Comparative Transcriptomic Analysis of Cerebellar Astrocytes across Developmental Stages and Brain Regions
Abstract
1. Introduction
2. Results
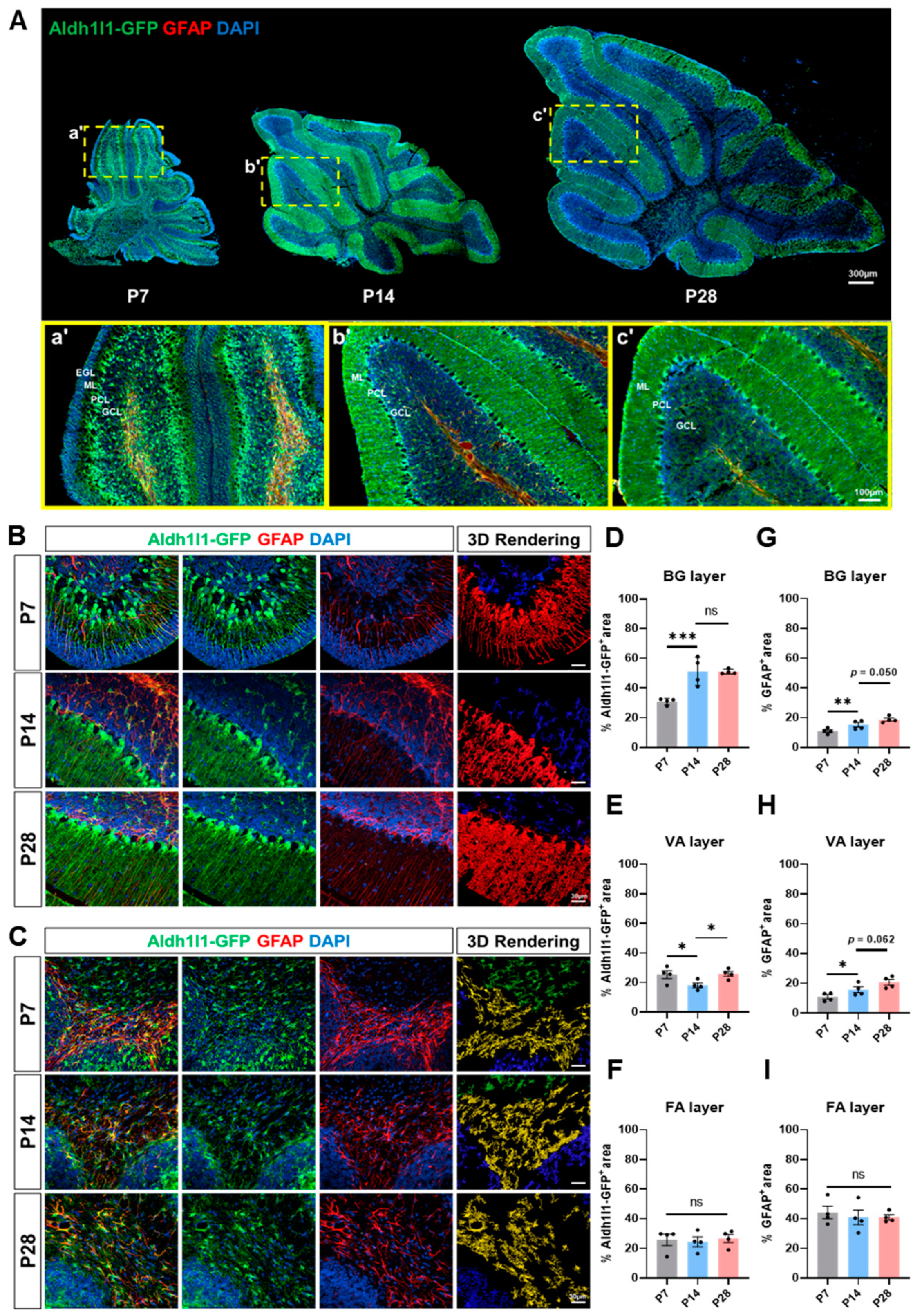
2.1. Dynamic Astrocyte Marker Expression Changes during Cerebellar Development
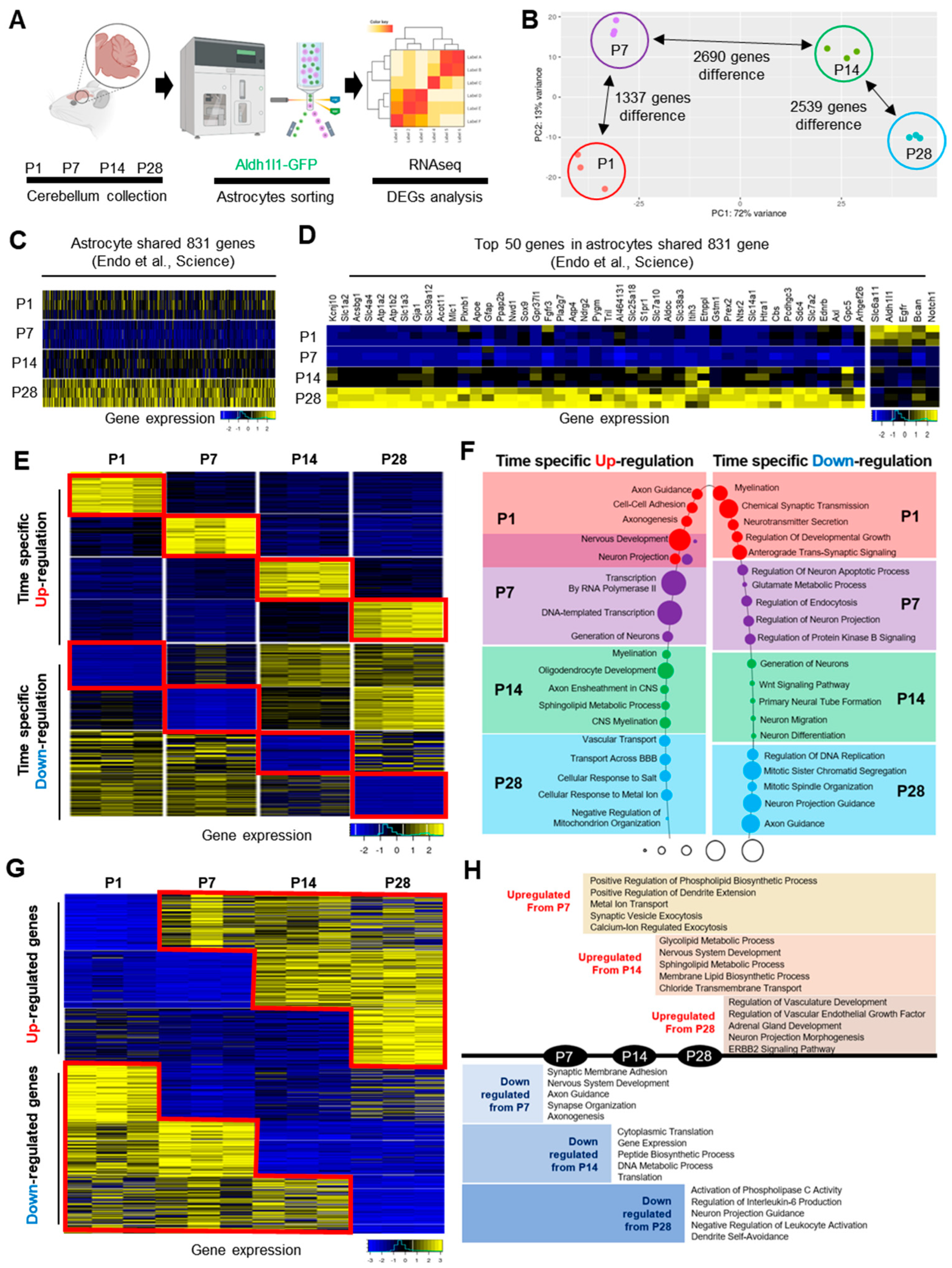
2.2. Stage-Specific Astrocytic Gene Expression Signatures during Postnatal Cerebellar Development
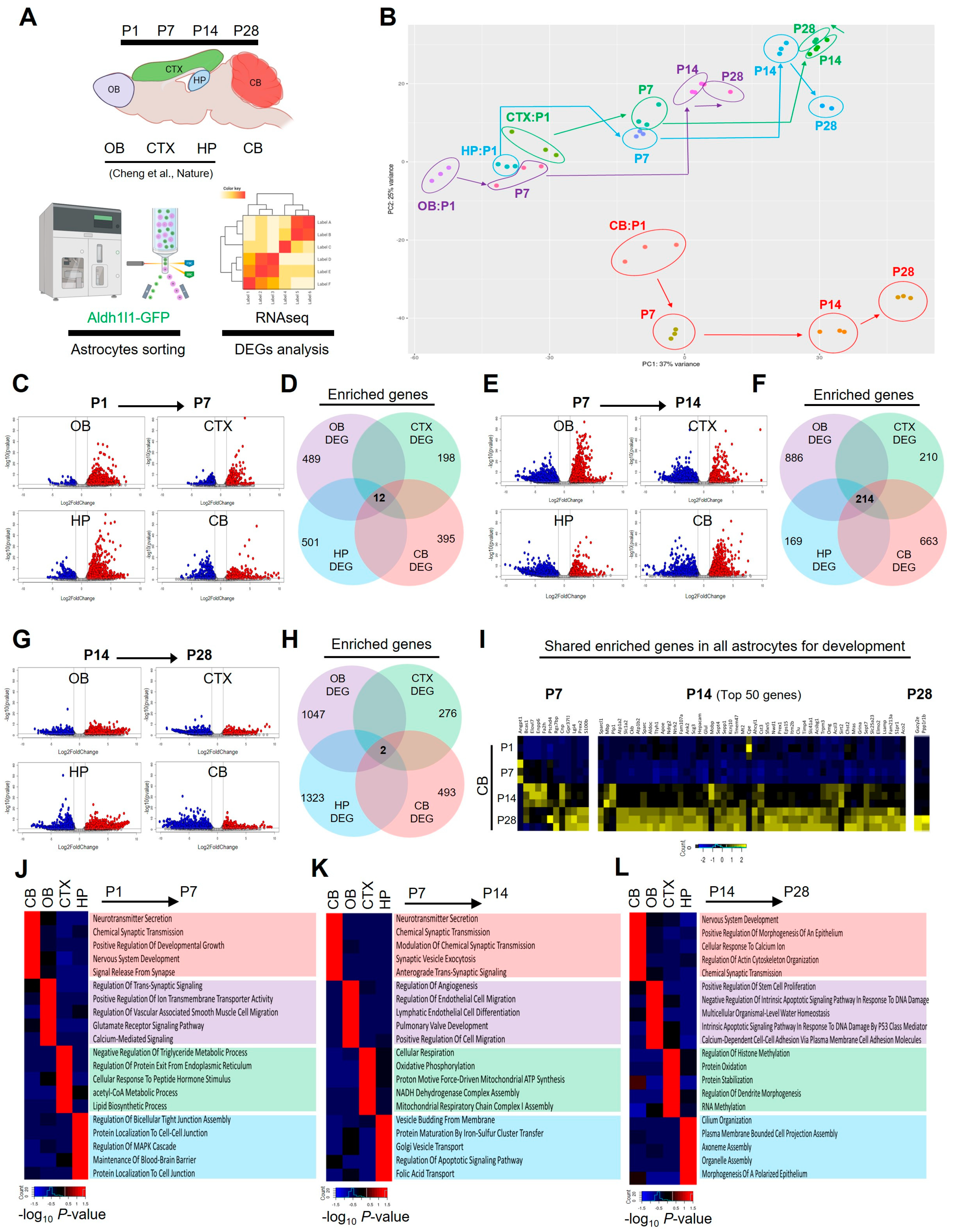
2.3. Region-Specific Astrocyte-Enriched Gene Signatures during Postnatal Development
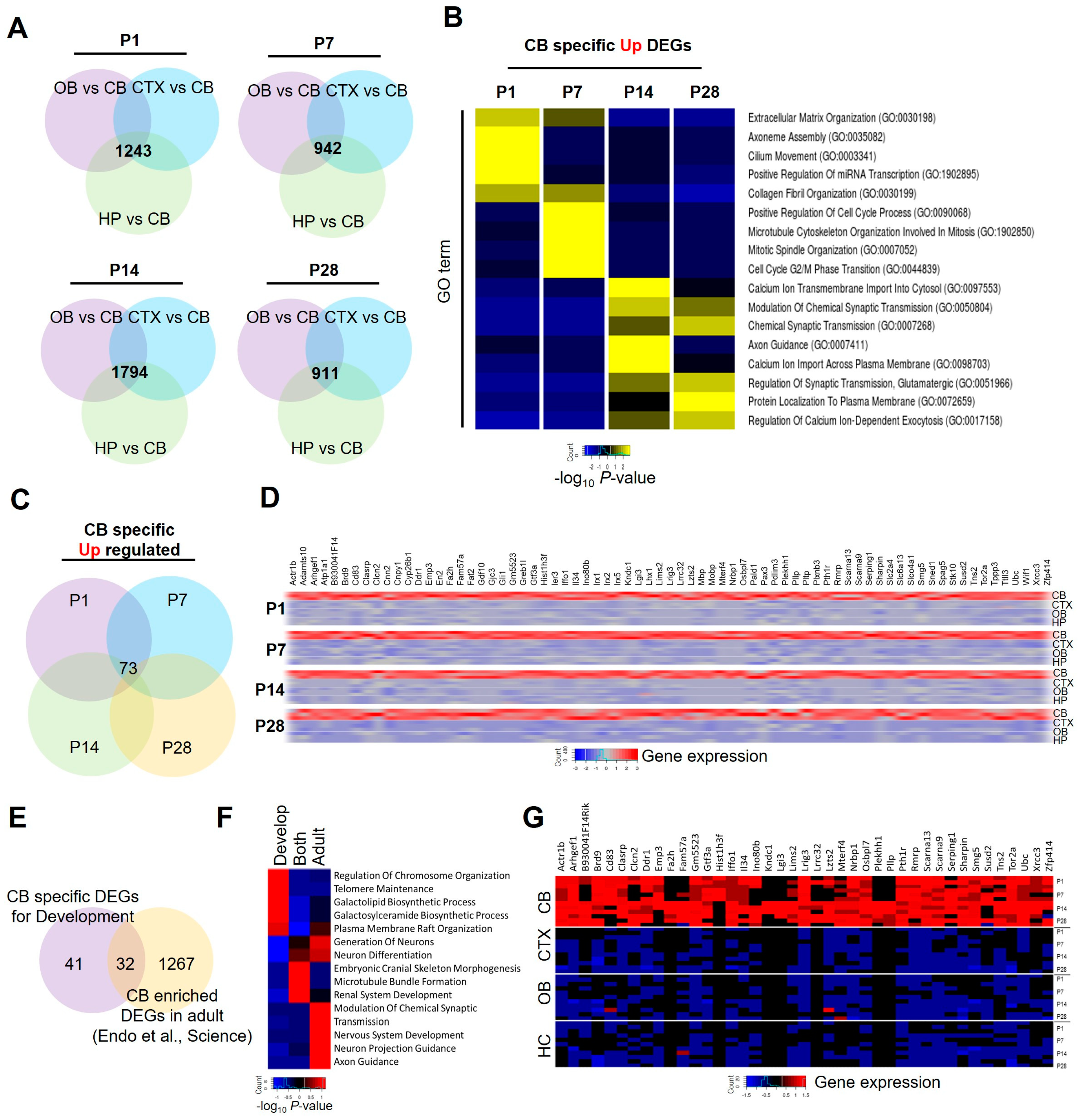
2.4. Identification of Cerebellar-Specific Enriched Astrocyte Gene Signatures during Postnatal Development
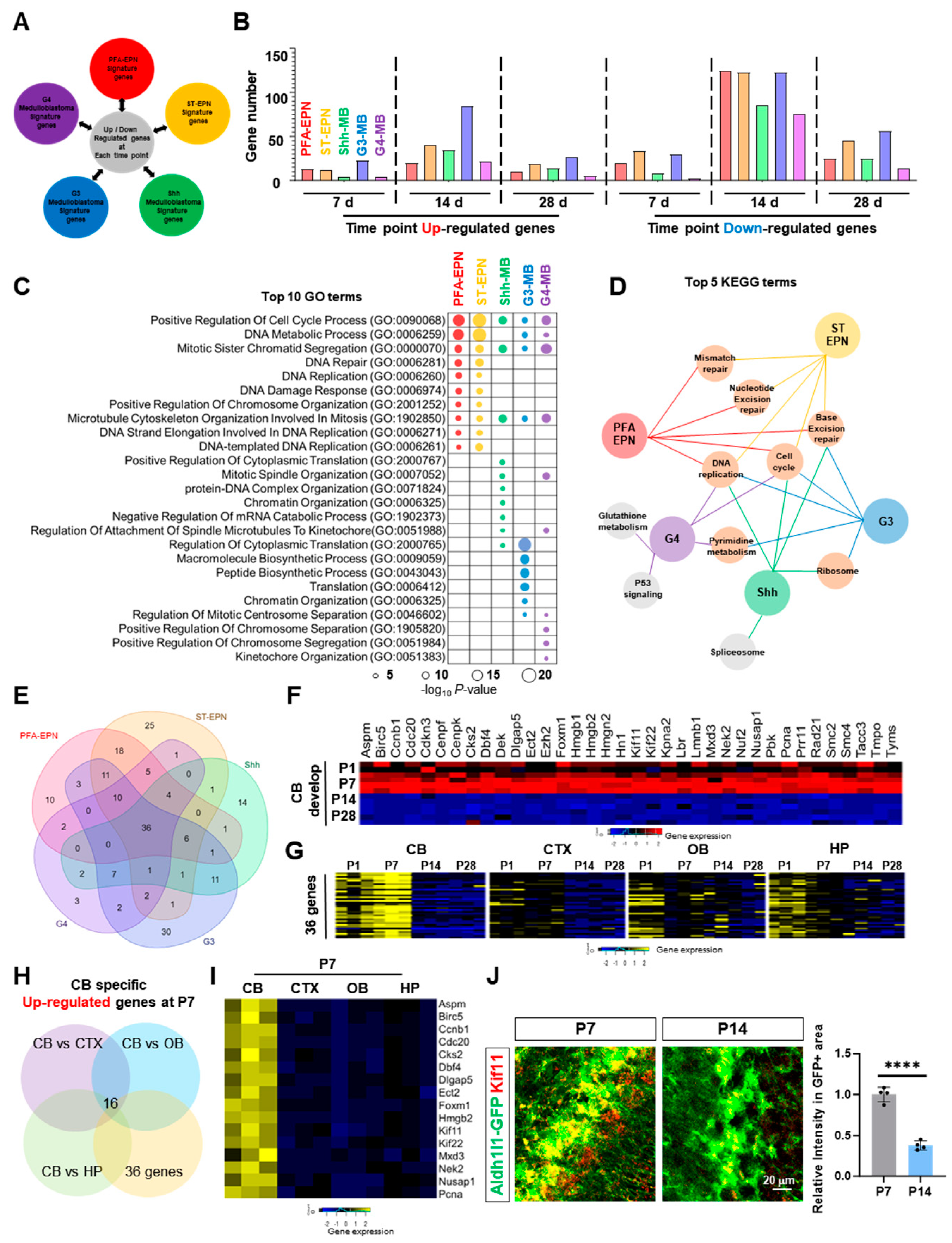
2.5. Identification of Cerebellar-Specific Gene Signatures Expressed in Pediatric Brain Tumors
3. Discussion
3.1. Distinct and Conserved Gene Signatures in the Transcriptome of Cerebellar Astrocytes during Development
3.2. Temporal Cerebellar Astrocyte Gene Signature: Implications for Pediatric Brain Tumors of Cerebellar Origin
4. Materials and Methods
4.1. Animals
4.2. Tissue Preparation
4.3. Immunofluorescent Staining
4.4. Confocal Imaging and Image Analysis
4.5. FACS Sorting
4.6. RNA Extraction and Library Preparation
4.7. RNA Sequencing
4.8. Bioinformatics Analysis
4.9. Statistical Analysis
4.10. Data Availability
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haim, L.B.; Rowitch, D.H. Functional Diversity of Astrocytes in Neural Circuit Regulation. Nat. Rev. Neurosci. 2017, 18, 31–41. [Google Scholar] [CrossRef]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte–Endothelial Interactions at the Blood–Brain Barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Dallérac, G.; Zapata, J.; Rouach, N. Versatile Control of Synaptic Circuits by Astrocytes: Where, When and How? Nat. Rev. Neurosci. 2018, 19, 729–743. [Google Scholar] [CrossRef]
