Abstract
Chemokines are integral components of the immune system and deeply involved in the pathogenesis and progression of inflammatory bowel disease (IBD) and colorectal cancer (CRC). Although a considerable amount of transcriptome data has been accumulated on these diseases, most of them are limited to a specific stage of the disease. The purpose of this study is to visually demonstrate the dynamic changes in chemokines across various stages of bowel diseases by integrating relevant datasets. Integrating the existing datasets for IBD and CRC, we compare the expression changes of chemokines across different pathological stages. This study collected 11 clinical databases from various medical centers around the world. Patients: Data of patient tissue types were classified into IBD, colorectal adenoma, primary carcinoma, metastasis, and healthy control according to the publisher’s annotation. The expression changes in chemokines in various pathological stages are statistically analyzed. The chemokines were clustered by different expression patterns. The chemokine family was clustered into four distinct expression patterns, which correspond to varying expression changes in different stages of colitis and tumor development. Certain chemokines and receptors associated with inflammation and tumorigenesis have been identified. Furthermore, it was confirmed that the 2,4,6-trinitrobenzenesulfonic acid (TNBS)-induced colitis model and the azoxymethane (AOM)/ dextran sulfate sodium (DSS)-induced colon cancer model shows stronger correlations with the clinical data in terms of chemokine expression levels. This study paints a panoramic picture of the expression profiles of chemokine families at multiple stages from IBD to advanced colon cancer, facilitating a comprehensive understanding of the regulation patterns of chemokines and guiding the direction of drug development. This study provides researchers with a clear atlas of chemokine expression in the pathological processes of inflammatory bowel disease and colon cancer.
1. Introduction
Chemokines are a class of cytokines that gradually evolve during the development of the central nervous system in chordates [1]. In humans, this family, according to the conserved cysteine residues, is subdivided into four subfamilies, CXC, CC, CX3C, and XC, with a total of 39 members. Similarly, their receptors are also classified into four subfamilies based on the ligands they bind to, encompassing 22 genes. The chemokine system, constituting the core regulatory mechanism for directed cell migration, plays an indispensable role in normal embryonic development and immune system function.
The chemokine system, as a key mechanism, plays a crucial role in mediating the infiltration of inflammatory cells [2]. Inflammatory bowel diseases (IBD), especially ulcerative colitis (UC) and Crohn’s disease (CD), are the most common intestinal diseases closely related to autoimmune defects. Significant changes in chemokine expression levels have become one of their important pathological characteristics [3]. It is noteworthy that chemokines play an active role in bacterial colitis, but they often become a factor aggravating the disease in IBD. Therefore, chemokines are regarded as important potential targets for colitis drug development [4,5].
Colon cancer is the third most common cancer type and the second leading cause of cancer death globally. There is a causal relationship between the occurrence of some colon cancers and a history of colitis. Chemokines serve as crucial regulators in the microenvironment of colon cancer, with the presence of two opposing forces, tumor-promoting and tumor-destructive chemokines [6,7], and the balance between them represents an important factor influencing prognosis. Targeting chemokines is one of the key directions in immunotherapy [8].
Based on the information provided above, the progression from colitis to primary tumor, and further to metastatic tumor, represents a continuous process that involves several stages, including inflammation, tumor immunity, immune escape, and tumor metastasis. The correlation between the expression and silencing of chemokines and pathological stages during this process holds significant importance for understanding and treating the disease [9]. Currently, there is a lack of comprehensive studies analyzing the expression profiles of chemokines throughout this process.
In this article, we systematically integrated multiple transcriptome data resources currently available on colitis, colonic adenoma, colorectal cancer, and distant metastatic cancer, and deeply explored the expression profile information of the chemokine family genes within them. By analyzing these data, we successfully identified a set of genes that are specific to inflammation, tumorigenesis, and metastasis. The mapping of this chemokine expression atlas not only presents a clear picture of the role of chemokines in the development and progression of colorectal cancer, but also provides powerful directional guidance for further elucidating the relevant mechanisms, exploring novel therapeutic targets, and driving drug development.
2. Results
2.1. Chemokine Family Exhibits Distinct Expression Patterns in Colonic Diseases
We integrated the expression change data of chemokine genes at specific disease stages from each dataset (Supplementary Figures S1–S6, Supplementary Data S1) and presented them in the form of a heatmap (Figure 1A). Each color block in the figure represents the ratio of significant change in a gene during a specific disease course compared to healthy controls, with red indicating upregulation and green indicating downregulation. Based on the change trends, we clustered the chemokine ligands into four categories, which will be elaborated in detail later.

Figure 1.
