Exposure to Secondhand Smoke Extract Increases Cisplatin Resistance in Head and Neck Cancer Cells
Abstract
1. Introduction
2. Results
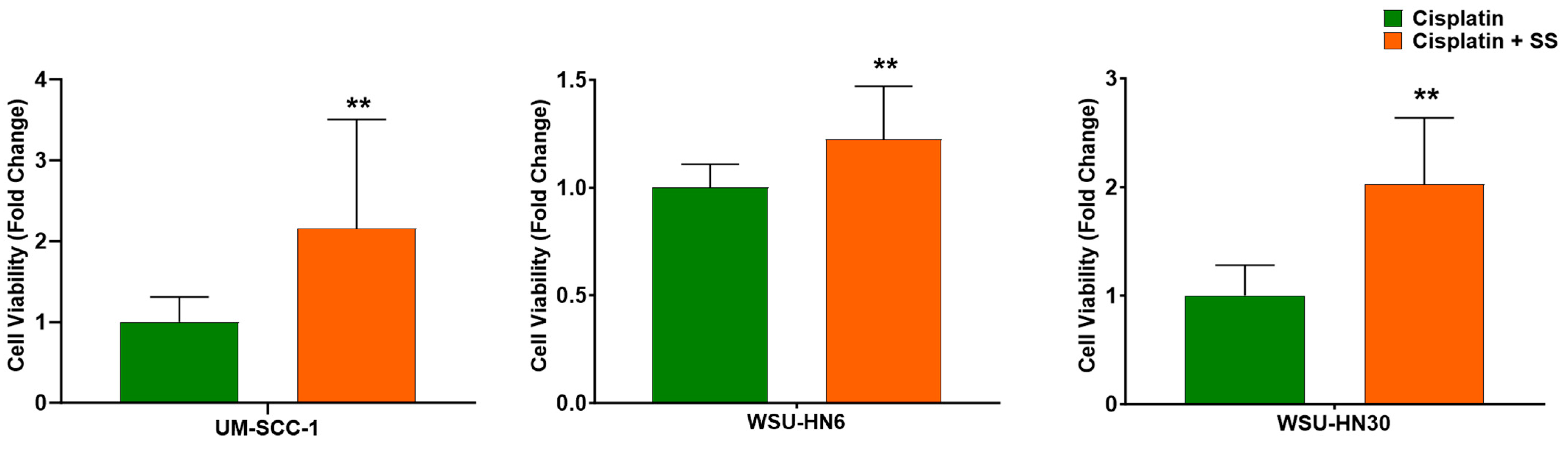
2.1. SS Smoke Extract Increases the Cell Viability of Cisplatin-Treated HNSCC Cells
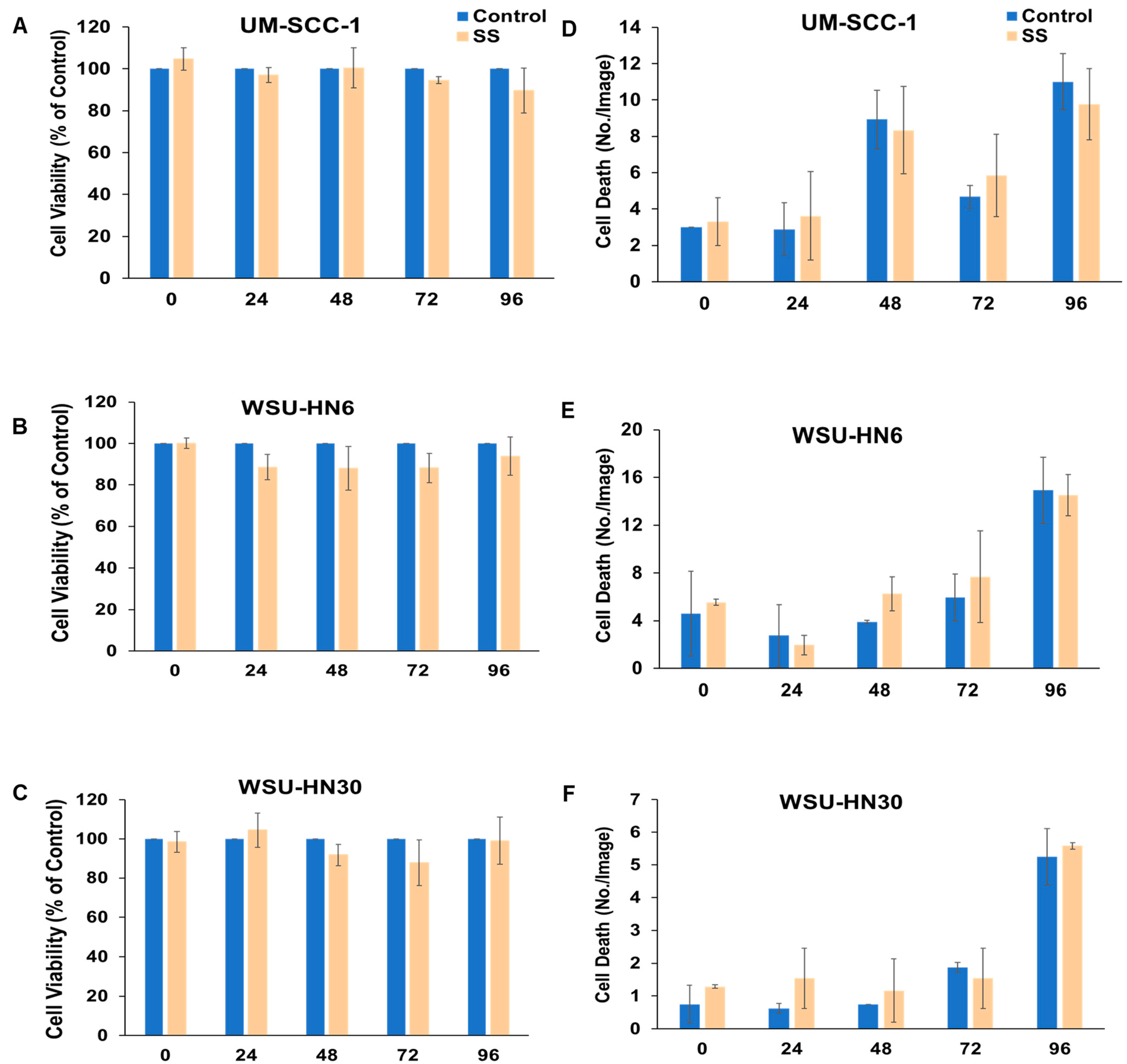
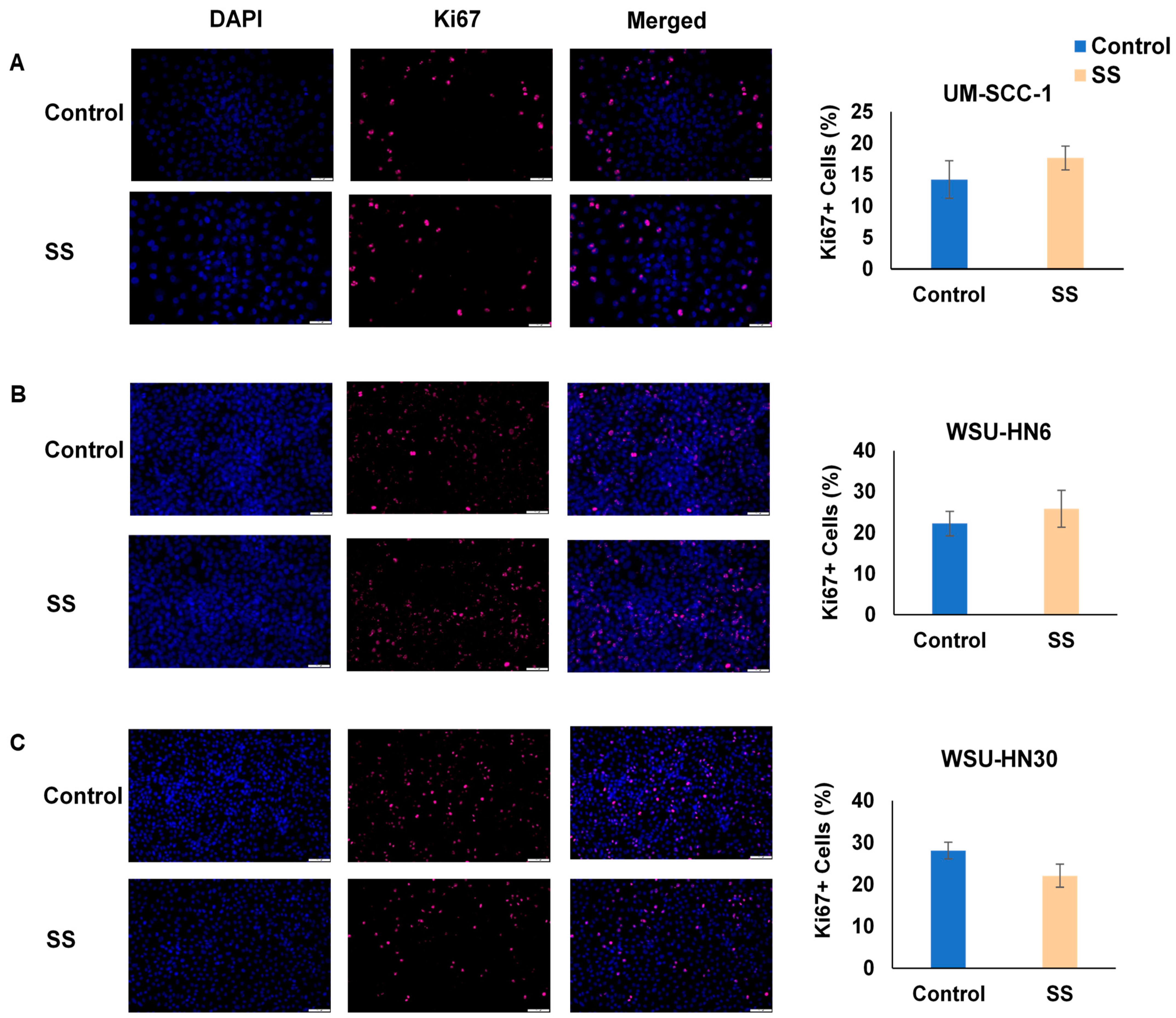
2.2. SS Smoke Extract Alone Does Not Alter HNSCC Cell Viability, Cell Death, or Cell Proliferation
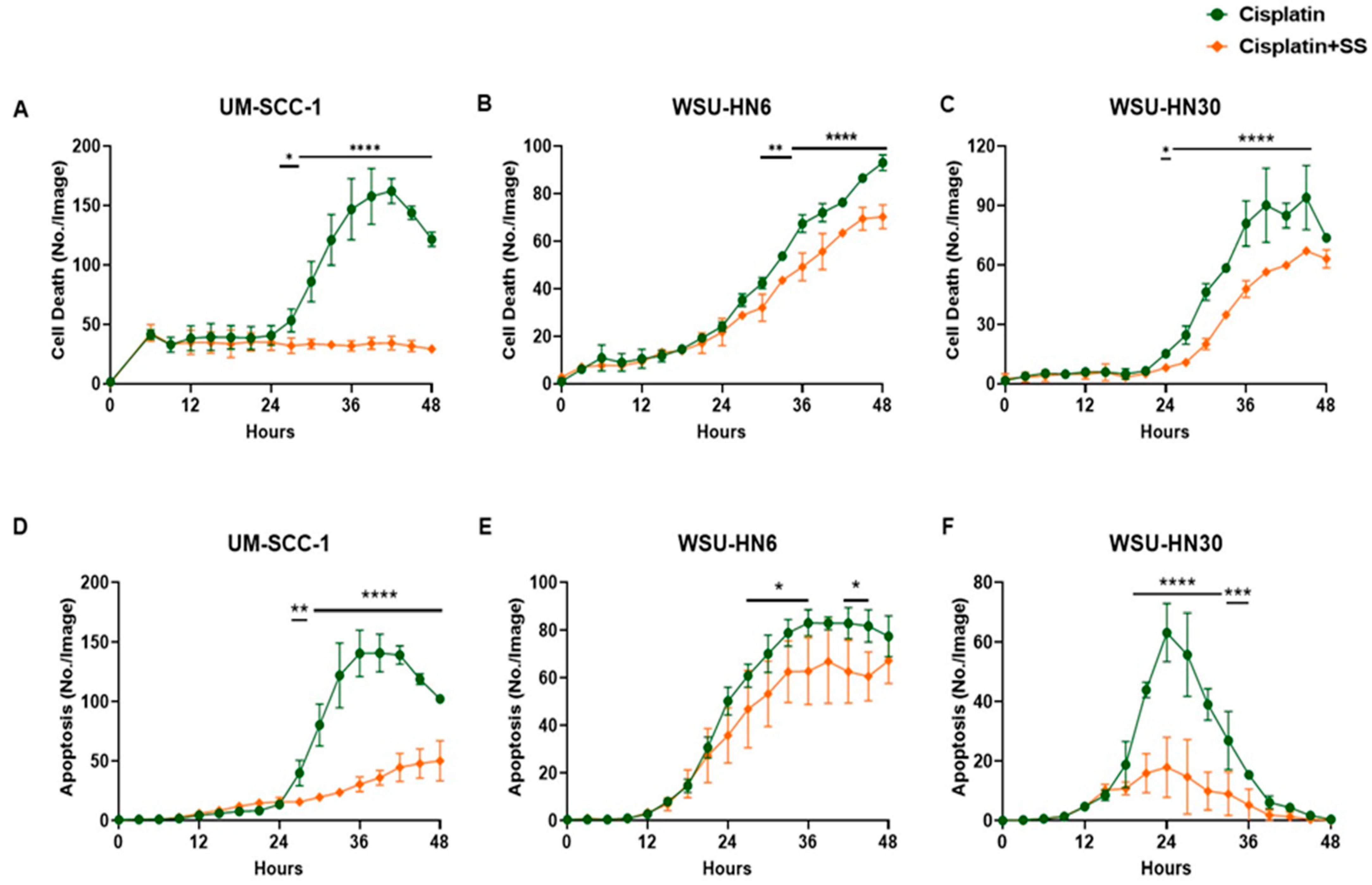
2.3. SS Smoke Extract Reduces Cisplatin-Induced HNSCC Cell Death and Apoptosis
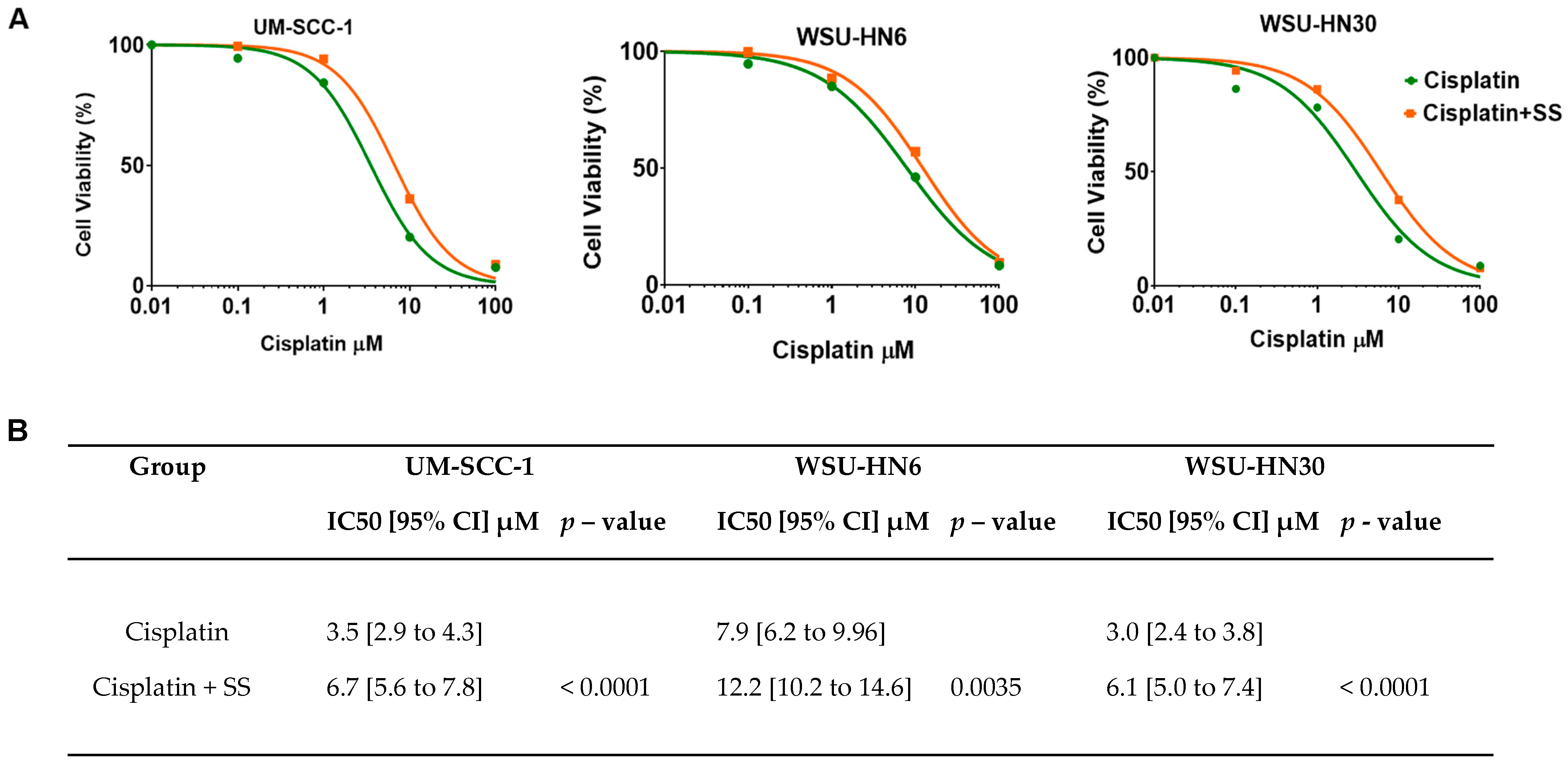
2.4. SS Smoke Extract Exposure Increases Cisplatin IC50 in HNSCC Cells
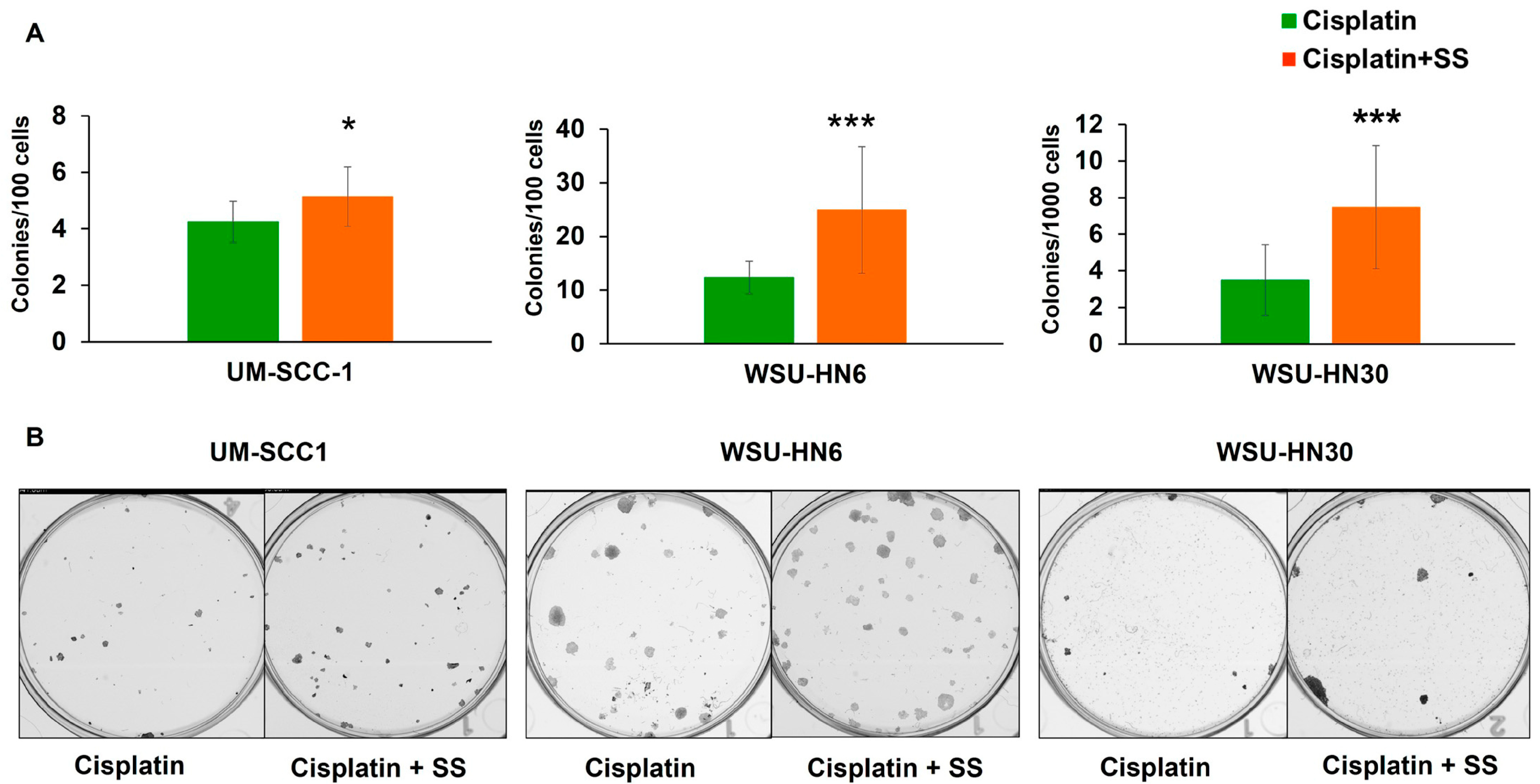
2.5. SS Smoke Extract Exposure during Cisplatin Treatment Increases the Clonogenic Survival of Head and Neck Cancer Cells
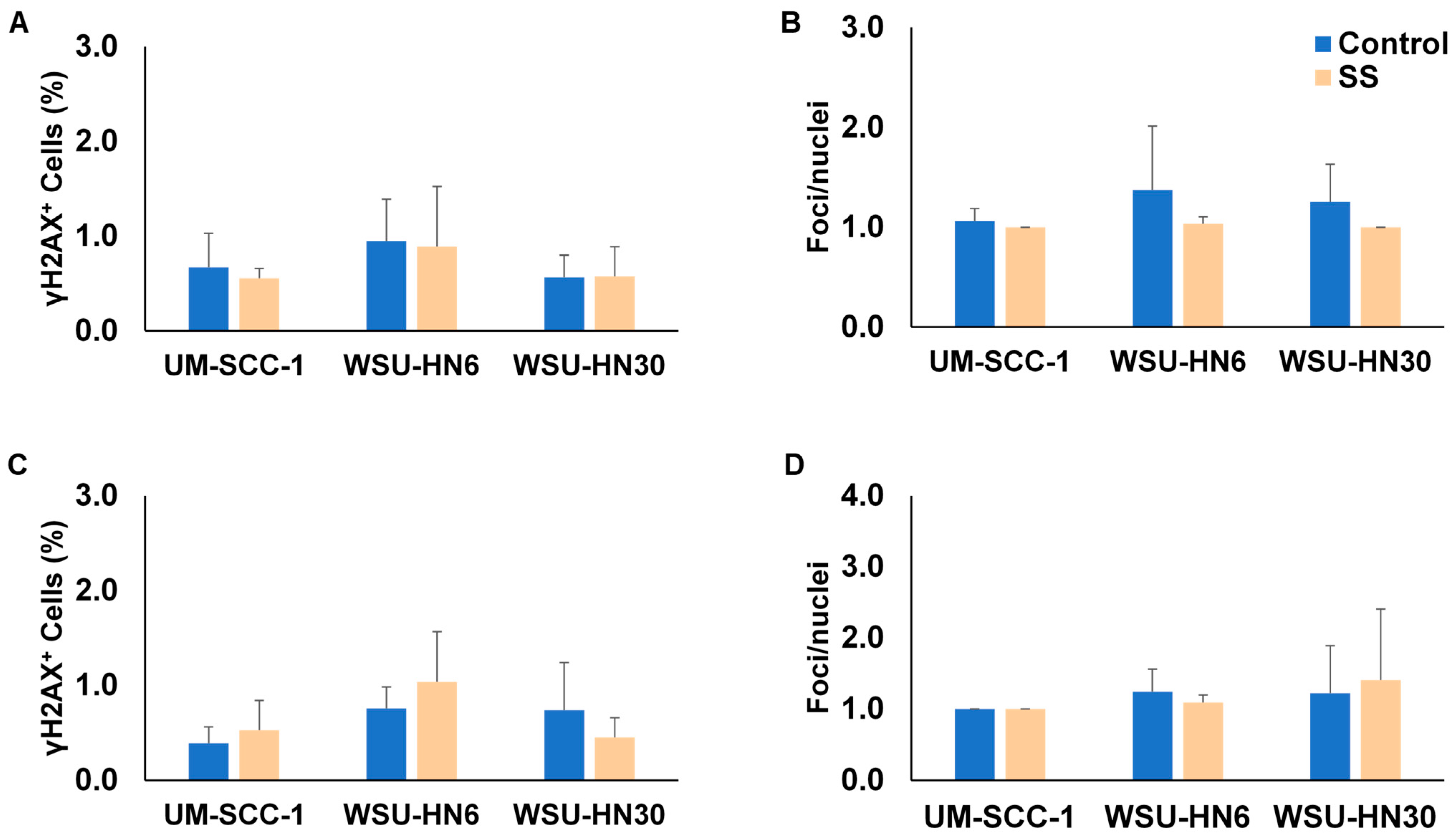
2.6. SS Smoke Exposure Does Not Upregulate the Main DNA Repair Pathways Involved in Cisplatin Adduct Reduction
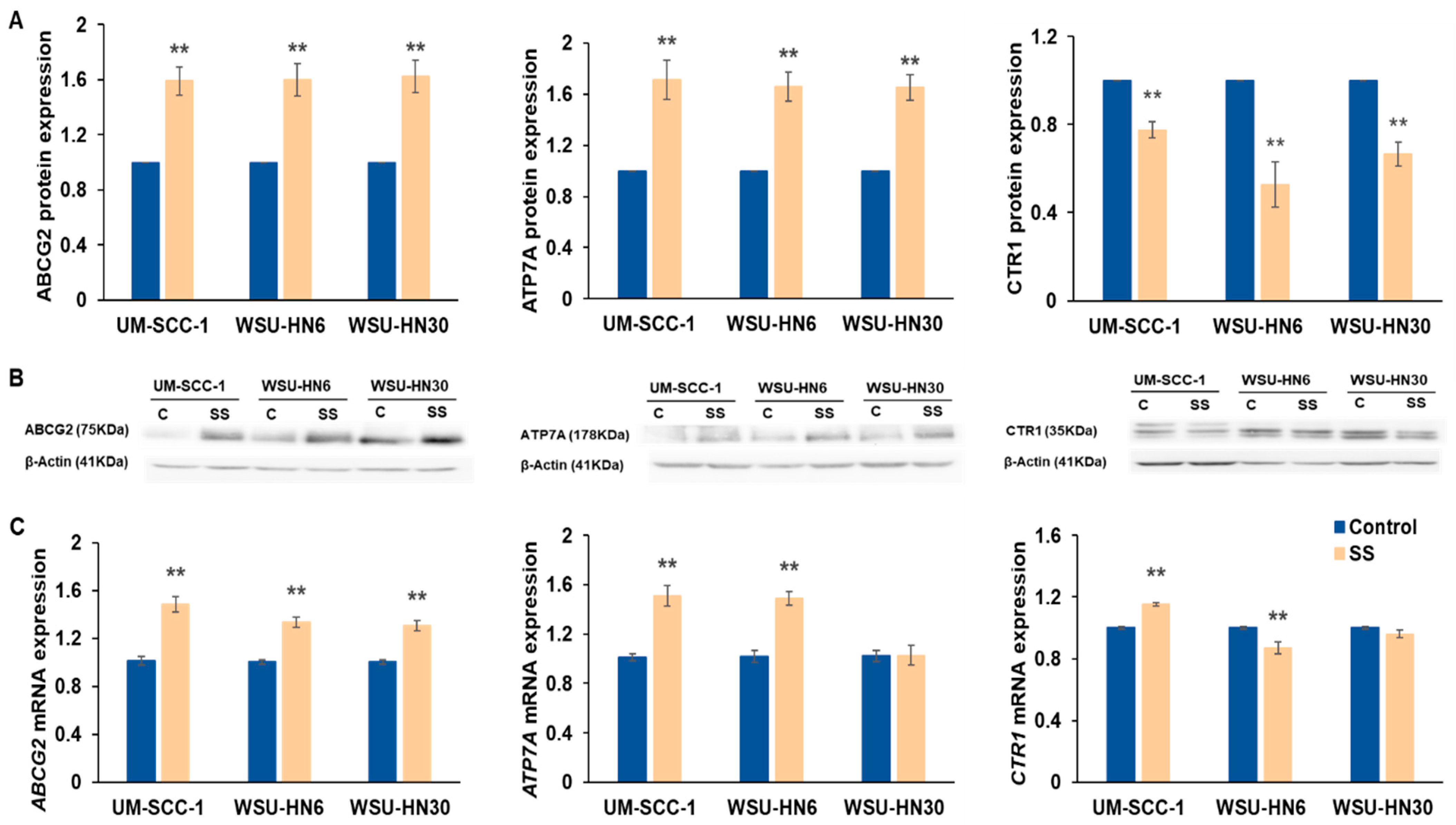
2.7. SS Smoke Extract Exposure Alters the Expression of Multidrug-Resistant Proteins
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Tobacco Smoke Extracts and Cisplatin Preparation
4.3. Cell Viability and Cell Proliferation Analysis
4.4. Cell Death and Apoptosis Analysis
4.5. Cisplatin Chemosensitivity Assay
4.6. Clonogenic Assay
4.7. Gamma H2AX Assay
4.8. Western Blot Analysis
4.9. RNA Extraction, cDNA Synthesis, and qPCR
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABCG2 | ATP-binding cassette subfamily G member 2 |
| ATP7A | ATPase copper transporting alpha |
| IC50 | Half-maximal inhibitory concentration |
| HNSCC | Head and neck squamous cell carcinoma |
| CTR1 | High affinity copper uptake protein 1 |
| SHS | Secondhand smoke |
| SS | Sidestream |
References
- World Health Organization. WHO Report on the Global Tobacco Epidemic, 2017: Monitoring Tobacco Use and Prevention Policies; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Hecht, S. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat. Rev. Cancer 2003, 3, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Husgafvel-Pursiainen, K. Genotoxicity of environmental tobacco smoke: A review. Mutat. Res. 2004, 567, 427–445. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.E. Cigarette Smoke Components and Disease: Cigarette Smoke Is More Than a Triad of Tar, Nicotine, and Carbon Monoxide. In Smoking and Tobacco Control Monograph; National Cancer Institute: Bethesda, MD, USA, 2013; Volume 7, pp. 59–75. [Google Scholar]
