TNFα-Related Chondrocyte Inflammation Models: A Systematic Review
Abstract
:1. Introduction
2. Results
2.1. Description of Studies
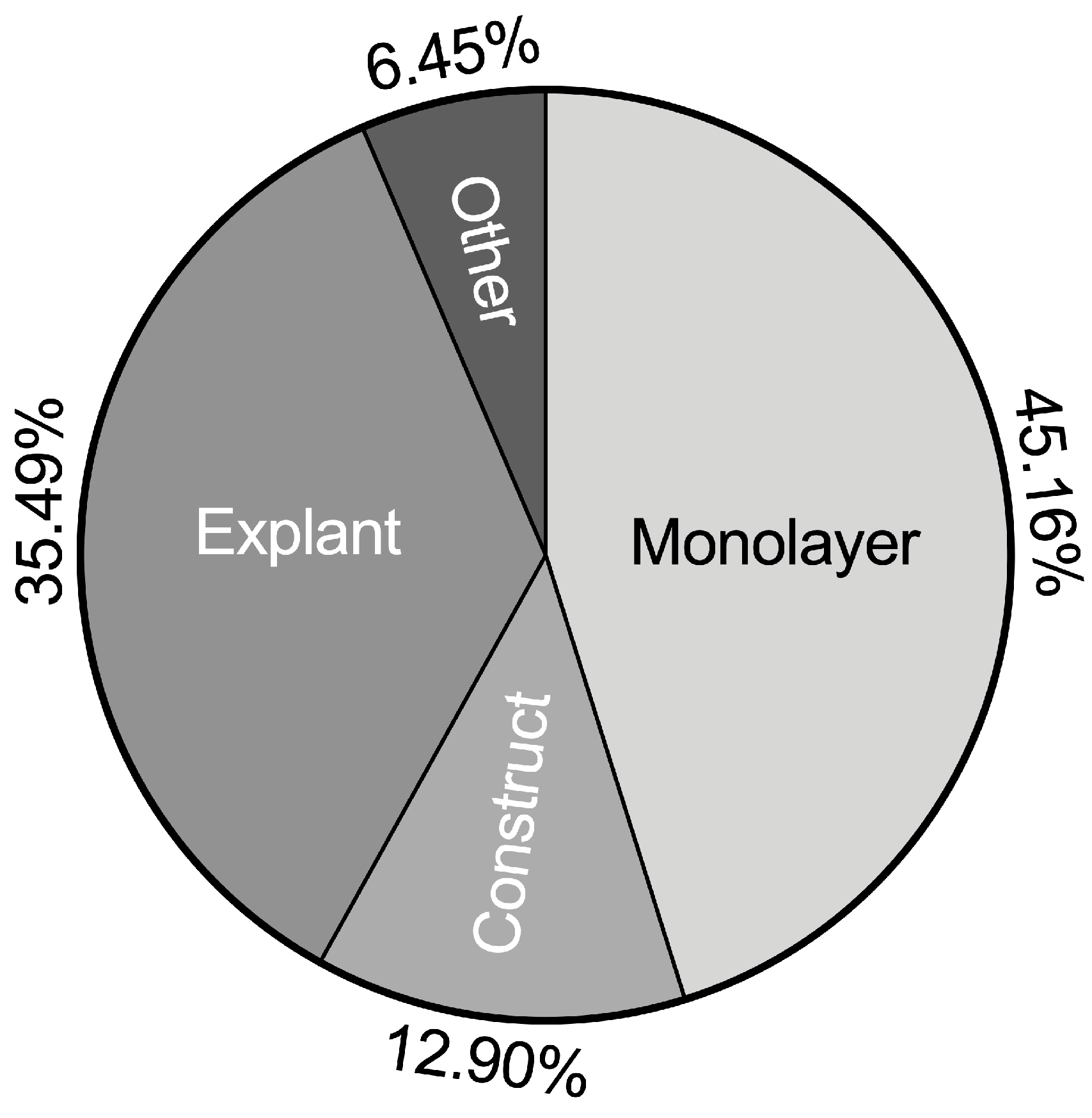
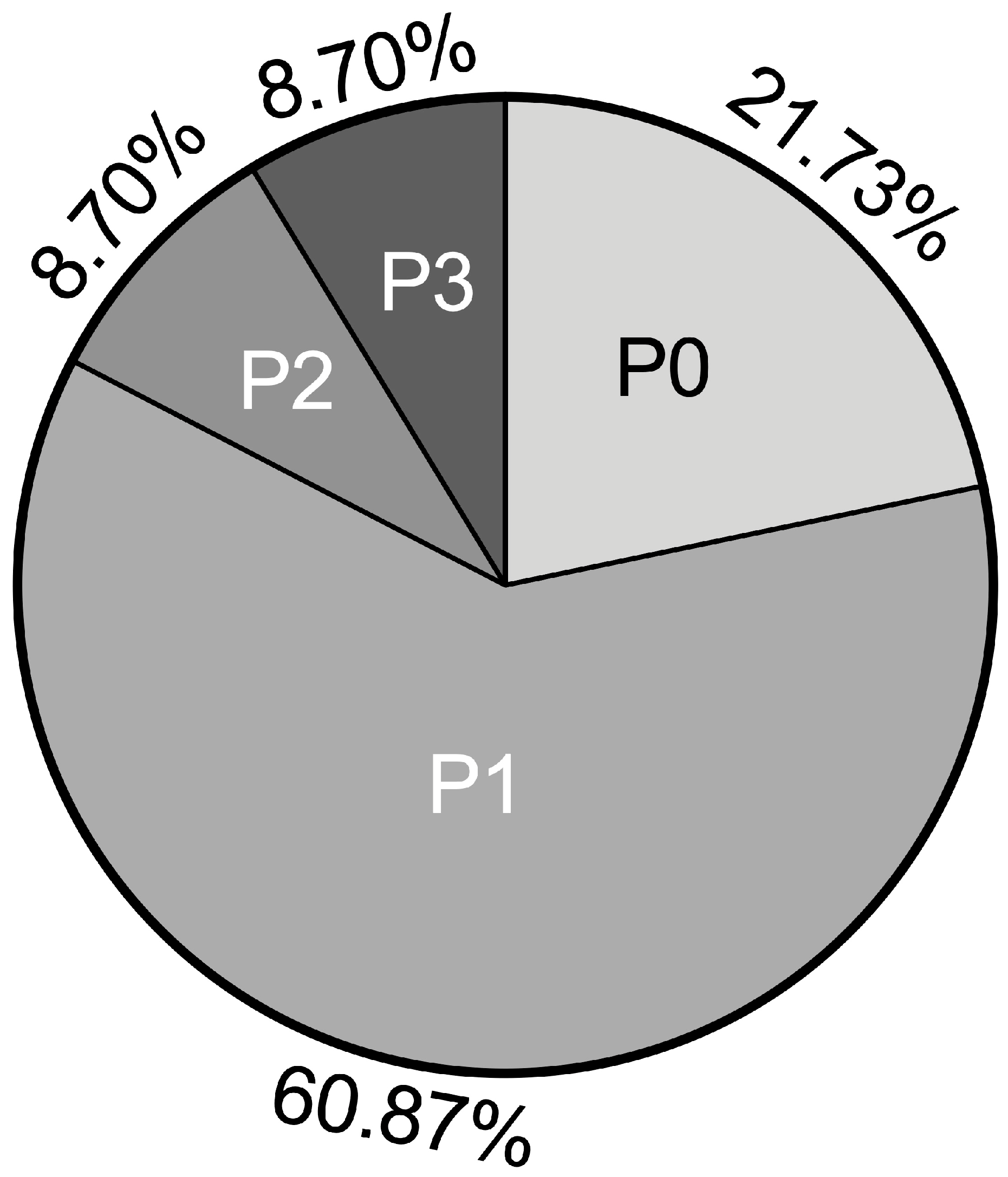
2.2. Classification of TNFα Chondrocyte Inflammation Models
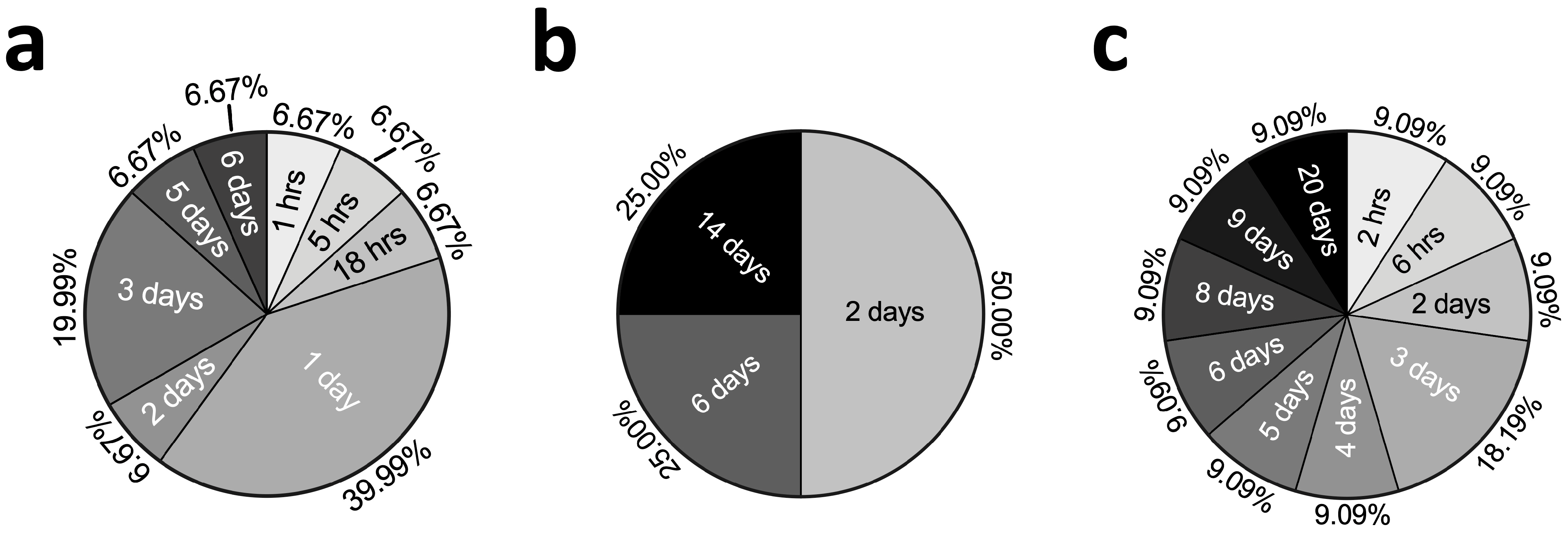
2.3. Concentrations and Stimulation Durations of TNFα
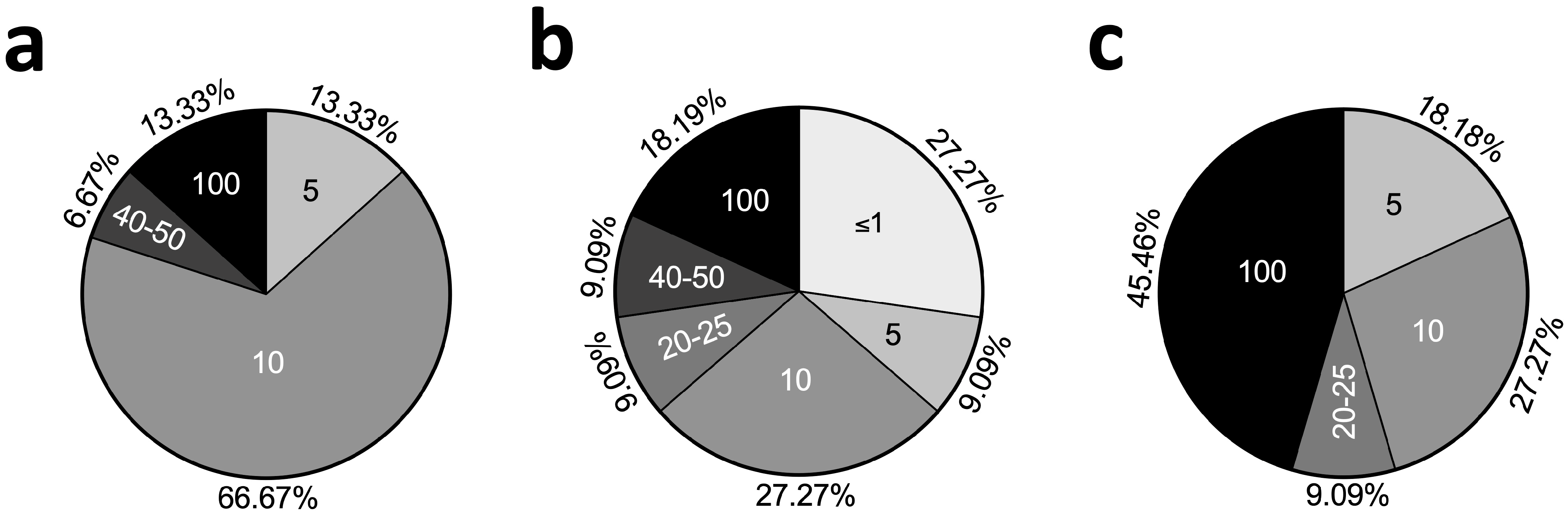
2.4. Passages Used in TNFα Chondrocyte Inflammation Models
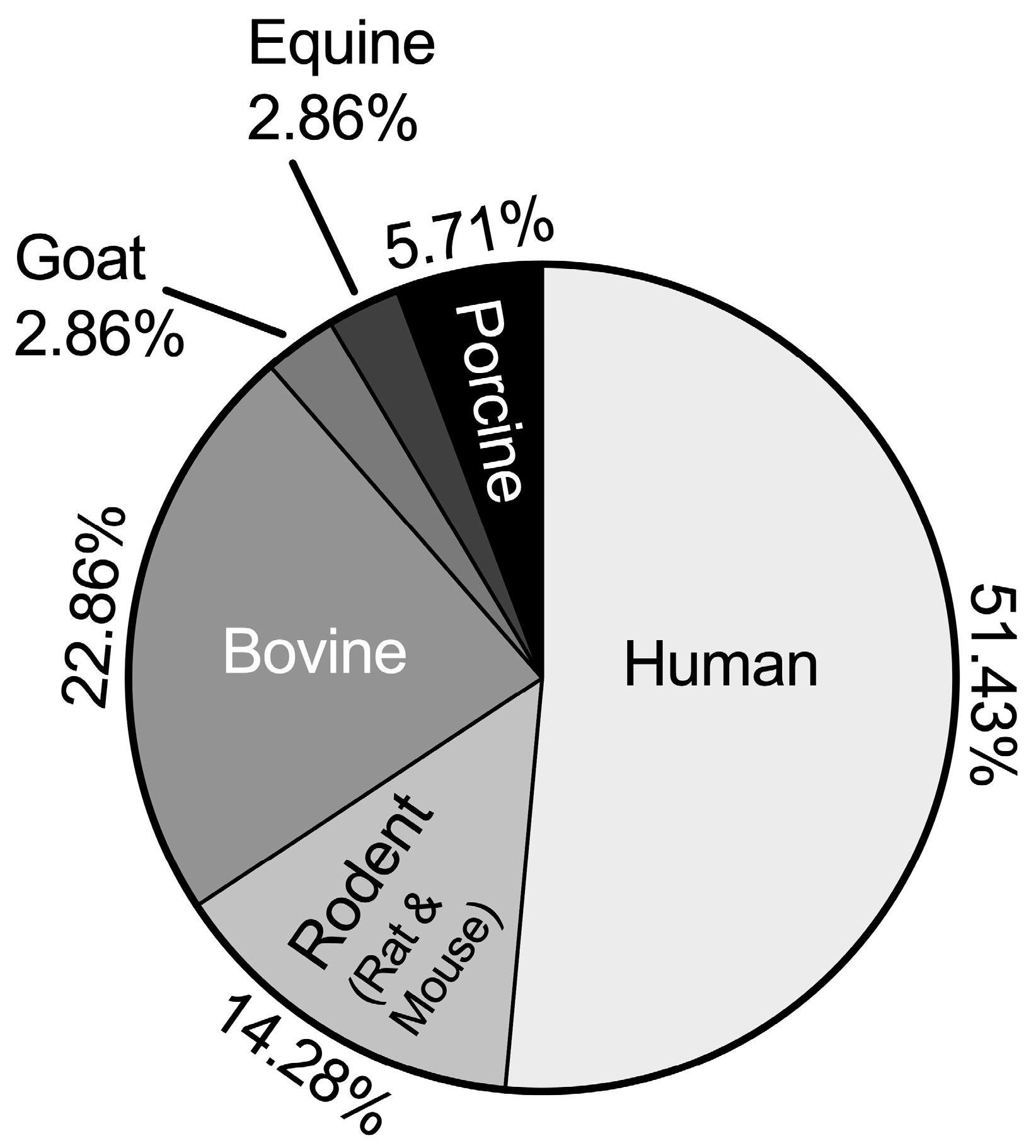
2.5. Species Distribution
2.6. Mechanical Stimuli
2.7. Common Methods of Evaluating Experimental Results
3. Discussion
3.1. TNFα Chondrocyte Inflammation—Differences in Available Models
3.2. High Diversity of Outcome Assessments in Chondrocyte Inflammation Models with TNFα
3.3. Mechanical Stimulation as Key for the Simulation of Physiologic Conditions
3.4. Limitations
4. Materials and Methods
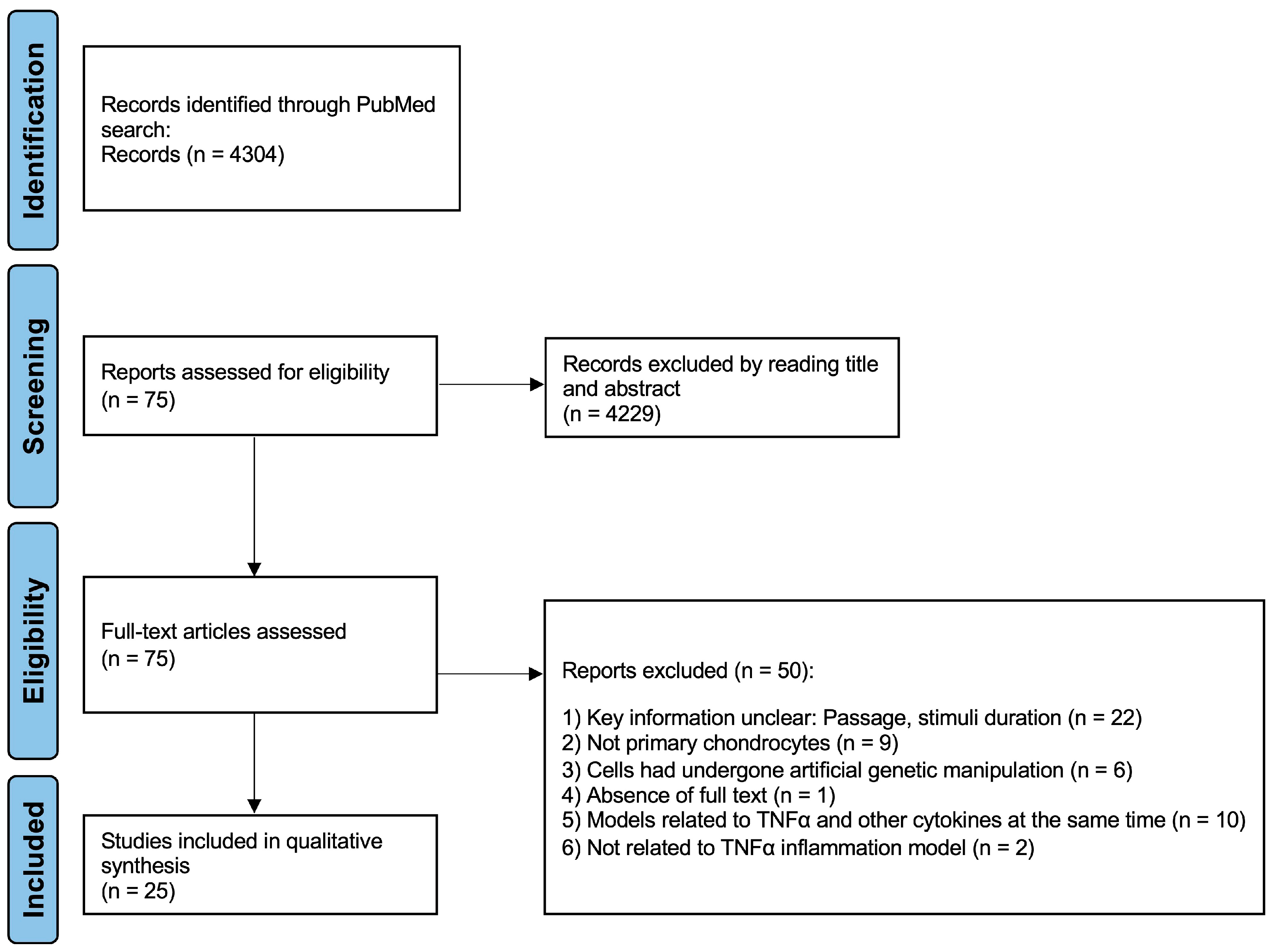
4.1. Exclusion and Inclusion Criteria and Study Selection
4.2. Data Collection Process
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baud, V.; Karin, M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001, 11, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, T.; Mitoma, H.; Harashima, S.-I.; Tsukamoto, H.; Shimoda, T. Transmembrane TNF-alpha: Structure, function and interaction with anti-TNF agents. Rheumatology 2010, 49, 1215–1228. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yu, M.; Hu, X.; Han, L.; Yang, K.; Ba, H.; Zhang, Z.; Yin, B.; Yang, X.-P.; Li, Z.; et al. STAT1 mediates transmembrane TNF-alpha-induced formation of death-inducing signaling complex and apoptotic signaling via TNFR1. Cell Death Differ. 2017, 24, 660–671. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.R. TNF-mediated inflammatory disease. J. Pathol. 2008, 214, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.-I.; Lee, A.-H.; Shin, H.-Y.; Song, H.-R.; Park, J.-H.; Kang, T.-B.; Lee, S.-R.; Yang, S.-H. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef]
- Smith, C.A.; Davis, T.; Anderson, D.; Solam, L.; Beckmann, M.P.; Jerzy, R.; Dower, S.K.; Cosman, D.; Goodwin, R.G. A Receptor for Tumor Necrosis Factor Defines an Unusual Family of Cellular and Viral Proteins. Science 1990, 248, 1019–1023. [Google Scholar] [CrossRef]
- Vandenabeele, P.; Declercq, W.; Beyaert, R.; Fiers, W. Two tumour necrosis factor receptors: Structure and function. Trends Cell Biol. 1995, 5, 392–399. [Google Scholar] [CrossRef]
- Catrina, A.I.; Klint, E.A.; Ernestam, S.; Catrina, S.; Makrygiannakis, D.; Botusan, I.R.; Klareskog, L.; Ulfgren, A. Anti-tumor necrosis factor therapy increases synovial osteoprotegerin expression in rheumatoid arthritis. Arthritis Rheum. 2006, 54, 76–81. [Google Scholar] [CrossRef]
- Wehling, N.; Palmer, G.D.; Pilapil, C.; Liu, F.; Wells, J.W.; Müller, P.E.; Evans, C.H.; Porter, R.M. Interleukin-1beta and tumor necrosis factor alpha inhibit chondrogenesis by human mesenchymal stem cells through NF-kappaB-dependent pathways. Arthritis Rheum. 2009, 60, 801–812. [Google Scholar] [CrossRef]
- Murakami, S.; Lefebvre, V.; de Crombrugghe, B. Potent inhibition of the master chondrogenic factor Sox9 gene by interleukin-1 and tumor necrosis factor-alpha. J. Biol. Chem. 2000, 275, 3687–3692. [Google Scholar] [CrossRef]
- Duprez, L.; Takahashi, N.; Van Hauwermeiren, F.; Vandendriessche, B.; Goossens, V.; Vanden Berghe, T.; Declercq, W.; Libert, C.; Cauwels, A.; Vandenabeele, P. RIP kinase-dependent necrosis drives lethal systemic inflammatory response syndrome. Immunity 2011, 35, 908–918. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Fadoju, D.; Rezvani, G.; De Luca, F. Stimulatory effects of insulin-like growth factor-I on growth plate chondrogenesis are mediated by nuclear factor-kappaB p65. J. Biol. Chem. 2008, 283, 34037–34044. [Google Scholar] [CrossRef] [PubMed]
- Oberst, A.; Dillon, C.P.; Weinlich, R.; McCormick, L.L.; Fitzgerald, P.; Pop, C.; Hakem, R.; Salvesen, G.S.; Green, D.R. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature 2011, 471, 363–367. [Google Scholar] [CrossRef] [PubMed]
- van Loo, G.; Bertrand, M.J.M. Death by TNF: A road to inflammation. Nat. Rev. Immunol. 2023, 23, 289–303. [Google Scholar] [CrossRef]