- Khakh, B.S.; Deneen, B. The Emerging Nature of Astrocyte Diversity. Annu. Rev. Neurosci. 2019, 42, 187–207. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-T.; Woo, J.; Deneen, B. Sculpting Astrocyte Diversity through Circuits and Transcription. Neuroscientist 2023, 29, 445–460. [Google Scholar] [CrossRef] [PubMed]
- Cahoy, J.D.; Emery, B.; Kaushal, A.; Foo, L.C.; Zamanian, J.L.; Christopherson, K.S.; Xing, Y.; Lubischer, J.L.; Krieg, P.A.; Krupenko, S.A.; et al. A Transcriptome Database for Astrocytes, Neurons, and Oligodendrocytes: A New Resource for Understanding Brain Development and Function. J. Neurosci. 2008, 28, 264–278. [Google Scholar] [CrossRef]
- John Lin, C.-C.; Yu, K.; Hatcher, A.; Huang, T.-W.; Lee, H.K.; Carlson, J.; Weston, M.C.; Chen, F.; Zhang, Y.; Zhu, W.; et al. Identification of Diverse Astrocyte Populations and Their Malignant Analogs. Nat. Neurosci. 2017, 20, 396–405. [Google Scholar] [CrossRef]
- Lozzi, B.; Huang, T.-W.; Sardar, D.; Huang, A.Y.-S.; Deneen, B. Regionally Distinct Astrocytes Display Unique Transcription Factor Profiles in the Adult Brain. Front. Neurosci. 2020, 14, 61. [Google Scholar] [CrossRef]
- Cuevas-Diaz Duran, R.; Wang, C.-Y.; Zheng, H.; Deneen, B.; Wu, J.Q. Brain Region-Specific Gene Signatures Revealed by Distinct Astrocyte Subpopulations Unveil Links to Glioma and Neurodegenerative Diseases. eNeuro 2019, 6, ENEURO.0288-18.2019. [Google Scholar] [CrossRef]
- Endo, F.; Kasai, A.; Soto, J.S.; Yu, X.; Qu, Z.; Hashimoto, H.; Gradinaru, V.; Kawaguchi, R.; Khakh, B.S. Molecular Basis of Astrocyte Diversity and Morphology across the CNS in Health and Disease. Science 2022, 378, eadc9020. [Google Scholar] [CrossRef]
