Aging, Neurodegenerative Disorders, and Cerebellum
Abstract
1. Introduction
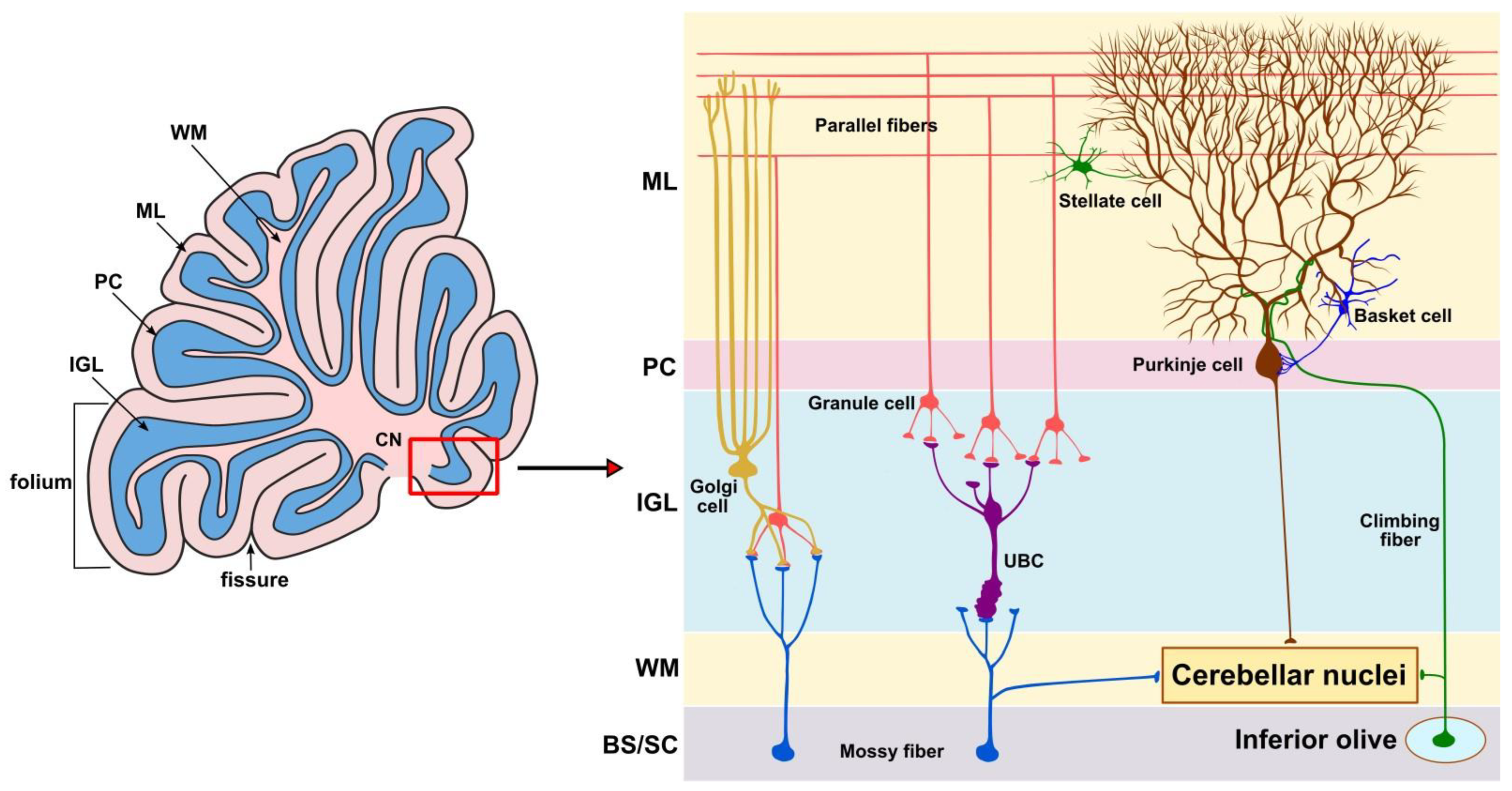
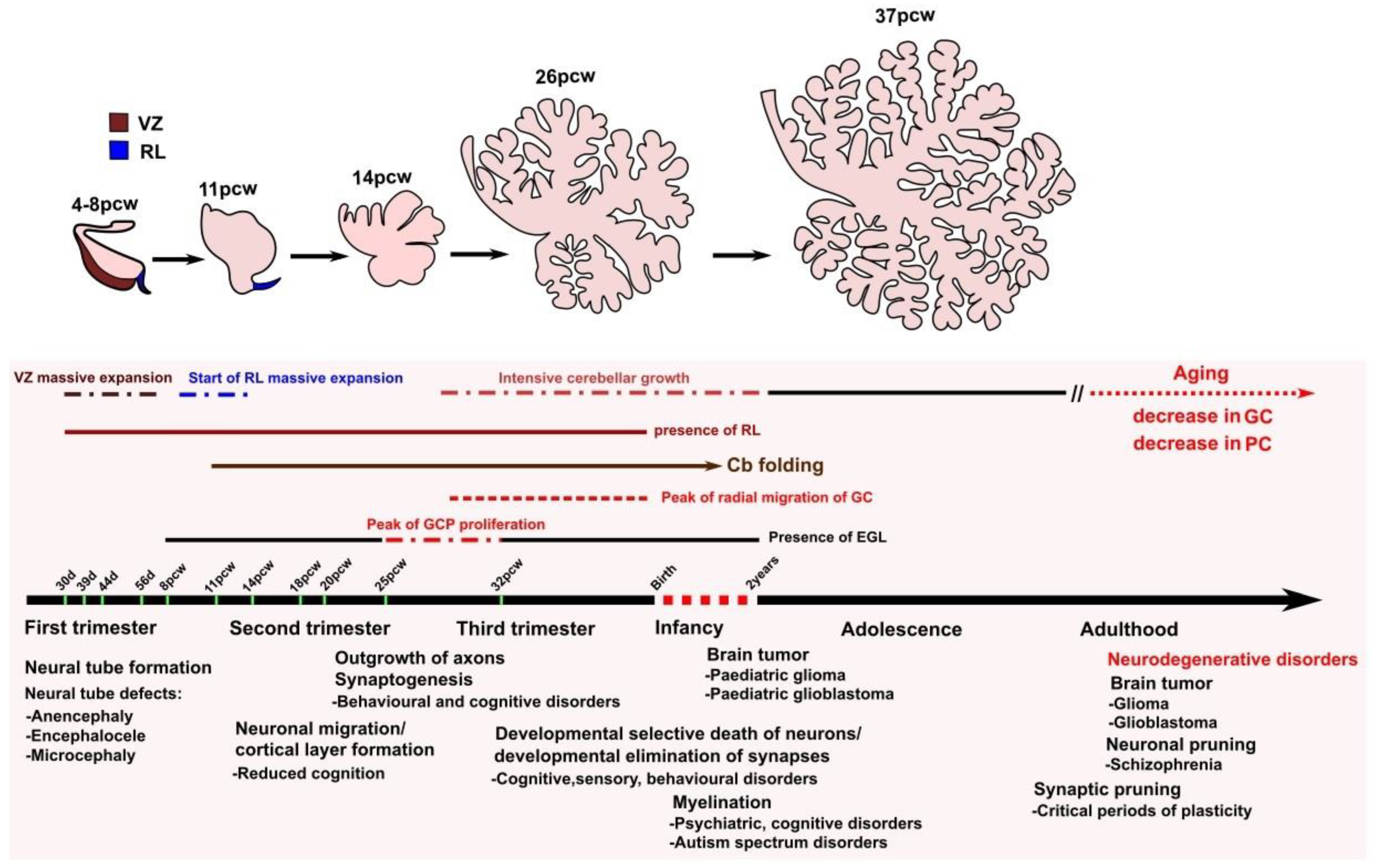
2. The Role of the Cerebellum in Aging
3. Role of the Cerebellum in Neurodegenerative Disorders
3.1. Alzheimer’s Disease (AD)
3.2. Parkinson’s Disease (PD)
3.3. Huntington’s Disease (HD)
3.4. Amyotrophic Lateral Sclerosis (ALS)
3.5. Friedreich’s Ataxia (FRDA)
3.6. Spinal Muscular Atrophy (SMA)
3.7. Juvenile Batten’s Disease (JBD)
3.8. Multiple Sclerosis (MS)
3.9. Vascular Dementia (VD)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Keefe, M.G.; Nowakowski, T.J. Evolutionary Expansion of Human Cerebellar Germinal Zones. Trends Neurosci. 2020, 43, 75–77. [Google Scholar] [CrossRef] [PubMed]
- Diedrichsen, J.; King, M.; Hernandez-Castillo, C.; Sereno, M.; Ivry, R.B. Universal Transform or Multiple Functionality? Understanding the Contribution of the Human Cerebellum across Task Domains. Neuron 2019, 102, 918–928. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; De Schutter, E. Recent data on the cerebellum require new models and theories. Curr. Opin. Neurobiol. 2023, 82, 102765. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Kloth, A.D.; Badura, A. The cerebellum, sensitive periods, and autism. Neuron 2014, 83, 518–532. [Google Scholar] [CrossRef] [PubMed]
- Chizhikov, V.V.; Millen, K.J. Neurogenesis in the cerebellum. In Comprehensive Developmental Neuroscience: Patterning and Cell Type Specification in the Developing CNS and PNS, 1st ed.; Rakic, P., Rubenstein, J., Eds.; Academic Press: Cambridge, MA, USA, 2013; Chapter 22; pp. 417–434. [Google Scholar] [CrossRef]
- Butts, T.; Green, M.J.; Wingate, R.T.J. Development of the cerebellum: Simple steps to make a ‘little brain’. Development 2014, 141, 4031–4041. [Google Scholar] [CrossRef]
- Marzban, H.; Del Bigio, M.R.; Alizadeh, J.; Ghavami, S.; Zachariah, R.M.; Rastegar, M. Cellular commitment in the developing cerebellum. Front. Cell Neurosci. 2015, 8, 450. [Google Scholar] [CrossRef] [PubMed]
- Iskusnykh, I.Y.; Fattakhov, N.; Buddington, R.K.; Chizhikov, V.V. Intrauterine growth restriction compromises cerebellar development by affecting radial migration of granule cells via the JamC/Pard3a molecular pathway. Exp. Neurol. 2021, 336, 113537. [Google Scholar] [CrossRef]
- Consalez, G.G.; Goldowitz, D.; Casoni, F.; Hawkes, R. Origins, Development, and Compartmentation of the Granule Cells of the Cerebellum. Front. Neural Circuits 2021, 14, 611841. [Google Scholar] [CrossRef]
- Goldowitz, D.; Hamre, K. The cells and molecules that make a cerebellum. Trends Neurosci. 1998, 21, 375–382. [Google Scholar] [CrossRef]
- Chizhikov, V.V.; Millen, K.J. Neurogenesis in the cerebellum. In Patterning and Cell Type Specification in the Developing CNS and PNS, 1st ed.; Rubenstein, J., Rakic, P., Chen, B., Kwan, K.Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Chapter 16; pp. 349–367. [Google Scholar] [CrossRef]
- Zang, Y.; Dieudonné, S.; De Schutter, E. Voltage- and Branch-Specific Climbing Fiber Responses in Purkinje Cells. Cell Rep. 2018, 24, 1536–1549. [Google Scholar] [CrossRef]
- Altman, J.; Bayer, S.A. Development of the Cerebellar System: In Relation to Its Evolution, Structure, and Functions, 1st ed.; CRC Press: New York, NY, USA, 1997; p. 783. [Google Scholar]
- Chizhikov, D.; Buddington, R.K.; Iskusnykh, I.Y. Effects of Phosphatidylserine Source of Docosahexaenoic Acid on Cerebellar Development in Preterm Pigs. Brain Sci. 2020, 10, 475. [Google Scholar] [CrossRef] [PubMed]
- Stoodley, C.J.; Limperopoulos, C. Structure–function relationships in the developing cerebellum: Evidence from early-life cerebellar injury and neurodevelopmental disorders. Semin. Fetal Neonatal Med. 2016, 21, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.J.; Carlson, E.S. Resistance, vulnerability and resilience: A review of the cognitive cerebellum in aging and neurodegenerative diseases. Neurobiol. Learn. Mem. 2020, 170, 106981. [Google Scholar] [CrossRef] [PubMed]
- Ouellette, J.; Lacoste, B. From Neurodevelopmental to Neurodegenerative Disorders: The Vascular Continuum. Front. Aging Neurosci. 2021, 13, 749026. [Google Scholar] [CrossRef] [PubMed]
- Starr, J.M. Ageing and epigenetics: Linking neurodevelopmental and neurodegenerative disorders. Dev. Med. Child Neurol. 2019, 61, 1134–1138. [Google Scholar] [CrossRef]
- Iskusnykh, I.Y.; Chizhikov, V.V. Cerebellar development after preterm birth. Front. Cell Dev. Biol. 2022, 10, 1068288. [Google Scholar] [CrossRef]
- Haldipur, P.; Millen, K.J.; Aldinger, K.A. Human Cerebellar Development and Transcriptomics: Implications for Neurodevelopmental Disorders. Annu. Rev. Neurosci. 2022, 45, 515–531. [Google Scholar] [CrossRef]
- Haniffa, M.; Taylor, D.; Linnarsson, S.; Aronow, B.J.; Bader, G.D.; Barker, R.A.; Camara, P.G.; Camp, J.G.; Chédotal, A.; Copp, A.; et al. Human Cell Atlas Developmental Biological Network. A roadmap for the Human Developmental Cell Atlas. Nature 2021, 597, 196–205. [Google Scholar] [CrossRef]
- Nakamura, H.; Sato, T.; Suzuki-Hirano, A. Isthmus organizer for mesencephalon and metencephalon. Dev. Growth Differ. 2008, 50 (Suppl. 1), S113–S118. [Google Scholar] [CrossRef]
- Sillitoe, R.V.; Joyner, A.L. Morphology, molecular codes, and circuitry produce the three-dimensional complexity of the cerebellum. Annu. Rev. Cell Dev. Biol. 2007, 23, 549–577. [Google Scholar] [CrossRef]