- Ahmad, R.; Sylvester, J.; Ahmad, M.; Zafarullah, M. Involvement of H-Ras and reactive oxygen species in proinflammatory cytokine-induced matrix metalloproteinase-13 expression in human articular chondrocytes. Arch. Biochem. Biophys. 2011, 507, 350–355. [Google Scholar] [CrossRef]
- Ben-Aderet, L.; Merquiol, E.; Fahham, D.; Kumar, A.; Reich, E.; Ben-Nun, Y.; Kandel, L.; Haze, A.; Liebergall, M.; Kosińska, M.K.; et al. Detecting cathepsin activity in human osteoarthritis via activity-based probes. Arthritis Res. Ther. 2015, 17, 1–14. [Google Scholar] [CrossRef]
- Guo, F.J.; Xiong, Z.; Lu, X.; Ye, M.; Han, X.; Jiang, R. ATF6 upregulates XBP1S and inhibits ER stress-mediated apoptosis in osteoarthritis cartilage. Cell. Signal. 2014, 26, 332–342. [Google Scholar] [CrossRef]
- Heinecke, L.F.; Grzanna, M.W.; Au, A.Y.; Mochal, C.A.; Rashmir-Raven, A.; Frondoza, C.G. Inhibition of cyclooxygenase-2 expression and prostaglandin E2 production in chondrocytes by avocado soybean unsaponifiables and epigallocatechin gallate. Osteoarthr. Cartil. 2010, 18, 220–227. [Google Scholar] [CrossRef]
- Imamura, T.; Imamura, C.; McAlinden, A.; Davies, S.R.; Iwamoto, Y.; Sandell, L.J. A novel tumor necrosis factor alpha-responsive CCAAT/enhancer binding protein site regulates expression of the cartilage-derived retinoic acid-sensitive protein gene in cartilage. Arthritis Rheum. 2008, 58, 1366–1376. [Google Scholar] [CrossRef]
- Kunisch, E.; Kinne, R.W.; Alsalameh, R.J.; Alsalameh, S. Pro-inflammatory IL-1beta and/or TNF-alpha up-regulate matrix metalloproteases-1 and -3 mRNA in chondrocyte subpopulations potentially pathogenic in osteoarthritis: In situ hybridization studies on a single cell level. Int. J. Rheum. Dis. 2016, 19, 557–566. [Google Scholar] [CrossRef]
- Madhavan, S.; Anghelina, M.; Sjostrom, D.; Dossumbekova, A.; Guttridge, D.C.; Agarwal, S. Biomechanical signals suppress TAK1 activation to inhibit NF-kappaB transcriptional activation in fibrochondrocytes. J. Immunol. 2007, 179, 6246–6254. [Google Scholar] [CrossRef] [PubMed]
- Qu, R.; Chen, X.; Wang, W.; Qiu, C.; Ban, M.; Guo, L.; Vasilev, K.; Chen, J.; Li, W.; Zhao, Y. Ghrelin protects against osteoarthritis through interplay with Akt and NF–kB signaling pathways. FASEB J. 2018, 32, 1044–1058. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Liu, Y.; Li, R. FSTL1 promotes inflammatory reaction and cartilage catabolism through interplay with NFkB signaling pathways in an in vitro ONFH model. Inflammation 2019, 42, 1491–1503. [Google Scholar] [CrossRef] [PubMed]
- Sirikaew, N.; Chomdej, S.; Tangyuenyong, S.; Tangjitjaroen, W.; Somgird, C.; Thitaram, C.; Ongchai, S. Proinflammatory cytokines and lipopolysaccharides up regulate MMP-3 and MMP-13 production in Asian elephant (Elephas maximus) chondrocytes: Attenuation by anti-arthritic agents. BMC Vet. Res. 2019, 15, 419. [Google Scholar] [CrossRef]
- Sommaggio, R.; Máñez, R.; Costa, C. TNF, pig CD86, and VCAM-1 identified as potential targets for intervention in xenotransplantation of pig chondrocytes. Cell Transplant. 2009, 18, 1381–1393. [Google Scholar] [CrossRef] [PubMed]
- Vaamonde-García, C.; Riveiro-Naveira, R.R.; Valcárcel-Ares, M.N.; Hermida-Carballo, L.; Blanco, F.J.; López-Armada, M.J. Mitochondrial dysfunction increases inflammatory responsiveness to cytokines in normal human chondrocytes. Arthritis Rheum. 2012, 64, 2927–2936. [Google Scholar] [CrossRef]
- Wang, W.; Li, J.; Li, F.; Peng, J.; Xu, M.; Shangguan, Y.; Li, Y.; Zhao, Y.; Qiu, C.; Qu, R.; et al. Scutellarin suppresses cartilage destruction in osteoarthritis mouse model by inhibiting the NF-kB and PI3K/AKT signaling pathways. Int. Immunopharmacol. 2019, 77, 105928. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Cao, Y.; Huang, T.; Song, D.X.; Tao, H.R. Therapeutic potential of hyaluronic acid/chitosan nanoparticles for the delivery of curcuminoid in knee osteoarthritis and an invitro evaluation in chondrocytes. Int. J. Mol. Med. 2018, 42, 2604–2614. [Google Scholar] [CrossRef]
- Weinmann, D.; Mueller, M.; Walzer, S.M.; Hobusch, G.M.; Lass, R.; Gahleitner, C.; Viernstein, H.; Windhager, R.; Toegel, S. Brazilin blocks catabolic processes in human osteoarthritic chondrocytes via inhibition of NFKB1/p50. J. Orthop. Res. 2018, 36, 2431–2438. [Google Scholar] [CrossRef]