- Sathyanesan, A.; Zhou, J.; Scafidi, J.; Heck, D.H.; Sillitoe, R.V.; Gallo, V. Emerging Connections between Cerebellar Development, Behaviour and Complex Brain Disorders. Nat. Rev. Neurosci. 2019, 20, 298–313. [Google Scholar] [CrossRef] [PubMed]
- Buckner, R.L. The Cerebellum and Cognitive Function: 25 Years of Insight from Anatomy and Neuroimaging. Neuron 2013, 80, 807–815. [Google Scholar] [CrossRef]
- Stoodley, C.J.; Valera, E.M.; Schmahmann, J.D. Functional Topography of the Cerebellum for Motor and Cognitive Tasks: An fMRI Study. NeuroImage 2012, 59, 1560–1570. [Google Scholar] [CrossRef] [PubMed]
- Leto, K.; Arancillo, M.; Becker, E.B.E.; Buffo, A.; Chiang, C.; Ding, B.; Dobyns, W.B.; Dusart, I.; Haldipur, P.; Hatten, M.E.; et al. Consensus Paper: Cerebellar Development. Cerebellum 2016, 15, 789–828. [Google Scholar] [CrossRef] [PubMed]
- Cerrato, V. Cerebellar Astrocytes: Much More Than Passive Bystanders In Ataxia Pathophysiology. J. Clin. Med. 2020, 9, 757. [Google Scholar] [CrossRef]
- Haldipur, P.; Millen, K.J.; Aldinger, K.A. Human Cerebellar Development and Transcriptomics: Implications for Neurodevelopmental Disorders. Annu. Rev. Neurosci. 2022, 45, 515–531. [Google Scholar] [CrossRef]
- Ito, M. Control of Mental Activities by Internal Models in the Cerebellum. Nat. Rev. Neurosci. 2008, 9, 304–313. [Google Scholar] [CrossRef]
- Woo, J.; Min, J.O.; Kang, D.-S.; Kim, Y.S.; Jung, G.H.; Park, H.J.; Kim, S.; An, H.; Kwon, J.; Kim, J.; et al. Control of Motor Coordination by Astrocytic Tonic GABA Release through Modulation of Excitation/Inhibition Balance in Cerebellum. Proc. Natl. Acad. Sci. USA 2018, 115, 5004–5009. [Google Scholar] [CrossRef]
