The Expression of miR-211-5p in Sentinel Lymph Node Metastases of Malignant Melanoma Is a Potential Marker for Poor Prognosis †
Abstract
:1. Introduction
2. Results
2.1. Patient Characteristics
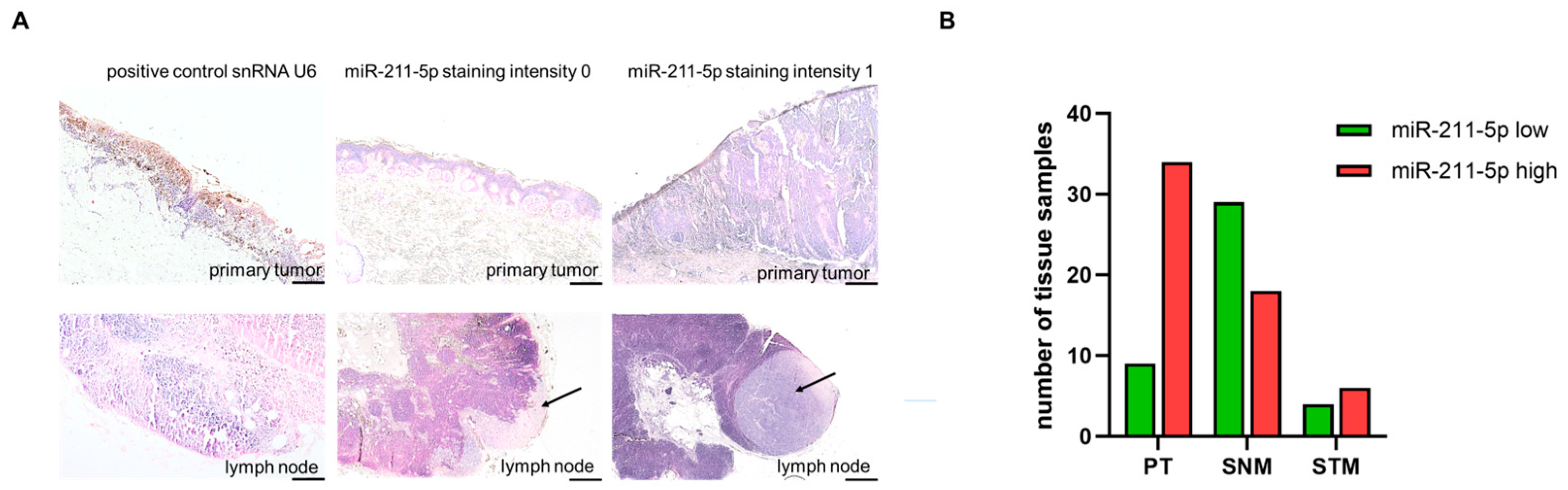
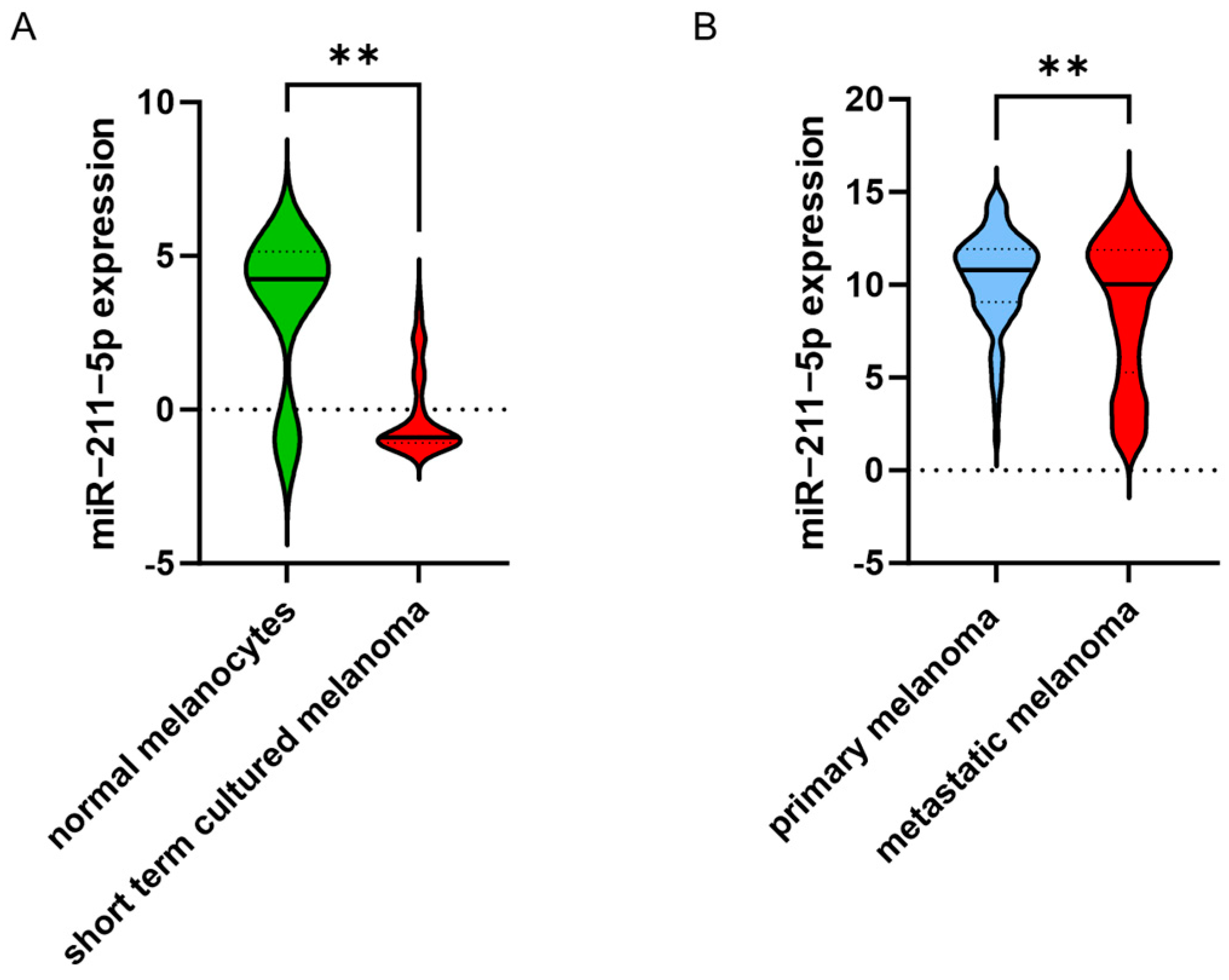
2.2. miR-211-5p Is More Frequently Highly Expressed in Primary Melanomas
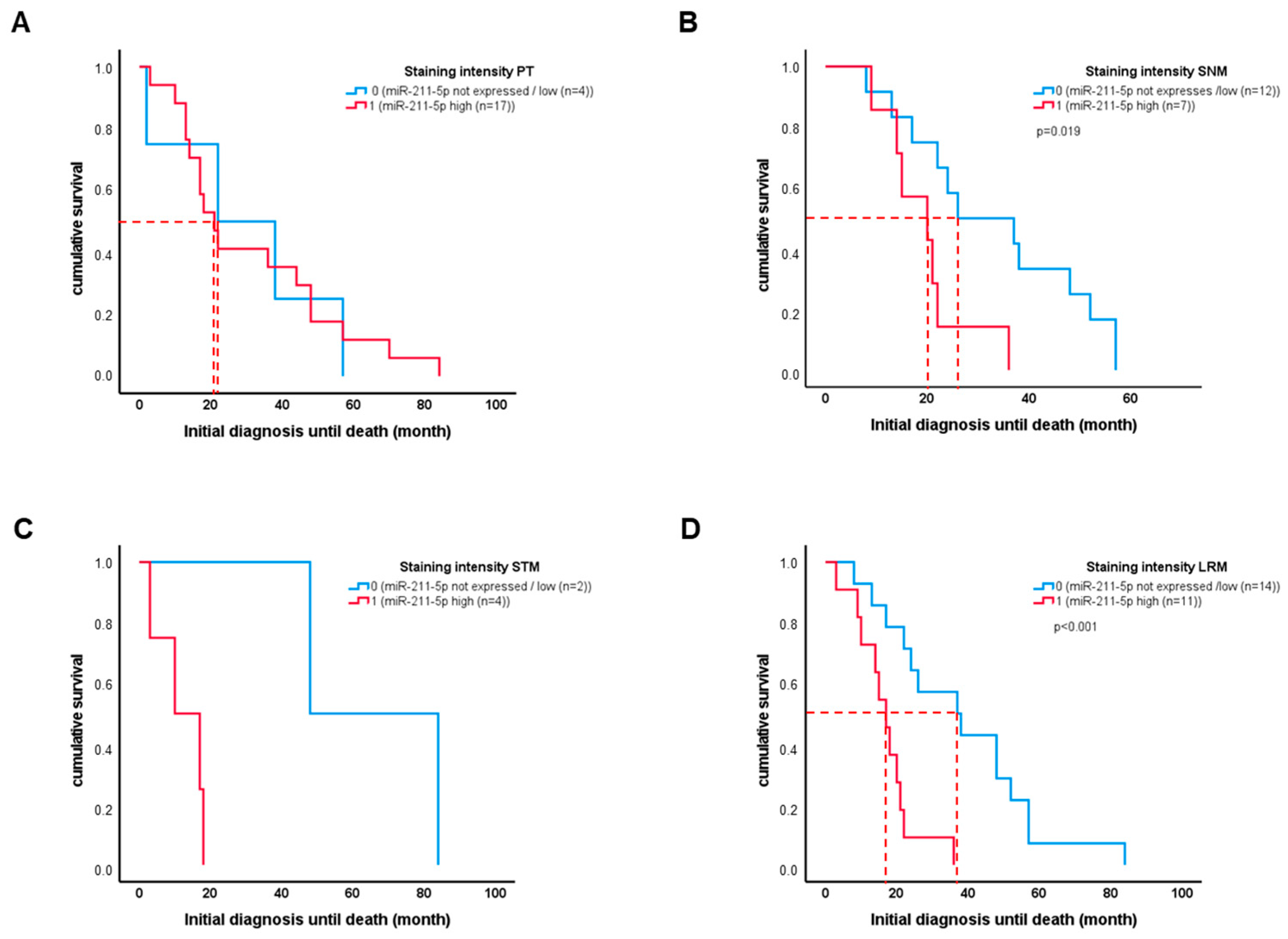
2.3. High miR-211-5p Expression in Sentinel Lymph Node Metastasis Is Associated with Worse Patient Outcome
2.4. In Silico Data Analysis
3. Discussion
4. Materials and Methods
4.1. Patient Samples
4.2. In Situ Hybridization
4.3. In Silico Dataset Analyses
4.4. Statistical Analyses
4.5. Ethics Approval
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leiter, U.; Keim, U.; Garbe, C. Epidemiology of Skin Cancer: Update 2019. Adv. Exp. Med. Biol. 2020, 1268, 123–139. [Google Scholar] [PubMed]
- Gerloff, D.; Kewitz-Hempel, S.; Hause, G.; Ehrenreich, J.; Golle, L.; Kingreen, T.; Sunderkötter, C. Comprehensive Analyses of miRNAs Revealed miR-92b-3p, miR-182-5p and miR-183-5p as Potential Novel Biomarkers in Melanoma-Derived Extracellular Vesicles. Front. Oncol. 2022, 12, 935816. [Google Scholar] [CrossRef] [PubMed]
- Lawrie, C.H.; Gal, S.; Dunlop, H.M.; Pushkaran, B.; Liggins, A.P.; Pulford, K.; Banham, A.H.; Pezzella, F.; Boultwood, J.; Wainscoat, J.S.; et al. Detection of elevated levels of tumour-associated microRNAs in serum of patients with diffuse large B-cell lymphoma. Br. J. Haematol. 2008, 141, 672–675. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D.-D.; Lv, J.; Wei, K.-L.; Feng, Z.-B.; Chen, J.-T.; Liu, K.-C.; Chen, G.; Luo, D.-Z. A nine-miRNA signature as a potential diagnostic marker for breast carcinoma: An integrated study of 1,110 cases. Oncol. Rep. 2017, 37, 3297–3304. [Google Scholar] [CrossRef]