- Woodell-May, J.; Matuska, A.; Oyster, M.; Welch, Z.; O’Shaughnessey, K.; Hoeppner, J. Autologous protein solution inhibits MMP-13 production by IL-1b and TNFa-stimulated human articular chondrocytes. J. Orthop. Res. 2011, 29, 1320–1326. [Google Scholar] [CrossRef]
- Zhi, L.Q.; Yao, S.X.; Liu, H.L.; Li, M.; Duan, N.; Ma, J.B. Hydroxytyrosol inhibits the inflammatory response of osteoarthritis chondrocytes via SIRT6-mediated autophagy. Mol. Med. Rep. 2017, 17, 4035–4042. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.-P.; Dai, B.-B.; Xie, Y.-Y.; Wu, X.-S.; Li, Y.; Wang, Z.-Q.; Zu, S.-Q.; Ge, J.-F.; Chen, F.-H. Interleukin-1b and tumor necrosis factor-a augment acidosis-induced rat articular chondrocyte apoptosis via nuclear factor-kappaB-dependent upregulation of ASIC1a channel. Biochim. Et Biophys. Acta(BBA)-Mol. Basis Dis. 2018, 1864, 162–177. [Google Scholar] [CrossRef] [PubMed]
- Žigon-Branc, S.; Barlič, A.; Knežević, M.; Jeras, M.; Vunjak-Novakovic, G. Testing the potency of anti-TNF-a and anti-IL-1b drugs using spheroid cultures of human osteoarthritic chondrocytes and donor-matched chondrogenically differentiated mesenchymal stem cells. Biotechnol. Prog. 2018, 34, 1045–1058. [Google Scholar] [CrossRef]
- Xiang, X.; Zhou, Y.; Sun, H.; Tan, S.; Lu, Z.; Huang, L.; Wang, W. Ivabradine abrogates TNF-a-induced degradation of articular cartilage matrix. Int. Immunopharmacol. 2019, 66, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Cillero-Pastor, B.; Ruiz-Romero, C.; Caramés, B.; López-Armada, M.J.; Blanco, F.J. Proteomic analysis by two-dimensional electrophoresis to identify the normal human chondrocyte proteome stimulated by tumor necrosis factor alpha and interleukin-1beta. Arthritis Rheum. 2010, 62, 802–814. [Google Scholar] [CrossRef] [PubMed]
- Wenger, R. Hydrostatic Pressure Increases Apoptosis in Cartilage-Constructs Produced From Human Osteoarthritic Chondrocytes. Front. Biosci. 2006, 11, 1690. [Google Scholar] [CrossRef] [PubMed]
- Olkkonen, J.; Kouri, V.P.; Hynninen, J.; Konttinen, Y.T.; Mandelin, J. Differentially expressed in chondrocytes 2 (DEC2) increases the expression of IL-1b and is abundantly present in synovial membrane in rheumatoid arthritis. PLoS ONE 2015, 10, e0145279. [Google Scholar] [CrossRef]
- Wang, L.; Yang, M.; Zhang, C.; Huang, F. The protective effects of dehydrocostus lactone against TNF-a-induced degeneration of extracellular matrix (ECM) in SW1353 cells. Aging 2020, 12, 17137–17149. [Google Scholar] [CrossRef]
- Huang, Y.; Jiang, T.; Chen, J.; Yin, G.Y.; Fan, J. Effects of kartogenin on the attenuated nucleus pulposus cell degeneration of intervertebral discs induced by interleukin-1b and tumor necrosis factor-a. Int. J. Mol. Med. 2017, 41, 749–756. [Google Scholar] [CrossRef]
- Jones, S.W.; Brockbank, S.M.V.; Clements, K.M.; Le Good, N.; Campbell, D.; Read, S.J.; Newham, P. Mitogen-activated protein kinase-activated protein kinase 2 (MK2) modulates key biological pathways associated with OA disease pathology. Osteoarthr. Cartil. 2009, 17, 124–131. [Google Scholar] [CrossRef]
- Rainbow, R.S.; Kwon, H.; Foote, A.T.; Preda, R.C.; Kaplan, D.L.; Zeng, L. Muscle cell-derived factors inhibit inflammatory stimuli-induced damage in hMSC-derived chondrocytes. Osteoarthr. Cartil. 2013, 21, 990–998. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Xu, W.; Yan, J.-L.; Yokota, H.; Na, S. Distinctive subcellular inhibition of cytokine-induced src by salubrinal and fluid flow. PLoS ONE 2014, 9, e105699. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Qu, L. The anti-fibrotic agent nintedanib protects chondrocytes against tumor necrosis factor-a (TNF-a)-induced extracellular matrix degradation. Bioengineered 2022, 13, 5318–5329. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Liu, L.; Xu, F.; Wu, X.; Yin, Z.; Dong, Y.; Qian, P. Feprazone ameliorates TNF-a-Induced loss of aggrecan via inhibition of the SOX-4/ADAMTS-5 signaling pathway. ACS Omega 2021, 6, 7638–7645. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Dong, Y.; Dong, H.; Cui, Y.; Du, Q.; Wang, X.; Li, L.; Zhang, H. Telmisartan mitigates TNF-a-Induced type II collagen reduction by upregulating SOX-9. ACS Omega 2021, 6, 11756–11761. [Google Scholar] [CrossRef]
- Kim, H.A.; Yeo, Y.; Jung, H.A.; Jung, Y.O.; Park, S.J.; Kim, S.J. Phase 2 enzyme inducer sulphoraphane blocks prostaglandin and nitric oxide synthesis in human articular chondrocytes and inhibits cartilage matrix degradation. Rheumatology 2012, 51, 1006–1016. [Google Scholar] [CrossRef]
