhang, J.; Gao, X.; Xu, Y.; Li, Y. Effects of simulated N deposition on photosynthesis and productivity of key plants from different functional groups of alpine meadow on Qinghai-Tibetan plateau. Environ. Pollut. 2019, 251, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tariq, A.; Zeng, F.; Graciano, C.; Zhang, B. Nitrogen application mitigates drought-induced metabolic changes in Alhagi sparsifolia seedlings by regulating nutrient and biomass allocation patterns. Plant Physiol. Biochem. 2020, 155, 828–841. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, J.; Liu, M.; Meng, Z.; Liu, K.; Sui, N. Nitrogen increases drought tolerance in maize seedlings. Funct. Plant Biol. 2019, 46, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Peguero, G.; Folch, E.; Liu, L.; Ogaya, R.; Penuelas, J. Divergent effects of drought and nitrogen deposition on microbial and arthropod soil communities in a Mediterranean forest. Eur. J. Soil Biol. 2021, 103, 103275. [Google Scholar] [CrossRef]
- Wang, Z.; Na, R.; Koziol, L.; Schellenberg, M.P.; Li, X.; Ta, N.; Jin, K.; Wang, H. Response of bacterial communities and plant-mediated soil processes to nitrogen deposition and precipitation in a desert steppe. Plant Soil 2020, 448, 277–297. [Google Scholar] [CrossRef]
- Ummenhofer, C.C.; Meehl, G.A. Extreme weather and climate events with ecological relevance: A review. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160135. [Google Scholar] [CrossRef]
- Han, L.; Zhang, Q.; Ma, P.; Jia, J.; Wang, J. The spatial distribution characteristics of a comprehensive drought risk index in southwestern China and underlying causes. Theor. Appl. Climatol. 2016, 124, 517–528. [Google Scholar] [CrossRef]
- Liu, F.; Xiao, X.; Qin, Y.; Yan, H.; Huang, J.; Wu, X.; Zhang, Y.; Zou, Z.; Doughty, R.B. Large spatial variation and stagnation of cropland gross primary production increases the challenges of sustainable grain production and food security in China. Sci. Total Environ. 2022, 811, 151408. [Google Scholar] [CrossRef]
- Cui, S.; Shi, Y.; Groffman, P.M.; Schlesinger, W.H.; Zhu, Y.-G. Centennial-scale analysis of the creation and fate of reactive nitrogen in China (1910–2010). Proc. Natl. Acad. Sci. USA 2013, 110, 2052–2057. [Google Scholar] [CrossRef] [PubMed]
- Aronson, E.L.; Goulden, M.L.; Allison, S.D. Greenhouse gas fluxes under drought and nitrogen addition in a Southern California grassland. Soil Biol. Biochem. 2019, 131, 19–27. [Google Scholar] [CrossRef]
- McNear, D., Jr. The Rhizosphere-Roots Soil and Everything in Between. Nat. Educ. Knowl. 2013, 4, 1. [Google Scholar]
- Liu, S.; He, F.; Kuzyakov, Y.; Xiao, H.; Hoang, D.T.T.; Pu, S.; Razavi, B.S. Nutrients in the rhizosphere: A meta-analysis of content, availability, and influencing factors. Sci. Total Environ. 2022, 826, 153908. [Google Scholar] [CrossRef]
- Lau, J.A.; Lennon, J.T. Rapid responses of soil microorganisms improve plant fitness in novel environments. Proc. Natl. Acad. Sci. USA 2012, 109, 14058–14062. [Google Scholar] [CrossRef]
- Kuppe, C.W.; Schnepf, A.; von Lieres, E.; Watt, M.; Postma, J.A. Rhizosphere models: Their concepts and application to plant-soil ecosystems. Plant Soil 2022, 474, 17–55. [Google Scholar] [CrossRef]
