Modulation of the Arabidopsis Starch Metabolic Network by the Cytosolic Acetyl-CoA Pathway in the Context of the Diurnal Illumination Cycle
Abstract
1. Introduction
2. Results
2.1. Phenotype of Antisense-ACLA Plants Is Dependent on the Length of the Growth Photoperiod
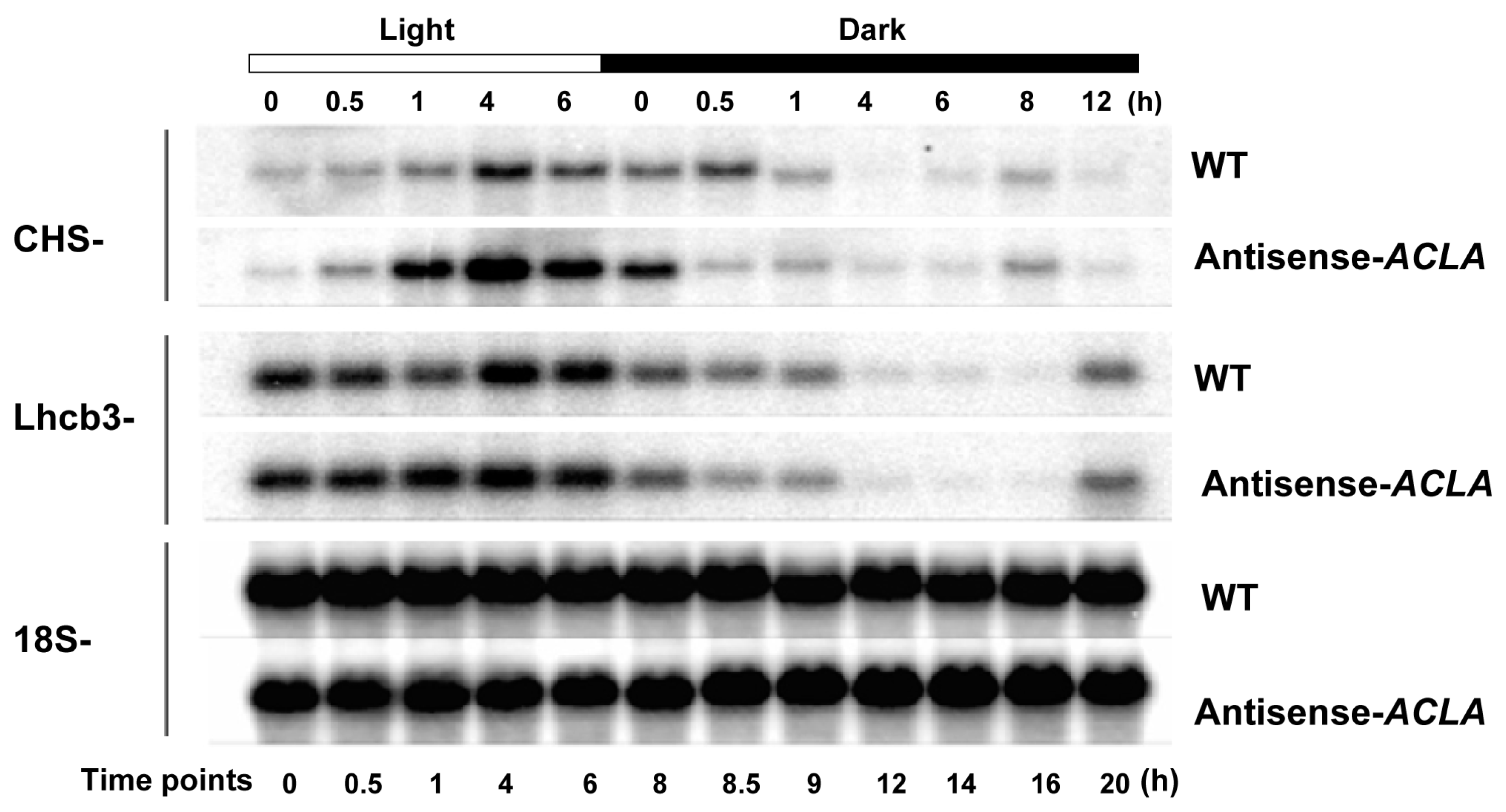
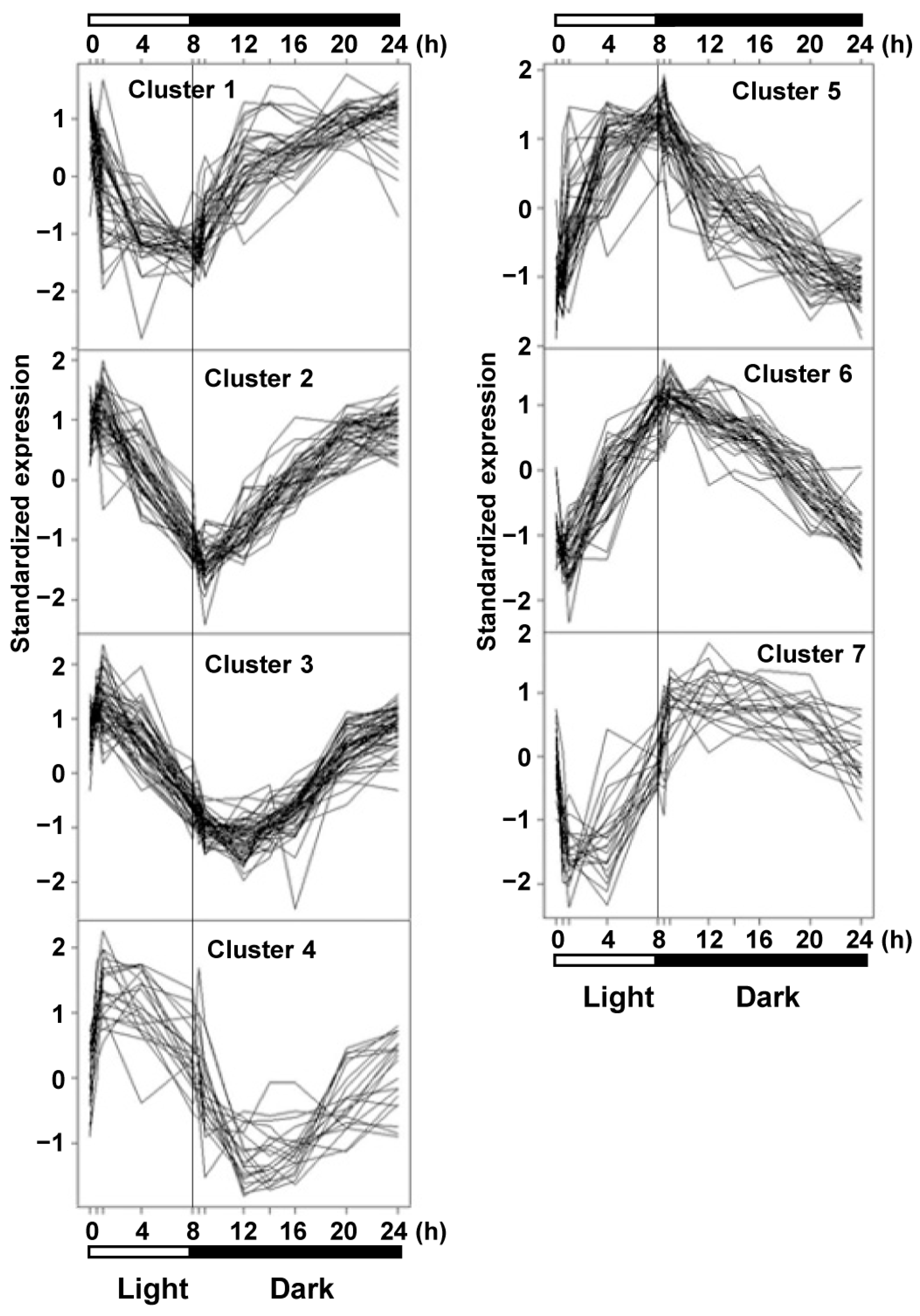
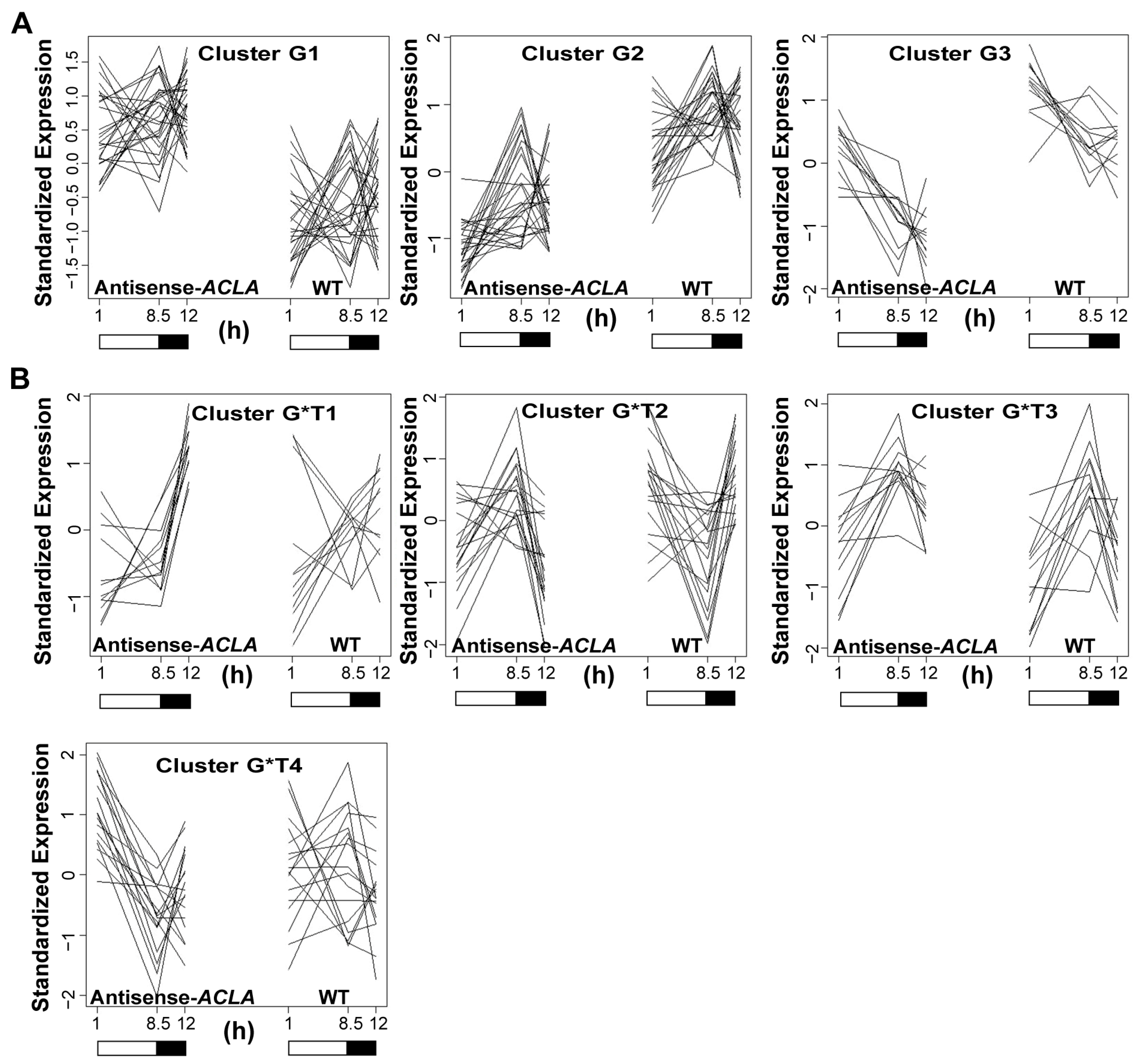
2.2. Fluctuation in Transcriptome under SD Diurnal Cycle: The Circadian Rhythm Is Not Altered by ACL Reduction