- Malemud, C.J.; Sun, Y.; Pearlman, E.; Ginley, N.M.; Awadallah, A.; Wisler, B.A.; Dennis, J.E. Monosodium urate and tumor necrosis factor-a increase apoptosis in human chondrocyte cultures. Rheumatology 2012, 2, 113. [Google Scholar] [CrossRef]
- López-Armada, M.J.; Caramés, B.; Martín, M.A.; Cillero-Pastor, B.; Lires-Dean, M.; Fuentes-Boquete, I.; Arenas, J.; Blanco, F.J. Mitochondrial activity is modulated by TNFalpha and IL-1beta in normal human chondrocyte cells. Osteoarthr. Cartil. 2006, 14, 1011–1022. [Google Scholar] [CrossRef]
- López-Armada, M.J.; Caramés, B.; Lires-Deán, M.; Cillero-Pastor, B.; Ruiz-Romero, C.; Galdo, F.; Blanco, F. Cytokines, tumor necrosis factor-alpha and interleukin-1beta, differentially regulate apoptosis in osteoarthritis cultured human chondrocytes. Osteoarthr. Cartil. 2006, 14, 660–669. [Google Scholar] [CrossRef]
- Boileau, C.; Amiable, N.; Martel-Pelletier, J.; Fahmi, H.; Duval, N.; Pelletier, J.P. Activation of proteinase-activated receptor 2 in human osteoarthritic cartilage upregulates catabolic and proinflammatory pathways capable of inducing cartilage degradation: A basic science study. Arthritis Res. Ther. 2007, 9, R121. [Google Scholar] [CrossRef]
- Tardif, G. Differential regulation of the bone morphogenic protein antagonist chordin in human normal and osteoarthritic chondrocytes. Ann. Rheum. Dis. 2006, 65, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Caramés, B.; López-Armada, M.J.; Cillero-Pastor, B.; Lires-Dean, M.; Vaamonde, C.; Galdo, F.; Blanco, F. Differential effects of tumor necrosis factor-alpha and interleukin-1beta on cell death in human articular chondrocytes. Osteoarthr. Cartil. 2008, 16, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Yik, J.H.N.; Hu, Z.; Kumari, R.; Christiansen, B.A.; Haudenschild, D.R. Cyclin-Dependent Kinase 9 Inhibition Protects Cartilage From the Catabolic Effects of Proinflammatory Cytokines. Arthritis Rheumatol. 2014, 66, 1537–1546. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Buckwalter, J.A.; Martin, J.A. DAMPs synergize with cytokines or fibronectin fragment on inducing chondrolysis but lose effect when acting alone. Mediat. Inflamm. 2017, 2017, 2642549. [Google Scholar] [CrossRef] [PubMed]
- Terkeltaub, R.; Yang, B.; Lotz, M.; Liu-Bryan, R. Chondrocyte AMP-activated protein kinase activity suppresses matrix degradation responses to proinflammatory cytokines interleukin-1b and tumor necrosis factor a. Arthritis Rheum. 2011, 63, 1928–1937. [Google Scholar] [CrossRef]
- Chen, H.; Shao, X.; Li, L.; Zheng, C.; Xu, X.; Hong, X.; Li, X.; Wu, M. Electroacupuncture serum inhibits TNF-a-mediated chondrocyte inflammation via the Ras-Raf-MEK1/2-ERK1/2 signaling pathway. Mol. Med. Rep. 2017, 16, 5807–5814. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, X.; Shang, W.; Xu, J.; Wang, X.; Hu, X.; Ao, Y.; Cheng, H. Proinflammatory Cytokines Stimulate Mitochondrial Superoxide Flashes in Articular Chondrocytes In Vitro and In Situ. PLoS ONE 2013, 8, e66444. [Google Scholar] [CrossRef]
- Djouad, F.; Rackwitz, L.; Song, Y.; Janjanin, S.; Tuan, R.S. ERK1/2 activation induced by inflammatory cytokines compromises effective host tissue integration of engineered cartilage. Tissue Eng. Part A 2009, 15, 2825–2835. [Google Scholar] [CrossRef]
- Chen, C.; Xie, J.; Rajappa, R.; Deng, L.; Fredberg, J.; Yang, L. Interleukin-1b and tumor necrosis factor-a increase stiffness and impair contractile function of articular chondrocytes. Acta Biochim. Biophys. Sin. 2015, 47, 121–129. [Google Scholar] [CrossRef]
- Mohanraj, B.; Huang, A.H.; Yeger–McKeever, M.J.; Schmidt, M.J.; Dodge, G.R.; Mauck, R.L. Chondrocyte and mesenchymal stem cell derived engineered cartilage exhibits differential sensitivity to pro–inflammatory cytokines. J. Orthop. Res. 2018, 36, 2901–2910. [Google Scholar] [CrossRef]
