A Review on Apple Pomace Bioactives for Natural Functional Food and Cosmetic Products with Therapeutic Health-Promoting Properties
Abstract
:1. Introduction
2. Materials and Methods
3. Apple Pomace Composition
3.1. Apple Pomace Macro-Constituents
3.2. Apple Pomace Micro-Constituents
4. Apple Pomace: Constituents with Health-Promoting Properties
4.1. Apple Pomace Polyphenols: Mechanisms of Action and Health Promotion
4.2. Additional Macro- and Micro-Constituents in Apple Pomace with the Potential to Promote Functional Health—Mechanisms of Action
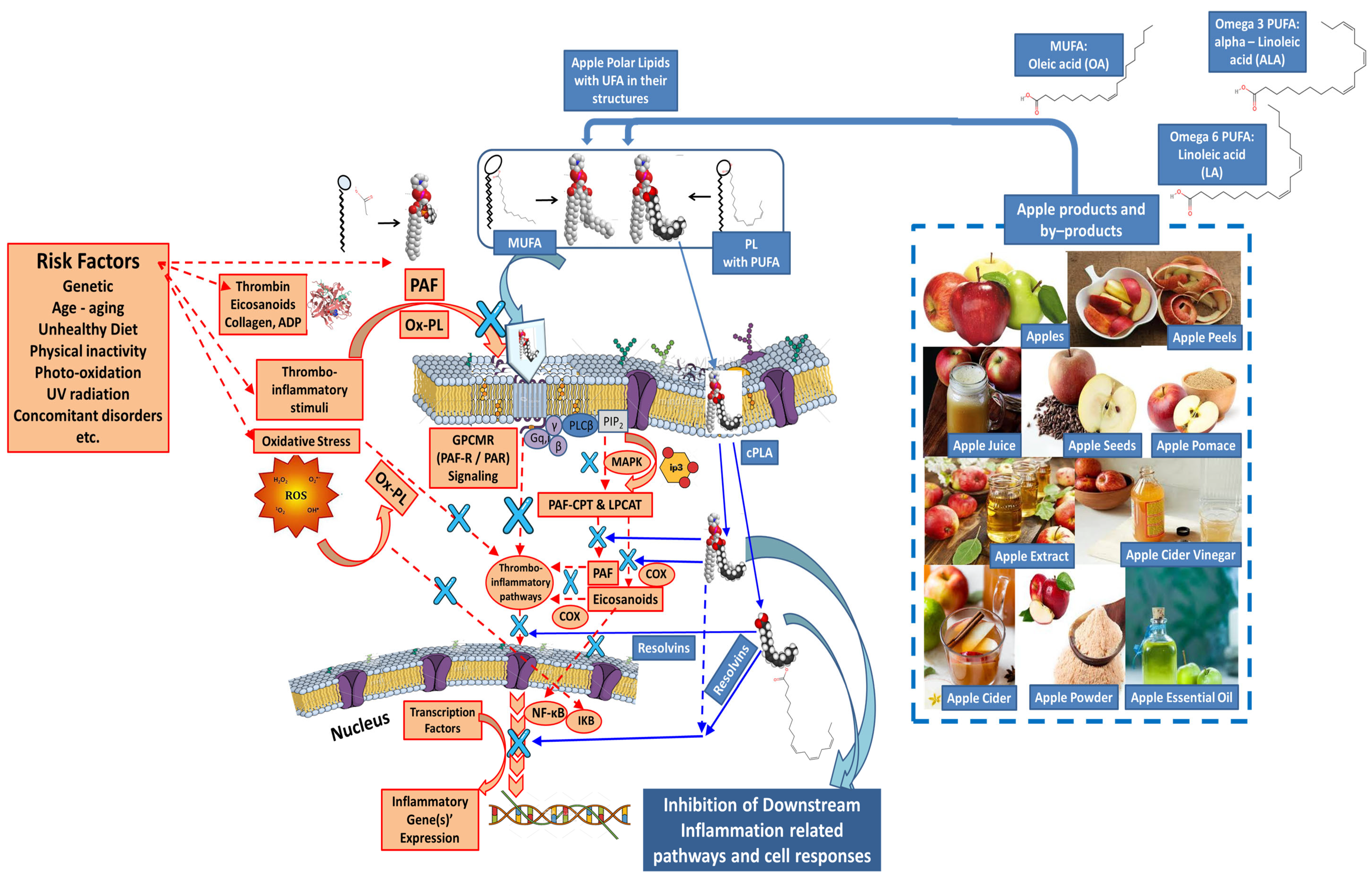
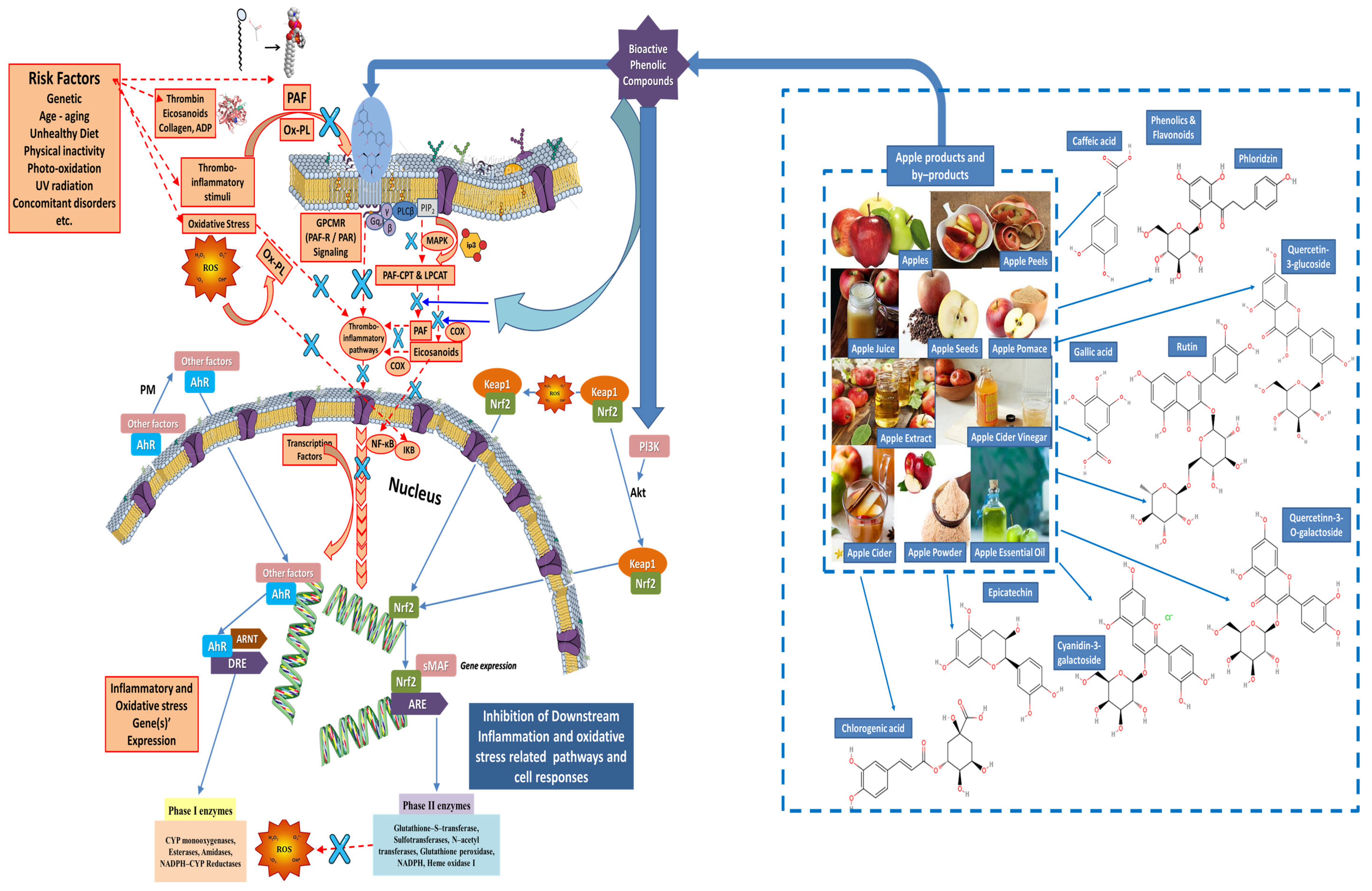
4.3. Mechanisms of Action of Natural Amphiphilic Bioactives (Rich-in-UFA Biofunctional Polar Lipids and Phenolic Bioactives) from Apple Products and By-Products against Oxidative Stress and Thrombo-Inflammatory Signaling
5. Apple Pomace: A Functional Ingredient or Substrate
5.1. Foods
5.2. Cosmeceuticals
5.2.1. Anti-Aging and Anti-Wrinkle Properties
5.2.2. Skin Protection
5.2.3. Skin Whitening
5.2.4. Antioxidant Properties
5.2.5. Antimicrobial Properties
5.2.6. Anti-Inflammatory Properties
5.3. Limitations of Apple Pomace Utilization
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Oyenihi, A.B.; Belay, Z.A.; Mditshwa, A.; Caleb, O.J. “An Apple a Day Keeps the Doctor Away”: The Potentials of Apple Bioactive Constituents for Chronic Disease Prevention. J. Food Sci. 2022, 87, 2291–2309. [Google Scholar] [CrossRef] [PubMed]
- Perussello, C.A.; Zhang, Z.; Marzocchella, A.; Tiwari, B.K. Valorization of Apple Pomace by Extraction of Valuable Compounds. Compr. Rev. Food Sci. Food Saf. 2017, 16, 776–796. [Google Scholar] [CrossRef] [PubMed]
- Karantonis, H.C.; Tsoupras, A.; Moran, D.; Zabetakis, I.; Nasopoulou, C. Chapter 5—Olive, Apple, and Grape Pomaces with Antioxidant and Anti-Inflammatory Bioactivities for Functional Foods. In Functional Foods and Their Implications for Health Promotion; Zabetakis, I., Tsoupras, A., Lordan, R., Ramji, D., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 131–159. ISBN 978-0-12-823811-0. [Google Scholar]
- Tsoupras, A.; Gkika, D.A.; Markopoulos, T.; Curran, R.; Scallon, C.; Karali, M.; Kyzas, G.Z. Apple Products (Apple Juice and Cider) and by-Products (Apple Pomace): Bioactive Compounds and Biological Properties. In Natural Products in Beverages: Botany, Phytochemistry, Pharmacology and Processing; Springer: Berlin/Heidelberg, Germany, 2024; pp. 1–42. [Google Scholar]
- Tsoupras, A.; Moran, D.; Lordan, R. Functional Properties of the Fermented Alcoholic Beverages: Apple Cider and Beer; Academic Press: Cambridge, MA, USA, 2023; pp. 319–339. ISBN 978-0-12-823811-0. [Google Scholar]
- Barreira, J.C.; Arraibi, A.A.; Ferreira, I.C. Bioactive and Functional Compounds in Apple Pomace from Juice and Cider Manufacturing: Potential Use in Dermal Formulations. Trends Food Sci. Technol. 2019, 90, 76–87. [Google Scholar] [CrossRef]
- Puangpraphant, S.; Cuevas-Rodríguez, E.-O.; Oseguera-Toledo, M. Anti-Inflammatory and Antioxidant Phenolic Compounds. In Current Advances for Development of Functional Foods Modulating Inflammation and Oxidative Stress; Elsevier: Amsterdam, The Netherlands, 2022; pp. 165–180. [Google Scholar]
- Feng, S.; Yi, J.; Li, X.; Wu, X.; Zhao, Y.; Ma, Y.; Bi, J. Systematic Review of Phenolic Compounds in Apple Fruits: Compositions, Distribution, Absorption, Metabolism, and Processing Stability. J. Agric. Food Chem. 2021, 69, 7–27. [Google Scholar] [CrossRef]
- Lyu, F.; Luiz, S.F.; Azeredo, D.R.P.; Cruz, A.G.; Ajlouni, S.; Ranadheera, C.S. Apple Pomace as a Functional and Healthy Ingredient in Food Products: A Review. Processes 2020, 8, 319. [Google Scholar] [CrossRef]