- Ambros, V. The functions of animal microRNAs. Nature 2004, 431, 350–355. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Gerloff, D.; Sunderkötter, C.; Wohlrab, J. Importance of microRNAs in Skin Oncogenesis and Their Suitability as Agents and Targets for Topical Therapy. Skin Pharmacol. Physiol. 2020, 33, 270–279. [Google Scholar] [CrossRef]
- Gebhardt, K.; Edemir, B.; Groß, E.; Nemetschke, L.; Kewitz-Hempel, S.; Moritz, R.K.C.; Sunderkötter, C.; Gerloff, D. BRAF/EZH2 Signaling Represses miR-129-5p Inhibition of SOX4 Thereby Modulating BRAFi Resistance in Melanoma. Cancers 2021, 13, 2393. [Google Scholar] [CrossRef]
- Levy, C.; Khaled, M.; Iliopoulos, D.; Janas, M.M.; Schubert, S.; Pinner, S.; Chen, P.-H.; Li, S.; Fletcher, A.L.; Yokoyama, S.; et al. Intronic miR-211 assumes the tumor suppressive function of its host gene in melanoma. Mol. Cell 2010, 40, 841–849. [Google Scholar] [CrossRef]
- Zeng, B.; Chen, Y.; Chen, H.; Zhao, Q.; Sun, Z.; Liu, D.; Li, X.; Zhang, Y.; Wang, J.; Xing, H.R. Exosomal miR-211-5p regulates glucose metabolism, pyroptosis, and immune microenvironment of melanoma through GNA15. Pharmacol. Res. 2023, 188, 106660. [Google Scholar] [CrossRef]
- Boyle, G.M.; Woods, S.L.; Bonazzi, V.F.; Stark, M.S.; Hacker, E.; Aoude, L.G.; Dutton-Regester, K.; Cook, A.L.; Sturm, R.A.; Hayward, N.K. Melanoma cell invasiveness is regulated by miR-211 suppression of the BRN2 transcription factor. Pigment Cell Melanoma Res. 2011, 24, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Margue, C.; Philippidou, D.; Reinsbach, S.E.; Schmitt, M.; Behrmann, I.; Kreis, S. New target genes of MITF-induced microRNA-211 contribute to melanoma cell invasion. PLoS ONE 2013, 8, e73473. [Google Scholar] [CrossRef] [PubMed]