- Ossendorff, R.; Grad, S.; Stoddart, M.J.; Alini, M.; Schmal, H.; Südkamp, N.; Salzmann, G.M. Autologous chondrocyte implantation in osteoarthritic surroundings: TNFa and its inhibition by adalimumab in a knee-specific bioreactor. Am. J. Sports Med. 2018, 46, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Tilwani, R.K.; Vessillier, S.; Pingguan-Murphy, B.; Lee, D.A.; Bader, D.L.; Chowdhury, T.T. Oxygen tension modulates the effects of TNFa in compressed chondrocytes. Inflamm. Res. 2017, 66, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Morris, K.J.; Cs-Szabo, G.; Cole, A.A. Characterization of TIMP-3 in human articular talar cartilage. Connect. Tissue Res. 2010, 51, 478–490. [Google Scholar] [CrossRef] [PubMed]
- Little, C.B.; Flannery, C.R.; Hughes, C.E.; Goodship, A.; Caterson, B. Cytokine induced metalloproteinase expression and activity does not correlate with focal susceptibility of articular cartilage to degeneration. Osteoarthr. Cartil. 2005, 13, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Little, C.B.; Flannery, C.R.; Hughes, C.E.; Mort, J.S.; Roughley, P.J.; Dent, C.; Caterson, B. Aggrecanase versus matrix metalloproteinases in the catabolism of the interglobular domain of aggrecan in vitro. Biochem. J. 1999, 344 Pt 1, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Bevill, S.L.; Boyer, K.A.; Andriacchi, T.P. The Regional Sensitivity of Chondrocyte Gene Expression to Coactive Mechanical Load and Exogenous TNF-α Stimuli. J. Biomech. Eng. 2014, 136, 0910051–0910057. [Google Scholar] [CrossRef]
- Stevens, A.L.; Wishnok, J.S.; Chai, D.H.; Grodzinsky, A.J.; Tannenbaum, S.R. A sodium dodecyl sulfate-polyacrylamide gel electrophoresis-liquid chromatography tandem mass spectrometry analysis of bovine cartilage tissue response to mechanical compression injury and the inflammatory cytokines tumor necrosis factor alpha and interleukin-1beta. Arthritis Rheum. 2008, 58, 489–500. [Google Scholar] [CrossRef]
- Sui, Y.; Lee, J.H.; DiMicco, M.A.; Vanderploeg, E.J.; Blake, S.M.; Hung, H.; Plaas, A.H.K.; James, I.E.; Song, X.; Lark, M.W.; et al. Mechanical injury potentiates proteoglycan catabolism induced by interleukin-6 with soluble interleukin-6 receptor and tumor necrosis factor alpha in immature bovine and adult human articular cartilage. Arthritis Rheum. 2009, 60, 2985–2996. [Google Scholar] [CrossRef]
- Shakibaei, M.; John, T.; Schulze-Tanzil, G.; Lehmann, I.; Mobasheri, A. Suppression of NF-kappaB activation by curcumin leads to inhibition of expression of cyclo-oxygenase-2 and matrix metalloproteinase-9 in human articular chondrocytes: Implications for the treatment of osteoarthritis. Biochem. Pharmacol. 2007, 73, 1434–1445. [Google Scholar] [CrossRef]
- Roman-Blas, J.A.; Stokes, D.G.; Jimenez, S.A. Modulation of TGF-beta signaling by proinflammatory cytokines in articular chondrocytes. Osteoarthr. Cartil. 2007, 15, 1367–1377. [Google Scholar] [CrossRef]
- Yun, K.; Choi, Y.D.; Nam, J.H.; Park, Z.; Im, S.-H. NF-kappaB regulates Lef1 gene expression in chondrocytes. Biochem. Biophys. Res. Commun. 2007, 357, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Saklatvala, J. Inflammatory signaling in cartilage: MAPK and NF-kappaB pathways in chondrocytes and the use of inhibitors for research into pathogenesis and therapy of osteoarthritis. Curr. Drug Targets 2007, 8, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.D.; Thoma, L.M.; Golightly, Y.M. Epidemiology of osteoarthritis. Osteoarthr. Cartil. 2022, 30, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Dai, A.; Yang, M.; Chen, S.; Deng, Z.; Li, L. p38MAPK Signaling Pathway in Osteoarthritis: Pathological and Therapeutic Aspects. J. Inflamm. Res. 2022, 15, 723–734. [Google Scholar] [CrossRef]
- Zhou, Q.; Ren, Q.; Jiao, L.; Huang, J.; Yi, J.; Chen, J.; Lai, J.; Ji, G.; Zheng, T. The potential roles of JAK/STAT signaling in the progression of osteoarthritis. Front. Endocrinol. 2022, 13, 1069057. [Google Scholar] [CrossRef]
- Saklatvala, J. Tumour necrosis factor alpha stimulates resorption and inhibits synthesis of proteoglycan in cartilage. Nature 1986, 322, 547–549. [Google Scholar] [CrossRef]
- Sun, K.; Luo, J.; Guo, J.; Yao, X.; Jing, X.; Guo, F. The PI3K/AKT/mTOR signaling pathway in osteoarthritis: A narrative review. Osteoarthr. Cartil. 2020, 28, 400–409. [Google Scholar] [CrossRef]
- Fu, L.; Zhang, L.; Zhang, X.; Chen, L.; Cai, Q.; Yang, X. Roles of oxygen level and hypoxia-inducible factor signaling pathway in cartilage, bone and osteochondral tissue engineering. Biomed. Mater. 2021, 16, 022006. [Google Scholar] [CrossRef]
- Johnson, C.I.; Argyle, D.J.; Clements, D.N. In vitro models for the study of osteoarthritis. Vet. J. 2016, 209, 40–49. [Google Scholar] [CrossRef]
