Pre- and Postnatal Vitamin A Deficiency Impairs Motor Skills without a Consistent Effect on Trace Mineral Status in Young Mice
Abstract
:1. Introduction
2. Results
2.1. Plasma and Hepatic Retinol Concentration
2.2. Clinical Manifestations
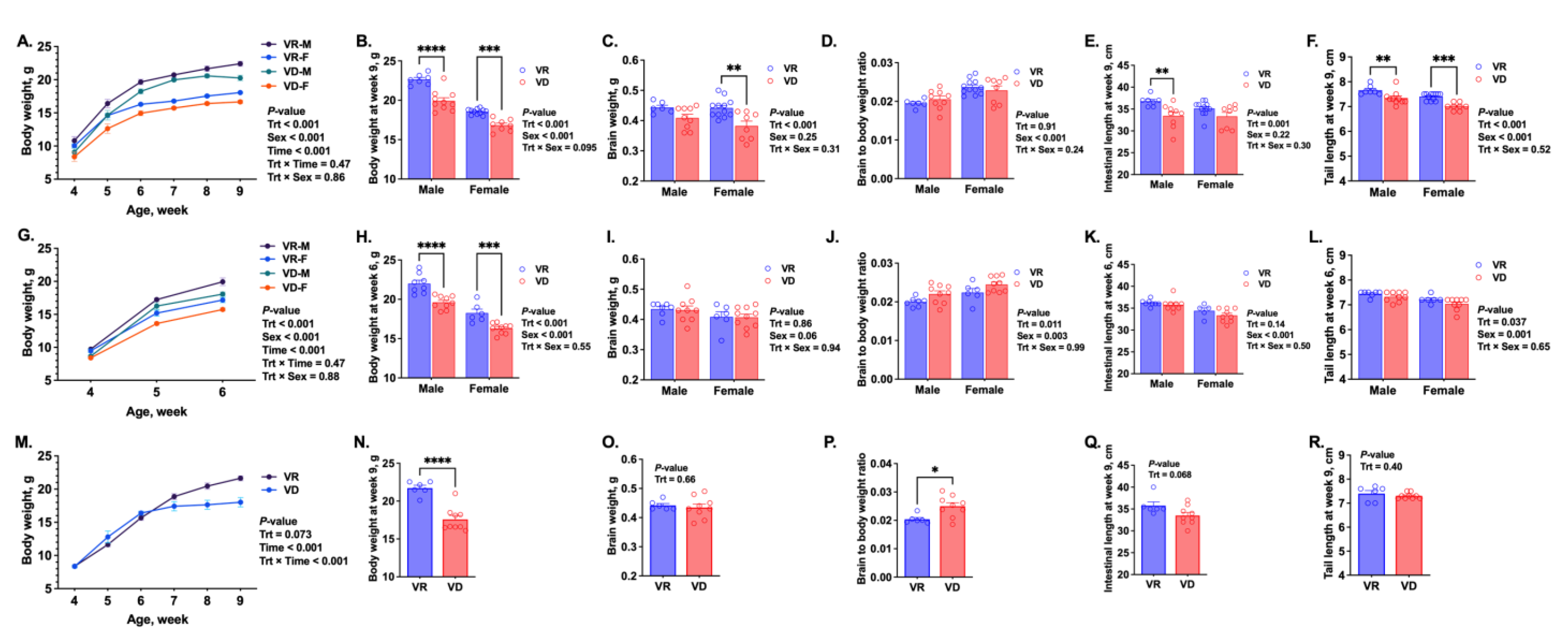
2.3. Growth and Development
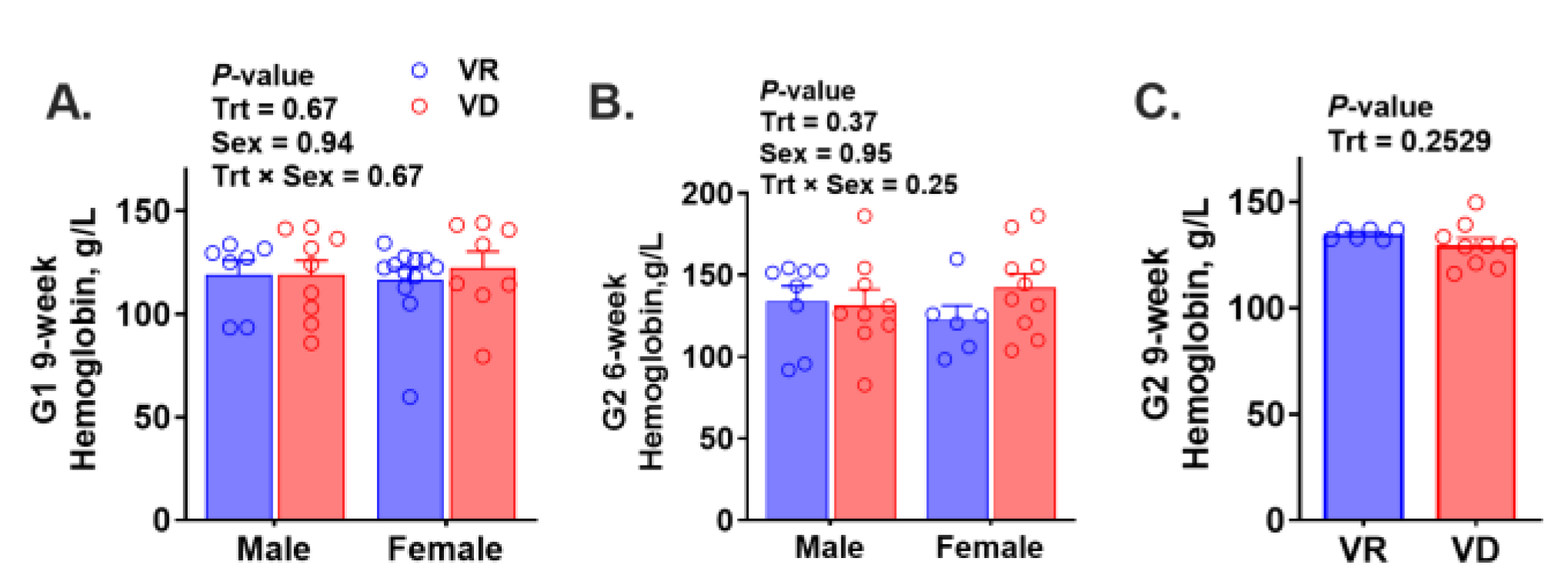
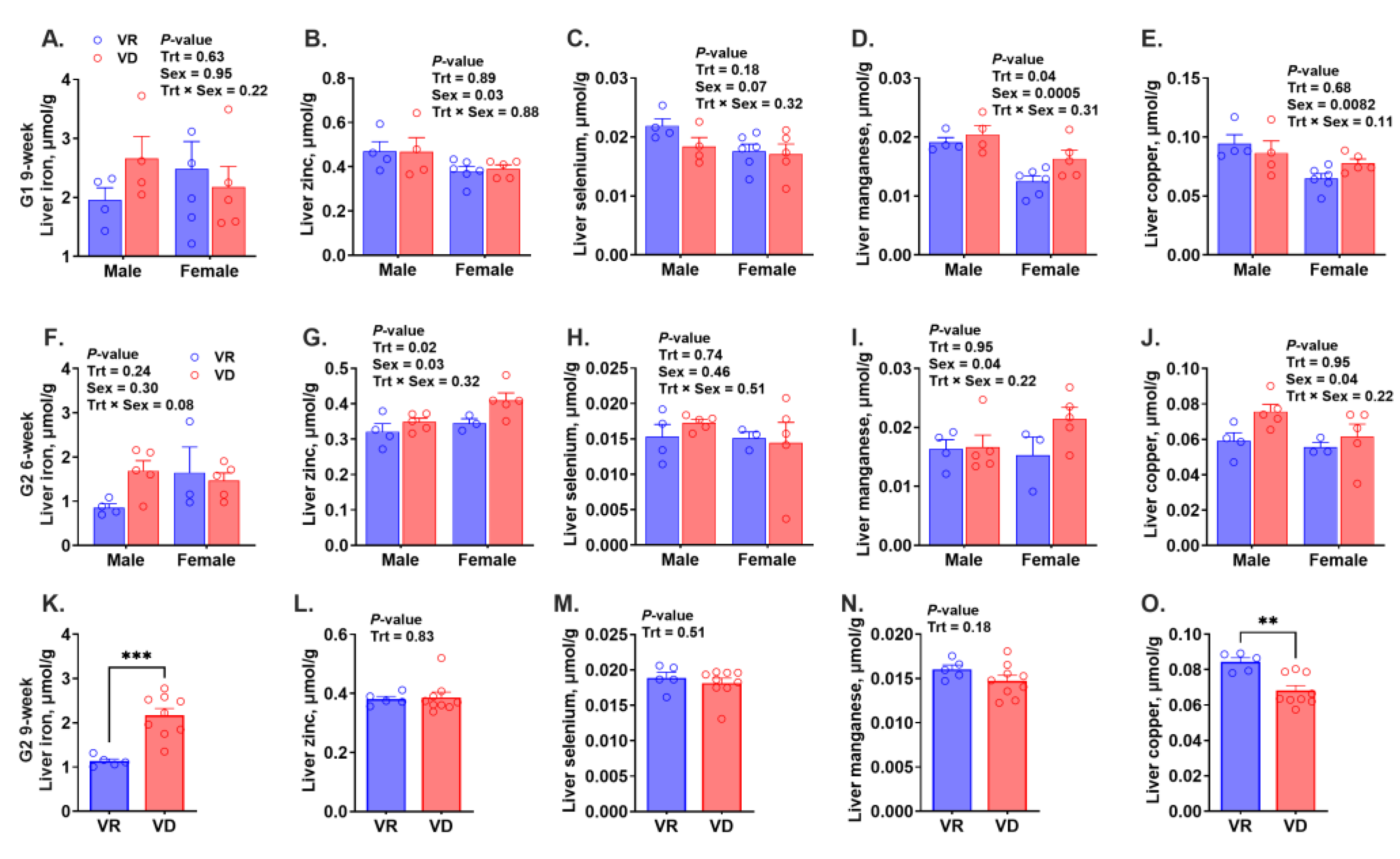
2.4. Risk of Anemia and Trace Mineral Concentrations in the Liver
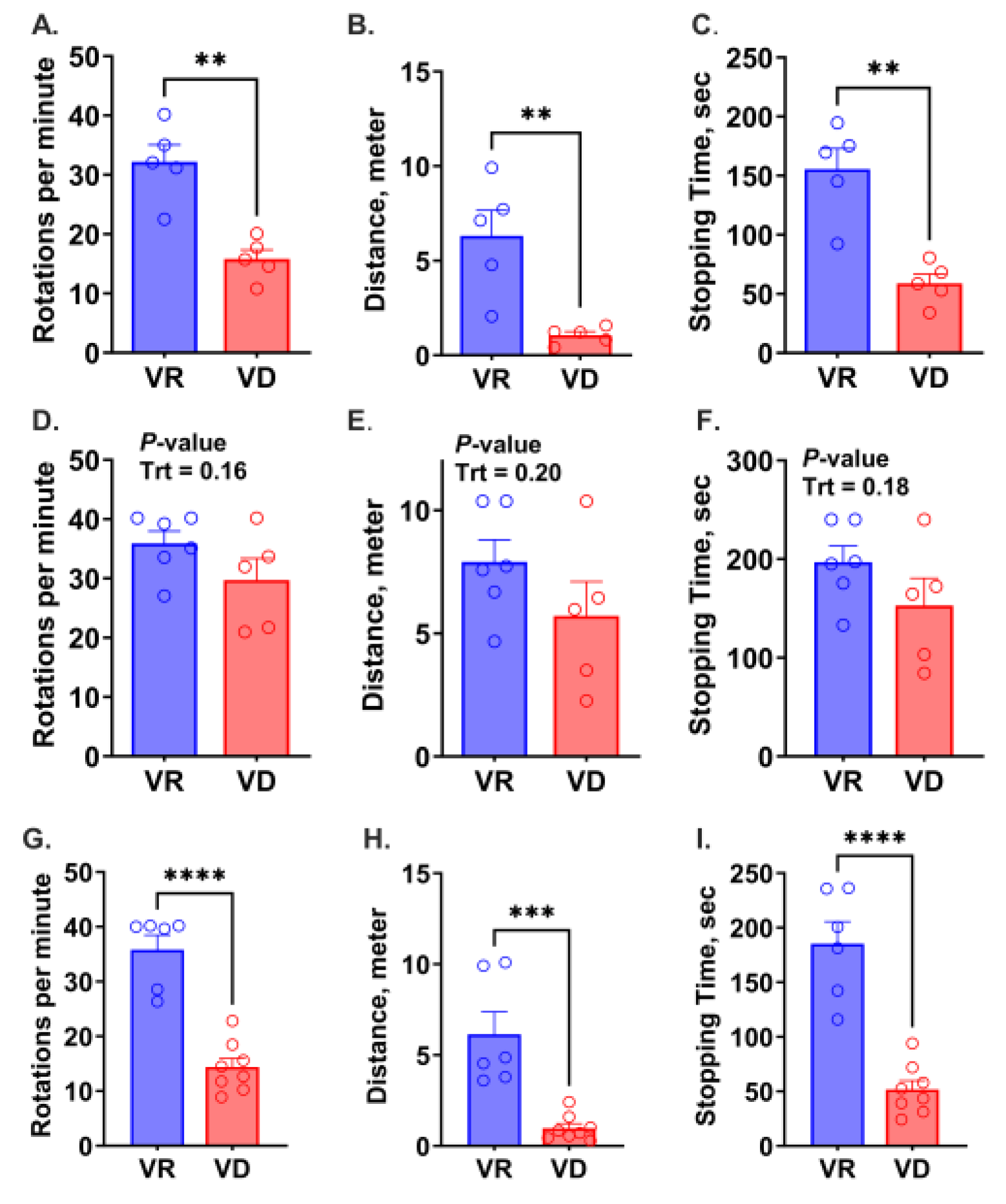
2.5. Motor Coordination
2.6. Grip Strength
2.7. Anxiety Behavior
2.8. Spatial Cognition
3. Discussion
3.1. Developmental Outcomes and Clinical Symptoms
3.2. Motor Incoordination
3.3. Trace Mineral Status and Risk of Anemia
3.4. Conclusions
4. Materials and Methods
4.1. Experimental Design
4.2. Elevated Plus Maze Test for Anxiety
4.3. Barnes Maze Test for Spatial Cognition
4.4. Grip Strength Test
4.5. Rotarod Test for Motor Function
4.6. Plasma and Liver Retinol Analysis
4.7. Trace Mineral Analysis
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bailey, R.L.; West, K.P., Jr.; Black, R.E. The Epidemiology of Global Micronutrient Deficiencies. Ann. Nutr. Metab. 2015, 66 (Suppl. S2), 22–33. [Google Scholar] [CrossRef] [PubMed]
- Demissie, T.; Ali, A.; Mekonen, Y.; Haider, J.; Umeta, M. Magnitude and distribution of vitamin A deficiency in Ethiopia. Food Nutr. Bull. 2010, 31, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Adeloye, D.; Li, S.; Zhao, D.; Ye, X.; Pan, Q.; Qiu, Y.; Zhang, R.; Rudan, I.; Group, G.H.E.R. The prevalence of vitamin A deficiency and its public health significance in children in low-and middle-income countries: A systematic review and modelling analysis. J. Glob. Health 2023, 13, 04084. [Google Scholar] [CrossRef]
- Haddad, L.J.; Ross, J.S. 5th report on the world nutrition situation: Nutrition for improved development outcomes. In Proceedings of the United Nations System Standing Committee on Nutrition (SCN), Geneva, Switzerland, 13–17 March 2004; Volume 5. [Google Scholar]