- Phares, D.J.; Collier, S.; Zheng, Z.; Jung, H.S. In-situ analysis of the gas-and particle-phase in cigarette smoke by chemical ionization TOF-MS. J. Aerosol Sci. 2017, 106, 132–141. [Google Scholar] [CrossRef]
- Löfroth, G. Environmental tobacco smoke: Overview of chemical composition and genotoxic components. Mutat. Res. Genet. Toxicol. 1989, 222, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Debra, J.; Brody, M.P.H.; Faust, E.; Tsai, J. Secondhand smoke exposure among nonsmoking adults: United States, 2015–2018. In NCHS Data Briefs; National Center for Health Statistics: Hyattsville, MD, USA, 2021. [Google Scholar]
- Tsai, J.; Homa, D.M.; Gentzke, A.S.; Mahoney, M.; Sharapova, S.R.; Sosnoff, C.S.; Caron, K.T.; Wang, L.; Melstrom, P.C.; Trivers, K.F. Exposure to secondhand smoke among nonsmokers-United States, 1988–2014. Morb. Mortal. Wkly. Rep. 2018, 67, 1342. [Google Scholar] [CrossRef] [PubMed]
- Max, W.; Sung, H.Y.; Shi, Y. Deaths from secondhand smoke exposure in the United States: Economic implications. Am. J. Public Health 2012, 102, 2173–2180. [Google Scholar] [CrossRef]
- Kim, A.; Ko, H.J.; Kwon, J.H.; Lee, J.M. Exposure to secondhand smoke and risk of cancer in never smokers: A meta-analysis of epidemiologic studies. Int. J. Environ. Res. Public Health 2018, 15, 1981. [Google Scholar] [CrossRef]
- Kim, C.H.; Lee, Y.C.; Hung, R.J.; Boffetta, P.; Xie, D.; Wampfler, J.A.; Cote, M.L.; Chang, S.C.; Ugolini, D.; Neri, M.; et al. Secondhand Tobacco Smoke Exposure and Lung Adenocarcinoma In Situ/Minimally Invasive Adenocarcinoma (AIS/MIA). Cancer Epidemiol. Biomark. Prev. 2015, 24, 1902–1906. [Google Scholar] [CrossRef]
- Mariano, L.C.; Warnakulasuryia, S.; Straif, K.; Monteiro, L. Secondhand smoke exposure and oral cancer risk: A systematic review and meta-analysis. Tob. Control 2021, 31, 597–601. [Google Scholar] [CrossRef]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef]