Expression profiles of the chemokine family in the pathological process of colitis to colon cancer. (A) Heatmap of expression changes. The percentage of significant changes was obtained by dividing the number of datasets with significant changes in expression levels at different pathological stages compared to healthy colon tissue by the total number of datasets in the database. The chemokine family was clustered into four groups based on the trends of expression changes. ELR: ELR motif + chemokine. (B) A scatter plot was created with the expression changes of chemokines in the IBD datasets as the x-axis and the average changes in the adenoma and adenocarcinoma datasets as the y-axis. Chemokines were divided into four groups (i, ii, iii and iv) based on the cut-off values of +1 and −1 for Log2FC in the two dimensions. The genes in these four groups correspond to the four clusters in (A). (C) Line graphs of expression changes of genes in the four clusters in (A) were illustrated during the colitis to colon cancer process.
In addition, we exhibited the expression levels of chemokines in IBD and tumor tissues through a scatter plot (Figure 1B). The average change in gene expression across multiple IBD datasets was taken as the x-axis, while the average change in chemokine expression across multiple adenoma and adenocarcinoma datasets was used as the y-axis. We established a two-fold change level as the criterion for grouping, dividing the chemokine family into four groups (group i, ii, iii, iv). These four groups correspond to the four clusters in Figure 1A.
Based on the average changes in gene expression from a representative dataset GSE4183, a line chart was plotted to visually illustrate the overall trend of gene expression levels in the four groups (Figure 1C).
Next, we will describe the characteristics of the expression profiles of these four groups individually.
(i) Neoplasm-upregulated chemokines: This group of chemokines includes CXCL1- 8, 11, and CCL20, 24. These chemokines are characterized by a significant increase in expression levels during colitis, and their expression remains high even after transitioning into the tumor stage (Figure 1A). Among them, ELR+ chemokines belong to this group, with CXCL8, CXCL3, and CXCL1 exhibiting the largest changes in expression levels.
(ii) Inflammation-specific chemokines: This group includes CXCL9, 10, 16, CCL2, 3, 4, 11, 18, 22. Compared to chemokines positively correlated with cell proliferation, these factors are significantly upregulated only during immune–inflammatory diseases. However, as the disease progresses to adenoma or later stages, their expression levels revert to levels close to those in healthy controls, displaying inflammation-specific expression patterns. Among them, CXCL9 and CXCL10 exhibit the most significant changes (Log2FC > 3).
(iii) Chemokines unrelated to colonic diseases: These chemokines belong to the remaining family members that do not show significant changes in expression levels, either in inflammatory bowel diseases or in tumor-related tissues.
(iv) Neoplasm-downregulated chemokines: This group of chemokines includes CXCL12, 13, 14, CCL5, 8, 13, 14, 15, 19, 21, 23, XCL1, 2. In contrast to factors positively correlated with proliferation, the expression levels of these chemokines are significantly downregulated in colorectal tumors. However, they do not exhibit significant changes during the IBD stage, demonstrating a pure tumor proliferation-specific negative correlation. Among them, CXCL12 exhibits the most significant change (Log2FC < −3).
2.2. Changes in the Expression of Chemokines in Metastatic Cancer
We compared the expression differences between metastatic cancer and primary cancer and found that, apart from a significant downregulation of CXCL14 and CCL28, as well as a significant upregulation of CXCL12 and CCL2, the expression levels of most chemokines did not achieve a twofold change (Figure 1A,B, Supplementary Table S2, Supplementary Data S2). CXCL14 gradually decreased throughout the disease process, with a particularly sharp decline in its expression level in metastatic tumor tissues (Figure 2A), making it rather exceptional among the entire chemokine family. CCL28 gene data were only available in the Sidra-LUMC dataset, revealing a significant downregulation in expression. CXCL12 gene expression was drastically downregulated in primary colon cancer (Log2FC = −3.1), but its expression level increased in metastatic cancer vs. primary, Log2FC = 1.03. CCL2 expression, on the other hand, was mildly downregulated in primary colon cancer but increased in metastatic cancer (Log2FC = 1.06 ± 0.36). These findings suggest that these genes play a role in the process of tumor metastasis.
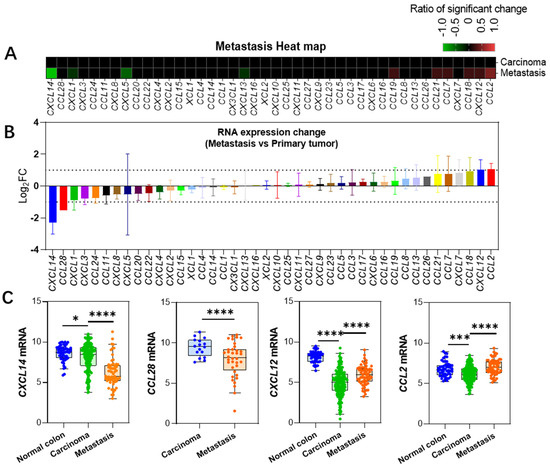
Figure 2.