- Ling, W.; Ma, B.; Zhang, W. Rhizosphere microbiology: Toward a clean and healthy soil environment. Front. Microbiol. 2022, 13, 991356. [Google Scholar] [CrossRef]
- Suman, J.; Rakshit, A.; Ogireddy, S.D.; Singh, S.; Gupta, C.; Chandrakala, J. Microbiome as a key player in sustainable agriculture and human health. Front. Soil Sci. 2022, 2, 821589. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Y.; Pan, K.; Zhu, T.; Li, W.; Zhang, L. Carbon and nitrogen metabolism in leaves and roots of dwarf bamboo (Fargesia denudata Yi) subjected to drought for two consecutive years during sprouting period. J. Plant Growth Regul. 2014, 33, 243–255. [Google Scholar] [CrossRef]
- Wu, S.; Tian, J.; Ren, T.; Wang, Y. Osmotic adjustment and antioxidant system regulated by nitrogen deposition improve photosynthetic and growth performance and alleviate oxidative damage in dwarf bamboo under drought stress. Front. Plant Sci. 2022, 13, 819071. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Huang, J. Reseach into Bomboo Species as Giant Panda’s Main Diet and its Progress. World Bamboo Ratt. 2005, 3, 1–6. [Google Scholar]
- Zhou, X.; Wang, J.; Zhang, H.; Wang, J.; Zhang, Y. Effect of elevated CO2 and nitrogen deposition on leaf nutrient quality of Fargesia rufa Yi. Acta Ecol. Sin. 2012, 32, 7644–7653. [Google Scholar] [CrossRef]
- Pieters, A.J.; Paul, M.J.; Lawlor, D.W. Low sink demand limits photosynthesis under Pi deficiency. J. Exp. Bot. 2001, 52, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Berhe, A.A.; Barnes, R.T.; Six, J.; Marín-Spiotta, E. Role of soil erosion in biogeochemical cycling of essential elements: Carbon, nitrogen, and phosphorus. Annu. Rev. Earth Planet. Sci. 2018, 46, 521–548. [Google Scholar] [CrossRef]
- Xu, N.; Guo, W.; Liu, J.; Du, N.; Wang, R. Increased nitrogen deposition alleviated the adverse effects of drought stress on Quercus variabilis and Quercus mongolica seedlings. Acta Physiol. Plant. 2015, 37, 107. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, L.; Lu, H.; Shao, Y.; Liu, S.; Fu, S. Drought promotes soil phosphorus transformation and reduces phosphorus bioavailability in a temperate forest. Sci. Total Environ. 2020, 732, 139295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, J.; Sayer, E.J.; Lambers, H.; Liu, Z.; Lu, X.; Li, Y.; Li, Y.; Li, H.; Wang, F. Nitrogen deposition enhances soil organic carbon and microbial residual carbon in a tropical forest. Plant Soil 2023, 484, 217–235. [Google Scholar] [CrossRef]
- Wang, Z.; Tao, T.; Wang, H.; Chen, J.; Small, G.E.; Johnson, D.; Chen, J.; Zhang, Y.; Zhu, Q.; Zhang, S. Forms of nitrogen inputs regulate the intensity of soil acidification. Glob. Change Biol. 2023, 29, 4044–4055. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, J.; Shi, Z.; Kang, B.; Tu, H.; Zhu, J.; Li, H. Nitrogen addition and drought affect nitrogen uptake patterns and biomass production of four urban greening tree species in North China. Sci. Total Environ. 2023, 893, 164893. [Google Scholar] [CrossRef]