- Cohen, N.; Fedewa, S.; Chen, A.Y. Epidemiology and Demographics of the Head and Neck Cancer Population. Oral Maxillofac. Surg. Clin. N. Am. 2018, 30, 381–395. [Google Scholar] [CrossRef] [PubMed]
- Jethwa, A.R.; Khariwala, S.S. Tobacco-related carcinogenesis in head and neck cancer. Cancer Metastasis Rev. 2017, 36, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Krutz, M.; Acharya, P.; Elliott, J.; Zhao, D.; Mhawej, R.; Queimado, L. Tobacco Cessation Following Laryngeal Cancer Diagnosis Predicts Response to Treatment and Laryngectomy-Free Survival. Otolaryngol. Head Neck Surg. 2023; ahead of print. [Google Scholar]
- Iocca, O.; Farcomeni, A.; Di Rocco, A.; Di Maio, P.; Golusinski, P.; Pardinas Lopez, S.; Savo, A.; Pellini, R.; Spriano, G. Locally advanced squamous cell carcinoma of the head and neck: A systematic review and Bayesian network meta-analysis of the currently available treatment options. Oral Oncol. 2018, 80, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Coinu, A.; Riboldi, V.; Borgonovo, K.; Ghilardi, M.; Cabiddu, M.; Lonati, V.; Sarti, E.; Barni, S. Concomitant platinum-based chemotherapy or cetuximab with radiotherapy for locally advanced head and neck cancer: A systematic review and meta-analysis of published studies. Oral Oncol. 2014, 50, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Manyanga, J.; Ganapathy, V.; Bouharati, C.; Mehta, T.; Sadhasivam, B.; Acharya, P.; Zhao, D.; Queimado, L. Electronic cigarette aerosols alter the expression of cisplatin transporters and increase drug resistance in oral cancer cells. Sci. Rep. 2021, 11, 1821. [Google Scholar] [CrossRef]
- Simon, F.; Schwenk-Zieger, S.; Becker, S.; Unger, K.; Gires, O.; Baumeister, P. Cigarette Smoke Reduces the Efficacy of Cisplatin in Head and Neck Cancer Cells–Role of ABCG2. Anticancer Res. 2020, 40, 1277–1284. [Google Scholar] [CrossRef]
- Choi, S.H.; Terrell, J.E.; Bradford, C.R.; Ghanem, T.; Spector, M.E.; Wolf, G.T.; Lipkus, I.M.; Duffy, S.A. Does Quitting Smoking Make a Difference Among Newly Diagnosed Head and Neck Cancer Patients? Nicotine Tob. Res. 2016, 18, 2216–2224. [Google Scholar] [CrossRef]