- Cope, P.J.; Ourradi, K.; Li, Y.; Sharif, M. Models of osteoarthritis: The good, the bad and the promising. Osteoarthr. Cartil. 2019, 27, 230. [Google Scholar] [CrossRef]
- Grad, S.; Eglin, D.; Alini, M.; Stoddart, M.J. Physical stimulation of chondrogenic cells in vitro: A review. Clin. Orthop. Relat. Res. 2011, 469, 2764–2772. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Patel, J.M.; Wise, B.C.; Bonnevie, E.D.; Mauck, R.L. A Systematic Review and Guide to Mechanical Testing for Articular Cartilage Tissue Engineering. Tissue Eng. Part C Methods 2019, 25, 593–608. [Google Scholar] [CrossRef] [PubMed]
- Reddi, A.H.; Becerra, J.; Andrades, J.A. Nanomaterials and hydrogel scaffolds for articular cartilage regeneration. Tissue Eng. Part B Rev. 2011, 17, 301–305. [Google Scholar] [CrossRef]
- van Haaften, E.E.; Ito, K.; van Donkelaar, C.C. The initial repair response of articular cartilage after mechanically induced damage. J. Orthop. Res. 2017, 35, 1265–1273. [Google Scholar] [CrossRef]
- Juhász, T.; Matta, C.; Somogyi, C.; Katona, E.; Takács, R.; Soha, R.F.; Szabó, I.A.; Cserháti, C.; Sződy, R.; Karácsonyi, Z.; et al. Mechanical loading stimulates chondrogenesis via the PKA/CREB-Sox9 and PP2A pathways in chicken micromass cultures. Cell. Signal. 2014, 26, 468–482. [Google Scholar] [CrossRef]
- McCoy, A.M. Animal Models of Osteoarthritis: Comparisons and Key Considerations. Vet. Pathol. 2015, 52, 803–818. [Google Scholar] [CrossRef]
- Huntley, J.S.; Simpson, A.H.; Hall, A.C. Use of non-degenerate human osteochondral tissue and confocal laser scanning microscopy for the study of chondrocyte death at cartilage surgery. Eur. Cells Mater. 2005, 9, 13–22. [Google Scholar] [CrossRef]
- Buckwalter, J.A.; Mankin, H.J.; Grodzinsky, A.J. Articular cartilage and osteoarthritis. Lect.-Am. Acad. Orthop. Surg. 2005, 54, 465–480. [Google Scholar]
- Athanasiou, K.A.; Darling, E.M.; Hu, J.C.; DuRaine, G.D.; Reddi, A.H. Articular Cartilage, 2nd ed.; Taylor & Francis: Abingdon, UK, 2016. [Google Scholar]
- Elahi, S.A.; Castro-Viñuelas, R.; Govaerts, A.; Lories, R.; Famaey, N.; Jonkers, I. Unconfined Compression Experimental Protocol for Cartilage Explants and Hydrogel Constructs: From Sample Preparation to Mechanical Characterization. Methods Mol. Biol. 2023, 2598, 271–287. [Google Scholar] [CrossRef]
- Olvera, D.; Daly, A.; Kelly, D.J. Mechanical Testing of Cartilage Constructs. Methods Mol. Biol. 2015, 1340, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Henrotin, Y.; Pesesse, L.; Sanchez, C. Identification of Mechanosensitive Genes in Chondrocytes and Osteoblasts and Their Role in OA Pathogenesis. In Mechanical Stretch and Cytokines; Kamkin, A., Kiseleva, I., Eds.; Mechanosensitivity in Cells and Tissues; Springer: Dordrecht, The Netherlands, 2012; pp. 223–233. [Google Scholar] [CrossRef]
- Fang, T.; Zhou, X.; Jin, M.; Nie, J.; Li, X. Molecular mechanisms of mechanical load-induced osteoarthritis. Int. Orthop. 2021, 45, 1125–1136. [Google Scholar] [CrossRef] [PubMed]
- Logerstedt, D.S.; Ebert, J.R.; MacLeod, T.D.; Heiderscheit, B.C.; Gabbett, T.J.; Eckenrode, B.J. Effects of and Response to Mechanical Loading on the Knee. Sports Med. 2022, 52, 201–235. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Frank, E.H.; Wang, Y.; Chubinskaya, S.; Huang, H.H.; Grodzinsky, A.J. Moderate dynamic compression inhibits pro-catabolic response of cartilage to mechanical injury, tumor necrosis factor-a and interleukin-6, but accentuates degradation above a strain threshold. Osteoarthr. Cartil. 2013, 21, 1933–1941. [Google Scholar] [CrossRef] [PubMed]
- Torzilli, P.A.; Bhargava, M.; Park, S.; Chen, C. Mechanical Load Inhibits Il-1 Induced Matrix Degradation in Articular Cartilage. Osteoarthr. Cartil. 2010, 18, 97. [Google Scholar] [CrossRef]
- Natenstedt, J.; Kok, A.C.; Dankelman, J.; Tuijthof, G.J. What quantitative mechanical loading stimulates in vitro cultivation best? J. Exp. Orthop. 2015, 2, 15. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ. 2009, 339, b2700. [Google Scholar] [CrossRef]
| Species | Authors | Origin | Age (Mean) | Concentration (ng/mL) | Passage | Duration | Main Findings |
|---|---|---|---|---|---|---|---|