- Dror, D.K.; Allen, L.H. Retinol-to-fat ratio and retinol concentration in human milk show similar time trends and associations with maternal factors at the population level: A systematic review and meta-analysis. Adv. Nutr. 2018, 9, 332S–346S. [Google Scholar] [CrossRef] [PubMed]
- Stoltzfus, R.; Underwood, B.A. Breast-milk vitamin A as an indicator of the vitamin A status of women and infants. Bull. World Health Organ. 1995, 73, 703. [Google Scholar]
- Bates, C. Vitamin A in pregnancy and lactation. Proc. Nutr. Soc. 1983, 42, 65–79. [Google Scholar] [CrossRef]
- Maria, A.; Pasatiempo, G.; Ross, A.C. Effects of food or nutrient restriction on milk vitamin A transfer and neonatal vitamin A stores in the rat. Br. J. Nutr. 1990, 63, 351–362. [Google Scholar] [CrossRef]
- Ross, A.C.; Gardner, E.M. The function of vitamin A in cellular growth and differentiation, and its roles during pregnancy and lactation. In Nutrient Regulation during Pregnancy, Lactation, and Infant Growth; Springer: Boston, MA, USA, 1994; pp. 187–200. [Google Scholar]
- Debier, C.; Larondelle, Y. Vitamins A and E: Metabolism, roles and transfer to offspring. Br. J. Nutr. 2005, 93, 153–174. [Google Scholar] [CrossRef]
- Li, Z.; Shen, J.; Wu, W.K.; Wang, X.; Liang, J.; Qiu, G.; Liu, J. Vitamin A deficiency induces congenital spinal deformities in rats. PLoS ONE 2012, 7, e46565. [Google Scholar] [CrossRef]
- Wilson, J.G.; Roth, C.B.; Warkany, J. An analysis of the syndrome of malformations induced by maternal vitamin A deficiency. Effects of restoration of vitamin A at various times during gestation. Am. J. Anat. 1953, 92, 189–217. [Google Scholar] [CrossRef]
- Lammer, E.J.; Chen, D.T.; Hoar, R.M.; Agnish, N.D.; Benke, P.J.; Braun, J.T.; Curry, C.J.; Fernhoff, P.M.; Grix, A.W., Jr.; Lott, I.T.; et al. Retinoic acid embryopathy. N. Engl. J. Med. 1985, 313, 837–841. [Google Scholar] [CrossRef] [PubMed]