- Mazar, J.; DeYoung, K.; Khaitan, D.; Meister, E.; Almodovar, A.; Goydos, J.; Ray, A.; Perera, R.J. The regulation of miRNA-211 expression and its role in melanoma cell invasiveness. PLoS ONE 2010, 5, e13779. [Google Scholar] [CrossRef]
- Babapoor, S.; Horwich, M.; Wu, R.; Levinson, S.; Gandhi, M.; Makkar, H.; Kristjansson, A.; Chang, M.; Dadras, S.S. microRNA in situ hybridization for miR-211 detection as an ancillary test in melanoma diagnosis. Mod. Pathol. 2016, 29, 461–475. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Martínez, M.; Benito-Jardón, L.; Alonso, L.; Koetz-Ploch, L.; Hernando, E.; Teixidó, J. miR-204-5p and miR-211-5p Contribute to BRAF Inhibitor Resistance in Melanoma. Cancer Res. 2018, 78, 1017–1030. [Google Scholar] [CrossRef]
- Lee, B.; Sahoo, A.; Sawada, J.; Marchica, J.; Sahoo, S.; Layng, F.I.A.L.; Finlay, D.; Mazar, J.; Joshi, P.; Komatsu, M.; et al. MicroRNA-211 Modulates the DUSP6-ERK5 Signaling Axis to Promote BRAF(V600E)-Driven Melanoma Growth In Vivo and BRAF/MEK Inhibitor Resistance. J. Investig. Dermatol. 2021, 141, 385–394. [Google Scholar] [CrossRef]
- Dror, S.; Sander, L.; Schwartz, H.; Sheinboim, D.; Barzilai, A.; Dishon, Y.; Apcher, S.; Golan, T.; Greenberger, S.; Barshack, I.; et al. Melanoma miRNA trafficking controls tumour primary niche formation. Nat. Cell Biol. 2016, 18, 1006–1017. [Google Scholar] [CrossRef]
- Xu, Y.; Brenn, T.; Brown, E.R.S.; Doherty, V.; Melton, D.W. Differential expression of microRNAs during melanoma progression: miR-200c, miR-205 and miR-211 are downregulated in melanoma and act as tumour suppressors. Br. J. Cancer 2012, 106, 553–561. [Google Scholar] [CrossRef]