Changes in gene expression during metastatic cancer progression. (A) Heatmap showing alterations in gene expression levels in metastatic cancers developed from primary colon cancer. (B) Changes in expression levels relative to primary colon cancer in metastatic cancers, presented as mean ± SD based on multiple datasets. Note that CCL26, CCL28, and CXCL16 are only present in one dataset, hence no standard deviation data is available. (C) Expression level data for four genes showing significant changes. Data for CXCL14, CXCL12, and CCL2 are derived from the GSE41258 dataset, while CCL28 data is from the Sidra-LUMC dataset [10]. *: p < 0.05, ***: p < 0.001, ****: p < 0.0001.
2.3. Changes in Receptor Gene Expression
We compared the changes in the expression levels of chemokine receptors during the colitis-to-colon cancer process (Figure 3A, Supplementary Data S3) and found that CXCR2 (Log2FC = 2.7 ± 1.2) and CCR1 (Log2FC = 1.36 ± 0.28) were significantly upregulated in all colitis databases, consistent with the expression trends of their ligands, CXCL1, 2, 3, 5, 6, 7, 8, and CCL3, 4. In contrast, CCR2 and CXCR5 were significantly downregulated during the colonic adenoma stage, with CCR2 (Log2FC = −1.02 ± 0.79) and CXCR5 (Log2FC = −1.42 ± 0.57). No significant changes were observed in the expression levels of other receptor genes. Figure 3B depicts the expression changes of the significant genes in the colitis, adenoma, and adenocarcinoma stages from dataset GSE4183, and the trends are consistent with the results from the integration of multiple datasets.
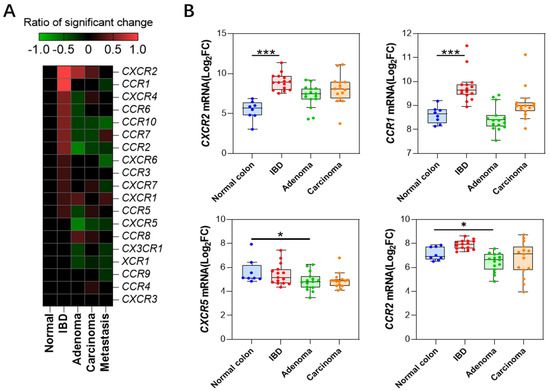
Figure 3.
Chemokine receptor expression profiles (A) Heatmap depicting changes in expression levels of chemokine receptors at different stages. (B) Expression profiles of four receptor genes with significant changes in expression levels (GSE4183 dataset). *: p < 0.05, ***: p < 0.001.
2.4. Correlation between Mouse Models of Colonic Diseases and Clinical Data
The commonly used mouse models for inflammatory bowel disease are acute colitis models induced by dextran sulfate sodium (DSS) or 2,4,6-trinitrobenzenesulfonic acid (TNBS). Based on the homology between humans and mice (see Supplementary Table S2, Stpplemental data S4), we analyzed the correlation between changes in chemokine levels in these two mouse colitis models (Table 1) and those in human immune-related bowel disease samples (Figure 4).

Figure 4.
Correlation between chemokine expression changes in mouse intestinal disease models and clinical databases. (A) Correlation between mouse TNBS colitis model (GSE13705) and human IBD data. (B) Correlation between mouse DSS colitis model (GSE22307) and human IBD data. (C) Correlation between mouse AOM-DSS colon cancer model (GSE31106) and human colon cancer data. (D) Correlation between spontaneous colon cancer in ApcMin/+/J mice (GSE43338) and human colon cancer databases.
The results revealed that there was a strong correlation between changes in chemokines in both the TNBS and DSS models and those in human IBD (Figure 4A,B, Supplementary Data S4). In the TNBS model, the correlation coefficients for changes in chemokines, excluding CXCL10 and 11, reached r = 0.73 (p < 0.0001).
Currently, the most commonly used model is the AOM-DSS model [11], while there is also the ApcMin/+/J mouse spontaneous colon cancer model [12]. We compared the correlation between the fold change of chemokine expression in these two models (Table 1) and that in the human colon cancer clinical databases. The results showed that the trend of chemokine changes in the AOM-DSS model highly matched the clinical data (r = 0.71 and p < 0.0001), except for CXCL12 and CXCL14 exhibiting significant differences. However, the correlation between the APC (Min) spontaneous colon cancer model and the clinical data was relatively weak (r = 0.37, p = 0.041).

Table 1.
The datasets used in this study.
Table 1.