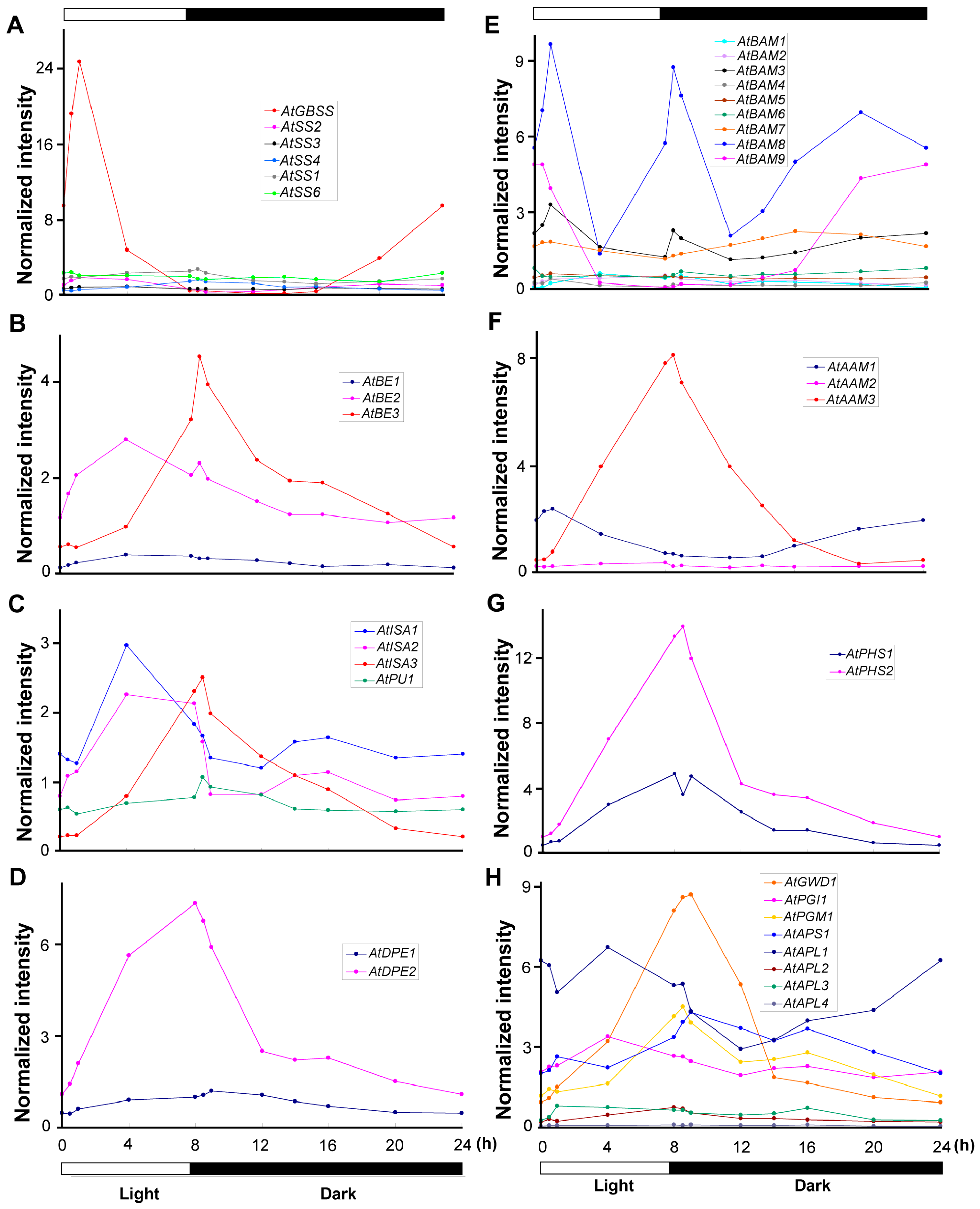
2.3. Expression of Genes Coding for Starch Biosynthesis and Degradation Enzymes in an SD Diurnal Cycle
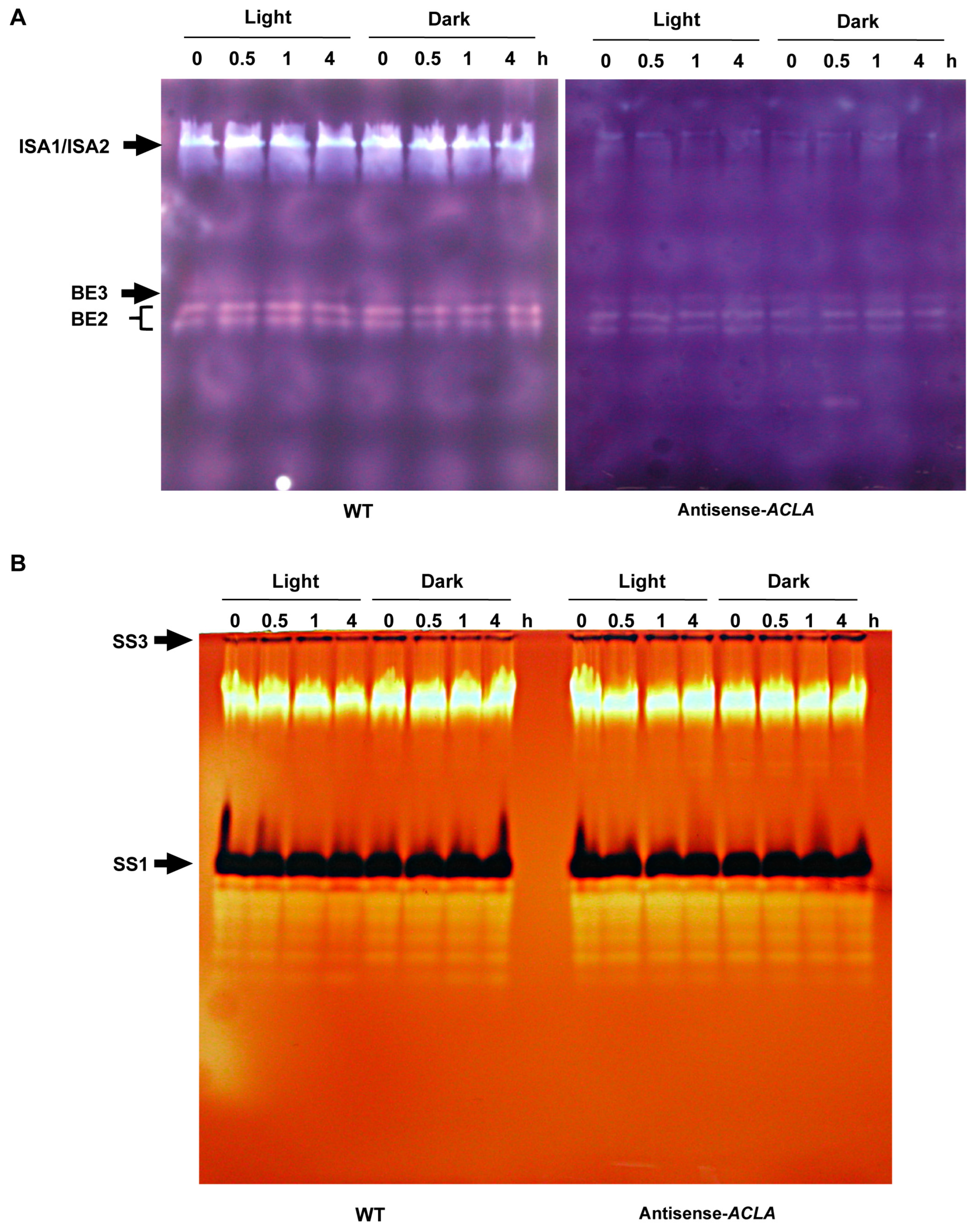
2.4. Antisense-ACLA Allele Affects Activities of Starch Metabolic Enzymes, without Affecting Their Diurnal Expression Pattern
2.5. The Antisense-ACLA Allele Causes Complex Alterations in Seedling Transcriptome
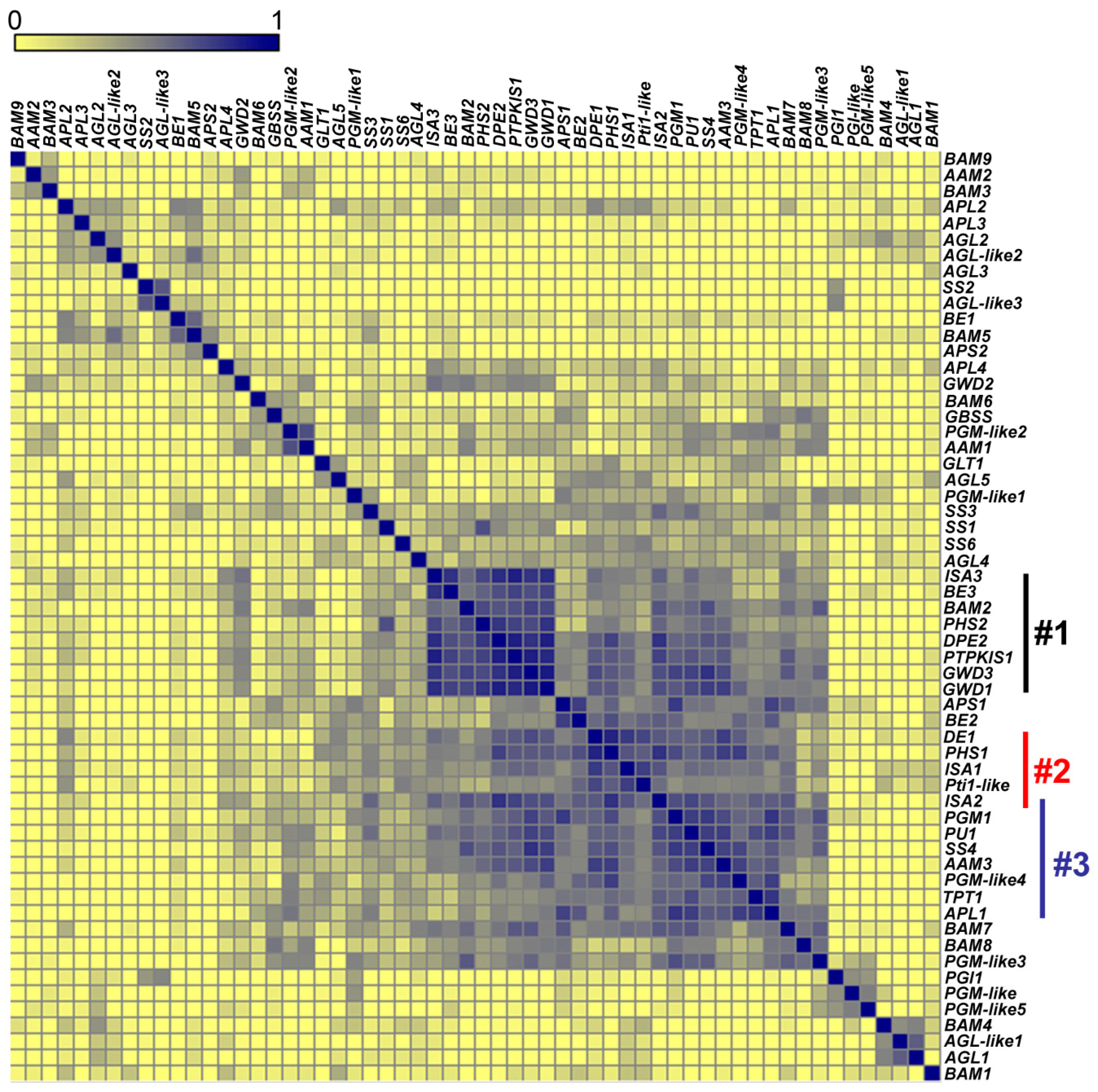
2.6. Co-Expression of Starch Metabolic Genes across Diverse Microarray Experiments
- Group 1:
- DPE2, GWD1, GWD3, PHS2, SEX4, ISA3, BAM2, and BE3.
- Group 2:
- The starch synthesis genes (ISA1 and ISA2), DPE1, PHS1, and the gene encoding Pti1-like protein.
- Group 3:
- ISA2, SS4, and APL1 (At5g19220) involved in starch synthesis; PU1 and AAM3 (chloroplastic, glycogen catabolism) linked to degradation; and triose phosphate/phosphate translocator gene (TPT1, At5g46110), phosphoglucomutase 1 (PGM1, At5g51820), and a PGM-like4 (At1g70820) critical to carbon flux.