- Yaguchi, Y.; Yu, T.; Ahmed, M.U.; Berry, M.; Mason, I.; Basson, M.A. Fibroblast growth factor (FGF) gene expression in the developing cerebellum suggests multiple roles for FGF signaling during cerebellar morphogenesis and development. Dev. Dyn. 2009, 238, 2058–2072. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Hibi, M. Development and evolution of cerebellar neural circuits. Dev. Growth Differ. 2012, 54, 373–389. [Google Scholar] [CrossRef] [PubMed]
- Leto, K.; Bartolini, A.; Yanagawa, Y.; Obata, K.; Magrassi, L.; Schilling, K.; Rossi, F. Laminar fate and phenotype specification of cerebellar GABAergic interneurons. J. Neurosci. 2009, 29, 7079–7091. [Google Scholar] [CrossRef]
- Wullimann, M.F.; Mueller, T.; Distel, M.; Babaryka, A.; Grothe, B.; Köster, R.W. The long adventurous journey of rhombic lip cells in jawed vertebrates: A comparative developmental analysis. Front. Neuroanat. 2011, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Leto, K.; Arancillo, M.; Becker, E.B.; Buffo, A.; Chiang, C.; Ding, B.; Dobyns, W.B.; Dusart, I.; Haldipur, P.; Hatten, M.E.; et al. Consensus Paper: Cerebellar Development. Cerebellum 2016, 15, 789–828. [Google Scholar] [CrossRef]
- Iskusnykh, I.Y.; Buddington, R.K.; Chizhikov, V.V. Preterm birth disrupts cerebellar development by affecting granule cell proliferation program and Bergmann glia. Exp. Neurol. 2018, 306, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; An, Y.; Carass, A.; Prince, J.L.; Resnick, S.M. Longitudinal analysis of regional cerebellum volumes during normal aging. NeuroImage 2020, 220, 117062. [Google Scholar] [CrossRef]
- Azizi, S.A. Role of the cerebellum in the phenotype of neurodegenerative diseases: Mitigate or exacerbate? Neurosci. Lett. 2021, 760, 136105. [Google Scholar] [CrossRef]
- Sargent, M.A.; Poskitt, K.J.; Roland, E.H.; Hill, A.; Hendson, G. Cerebellar vermian atrophy after neonatal hypoxic-ischemic encephalopathy. AJNR Am. J. Neuroradiol. 2004, 25, 1008–1015. [Google Scholar]
- Tam, E.W.Y. Cerebellar injury in preterm infants. Handb. Clin. Neurol. 2018, 155, 49–59. [Google Scholar] [CrossRef]
- Ottolini, K.M.; Andescavage, N.; Kapse, K.; Jacobs, M.; Murnick, J.; VanderVeer, R.; Basu, S.; Said, M.; Limperopoulos, C. Early Lipid Intake Improves Cerebellar Growth in Very Low-Birth-Weight Preterm Infants. JPEN J. Parenter. Enter. Nutr. 2021, 45, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, K.; Tanda, K.; Nishimura, A.; Kinoshita, D.; Nishimoto, M.; Kizaki, Z.; Ohno, K. Morphological changes in the pons and cerebellum during the first two weeks in term infants with pontine and cerebellar injury and profound neonatal asphyxia. Acta Radiol. 2022, 63, 1110–1117. [Google Scholar] [CrossRef] [PubMed]
- Hedden, T.; Gabrieli, J.D. Insights into the ageing mind: A view from cognitive neuroscience. Nat. Rev. Neurosci. 2004, 5, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Goble, D.J.; Coxon, J.P.; Van Impe, A.; De Vos, J.; Wenderoth, N.; Swinnen, S.P. The neural control of bimanual movements in the elderly: Brain regions exhibiting age-related increases in activity, frequency-induced neural modulation, and task-specific compensatory recruitment. Hum. Brain Mapp. 2010, 31, 1281–1295. [Google Scholar] [CrossRef] [PubMed]
- Boisgontier, M.P.; Cheval, B.; van Ruitenbeek, P.; Cuypers, K.; Leunissen, I.; Sunaert, S.; Meesen, R.; Zivari Adab, H.; Renaud, O.; Swinnen, S.P. Cerebellar gray matter explains bimanual coordination performance in children and older adults. Neurobiol. Aging 2018, 65, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Goodall, E.F.; Wang, C.; Simpson, J.E.; Baker, D.J.; Drew, D.R.; Heath, P.R.; Saffrey, M.J.; Romero, I.A.; Wharton, S.B. Age-associated changes in the blood-brain barrier: Comparative studies in human and mouse. Neuropathol. Appl. Neurobiol. 2018, 44, 328–340, Erratum in Neuropathol. Appl. Neurobiol. 2018, 44, 747–748. [Google Scholar] [CrossRef] [PubMed]
- Nazeri, A.; Chakravarty, M.M.; Rotenberg, D.J.; Rajji, T.K.; Rathi, Y.; Michailovich, O.V.; Voineskos, A.N. Functional consequences of neurite orientation dispersion and density in humans across the adult lifespan. J. Neurosci. 2015, 35, 1753–1762. [Google Scholar] [CrossRef]
- Childs, R.; Gamage, R.; Münch, G.; Gyengesi, E. The effect of aging and chronic microglia activation on the morphology and numbers of the cerebellar Purkinje cells. Neurosci. Lett. 2021, 751, 135807. [Google Scholar] [CrossRef]
- Zheng, H.; Jiang, L.; Tsuduki, T.; Conrad, M.; Toyokuni, S. Embryonal erythropoiesis and aging exploit ferroptosis. Redox Biol. 2021, 48, 102175. [Google Scholar] [CrossRef]
- Oh, G.; Ebrahimi, S.; Wang, S.C.; Cortese, R.; Kaminsky, Z.A.; Gottesman, I.I.; Burke, J.R.; Plassman, B.L.; Petronis, A. Epigenetic assimilation in the aging human brain. Genome Biol. 2016, 17, 76. [Google Scholar] [CrossRef]