The datasets used in this study.
| Clinical Data | ||||||
|---|---|---|---|---|---|---|
| Dataset Accession No. or Dataset No. | Sample Number | Ref. | ||||
| Control | IBD | Adenoma | Primary Carcinoma | Metastasis Carcinoma | ||
| GSE49355 | 18 | 20 | 19 | Del Rio et al. [13] | ||
| GSE41258 | 54 | 186 | 67 a | Sheffer et al. [14] | ||
| GSE4183 | 7 | 15 | 15 | 15 | Galamb et al. [15] | |
| GSE37364 | 38 | 29 | 14 | 13 | Galamb et al. [16] | |
| GSE23878 | 24 | 35 | Uddini et al. [17] | |||
| GDS2947 b | 32 | 32 | Sabates et al. [18] | |||
| GDS4382 b | 17 | 17 | Khamas et al. [19] | |||
| GDS3119 | 5 | 8 | Olsen et al. [20] | |||
| GSE36807 | 7 | 15 c | Montero-Melendez et al. [21] | |||
| Sidra-LUMC | 69 | 36 | Roelands et al. [10] | |||
| GSE14580 | 6 | 24 | Arijs et al. [22] | |||
| Mouse Model | ||||||
| Dataset | Model | Healthy | Disease | |||
| GSE22307 | DSS-induced UC model | 5 | 5 | Fang et al. [23] | ||
| GSE13705 | TNBS-induced IBD model | 3 | 3 | Billerey-Larmonier et al. [24] | ||
| GSE31106 | AOM-DSS-induced CAC model | 3 | 3 | Tang et al. [25] | ||
| GSE43338 | C57BL/6J-ApcMin/+/J spontaneous CRC | 3 | 5 | Neufert et al. [26] | ||
a 47 liver metastasis and 20 lung metastasis; b. paired samples; c. only UC were included.
3. Discussion
IBD is a significant intestinal disease, and chemokine-mediated immune cell infiltration is one of its major pathological processes [27]. CRC is the third most diagnosed cancer and the second leading cause of cancer death worldwide, and chemokine-mediated regulation of the tumor microenvironment plays a crucial role in patients’ prognosis. There is a certain causal relationship between IBD and CRC [7], and studying them as a whole holds significant theoretical and practical implications for understanding the pathogenesis of colorectal cancer.
The highlight of this study is the collection and comprehensive analysis of existing multiple transcriptomics data on the chemokine family across different pathological stages of colitis–colorectal cancer, resulting in a continuous expression profile. The entire process is divided into four stages: immunological bowel disease, adenoma, primary colorectal cancer, and metastatic cancer, with each stage encompassing three to six datasets. This approach primarily serves to avoid statistical biases in the expression patterns of certain genes that may arise from the random error of a single dataset. More importantly, there is currently no single dataset that covers all there pathological stages. By integrating various scattered datasets, we have obtained a comprehensive dynamic picture of chemokine expression levels.
This study yielded several significant results. Firstly, chemokines during the IBD stage can be clearly divided into two groups, with one group showing significant upregulation, while the other group remains unchanged comparing to the controls. This finding holds significant importance for understanding the occurrence mechanisms of colitis and for drug development. Analysis of the expression profile reveals that numerous chemokines are involved in IBD, potentially indicating a degree of redundancy among them. Therefore, antagonizing a single chemokine may not produce significant therapeutic effects. For example, Eli Lilly developed a neutralizing antibody, Eltrekibart (LY-3041658), targeted against ELR+ chemokines for the treatment of inflammatory bowel disease [28]. This antibody is capable of simultaneously antagonizing the activities of seven chemokines with the ELR motif, namely CXCL1, 2, 3, 5, 6, 7, and 8 (their corresponding receptors are CXCR1 and CXCR2). However, the study was terminated due to undisclosed reasons, and the authors speculate that it may be because the antibody did not achieve the expected therapeutic effect. Given the upregulation of numerous chemokines in IBD, it may be necessary to consider simultaneously interfering with multiple pathways to overcome the functional redundancy of chemokines.
Secondly, the genes that undergo significant changes in colon cancer can be classified into upregulated and downregulated groups. We defined the genes in the former group as neoplasm-upregulated chemokines (NUCs, Figure 1), where all ELR + chemokines are clustered. The latter group was referred to as neoplasm-downregulated chemokines (NDCs). According to the clonal selection theory of cancer cells, the expression regulation of NUCs and NDCs in tumors may represent clonal traits that are fixed and facilitate tumor proliferation or immune escape. Intervening in these traits may become a potential approach for the treatment of colon cancer.
CXCL14 was clustered within the neoplasm-downregulated chemokines cluster, but its sharp downregulation during tumor metastasis particularly stands out (Figure 3). It is the only gene that is significantly downregulated in all datasets comparing metastatic cancers to primary cancers, with an average downregulation fold change of Log2FC = 2.3 ± 0.7. This suggests that CXCL14 silencing plays an important role in the process of colon cancer metastasis, as mentioned in a previous study [29].