- Szabo, K.; Mitrea, L.; Călinoiu, L.F.; Teleky, B.-E.; Martău, G.A.; Plamada, D.; Pascuta, M.S.; Nemeş, S.-A.; Varvara, R.-A.; Vodnar, D.C. Natural Polyphenol Recovery from Apple-, Cereal-, and Tomato-Processing by-Products and Related Health-Promoting Properties. Molecules 2022, 27, 7977. [Google Scholar] [CrossRef] [PubMed]
- Ben-Othman, S.; Jõudu, I.; Bhat, R. Bioactives from Agri-Food Wastes: Present Insights and Future Challenges. Molecules 2020, 25, 510. [Google Scholar] [CrossRef]
- Tsoupras, A.; Moran, D.; Byrne, T.; Ryan, J.; Barrett, L.; Traas, C.; Zabetakis, I. Anti-Inflammatory and Anti-Platelet Properties of Lipid Bioactives from Apple Cider by-Products. Molecules 2021, 26, 2869. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.; Sales, H.; Pontes, R.; Nunes, J.; Gouveia, I. Food Wastes and Microalgae as Sources of Bioactive Compounds and Pigments in a Modern Biorefinery: A Review. Antioxidants 2023, 12, 328. [Google Scholar] [CrossRef]
- Rabetafika, H.N.; Bchir, B.; Blecker, C.; Richel, A. Fractionation of Apple By-Products as Source of New Ingredients: Current Situation and Perspectives. Trends Food Sci. Technol. 2014, 40, 99–114. [Google Scholar] [CrossRef]
- Ashok, P.K.; Upadhyaya, K. Tannins Are Astringent. J. Pharmacogn. Phytochem. 2012, 1, 45–50. [Google Scholar]
- Sette, P.; Fernandez, A.; Soria, J.; Rodriguez, R.; Salvatori, D.; Mazza, G. Integral Valorization of Fruit Waste from Wine and Cider Industries. J. Clean. Prod. 2020, 242, 118486. [Google Scholar] [CrossRef]
- Hobbi, P.; Okoro, O.V.; Hajiabbas, M.; Hamidi, M.; Nie, L.; Megalizzi, V.; Musonge, P.; Dodi, G.; Shavandi, A. Chemical Composition, Antioxidant Activity and Cytocompatibility of Polyphenolic Compounds Extracted from Food Industry Apple Waste: Potential in Biomedical Application. Molecules 2023, 28, 675. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Khan, S.T. Potential Industrial Use of Compounds from By-Products of Fruits and Vegetables. In Health and Safety Aspects of Food Processing Technologies; Malik, A., Erginkaya, Z., Erten, H., Eds.; Springer: Cham, Switzerland, 2019; pp. 273–307. [Google Scholar] [CrossRef]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for Extraction and Isolation of Natural Products: A Comprehensive Review. Chin. Med. 2018, 13, 1–26. [Google Scholar] [CrossRef]
- Zhang, H.-F.; Yang, X.-H.; Wang, Y. Microwave Assisted Extraction of Secondary Metabolites from Plants: Current Status and Future Directions. Trends Food Sci. Technol. 2011, 22, 672–688. [Google Scholar] [CrossRef]
- Tsoupras, A.; Moran, D.; Shiels, K.; Saha, S.K.; Abu-Reidah, I.M.; Thomas, R.H.; Redfern, S. Enrichment of Whole-Grain Breads with Food-Grade Extracted Apple Pomace Bioactives Enhanced Their Anti-Inflammatory, Antithrombotic and Anti-Oxidant Functional Properties. Antioxidants 2024, 13, 225. [Google Scholar] [CrossRef]
- Tsoupras, A.; Moran, D.; Pleskach, H.; Durkin, M.; Traas, C.; Zabetakis, I. Beneficial Anti-Platelet and Anti-Inflammatory Properties of Irish Apple Juice and Cider Bioactives. Foods 2021, 10, 412. [Google Scholar] [CrossRef]
- Moran, D.; Fleming, M.; Daly, E.; Gaughan, N.; Zabetakis, I.; Traas, C.; Tsoupras, A. Anti-Platelet Properties of Apple Must/Skin Yeasts and of Their Fermented Apple Cider Products. Beverages 2021, 7, 54. [Google Scholar] [CrossRef]
- Dutta-Roy, A.K.; Crosbie, L.; Gordon, M.J. Effects of Tomato Extract on Human Platelet Aggregation in Vitro. Platelets 2001, 12, 218–227. [Google Scholar] [PubMed]
- Fărcaș, A.C.; Socaci, S.A.; Chiș, M.S.; Dulf, F.V.; Podea, P.; Tofană, M. Analysis of Fatty Acids, Amino Acids and Volatile Profile of Apple by-Products by Gas Chromatography-Mass Spectrometry. Molecules 2022, 27, 1987. [Google Scholar] [CrossRef]