- Hermans, S.M.; Buckley, H.L.; Case, B.S.; Curran-Cournane, F.; Taylor, M.; Lear, G. Using soil bacterial communities to predict physico-chemical variables and soil quality. Microbiome 2020, 8, 79. [Google Scholar] [CrossRef]
- Xia, Q.; Rufty, T.; Shi, W. Soil microbial diversity and composition: Links to soil texture and associated properties. Soil Biol. Biochem. 2020, 149, 107953. [Google Scholar] [CrossRef]
- Tajik, S.; Ayoubi, S.; Lorenz, N. Soil microbial communities affected by vegetation, topography and soil properties in a forest ecosystem. Appl. Soil Ecol. 2020, 149, 103514. [Google Scholar] [CrossRef]
- Shu, X.; Zhang, K.; Zhang, Q.; Wang, W. Changes in the composition of rhizosphere bacterial communities in response to soil types and acid rain. J. Environ. Manag. 2023, 325, 116493. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Cai, J.; Yang, S.; Li, S.; Shao, X.; Fu, C.; Li, C.; Deng, Y.; Huang, J.; Ruan, Y. Partial substitution of chemical fertilizer with organic fertilizer and slow-release fertilizer benefits soil microbial diversity and pineapple fruit yield in the tropics. Appl. Soil Ecol. 2023, 189, 104974. [Google Scholar] [CrossRef]
- Ijaz, F.; Ijaz, M.F.; Javed, H.; Amin, H.A.; Zafar, H.; Hamza, A.; Saleem, M.U.; Mujeeb, F.; Ehsan, S.; Alvi, A. Co-inoculation of Bradyrhizobium and Phosphate Solubilizing Microbes on Growth Promotion of Groundnut Under Rain-fed Conditions. J. Appl. Res. Plant Sci. 2023, 4, 348–355. [Google Scholar] [CrossRef]
- Gogoi, N.; Baruah, K.K.; Meena, R.S. Grain legumes: Impact on soil health and agroecosystem. In Legumes for Soil Health and Sustainable Management; Springer: Singapore, 2018; pp. 511–539. [Google Scholar]
- Zhang, Z.; Tariq, A.; Zeng, F.; Graciano, C.; Sun, F.; Chai, X.; Ahmed, Z. Nitrogen and water addition regulate fungal community and microbial co-occurrence network complexity in the rhizosphere of Alhagi sparsifolia seedlings. Appl. Soil Ecol. 2021, 164, 103940. [Google Scholar] [CrossRef]
- Li, Z.; Peng, Q.; Dong, Y.; Guo, Y. The influence of increased precipitation and nitrogen deposition on the litter decomposition and soil microbial community structure in a semiarid grassland. Sci. Total Environ. 2022, 844, 157115. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xie, J.; Zhang, Y.; Dong, L.; Shangguan, Z.; Deng, L. Interactive effects of nitrogen and water addition on soil microbial resource limitation in a temperate desert shrubland. Plant Soil 2022, 475, 361–378. [Google Scholar] [CrossRef]
- Deng, L.; Peng, C.; Kim, D.-G.; Li, J.; Liu, Y.; Hai, X.; Liu, Q.; Huang, C.; Shangguan, Z.; Kuzyakov, Y. Drought effects on soil carbon and nitrogen dynamics in global natural ecosystems. Earth-Sci. Rev. 2021, 214, 103501. [Google Scholar] [CrossRef]
- Gao, D.; Bai, E.; Li, M.; Zhao, C.; Yu, K.; Hagedorn, F. Responses of soil nitrogen and phosphorus cycling to drying and rewetting cycles: A meta-analysis. Soil Biol. Biochem. 2020, 148, 107896. [Google Scholar] [CrossRef]
- Du, Y.; Zhao, Q.; Chen, L.; Yao, X.; Xie, F. Effect of drought stress at reproductive stages on growth and nitrogen metabolism in soybean. Agronomy 2020, 10, 302. [Google Scholar] [CrossRef]