Compared to the drastic changes in ligand expression, the expression of receptor genes does not exhibit remarkable variations. Only four receptors, namely CXCR2, CCR1, CXCR5, and CCR2, showed significant changes in over half of the datasets in a certain pathological stage. Among them, CXCR2 was drastically upregulated in the IBD and adenoma stage, which is corroborated by the general upregulation of its ligands, CXCL1-3 and CXCL5-8 (Figure 1). This suggests that immune cells related to the CXCR2 axis are deeply involved in these pathological processes. Previous studies have shown that neutralizing CXCR2 can inhibit neutrophil-mediated colitis [30]; furthermore, genetic knockout of the CXCR2 gene is capable of inhibiting the formation of primary colon cancer in mice [31]. Similarly, CCR1, as a common receptor for numerous CCL ligand family members (CCL3, CCL5, 7, 8, CCL13-16, CCL23), also exhibits an extremely significant upregulation in the IBD stage. However, interestingly, among these ligands, only CCL3 shows a significant upregulation in IBD (Figure 1), indicating that the CCL3-CCR1 axis plays a crucial role in this pathological process, which is consistent with previous studies [32]. In addition, there are two other significantly downregulated receptor genes, CXCR5 and CCR2, which both show a marked downregulation in adenoma samples. This downregulation echoes the corresponding decrease in their respective ligands, CXCL13 and CCL2. Previous studies have indicated that the CXCL13-CXCR5 axis is related to the prognosis of colon cancer [33]. CCL2 is downregulated in adenoma but significantly upregulated in metastasis samples (Figure 2), which aligns with previous findings that it promotes liver metastasis [34,35].
Finally, appropriate animal models are of great significance for drug development in preclinical studies. Given that chemokine-targeted drug development necessitates the utilization of suitable mouse models, we examined the correlation between chemokines in various mouse models of IBD and primary colon cancer and clinical samples of human colon cancer. Through the comparison of correlation coefficients, we determined that colitis induced by TNBS chemical challenge and colon cancer generated through the AOM-DSS protocol exhibit closer chemokine expression profiles to clinical samples (Figure 4). As a result, we may prioritize these two models in future research endeavors.
This study also has its limitations. Firstly, we identified genes with significantly different expression levels by using the criteria of both a t-test Q-value < 0.05 and an expression change greater than two-fold (|Log2FC | > 1). However, there are some genes that own a | Log2FC | < 1 and have a Q-value significantly less than 0.05. The correlation of these genes with the disease may have been underestimated in this study. Readers interested in this aspect can refer to the Supplementary Materials for detail data.
Secondly, to make the results simpler and more readable, we treated Crohn’s disease and ulcerative colitis collectively as IBD in our study. Although Crohn’s disease and ulcerative colitis share some similarities, they are two distinct diseases with different causes. Additionally, we combined liver metastasis and lung metastasis samples as metastatic samples for analysis. These processing procedures may have overlooked some disease-specific genes.
In conclusion, this study has drawn a panoramic picture of the expression profiles of chemokine families at multiple stages from colitis to advanced colon cancer, through the integration of transcriptomics data. These findings provide researchers with a more macro-level perspective, facilitating a comprehensive understanding of the regulation patterns of chemokines and guiding the direction of drug development.
4. Materials and Methods
4.1. Data Collection
This study comprehensively integrated multiple datasets encompassing colitis, colonic adenomas, colonic primary tumors, and metastatic tumors (as shown in Table 1), aiming to delve into the critical molecular mechanisms underlying the occurrence and progression of the disease. During the data processing, we focused on extracting the expression profile data of chemokine genes and carefully summarizing them, as detailed in Supplementary Materials S1. It needs to be emphasized that in cases where multiple probes existed for certain genes, we adopted the probe with the strongest signal as valid data for subsequent analysis (Supplementary Table S1).
4.2. Data Analysis
Across different datasets, publishers provided varying annotations for the same type of samples. Based on the annotations provided by the dataset publishers regarding the disease stage of the samples, the data are categorized into five primary groups: (1) healthy controls, including samples annotated as “none”, “control”, “normal”, and “normal colonic mucosa”; (2) IBD, encompassing samples annotated as “IBD”, “UC”, and “CD”; (3) colon adenoma; (4) primary colon cancer, comprising samples annotated as “colorectal cancer”, “primary tumor”, and “carcinoma”; (5) metastatic cancer, including samples annotated as “metastatic cancer”, “liver metastasis”, and “lung metastasis”.
To ensure the comparability of the data and the accuracy of the analysis, we converted the linear numerical values in the dataset to logarithmic values (Log2), since the log-converted data are closer to a normal distribution. For the differential analysis, we selected the appropriate t-test method based on the source and type of samples. For paired data, which refers to samples taken from different tumor stages of the same patient, we employed the paired t-test to reveal the dynamic changes in gene expression during disease progression. For unpaired data, we used the unpaired Student t-test to assess the differences in gene expression between different disease groups. In all t-tests, we assumed equal variance and adopted a two-tailed test. Additionally, to control the false positive rate, we also applied the Benjamini–Hochberg method to transform the p-values to Q-values. Statistical significance: *: p < 0.05, **: p < 0.01, ***: p < 0.001, ****: p < 0.0001.
4.3. Graphical Representation
Volcano Plot: We created a volcano plot to visually illustrate the changes in gene expression. In the volcano plot, the horizontal axis represents the mean difference in gene expression (denoted as Log2FC), while the vertical axis employs the -Log10Q value. This setup facilitates the rapid identification of genes with significant differences, specifically those that demonstrate both significant changes in expression levels and high statistical reliability.