- Li, C.; Saliba, N.B.; Martin, H.; Losurdo, N.A.; Kolahdouzan, K.; Siddiqui, R.; Medeiros, D.; Li, W. Purkinje Cell Dopaminergic Inputs to Astrocytes Regulate Cerebellar-Dependent Behavior. Nat. Commun. 2023, 14, 1613. [Google Scholar] [CrossRef]
- Kanner, S.; Goldin, M.; Galron, R.; Ben Jacob, E.; Bonifazi, P.; Barzilai, A. Astrocytes Restore Connectivity and Synchronization in Dysfunctional Cerebellar Networks. Proc. Natl. Acad. Sci. USA 2018, 115, 8025–8030. [Google Scholar] [CrossRef]
- Buffo, A.; Rossi, F. Origin, Lineage and Function of Cerebellar Glia. Prog. Progress. Neurobiol. 2013, 109, 42–63. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-T.; Luna-Figueroa, E.; Woo, J.; Chen, H.-C.; Lee, Z.-F.; Harmanci, A.S.; Deneen, B. Inhibitory Input Directs Astrocyte Morphogenesis through Glial GABABR. Nature 2023, 617, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Vladoiu, M.C.; El-Hamamy, I.; Donovan, L.K.; Farooq, H.; Holgado, B.L.; Sundaravadanam, Y.; Ramaswamy, V.; Hendrikse, L.D.; Kumar, S.; Mack, S.C.; et al. Childhood Cerebellar Tumours Mirror Conserved Fetal Transcriptional Programs. Nature 2019, 572, 67–73. [Google Scholar] [CrossRef]
- Pajtler, K.W.; Witt, H.; Sill, M.; Jones, D.T.W.; Hovestadt, V.; Kratochwil, F.; Wani, K.; Tatevossian, R.; Punchihewa, C.; Johann, P.; et al. Molecular Classification of Ependymal Tumors across All CNS Compartments, Histopathological Grades, and Age Groups. Cancer Cell 2015, 27, 728–743. [Google Scholar] [CrossRef] [PubMed]
- Northcott, P.A.; Robinson, G.W.; Kratz, C.P.; Mabbott, D.J.; Pomeroy, S.L.; Clifford, S.C.; Rutkowski, S.; Ellison, D.W.; Malkin, D.; Taylor, M.D.; et al. Medulloblastoma. Nat. Rev. Dis. Primers 2019, 5, 11. [Google Scholar] [CrossRef]