- Rothman, K.J.; Moore, L.L.; Singer, M.R.; Nguyen, U.S.; Mannino, S.; Milunsky, A. Teratogenicity of high vitamin A intake. N. Engl. J. Med. 1995, 333, 1369–1373. [Google Scholar] [CrossRef] [PubMed]
- Maden, M. Retinoic acid in the development, regeneration and maintenance of the nervous system. Nat. Rev. Neurosci. 2007, 8, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Misner, D.L.; Jacobs, S.; Shimizu, Y.; de Urquiza, A.M.; Solomin, L.; Perlmann, T.; De Luca, L.M.; Stevens, C.F.; Evans, R.M. Vitamin A deprivation results in reversible loss of hippocampal long-term synaptic plasticity. Proc. Natl. Acad. Sci. USA 2001, 98, 11714–11719. [Google Scholar] [CrossRef]
- Buckley, G.J.; Murray-Kolb, L.E.; Khatry, S.K.; Leclerq, S.C.; Wu, L.; West, K.P., Jr.; Christian, P. Cognitive and motor skills in school-aged children following maternal vitamin A supplementation during pregnancy in rural Nepal: A follow-up of a placebo-controlled, randomised cohort. BMJ Open 2013, 3, e002000. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, X.; Wei, X.-p.; Qu, P.; Liu, Y.-x.; Li, T.-y. Antioxidant vitamin status during pregnancy in relation to cognitive development in the first two years of life. Early Hum. Dev. 2009, 85, 421–427. [Google Scholar] [CrossRef]
- Humphrey, J.H.; Agoestina, T.; Juliana, A.; Septiana, S.; Widjaja, H.; Cerreto, M.C.; Wu, L.S.; Ichord, R.N.; Katz, J.; West, K.P., Jr. Neonatal vitamin A supplementation: Effect on development and growth at 3 y of age. Am. J. Clin. Nutr. 1998, 68, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Muthayya, S.; Rah, J.H.; Sugimoto, J.D.; Roos, F.F.; Kraemer, K.; Black, R.E. The global hidden hunger indices and maps: An advocacy tool for action. PLoS ONE 2013, 8, e67860. [Google Scholar] [CrossRef]
- Alaofè, H.; Burney, J.; Naylor, R.; Taren, D. Prevalence of anaemia, deficiencies of iron and vitamin A and their determinants in rural women and young children: A cross-sectional study in Kalalé district of northern Benin. Public Health Nutr. 2017, 20, 1203–1213. [Google Scholar] [CrossRef]
