Protective Potential of Cicerbita alpina Leaf Extract on Metabolic Disorders and Oxidative Stress in Model Animals
Abstract
:1. Introduction
2. Results and Discussion
2.1. Glucose Concentration in the Blood
2.2. Serum Biochemical Parameters
2.3. Oxidative Stress Markers and Antioxidant Enzymes Activity in the Liver
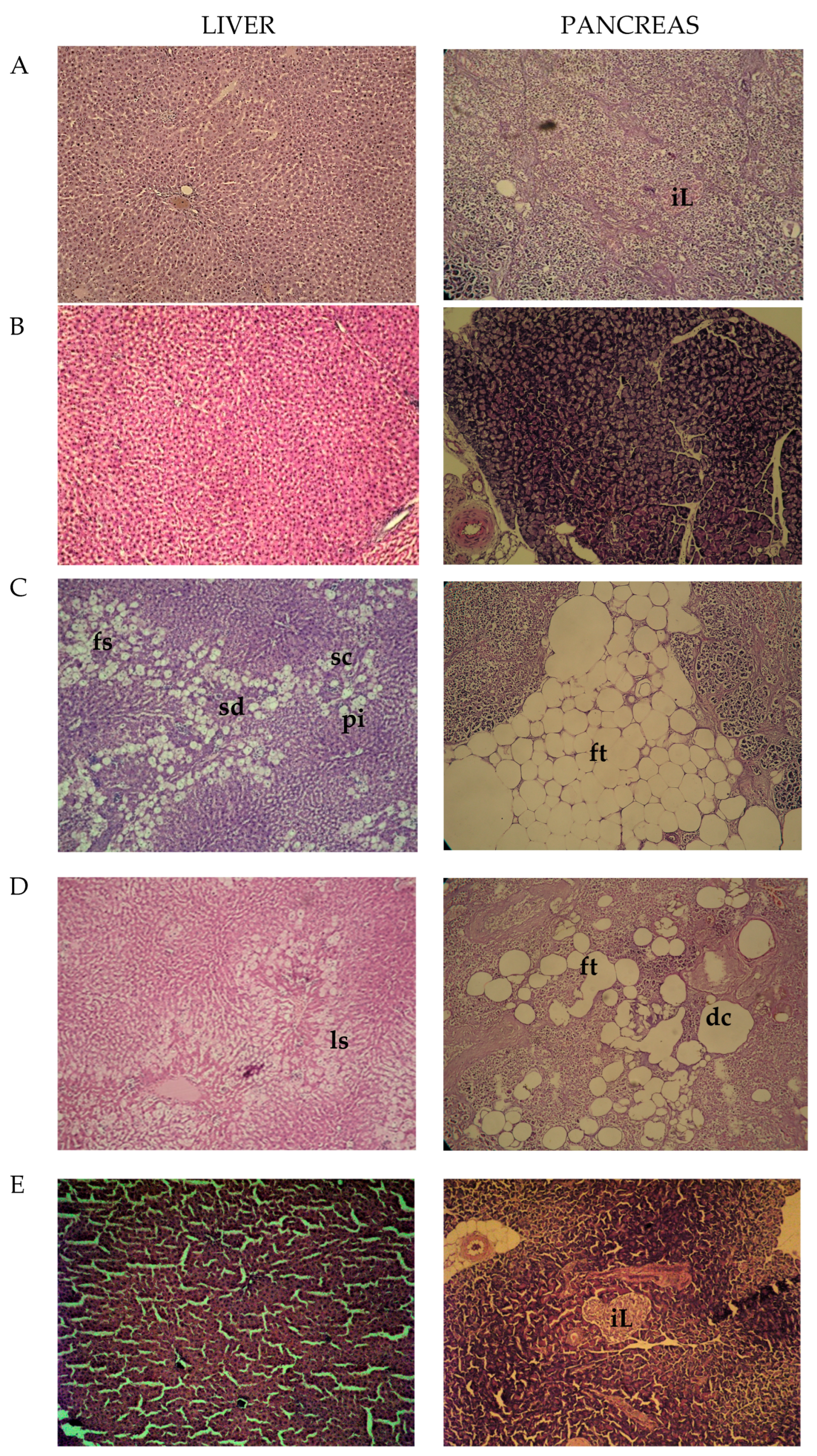
2.4. Histopathological Observations
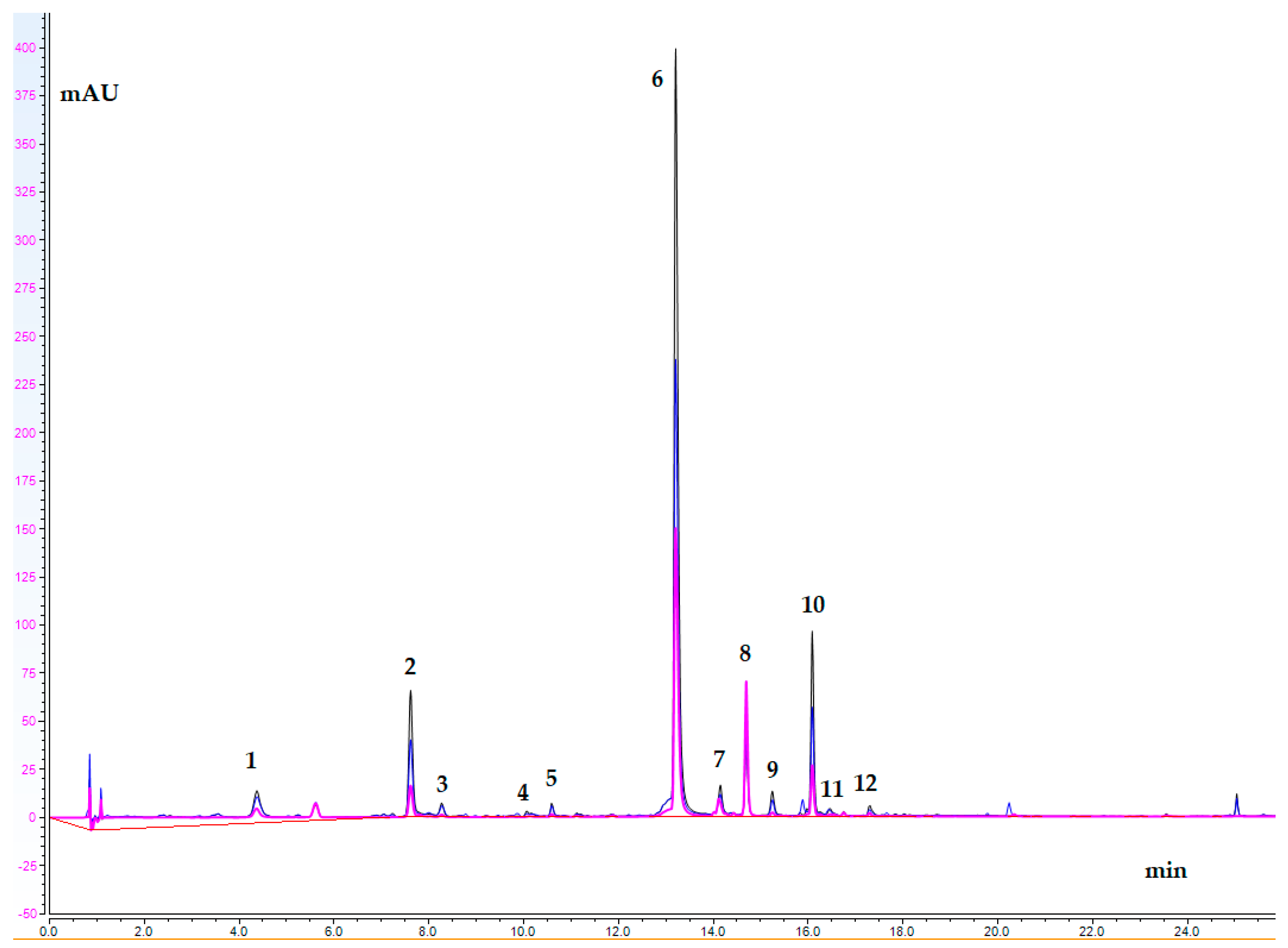
2.5. UHPLC-DAD Study
3. Materials and Methods
3.1. Plant Material and Sample Extraction
3.2. Chemicals
3.3. Animals
3.4. Induction of Diabetes Type 2
3.5. Experimental Design
3.6. Serum Biochemical Investigation and Oxidative Stress Markers
3.7. Histopathological Exploration
3.8. UHPLC-DAD Analysis
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Funk, V.A.; Susanna, A.; Stussey, T.F.; Bayer, R.J. (Eds.) Systematics, Evolution, and Biogeography of the Compositae; IAPT, International Association for Plant Taxonomy: Vienna, Austria, 2009; ISBN 978-3-9501754-3-1. [Google Scholar]
- Fusani, P.; Zidorn, C. Phenolics and a Sesquiterpene Lactone in the Edible Shoots of Cicerbita alpina (L.) Wallroth. J. Food Compos. Anal. 2010, 23, 658–663. [Google Scholar] [CrossRef]
- Appendino, G.; Tettamanzi, P.; Gariboldi, P. Sesquiterpene Lactones and Furanocoumarins from Cicerbita alpina. Phytochemistry 1991, 30, 1319–1320. [Google Scholar] [CrossRef]
- Djordjević, I.; Tešević, V.; Janaćković, P.; Milosavljević, S.; Vajs, V. Sesquiterpene Lactones from Cicerbita alpina. Biochem. Syst. Ecol. 2004, 32, 209–210. [Google Scholar] [CrossRef]
- Zidorn, C.; Schwaha, R.; Ellmerer, E.; Stuppner, H. On the Occurrence of Sonchuside A in Cicerbita alpina and Its Chemosystematic Significance. J. Serbian Chem. Soc. 2005, 70, 171–175. [Google Scholar] [CrossRef]
- Zheleva-Dimitrova, D.; Petrova, A.; Zengin, G.; Sinan, K.I.; Balabanova, V.; Joubert, O.; Zidorn, C.; Voynikov, Y.; Simeonova, R.; Gevrenova, R. Metabolite Profiling and Bioactivity of Cicerbita alpina (L.) Wallr. (Asteraceae, Cichorieae). Plants 2023, 12, 1009. [Google Scholar] [CrossRef]
- Michalska, K.; Stojakowska, A.; Malarz, J.; Doležalová, I.; Lebeda, A.; Kisiel, W. Systematic Implications of Sesquiterpene Lactones in Lactuca Species. Biochem. Syst. Ecol. 2009, 37, 174–179. [Google Scholar] [CrossRef]