- Gojo, J.; Englinger, B.; Jiang, L.; Hübner, J.M.; Shaw, M.L.; Hack, O.A.; Madlener, S.; Kirchhofer, D.; Liu, I.; Pyrdol, J.; et al. Single-Cell RNA-Seq Reveals Cellular Hierarchies and Impaired Developmental Trajectories in Pediatric Ependymoma. Cancer Cell 2020, 38, 44–59. [Google Scholar] [CrossRef] [PubMed]
- Nagai, J.; Yu, X.; Papouin, T.; Cheong, E.; Freeman, M.R.; Monk, K.R.; Hastings, M.H.; Haydon, P.G.; Rowitch, D.; Shaham, S.; et al. Behaviorally Consequential Astrocytic Regulation of Neural Circuits. Neuron 2021, 109, 576–596. [Google Scholar] [CrossRef]
- Chai, H.; Diaz-Castro, B.; Shigetomi, E.; Monte, E.; Octeau, J.C.; Yu, X.; Cohn, W.; Rajendran, P.S.; Vondriska, T.M.; Whitelegge, J.P.; et al. Neural Circuit-Specialized Astrocytes: Transcriptomic, Proteomic, Morphological, and Functional Evidence. Neuron 2017, 95, 531–549. [Google Scholar] [CrossRef]
- Yu, X.; Nagai, J.; Marti-Solano, M.; Soto, J.S.; Coppola, G.; Babu, M.M.; Khakh, B.S. Context-Specific Striatal Astrocyte Molecular Responses Are Phenotypically Exploitable. Neuron 2020, 108, 1146–1162.e10. [Google Scholar] [CrossRef]
- Morel, L.; Chiang, M.S.R.; Higashimori, H.; Shoneye, T.; Iyer, L.K.; Yelick, J.; Tai, A.; Yang, Y. Molecular and Functional Properties of Regional Astrocytes in the Adult Brain. J. Neurosci. 2017, 37, 8706–8717. [Google Scholar] [CrossRef]
- Tsai, H.-H.; Li, H.; Fuentealba, L.C.; Molofsky, A.V.; Taveira-Marques, R.; Zhuang, H.; Tenney, A.; Murnen, A.T.; Fancy, S.P.J.; Merkle, F.; et al. Regional Astrocyte Allocation Regulates CNS Synaptogenesis and Repair. Science 2012, 337, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Molofsky, A.V.; Kelley, K.W.; Tsai, H.