3. Discussion
4. Materials and Methods
4.1. Plant Materials, Growth Conditions, and Experimental Design
4.2. Starch Quantification
4.3. RNA Blot Analysis
4.4. In Gel Enzyme Activity Analyses of Starch Metabolic Enzymes
4.5. GeneChip Analysis of SD Experiments
4.6. Clustering
4.7. Correlation Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feugier, F.G.; Satake, A. Dynamical feedback between circadian clock and sucrose availability explains adaptive response of starch metabolism to various photoperiods. Front. Plant Sci. 2013, 3, 305. [Google Scholar] [CrossRef] [PubMed]
- Gibon, Y.; Blasing, O.E.; Palacios-Rojas, N.; Pankovic, D.; Hendriks, J.H.; Fisahn, J.; Hohne, M.; Gunther, M.; Stitt, M. Adjustment of diurnal starch turnover to short days: Depletion of sugar during the night leads to a temporary inhibition of carbohydrate utilization, accumulation of sugars and post-translational activation of ADP-glucose pyrophosphorylase in the following light period. Plant J. 2004, 39, 847–862. [Google Scholar] [PubMed]
- Halford, N.; Curtis, T.; Muttucumaru, N.; Postles, J.; Mottram, D. Sugars in crop plants. Ann. Appl. Biol. 2011, 158, 1–25. [Google Scholar] [CrossRef]
- Fox, T.C.; Geiger, D.R. Effects of decreased net carbon exchange on carbohydrate metabolism in sugar beet source leaves. Plant Physiol. 1984, 76, 763–768. [Google Scholar] [CrossRef]
- Smith, A.M.; Zeeman, S.C.; Smith, S.M. Starch degradation. Annu. Rev. Plant Biol. 2005, 56, 73–98. [Google Scholar] [CrossRef]
- Niu, S.; Li, X.-Q.; Tang, R.; Zhang, G.; Li, X.; Cui, B.; Mikitzel, L.; Haroon, M. Starch granule sizes and degradation in sweet potatoes during storage. Postharvest Biol. Technol. 2019, 150, 137–147. [Google Scholar] [CrossRef]
- Smith, A.M. The biosynthesis of starch granules. Biomacromolecules 2001, 2, 335–341. [Google Scholar] [CrossRef]
- Szydlowski, N.; Ragel, P.; Raynaud, S.; Lucas, M.M.; Roldán, I.; Montero, M.; Muñoz, F.J.; Ovecka, M.; Bahaji, A.; Planchot, V. Starch granule initiation in Arabidopsis requires the presence of either class IV or class III starch synthases. Plant Cell 2009, 21, 2443–2457. [Google Scholar] [CrossRef]
- Lu, K.-J.; Pfister, B.; Jenny, C.; Eicke, S.; Zeeman, S.C. Distinct functions of STARCH SYNTHASE 4 domains in starch granule formation. Plant Physiol. 2018, 176, 566–581. [Google Scholar] [CrossRef]
- Crumpton-Taylor, M.; Pike, M.; Lu, K.J.; Hylton, C.M.; Feil, R.; Eicke, S.; Lunn, J.E.; Zeeman, S.C.; Smith, A.M. Starch synthase 4 is essential for coordination of starch granule formation with chloroplast division during Arabidopsis leaf expansion. New Phytol. 2013, 200, 1064–1075. [Google Scholar] [CrossRef]
- Wang, J.; Hu, P.; Lin, L.; Chen, Z.; Liu, Q.; Wei, C. Gradually decreasing starch branching enzyme expression is responsible for the formation of heterogeneous starch granules. Plant Physiol. 2018, 176, 582–595. [Google Scholar] [CrossRef] [PubMed]
- Mérida, A.; Fettke, J. Starch granule initiation in Arabidopsis thaliana chloroplasts. Plant J. 2021, 107, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Compart, J.; AL-Rawi, S.A.; Mahto, H.; Ahmad, A.M.; Fettke, J. LIKE EARLY STARVATION 1 alters the glucan structures at the starch granule surface and thereby influences the action of both starch-synthesizing and starch-degrading enzymes. Plant J. 2022, 111, 819–835. [Google Scholar] [CrossRef]
- Shen, L.; Li, J.; Li, Y. Resistant starch formation in rice: Genetic regulation and beyond. Plant Commun. 2022, 3, 100329. [Google Scholar] [CrossRef]
- Bürgy, L.; Eicke, S.; Kopp, C.; Jenny, C.; Lu, K.J.; Escrig, S.; Meibom, A.; Zeeman, S.C. Coalescence and directed anisotropic growth of starch granule initials in subdomains of Arabidopsis thaliana chloroplasts. Nature Commun. 2021, 12, 1–10. [Google Scholar] [CrossRef]
- Geiger, D.R.; Servaites, J.C. Diurnal regulation of photosynthetic carbon metabolism in C3 plants. Annu. Rev. Plant Biol. 1994, 45, 235–256. [Google Scholar] [CrossRef]
- Matt, P.; Schurr, U.; Klein, D.; Krapp, A.; Stitt, M. Growth of tobacco in short-day conditions leads to high starch, low sugars, altered diurnal changes in the Nia transcript and low nitrate reductase activity, and inhibition of amino acid synthesis. Planta 1998, 207, 27–41. [Google Scholar] [CrossRef]
- Zeeman, S.C.; Northrop, F.; Smith, A.M.; Rees, T.A. A starch-accumulating mutant of Arabidopsis thaliana deficient in a chloroplastic starch-hydrolysing enzyme. Plant J. 1998, 15, 357–365. [Google Scholar] [CrossRef]
- Fernandez, O.; Ishihara, H.; George, G.M.; Mengin, V.; Flis, A.; Sumner, D.; Arrivault, S.; Feil, R.; Lunn, J.E.; Zeeman, S.C. Leaf starch turnover occurs in long days and in falling light at the end of the day. Plant Physiol. 2017, 174, 2199–2212. [Google Scholar] [CrossRef]
- Abt, M.R.; Pfister, B.; Sharma, M.; Eicke, S.; Bürgy, L.; Neale, I.; Seung, D.; Zeeman, S.C. STARCH SYNTHASE5, a noncanonical starch synthase-like protein, promotes starch granule initiation in Arabidopsis. Plant Cell 2020, 32, 2543–2565. [Google Scholar] [CrossRef]
- Lu, Y.; Gehan, J.P.; Sharkey, T.D. Daylength and circadian effects on starch degradation and maltose metabolism. Plant Physiol. 2005, 138, 2280–2291. [Google Scholar] [CrossRef] [PubMed]
- Baerenfaller, K.; Massonnet, C.; Hennig, L.; Russenberger, D.; Sulpice, R.; Walsh, S.; Stitt, M.; Granier, C.; Gruissem, W. A long photoperiod relaxes energy management in Arabidopsis leaf six. Curr. Plant Biol. 2015, 2, 34–45. [Google Scholar] [CrossRef]
- Koch, K.E. Carbohydrate-modulated gene expression in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 509–540. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.S.; Bao, Q.X.; Mu, X.R.; Tong, C.; Cao, X.Y.; Huang, J.J.; Xue, L.N.; Liu, C.Y.; Fei, Y.; Loake, G.J. Glucose- and sucrose-signaling modules regulate the Arabidopsis juvenile-to-adult phase transition. Cell Rep. 2021, 36, 109348. [Google Scholar] [CrossRef] [PubMed]
- Neff, M.M.; Fankhauser, C.; Chory, J. Light: An indicator of time and place. Genes Dev. 2000, 14, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Horrer, D.; Flutsch, S.; Pazmino, D.; Matthews, J.S.; Thalmann, M.; Nigro, A.; Leonhardt, N.; Lawson, T.; Santelia, D. Blue light induces a distinct starch degradation pathway in guard cells for stomatal opening. Curr. Biol. 2016, 26, 362–370. [Google Scholar] [CrossRef]
- Mengin, V.; Pyl, E.T.; Alexandre Moraes, T.; Sulpice, R.; Krohn, N.; Encke, B.; Stitt, M. Photosynthate partitioning to starch in Arabidopsis thaliana is insensitive to light intensity but sensitive to photoperiod due to a restriction on growth in the light in short photoperiods. Plant Cell Environ. 2017, 40, 2608–2627. [Google Scholar] [CrossRef]
- Harmer, S.L.; Hogenesch, J.B.; Straume, M.; Chang, H.S.; Han, B.; Zhu, T.; Wang, X.; Kreps, J.A.; Kay, S.A. Orchestrated transcription of key pathways in Arabidopsis by the circadian clock. Science 2000, 290, 2110–2113. [Google Scholar] [CrossRef]