- Bertoni-Freddari, C.; Fattoretti, P.; Giorgetti, B.; Solazzi, M.; Balietti, M.; Di Stefano, G.; Casoli, T. Decay of Mitochondrial Metabolic Competence in the Aging Cerebellum. Ann. N. Y. Acad. Sci. 2004, 1019, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Popa-Wagner, A.; Sandu, R.E.; Cristin, C.; Uzoni, A.; Welle, K.A.; Hryhorenko, J.R.; Ghaemmaghami, S. Increased Degradation Rates in the Components of the Mitochondrial Oxidative Phosphorylation Chain in the Cerebellum of Old Mice. Front. Aging Neurosci. 2018, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Santaniello, S. Role of cerebellar GABAergic dysfunctions in the origins of essential tremor. Proc. Natl. Acad. Sci. USA 2019, 116, 13592–13601. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Verkhratsky, A.; Toescu, E.C. Changes in Mitochondrial Status Associated with Altered Ca2+ Homeostasis in Aged Cerebellar Granule Neurons in Brain Slices. J. Neurosci. 2002, 22, 10761–10771. [Google Scholar] [CrossRef]
- Turpeenoja, L.; Villa, R.F.; Magri, G.; Giuffrida Stella, A.M. Changes of mitochondrial membrane proteins in rat cerebellum during aging. Neurochem. Res. 1988, 13, 859–865. [Google Scholar] [CrossRef]
- Balietti, M.; Giorgetti, B.; Di Stefano, G.; Casoli, T.; Platano, D.; Solazzi, M.; Bertoni-Freddari, C.; Aicardi, G.; Lattanzio, F.; Fattoretti, P. A ketogenic diet increases succinic dehydrogenase (SDH) activity and recovers age-related decrease in numeric density of SDH-positive mitochondria in cerebellar Purkinje cells of late-adult rats. Micron 2010, 41, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Gostomska-Pampuch, K.; Drulis-Fajdasz, D.; Gizak, A.; Wiśniewski, J.R.; Rakus, D. Absolute Proteome Analysis of Hippocampus, Cortex and Cerebellum in Aged and Young Mice Reveals Changes in Energy Metabolism. Int. J. Mol. Sci. 2021, 22, 6188. [Google Scholar] [CrossRef]
- Haduch, A.; Danek, P.J.; Kuban, W.; Pukło, R.; Alenina, N.; Gołębiowska, J.; Popik, P.; Bader, M.; Daniel, W.A. Cytochrome P450 2D (CYP2D) enzyme dysfunction associated with aging and serotonin deficiency in the brain and liver of female Dark Agouti rats. Neurochem. Int. 2022, 152, 105223. [Google Scholar] [CrossRef]
- Giannini, G.; Sorrentino, V. Molecular structure and tissue distribution of ryanodine receptors calcium channels. Med. Res. Rev. 1995, 15, 313–323. [Google Scholar] [CrossRef]
- Mori, F.; Fukaya, M.; Abe, H.; Wakabayashi, K.; Watanabe, M. Developmental changes in expression of the three ryanodine receptor mRNAs in the mouse brain. Neurosci. Lett. 2000, 285, 57–60. [Google Scholar] [CrossRef]
- Gkanatsiou, E.; Nilsson, J.; Toomey, C.E.; Vrillon, A.; Kvartsberg, H.; Portelius, E.; Zetterberg, H.; Blennow, K.; Brinkmalm, A.; Lashley, T.; et al. Amyloid pathology and synaptic loss in pathological aging. J. Neurochem. 2021, 159, 258–272. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, P.H.C.; Osburn, S.C.; Godwin, J.S.; Roberts, M.D.; Kavazis, A.N. Effects of aging and long-term physical activity on mitochondrial physiology and redox state of the cortex and cerebellum of female rats. Physiol. Rep. 2022, 10, e15542. [Google Scholar] [CrossRef] [PubMed]
- Caron, P.C.; Unsworth, B.R. Alteration of the activity and molecular from of thymidine kinase during development and aging in the mouse cerebellum. Mech. Ageing Dev. 1978, 8, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.I.; Rivas, L.; Lacruz, C.; Toledano, A. Astroglial cell subtypes in the cerebella of normal adults, elderly adults, and patients with Alzheimer’s disease: A histological and immunohistochemical comparison. Glia 2015, 63, 287–312. [Google Scholar] [CrossRef] [PubMed]
- Maurya, S.K.; Mishra, R. Pax6 interacts with Iba1 and shows age-associated alterations in brain of aging mice. J. Chem. Neuroanat. 2017, 82, 60–64. [Google Scholar] [CrossRef] [PubMed]
- El Hajj, A.; Yen, F.T.; Oster, T.; Malaplate, C.; Pauron, L.; Corbier, C.; Lanhers, M.C.; Claudepierre, T. Age-related changes in regiospecific expression of Lipolysis Stimulated Receptor (LSR) in mice brain. PLoS ONE 2019, 14, e0218812. [Google Scholar] [CrossRef] [PubMed]
- Mathiesen, C.; Brazhe, A.; Thomsen, K.; Lauritzen, M. Spontaneous calcium waves in Bergman glia increase with age and hypoxia and may reduce tissue oxygen. J. Cereb. Blood Flow Metab. 2013, 33, 161–169. [Google Scholar] [CrossRef]
- Murru, S.; Hess, S.; Barth, E.; Almajan, E.R.; Schatton, D.; Hermans, S.; Brodesser, S.; Langer, T.; Kloppenburg, P.; Rugarli, E.I. Astrocyte-specific deletion of the mitochondrial m-AAA protease reveals glial contribution to neurodegeneration. Glia 2019, 67, 1526–1541. [Google Scholar] [CrossRef]
- Dlugos, C.A.; Pentney, R.J. Morphometric analyses of Purkinje and granule cells in aging F344 rats. Neurobiol. Aging 1994, 15, 435–440. [Google Scholar] [CrossRef]
- Fan, W.J.; Yan, M.C.; Wang, L.; Sun, Y.Z.; Deng, J.B.; Deng, J.X. Synaptic aging disrupts synaptic morphology and function in cerebellar Purkinje cells. Neural Regen. Res. 2018, 13, 1019–1025. [Google Scholar] [CrossRef]
- Vázquez-Hernández, N.; Martínez-Torres, N.I.; González-Burgos, I. Plastic changes to dendritic spines in the cerebellar and prefrontal cortices underlie the decline in motor coordination and working memory during successful aging. Behav. Brain Res. 2021, 400, 113014. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.O.; Skalicky, M.; Viidik, A. Does long-term physical exercise counteract age-related Purkinje cell loss? A stereological study of rat cerebellum. J. Comp. Neurol. 2000, 428, 2013–2222. [Google Scholar] [CrossRef]
- Ivy, G.O.; Gurd, J.W. A proteinase inhibitor model of lipofuscin formation. In Lipofuscin–1987: State of the Art, 1st ed.; Zs-Nagy, I., Ed.; Elsevier: Amsterdam, The Netherlands, 1988; pp. 83–108. [Google Scholar]