- Palafox, N.A.; Gamble, M.V.; Dancheck, B.; Ricks, M.O.; Briand, K.; Semba, R.D. Vitamin A deficiency, iron deficiency, and anemia among preschool children in the Republic of the Marshall Islands. Nutrition 2003, 19, 405–408. [Google Scholar] [CrossRef]
- Muñoz, E.C.; Rosado, J.L.; López, P.; Furr, H.C.; Allen, L.H. Iron and zinc supplementation improves indicators of vitamin A status of Mexican preschoolers. Am. J. Clin. Nutr. 2000, 71, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Christian, P.; West, K.P., Jr. Interactions between zinc and vitamin A: An update. Am. J. Clin. Nutr. 1998, 68, 435S–441S. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.T.; Green, J.B.; Beard, J.L.; Green, M.H. Kinetic analysis shows that iron deficiency decreases liver vitamin A mobilization in rats. J. Nutr. 2000, 130, 1291–1296. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wei, C.H.; Green, M.H.; Ross, A.C. Dietary Iron Repletion Stimulates Hepatic Mobilization of Vitamin A in Previously Iron-Deficient Rats as Determined by Model-Based Compartmental Analysis. J. Nutr. 2020, 150, 1982–1988. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wei, C.H.; Xiao, X.; Green, M.H.; Ross, A.C. Perturbed Vitamin A Status Induced by Iron Deficiency Is Corrected by Iron Repletion in Rats with Pre-Existing Iron Deficiency. J. Nutr. 2020, 150, 1989–1995. [Google Scholar] [CrossRef]
- Berzin, N.I.; Bauman, V.K. Vitamin-A-dependent zinc-binding protein and intestinal absorption of Zn in chicks. Br. J. Nutr. 1987, 57, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha, M.S.B.; Siqueira, E.M.A.; Trindade, L.S.; Arruda, S.F. Vitamin A deficiency modulates iron metabolism via ineffective erythropoiesis. J. Nutr. Biochem. 2014, 25, 1035–1044. [Google Scholar] [CrossRef]