- Tsoupras, A.B.; Iatrou, C.; Frangia, C.; Demopoulos, C.A. The Implication of Platelet Activating Factor in Cancer Growth and Metastasis: Potent Beneficial Role of PAF-Inhibitors and Antioxidants. Infect. Disord. Drug Targets Disord. 2009, 9, 390–399. [Google Scholar] [CrossRef]
- Tsoupras, A.; Lordan, R.; Zabetakis, I. Chapter 3—Inflammation and Cardiovascular Diseases. In The Impact of Nutrition and Statins on Cardiovascular Diseases; Zabetakis, I., Lordan, R., Tsoupras, A., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 53–117. ISBN 978-0-12-813792-5. [Google Scholar]
- Jackson, C.; Shukla, V.; Kolba, N.; Agarwal, N.; Padilla-Zakour, O.I.; Tako, E. Empire Apple (Malus domestica) Juice, Pomace, and Pulp Modulate Intestinal Functionality, Morphology, and Bacterial Populations in Vivo (Gallus gallus). Nutrients 2022, 14, 4955. [Google Scholar] [CrossRef] [PubMed]
- Fernandez de Palencia, P.; Werning, M.L.; Sierra-Filardi, E.; Duenas, M.T.; Irastorza, A.; Corbí, A.L.; López, P. Probiotic Properties of the 2-Substituted (1, 3)-β-D-Glucan-Producing Bacterium Pediococcus Parvulus 2.6. Appl. Environ. Microbiol. 2009, 75, 4887–4891. [Google Scholar] [CrossRef] [PubMed]
- Cerda-Tapia, A.; Pérez-Chabela, M.D.L.; Pérez-Álvarez, J.Á.; Fernández-López, J.; Viuda-Martos, M. Valorization of Pomace Powder Obtained from Native Mexican Apple (Malus domestica Var. Rayada): Chemical, Techno-Functional and Antioxidant Properties. Plant Foods Hum. Nutr. 2015, 70, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Buljeta, I.; Nosić, M.; Pichler, A.; Ivić, I.; Šimunović, J.; Kopjar, M. Apple Fibers as Carriers of Blackberry Juice Polyphenols: Development of Natural Functional Food Additives. Molecules 2022, 27, 3029. [Google Scholar] [CrossRef]
- Kuś, P.M.; Jerković, I.; Tuberoso, C.I.G.; Šarolić, M. The Volatile Profiles of a Rare Apple (Malus domestica Borkh.) Honey: Shikimic Acid-pathway Derivatives, Terpenes, and Others. Chem. Biodivers. 2013, 10, 1638–1652. [Google Scholar] [CrossRef]
- Eliopoulos, C.; Markou, G.; Langousi, I.; Arapoglou, D. Reintegration of Food Industry By-Products: Potential Applications. Foods 2022, 11, 3743. [Google Scholar] [CrossRef]
- Iqbal, A.; Schulz, P.; Rizvi, S.S. Valorization of Bioactive Compounds in Fruit Pomace from Agro-Fruit Industries: Present Insights and Future Challenges. Food Biosci. 2021, 44, 101384. [Google Scholar] [CrossRef]
- Tsoupras, A.; Lordan, R.; Zabetakis, I. Inflammation, Not Cholesterol, Is a Cause of Chronic Disease. Nutrients 2018, 10, 604. [Google Scholar] [CrossRef]
- Tsoupras, A.; Adamantidi, T.; Finos, M.A.; Philippopoulos, A.; Detopoulou, P.; Tsopoki, I.; Kynatidou, M.; Demopoulos, C.A. Re-Assessing the Role of Platelet Activating Factor and Its Inflammatory Signaling and Inhibitors in Cancer and An-Ti-Cancer Strategies. Front. Biosci. Landmark 2024, 29, 345. [Google Scholar]
- Han, X.; Shen, T.; Lou, H. Dietary Polyphenols and Their Biological Significance. Int. J. Mol. Sci. 2007, 8, 950–988. [Google Scholar] [CrossRef]
- Luo, J.; Si, H.; Jia, Z.; Liu, D. Dietary Anti-Aging Polyphenols and Potential Mechanisms. Antioxidants 2021, 10, 283. [Google Scholar] [CrossRef] [PubMed]
- Heydarzadeh, S.; Kia, S.K.; Zarkesh, M.; Pakizehkar, S.; Hosseinzadeh, S.; Hedayati, M. The Cross-Talk between Polyphenols and the Target Enzymes Related to Oxidative Stress-Induced Thyroid Cancer. Oxidative Med. Cell. Longev. 2022, 2022, 2724324. [Google Scholar] [CrossRef] [PubMed]
- Palai, S.; Rudrapal, M. Nanodeliveries of Food Polyphenols as Nutraceuticals. In Polyphenols; John Wiley & Sons (Wiley): Hoboken, NJ, USA, 2023; pp. 155–175. ISBN 978-1-394-18886-4. [Google Scholar]
- Gumul, D.; Kruczek, M.; Ivanišová, E.; Słupski, J.; Kowalski, S. Apple Pomace as an Ingredient Enriching Wheat Pasta with Health-Promoting Compounds. Foods 2023, 12, 804. [Google Scholar] [CrossRef] [PubMed]