Heatmap: To further analyze gene expression patterns in depth, we generated a heatmap. In creating the heatmap, we established strict screening criteria: only genes with a -Log10Q value greater than 1.3 (i.e., a Q-value < 0.05) and an | Log2FC | > 1 were considered to have a significantly different expression. Based on these criteria, genes with a significantly increased expression were assigned a score of 1, while genes with a significantly decreased expression were assigned a score of −1. Genes without significant changes were scored as 0. Subsequently, we calculated the arithmetic mean of scores across different datasets for the same disease stage, yielding a final expression change score for each gene at that stage. For instance, CCL20 exhibits a significant increase (LogFc > 2, Q-value < 0.05) in 3 out of 4 IBD databases; we define the “ratio of significant difference” as 3/4 = 0.75 in IBD stage. This value was then used to construct the heatmap. Finally, we used SPSS software (v25) to perform a clustering analysis on the gene score matrix, generating a heatmap that visually displays the gene expression patterns (as shown in Figure 1A).
Scatter Plot: In the process of creating the scatter plot, we first calculated the mean fold change (FC) of every gene across the IBD datasets, which served as the horizontal axis. Subsequently, we computed the average gene variation for both the adenoma dataset and the adenocarcinoma dataset, and then took the arithmetic average of these two means as a representative of the gene changes in the tumor, which was plotted on the vertical axis. Through this approach, we were able to compare and visualize the differences and trends in gene expression changes between IBD and tumor stages (as depicted in Figure 1B).
Line chart of gene expression: To systematically demonstrate the changes in gene expression across different pathological stages within each dataset, we have also constructed a line chart that tracks gene variation. In these line charts, the arithmetic means of gene variation for each dataset at different pathological stages were calculated. By connecting the points, we clearly illustrated the dynamic process of gene expression changes over the course of the disease progression.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms251910857/s1.
Author Contributions
Conceptualization, J.L.; methodology, J.L. and Y.W.; validation, Y.Z. and J.L.; investigation, Y.Z., Y.J. and Y.N.; data curation, S.W.; writing J.L.; supervision, B.M.; project administration, J.L.; funding acquisition, B.M. and Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
B. Ma is thankful for support from the National Natural Science Foundation of China (Grant No. 32171246) and the Shanghai Municipal Government Science Innovation grant 21JC1403700. Y. Wang is grateful for support from grants from the National Natural Science Foundation of China (No. 32200531).
Institutional Review Board Statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare that they have NO affiliations with or involvement in any organization or entity with any financial interest in the subject matter or materials discussed in this manuscript.
Abbreviations
AOM, Azoxymethane; CAC, colitis-associated colorectal cancer; CD, Crohn’s disease; CRC, colorectal cancer; DSS, dextran sulfate sodium; ELR, Glu-Leu-Arg motif; FC, fold change; IBD, inflammatory bowel disease; NUCs, neoplasm-upregulated chemokines; NDCs, neoplasm-downregulated chemokines; TNBS, 2,4,6-trinitrobenzenesulfonic acid; UC, ulcerative colitis.
References
- Huising, M.O.; Stet, R.J.; Kruiswijk, C.P.; Savelkoul, H.F.; Lidy Verburg-van Kemenade, B.M. Molecular evolution of CXC chemokines: Extant CXC chemokines originate from the CNS. Trends Immunol. 2003, 24, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.E.; Nibbs, R.J.B. A guide to chemokines and their receptors. FEBS J. 2018, 285, 2944–2971. [Google Scholar] [CrossRef] [PubMed]
- Mello, J.D.C.; Gomes, L.E.M.; Silva, J.F.; Siqueira, N.S.N.; Pascoal, L.B.; Martinez, C.A.R.; Ayrizono, M.L.S.; Leal, R.F. The role of chemokines and adipokines as biomarkers of Crohn’s disease activity: A systematic review of the literature. Am. J. Transl. Res. 2021, 13, 8561–8574. [Google Scholar] [PubMed]