- Kewitz-Hempel, S.; Windisch, N.; Hause, G.; Müller, L.; Sunderkötter, C.; Gerloff, D. Extracellular vesicles derived from melanoma cells induce carcinoma-associated fibroblasts via miR-92b-3p mediated downregulation of PTEN. J. Extracell. Vesicles 2024, 13, e12509. [Google Scholar] [CrossRef]
- Sahoo, A.; Lee, B.; Boniface, K.; Seneschal, J.; Sahoo, S.K.; Seki, T.; Wang, C.; Das, S.; Han, X.; Steppie, M.; et al. MicroRNA-211 Regulates Oxidative Phosphorylation and Energy Metabolism in Human Vitiligo. J. Investig. Dermatol. 2004, 137, 1965–1974. [Google Scholar] [CrossRef]
- Steingrímsson, E.; Copeland, N.G.; Jenkins, N.A. Melanocytes and the microphthalmia transcription factor network. Annu. Rev. Genet. 2004, 38, 365–411. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.J.; Du, J.; Rowan, S.; Hershey, C.L.; Widlund, H.R.; Fisher, D.E.; Chu, F.-F.; Esworthy, R.S.; Chu, P.G.; Longmate, J.A.; et al. Transcriptional regulation of the melanoma prognostic marker melastatin (TRPM1) by MITF in melanocytes and melanoma. Cancer Res. 2004, 64, 509–516. [Google Scholar] [CrossRef]
- Garraway, L.A.; Widlund, H.R.; Rubin, M.A.; Getz, G.; Berger, A.J.; Ramaswamy, S.; Beroukhim, R.; Milner, D.A.; Granter, S.R.; Du, J.; et al. Integrative genomic analyses identify MITF as a lineage survival oncogene amplified in malignant melanoma. Nature 2005, 436, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, J.S.; Hölzel, M.; Lambert, J.; Buffa, F.M.; Goding, C.R. The MITF regulatory network in melanoma. Pigment. Cell Melanoma Res. 2022, 35, 517–533. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Ashktorab, H.; Pang, X.; Zhao, Y.; Sha, W.; Liu, Y.; Gu, X. MicroRNA-211 expression promotes colorectal cancer cell growth in vitro and in vivo by targeting tumor sup-pressor CHD5. PLoS ONE 2012, 7, e29750. [Google Scholar]