-H.; Redmond, S.A.; Chang, S.M.; Madireddy, L.; Chan, J.R.; Baranzini, S.E.; Ullian, E.M.; Rowitch, D.H. Astrocyte-Encoded Positional Cues Maintain Sensorimotor Circuit Integrity. Nature 2014, 509, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Patapoutian, A.; Reichardt, L.F. Roles of Wnt Proteins in Neural Development and Maintenance. Curr. Opin. Neurobiol. 2000, 10, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Cerminara, N.L.; Lang, E.J.; Sillitoe, R.V.; Apps, R. Redefining the Cerebellar Cortex as an Assembly of Non-Uniform Purkinje Cell Microcircuits. Nat. Rev. Neurosci. 2015, 16, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Van Essen, M.J.; Nayler, S.; Becker, E.B.E.; Jacob, J. Deconstructing Cerebellar Development Cell by Cell. PLoS Genet. 2020, 16, e1008630. [Google Scholar] [CrossRef] [PubMed]
- Edamakanti, C.R.; Do, J.; Didonna, A.; Martina, M.; Opal, P. Mutant Ataxin1 Disrupts Cerebellar Development in Spinocerebellar Ataxia Type 1. J. Clin. Investig. 2018, 128, 2252–2265. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.-H.; Kloth, A.D.; Badura, A. The Cerebellum, Sensitive Periods, and Autism. Neuron 2014, 83, 518–532. [Google Scholar] [CrossRef]
- The FANTOM Consortium; Ha, T.J.; Zhang, P.G.Y.; Robert, R.; Yeung, J.; Swanson, D.J.; Mathelier, A.; Wasserman, W.W.; Im, S.; Itoh, M.; et al. Identification of Novel Cerebellar Developmental Transcriptional Regulators with Motif Activity Analysis. BMC Genomics 2019, 20, 718. [Google Scholar] [CrossRef]
- Jun, S.; Kim, M.; Park, H.; Hwang, E.; Yamamoto, Y.; Tanaka-Yamamoto, K. Organization of Purkinje Cell Development by Neuronal MEGF11 in Cerebellar Granule Cells. Cell Reports 2023, 42, 113137. [Google Scholar] [CrossRef]
- Jenseit, A.; Camgöz, A.; Pfister, S.M.; Kool, M. EZHIP: A New Piece of the Puzzle towards Understanding Pediatric Posterior Fossa Ependymoma. Acta Neuropathol. 2022, 143, 1–13. [Google Scholar] [CrossRef]