- Liu, X.; Wu, Y.; Sun, R.; Hu, S.; Qiao, Z.; Wang, S.; Mi, X. NH4+-N/NO3−-N ratio controlling nitrogen transformation accompanied with NO2−-N accumulation in the oxic-anoxic transition zone. Environ. Res. 2020, 189, 109962. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Qin, Y.; Wang, Y.; Liu, S.; Yu, B.; Song, Y.; Wang, X.; Zhu, G. Dissimilatory nitrate/nitrite reduction to ammonium (DNRA) pathway dominates nitrate reduction processes in rhizosphere and non-rhizosphere of four fertilized farmland soil. Environ. Res. 2020, 186, 109612. [Google Scholar] [CrossRef]
- Bowen, J.L.; Spivak, A.C.; Bernhard, A.E.; Fulweiler, R.W.; Giblin, A.E. Salt marsh nitrogen cycling: Where land meets sea. Trends Microbiol. 2023, 32, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Richardson, D.; Felgate, H.; Watmough, N.; Thomson, A.; Baggs, E. Mitigating release of the potent greenhouse gas N2O from the nitrogen cycle–could enzymic regulation hold the key? Trends Biotechnol. 2009, 27, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, E.M.; Franklin, R.B. Resource effects on denitrification are mediated by community composition in tidal freshwater wetlands soils. Environ. Microbiol. 2015, 17, 1520–1532. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, C.; Zheng, M.; Jiang, L.; Luo, Y. Patterns and mechanisms of responses by soil microbial communities to nitrogen addition. Soil Biol. Biochem. 2017, 115, 433–441. [Google Scholar] [CrossRef]
- Guo, G.; Kong, W.; Liu, J.; Zhao, J.; Du, H.; Zhang, X.; Xia, P. Diversity and distribution of autotrophic microbial community along environmental gradients in grassland soils on the Tibetan Plateau. Appl. Microbiol. Biotechnol. 2015, 99, 8765–8776. [Google Scholar] [CrossRef]
- He, W.; Zhang, M.; Jin, G.; Sui, X.; Zhang, T.; Song, F. Effects of nitrogen deposition on nitrogen-mineralizing enzyme activity and soil microbial community structure in a Korean pine plantation. Microb. Ecol. 2021, 81, 410–424. [Google Scholar] [CrossRef]
- Guoyong, Y.; Yajuan, X.; Shijie, H.; Zhang, J.; Qinggui, W.; Changcheng, M. Long-time precipitation reduction and nitrogen deposition increase alter soil nitrogen dynamic by influencing soil bacterial communities and functional groups. Pedosphere 2020, 30, 363–377. [Google Scholar]
- Bahulikar, R.A.; Chaluvadi, S.R.; Torres-Jerez, I.; Mosali, J.; Bennetzen, J.L.; Udvardi, M. Nitrogen fertilization reduces nitrogen fixation activity of diverse diazotrophs in switchgrass roots. Phytobiomes J. 2021, 5, 80–87. [Google Scholar] [CrossRef]
- Li, W.; Xie, L.; Zhao, C.; Hu, X.; Yin, C. Nitrogen fertilization increases soil microbial biomass and alters microbial composition especially under low soil water availability. Microb. Ecol. 2023, 86, 536–548. [Google Scholar] [CrossRef] [PubMed]
- Kuerban, M.; Cong, W.-F.; Jing, J.; Bezemer, T.M. Microbial soil legacies of crops under different water and nitrogen levels determine succeeding crop performance. Plant Soil 2023, 485, 167–180. [Google Scholar] [CrossRef]