- Mendes, J.F.R.; Siqueira, E.M.D.A.; de Brito e Silva, J.G.M.; Arruda, S.F. Vitamin A deficiency modulates iron metabolism independent of hemojuvelin (Hfe2) and bone morphogenetic protein 6 (Bmp6) transcript levels. Genes Nutr. 2016, 11, 1. [Google Scholar] [CrossRef]
- Corcoran, J.; So, P.L.; Maden, M. Absence of retinoids can induce motoneuron disease in the adult rat and a retinoid defect is present in motoneuron disease patients. J. Cell Sci. 2002, 115 Pt 24, 4735–4741. [Google Scholar] [CrossRef]
- Carta, M.; Stancampiano, R.; Tronci, E.; Collu, M.; Usiello, A.; Morelli, M.; Fadda, F. Vitamin A deficiency induces motor impairments and striatal cholinergic dysfunction in rats. Neuroscience 2006, 139, 1163–1172. [Google Scholar] [CrossRef]
- McCarthy, P.T.; Cerecedo, L.R. Vitamin A Deficiency in the Mouse1. J. Nutr. 1952, 46, 361–376. [Google Scholar] [CrossRef] [PubMed]
- Annu, K.; Cline, C.; Yasuda, K.; Ganguly, S.; Pesch, A.; Cooper, B.; Janke, L.; Payton, M.; Mukherjee, K.; Surman, S.L.; et al. Role of Vitamins A and D in BCR-ABL Arf(−/−) Acute Lymphoblastic Leukemia. Sci. Rep. 2020, 10, 2359. [Google Scholar] [CrossRef]
- Meneses, F.; Trugo, N.M. Retinol, β-carotene, and lutein+ zeaxanthin in the milk of Brazilian nursing women: Associations with plasma concentrations and influences of maternal characteristics. Nutr. Res. 2005, 25, 443–451. [Google Scholar] [CrossRef]
- Maden, M. Retinoid signalling in the development of the central nervous system. Nat. Rev. Neurosci. 2002, 3, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Hollyday, M. Organization of motor pools in the chick lumbar lateral motor column. J. Comp. Neurol. 1980, 194, 143–170. [Google Scholar] [CrossRef]
- Landmesser, L. The distribution of motoneurones supplying chick hind limb muscles. J. Physiol. 1978, 284, 371–389. [Google Scholar] [CrossRef]
- Sockanathan, S.; Jessell, T.M. Motor neuron-derived retinoid signaling specifies the subtype identity of spinal motor neurons. Cell 1998, 94, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Wuarin, L.; Sidell, N. Differential susceptibilities of spinal cord neurons to retinoic acid-induced survival and differentiation. Dev. Biol. 1991, 144, 429–435. [Google Scholar] [CrossRef]