- Ivy, G.O.; Schottler, F.; Wenzel, J.; Baudry, M.; Lynch, G. Inhibitors of lysosomal enzymes: Accumulation of lipofuscin-like dense bodies in the brain. Science 1984, 226, 985–987. [Google Scholar] [CrossRef] [PubMed]
- Ivy, G.O.; Kanai, S.; Ohta, M.; Smith, G.; Sato, Y.; Kobayashi, M.; Kitani, K. Lipofuscin-like substances accumulate rapidly in brain, retina and internal organs with cysteine protease inhibition. In Lipofuscin and Ceroid Pigments, 1st ed.; Porta, E.A., Ed.; Plenum Press: New York, NY, USA, 1990; pp. 31–47. [Google Scholar]
- Oenzil, F.; Kishikawa, M.; Mizuno, T.; Nakano, M. Age-related accumulation of lipofuscin in 3 different regions of rat brain. Mech. Age Dev. 1994, 76, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Gilissen, E.P.; Staneva-Dobrovski, L. Distinct types of lipofuscin pigment in the hippocampus and cerebellum of aged cheirogaleid primates. Anat. Rec. 2013, 296, 1895–1906. [Google Scholar] [CrossRef] [PubMed]
- Andersen, B.B.; Gundersen, H.J.; Pakkenberg, B. Aging of the human cerebellum: A stereological study. J. Comp. Neurol. 2003, 466, 356–365. [Google Scholar] [CrossRef]
- Gilissen, E.P.; Leroy, K.; Yilmaz, Z.; Kövari, E.; Bouras, C.; Boom, A.; Poncelet, L.; Erwin, J.M.; Sherwood, C.C.; Hof, P.R.; et al. A neuronal aging pattern unique to humans and common chimpanzees. Brain Struct. Funct. 2016, 221, 647–664. [Google Scholar] [CrossRef]
- Chung, Y.H.; Shin, C.; Kim, M.J.; Lee, B.; Park, K.H.; Cha, C.I. Immunocytochemical study on the distribution of p53 in the hippocampus and cerebellum of the aged rat. Brain Res. 2000, 885, 137–141. [Google Scholar] [CrossRef]
- de Graaf, E.L.; Vermeij, W.P.; de Waard, M.C.; Rijksen, Y.; van der Pluijm, I.; Hoogenraad, C.C.; Hoeijmakers, J.H.; Altelaar, A.F.; Heck, A.J. Spatio-temporal analysis of molecular determinants of neuronal degeneration in the aging mouse cerebellum. Mol. Cell Proteom. 2013, 12, 1350–1362. [Google Scholar] [CrossRef]
- Moreno-Baylach, M.J.; Felipo, V.; Männistö, P.T.; García-Horsman, J.A. Expression and traffic of cellular prolyl oligopeptidase are regulated during cerebellar granule cell differentiation, maturation, and aging. Neuroscience 2008, 156, 580–585. [Google Scholar] [CrossRef]
- Zhang, H.; Kuang, X.L.; Chang, Y.; Lu, J.; Jiang, H.; Wu, S. Reduced serine racemase expression in aging rat cerebellum is associated with oxidative DNA stress and hypermethylation in the promoter. Brain Res. 2015, 1629, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Bravo, J.I.; Son, J.M.; Lee, C.; Benayoun, B.A. Remodeling of the H3 nucleosomal landscape during mouse aging. Transl. Med. Aging 2020, 4, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Fries, G.R.; Bauer, I.E.; Scaini, G.; Wu, M.J.; Kazimi, I.F.; Valvassori, S.S.; Zunta-Soares, G.; Walss-Bass, C.; Soares, J.C.; Quevedo, J. Accelerated epigenetic aging and mitochondrial DNA copy number in bipolar disorder. Transl. Psychiatry 2017, 7, 1283. [Google Scholar] [CrossRef]
- Lardenoije, R.; van den Hove, D.L.A.; Vaessen, T.S.J.; Iatrou, A.; Meuwissen, K.P.V.; van Hagen, B.T.J.; Kenis, G.; Steinbusch, H.W.M.; Schmitz, C.; Rutten, B.P.F. Epigenetic modifications in mouse cerebellar Purkinje cells: Effects of aging, caloric restriction, and overexpression of superoxide dismutase 1 on 5-methylcytosine and 5-hydroxymethylcytosine. Neurobiol. Aging 2015, 36, 3079–3089. [Google Scholar] [CrossRef] [PubMed]
- Iacopino, A.M.; Rhoten, W.B.; Christakos, S. Calcium binding protein (calbindin-D28k) gene expression in the developing and aging mouse cerebellum. Brain Res. Mol. Brain Res. 1990, 8, 283–290. [Google Scholar] [CrossRef]
- Pires, R.S.; Real, C.C.; Folador, T.S.; Tellini, N.R.; Torrão, A.S.; Britto, L.R. Differential response of AMPA and NMDA glutamate receptors of Purkinje cells to aging of the chicken cerebellum. Neurosci. Lett. 2010, 478, 146–149. [Google Scholar] [CrossRef]
- Fattoretti, P.; Bertoni-Freddari, C.; Caselli, U.; Paoloni, R.; Meier-Ruge, W. Morphologic changes in cerebellar mitochondria during aging. Anal. Quant. Cytol. Histol. 1996, 18, 205–208. [Google Scholar]
- Fattoretti, P.; Bertoni-Freddari, C.; Caselli, U.; Paoloni, R.; Meier-Ruge, W. Impaired succinic dehydrogenase activity of rat Purkinje cell mitochondria during aging. Mech. Ageing Dev. 1998, 101, 175–182. [Google Scholar] [CrossRef]
- Ogata, R.; Ikari, K.; Hayashi, M.; Tamai, K.; Tagawa, K. Age-related changes in the Purkinje’s cells in the rat cerebellar cortex: A quantitative electron microscopic study. Folia Psychiatr. Neurol. JPN 1984, 38, 159–167. [Google Scholar] [CrossRef]
- Nosal, G. Neuronal involution during ageing. Ultrastructural study in the rat cerebellum. Mech. Ageing Dev. 1979, 10, 295–314. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, Q.; Hua, T. Aging of cerebellar Purkinje cells. Cell Tissue Res. 2010, 341, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, R.; Gatt, A.; Fratta, P.; Lashley, T.; Bampton, A. HnRNP K mislocalisation in neurons of the dentate nucleus is a novel neuropathological feature of neurodegenerative disease and ageing. Neuropathol. Appl. Neurobiol. 2022, 48, e12793. [Google Scholar] [CrossRef] [PubMed]