- Taylor, M.D.; Poppleton, H.; Fuller, C.; Su, X.; Liu, Y.; Jensen, P.; Magdaleno, S.; Dalton, J.; Calabrese, C.; Board, J.; et al. Radial Glia Cells Are Candidate Stem Cells of Ependymoma. Cancer Cell 2005, 8, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.S.; Bihannic, L.; Gudenas, B.L.; Haldipur, P.; Tao, R.; Gao, Q.; Li, Y.; Aldinger, K.A.; Iskusnykh, I.Y.; Chizhikov, V.V.; et al. Unified Rhombic Lip Origins of Group 3 and Group 4 Medulloblastoma. Nature 2022, 609, 1012–1020. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.H.; Nelson, A.L.; Algon, S.A.; Graves, O.; Sturla, L.M.; Goumnerova, L.C.; Rowitch, D.H.; Segal, R.A.; Pomeroy, S.L. Medulloblastoma Tumorigenesis Diverges from Cerebellar Granule Cell Differentiation in Patched Heterozygous Mice. Developmental Biology 2003, 263, 50–66. [Google Scholar] [CrossRef] [PubMed]
- Hendrikse, L.D.; Haldipur, P.; Saulnier, O.; Millman, J.; Sjoboen, A.H.; Erickson, A.W.; Ong, W.; Gordon, V.; Coudière-Morrison, L.; Mercier, A.L.; et al. Failure of Human Rhombic Lip Differentiation Underlies Medulloblastoma Formation. Nature 2022, 609, 1021–1028. [Google Scholar] [CrossRef]
- Gibson, P.; Tong, Y.; Robinson, G.; Thompson, M.C.; Currle, D.S.; Eden, C.; Kranenburg, T.A.; Hogg, T.; Poppleton, H.; Martin, J.; et al. Subtypes of Medulloblastoma Have Distinct Developmental Origins. Nature 2010, 468, 1095–1099. [Google Scholar] [CrossRef]
- Huang, A.Y.-S.; Woo, J.; Sardar, D.; Lozzi, B.; Bosquez Huerta, N.A.; Lin, C.-C.J.; Felice, D.; Jain, A.; Paulucci-Holthauzen, A.; Deneen, B. Region-Specific Transcriptional Control of Astrocyte Function Oversees Local Circuit Activities. Neuron 2020, 106, 992–1008.e9. [Google Scholar] [CrossRef]