- Izumi, M. Roles of the Clock in Controlling Starch Metabolism. Plant Physiol. 2019, 179, 1441–1443. [Google Scholar] [CrossRef]
- Baier, M.; Hemmann, G.; Holman, R.; Corke, F.; Card, R.; Smith, C.; Rook, F.; Bevan, M.W. Characterization of mutants in Arabidopsis showing increased sugar-specific gene expression, growth, and developmental responses. Plant Physiol. 2004, 134, 81–91. [Google Scholar] [CrossRef]
- Coruzzi, G.; Bush, D.R. Nitrogen and carbon nutrient and metabolite signaling in plants. Plant Physiol. 2001, 125, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Haro, D.; Marrero, P.F.; Relat, J. Nutritional regulation of gene expression: Carbohydrate-, Fat- and Amino Acid-Dependent modulation of transcriptional activity. Int. J. Mol. Sci. 2019, 20, 1386. [Google Scholar] [CrossRef]
- Fatland, B.L.; Nikolau, B.J.; Wurtele, E.S. Reverse genetic characterization of cytosolic acetyl-CoA generation by ATP-citrate lyase in Arabidopsis. Plant Cell. 2005, 17, 182–203. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.; van Deenen, N.; Magliano, P.; Frahm, L.; Forestier, E.; Nawrath, C.; Schaller, H.; Gronover, C.S.; Prüfer, D.; Poirier, Y. ATP citrate lyase activity is post-translationally regulated by sink strength and impacts the wax, cutin and rubber biosynthetic pathways. Plant J. 2014, 79, 270–284. [Google Scholar] [CrossRef]
- Zhong, R.; Cui, D.; Richardson, E.A.; Phillips, D.R.; Azadi, P.; Lu, G.; Ye, Z.-H. Cytosolic Acetyl-CoA generated by ATP-citrate lyase is essential for acetylation of cell wall polysaccharides. Plant Cell Physiol. 2020, 61, 64–75. [Google Scholar] [CrossRef]
- Fatland, B.L.; Ke, J.; Anderson, M.D.; Mentzen, W.I.; Cui, L.W.; Allred, C.C.; Johnston, J.L.; Nikolau, B.J.; Wurtele, E.S. Molecular characterization of a heteromeric ATP-citrate lyase that generates cytosolic acetyl-coenzyme A in Arabidopsis. Plant Physiol. 2002, 130, 740–756. [Google Scholar] [CrossRef]
- Chen, C.; Li, C.; Wang, Y.; Renaud, J.; Tian, G.; Kambhampati, S.; Saatian, B.; Nguyen, V.; Hannoufa, A.; Marsolais, F. Cytosolic acetyl-CoA promotes histone acetylation predominantly at H3K27 in Arabidopsis. Nat. Plants 2017, 3, 814–824. [Google Scholar] [CrossRef]
- Smith, S.M.; Fulton, D.C.; Chia, T.; Thorneycroft, D.; Chapple, A.; Dunstan, H.; Hylton, C.; Zeeman, S.C.; Smith, A.M. Diurnal changes in the transcriptome encoding enzymes of starch metabolism provide evidence for both transcriptional and posttranscriptional regulation of starch metabolism in Arabidopsis leaves. Plant Physiol. 2004, 136, 2687–2699. [Google Scholar] [CrossRef]
- Myers, A.M.; Morell, M.K.; James, M.G.; Ball, S.G. Recent progress toward understanding biosynthesis of the amylopectin crystal. Plant Physiol. 2000, 122, 989–997. [Google Scholar] [CrossRef]
- Tenorio, G.; Orea, A.; Romero, J.M.; Merida, A. Oscillation of mRNA level and activity of granule-bound starch synthase I in Arabidopsis leaves during the day/night cycle. Plant Mol. Biol. 2003, 51, 949–958. [Google Scholar] [CrossRef]
- David, L.C.; Lee, S.K.; Bruderer, E.; Abt, M.R.; Fischer-Stettler, M.; Tschopp, M.A.; Solhaug, E.M.; Sanchez, K.; Zeeman, S.C. BETA-AMYLASE9 is a plastidial nonenzymatic regulator of leaf starch degradation. Plant Physiol. 2022, 188, 191–207. [Google Scholar] [CrossRef] [PubMed]
- Baroja-Fernandez, E.; Munoz, F.J.; Zandueta-Criado, A.; Moran-Zorzano, M.T.; Viale, A.M.; Alonso-Casajus, N.; Pozueta-Romero, J. Most of ADP x glucose linked to starch biosynthesis occurs outside the chloroplast in source leaves. Proc. Natl. Acad. Sci. USA 2004, 101, 13080–13085. [Google Scholar] [CrossRef] [PubMed]
- Cross, J.M.; Clancy, M.; Shaw, J.R.; Greene, T.W.; Schmidt, R.R.; Okita, T.W.; Hannah, L.C. Both subunits of ADP-glucose pyrophosphorylase are regulatory. Plant Physiol. 2004, 135, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, J.H.; Kolbe, A.; Gibon, Y.; Stitt, M.; Geigenberger, P. ADP-glucose pyrophosphorylase is activated by posttranslational redox-modification in response to light and to sugars in leaves of Arabidopsis and other plant species. Plant Physiol. 2003, 133, 838–849. [Google Scholar] [CrossRef]
- Dinges, J.R.; Colleoni, C.; Myers, A.M.; James, M.G. Molecular structure of three mutations at the maize sugary1 locus and their allele-specific phenotypic effects. Plant Physiol. 2001, 125, 1406–1418. [Google Scholar] [CrossRef]
- Kakefuda, G.; Duke, S.H. Electrophoretic transfer as a technique for the detection and identification of plant amylolytic enzymes in polyacrylamide gels. Plant Physiol. 1984, 75, 278–280. [Google Scholar] [CrossRef]
- Lu, K.J.; Streb, S.; Meier, F.; Pfister, B.; Zeeman, S.C. Molecular genetic analysis of glucan branching enzymes from plants and bacteria in arabidopsis reveals marked differences in their functions and capacity to mediate starch granule formation. Plant Physiol. 2015, 169, 1638–1655. [Google Scholar] [CrossRef]
- Dumez, S.; Wattebled, F.; Dauvillee, D.; Delvalle, D.; Planchot, V.; Ball, S.G.; D’Hulst, C. Mutants of Arabidopsis lacking starch branching enzyme II substitute plastidial starch synthesis by cytoplasmic maltose accumulation. Plant Cell 2006, 18, 2694–2709. [Google Scholar] [CrossRef]
- Cao, H.; Imparl-Radosevich, J.; Guan, H.; Keeling, P.L.; James, M.G.; Myers, A.M. Identification of the soluble starch synthase activities of maize endosperm. Plant Physiol. 1999, 120, 205–216. [Google Scholar] [CrossRef]
- Hayashi, M.; Crofts, N.; Oitome, N.F.; Fujita, N. Analyses of starch biosynthetic protein complexes and starch properties from developing mutant rice seeds with minimal starch synthase activities. BMC Plant Biol. 2018, 18, 59. [Google Scholar] [CrossRef]
- Zhang, X.; Szydlowski, N.; Delvalle, D.; D’Hulst, C.; James, M.G.; Myers, A.M. Overlapping functions of the starch synthases SSII and SSIII in amylopectin biosynthesis in Arabidopsis. BMC Plant Biol. 2008, 8, 96. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Myers, A.M.; James, M.G. Mutations affecting starch synthase III in Arabidopsis alter leaf starch structure and increase the rate of starch synthesis. Plant Physiol. 2005, 138, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, L.; Rousseeuw, P.J. An Introduction to Cluster Analysis; John Wiley and Sons, Incorporated: Hoboken, NJ, USA, 1990. [Google Scholar]
- Storey, J.D.; Tibshirani, R. Statistical significance for genomewide studies. Proc. Natl. Acad. Sci. USA 2003, 100, 9440–9445. [Google Scholar] [CrossRef] [PubMed]
- Wurtele, E.S.; Li, J.; Diao, L.; Zhang, H.; Foster, C.M.; Fatland, B.; Dickerson, J.; Brown, A.; Cox, Z.; Cook, D. MetNet: Software to build and model the biogenetic lattice of Arabidopsis. Comp. Funct. Genom. 2003, 4, 239–245. [Google Scholar] [CrossRef]
- Mentzen, W.I. From Pathway to Regulon in Arabidopsis; Iowa State University: Ames, IA, USA, 2006. [Google Scholar]