- Tsukada, M.; Yamazaki, Y.; Koizumi, A. Changes in nitric oxide synthase activities in the cerebellum during development and aging of C57BL/6 mice. Tohoku J. Exp. Med. 1995, 176, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, K.; Tripathi, R.; Mishra, R. Age-dependent alterations in expression and co-localization of Pax6 and Ras-GAP in brain of aging mice. J. Chem. Neuroanat. 2018, 92, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Barral, S.; Beltramo, R.; Salio, C.; Aimar, P.; Lossi, L.; Merighi, A. Phosphorylation of histone H2AX in the mouse brain from development to senescence. Int. J. Mol. Sci. 2014, 15, 1554–1573. [Google Scholar] [CrossRef]
- Braak, H.; Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991, 82, 239–259. [Google Scholar] [CrossRef]
- Thal, D.R.; Rüb, U.; Orantes, M.; Braak, H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology 2002, 58, 1791–1800. [Google Scholar] [CrossRef]
- Blennow, K.; Bogdanovic, N.; Alafuzoff, I.; Ekman, R.; Davidsson, P. Synaptic pathology in Alzheimer’s disease: Relation to severity of dementia, but not to senile plaques, neurofibrillary tangles, or the ApoE4 allele. J. Neural Transm. 1996, 103, 603–618. [Google Scholar] [CrossRef]
- Kim, H.J.; Chae, S.C.; Lee, D.K.; Chromy, B.; Lee, S.C.; Park, Y.C.; Klein, W.L.; Krafft, G.A.; Hong, S.T. Selective neuronal degeneration induced by soluble oligomeric amyloid beta protein. FASEB J. 2003, 17, 118–120. [Google Scholar] [CrossRef]
- McLaren, D.G.; Sreenivasan, A.; Diamond, E.L.; Mitchell, M.B.; Van Dijk, K.R.; Deluca, A.N.; O’Brien, J.L.; Rentz, D.M.; Sperling, R.A.; Atri, A. Tracking cognitive change over 24 weeks with longitudinal functional magnetic resonance imaging in Alzheimer’s disease. Neurodegener. Dis. 2012, 9, 176–186. [Google Scholar] [CrossRef]
- Perovnik, M.; Tomše, P.; Jamšek, J.; Emeršič, A.; Tang, C.; Eidelberg, D.; Trošt, M. Identification and validation of Alzheimer’s disease-related metabolic brain pattern in biomarker confirmed Alzheimer’s dementia patients. Sci. Rep. 2022, 12, 11752. [Google Scholar] [CrossRef]
- Bianchini, F.; Di Vita, A.; Palermo, L.; Piccardi, L.; Blundo, C.; Guariglia, C. A Selective Egocentric Topographical Working Memory Deficit in the Early Stages of Alzheimer’s Disease: A Preliminary Study. Am. J. Alzheimers Dis. Other Demen. 2014, 29, 749–754. [Google Scholar] [CrossRef]
- Mirino, P.; Pecchinenda, A.; Boccia, M.; Capirchio, A.; D’Antonio, F.; Guariglia, C. Cerebellum-Cortical Interaction in Spatial Navigation and Its Alteration in Dementias. Brain Sci. 2022, 12, 523. [Google Scholar] [CrossRef] [PubMed]
- Abyadeh, M.; Tofigh, N.; Hosseinian, S.; Hasan, M.; Amirkhani, A.; Fitzhenry, M.J.; Gupta, V.; Chitranshi, N.; Salekdeh, G.H.; Haynes, P.A.; et al. Key Genes and Biochemical Networks in Various Brain Regions Affected in Alzheimer’s Disease. Cells 2022, 11, 987. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; van Blitterswijk, M.; Allen, M.; Carrasquillo, M.M.; Reddy, J.S.; Wang, X.; Beach, T.G.; Dickson, D.W.; Ertekin-Taner, N.; Asmann, Y.W.; et al. TMEM106B haplotypes have distinct gene expression patterns in aged brain. Mol. Neurodegener. 2018, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Wegiel, J.; Wisniewski, H.M.; Dziewiatkowski, J.; Badmajew, E.; Tarnawski, M.; Reisberg, B.; Mlodzik, B.; De Leon, M.J.; Miller, D.C. Cerebellar atrophy in Alzheimer’s disease-clinicopathological correlations. Brain Res. 1999, 818, 41–50. [Google Scholar] [CrossRef]
- Guo, C.C.; Tan, R.; Hodges, J.R.; Hu, X.; Sami, S.; Hornberger, M. Network-Selective Vulnerability of the Human Cerebellum to Alzheimer’s Disease and Frontotemporal Dementia. Brain 2016, 139, 1527–1538. [Google Scholar] [CrossRef]
- Singh-Bains, M.K.; Linke, V.; Austria, M.D.R.; Tan, A.Y.S.; Scotter, E.L.; Mehrabi, N.F.; Faull, R.L.M.; Dragunow, M. Altered microglia and neurovasculature in the Alzheimer’s disease cerebellum. Neurobiol. Dis. 2019, 132, 104589. [Google Scholar] [CrossRef]
- Pellegrini, C.; Pirazzini, C.; Sala, C.; Sambati, L.; Yusipov, I.; Kalyakulina, A.; Ravaioli, F.; Kwiatkowska, K.M.; Durso, D.F.; Ivanchenko, M.; et al. A Meta-Analysis of Brain DNA Methylation Across Sex, Age, and Alzheimer’s Disease Points for Accelerated Epigenetic Aging in Neurodegeneration. Front. Aging Neurosci. 2021, 13, 639428. [Google Scholar] [CrossRef]
- Jacobs, H.I.L.; Hopkins, D.A.; Mayrhofer, H.C.; Bruner, E.; van Leeuwen, F.W.; Raaijmakers, W.; Schmahmann, J.D. The cerebellum in Alzheimer’s disease: Evaluating its role in cognitive decline. Brain 2018, 141, 37–47. [Google Scholar] [CrossRef]
- Cheron, G.; Ristori, D.; Marquez-Ruiz, J.; Cebolla, A.M.; Ris, L. Electrophysiological alterations of the Purkinje cells and deep cerebellar neurons in a mouse model of Alzheimer disease (electrophysiology on cerebellum of AD mice). Eur. J. Neurosci. 2022, 56, 5547–5563. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Hong, S.; De Schutter, E. Firing rate-dependent phase responses of Purkinje cells support transient oscillations. eLife 2020, 9, e60692. [Google Scholar] [CrossRef] [PubMed]