- Gorjanović, S.; Micić, D.; Pastor, F.; Tosti, T.; Kalušević, A.; Ristić, S.; Zlatanović, S. Evaluation of Apple Pomace Flour Obtained Industrially by Dehydration as a Source of Biomolecules with Antioxidant, Antidiabetic and Antiobesity Effects. Antioxidants 2020, 9, 413. [Google Scholar] [CrossRef]
- Zlatanović, S.; Kalušević, A.; Micić, D.; Laličić-Petronijević, J.; Tomić, N.; Ostojić, S.; Gorjanović, S. Functionality and Storability of Cookies Fortified at the Industrial Scale with up to 75% of Apple Pomace Flour Produced by Dehydration. Foods 2019, 8, 561. [Google Scholar] [CrossRef]
- Kruczek, M.; Gumul, D.; Korus, A.; Buksa, K.; Ziobro, R. Phenolic Compounds and Antioxidant Status of Cookies Supplemented with Apple Pomace. Antioxidants 2023, 12, 324. [Google Scholar] [CrossRef]
- Valková, V.; Ďúranová, H.; Havrlentová, M.; Ivanišová, E.; Mezey, J.; Tóthová, Z.; Gabríny, L.; Kačániová, M. Selected Physico-Chemical, Nutritional, Antioxidant and Sensory Properties of Wheat Bread Supplemented with Apple Pomace Powder as a by-Product from Juice Production. Plants 2022, 11, 1256. [Google Scholar] [CrossRef]
- Popescu, L.; Ceșco, T.; Gurev, A.; Ghendov-Mosanu, A.; Sturza, R.; Tarna, R. Impact of Apple Pomace Powder on the Bioactivity, and the Sensory and Textural Characteristics of Yogurt. Foods 2022, 11, 3565. [Google Scholar] [CrossRef]
- Jung, J.; Cavender, G.; Zhao, Y. Impingement Drying for Preparing Dried Apple Pomace Flour and Its Fortification in Bakery and Meat Products. J. Food Sci. Technol. 2015, 52, 5568–5578. [Google Scholar] [CrossRef]
- Munekata, P.E.; Pateiro, M.; Domínguez, R.; Nieto, G.; Kumar, M.; Dhama, K.; Lorenzo, J.M. Bioactive Compounds from Fruits as Preservatives. Foods 2023, 12, 343. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Kristo, E.; LaPointe, G. The Effect of Apple Pomace on the Texture, Rheology and Microstructure of Set Type Yogurt. Food Hydrocoll. 2019, 91, 83–91. [Google Scholar] [CrossRef]
- Mileriene, J.; Serniene, L.; Kasparaviciene, B.; Lauciene, L.; Kasetiene, N.; Zakariene, G.; Kersiene, M.; Leskauskaite, D.; Viskelis, J.; Kourkoutas, Y. Exploring the Potential of Sustainable Acid Whey Cheese Supplemented with Apple Pomace and GABA-Producing Indigenous Lactococcus Lactis Strain. Microorganisms 2023, 11, 436. [Google Scholar] [CrossRef] [PubMed]
- Bortolini, D.G.; Benvenutti, L.; Demiate, I.M.; Nogueira, A.; Alberti, A.; Zielinski, A.A.F. A New Approach to the Use of Apple Pomace in Cider Making for the Recovery of Phenolic Compounds. LWT 2020, 126, 109316. [Google Scholar] [CrossRef]
- Benvenutti, L.; Bortolini, D.G.; Fischer, T.E.; Zardo, D.M.; Nogueira, A.; Zielinski, A.A.F.; Alberti, A. Bioactive Compounds Recovered from Apple Pomace as Ingredient in Cider Processing: Monitoring of Compounds during Fermentation. J. Food Sci. Technol. 2022, 59, 3349–3358. [Google Scholar] [CrossRef]
- Di Lorenzo, R.; Maisto, M.; Ricci, L.; Piccolo, V.; Marzocchi, A.; Greco, G.; Tenore, G.C.; Laneri, S. Annurca Apple Oleolite as Functional Ingredient for the Formulation of Cosmetics with Skin-Antiaging Activity. Int. J. Mol. Sci. 2024, 25, 1677. [Google Scholar] [CrossRef]
- Cho, Y.-J.; Lee, E.-H.; Yoo, J.; Kwon, S.-I.; Choi, H.W.; Kang, I.-K. Analysis of Functional Properties in the New Korean Apple Cultivar Arisoo. Hortic. Environ. Biotechnol. 2019, 60, 787–795. [Google Scholar] [CrossRef]
- Lee, E.-H.; Cho, E.-B.; Kim, B.-O.; Jung, H.-Y.; Lee, S.-Y.; Yoo, J.; Kang, I.-K.; Cho, Y.-J. Functional Properties of Newly-Bred ‘Summer King’Apples. Hortic. Sci. Technol. 2020, 38, 405–417. [Google Scholar]