- Fazel, A.; Quabius, E.S.; Gonzales-donate, M.; Laudien, M.; Herzog, A.; Kress, K.; Schleicher, T.; Fabian, A.; Huber, K.; Hoffmann, M. Alteration of smoking habit at time of first diagnosis influences survival of patients with HNSCC. Mol. Clin. Oncol. 2020, 13, 50. [Google Scholar] [CrossRef]
- Krutz, M.; Acharya, P.; Chissoe, G.; Raj, V.; Driskill, L.; Krempl, G.; Zhao, D.; Mhawej, R.; Queimado, L. Tobacco cessation after head and neck cancer diagnosis is an independent predictor of treatment response and long-term survival. Oral Oncol. 2022, 134, 106072. [Google Scholar] [CrossRef]
- Idris, S.; Baqays, A.; Isaac, A.; Chau, J.K.; Calhoun, K.H.; Seikaly, H. The effect of second hand smoke in patients with squamous cell carcinoma of the head and neck. J. Otolaryngol. Head Neck Surg. 2019, 48, 33. [Google Scholar] [CrossRef]
- Jarvis, M.; Russell, M.; Feyerabend, C. Absorption of nicotine and carbon monoxide from passive smoking under natural conditions of exposure. Thorax 1983, 38, 829–833. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, T.; Orr, J.; Grendys, E.; Edwards, R.; Krivak, T.C.; Holloway, R.; Moore, R.G.; Puls, L.; Tillmanns, T.; Schink, J.C.; et al. A prospective study evaluating the clinical relevance of a chemoresponse assay for treatment of patients with persistent or recurrent ovarian cancer. Gynecol. Oncol. 2013, 131, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Munshi, A.; Hobbs, M.; Meyn, R.E. Clonogenic Cell Survival Assay. Methods Mol. Med. 2005, 110, 21–28. [Google Scholar] [PubMed]
- Zhou, J.; Kang, Y.; Chen, L.; Wang, H.; Liu, J.; Zeng, S.; Yu, L. The Drug-Resistance Mechanisms of Five Platinum-Based Antitumor Agents. Front. Pharmacol. 2020, 11, 343. [Google Scholar] [CrossRef]
- Galluzzi, L.; Senovilla, L.; Vitale, I.; Michels, J.; Martins, I.; Kepp, O.; Castedo, M.; Kroemer, G. Molecular mechanisms of cisplatin resistance. Oncogene 2012, 31, 1869–1883. [Google Scholar] [CrossRef] [PubMed]
- Rocha, C.R.R.; Silva, M.M.; Quinet, A.; Cabral-Neto, J.B.; Menck, C.F.M. DNA repair pathways and cisplatin resistance: An intimate relationship. Clinics 2018, 73, e478s. [Google Scholar] [CrossRef]
- Yimit, A.; Adebali, O.; Sancar, A.; Jiang, Y. Differential damage and repair of DNA-adducts induced by anti-cancer drug cisplatin across mouse organs. Nat. Commun. 2019, 10, 309. [Google Scholar] [CrossRef]