- Picman, A.K. Biological Activities of Sesquiterpene Lactones. Biochem. Syst. Ecol. 1986, 14, 255–281. [Google Scholar] [CrossRef]
- Wesołowska, A.; Nikiforuk, A.; Michalska, K.; Kisiel, W.; Chojnacka-Wójcik, E. Analgesic and Sedative Activities of Lactucin and Some Lactucin-like Guaianolides in Mice. J. Ethnopharmacol. 2006, 107, 254–258. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-Antioxidant Activity Relationships of Flavonoids and Phenolic Acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Spitaler, R.; Schlorhaufer, P.D.; Ellmerer, E.P.; Merfort, I.; Bortenschlager, S.; Stuppner, H.; Zidorn, C. Altitudinal Variation of Secondary Metabolite Profiles in Flowering Heads of Arnica Montana Cv. ARBO. Phytochemistry 2006, 67, 409–417. [Google Scholar] [CrossRef]
- Lee, J.; Scagel, C.F. Chicoric Acid: Chemistry, Distribution, and Production. Front. Chem. 2013, 1, 40. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Sun, Q.; Park, Y. The Bioactive Effects of Chicoric Acid As a Functional Food Ingredient. J. Med. Food 2019, 22, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Azay-Milhau, J.; Ferrare, K.; Leroy, J.; Aubaterre, J.; Tournier, M.; Lajoix, A.-D.; Tousch, D. Antihyperglycemic Effect of a Natural Chicoric Acid Extract of Chicory (Cichorium Intybus L.): A Comparative in Vitro Study with the Effects of Caffeic and Ferulic Acids. J. Ethnopharmacol. 2013, 150, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Zhang, X.; Niu, Y.; Diao, Z.; Ren, B.; Li, X.; Liu, Z.; Liu, X. Cichoric Acid Improved Hyperglycaemia and Restored Muscle Injury via Activating Antioxidant Response in MLD-STZ-Induced Diabetic Mice. Food Chem. Toxicol. 2017, 107, 138–149. [Google Scholar] [CrossRef]
- Pellati, F.; Benvenuti, S.; Magro, L.; Melegari, M.; Soragni, F. Analysis of Phenolic Compounds and Radical Scavenging Activity of Echinacea Spp. J. Pharm. Biomed. Anal. 2004, 35, 289–301. [Google Scholar] [CrossRef]
- Parr, A.J.; Bolwell, G.P. Phenols in the Plant and in Man. The Potential for Possible Nutritional Enhancement of the Diet by Modifying the Phenols Content or Profile. J. Sci. Food Agric. 2000, 80, 985–1012. [Google Scholar] [CrossRef]