- Mendelsohn, A.I.; Dasen, J.S.; Jessell, T.M. Divergent Hox Coding and Evasion of Retinoid Signaling Specifies Motor Neurons Innervating Digit Muscles. Neuron 2017, 93, 792–805.E4. [Google Scholar] [CrossRef]
- McGrath, N.; Bellinger, D.; Robins, J.; Msamanga, G.I.; Tronick, E.; Fawzi, W.W. Effect of maternal multivitamin supplementation on the mental and psychomotor development of children who are born to HIV-1-infected mothers in Tanzania. Pediatrics 2006, 117, e216–e225. [Google Scholar] [CrossRef]
- Chiang, M.Y.; Misner, D.; Kempermann, G.; Schikorski, T.; Giguère, V.; Sucov, H.M.; Gage, F.H.; Stevens, C.F.; Evans, R.M. An essential role for retinoid receptors RARbeta and RXRgamma in long-term potentiation and depression. Neuron 1998, 21, 1353–1361. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D.; Bloem, M.W. The anemia of vitamin A deficiency: Epidemiology and pathogenesis. Eur. J. Clin. Nutr. 2002, 56, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar]
- Staab, D.B.; Hodges, R.E.; Metcalf, W.K.; Smith, J.L. Relationship between Vitamin A and Iron in the Liver. J. Nutr. 1984, 114, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Mehdad, A.; Siqueira, E.M.; Arruda, S.F. Effect of vitamin A deficiency on iron bioavailability. Ann. Nutr. Metab. 2010, 57, 35–39. [Google Scholar] [CrossRef]
- Da Cunha, M.S.B.; Campos Hankins, N.A.; Arruda, S.F. Effect of vitamin A supplementation on iron status in humans: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2019, 59, 1767–1781. [Google Scholar] [CrossRef]
- Arruda, S.F.; de Almeida Siqueira, E.M.; de Valência, F.F. Vitamin A deficiency increases hepcidin expression and oxidative stress in rat. Nutrition 2009, 25, 472–478. [Google Scholar] [CrossRef]