- Seidel, K.; Bouzrou, M.; Heidemann, N.; Krüger, R.; Schöls, L.; den Dunnen, W.F.A.; Korf, H.W.; Rüb, U. Involvement of the cerebellum in Parkinson disease and dementia with Lewy bodies. Ann. Neurol. 2017, 81, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Xuan, M.; Gu, Q.; Xu, X.; Huang, P.; Wang, N.; Shen, Z.; Xu, J.; Luo, W.; Zhang, M. Influence of regional iron on the motor impairments of Parkinson’s disease: A quantitative susceptibility mapping study. J. Magn. Reason. Imaging 2017, 45, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Su, W.; Li, S.; Li, C.; Wang, R.; Chen, M.; Chen, H. Cerebellar atrophy in different subtypes of Parkinson’s disease. J. Neurol. Sci. 2018, 392, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Hett, K.; Lyu, I.; Trujillo, P.; Lopez, A.M.; Aumann, M.; Larson, K.E.; Hedera, P.; Dawant, B.; Landman, B.A.; Claassen, D.O.; et al. Anatomical texture patterns identify cerebellar distinctions between essential tremor and Parkinson’s disease. Hum. Brain Mapp. 2021, 42, 2322–2331. [Google Scholar] [CrossRef] [PubMed]
- Gardoni, A.; Agosta, F.; Sarasso, E.; Basaia, S.; Canu, E.; Leocadi, M.; Castelnovo, V.; Tettamanti, A.; Volontè, M.A.; Filippi, M. Cerebellar alterations in Parkinson’s disease with postural instability and gait disorders. J. Neurol. 2023, 270, 1735–1744. [Google Scholar] [CrossRef]
- Lopez, A.M.; Trujillo, P.; Hernandez, A.B.; Lin, Y.C.; Kang, H.; Landman, B.A.; Englot, D.J.; Dawant, B.M.; Konrad, P.E.; Claassen, D.O. Structural Correlates of the Sensorimotor Cerebellum in Parkinson’s Disease and Essential Tremor. Mov. Disord. 2020, 35, 1181–1188. [Google Scholar] [CrossRef]
- Sadeghi, F.; Pötter-Nerger, M.; Grimm, K.; Gerloff, C.; Schulz, R.; Zittel, S. Smaller Cerebellar Lobule VIIb is Associated with Tremor Severity in Parkinson’s Disease. Cerebellum 2023. [Google Scholar] [CrossRef]
- Chen, Z.; He, C.; Zhang, P.; Cai, X.; Huang, W.; Chen, X.; Xu, M.; Wang, L.; Zhang, Y. Abnormal cerebellum connectivity patterns related to motor subtypes of Parkinson’s disease. J. Neural Transm. 2023, 130, 549–560. [Google Scholar] [CrossRef]
- Maiti, B.; Rawson, K.S.; Tanenbaum, A.B.; Koller, J.M.; Snyder, A.Z.; Campbell, M.C.; Earhart, G.M.; Perlmutter, J.S. Functional Connectivity of Vermis Correlates with Future Gait Impairments in Parkinson’s Disease. Mov. Disord. 2021, 36, 2559–2568. [Google Scholar] [CrossRef] [PubMed]
- Palmer, W.C.; Cholerton, B.A.; Zabetian, C.P.; Montine, T.J.; Grabowski, T.J.; Rane, S. Resting-State Cerebello-Cortical Dysfunction in Parkinson’s Disease. Front. Neurol. 2021, 11, 594213. [Google Scholar] [CrossRef]
- Basaia, S.; Agosta, F.; Francia, A.; Cividini, C.; Balestrino, R.; Stojkovic, T.; Stankovic, I.; Markovic, V.; Sarasso, E.; Gardoni, A.; et al. Cerebro-cerebellar motor networks in clinical subtypes of Parkinson’s disease. NPJ Park. Dis. 2022, 8, 113. [Google Scholar] [CrossRef]
- Bosch, T.J.; Groth, C.; Eldridge, T.A.; Gnimpieba, E.Z.; Baugh, L.A.; Singh, A. Altered Cerebellar Oscillations in Parkinson’s Disease Patients during Cognitive and Motor Tasks. Neuroscience 2021, 475, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Bosch, T.J.; Espinoza, A.I.; Singh, A. Cerebellar oscillatory dysfunction during lower-limb movement in Parkinson’s disease with freezing of gait. Brain Res. 2023, 1808, 148334. [Google Scholar] [CrossRef]
- Sako, W.; Abe, T.; Matsumoto, Y.; Nakamura, K.; Haji, S.; Osaki, Y.; Harada, M.; Izumi, Y. The Cerebellum Is a Common Key for Visuospatial Execution and Attention in Parkinson’s Disease. Diagnostics 2021, 11, 1042. [Google Scholar] [CrossRef] [PubMed]
- Blum, D.; la Fougère, C.; Pilotto, A.; Maetzler, W.; Berg, D.; Reimold, M.; Liepelt-Scarfone, I. Hypermetabolism in the cerebellum and brainstem and cortical hypometabolism are independently associated with cognitive impairment in Parkinson’s disease. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2387–2395. [Google Scholar] [CrossRef]
- Riou, A.; Houvenaghel, J.F.; Dondaine, T.; Drapier, S.; Sauleau, P.; Drapier, D.; Duprez, J.; Guillery, M.; Le Jeune, F.; Verin, M.; et al. Functional Role of the Cerebellum in Parkinson Disease: A PET Study. Neurology 2021, 96, e2874–e2884. [Google Scholar] [CrossRef]
- Padron-Rivera, G.; Diaz, R.; Vaca-Palomares, I.; Ochoa, A.; Hernandez-Castillo, C.R.; Fernandez-Ruiz, J. Cerebellar Degeneration Signature in Huntington’s Disease. Cerebellum 2021, 20, 942–945. [Google Scholar] [CrossRef]
- Fennema-Notestine, C.; Archibald, S.L.; Jacobson, M.W.; Corey-Bloom, J.; Paulsen, J.S.; Peavy, G.M.; Gamst, A.C.; Hamilton, J.M.; Salmon, D.P.; Jernigan, T.L. In vivo evidence of cerebellar atrophy and cerebral white matter loss in Huntington disease. Neurology 2004, 63, 989–995. [Google Scholar] [CrossRef]
- Singh-Bains, M.K.; Mehrabi, N.F.; Sehji, T.; Austria, M.D.R.; Tan, A.Y.S.; Tippett, L.J.; Dragunow, M.; Waldvogel, H.J.; Faull, R.L.M. Cerebellar degeneration correlates with motor symptoms in Huntington disease. Ann. Neurol. 2019, 85, 396–405. [Google Scholar] [CrossRef]
- Ishikawa, A.; Oyanagi, K.; Tanaka, K.; Igarashi, S.; Sato, T.; Tsuji, S. A non-familial Huntington’s disease patient with grumose degeneration in the dentate nucleus. Acta Neurol. Scand. 1999, 99, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.; Chen, C.Y.; Jonson, M.; Kaczmarczyk, L.; Magadi, S.S.; Jackson, W.S. Cerebellar granule neurons induce Cyclin D1 before the onset of motor symptoms in Huntington’s disease mice. Acta Neuropathol. Commun. 2023, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Chipika, R.H.; Mulkerrin, G.; Pradat, P.F.; Murad, A.; Ango, F.; Raoul, C.; Bede, P. Cerebellar pathology in motor neuron disease: Neuroplasticity and neurodegeneration. Neural Regen Res. 2022, 17, 2335–2341. [Google Scholar] [CrossRef]