- Hagh-Doust, N.; Mikryukov, V.; Anslan, S.; Bahram, M.; Puusepp, R.; Dulya, O.; Tedersoo, L. Effects of nitrogen deposition on carbon and nutrient cycling along a natural soil acidity gradient as revealed by metagenomics. New Phytol. 2023, 238, 2607–2620. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Wang, J.; Dijkstra, F.A.; Lei, S.; Zhang, L.; Wang, X.; Liu, G.; Zhang, C. Nitrogen enrichment stimulates rhizosphere multi-element cycling genes via mediating plant biomass and root exudates. Soil Biol. Biochem. 2024, 190, 109306. [Google Scholar] [CrossRef]
- Yang, X.; Ni, K.; Shi, Y.; Yi, X.; Ji, L.; Wei, S.; Jiang, Y.; Zhang, Y.; Cai, Y.; Ma, Q. Metagenomics reveals N-induced changes in carbon-degrading genes and microbial communities of tea (Camellia sinensis L.) plantation soil under long-term fertilization. Sci. Total Environ. 2023, 856, 159231. [Google Scholar] [CrossRef]
- Khalili, B.; Ogunseitan, O.A.; Goulden, M.L.; Allison, S.D. Interactive effects of precipitation manipulation and nitrogen addition on soil properties in California grassland and shrubland. Appl. Soil Ecol. 2016, 107, 144–153. [Google Scholar] [CrossRef]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The interplay between microbial communities and soil properties. Nat. Rev. Microbiol. 2024, 22, 226–239. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhang, J.; Ma, T.; Lv, W.; Zhang, Z.; Shen, Y.; Yang, Q.; Wang, X.; Li, J.; Xiang, Q. Effects of short-term drought, nitrogen application and their interactions on the composition and functional genes of soil microbial communities in alfalfa grassland on the Loess Plateau. Front. Sustain. Food Syst. 2023, 7, 1332683. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Gao, X.; Wang, Q.; Cui, M.; Zhang, D.; Guo, P. Unveiling the driving role of pH on community stability and function during lignocellulose degradation in paddy soil. Front. Microbiol. 2024, 15, 1338842. [Google Scholar] [CrossRef]
- Xiang, J.; Gu, J.; Wang, G.; Bol, R.; Yao, L.; Fang, Y.; Zhang, H. Soil pH controls the structure and diversity of bacterial communities along elevational gradients on Huangshan, China. Eur. J. Soil Biol. 2024, 120, 103586. [Google Scholar] [CrossRef]
- Lü, C.Q.; Tian, H.Q. Spatial and temporal patterns of nitrogen deposition in China: Synthesis of observational data. J. Geophys. Res. Atmos. 2007, 112, D22S05. [Google Scholar] [CrossRef]
- Xu, Z.Z.; Zhou, G.S.; Shimizu, H. Are plant growth and photosynthesis limited by pre-drought following rewatering in grass? J. Exp. Bot. 2009, 60, 3737–3749. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.G.; Wang, Y.J.; Pan, K.W.; Wang, Q.W.; Liang, J.; Jin, Y.Q.; Tariq, A. The synergistic responses of different photoprotective pathways in dwarf bamboo (Fargesia rufa) to drought and subsequent rewatering. Front. Plant Sci. 2017, 8, 489. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Noguchi, H.; Park, J.; Takagi, T. MetaGene: Prokaryotic gene finding from environmental genome shotgun sequences. Nucleic Acids Res. 2006, 34, 5623–5630. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]

| Treatments | Raw Reads | Clean Reads | Clean Per | Contigs | Bases (bp) | N50 (bp) N90 (bp) | Max (bp) Min (bp) | ||
|---|---|---|---|---|---|---|---|---|---|