- Mejia, L.A.; Hodges, R.E.; Rucker, R.B. Role of Vitamin A in the Absorption, Retention and Distribution of Iron in the Rat. J. Nutr. 1979, 109, 129–137. [Google Scholar] [CrossRef]
- Mejia, L.A.; Hodges, R.E.; Rucker, R.B. Clinical signs of anemia in vitamin A-deficient rats. Am. J. Clin. Nutr. 1979, 32, 1439–1444. [Google Scholar] [CrossRef]
- Roodenburg, A.C.; West, C.E.; Hovenierl, R.; Beynen, A.C. Supplemental vitamin A enhances the recovery from iron deficiency in rats with chronic vitamin A deficiency. Br. J. Nutr. 1996, 75, 623–636. [Google Scholar] [CrossRef]
- Rutkowsky, J.M.; Roland, Z.; Valenzuela, A.; Nguyen, A.B.; Park, H.H.; Six, N.; Dursun, I.; Kim, K.; Lein, P.J.; Ramsey, J.J. The impact of continuous and intermittent ketogenic diets on cognitive behavior, motor function, and blood lipids in TgF344-AD rats. Aging 2024, 16, 5811–5828. [Google Scholar] [CrossRef] [PubMed]
- Bieri, J.G.; Tolliver, T.J.; Catignani, G.L. Simultaneous determination of alpha-tocopherol and retinol in plasma or red cells by high pressure liquid chromatography. Am. J. Clin. Nutr. 1979, 32, 2143–2149. [Google Scholar] [CrossRef] [PubMed]
- Handelman, G.J.; Shen, B.; Krinsky, N.I. High resolution analysis of carotenoids in human plasma by high-performance liquid chromatography. Methods Enzym. 1992, 213, 336–346. [Google Scholar]
| Gestation, Week | Sex | Trt | Plasma Retinol, µmol/L | p-Value Plasma | Liver Retinol, µmol/g Tissue | p-Value Liver | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Trt | Sex | Trt × Sex | Trt | Sex | Trt × Sex | |||||
| G1, week 9 | Male | VR | 0.687 ± 0.099 | <0.001 | 0.12 | 0.35 | 0.289 ± 0.114 | 0.0002 | 0.8268 | 0.8268 |
| VD | 0.031 ± 0.031 | N.D. | ||||||||
| Female | VR | 0.569 ± 0.024 | 0.264 ± 0.057 | |||||||
| VD | N.D. | N.D. | ||||||||
| G2, week 6 | Male | VR | 0.649 ± 0.089 | <0.001 | 0.85 | 0.89 | 0.101 ± 0.020 | 0.0001 | 0.7921 | 0.7921 |
| VD | 0.030 ± 0.003 | N.D. | ||||||||
| Female | VR | 0.651 ± 0.017 | 0.096 ± 0.009 | |||||||
| VD | 0.045 ± 0.007 | N.D. | ||||||||