- Schuler, P.J.; Trellakis, S.; Greve, J.; Bas, M.; Bergmann, C.; Bölke, E.; Lehnerdt, G.; Mattheis, S.; Albers, A.E.; Brandau, S.; et al. In vitro chemosensitivity of head and neck cancer cell lines. Eur. J. Med. Res. 2010, 15, 337–344. [Google Scholar] [CrossRef]
- Sharma, P.; Sane, N.; Anand, S.D.; Marimutthu, P.; Benegal, V. Assessment of cotinine in urine and saliva of smokers, passive smokers, and nonsmokers: Method validation using liquid chromatography and mass spectrometry. Indian J. Psychiatry 2019, 61, 270–276. [Google Scholar] [CrossRef]
- Mahabee-Gittens, E.M.; Merianos, A.L.; Matt, G.E. Preliminary evidence that high levels of nicotine on children’s hands may contribute to overall tobacco smoke exposure. Tob. Control 2018, 27, 217–219. [Google Scholar] [CrossRef]
- Thomson, G.; Wilson, N.; Edwards, R. At the frontier of tobacco control: A brief review of public attitudes toward smoke-free outdoor places. Nicotine Tob. Res. 2009, 11, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Teneggi, V.; Squassante, L.; Iavarone, L.; Milleri, S.; Bye, A.; Gomeni, R. Correlation and predictive performances of saliva and plasma nicotine concentration on tobacco withdrawal-induced craving. Br. J. Clin. Pharmacol. 2002, 54, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Robson, N.; Bond, A.; Wolff, K. Salivary Nicotine and Cotinine Concentrations in Unstimulated and Stimulated Saliva. Afr. J. Pharm. Pharmacol. 2010, 4, 61–65. [Google Scholar]
- Wong, L.S.; Green, H.M.; Feugate, J.E.; Yadav, M.; Nothnagel, E.A.; Martins-Green, M. Effects of “second-hand” smoke on structure and function of fibroblasts, cells that are critical for tissue repair and remodeling. BMC Cell Biol. 2004, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Hwang, K.A.; Choi, D.W.; Choi, K.C. The cigarette smoke components induced the cell proliferation and epithelial to mesenchymal transition via production of reactive oxygen species in endometrial adenocarcinoma cells. Food Chem. Toxicol. 2018, 121, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.C.; Tsai, K.Y.; Su, Y.F.; Chien, C.Y.; Chen, Y.C.; Wu, Y.C.; Liu, S.Y.; Shieh, Y.S. α7-Nicotine acetylcholine receptor mediated nicotine induced cell survival and cisplatin resistance in oral cancer. Arch. Oral Biol. 2020, 111, 104653. [Google Scholar] [CrossRef] [PubMed]