- Trentini, M.; Zanolla, I.; Zanotti, F.; Tiengo, E.; Licastro, D.; Dal Monego, S.; Lovatti, L.; Zavan, B. Apple Derived Exosomes Improve Collagen Type I Production and Decrease MMPs during Aging of the Skin through Downregulation of the NF-κB Pathway as Mode of Action. Cells 2022, 11, 3950. [Google Scholar] [CrossRef]
- Stojiljković, D.; Tadić, V.; Stanković, M.; Roganović, S.; Arsić, I. Standardized Extract of Wild Apple Fruit in Alkyl-polyglucoside-based Cosmetic Cream–Estimation of Stability, Safety, Antioxidant Activity and Efficiency. Int. J. Cosmet. Sci. 2018, 40, 285–294. [Google Scholar] [CrossRef]
- Martins, R.M.; de Assis Dias Alves, G.; de Siqueira Martins, S.; de Freitas, L.A.P.; Rochette, P.J.; Moulin, V.J.; Fonseca, M.J.V. Apple Extract (Malus Sp.) and Rutin as Photochemopreventive Agents: Evaluation of Ultraviolet b-Induced Alterations on Skin Biopsies and Tissue-Engineered Skin. Rejuvenation Res. 2020, 23, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Kitayama, Y.; Kanazawa, T.; Okabe, T.; Morita, Y.; Fukui, T.; Yamamoto, T.; Nakamura, S. Development of Extracts from Aomori Hiba and Apple Polyphenol as Cosmetics Materials. Trans.-Mater. Res. Soc. Jpn. 2006, 31, 965. [Google Scholar]
- Cossignani, L.; Ianni, F.; Blasi, F.; Pollini, L.; Di Michele, A.; Pagano, C.; Ricci, M.; Perioli, L. Effect of Different Drying Treatments and Sieving on Royal Gala Apple Pomace, a Thickening Agent with Antioxidant Properties. Plants 2023, 12, 906. [Google Scholar] [CrossRef] [PubMed]
- Opriş, O.; Lung, I.; Soran, M.-L.; Stegarescu, A.; Cesco, T.; Ghendov-Mosanu, A.; Podea, P.; Sturza, R. Efficient Extraction of Total Polyphenols from Apple and Investigation of Its SPF Properties. Molecules 2022, 27, 1679. [Google Scholar] [CrossRef] [PubMed]
- Butkevičiūtė, A.; Ramanauskienė, K.; Janulis, V. Formulation of Gels and Emulgels with Malus Domestica Borkh: Apple Extracts and Their Biopharmaceutical Evaluation In Vitro. Antioxidants 2022, 11, 373. [Google Scholar] [CrossRef]
- Maisto, M.; Piccolo, V.; Novellino, E.; Schiano, E.; Iannuzzo, F.; Ciampaglia, R.; Summa, V.; Tenore, G.C. Optimization of Ursolic Acid Extraction in Oil from Annurca Apple to Obtain Oleolytes with Potential Cosmeceutical Application. Antioxidants 2023, 12, 224. [Google Scholar] [CrossRef]
- Nagaki, M.; Goto, Y.; Yamanouchi, K.; Kudo, S.; Chounan, Y. Phytochemical Profile and Antioxidant Activity of the Leaf of Aomori Hiba (Thujopsis dolabrata SEIB. et ZUCC. var. hondai MAKINO). J. Hirosaki Univ. Health Welfare 2018, 9, 1–6. Available online: https://www.researchgate.net/publication/324025610_Phytochemical_Profile_and_Antioxidant_Activity_of_the_Leaf_of_Aomori_Hiba_Thujopsis_dolabrata_SEIB_et_ZUCC_var_hondai_MAKINO (accessed on 6 October 2024).
- de Oliveira Raphaelli, C.; Azevedo, J.G.; dos Santos Pereira, E.; Vinholes, J.R.; Camargo, T.M.; Hoffmann, J.F.; Ribeiro, J.A.; Vizzotto, M.; Rombaldi, C.V.; Wink, M.R. Phenolic-Rich Apple Extracts Have Photoprotective and Anti-Cancer Effect in Dermal Cells. Phytomedicine Plus 2021, 1, 100112. [Google Scholar] [CrossRef]
- Khayatan, D.; Nilforoushzadeh, M.A.; Ahmadi Ashtiani, H.R.; Hashemian, F. Effect of Apple (Malus domestica) Stem Cells on UVB-induced Damage Skin with Anti-inflammatory Properties: An In Vivo Study. Adv. Mater. Sci. Eng. 2022, 2022, 2417766. [Google Scholar] [CrossRef]
- Park, H.R.; Kim, J.K.; Lee, J.K.; Choi, B.R.; Ku, S.K.; Jegal, K.H. The Protective Effects of Unripe Apple (Malus pumila) Extract on Ultraviolet B-Induced Skin Photoaging Mouse Model. Appl. Sci. 2023, 13, 4788. [Google Scholar] [CrossRef]
- Stojiljković, D.; Arsić, I.; Tadić, V. Extracts of Wild Apple Fruit (Malus sylvestris (L.) Mill., Rosaceae) as a Source of Antioxidant Substances for Use in Production of Nutraceuticals and Cosmeceuticals. Ind. Crops Prod. 2016, 80, 165–176. [Google Scholar] [CrossRef]