| Well-watered, −N | 99,089,384 | 97,764,518 | 98.66% | 601,083 | 326,882,166 | 557 | 337 | 20,153 | 300 |
| 83,740,618 | 82,770,650 | 98.84% | 432,627 | 225,993,005 | 532 | 335 | 8520 | 300 | |
| 93,404,480 | 92,337,966 | 98.86% | 596,467 | 319,518,642 | 547 | 335 | 43,654 | 300 | |
| Well-watered, +N | 95,058,150 | 94,005,278 | 98.90% | 468,145 | 232,774,876 | 501 | 332 | 7784 | 300 |
| 97,124,186 | 96,005,208 | 98.85% | 484,206 | 240,004,615 | 499 | 332 | 6819 | 300 | |
| 96,808,852 | 95,707,126 | 98.86% | 489,179 | 240,032,836 | 497 | 332 | 8035 | 300 | |
| Water-stressed, −N | 92,819,990 | 101,910,784 | 98.85% | 507,764 | 249,379,261 | 492 | 330 | 9944 | 300 |
| 94,154,460 | 931,12,574 | 98.90% | 520,176 | 268,941,072 | 522 | 334 | 10,380 | 300 | |
| 103,093,822 | 91,759,092 | 98.86% | 553,203 | 289,580,691 | 531 | 335 | 13,386 | 300 | |
| Water-stressed, +N | 92,969,798 | 91,811,334 | 98.75% | 451,016 | 222,705,758 | 500 | 331 | 5748 | 300 |
| 93,749,160 | 94,866,738 | 98.81% | 549,649 | 286,257,660 | 528 | 335 | 7542 | 300 | |
| 96,007,016 | 92,545,234 | 98.72% | 440,843 | 216,002,776 | 496 | 331 | 6582 | 300 | |
| Diversity Index | Well-Watered | Water-Stressed | W | N | W × N | ||
|---|---|---|---|---|---|---|---|
| −N | +N | −N | +N | ||||
| Shannon | 6.74 ± 0.02 ab | 6.75 ± 0.02 a | 6.76 ± 0.01 a | 6.37 ± 0.02 b | ns | ns | * |
| Simpson | 0.99 ± 0.00 b | 0.99 ± 0.00 a | 0.99 ± 0.00 ab | 0.99 ± 0.00 ab | ns | ns | * |
| Chao 1 | 11,827.66 ± 29.48 a | 11,794.67 ± 49.17 a | 11,858 ± 39.94 a | 11,728 ± 53.11 a | ns | ns | ns |
| ACE | 11,827.67 ± 29.48 a | 11,794.67 ± 49.17 a | 11,858 ± 39.94 a | 11,728 ± 53.11 a | ns | ns | ns |
| Genes | Well-Watered | Water-Stressed | W | N | W × N | ||
|---|---|---|---|---|---|---|---|
| −N | +N | −N | +N | ||||
| nirA | 1.87 × 10−4 a | 1.60 × 10−4 a | 1.72 × 10−4 a | 1.45 × 10−4 a | ns | ns | ns |
| nirK | 1.88 × 10−4 a | 1.52 × 10−4 b | 1.65 × 10−4 ab | 1.45 × 10−4 b | ns | * | ns |
| nirS | 4.34 × 10−6 a | 2.89 × 10−6 a | 2.75 × 10−6 a | 2.31 × 10−6 a | ns | ns | ns |
| narG | 1.95 × 10−4 a | 1.56 × 10−4 a | 1.74 × 10−4 a | 1.53 × 10−4 a | ns | ns | ns |
| nosZ | 4.34 × 10−5 a | 2.86 × 10−5 b | 3.09 × 10−5 b | 2.98 × 10−5 b | ns | * | * |
| napA | 1.53 × 10−4 a | 1.20 × 10−4 a | 1.36 × 10−4 a | 1.24 × 10−4 a | ns | ns | ns |
| nifH | 4.77 × 10−6 a | 2.31 × 10−6 b | 5.35 × 10−6 a | 2.02 × 10−6 b | ns | * | ns |
| norB | 2.00 × 10−4 a | 1.30 × 10−4 b | 1.75 × 10−4 ab | 1.36 × 10−4 b | ns | * | ns |
| amoA | 1.69 × 10−5 a | 1.40 × 10−5 a | 1.46 × 10−5 a | 1.23 × 10−5 a | ns | ns | ns |
| nrfA | 1.01 × 10−4 b | 7.91 × 10−5 a | 1.03 × 10−4 b | 7.69 × 10−5 a | ns | * | ns |
| Microbial Functional Genes Involved in the Carbon Cycle | Well-Watered | Water-Stressed | W | N | W × N | ||
|---|---|---|---|---|---|---|---|
| −N | +N | −N | +N | ||||
| Butanoate metabolism | 2.28 × 10−2 a | 2.32 × 10−2 a | 2.31 × 10−2 a | 2.31 × 10−2 a | ns | ns | ns |
| C5-Branched dibasic acid metabolism | 7.94 × 10−3 b | 8.40 × 10−3 a | 8.17 × 10−3 ab | 8.43 × 10−3 a | ns | ** | ns |
| Citrate cycle | 2.21 × 10−2 b | 2.37 × 10−2 a | 2.29 × 10−2 ab | 2.35 × 10−2 a | ns | ** | ns |