- Koriem, K.M.M. Caftaric Acid: An Overview on Its Structure, Daily Consumption, Bioavailability and Pharmacological Effects. Biointerface Res. Appl. Chem. 2020, 10, 5616–5623. [Google Scholar] [CrossRef]
- Yan, Y.; Zhou, X.; Guo, K.; Zhou, F.; Yang, H. Use of Chlorogenic Acid against Diabetes Mellitus and Its Complications. J. Immunol. Res. 2020, 2020, 680508. [Google Scholar] [CrossRef]
- Zuñiga, L.Y.; Aceves-de La Mora, M.C.A.; González-Ortiz, M.; Ramos-Núñez, J.L.; Martínez-Abundis, E. Effect of Chlorogenic Acid Administration on Glycemic Control, Insulin Secretion, and Insulin Sensitivity in Patients with Impaired Glucose Tolerance. J. Med. Food 2018, 21, 469–473. [Google Scholar] [CrossRef]
- Kim, J.-S.; Lee, H.; Jung, C.H.; Lee, S.-J.; Ha, T.-Y.; Ahn, J. Chicoric Acid Mitigates Impaired Insulin Sensitivity by Improving Mitochondrial Function. Biosci. Biotechnol. Biochem. 2018, 82, 1197–1206. [Google Scholar] [CrossRef]
- Meng, S.; Cao, J.; Feng, Q.; Peng, J.; Hu, Y. Roles of Chlorogenic Acid on Regulating Glucose and Lipids Metabolism: A Review. Evid.-Based Complement. Altern. Med. 2013, 2013, 801457. [Google Scholar] [CrossRef] [PubMed]
- Sharma, T.; Singh, I.; Singh, N.; Vardey, G.; Kumawat, M.; Ghalaut, V.; Shankar, V. Antioxidant Enzymes and Lipid Peroxidation in Type 2 Diabetes Mellitus Patients with and without Nephropathy. N. Am. J. Med. Sci. 2013, 5, 213. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, J.; Nafizah, A.N.; Zariyantey, A.H.; Budin, S. Mechanisms of Diabetes-Induced Liver Damage: The Role of Oxidative Stress and Inflammation. SQUMJ 2016, 16, e132–e141. [Google Scholar] [CrossRef]
- Carr, R.M.; Oranu, A.; Khungar, V. Nonalcoholic Fatty Liver Disease. Gastroenterol. Clin. N. Am. 2016, 45, 639–652. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.W.; Hsu, A.; Tan, B.K.H. Anti-Diabetic and Anti-Lipidemic Effects of Chlorogenic Acid Are Mediated by Ampk Activation. Biochem. Pharmacol. 2013, 85, 1341–1351. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Jian, T.; Li, J.; Lv, H.; Tong, B.; Li, J.; Meng, X.; Ren, B.; Chen, J. Chicoric Acid Ameliorates Nonalcoholic Fatty Liver Disease via the AMPK/Nrf2/NFκB Signaling Pathway and Restores Gut Microbiota in High-Fat-Diet-Fed Mice. Oxidative Med. Cell. Longev. 2020, 2020, 9734560. [Google Scholar] [CrossRef]