- Ewels, P.; Magnusson, M.; Lundin, S.; Käller, M. MultiQC: Summarize Analysis Results for Multiple Tools and Samples in a Single Report. Bioinformatics 2016, 32, 3047–3048. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast Universal RNA-Seq Aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Lawrence, M.; Huber, W.; Pagès, H.; Aboyoun, P.; Carlson, M.; Gentleman, R.; Morgan, M.T.; Carey, V.J. Software for Computing and Annotating Genomic Ranges. PLoS Comput. Biol. 2013, 9, e1003118. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, W.; Choi, D.-J.; Yu, K.; Williamson, M.R.; Murali, S.; Ko, Y.; Woo, J.; Deneen, B. Comparative Transcriptomic Analysis of Cerebellar Astrocytes across Developmental Stages and Brain Regions. Int. J. Mol. Sci. 2024, 25, 1021. https://doi.org/10.3390/ijms25021021
Kwon W, Choi D-J, Yu K, Williamson MR, Murali S, Ko Y, Woo J, Deneen B. Comparative Transcriptomic Analysis of Cerebellar Astrocytes across Developmental Stages and Brain Regions. International Journal of Molecular Sciences. 2024; 25(2):1021. https://doi.org/10.3390/ijms25021021
Chicago/Turabian StyleKwon, Wookbong, Dong-Joo Choi, Kwanha Yu, Michael R. Williamson, Sanjana Murali, Yeunjung Ko, Junsung Woo, and Benjamin Deneen. 2024. "Comparative Transcriptomic Analysis of Cerebellar Astrocytes across Developmental Stages and Brain Regions" International Journal of Molecular Sciences 25, no. 2: 1021. https://doi.org/10.3390/ijms25021021
APA StyleKwon, W., Choi, D.-J., Yu, K., Williamson, M. R., Murali, S., Ko, Y., Woo, J., & Deneen, B. (2024). Comparative Transcriptomic Analysis of Cerebellar Astrocytes across Developmental Stages and Brain Regions. International Journal of Molecular Sciences, 25(2), 1021. https://doi.org/10.3390/ijms25021021