| Fructose and mannose metabolism | 7.83 × 10−3 b | 8.16 × 10−3 a | 7.96 × 10−3 ab | 8.18 × 10−3 a | ns | * | ns |
| Galactose metabolism | 6.26 × 10−3 a | 6.16 × 10−3 b | 6.19 × 10−3 ab | 6.27 × 10−3 a | ns | ns | * |
| Glycolysis/Gluconeogenesis | 2.63 × 10−2 b | 2.71 × 10−2 a | 2.67 × 10−2 ab | 2.71 × 10−2 a | ns | ** | ns |
| Glyoxylate and dicarboxylate metabolism | 2.81 × 10−2 b | 2.99 × 10−2 a | 2.91 × 10−2 ab | 3.03 × 10−2 a | ns | * | ns |
| Inositol phosphate metabolism | 3.59 × 10−3 b | 3.61 × 10−3 b | 3.66 × 10−3 b | 3.83 × 10−3 a | * | ns | ns |
| Pentose and glucuronate interconversions | 3.82 × 10−3 b | 4.09 × 10−3 a | 3.97 × 10−3 ab | 4.09 × 10−3 a | ns | * | ns |
| Pentose phosphate pathway | 1.78 × 10−2 a | 1.80 × 10−2 a | 1.78 × 10−2 a | 1.77 × 10−2 a | ns | ns | ns |
| Propanoate metabolism | 2.13 × 10−2 b | 2.25 × 10−2 a | 2.19 × 10−2 ab | 2.25 × 10−2 a | ns | * | ns |
| Pyruvate metabolism | 3.07 × 10−2 b | 3.21 × 10−2 a | 3.14 × 10−2 ab | 3.20 × 10−2 a | ns | ** | ns |
| Starch and sucrose metabolism | 1.62 × 10−2 a | 1.56 × 10−2 b | 1.57 × 10−2 b | 1.58 × 10−2 ab | ns | n | * |
| Carbon fixation in photosynthetic organisms | 1.07 × 10−2 b | 1.12 × 10−2 a | 1.10 × 10−2 ab | 1.10 × 10−2 ab | ns | * | ns |
| Carbon fixation pathways in prokaryotes | 3.29 × 10−2 b | 3.37 × 10−2 ab | 3.33 × 10−2 ab | 3.38 × 10−2 a | ns | * | ns |
| Microbial Functional Genes Involved in Carbon Degrading Enzymes | Well-Watered | Water-Stressed | W | N | W × N | ||
|---|---|---|---|---|---|---|---|
| −N | +N | −N | +N | ||||
| Cellulose Degradation | 1.56 × 10−3 a | 1.58 × 10−3 a | 1.54 × 10−3 a | 1.58 × 10−3 a | ns | ns | ns |
| Cellobiose Transport | 2.04 × 10−5 c | 4.13 × 10−5 ab | 3.19 × 10−5 cb | 5.06 × 10−5 a | ns | ** | ns |
| Hemi-cellulose Degradation | 2.16 × 10−4 a | 2.31 × 10−4 a | 1.77 × 10−4 b | 2.38 × 10−4 a | * | ** | ns |
| Chitin Degradation | 1.08 × 10−4 a | 1.06 × 10−4 a | 1.11 × 10−4 a | 9.80 × 10−5 a | ns | ns | ns |
| Sugar Utilization | 1.70 × 10−3 a | 1.48 × 10−3 a | 1.61 × 10−3 a | 1.57 × 10−3 a | ns | ns | ns |
| Sugar Transporters | 6.66 × 10−3 a | 5.46 × 10−3 b | 6.29 × 10−3 ab | 5.91 × 10−3 ab | ns | * | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, J.; Zhang, N.; Li, J.; Zhu, Y.; Cao, T.; Wang, Y. Unveiling the Hidden Responses: Metagenomic Insights into Dwarf Bamboo (Fargesia denudata) Rhizosphere under Drought and Nitrogen Challenges. Int. J. Mol. Sci. 2024, 25, 10790. https://doi.org/10.3390/ijms251910790
Xiang J, Zhang N, Li J, Zhu Y, Cao T, Wang Y. Unveiling the Hidden Responses: Metagenomic Insights into Dwarf Bamboo (Fargesia denudata) Rhizosphere under Drought and Nitrogen Challenges. International Journal of Molecular Sciences. 2024; 25(19):10790. https://doi.org/10.3390/ijms251910790
Chicago/Turabian StyleXiang, Jun, Nannan Zhang, Jiangtao Li, Yue Zhu, Tingying Cao, and Yanjie Wang. 2024. "Unveiling the Hidden Responses: Metagenomic Insights into Dwarf Bamboo (Fargesia denudata) Rhizosphere under Drought and Nitrogen Challenges" International Journal of Molecular Sciences 25, no. 19: 10790. https://doi.org/10.3390/ijms251910790