- Xiao, H.; Xie, G.; Wang, J.; Hou, X.; Wang, X.; Wu, W.; Liu, X. Chicoric Acid Prevents Obesity by Attenuating Hepatic Steatosis, Inflammation and Oxidative Stress in High-Fat Diet-Fed Mice. Food Res. Int. 2013, 54, 345–353. [Google Scholar] [CrossRef]
- Ahmed, N. Advanced Glycation Endproducts—Role in Pathology of Diabetic Complications. Diabetes Res. Clin. Pract. 2005, 67, 3–21. [Google Scholar] [CrossRef]
- Kesavulu, M.M.; Giri, R.; Kameswara Rao, B.; Apparao, C. Lipid Peroxidation and Antioxidant Enzyme Levels in Type 2 Diabetics with Microvascular Complications. Diabetes Metab. 2000, 26, 387–392. [Google Scholar]
- Slatter, D.A.; Bolton, C.H.; Bailey, A.J. The Importance of Lipid-Derived Malondialdehyde in Diabetes Mellitus. Diabetologia 2000, 43, 550–557. [Google Scholar] [CrossRef]
- Baynes, J.W.; Thorpe, S.R. Role of Oxidative Stress in Diabetic Complications: A New Perspective on an Old Paradigm. Diabetes 1999, 48, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Waggiallah, H.; Alzohairy, M. The Effect of Oxidative Stress on Human Red Cells Glutathione Peroxidase, Glutathione Reductase Level, and Prevalence of Anemia among Diabetics. N. Am. J. Med. Sci. 2011, 3, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Arai, K.; Iizuka, S.; Tada, Y.; Oikawa, K.; Taniguchi, N. Increase in the Glucosylated Form of Erythrocyte Cu-Zn-Superoxide Dismutase in Diabetes and Close Association of the Nonenzymatic Glucosylation with the Enzyme Activity. Biochim. Biophys. Acta (BBA)-Gen. Subj. 1987, 924, 292–296. [Google Scholar] [CrossRef]
- Blum, J.; Fridovich, I. Inactivation of Glutathione Peroxidase by Superoxide Radical. Arch. Biochem. Biophys. 1985, 240, 500–508. [Google Scholar] [CrossRef]
- Parveen, K.; Khan, M.R.; Mujeeb, M.; Siddiqui, W.A. Protective Effects of Pycnogenol® on Hyperglycemia-Induced Oxidative Damage in the Liver of Type 2 Diabetic Rats. Chem.-Biol. Interact. 2010, 186, 219–227. [Google Scholar] [CrossRef]