| Human | Kim et al. [46] | Knee OA | 69.5 y | 10 | 1 | 24 h | Sulforaphane (SFN) hindered the synthesis of prostaglandin and nitric oxide in human articular chondrocytes, thereby preventing the degradation of cartilage matrix. |
| Human | Malemud et al. [47] | OA Adult knee Juvenile knee | NA NA 12 y | 10 | 1 | 1 h | TNFα could increase apoptosis in normal human chondrocytes, OA chondrocytes and human juvenile chondrocyte pellet cultures, but not in chondrocyte pellet cultures initiated from MSCs. |
| Human | López-Armada et al. [48] | Healthy knee | 59 y | 10 | 1 | 6 days | TNFα and IL1β impacted the mitochondrial function of human articular chondrocytes, and the inhibition of complex I may be a factor in the cartilage breakdown caused by these cytokines. |
| Human | López-Armada et al. [49] | Hip OA | NA | 10 | 1 | 7 days | TNFα and IL-1β exerted distinct influences on the apoptotic pathway in human chondrocytes, with this variation being contingent upon the levels of PGE2 and caspase-8. |
| Human | Boileau et al. [50] | Healthy knee Knee OA | 52 y 76 y | 5 | 1 | 72 h | Activation of PAR-2 in osteoarthritic cartilage influenced disease pathways, making PAR-2 antagonists promising for OA treatment. |
| Human | Tardif et al. [51] | Healthy cartilage OA | 66 y 71 y | 5 | 1 | 24 h | Chordin was regulated differently in normal and osteoarthritic human chondrocytes. |
| Human | Caramés et al. [52] | Healthy cartilage | NA | 10 | 1 | 24 h | TNFα and IL-1β regulated apoptosis differently in human chondrocytes. |
| Human | Yik et al. [53] | Knee OA | 44–80 y | 10 | 0 | 5 h | CDK-9 activity was required for the primary inflammatory response in chondrocytes, CDK-9 inhibition provides protection of cartilage against catabolism effects of proinflammatory cytokines. |
| Human | Ding et al. [54] | Healthy ankle | 45 y | 100 | 2 | 24 h | DAMPs induced chondrolysis with cytokines or fibronectin fragment, but lost their effects when acting alone. |
| Human | Terkeltaub et al. [55] | Healthy knee OA knee | NA NA | 10 | 1 | 18 h | Maintaining AMPK activation protected the cartilage matrix from deterioration caused by inflammation. |
| Rat | Chen et al. [56] | Wild type | 4 w | 10 | 3 | 72 h | Electroacupuncture serum inhibits TNFα mediated chondrocyte inflammation via the Ras-Raf-MEK1/2-ERK1/2 signaling pathway. |
| Mouse | Cao et al. [57] | Wild type Transgenic | 4 w 4 w | 10 | 1 | 1 h | TNFα stimulated mitochondrial superoxide flash activity by 2-fold in vitro and 5-fold in situ. Mitochondria are a significant source of cellular oxidants. |
| Bovine | Ding et al. [54] | Healthy stifle joint | NA | 100 | 1 | 24 h | DAMPs induced chondrolysis with cytokines or fibronectin fragment, but lost their effects when acting alone. |
| Bovine | Djouad et al. [58] | Carpometacarpal joint (healthy) | 6 m | 10 | 1 | 72 h | Proinflammation cytokines had devastating effects on the cartilage constructs and those effects could be inhibited by the blockade of ERK signaling pathway. |
| Goat | Chen et al. [59] | Healthy knee | NA | 40 | 1 | 24 h | Proinflammatory cytokine could change the mechanical properties of chondrocyte in vitro. |
| Species | Authors | Origin | Age (Mean) | Model | Concentration (ng/mL) | Passage | Duration | Main Findings |
|---|---|---|---|---|---|---|---|---|
| Bovine | Mohanraj et al. [60] | Healthy knee | 2–6 m | Agarose construct + TNFα | 1, 5, 10 | 0 | 6 days | MSC-derived constructs were more easily influenced by the inflammatory environment compared to chondrocyte-derived constructs. |
| Bovine | Ossendorff et al. [61] | Healthy fetlock joint | 5–7 m | PolyurethanePU scaffolds + TNFα | 20 | 3 | 14 days | TNFα had negative effects on chondrogenesis under simulated ACI conditions. Dynamic load and adalimumab inhibited those effects. |
| Bovine | Tilwani et al. [62] | Healthy cartilage | <18 m | Agarose construct + TNFα | 0.1, 10, 100 | 0 | 48 h | TNF enhanced NO, PGE2, and MMP activity at 5% oxygen tension, and dynamic compression might counteract this impact. |
| Human | Morris et al. [63] | Healthy knee and ankle | 65 y | alginate beads + TNFα | 1, 10, 50, 100 | 0 | 48 h | TIMP3 may serve a chondroprotective role and it may be necessary to bind to ECM for its full function. |
| Species | Authors | Origin | Age | Concentration (ng/mL) | Duration | Main Findings |
|---|---|---|---|---|---|---|