- Kabiljo, R.; Iacoangeli, A.; Al-Chalabi, A.; Rosenzweig, I. Amyotrophic lateral sclerosis and cerebellum. Sci. Rep. 2022, 12, 12586. [Google Scholar] [CrossRef] [PubMed]
- Bede, P.; Chipika, R.H.; Christidi, F.; Hengeveld, J.C.; Karavasilis, E.; Argyropoulos, G.D.; Lope, J.; Li Hi Shing, S.; Velonakis, G.; Dupuis, L.; et al. Genotype-associated cerebellar profiles in ALS: Focal cerebellar pathology and cerebro-cerebellar connectivity alterations. J. Neurol. Neurosurg. Psychiatry 2021, 92, 1197–1205. [Google Scholar] [CrossRef] [PubMed]
- Khan, W.; Corben, L.A.; Bilal, H.; Vivash, L.; Delatycki, M.B.; Egan, G.F.; Harding, I.H. Neuroinflammation in the Cerebellum and Brainstem in Friedreich Ataxia: An [18F]-FEMPA PET Study. Mov. Disord. 2022, 37, 218–224. [Google Scholar] [CrossRef]
- Lindig, T.; Bender, B.; Kumar, V.J.; Hauser, T.K.; Grodd, W.; Brendel, B.; Just, J.; Synofzik, M.; Klose, U.; Scheffler, K.; et al. Pattern of Cerebellar Atrophy in Friedreich’s Ataxia-Using the SUIT Template. Cerebellum 2019, 18, 435–447. [Google Scholar] [CrossRef]
- Cocozza, S.; Costabile, T.; Pontillo, G.; Lieto, M.; Russo, C.; Radice, L.; Pane, C.; Filla, A.; Brunetti, A.; Saccà, F. Cerebellum and cognition in Friedreich ataxia: A voxel-based morphometry and volumetric MRI study. J. Neurol. 2020, 267, 350–358. [Google Scholar] [CrossRef]
- Kerestes, R.; Cummins, H.; Georgiou-Karistianis, N.; Selvadurai, L.P.; Corben, L.A.; Delatycki, M.B.; Egan, G.F.; Harding, I.H. Reduced cerebello-cerebral functional connectivity correlates with disease severity and impaired white matter integrity in Friedreich ataxia. J. Neurol. 2023, 270, 2360–2369. [Google Scholar] [CrossRef]
- Mercado-Ayón, E.; Warren, N.; Halawani, S.; Rodden, L.N.; Ngaba, L.; Dong, Y.N.; Chang, J.C.; Fonck, C.; Mavilio, F.; Lynch, D.R.; et al. Cerebellar Pathology in an Inducible Mouse Model of Friedreich Ataxia. Front. Neurosci. 2022, 16, 819569. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, H.P.; Härle, M.; Koelfen, W.; Nissen, K.-H. Childhood progressive spinal muscular atrophy with facioscapulo-humeral predominance, sensory and autonomic involvement and optic atrophy. Brain Dev. 1994, 16, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Harding, B.N.; Kariya, S.; Monani, U.R.; Chung, W.K.; Benton, M.; Yum, S.W.; Tennekoon, G.; Finkel, R.S. Spectrum of neuropathophysiology in spinal muscular atrophy type I. J. Neuropathol. Exp. Neurol. 2015, 74, 15–24. [Google Scholar] [CrossRef] [PubMed]
- de Borba, F.C.; Querin, G.; França, M.C., Jr.; Pradat, P.F. Cerebellar degeneration in adult spinal muscular atrophy patients. J. Neurol. 2020, 267, 2625–2631. [Google Scholar] [CrossRef] [PubMed]
- Tharaneetharan, A.; Cole, M.; Norman, B.; Romero, N.C.; Wooltorton, J.R.A.; Harrington, M.A.; Sun, J. Functional Abnormalities of Cerebellum and Motor Cortex in Spinal Muscular Atrophy Mice. Neuroscience 2021, 452, 78–97. [Google Scholar] [CrossRef] [PubMed]
- Studniarczyk, D.; Needham, E.L.; Mitchison, H.M.; Farrant, M.; Cull-Candy, S.G. Altered Cerebellar Short-Term Plasticity but No Change in Postsynaptic AMPA-Type Glutamate Receptors in a Mouse Model of Juvenile Batten Disease. Eneuro 2018, 5, ENEURO.0387-17.2018. [Google Scholar] [CrossRef]
- Nardocci, N.; Verga, M.L.; Binelli, S.; Zorzi, G.; Angelini, L.; Bugiani, O. Neuronal ceroid-lipofuscinosis: A clinical and morphological study of 19 patients. Am. J. Med. Genet. 1995, 57, 137–141. [Google Scholar] [CrossRef]
- Raininko, R.; Santavuori, P.; Heiskala, H.; Sainio, K.; Palo, J. CT findings in neuronal ceroid lipofuscinoses. Neuropediatrics 1990, 21, 95–101. [Google Scholar] [CrossRef]
- Kovács, A.D.; Weimer, J.M.; Pearce, D.A. Selectively increased sensitivity of cerebellar granule cells to AMPA receptor-mediated excitotoxicity in a mouse model of Batten disease. Neurobiol. Dis. 2006, 22, 575–585. [Google Scholar] [CrossRef]
- Weimer, J.M.; Benedict, J.W.; Getty, A.L.; Pontikis, C.C.; Lim, M.J.; Cooper, J.D.; Pearce, D.A. Cerebellar defects in a mouse model of juvenile neuronal ceroid lipofuscinosis. Brain Res. 2009, 1266, 93–107. [Google Scholar] [CrossRef]
- Tommasin, S.; Iakovleva, V.; Rocca, M.A.; Giannì, C.; Tedeschi, G.; De Stefano, N.; Pozzilli, C.; Filippi, M.; Pantano, P. INNI Network. Relation of sensorimotor and cognitive cerebellum functional connectivity with brain structural damage in patients with multiple sclerosis and no disability. Eur. J. Neurol. 2022, 29, 2036–2046. [Google Scholar] [CrossRef] [PubMed]
- Rota, V.; Perucca, L.; Simone, A.; Tesio, L. Walk ratio (step length/cadence) as a summary index of neuromotor control of gait: Application to multiple sclerosis. Int. J. Rehabil. Res. 2011, 34, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Kalron, A.; Menascu, S.; Givon, U.; Dolev, M.; Achiron, A. Is the walk ratio a window to the cerebellum in multiple sclerosis? A structural magnetic resonance imaging study. Eur. J. Neurol. 2020, 27, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Bonacchi, R.; Meani, A.; Pagani, E.; Marchesi, O.; Filippi, M.; Rocca, M.A. The role of cerebellar damage in explaining disability and cognition in multiple sclerosis phenotypes: A multiparametric MRI study. J. Neurol. 2022, 269, 3841–3857. [Google Scholar] [CrossRef] [PubMed]