- Vizzotto, M.; da Rosa Fetter, M.; Couto Pereira, M.; Dutra Corbelini, D. Apple: Extraction Optimization of Antioxidant Compounds and Determination of Total Phenolic Amount and Antioxidant Activity on Product and Co-Product. Acta Hortic. 2010, 939, 331–336. [Google Scholar] [CrossRef]
- Ivanišová, E.; Frančáková, H.; Ritschlová, P.; Dráb, Š.; Solgajová, M.; Tokár, M. Biological Activity of Apple Juice Enriched by Herbal Extracts. J. Microbiol. Biotechnol. Food Sci. 2015, 4, 69–73. [Google Scholar] [CrossRef]
- Park, S.; Park, S.-K. Anti-Oxidant and Anti-Aging Effects of Phlorizin Are Mediated by DAF-16-Induced Stress Response and Autophagy in Caenorhabditis Elegans. Antioxidants 2022, 11, 1996. [Google Scholar] [CrossRef] [PubMed]
- Hyun, S.B.; Bae, S.; Hyun, C.-G. Antioxidant Activities of Jeju Wax Apple (Syzygium samarangense) and Safety of Human Keratinocytes and Primary Skin Irritation Test. Cosmetics 2020, 7, 39. [Google Scholar] [CrossRef]
- Lin, Y.-P.; Hsu, F.-L.; Chen, C.-S.; Chern, J.-W.; Lee, M.-H. Constituents from the Formosan Apple Reduce Tyrosinase Activity in Human Epidermal Melanocytes. Phytochemistry 2007, 68, 1189–1199. [Google Scholar] [CrossRef]
- Shoji, T.; Masumoto, S.; Moriichi, N.; Ohtake, Y.; Kanda, T. Administration of Apple Polyphenol Supplements for Skin Conditions in Healthy Women: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2020, 12, 1071. [Google Scholar] [CrossRef]
- Butkeviciute, A.; Ramanauskiene, K.; Valdimaras, J. Release of Apple Extract And Phenolic Compounds From Five Semi-Solid Formulations. Sci. Collect. InterConf. 2022, 23, 209–214. [Google Scholar] [CrossRef]
- Naveed, A.; Muhammad, H.; Khan, H.M.S.; Gulfishan, F.; Rasool, M.; Ahmad, T.; Nazir, S. Formulation and In Vitro Evaluation of a Cosmetic Emulsion Containing Apple Juice Extract. Asian J. Chem. 2010, 22, 7235–7242. [Google Scholar]
- Bonarska-Kujawa, D.; Cyboran, S.; Oszmiański, J.; Kleszczyńska, H. Extracts from Apple Leaves and Fruits as Effective Antioxidants. J. Med. Plants Res. 2011, 5, 2339–2347. [Google Scholar]
- Grigoras, C.G.; Destandau, E.; Fougère, L.; Elfakir, C. Evaluation of Apple Pomace Extracts as a Source of Bioactive Compounds. Ind. Crops Prod. 2013, 49, 794–804. [Google Scholar] [CrossRef]
- Hastuti, R.T.; Rakhmayanti, R.D. Antioxidant Activity of Peel Off Mask Preparation with Green Apple (Malus domesticus) Juice and Ultrasonic Extraction; Atlantis Press: Paris, France, 2022; pp. 3–10. [Google Scholar]
- Massini, L.; Rico, D.; Martin-Diana, A.B.; Barry-Ryan, C. Apple Peel Flavonoids as Natural Antioxidants for Vegetable Juice Applications. Eur. Food Res. Technol. 2016, 242, 1459–1469. [Google Scholar] [CrossRef]
- Xu, Z.-R.; Li, J.-Y.; Dong, X.-W.; Tan, Z.-J.; Wu, W.-Z.; Xie, Q.-M.; Yang, Y.-M. Apple Polyphenols Decrease Atherosclerosis and Hepatic Steatosis in ApoE−/− Mice through the ROS/MAPK/NF-κB Pathway. Nutrients 2015, 7, 7085–7105. [Google Scholar] [CrossRef]
- Arraibi, A.A.; Liberal, Â.; Dias, M.I.; Alves, M.J.; Ferreira, I.C.; Barros, L.; Barreira, J.C. Chemical and Bioactive Characterization of Spanish and Belgian Apple Pomace for Its Potential Use as a Novel Dermocosmetic Formulation. Foods 2021, 10, 1949. [Google Scholar] [CrossRef] [PubMed]
- Baldisserotto, A.; Malisardi, G.; Scalambra, E.; Andreotti, E.; Romagnoli, C.; Vicentini, C.B.; Manfredini, S.; Vertuani, S. Synthesis, Antioxidant and Antimicrobial Activity of a New Phloridzin Derivative for Dermo-Cosmetic Applications. Molecules 2012, 17, 13275–13289. [Google Scholar] [CrossRef]
- Skinner, R.C.; Gigliotti, J.C.; Ku, K.-M.; Tou, J.C. A Comprehensive Analysis of the Composition, Health Benefits, and Safety of Apple Pomace. Nutr. Rev. 2018, 76, 893–909. [Google Scholar] [CrossRef]

| Concentration (%) 1 | Major Compounds | |
|---|---|---|
| Macro-constituents | ||