- Lau, L.M.S.; Mayoh, C.; Xie, J.; Barahona, P.; MacKenzie, K.L.; Wong, M.; Kamili, A.; Tsoli, M.; Failes, T.W.; Kumar, A.; et al. In vitro and in vivo drug screens of tumor cells identify novel therapies for high-risk child cancer. EMBO Mol. Med. 2022, 14, e14608. [Google Scholar] [CrossRef] [PubMed]
- Fountzilas, G.; Kosmidis, P.; Avramidis, V.; Nikolaou, A.; Kalogera-Fountzila, A.; Makrantonakis, P.; Bacoyiannis, C.; Samantas, E.; Skarlos, D.; Daniilidis, J. Long-term survival data and prognostic factors of a complete response to chemotherapy in patients with head and neck cancer treated with platinum-based induction chemotherapy: A hellenic co-operative oncology group study. Med. Pediatr. Oncol. 1997, 28, 401–410. [Google Scholar] [CrossRef]
- Chen, A.M.; Chen, L.M.; Vaughan, A.; Sreeraman, R.; Farwell, D.G.; Luu, Q.; Lau, D.H.; Stuart, K.; Purdy, J.A.; Vijayakumar, S. Tobacco smoking during radiation therapy for head-and-neck cancer is associated with unfavorable outcome. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 414–419. [Google Scholar] [CrossRef]
- Browman, G.P.; Wong, G.; Hodson, I.; Sathya, J.; Russell, R.; McAlpine, L.; Skingley, P.; Levine, M.N. Influence of cigarette smoking on the efficacy of radiation therapy in head and neck cancer. N. Engl. J. Med. 1993, 328, 159–163. [Google Scholar] [CrossRef]
- Huang, D.; Savage, S.R.; Calinawan, A.P.; Lin, C.; Zhang, B.; Wang, P.; Starr, T.K.; Birrer, M.J.; Paulovich, A.G. A highly annotated database of genes associated with platinum resistance in cancer. Oncogene 2021, 40, 6395–6405. [Google Scholar] [CrossRef] [PubMed]
- da Silva, S.D.; Hier, M.; Mlynarek, A.; Kowalski, L.P.; Alaoui-Jamali, M.A. Recurrent oral cancer: Current and emerging therapeutic approaches. Front. Pharmacol. 2012, 3, 149. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Wang, H.T.; Weng, M.W.; Chin, C.; Huang, W.; Lepor, H.; Wu, X.R.; Rom, W.N.; Chen, L.C.; Tang, M.S. Cigarette side-stream smoke lung and bladder carcinogenesis: Inducing mutagenic acrolein-DNA adducts, inhibiting DNA repair and enhancing anchorage-independent-growth cell transformation. Oncotarget 2015, 6, 33226–33236. [Google Scholar] [CrossRef] [PubMed]