- Zhang, R.B.; Dong, L.C.; Shen, Y.; Li, H.Y.; Huang, Q.; Yu, S.G.; Wu, Q.F. Electroacupuncture alleviates ulcerative colitis by targeting CXCL1: Evidence from the transcriptome and validation. Front. Immunol. 2023, 14, 1187574. [Google Scholar] [CrossRef]
- Sitaru, S.; Budke, A.; Bertini, R.; Sperandio, M. Therapeutic inhibition of CXCR1/2: Where do we stand? Intern. Emerg. Med. 2023, 18, 1647–1664. [Google Scholar] [CrossRef]
- Balkwill, F.R.; Mantovani, A. Cancer-related inflammation: Common themes and therapeutic opportunities. Semin. Cancer Biol. 2012, 22, 33–40. [Google Scholar] [CrossRef]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Markl, F.; Huynh, D.; Endres, S.; Kobold, S. Utilizing chemokines in cancer immunotherapy. Trends Cancer 2022, 8, 670–682. [Google Scholar] [CrossRef]
- Bhat, A.A.; Nisar, S.; Singh, M.; Ashraf, B.; Masoodi, T.; Prasad, C.P.; Sharma, A.; Maacha, S.; Karedath, T.; Hashem, S.; et al. Cytokine- and chemokine-induced inflammatory colorectal tumor microenvironment: Emerging avenue for targeted therapy. Cancer Commun. 2022, 42, 689–715. [Google Scholar] [CrossRef]
- Roelands, J.; Kuppen, P.J.K.; Ahmed, E.I.; Mall, R.; Masoodi, T.; Singh, P.; Monaco, G.; Raynaud, C.; de Miranda, N.; Ferraro, L.; et al. An integrated tumor, immune and microbiome atlas of colon cancer. Nat. Med. 2023, 29, 1273–1286. [Google Scholar] [CrossRef]
- Dzhalilova, D.; Zolotova, N.; Fokichev, N.; Makarova, O. Murine models of colorectal cancer: The azoxymethane (AOM)/dextran sulfate sodium (DSS) model of colitis-associated cancer. PeerJ 2023, 11, e16159. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Sui, H.; Fang, F.; Li, Q.; Li, B. The application of Apc(Min/+) mouse model in colorectal tumor researches. J. Cancer Res. Clin. Oncol. 2019, 145, 1111–1122. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, M.; Mollevi, C.; Vezzio-Vie, N.; Bibeau, F.; Ychou, M.; Martineau, P. Specific extracellular matrix remodeling signature of colon hepatic metastases. PLoS ONE 2013, 8, e74599. [Google Scholar] [CrossRef] [PubMed]
- Sheffer, M.; Bacolod, M.D.; Zuk, O.; Giardina, S.F.; Pincas, H.; Barany, F.; Paty, P.B.; Gerald, W.L.; Notterman, D.A.; Domany, E. Association of survival and disease progression with chromosomal instability: A genomic exploration of colorectal cancer. Proc. Natl. Acad. Sci. USA 2009, 106, 7131–7136. [Google Scholar] [CrossRef] [PubMed]
- Galamb, O.; Gyorffy, B.; Sipos, F.; Spisak, S.; Nemeth, A.M.; Miheller, P.; Tulassay, Z.; Dinya, E.; Molnar, B. Inflammation, adenoma and cancer: Objective classification of colon biopsy specimens with gene expression signature. Dis. Markers 2008, 25, 1–16. [Google Scholar] [CrossRef]
- Galamb, O.; Wichmann, B.; Sipos, F.; Spisak, S.; Krenacs, T.; Toth, K.; Leiszter, K.; Kalmar, A.; Tulassay, Z.; Molnar, B. Dysplasia-carcinoma transition specific transcripts in colonic biopsy samples. PLoS ONE 2012, 7, e48547. [Google Scholar] [CrossRef]
- Uddin, S.; Ahmed, M.; Hussain, A.; Abubaker, J.; Al-Sanea, N.; AbdulJabbar, A.; Ashari, L.H.; Alhomoud, S.; Al-Dayel, F.; Jehan, Z.; et al. Genome-wide expression analysis of Middle Eastern colorectal cancer reveals FOXM1 as a novel target for cancer therapy. Am. J. Pathol. 2011, 178, 537–547. [Google Scholar] [CrossRef]
- Sabates-Bellver, J.; Van der Flier, L.G.; de Palo, M.; Cattaneo, E.; Maake, C.; Rehrauer, H.; Laczko, E.; Kurowski, M.A.; Bujnicki, J.M.; Menigatti, M.; et al. Transcriptome profile of human colorectal adenomas. Mol. Cancer Res. 2007, 5, 1263–1275. [Google Scholar] [CrossRef]
- Khamas, A.; Ishikawa, T.; Shimokawa, K.; Mogushi, K.; Iida, S.; Ishiguro, M.; Mizushima, H.; Tanaka, H.; Uetake, H.; Sugihara, K. Screening for epigenetically masked genes in colorectal cancer Using 5-Aza-2’-deoxycytidine, microarray and gene expression profile. Cancer Genom. Proteom. 2012, 9, 67–75. [Google Scholar]
- Olsen, J.; Gerds, T.A.; Seidelin, J.B.; Csillag, C.; Bjerrum, J.T.; Troelsen, J.T.; Nielsen, O.H. Diagnosis of ulcerative colitis before onset of inflammation by multivariate modeling of genome-wide gene expression data. Inflamm. Bowel Dis. 2009, 15, 1032–1038. [Google Scholar] [CrossRef]