- Craigon, D.J.; James, N.; Okyere, J.; Higgins, J.; Jotham, J.; May, S. NASCArrays: A repository for microarray data generated by NASC’s transcriptomics service. Nucleic Acids Res. 2004, 32, D575–D577. [Google Scholar] [CrossRef]
- Singh, U.; Hur, M.; Dorman, K.; Wurtele, E.S. MetaOmGraph: A workbench for interactive exploratory data analysis of large expression datasets. Nucleic Acids Res. 2020, 48, e23. [Google Scholar] [CrossRef]
- Hussain, H.; Mant, A.; Seale, R.; Zeeman, S.; Hinchliffe, E.; Edwards, A.; Hylton, C.; Bornemann, S.; Smith, A.M.; Martin, C.; et al. Three isoforms of isoamylase contribute different catalytic properties for the debranching of potato glucans. Plant Cell 2003, 15, 133–149. [Google Scholar] [CrossRef]
- Smith, A.M.; Zeeman, S.C.; Thorneycroft, D.; Smith, S.M. Starch mobilization in leaves. J. Exp. Bot. 2003, 54, 577–583. [Google Scholar] [CrossRef]
- Niittyla, T.; Messerli, G.; Trevisan, M.; Chen, J.; Smith, A.M.; Zeeman, S.C. A previously unknown maltose transporter essential for starch degradation in leaves. Science 2004, 303, 87–89. [Google Scholar] [CrossRef]
- Zhou, J.; Loh, Y.-T.; Bressan, R.A.; Martin, G.B. The tomato gene Pti1 encodes a serine/threonine kinase that is phosphorylated by Pto and is involved in the hypersensitive response. Cell 1995, 83, 925–935. [Google Scholar] [CrossRef]
- Bogdanove, A.J.; Martin, G.B. AvrPto-dependent Pto-interacting proteins and AvrPto-interacting proteins in tomato. Proc. Natl. Acad. Sci. USA 2000, 97, 8836–8840. [Google Scholar] [CrossRef] [PubMed]
- Riemenschneider, A.; Wegele, R.; Schmidt, A.; Papenbrock, J. Isolation and characterization of ad-cysteine desulfhydrase protein from Arabidopsis thaliana. FEBS J. 2005, 272, 1291–1304. [Google Scholar] [CrossRef] [PubMed]
- Chia, T.; Thorneycroft, D.; Chapple, A.; Messerli, G.; Chen, J.; Zeeman, S.C.; Smith, S.M.; Smith, A.M. A cytosolic glucosyltransferase is required for conversion of starch to sucrose in Arabidopsis leaves at night. Plant J. 2004, 37, 853–863. [Google Scholar] [CrossRef]
- Lu, Y.; Sharkey, T.D. The role of amylomaltase in maltose metabolism in the cytosol of photosynthetic cells. Planta 2004, 218, 466–473. [Google Scholar] [CrossRef]
- Fordham-Skelton, A.P.; Chilley, P.; Lumbreras, V.; Reignoux, S.; Fenton, T.R.; Dahm, C.C.; Pages, M.; Gatehouse, J.A. A novel higher plant protein tyrosine phosphatase interacts with SNF1-related protein kinases via a KIS (kinase interaction sequence) domain. Plant J. 2002, 29, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Halford, N.G.; Hardie, D.G. SNF1-related protein kinases: Global regulators of carbon metabolism in plants? Plant Mol. Biol. 1998, 37, 735–748. [Google Scholar] [CrossRef]
- Schaffer, R.; Landgraf, J.; Accerbi, M.; Simon, V.; Larson, M.; Wisman, E. Microarray analysis of diurnal and circadian-regulated genes in Arabidopsis. Plant Cell 2001, 13, 113–123. [Google Scholar] [CrossRef]
- Niittyla, T.; Comparot-Moss, S.; Lue, W.L.; Messerli, G.; Trevisan, M.; Seymour, M.D.; Gatehouse, J.A.; Villadsen, D.; Smith, S.M.; Chen, J.; et al. Similar protein phosphatases control starch metabolism in plants and glycogen metabolism in mammals. J. Biol. Chem. 2006, 281, 11815–11818. [Google Scholar] [CrossRef]
- Eisen, M.B.; Spellman, P.T.; Brown, P.O.; Botstein, D. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. USA 1998, 95, 14863–14868. [Google Scholar] [CrossRef]
- Segal, E.; Shapira, M.; Regev, A.; Pe’er, D.; Botstein, D.; Koller, D.; Friedman, N. Module networks: Identifying regulatory modules and their condition-specific regulators from gene expression data. Nat. Genet. 2003, 34, 166–176. [Google Scholar] [CrossRef]
- Stuart, J.M.; Segal, E.; Koller, D.; Kim, S.K. A gene-coexpression network for global discovery of conserved genetic modules. Science 2003, 302, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Razin, S.V.; Ioudinkova, E.S.; Kantidze, O.L.; Iarovaia, O.V. Co-Regulated genes and gene clusters. Genes 2021, 12, 907. [Google Scholar] [CrossRef] [PubMed]
- Keeling, P.L.; Myers, A.M. Biochemistry and genetics of starch synthesis. Annu. Rev. Food Sci. Technol. 2010, 1, 271–303. [Google Scholar] [CrossRef] [PubMed]
- Zeeman, S.C.; Smith, S.M.; Smith, A.M. The diurnal metabolism of leaf starch. Biochem. J. 2007, 401, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Stitt, M.; Muller, C.; Matt, P.; Gibon, Y.; Carillo, P.; Morcuende, R.; Scheible, W.R.; Krapp, A. Steps towards an integrated view of nitrogen metabolism. J. Exp. Bot. 2002, 53, 959–970. [Google Scholar] [CrossRef]
- Browse, J.; Roughan, P.G.; Slack, C.R. Light control of fatty acid synthesis and diurnal fluctuations of fatty acid composition in leaves. Biochem. J. 1981, 196, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Gilmour, S.J.; Sebolt, A.M.; Salazar, M.P.; Everard, J.D.; Thomashow, M.F. Overexpression of the Arabidopsis CBF3 transcriptional activator mimics multiple biochemical changes associated with cold acclimation. Plant Physiol. 2000, 124, 1854–1865. [Google Scholar] [CrossRef]
- Liu, Y.; Dang, P.; Liu, L.; He, C. Cold acclimation by the CBF-COR pathway in a changing climate: Lessons from Arabidopsis thaliana. Plant Cell Rep. 2019, 38, 511–519. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, W.Y.; Kwak, K.J.; Oh, S.H.; Han, Y.S.; Kang, H. Glycine-rich RNA-binding proteins are functionally conserved in Arabidopsis thaliana and Oryza sativa during cold adaptation process. J. Exp. Bot. 2010, 61, 2317–2325. [Google Scholar] [CrossRef]
- Davletova, S.; Rizhsky, L.; Liang, H.; Shengqiang, Z.; Oliver, D.J.; Coutu, J.; Shulaev, V.; Schlauch, K.; Mittler, R. Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell 2005, 17, 268–281. [Google Scholar] [CrossRef]
- Barros, J.; Escamilla-Trevino, L.; Song, L.; Rao, X.; Serrani-Yarce, J.C.; Palacios, M.D.; Engle, N.; Choudhury, F.K.; Tschaplinski, T.J.; Venables, B.J.; et al. 4-Coumarate 3-hydroxylase in the lignin biosynthesis pathway is a cytosolic ascorbate peroxidase. Nat. Commun. 2019, 10, 1994. [Google Scholar] [CrossRef] [PubMed]
- Bechtold, U.; Murphy, D.J.; Mullineaux, P.M. Arabidopsis peptide methionine sulfoxide reductase2 prevents cellular oxidative damage in long nights. Plant Cell 2004, 16, 908–919. [Google Scholar] [CrossRef] [PubMed]
- Rey, P.; Tarrago, L. Physiological Roles of Plant Methionine Sulfoxide Reductases in Redox Homeostasis and Signaling. Antioxidants 2018, 7, 114. [Google Scholar] [CrossRef]
- Fichtner, F.; Lunn, J.E. The Role of Trehalose 6-Phosphate (Tre6P) in plant metabolism and development. Annu. Rev. Plant Biol. 2021, 72, 737–760. [Google Scholar] [CrossRef] [PubMed]
- Hwang, G.; Kim, S.; Cho, J.Y.; Paik, I.; Kim, J.I.; Oh, E. Trehalose-6-phosphate signaling regulates thermoresponsive hypocotyl growth in Arabidopsis thaliana. EMBO Rep. 2019, 20, e47828. [Google Scholar] [CrossRef] [PubMed]