- Chu, T.H.; Yang, C.C.; Liu, C.J.; Lui, M.T.; Lin, S.C.; Chang, K.W. miR-211 promotes the progression of head and neck carcinomas by targeting TGFbetaRII. Cancer Lett. 2013, 337, 115–124. [Google Scholar] [CrossRef]
- Giovannetti, E.; van der Velde, A.; Funel, N.; Vasile, E.; Perrone, V.; Leon, L.G.; De Lio, N.; Avan, A.; Caponi, S.; Pollina, L.E.; et al. High-throughput microRNA (miRNAs) arrays unravel the prognostic role of MiR-211 in pancreatic cancer. PLoS ONE 2012, 7, e49145. [Google Scholar] [CrossRef]
- Xia, B.; Yang, S.; Liu, T.; Lou, G. miR-211 suppresses epithelial ovarian cancer proliferation and cell-cycle progression by targeting Cyclin D1 and CDK6. Mol. Cancer 2015, 14, 57. [Google Scholar] [CrossRef]
- Chen, L.; He, Y.; Zhu, J.; Zhao, S.; Qi, S.; Chen, X.; Zhang, H.; Ni, Z.; Zhou, Y.; Chen, G.; et al. The roles and mechanism of m6A RNA methylation regulators in cancer immunity. Biomed. Pharmacother. 2023, 163, 114839. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Q.; Pan, R.; Wang, Q.; Zhu, X.; Yuan, C.; Cai, F.; Gao, Y.; Cui, Y. Regulatory roles of three miRNAs on allergen mRNA expression in Tyrophagus putrescentiae. Allergy 2022, 77, 469–482. [Google Scholar] [CrossRef]


| Number of patients (%) | 76 (100) |
| Age at diagnosis (median (range)) | 70.5 (21–87) |
| Sex | Male: 45/Female: 31 |
| Died of tumor (%) | 29 (38.2) |
| Overall survival (month) from first diagnosis to follow-up (mean ± sd) | 54.5 ± 40.5 |
| Breslow Index (mm) (mean ± sd) | 4.7 ± 4.2 |
| BRAF status (%) | |
| Wildtype | 5 (6.6) |
| BRAF V600 | 6 (7.9) |
