Differential Effects of Aripiprazole on Electroencephalography-Recorded Gamma-Band Auditory Steady-State Response, Spontaneous Gamma Oscillations and Behavior in a Schizophrenia Rat Model
Abstract
:1. Introduction
2. Results
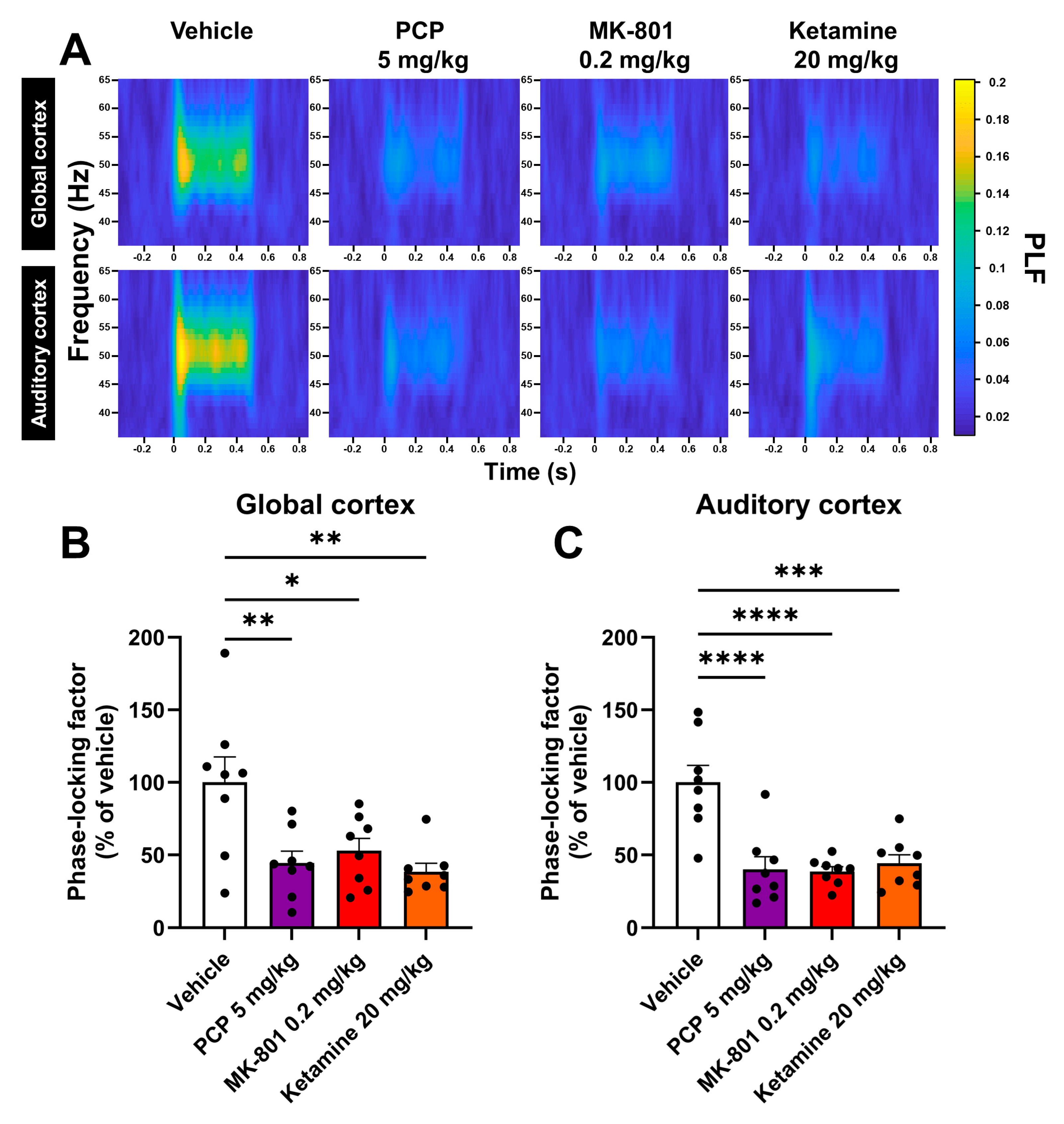
2.1. PCP, MK-801 and Ketamine Reduce the Phase Synchrony of the ASSR
2.2. PCP, MK-801 and Ketamine Increase Locomotor Activity and Decrease Social Interaction
2.3. Aripiprazole Has No Effect on Changes Provoked by MK-801 on Evoked Gamma Oscillations
2.4. Aripiprazole Does Not Normalize the Increase in Spontaneous Gamma Oscillations Provoked by MK-801
2.5. Aripiprazole Alleviates MK-801-Induced Hyperactivity but Not Social Deficit
3. Discussion
4. Materials and Methods
4.1. Animals
- Batch A: animals used for both EEG experiments (n = 12 implanted rats weighing between 207 and 293 g on the day of surgery).
- Batch B: animals used for the behavioral experiments described in Section 2.2 (n = 40 rats weighing between 226 and 298 g on the first day of the locomotor activity evaluation and between 240 and 314 g on the day of the social interaction evaluation) (Figure 2).