- Avonce, N.; Leyman, B.; Mascorro-Gallardo, J.O.; Van Dijck, P.; Thevelein, J.M.; Iturriaga, G. The Arabidopsis trehalose-6-P synthase AtTPS1 gene is a regulator of glucose, abscisic acid, and stress signaling. Plant Physiol. 2004, 136, 3649–3659. [Google Scholar] [CrossRef]
- Bowman, J. Reproductive structures in Arabidopsis. In Arabidopsis: An Atlas of Morphology and Development; Springer: Berlin/Heidelberg, Germany, 1994; pp. 156–161. [Google Scholar]
- Stessman, D.; Miller, A.; Spalding, M.; Rodermel, S. Regulation of photosynthesis during Arabidopsis leaf development in continuous light. Photosynth. Res. 2002, 72, 27–37. [Google Scholar] [CrossRef]
- Li, L.; Foster, C.M.; Gan, Q.; Nettleton, D.; James, M.G.; Myers, A.M.; Wurtele, E.S. Identification of the novel protein QQS as a component of the starch metabolic network in Arabidopsis leaves. Plant J. 2009, 58, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Ke, J.; Wen, T.N.; Nikolau, B.J.; Wurtele, E.S. Coordinate regulation of the nuclear and plastidic genes coding for the subunits of the heteromeric acetyl-coenzyme A carboxylase. Plant Physiol. 2000, 122, 1057–1071. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. B Stat. Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Tibshirani, R.; Walther, G.; Hastie, T. Estimating the number of clusters in a data set via the gap statistic. J. R. Stat. Soc. Ser. B 2001, 63, 411–423. [Google Scholar] [CrossRef]
- Team, R.C. R: A language and environment for statistical computing. Found. Stat. Comput. Vienna Austria 2013. [Google Scholar]
- Pavlidis, P.; Noble, W.S. Matrix2png: A utility for visualizing matrix data. Bioinformatics 2003, 19, 295–296. [Google Scholar] [CrossRef]
- Pfister, B.; Lu, K.J.; Eicke, S.; Feil, R.; Lunn, J.E.; Streb, S.; Zeeman, S.C. Genetic Evidence That Chain Length and Branch Point Distributions Are Linked Determinants of Starch Granule Formation in Arabidopsis. Plant Physiol. 2014, 165, 1457–1474. [Google Scholar] [CrossRef]
- Vandromme, C.; Spriet, C.; Putaux, J.L.; Dauvillee, D.; Courseaux, A.; D’Hulst, C.; Wattebled, F. Further insight into the involvement of PII1 in starch granule initiation in Arabidopsis leaf chloroplasts. New Phytol. 2023, 239, 132–145. [Google Scholar] [CrossRef]
- Wang, X.; Xue, L.; Sun, J.; Zuo, J. The Arabidopsis BE1 gene, encoding a putative glycoside hydrolase localized in plastids, plays crucial roles during embryogenesis and carbohydrate metabolism. J. Integr. Plant Biol. 2010, 52, 273–288. [Google Scholar] [CrossRef]
- Bossi, F.; Cordoba, E.; Dupre, P.; Mendoza, M.S.; Roman, C.S.; Leon, P. The Arabidopsis ABA-INSENSITIVE (ABI) 4 factor acts as a central transcription activator of the expression of its own gene, and for the induction of ABI5 and SBE2.2 genes during sugar signaling. Plant J. 2009, 59, 359–374. [Google Scholar] [CrossRef]
- Boyer, L.; Roussel, X.; Courseaux, A.; Ndjindji, O.M.; Lancelon-Pin, C.; Putaux, J.L.; Tetlow, I.J.; Emes, M.J.; Pontoire, B.; C, D.H.; et al. Expression of Escherichia coli glycogen branching enzyme in an Arabidopsis mutant devoid of endogenous starch branching enzymes induces the synthesis of starch-like polyglucans. Plant Cell Environ. 2016, 39, 1432–1447. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, W.; Wang, Y.; Jin, J.; Xu, H.; Fu, Y.; Shan, Z.; Wang, X.; Teng, X.; Li, X.; et al. Rice LIKE EARLY STARVATION1 cooperates with FLOURY ENDOSPERM6 to modulate starch biosynthesis and endosperm development. Plant Cell 2024, 36, 1892–1912. [Google Scholar] [CrossRef]
- Nagler, M.; Nukarinen, E.; Weckwerth, W.; Nagele, T. Integrative molecular profiling indicates a central role of transitory starch breakdown in establishing a stable C/N homeostasis during cold acclimation in two natural accessions of Arabidopsis thaliana. BMC Plant Biol. 2015, 15, 284. [Google Scholar] [CrossRef]
- Delatte, T.; Umhang, M.; Trevisan, M.; Eicke, S.; Thorneycroft, D.; Smith, S.M.; Zeeman, S.C. Evidence for distinct mechanisms of starch granule breakdown in plants. J. Biol. Chem. 2006, 281, 12050–12059. [Google Scholar] [CrossRef] [PubMed]
- Backstrom, S.; Elfving, N.; Nilsson, R.; Wingsle, G.; Bjorklund, S. Purification of a plant mediator from Arabidopsis thaliana identifies PFT1 as the Med25 subunit. Mol. Cell 2007, 26, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ahmad, A.M.; Zhong, Y.; Ding, L.; Blennow, A.; Fettke, J. Starch phosphorylation regulates starch granule morphological homogeneity in Arabidopsis thaliana. Plant Physiol. 2024, 194, 2600–2615. [Google Scholar] [CrossRef] [PubMed]
- Monroe, J.D.; Storm, A.R.; Badley, E.M.; Lehman, M.D.; Platt, S.M.; Saunders, L.K.; Schmitz, J.M.; Torres, C.E. beta-Amylase1 and beta-amylase3 are plastidic starch hydrolases in Arabidopsis that seem to be adapted for different thermal, pH, and stress conditions. Plant Physiol. 2014, 166, 1748–1763. [Google Scholar] [CrossRef]
- Soyk, S.; Simkova, K.; Zurcher, E.; Luginbuhl, L.; Brand, L.H.; Vaughan, C.K.; Wanke, D.; Zeeman, S.C. The Enzyme-Like domain of Arabidopsis nuclear beta-Amylases is critical for dna sequence recognition and transcriptional activation. Plant Cell 2014, 26, 1746–1763. [Google Scholar] [CrossRef]
- Schreier, T.B.; Umhang, M.; Lee, S.K.; Lue, W.L.; Shen, Z.; Silver, D.; Graf, A.; Muller, A.; Eicke, S.; Stadler-Waibel, M.; et al. LIKE SEX4 1 acts as a beta-Amylase-Binding Scaffold on starch granules during starch degradation. Plant Cell 2019, 31, 2169–2186. [Google Scholar] [CrossRef]
- Krokida, A.; Delis, C.; Geisler, K.; Garagounis, C.; Tsikou, D.; Pena-Rodriguez, L.M.; Katsarou, D.; Field, B.; Osbourn, A.E.; Papadopoulou, K.K. A metabolic gene cluster in Lotus japonicus discloses novel enzyme functions and products in triterpene biosynthesis. New Phytol. 2013, 200, 675–690. [Google Scholar] [CrossRef]
- Doyle, E.A.; Lane, A.M.; Sides, J.M.; Mudgett, M.B.; Monroe, J.D. An alpha-amylase (At4g25000) in Arabidopsis leaves is secreted and induced by biotic and abiotic stress. Plant Cell Environ. 2007, 30, 388–398. [Google Scholar] [CrossRef]
- Schopper, S.; Muhlenbock, P.; Sorensson, C.; Hellborg, L.; Lenman, M.; Widell, S.; Fettke, J.; Andreasson, E. Arabidopsis cytosolic alpha-glycan phosphorylase, PHS2, is important during carbohydrate imbalanced conditions. Plant Biol. 2015, 17, 74–80. [Google Scholar] [CrossRef]
- Liu, H.C.; Chen, H.C.; Huang, T.H.; Lue, W.L.; Chen, J.; Suen, D.F. Cytosolic phosphoglucose isomerase is essential for microsporogenesis and embryogenesis in Arabidopsis. Plant Physiol. 2023, 191, 177–198. [Google Scholar] [CrossRef]
- Flutsch, S.; Horrer, D.; Santelia, D. Starch biosynthesis in guard cells has features of both autotrophic and heterotrophic tissues. Plant Physiol. 2022, 189, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Funfgeld, M.; Wang, W.; Ishihara, H.; Arrivault, S.; Feil, R.; Smith, A.M.; Stitt, M.; Lunn, J.E.; Niittyla, T. Sucrose synthases are not involved in starch synthesis in Arabidopsis leaves. Nat. Plants 2022, 8, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.L.; Lue, W.L.; Lu, K.J.; Hsieh, M.H.; Wang, S.M.; Chen, J. Starch synthesis in Arabidopsis is achieved by spatial cotranscription of core starch metabolism genes. Plant Physiol. 2009, 151, 1582–1595. [Google Scholar] [CrossRef] [PubMed]