| Ash | 1.5–2.5 | |
| Carbohydrates | 45.1–83.8 | Galactose, Glucose, Fructose |
| Dietary Fibers | 14.5–65.0 | Cellulose, hemicellulose, lignin |
| Lipids | 0.6–4.2 | |
| Moisture | 4.4–10.5 | |
| Protein | 1.2–4.7 | |
| Micro-constituents | ||
| Anthocyanins | 0.005–0.013 | Cyanidin-3-O-galactoside |
| Dihydrochalcones | 0.069–0.254 | Phloretein, Phlorizin |
| Flavonoids | 0.215–0.373 | Epicatechin, glycoconjugates, guercetin, isorhamnetin, kaempferol, procyanidin B2, rhamnetin |
| Phenolic Acids | 0.052–0.154 | Caffeic acid, chlorogenic acid, p-coumaric acid, p-coumaroyl-quinic acid, ferulic acid, sinapic acid |
| Foods Functional Compounds | Aims | Results | References |
|---|---|---|---|
| Wheat Pasta | |||
| Apple pomace | Enrichment | Enrichment with phenolic acids, quercetin derivatives, flavon-3-ols, dihydrochalcones, dietary fiber | [41] |
| Cookies | |||
| Apple pomace powder | 50% | Increase in phenolic content and enhance of the nutritional profile | [42,43] |
| Gluten-free Cookies | |||
| Apple pomace | Enrichment | Increase in levels of phenolic acids, quercetin derivatives, flavan-3-ols, dihydrochalcones | [44] |
| Wheat Bread | |||
| Apple pomace powder | Enrichment | Improvements in various physico-chemical, nutritional, antioxidant, and sensory properties | [45] |
| Yogurts | |||
| Apple pomace | Enrichment |
| [49] |
| Cheese | |||
| Apple pomace | Enrichment |
| [50] |
| Hypothesis—Intervention | Study Design/Parameters Examined | Main Findings | Year of Study | Reference |
|---|---|---|---|---|
| In vitro and/or ex vitro studies | ||||
| This research was dedicated to the examination of apple extracts and apple polyphenols |
|
| 2006 | [59] |
| The effect of different drying treatments and sieving on Royal Gala apple pomace was investigated |
|
| 2023 | [60] |
| The purpose of this study was to evaluate the sun protection factor (SPF) of cosmetic emulsions with the addition of hydroalcoholic apple extract |
|
| 2022 | [61] |
| The purpose of this research was to formulate gels and emulgels containing a complex of phenolic compounds of apple extracts and to perform a biopharmaceutical evaluation of semi-solid pharmaceutical forms |
|
| 2020 | [62] |
| In vivo study | ||||
| The Annouka apple (AA) extract, rich in (UA) and sunflower oil (AAO) containing 784.40 ± 7.579 µg/mL of UA), was evaluated to inhibit porcine elastase enzymatic reactions via a validated spectrophotometric method |
|
| 2024 | [53] |
| Hypothesis—Intervention | Study Design/Parameters Examined | Main Findings | Year of Study | Reference |
|---|---|---|---|---|
| In vitro and/or ex vitro studies | ||||
| The purpose of this study was to evaluate the sun protection factor (SPF) of cosmetic emulsions with the addition of hydroalcoholic apple extract | The determination of each hydromodule, alcoholic extract obtained by sonication, reflux and several experimental parameters, as well as TPC, antioxidant capacity, SPF determination, and the incorporation of the apple extract in a sunscreen emulsion and characterization, were carried out |
| 2022 | [61] |
| The purpose of this research was to formulate gels and emulgels containing a complex of phenolic compounds of apple extracts and to perform a biopharmaceutical evaluation of semi-solid pharmaceutical forms |
|
| 2020 | [62] |
| The aim of this study was to select a suitable extraction method and extraction agents which will provide the wild apple fruits extracts with the highest content of polyphenolic compounds and the most pronounced antioxidant activity |
|
| 2016 | [68] |
| Some products and coproducts were analyzed, such as the cultivar ‘Fuji’, apple powder, concentrate apple juice and fruits from the pollination apple (Malus everest) plant |
|