- Montero-Melendez, T.; Llor, X.; Garcia-Planella, E.; Perretti, M.; Suarez, A. Identification of novel predictor classifiers for inflammatory bowel disease by gene expression profiling. PLoS ONE 2013, 8, e76235. [Google Scholar] [CrossRef] [PubMed]
- Arijs, I.; Li, K.; Toedter, G.; Quintens, R.; Van Lommel, L.; Van Steen, K.; Leemans, P.; De Hertogh, G.; Lemaire, K.; Ferrante, M.; et al. Mucosal gene signatures to predict response to infliximab in patients with ulcerative colitis. Gut 2009, 58, 1612–1619. [Google Scholar] [CrossRef] [PubMed]
- Fang, K.; Bruce, M.; Pattillo, C.B.; Zhang, S.; Stone, R., 2nd; Clifford, J.; Kevil, C.G. Temporal genomewide expression profiling of DSS colitis reveals novel inflammatory and angiogenesis genes similar to ulcerative colitis. Physiol. Genom. 2011, 43, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Billerey-Larmonier, C.; Uno, J.K.; Larmonier, N.; Midura, A.J.; Timmermann, B.; Ghishan, F.K.; Kiela, P.R. Protective effects of dietary curcumin in mouse model of chemically induced colitis are strain dependent. Inflamm. Bowel Dis. 2008, 14, 780–793. [Google Scholar] [CrossRef]
- Tang, A.; Li, N.; Li, X.; Yang, H.; Wang, W.; Zhang, L.; Li, G.; Xiong, W.; Ma, J.; Shen, S. Dynamic activation of the key pathways: Linking colitis to colorectal cancer in a mouse model. Carcinogenesis 2012, 33, 1375–1383. [Google Scholar] [CrossRef]
- Neufert, C.; Becker, C.; Tureci, O.; Waldner, M.J.; Backert, I.; Floh, K.; Atreya, I.; Leppkes, M.; Jefremow, A.; Vieth, M.; et al. Tumor fibroblast-derived epiregulin promotes growth of colitis-associated neoplasms through ERK. J. Clin. Investig. 2013, 123, 1428–1443. [Google Scholar] [CrossRef] [PubMed]
- Francescone, R.; Hou, V.; Grivennikov, S.I. Cytokines, IBD, and colitis-associated cancer. Inflamm. Bowel Dis. 2015, 21, 409–418. [Google Scholar] [CrossRef]
- Boyles, J.S.; Beidler, C.B.; Strifler, B.A.; Girard, D.S.; Druzina, Z.; Durbin, J.D.; Swearingen, M.L.; Lee, L.N.; Kikly, K.; Chintharlapalli, S.; et al. Discovery and characterization of a neutralizing pan-ELR+CXC chemokine monoclonal antibody. mAbs 2020, 12, 1831880. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Niu, Y.; Ma, B.; Li, J. Data Mining Suggests That CXCL14 Gene Silencing in Colon Cancer Is Due to Promoter Methylation. Int. J. Mol. Sci. 2023, 24, 16027. [Google Scholar] [CrossRef]
- Zhu, F.; He, H.; Fan, L.; Ma, C.; Xu, Z.; Xue, Y.; Wang, Y.; Zhang, C.; Zhou, G. Blockade of CXCR2 suppresses proinflammatory activities of neutrophils in ulcerative colitis. Am. J. Transl. Res. 2020, 12, 5237–5251. [Google Scholar]
- Katoh, H.; Wang, D.; Daikoku, T.; Sun, H.; Dey, S.K.; Dubois, R.N. CXCR2-expressing myeloid-derived suppressor cells are essential to promote colitis-associated tumorigenesis. Cancer Cell 2013, 24, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Ramos, C.D.; Canetti, C.; Souto, J.T.; Silva, J.S.; Hogaboam, C.M.; Ferreira, S.H.; Cunha, F.Q. MIP-1alpha[CCL3] acting on the CCR1 receptor mediates neutrophil migration in immune inflammation via sequential release of TNF-alpha and LTB4. J. Leukoc. Biol. 2005, 78, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.W.; Xia, S.H.; Yin, Y.; Jin, L.F.; Pu, Y.; Hua, D.; Wu, H.R. Expression features of CXCR5 and its ligand, CXCL13 associated with poor prognosis of advanced colorectal cancer. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 1916–1924. [Google Scholar] [PubMed]
- Zhao, L.; Lim, S.Y.; Gordon-Weeks, A.N.; Tapmeier, T.T.; Im, J.H.; Cao, Y.; Beech, J.; Allen, D.; Smart, S.; Muschel, R.J. Recruitment of a myeloid cell subset (CD11b/Gr1 mid) via CCL2/CCR2 promotes the development of colorectal cancer liver metastasis. Hepatology 2013, 57, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Boughriba, R.; Sahraoui, G.; Chaar, I.; Weslati, M.; Ayed, K.; Ounissi, D.; Hazgui, M.; Bouraoui, S.; Gati, A. Significant association of MCP1 rs1024611 and CCR2 rs1799864 polymorphisms with colorectal cancer and liver metastases susceptibility and aggressiveness: A case-control study. Cytokine 2023, 167, 156193. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