- Likidlilid, A.; Patchanans, N.; Peerapatdit, T.; Sriratanasathavorn, C. Lipid Peroxidation and Antioxidant Enzyme Activities in Erythrocytes of Type 2 Diabetic Patients. J. Med. Assoc. Thail. 2010, 93, 682–693. [Google Scholar]
- Thygesen, L.; Thulin, J.; Mortensen, A.; Skibsted, L.H.; Molgaard, P. Antioxidant Activity of Cichoric Acid and Alkamides from Echinacea Purpurea, Alone and in Combination. Food Chem. 2007, 101, 74–81. [Google Scholar] [CrossRef]
- Jabłońska-Trypuć, A.; Krętowski, R.; Kalinowska, M.; Świderski, G.; Cechowska-Pasko, M.; Lewandowski, W. Possible Mechanisms of the Prevention of Doxorubicin Toxicity by Cichoric Acid—Antioxidant Nutrient. Nutrients 2018, 10, 44. [Google Scholar] [CrossRef]
- Li, J.; Zhao, C.; Wei, L.; Li, X.; Liu, F.; Zhang, M.; Liu, X.; Wang, Y. Preservation of Cichoric Acid Antioxidant Properties Loaded in Heat Treated Lactoferrin Nanoparticles. Molecules 2018, 23, 2678. [Google Scholar] [CrossRef]
- Mihaylova, R.; Gevrenova, R.; Petrova, A.; Savov, Y.; Zheleva-Dimitrova, D.; Balabanova, V.; Momekov, G.; Simeonova, R. Mitigating Effects of Tanacetum balsamita L. on Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD). Plants 2024, 13, 2086. [Google Scholar] [CrossRef]
- Pari, L.; Karthikesan, K.; Menon, V.P. Comparative and Combined Effect of Chlorogenic Acid and Tetrahydrocurcumin on Antioxidant Disparities in Chemical Induced Experimental Diabetes. Mol. Cell. Biochem. 2010, 341, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Kurt, H. Comparative Therapeutic Potentials of Acarbose and a Formulated Herbal Extract on Type 2 Diabetic Rats. Afr. J. Pharm. Pharmacol. 2012, 6, 2194–2204. [Google Scholar] [CrossRef]
- Héctor Polizio, A.; Peña, C. Effects of Angiotensin II Type 1 Receptor Blockade on the Oxidative Stress in Spontaneously Hypertensive Rat Tissues. Regul. Pept. 2005, 128, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Bump, E.A.; Taylor, Y.C.; Brown, J.M. Role of Glutathione in the Hypoxic Cell Cytotoxicity of Misonidazole. Cancer Res. 1983, 43, 997–1002. [Google Scholar]
- Tappel, A.L. Hydroperoxides. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1978; Volume 52, pp. 506–513. ISBN 978-0-12-181952-1. [Google Scholar]