- Schoonheim, M.M.; Douw, L.; Broeders, T.A.; Eijlers, A.J.; Meijer, K.A.; Geurts, J.J. The cerebellum and its network: Disrupted static and dynamic functional connectivity patterns and cognitive impairment in multiple sclerosis. Mult. Scler. 2021, 27, 2031–2039. [Google Scholar] [CrossRef] [PubMed]
- Gera, G.; Fling, B.W.; Horak, F.B. Cerebellar White Matter Damage Is Associated With Postural Sway Deficits in People With Multiple Sclerosis. Arch. Phys. Med. Rehabil. 2020, 101, 258–264. [Google Scholar] [CrossRef]
- Prosperini, L.; Fanelli, F.; Petsas, N.; Sbardella, E.; Tona, F.; Raz, E.; Fortuna, D.; De Angelis, F.; Pozzilli, C.; Pantano, P. Multiple sclerosis: Changes in microarchitecture of white matter tracts after training with a video game balance board. Radiology 2014, 273, 529–538. [Google Scholar] [CrossRef]
- Sui, R.; Zhang, L. Cerebellar dysfunction may play an important role in vascular dementia. Med. Hypotheses 2012, 78, 162–165. [Google Scholar] [CrossRef]
- Schaefer, A.; Quinque, E.M.; Kipping, J.A.; Arélin, K.; Roggenhofer, E.; Frisch, S.; Villringer, A.; Mueller, K.; Schroeter, M.L. Early small vessel disease affects frontoparietal and cerebellar hubs in close correlation with clinical symptoms—A resting-state fMRI study. J. Cereb. Blood Flow. Metab. 2014, 34, 1091–1095. [Google Scholar] [CrossRef]
- Ruan, Z.; Gao, L.; Li, S.; Yu, M.; Rao, B.; Sun, W.; Zhou, X.; Li, Y.; Song, X.; Xu, H. Functional abnormalities of the cerebellum in vascular mild cognitive impairment. Brain Imaging Behav. 2023, 17, 530–540. [Google Scholar] [CrossRef]
- Acharya, A.; Ren, P.; Yi, L.; Tian, W.; Liang, X. Structural atrophy and functional dysconnectivity patterns in the cerebellum relate to cerebral networks in svMCI. Front. Neurosci. 2023, 16, 1006231. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, N.T.; Decarli, C. Vascular dementia: Emerging trends. Semin. Neurol. 2007, 27, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Palesi, F.; De Rinaldis, A.; Vitali, P.; Castellazzi, G.; Casiraghi, L.; Germani, G.; Bernini, S.; Anzalone, N.; Ramusino, M.C.; Denaro, F.M.; et al. Specific Patterns of White Matter Alterations Help Distinguishing Alzheimer’s and Vascular Dementia. Front. Neurosci. 2018, 12, 274. [Google Scholar] [CrossRef]
- Yoon, C.W.; Seo, S.W.; Park, J.S.; Kwak, K.C.; Yoon, U.; Suh, M.K.; Kim, G.H.; Shin, J.S.; Kim, C.H.; Noh, Y.; et al. Cerebellar atrophy in patients with subcortical-type vascular cognitive impairment. Cerebellum 2013, 12, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Pantel, J.; Schröder, J.; Essig, M.; Jauss, M.; Schneider, G.; Eysenbach, K.; von Kummer, R.; Baudendistel, K.; Schad, L.R.; Knopp, M.V. In vivo quantification of brain volumes in subcortical vascular dementia and Alzheimer’s disease. An MRI-based study. Dement. Geriatr. Cogn. Disord. 1998, 9, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Meguro, K.; Yamaguchi, S.; Yamazaki, H.; Itoh, M.; Yamaguchi, T.; Matsui, H.; Sasaki, H. Cortical glucose metabolism in psychiatric wandering patients with vascular dementia. Psychiatry Res. 1996, 67, 71–80. [Google Scholar] [CrossRef]
- Baloyannis, S.J. Pathological alterations of the climbing fibres of the cerebellum in vascular dementia: A Golgi and electron microscope study. J. Neurol. Sci. 2007, 257, 56–61. [Google Scholar] [CrossRef]
- Mielke, R.; Herholz, K.; Grond, M.; Kessler, J.; Heiss, W. Severity of Vascular Dementia Is Related to Volume of Metabolically Impaired Tissue. Arch. Neurol. 1992, 49, 909–913. [Google Scholar] [CrossRef]
- De Reuck, J.L.; Deramecourt, V.; Auger, F.; Durieux, N.; Cordonnier, C.; Devos, D.; Defebvre, L.; Moreau, C.; Capparos-Lefebvre, D.; Pasquier, F.; et al. The significance of cortical cerebellar microbleeds and microinfarcts in neurodegenerative and cerebrovascular diseases. A post-mortem 7.0-tesla magnetic resonance study with neuropathological correlates. Cerebrovasc. Dis. 2015, 39, 138–143. [Google Scholar] [CrossRef]
- Poh, L.; Razak, S.M.B.A.; Lim, H.M.; Lai, M.K.P.; Chen, C.L.; Lim, L.H.K.; Arumugam, T.V.; Fann, D.Y. AIM2 inflammasome mediates apoptotic and pyroptotic death in the cerebellum following chronic hypoperfusion. Exp. Neurol. 2021, 346, 113856. [Google Scholar] [CrossRef]
- Cui, Y.; Jin, X.; Choi, D.J.; Choi, J.Y.; Kim, H.S.; Hwang, D.H.; Kim, B.G. Axonal degeneration in an in vitro model of ischemic white matter injury. Neurobiol. Dis. 2020, 134, 104672. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iskusnykh, I.Y.; Zakharova, A.A.; Kryl’skii, E.D.; Popova, T.N. Aging, Neurodegenerative Disorders, and Cerebellum. Int. J. Mol. Sci. 2024, 25, 1018. https://doi.org/10.3390/ijms25021018
Iskusnykh IY, Zakharova AA, Kryl’skii ED, Popova TN. Aging, Neurodegenerative Disorders, and Cerebellum. International Journal of Molecular Sciences. 2024; 25(2):1018. https://doi.org/10.3390/ijms25021018
Chicago/Turabian StyleIskusnykh, Igor Y., Anastasia A. Zakharova, Evgenii D. Kryl’skii, and Tatyana N. Popova. 2024. "Aging, Neurodegenerative Disorders, and Cerebellum" International Journal of Molecular Sciences 25, no. 2: 1018. https://doi.org/10.3390/ijms25021018
APA StyleIskusnykh, I. Y., Zakharova, A. A., Kryl’skii, E. D., & Popova, T. N. (2024). Aging, Neurodegenerative Disorders, and Cerebellum. International Journal of Molecular Sciences, 25(2), 1018. https://doi.org/10.3390/ijms25021018