| G2, week 9 | Pooled | VR | 0.746 ± 0.062 | <0.001 | 0.115 ± 0.033 | 0.0004 | ||||
| VD | 0.020 ± 0.005 | N.D. | ||||||||
| G1, 9 Week | G2, 6 Week | G2, 9 Week | ||||
|---|---|---|---|---|---|---|
| Symptoms and Mortality | VR | VD | VR | VD | VR | VD |
| Microphthalmia, n | 1 | 1 | 0 | 0 | 0 | 1 |
| Ocular symptom, n | 0 | 3 | 0 | 0 | 0 | 0 |
| Mortality, n | 0 | 2 | 0 | 0 | 0 | 1 |
| Motor anomaly, n | 0 | 6 | 0 | 0 | 0 | 3 |
| Total, n | 1 | 12 | 0 | 0 | 0 | 5 |
| Risk, % of total animals | 5.2% | 63.2% | 0% | 0% | 0 | 50% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arballo, J.; Rutkowsky, J.M.; Haskell, M.J.; De Las Alas, K.; Engle-Stone, R.; Du, X.; Ramsey, J.J.; Ji, P. Pre- and Postnatal Vitamin A Deficiency Impairs Motor Skills without a Consistent Effect on Trace Mineral Status in Young Mice. Int. J. Mol. Sci. 2024, 25, 10806. https://doi.org/10.3390/ijms251910806
Arballo J, Rutkowsky JM, Haskell MJ, De Las Alas K, Engle-Stone R, Du X, Ramsey JJ, Ji P. Pre- and Postnatal Vitamin A Deficiency Impairs Motor Skills without a Consistent Effect on Trace Mineral Status in Young Mice. International Journal of Molecular Sciences. 2024; 25(19):10806. https://doi.org/10.3390/ijms251910806
Chicago/Turabian StyleArballo, Joseph, Jennifer M. Rutkowsky, Marjorie J. Haskell, Kyla De Las Alas, Reina Engle-Stone, Xiaogu Du, Jon J. Ramsey, and Peng Ji. 2024. "Pre- and Postnatal Vitamin A Deficiency Impairs Motor Skills without a Consistent Effect on Trace Mineral Status in Young Mice" International Journal of Molecular Sciences 25, no. 19: 10806. https://doi.org/10.3390/ijms251910806
APA StyleArballo, J., Rutkowsky, J. M., Haskell, M. J., De Las Alas, K., Engle-Stone, R., Du, X., Ramsey, J. J., & Ji, P. (2024). Pre- and Postnatal Vitamin A Deficiency Impairs Motor Skills without a Consistent Effect on Trace Mineral Status in Young Mice. International Journal of Molecular Sciences, 25(19), 10806. https://doi.org/10.3390/ijms251910806