- Sarker, A.H.; Chatterjee, A.; Williams, M.; Lin, S.; Havel, C.; Jacob Iii, P.; Boldogh, I.; Hazra, T.K.; Talbot, P.; Hang, B. NEIL2 Protects against Oxidative DNA Damage Induced by Sidestream Smoke in Human Cells. PLoS ONE 2014, 9, e90261. [Google Scholar] [CrossRef] [PubMed]
- Ganapathy, V.; Ramachandran, I.; Rubenstein, D.A.; Queimado, L. Detection of in vivo DNA damage induced by very low doses of mainstream and sidestream smoke extracts using a novel assay. Am. J. Prev. Med. 2015, 48, S102–S110. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhu, S.; Shanbhag, V.; Wang, Y.; Lee, J.; Petris, M. A Role for The ATP7A Copper Transporter in Tumorigenesis and Cisplatin Resistance. J. Cancer 2017, 8, 1952–1958. [Google Scholar] [CrossRef]
- Lukanović, D.; Herzog, M.; Kobal, B.; Černe, K. The contribution of copper efflux transporters ATP7A and ATP7B to chemoresistance and personalized medicine in ovarian cancer. Biomed. Pharmacother. 2020, 129, 110401. [Google Scholar] [CrossRef]
- Ebert, B.; Seidel, A.; Lampen, A. Identification of BCRP as transporter of benzo[a]pyrene conjugates metabolically formed in Caco-2 cells and its induction by Ah-receptor agonists. Carcinogenesis 2005, 26, 1754–1763. [Google Scholar] [CrossRef]
- Doyle, L.A.; Ross, D.D. Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2). Oncogene 2003, 22, 7340–7358. [Google Scholar] [CrossRef]
- Queimado, L.; Lopes, C.; Du, F.; Martins, C.; Fonseca, I.; Bowcock, A.M.; Soares, J.; Lovett, M. In vitro transformation of cell lines from human salivary gland tumors. Int. J. Cancer 1999, 81, 793–798. [Google Scholar] [CrossRef]
- Cardinali, M.; Pietraszkiewicz, H.; Ensley, J.F.; Robbins, K.C. Tyrosine phosphorylation as a marker for aberrantly regulated growth-promoting pathways in cell lines derived from head and neck malignancies. Int. J. Cancer 1995, 61, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Krause, C.J.; Carey, T.E.; Ott, R.W.; Hurbis, C.; McClatchey, K.D.; Regezi, J.A. Human squamous cell carcinoma. Establishment and characterization of new permanent cell lines. Arch. Otolaryngol. 