- Aebi, H. Catalase. In Methods of Enzymatic Analysis; Elsevier: Amsterdam, The Netherlands, 1974; pp. 673–684. ISBN 978-0-12-091302-2. [Google Scholar]
- Misra, H.P.; Fridovich, I. The Role of Superoxide Anion in the Autoxidation of Epinephrine and a Simple Assay for Superoxide Dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques, 5th ed.; Churchill Livingstone Publications: Edinburgh, UK, 2002; Available online: http://www.sciepub.com/reference/141095 (accessed on 20 April 2019).
| Groups | Week 1 | Week 2 | Week 3 | Week 4 |
|---|---|---|---|---|
| Control | 5.9 ± 0.35 | 5.8 ± 0.30 | 5.8 ± 0.31 | 5.6 ± 0.19 |
| ECAld | 5.9 ± 0.23 | 5.7 ± 0.24 | 6.1 ± 0.11 | 6.0 ± 0.55 |
| ECAhd | 5.7 ± 0.29 | 5.8 ± 0.32 | 6.0 ± 0.17 | 6.1 ± 0.34 |
| DMT2 | 6.0 ± 0.21 | 7.7 ± 0.52 ** | 8.2 ± 0.42 *** | 9.2 ± 0.32 *** |
| DMT2 + Acarb | 5.8 ± 0.24 | 7.4 ± 0.29 * | 7.8 ± 0.31 *** | 6.9 ± 0.22 **+++ |
| DMT2 + ECAld | 5.8 ± 0.13 | 7.2 ± 0.18 ** | 8.1 ± 0.16 *** | 7.1 ± 0.14 ***+++ |
| DMT2 + ECAhd | 6.0 ± 0.29 | 7.2 ± 0.11 * | 7.6 ± 0.23 *** | 6.6 ± 0.15 **+++# |
| Parameter | Controls | ECAld | ECAhd | DMT2 | DMT2 + Acarb | DMT2 + ECAld | DMT2 + ECAhd |
|---|---|---|---|---|---|---|---|
| Cholesterol mmol/L | 1.51 ± 0.06 | 1.52 ± 0.08 | 1.53 ± 0.07 | 2.29 ± 0.07 *** | 1.85 ± 0.061 ***+++ | 1.77 ± 0.10 **++ | 1.66 ± 0.14 *++# |
| Triglycerides mmol/L | 0.54 ± 0.03 | 0.52 ± 0.03 | 0.50 ± 0.08 | 1.30 ± 0.06 *** | 0.60 ± 0.05 ***+++ | 0.62 ± 0.04 *+++ | 0.59 ± 0.04 +++ |
| ASAT U/L | 88.8 ± 5.7 | 90.5 ± 3.1 | 87.4 ± 3.85 | 143.8 ± 17.8 *** | 139.8 ± 16.9 *** | 121.2 ± 11.5 **+ | 111.0 ± 6.32 ***+# |
| ALAT U/L | 65.6 ± 6.5 | 66.2 ± 10.6 | 69.6 ± 4.8 | 160.6 ± 9.99 *** | 147.6 ± 12.7 *** | 128.4 ± 13.7 **++# | 133.2 ± 17.1 **++ |
| Urea mmol/L | 6.31 ± 0.65 | 6.67 ± 0.87 | 6.61 ± 0.81 | 14.2 ± 0.75 *** | 12.0 ± 0.32 ***+ | 12.6 ± 0.60 ***+ | 12.3 ± 0.9 ***+ |
| Creatinine µmol/L | 52.9 ± 2.23 | 50.2 ± 1.16 | 51.9 ± 1.68 | 65.5 ± 3.23 ** | 51.1 ± 2.4 ++ | 50.1 ± 1.5 +++ | 52.2 ± 2.25 ++ |
| Parameter | Controls | ECAld | ECAhd | DMT2 | DMT2 + Acarb | DMT2 + ECAld | DMT2 + ECAhd |
|---|---|---|---|---|---|---|---|