| Human | Boileau et al. [50] | Healthy knee OA knee | 52 y 76 y | 5 | 2 days | PAR-2 activation had been linked to catabolic and inflammatory processes associated with OA development. |
| Human | Kim et al. [46] | OA knee | 69.5 y | 10 | 9 days | Sulphoraphane (SFN) inhibited multiple catabolic mechanisms in cartilage. |
| Human | Tardif et al. [51] | Healthy cartilage OA Cartilage | 66 71 | 5 | 3 days | Chordin was regulated differently in normal and osteoarthritic human chondrocytes. |
| Mouse | Cao et al. [57] | Hip and Knee wild type/transgenic | 4 w | 10 | 2 h | TNFα stimulated mitochondrial superoxide flash activity by 2-fold in vitro and 5-fold in situ. Mitochondria are a significant source of cellular oxidants. |
| Mouse | Terkeltaub et al. [55] | Healthy Femoral Head, wild-type | 2 m | 10 | 3 days | Maintaining AMPK activation protected the cartilage matrix from deterioration caused by inflammation. |
| Horse | Little et al. [64] | Healthy fetlock joint | 2–12 y | 100 | 4 days | Regional difference in response to catabolic cytokines was unlikely to be responsible for the initiation of focal cartilage degeneration in osteoarthritis. |
| Bovine/Porcine | Little et al. [65] | Healthy fetlock joints | 2 w 3–6 m | 100 | 4–20 days (Bovine, Porcine) | In vitro, aggrecan produced from normal and OA cartilage in response to TNFα is cleaved by aggrecanase rather than MMPs. |
| Porcine | Bevill et al. [66] | Healthy knee | 6–8 m | 100 | 6 h | The core and peripheral areas of tibial cartilage have distinct gene expression responses to mechanical strain and TNFα. |
| Bovine | Stevens et al. [67] | Healthy knee | 2–3 w | 100 | 5 days | Overload compression damaged the cartilage matrix and disrupted the cell membrane. IL-1 and TNFα stimulate chondrocytes to release proteins linked with innate immune and stress responses, which may aid in host defense against infections and protect cells from stress-induced damage. |
| Bovine Human | Sui et al. [68] | Healthy knee Healty ankle | 1–2 w 26–61 y | 25 (Bovine) 100 (Human) | 6 days (Bovine) 8 days (Human) | Mechanical stimulation may enhance the catabolic effects of proinflammatory cytokine. In addition, IL-6/sIL-6R works in tandem with TNFα to cause cartilage deterioration. |
| Species | Authors | Model | Origin | Age | Concentration (ng/mL) | Passage | Duration | Main Findings |
|---|---|---|---|---|---|---|---|---|
| Human | Shakibaei et al. [69] | 3D to 2D: chondrocytes migrated from alginate bead construct to monolayer culture + TNFα | Hip joint (femoral neck fracture) | NA | 10 | 2 | 24 h | Curcumin reduced TNF-induced COX-2 and MMP-9 expression in chondrocytes. |
| Human | Roman-Blas et al. [70] | 2D: Suspension culture + TNFα | OA knee | NA | 10 | 0 | 2 h | IL-1β or TNFα suppressed Smad3/4 DNA-binding activity. IL-1β or TNFα suppression of TGF-β signaling pathways may no related to Smad7. |
| Human | Malemud et al. [47] | 3D: Chondrocytes pellet + recombinant human TNFα | OA knee adult knee juvenile knee | NA NA 12 y | 10, 20, 50 | 1 | 48 h | TNFα could increase apoptosis in normal human chondrocytes, OA chondrocytes and human juvenile chondrocyte pellet cultures, but not in chondrocyte pellet cultures initiated from MSCs. |
| Bovine | Djouad et al. [58] | 3D: Agarose construct + cartilage ring | Healthy fetlock joint | 6 m | 1, 10 | 1 | 28 days | Proinflammation cytokines had devastating effects on the cartilage constructs and those effects could be inhibited by the blockade of ERK signaling pathway. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Kurth, S.; Burger, C.; Wirtz, D.C.; Schildberg, F.A.; Ossendorff, R. TNFα-Related Chondrocyte Inflammation Models: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 10805. https://doi.org/10.3390/ijms251910805
Wang S, Kurth S, Burger C, Wirtz DC, Schildberg FA, Ossendorff R. TNFα-Related Chondrocyte Inflammation Models: A Systematic Review. International Journal of Molecular Sciences. 2024; 25(19):10805. https://doi.org/10.3390/ijms251910805
Chicago/Turabian StyleWang, Su, Sarah Kurth, Christof Burger, Dieter C. Wirtz, Frank A. Schildberg, and Robert Ossendorff. 2024. "TNFα-Related Chondrocyte Inflammation Models: A Systematic Review" International Journal of Molecular Sciences 25, no. 19: 10805. https://doi.org/10.3390/ijms251910805