- Ventriglia, T.; Ballicora, M.A.; Crevillen, P.; Preiss, J.; Romero, J.M. Regulatory properties of potato-Arabidopsis hybrid ADP-glucose pyrophosphorylase. Plant Cell Physiol. 2007, 48, 875–880. [Google Scholar] [CrossRef]
- Ventriglia, T.; Kuhn, M.L.; Ruiz, M.T.; Ribeiro-Pedro, M.; Valverde, F.; Ballicora, M.A.; Preiss, J.; Romero, J.M. Two Arabidopsis ADP-glucose pyrophosphorylase large subunits (APL1 and APL2) are catalytic. Plant Physiol. 2008, 148, 65–76. [Google Scholar] [CrossRef]

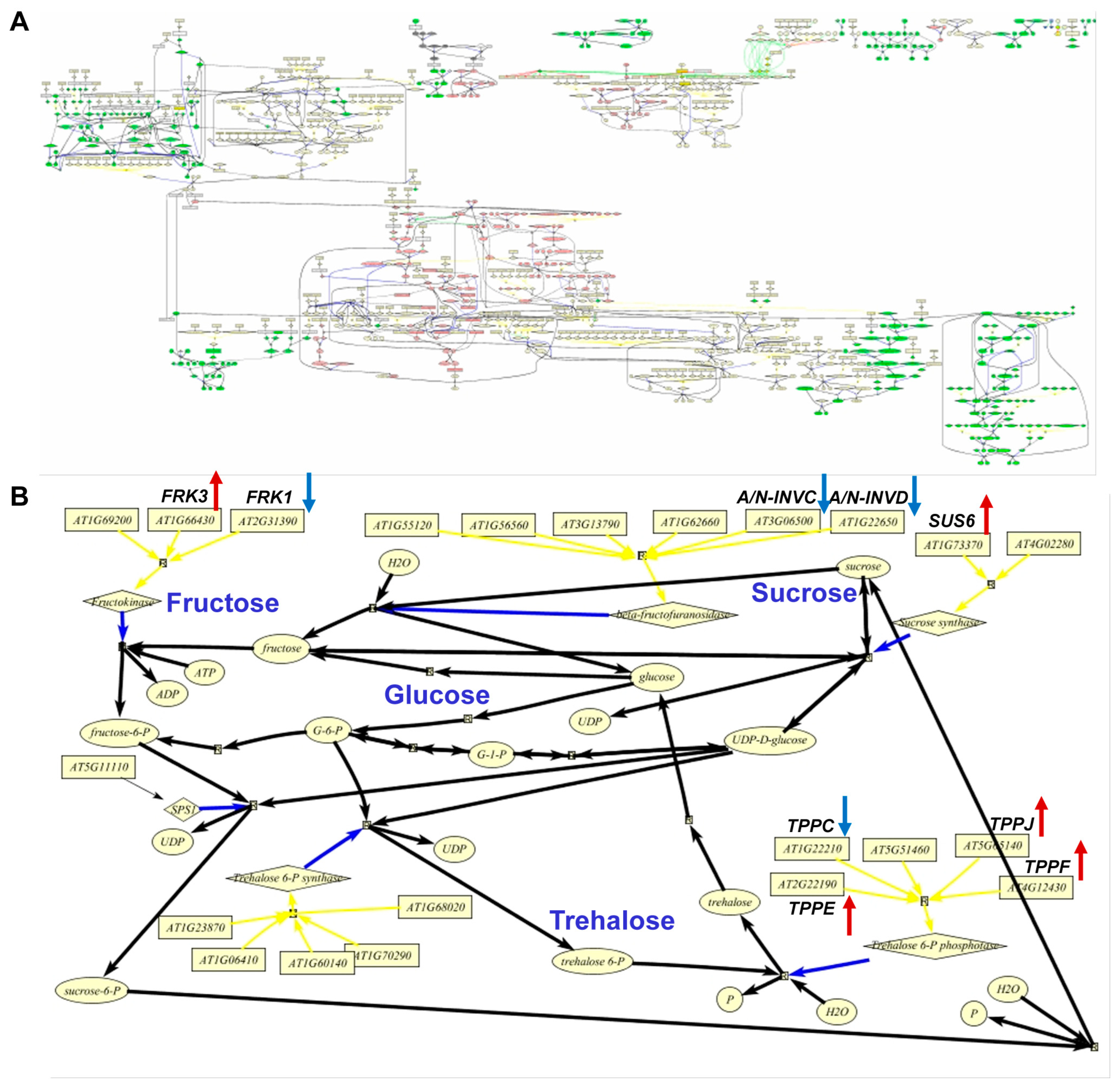

| Pathways with Up-Regulated Genes (41/173) | Pathways with Down-Regulated Genes (71/227) |
|---|---|
| Cell wall metabolism (7/24) | Cell wall metabolism (17/42) |
| Xylose degradation (1/3) | Mannose degradation (1/2) |
| Xylulose-monophosphate cycle (1/4) | GDP-D-rhamnose biosynthesis (5/16) |
| D-arabinose catabolism (1/4) | (Deoxy)ribose phosphate metabolism (4/10) |
| Dolichyl-diphosphooligosaccharide biosynthesis (2/10) | Glucosinolate biosynthesis from homomethionine (2/5) |
| Sinapate ester biosynthesis (2/3) | Monoterpene biosynthesis (2/3) |
| Fatty acid elongation (12/32) | Phenylpropanoid biosynthesis (3/6) |
| Fatty acid elongation (3/5) | Fatty acid oxidation (2/17) |
| Fatty acid omega-oxidation (1/2) | Fatty acid beta-oxidation (2/17) |
| Cutin biosynthesis (1/1) | Sulfur containing amino acid biosynthesis (17/63) |
| Mannosyl-chito-dolichol biosynthesis (2/3) | Sulfur assimilation (6/21) |
| Mevalonate pathway (5/21) | Homocysteine and cysteine interconversion (3/8) |
| Amino acid metabolism (8/33) | Cysteine biosynthesis (2/13) |
| Phenylalanine biosynthesis I (2/3) | Methionine biosynthesis (3/12) |
| Tyrosine biosynthesis II (2/4) | Threonine biosynthesis from homoserine (3/9) |
| Lysine biosynthesis (2/19) | Hormone metabolism and signal transduction (27/74) |
| Serine biosynthesis (2/7) | Jasmonic acid biosynthesis (5/18) |
| Hormone metabolism (2/8) | Abscisic acid biosynthesis (4/6) |
| Brassinosteroid biosynthesis (2/8) | Cytokinins degradation (3/6) |
| Vitamins (3/11) | Brassinosteroids signaling (4/12) |
| Biotin biosynthesis (3/11) | APX1 signal transduction pathway (5/13) |
| Other (9/65) | Lipoxygenase pathway (4/11) |
| Chlorophyll Biosynthesis (3/17) | Autophagy (2/8) |
| Glyoxylate cycle (2/17) | Other (8/31) |
| Glucose 1-phosphate metabolism (1/4) | Glutamate degradation III (1/3) |
| De novo biosynthesis of pyrimidine ribonucleotides (3/27) | Tryptophan biosynthesis (7/28) |
| Genes Up-Regulated | Genes Down-Regulated |
|---|---|
| Starch synthesis | Starch degradation |
| ADPGlu-PP (APL4, At4g39210) 2 (L1) | AAM2 (At4g25000)-2 (L1) |
| ADPGlu-PP (APL3, At2g21590) 3.4 (D0.5) | BAM1 (At4g15210)-3 (D0.5) |
| Trehalose | Trehalose |
| Trehalose-6-P phosphatase (At5g65140) 3 (L1) | Trehalose-6-P phosphatase (At1g22210)-3 (L1, D4) |
| Trehalose-6-P phosphatase (At4g12430) 2.1 (D0.5) | |
| Cell wall synthesis | Cell wall synthesis |
| UDP-galactose 4-epimerase (At2g34850) 6.2 (D0.5) | UDP-glucose/galactose 4-epimerase-like protein (At4g20460)-2 (D0.5) |
| Cellulose synthase like gene (AtCslA10, At1g24070) 3.2 (D0.5) | Mannose-6-phosphate isomerase 1 (At3g02570)-2 (D0.5) |
| Cellulose synthase like gene (AtCslA03, At1g23480) 2 (D4) | Rhamnosyltransferase, hemicellulose (At1g64910)-2 (D0.5) |
| Cell wall modification | Cell wall modification |
| Beta-expansin (EXPB1, At2g20750) 2 (L1) | Xyloglucan endotransglycosylase (At5g65730)-2 (L1) |
| Beta-expansin (EXPB3, At4g28250) 3 (L1) 2.4 (D0.5) | Pectinesterase (At2g26440)-3 (D0.5) |
| Invertase/pectin methylesterase inhibitor (At2g47670) 3.7 (D0.5) | Pectinesterase (At4g33220)-2 (D0.5) |
| Expansin, putative (EXP9, At5g02260) 3 (D4) | Pectinesterase (At2g47550)-2 (D4) |
| Xyloglucan endotransglycosylase 1 (At3g23730) 5 (D4) | Pectinacetylesterase (At3g09410)-2 (D4) |
| Xyloglucan endotransglycosylase (At4g03210) 5 (D4) | Invertase/pectin methylesterase inhibitor (At1g10770)-3 (D4) |
| Xyloglucan endotransglycosylase (At1g10550) 4 (D4) | |
| Endo-xyloglucan transferase (XTR7) (At4g14130) 3 (D4) | |
| Pectin methylesterase (At5g47500) 2 (D4) | |
| Invertase/pectin methylesterase inhibitor (At3g47380) 2 (D4) | |
| Cell wall degradation | Cell wall degradation |
| (1-4)-beta-mannan endohydrolase (At5g66460) 3 (L1) | Xyloglucan endo-1,4-beta-D-glucanase (SEN4, At4g30270)-4 (L1) |
| Polygalacturonase (At1g10640) 3 (L1) | Xyloglucan endo-1,4-beta-D glucanase (XTR-3, At4g30280)-3 (L1) |
| Pectate lyase (At1g67750) 2 (L1) 2.1 (D0.5) | Pectate lyase (At4g13210)-2 (L1) |
| Beta-xylosidase (At1g02640) 2 (D4) | Polygalacturonase inhibiting protein 2 (PGIP2, At5g06870)-2 (D0.5) |
| Xyloglucan endo-1,4-beta-D-glucanase (XTR-6, At4g25810)-2 (D0.5) | |
| Xyloglucan endo-1,4-beta-D-glucanase (At5g48070)-2 (D4) |
| Gene | Correlation Coefficient | Locus ID |
|---|---|---|
| ISA1 | 1.00 | At2g39930 |
| ISA2 | 0.74 | At1g03310 |
| Pti1 | 0.73 | At1g26150 |
| DPE1 | 0.72 | At5g64860 |
| Expressed protein | 0.72 | At4g10470 |
| RCP1/MEX1 | 0.68 | At5g17520 |
| Desulfhydrase | 0.67 | At1g48420 |
| Expressed protein | 0.67 | At3g60810 |
| PHS1 | 0.66 | At3g29320 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Foster, C.M.; Mentzen, W.I.; Tanvir, R.; Meng, Y.; Nikolau, B.J.; Wurtele, E.S.; Li, L. Modulation of the Arabidopsis Starch Metabolic Network by the Cytosolic Acetyl-CoA Pathway in the Context of the Diurnal Illumination Cycle. Int. J. Mol. Sci. 2024, 25, 10850. https://doi.org/10.3390/ijms251910850
Wang L, Foster CM, Mentzen WI, Tanvir R, Meng Y, Nikolau BJ, Wurtele ES, Li L. Modulation of the Arabidopsis Starch Metabolic Network by the Cytosolic Acetyl-CoA Pathway in the Context of the Diurnal Illumination Cycle. International Journal of Molecular Sciences. 2024; 25(19):10850. https://doi.org/10.3390/ijms251910850
Chicago/Turabian StyleWang, Lei, Carol M. Foster, Wieslawa I. Mentzen, Rezwan Tanvir, Yan Meng, Basil J. Nikolau, Eve Syrkin Wurtele, and Ling Li. 2024. "Modulation of the Arabidopsis Starch Metabolic Network by the Cytosolic Acetyl-CoA Pathway in the Context of the Diurnal Illumination Cycle" International Journal of Molecular Sciences 25, no. 19: 10850. https://doi.org/10.3390/ijms251910850
APA StyleWang, L., Foster, C. M., Mentzen, W. I., Tanvir, R., Meng, Y., Nikolau, B. J., Wurtele, E. S., & Li, L. (2024). Modulation of the Arabidopsis Starch Metabolic Network by the Cytosolic Acetyl-CoA Pathway in the Context of the Diurnal Illumination Cycle. International Journal of Molecular Sciences, 25(19), 10850. https://doi.org/10.3390/ijms251910850