| 2012 | [69] |
| The aim of this study was to determine antioxidant activity, total polyphenol and flavonoid content of apple juice enriched by water herbal extracts. Furthermore, the sensory traits of enriched apple juice were evaluated and addressed |
|
| 2015 | [70] |
| In vivo studies | ||||
| This study highlighted the mechanisms that flavonoids may promote survival or extend lifespan in some model organisms such as fruit flies, worms, and mice |
|
| 2022 | [71] |
| In this study, various assays, i.e., the DPPH radical scavenging assay and the ABTS assay, were performed to evaluate the antioxidant activity of the extracts of leaf and branch of S. samarangense, and the safety of the extracts was shown via the MTT assay and human skin primary irritation test |
|
| 2020 | [72] |
| Hypothesis—Intervention | Study Design/Parameters Examined | Main Findings | Year of Study | References |
|---|---|---|---|---|
| In vitro and/or ex vitro studies | ||||
| The purpose of this research was to formulate gels and emulgels containing a complex of phenolic compounds of apple extracts and to perform a biopharmaceutical evaluation of semi-solid pharmaceutical forms |
|
| 2020 | [62] |
| This study used an experimental method from gel mask formulation with juice and the ultrasonic extraction of green apple fruit |
|
| 2022 | [79] |
| This study investigated the use of phenolic compounds extracted from the new Korean apple cultivar Arisoo in cosmetics and food additives |
|
| 2019 | [54] |
| The aim of this study is to prepare a stable water and oil emulsion (w/o) from the extract (3%) of Malus domestica containing high percentage of antioxidant activity |
|
| 2010 | [76] |
| This study aims at assessing the antioxidant capacity of apple peel flavonoids in vegetable juices as the food model and evaluating the contribution of the hydrophilic and lipophilic antioxidants to the total antioxidant capacity equivalent to ascorbic acid using DPPH and FRAP assays |
|
| 2016 | [80] |
| In vivo studies | ||||
| This study highlighted the mechanisms that flavonoids may promote survival or extend lifespan in some model organisms such as fruit flies, worms, and mice |
|
| 2022 | [71] |
| In this study, various assays,, i.e., DPPH radical scavenging assay and the ABTS assay, were performed to evaluate the antioxidant activity of the extracts of leaf and branch of S. samarangense, and the safety of the extracts was shown via the MTT assay and human skin primary irritation test |
|
| 2020 | [72] |
| The effects of apple polyphenols on hyperlipidemia, atherosclerosis, hepatic steatosis and endothelial function and investigated the potential mechanisms, were evaluated |
|
| 2015 | [81] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vandorou, M.; Plakidis, C.; Tsompanidou, I.M.; Adamantidi, T.; Panagopoulou, E.A.; Tsoupras, A. A Review on Apple Pomace Bioactives for Natural Functional Food and Cosmetic Products with Therapeutic Health-Promoting Properties. Int. J. Mol. Sci. 2024, 25, 10856. https://doi.org/10.3390/ijms251910856
Vandorou M, Plakidis C, Tsompanidou IM, Adamantidi T, Panagopoulou EA, Tsoupras A. A Review on Apple Pomace Bioactives for Natural Functional Food and Cosmetic Products with Therapeutic Health-Promoting Properties. International Journal of Molecular Sciences. 2024; 25(19):10856. https://doi.org/10.3390/ijms251910856
Chicago/Turabian StyleVandorou, Maria, Christos Plakidis, Ilektra Maria Tsompanidou, Theodora Adamantidi, Eirini A. Panagopoulou, and Alexandros Tsoupras. 2024. "A Review on Apple Pomace Bioactives for Natural Functional Food and Cosmetic Products with Therapeutic Health-Promoting Properties" International Journal of Molecular Sciences 25, no. 19: 10856. https://doi.org/10.3390/ijms251910856