1981, 107, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, D.; Jesty, J.; Bluestein, D. Differences between mainstream and sidestream cigarette smoke extracts and nicotine in the activation of platelets under static and flow conditions. Circulation 2004, 109, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Ganapathy, V.; Manyanga, J.; Brame, L.; McGuire, D.; Sadhasivam, B.; Floyd, E.; Rubenstein, D.A.; Ramachandran, I.; Wagener, T.; Queimado, L. Electronic cigarette aerosols suppress cellular antioxidant defenses and induce significant oxidative DNA damage. PLoS ONE 2017, 12, e0177780. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadhasivam, B.; Manyanga, J.; Ganapathy, V.; Acharya, P.; Bouharati, C.; Chinnaiyan, M.; Mehta, T.; Mathews, B.; Castles, S.; Rubenstein, D.A.; et al. Exposure to Secondhand Smoke Extract Increases Cisplatin Resistance in Head and Neck Cancer Cells. Int. J. Mol. Sci. 2024, 25, 1032. https://doi.org/10.3390/ijms25021032
Sadhasivam B, Manyanga J, Ganapathy V, Acharya P, Bouharati C, Chinnaiyan M, Mehta T, Mathews B, Castles S, Rubenstein DA, et al. Exposure to Secondhand Smoke Extract Increases Cisplatin Resistance in Head and Neck Cancer Cells. International Journal of Molecular Sciences. 2024; 25(2):1032. https://doi.org/10.3390/ijms25021032
Chicago/Turabian StyleSadhasivam, Balaji, Jimmy Manyanga, Vengatesh Ganapathy, Pawan Acharya, Célia Bouharati, Mayilvanan Chinnaiyan, Toral Mehta, Basil Mathews, Samuel Castles, David A. Rubenstein, and et al. 2024. "Exposure to Secondhand Smoke Extract Increases Cisplatin Resistance in Head and Neck Cancer Cells" International Journal of Molecular Sciences 25, no. 2: 1032. https://doi.org/10.3390/ijms25021032
APA StyleSadhasivam, B., Manyanga, J., Ganapathy, V., Acharya, P., Bouharati, C., Chinnaiyan, M., Mehta, T., Mathews, B., Castles, S., Rubenstein, D. A., Tackett, A. P., Zhao, Y. D., Ramachandran, I., & Queimado, L. (2024). Exposure to Secondhand Smoke Extract Increases Cisplatin Resistance in Head and Neck Cancer Cells. International Journal of Molecular Sciences, 25(2), 1032. https://doi.org/10.3390/ijms25021032