| Unknown | 65 (85.5) |
| TNM classification (%) | |
| T1 | 6 (7.9) |
| T2 | 15 (19.7) |
| T3 | 22 (28.9) |
| T4 | 14 (18.4) |
| Pathological stage AJCC 2009 at first diagnosis (%) | |
| IA | 2 (2.6) |
| IB | 1 (1.3) |
| IIA | 3 (3.9) |
| IIB | 0 (0) |
| IIC | 0 (0) |
| IIIA | 1 (1.3) |
| IIIB | 32 (42.1) |
| IIIC | 6 (7.9) |
| III (unknown) | 10 (13.2) |
| IV | 21 (27.6) |
| Total (%) | ISH 0/Low (%) | ISH 1/High (%) | n.e. (%) | |
|---|---|---|---|---|
| Tissue number | 109 (100) | |||
| Primary tumor (PT) | 48 (44) | 9 (18.8) | 34 (70.8) * | 5 (10.4) |
| Sentinel node metastases (SNMs) | 51 (47) | 29 (56.9) | 18 (35.3) * | 4 (7.8) |
| Soft tissue metastases (STMs) | 10 (9) | 4 (40.0) | 6 (60.0) | 0 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moritz, R.K.C.; Ebelt, N.; Rattay, T.; Ehrenreich, J.; Sunderkötter, C.; Gerloff, D. The Expression of miR-211-5p in Sentinel Lymph Node Metastases of Malignant Melanoma Is a Potential Marker for Poor Prognosis. Int. J. Mol. Sci. 2024, 25, 10859. https://doi.org/10.3390/ijms251910859
Moritz RKC, Ebelt N, Rattay T, Ehrenreich J, Sunderkötter C, Gerloff D. The Expression of miR-211-5p in Sentinel Lymph Node Metastases of Malignant Melanoma Is a Potential Marker for Poor Prognosis. International Journal of Molecular Sciences. 2024; 25(19):10859. https://doi.org/10.3390/ijms251910859
Chicago/Turabian StyleMoritz, Rose Kathrin Caroline, Nicole Ebelt, Tina Rattay, Jovine Ehrenreich, Cord Sunderkötter, and Dennis Gerloff. 2024. "The Expression of miR-211-5p in Sentinel Lymph Node Metastases of Malignant Melanoma Is a Potential Marker for Poor Prognosis" International Journal of Molecular Sciences 25, no. 19: 10859. https://doi.org/10.3390/ijms251910859