| MDA 1 | 3.41 ± 0.29 | 3.59 ± 0.32 | 3.51 ± 0.27 | 5.15 ± 0.23 *** | 4.68 ± 0.42 ** | 3.82 ± 0.27 **++# | 3.62 ± 0.2 *++## |
| GSH 1 | 6.64 ± 0.55 | 7.0 ± 0.14 | 7.26 ± 0.24 | 4.11 ± 0.3 ** | 4.38 ± 0.24 ** | 4.65 ± 0.35 * | 5.24 ± 0.13 *++## |
| GPx 2 | 2.92 ± 0.28 | 2.86 ±0.29 | 2.89 ± 0.18 | 1.39 ± 0.15 *** | 1.53 ± 0.19 ** | 1.85 ± 0.11 **++# | 2.36 ± 0.17 *++## |
| CAT 2 | 5.70 ± 0.14 | 5.75 ± 0.29 | 5.5 ± 0.29 | 6.8 ± 0.27 ** | 6.66 ± 0.41 ** | 7.31 ± 0.13 ***+# | 7.31 ± 0.12 *,***+# |
| SOD 2 | 1.66 ± 0.1 | 1.68 ± 0.07 | 1.73 ± 0.06 | 1.15 ± 0.11 ** | 1.35 ± 0.14 ** | 1.71 ± 0.12 ++## | 1.79 ± 0.19 ++# |
| No | Analyte | tR | Content (mg/g de) |
|---|---|---|---|
| 1. | Caftaric acid | 4.35 | 11.364 ± 2.09 |
| 2. | Chlorogenic acid | 7.60 | 9.248 ± 0.050 |
| 3. | ATA I | 8.26 | 2.998 ± 0.336 |
| 4. | ATA II | 10.06 | 1.549 ± 0.086 |
| 5. | ATA III | 10.59 | 2.538 ± 0.047 |
| 6. | Cichoric acid | 13.21 | 91.930 ± 4.642 |
| 7. | AQA I | 14.15 | 5.032 ± 0.116 |
| 8. | Luteolin 7-O-glucoside | 14.70 | 3.006 ± 0.068 |
| 9. | 3,4-diCQA | 15.25 | 2.627 ± 0.045 |
| 10. | 1,5-diCQA | 16.09 | 9.455 ± 0.434 |
| 11. | 3,5-diCQA | 16.47 | 0.370 ± 0.005 |
| 12. | AQA II | 17.30 | 0.909 ± 0.038 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheleva-Dimitrova, D.; Petrova, A.; Savov, Y.; Gevrenova, R.; Balabanova, V.; Momekov, G.; Simeonova, R. Protective Potential of Cicerbita alpina Leaf Extract on Metabolic Disorders and Oxidative Stress in Model Animals. Int. J. Mol. Sci. 2024, 25, 10851. https://doi.org/10.3390/ijms251910851
Zheleva-Dimitrova D, Petrova A, Savov Y, Gevrenova R, Balabanova V, Momekov G, Simeonova R. Protective Potential of Cicerbita alpina Leaf Extract on Metabolic Disorders and Oxidative Stress in Model Animals. International Journal of Molecular Sciences. 2024; 25(19):10851. https://doi.org/10.3390/ijms251910851
Chicago/Turabian StyleZheleva-Dimitrova, Dimitrina, Alexandra Petrova, Yonko Savov, Reneta Gevrenova, Vessela Balabanova, Georgi Momekov, and Rumyana Simeonova. 2024. "Protective Potential of Cicerbita alpina Leaf Extract on Metabolic Disorders and Oxidative Stress in Model Animals" International Journal of Molecular Sciences 25, no. 19: 10851. https://doi.org/10.3390/ijms251910851