- Batch C: animals used for the behavioral experiments described in Section 2.5 (n = 70 rats weighing between 291 and 444 g on the first day of the locomotor activity evaluation and between 404 and 508 g on the day of the social interaction evaluation) (Figure 5 and Figure 6).
4.1.1. Batch A: Animals Used for EEG Experiments
4.1.2. Batches B and C: Animals Used for Behavioral Experiments
4.2. Drugs and Dosing Schemes for Each Test
4.2.1. Drugs
4.2.2. Dosing Schemes for Each Test
4.3. Surgical Implantation of the Telemetry Transmitter
- -
- Channel #1, global cortex: AP: +2.0 mm and ML: −1.5 mm from bregma for the active electrode, which is therefore located at the level of the frontal cortex, with a reference on the right cerebellum, i.e., AP: −11.0 mm and ML: −3.0 mm from bregma.
- -
- Channel #2, auditory cortex: AP: −4.8 mm and ML: +7 mm from bregma for the active electrode, which is at the level of the auditory cortex, with a reference on the left cerebellum, i.e., AP: −11.0 mm and ML: +3.0 mm from bregma.
4.4. EEG Recording
4.4.1. ASSR Recording
4.4.2. Recording of Spontaneous EEG for qEEG Analysis
4.5. EEG Signal Processing
4.5.1. ASSR Analysis
4.5.2. qEEG Analysis
4.6. Behavioral Evaluation
4.6.1. Locomotor Activity Evaluation (Actimetry)
4.6.2. Social Interaction Evaluation
4.7. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Owen, M.J.; Sawa, A.; Mortensen, P.B. Schizophrenia. Lancet Lond. Engl. 2016, 388, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Correll, C.U.; Schooler, N.R. Negative Symptoms in Schizophrenia: A Review and Clinical Guide for Recognition, Assessment, and Treatment. Neuropsychiatr. Dis. Treat. 2020, 16, 519–534. [Google Scholar] [CrossRef]
- McCutcheon, R.A.; Keefe, R.S.E.; McGuire, P.K. Cognitive Impairment in Schizophrenia: Aetiology, Pathophysiology, and Treatment. Mol. Psychiatry 2023, 28, 1902–1918. [Google Scholar] [CrossRef]
- Kas, M.J.; Penninx, B.; Sommer, B.; Serretti, A.; Arango, C.; Marston, H. A Quantitative Approach to Neuropsychiatry: The Why and the How. Neurosci. Biobehav. Rev. 2019, 97, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Drinkenburg, W.H.I.M.; Ruigt, G.S.F.; Ahnaou, A. Pharmaco-EEG Studies in Animals: An Overview of Contemporary Translational Applications. Neuropsychobiology 2015, 72, 151–164. [Google Scholar] [CrossRef] [PubMed]
- McNally, J.M.; McCarley, R.W. Gamma Band Oscillations: A Key to Understanding Schizophrenia Symptoms and Neural Circuit Abnormalities. Curr. Opin. Psychiatry 2016, 29, 202–210. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, B.F.; Vohs, J.L.; Krishnan, G.P.; Rass, O.; Hetrick, W.P.; Morzorati, S.L. The Auditory Steady-State Response (ASSR): A Translational Biomarker for Schizophrenia. Suppl. Clin. Neurophysiol. 2013, 62, 101–112. [Google Scholar] [CrossRef]
- Onitsuka, T.; Tsuchimoto, R.; Oribe, N.; Spencer, K.M.; Hirano, Y. Neuronal Imbalance of Excitation and Inhibition in Schizophrenia: A Scoping Review of Gamma-Band ASSR Findings. Psychiatry Clin. Neurosci. 2022, 76, 610–619. [Google Scholar] [CrossRef]
- Tada, M.; Kirihara, K.; Koshiyama, D.; Nagai, T.; Fujiouka, M.; Usui, K.; Satomura, Y.; Koike, S.; Sawada, K.; Matsuoka, J.; et al. Alterations of Auditory-Evoked Gamma Oscillations Are More Pronounced than Alterations of Spontaneous Power of Gamma Oscillation in Early Stages of Schizophrenia. Transl. Psychiatry 2023, 13, 218. [Google Scholar] [CrossRef]
- Grent-’t-Jong, T.; Gross, J.; Goense, J.; Wibral, M.; Gajwani, R.; Gumley, A.I.; Lawrie, S.M.; Schwannauer, M.; Schultze-Lutter, F.; Navarro Schröder, T.; et al. Resting-State Gamma-Band Power Alterations in Schizophrenia Reveal E/I-Balance Abnormalities across Illness-Stages. eLife 2018, 7, e37799. [Google Scholar] [CrossRef]
- Tanaka-Koshiyama, K.; Koshiyama, D.; Miyakoshi, M.; Joshi, Y.B.; Molina, J.L.; Sprock, J.; Braff, D.L.; Light, G.A. Abnormal Spontaneous Gamma Power Is Associated With Verbal Learning and Memory Dysfunction in Schizophrenia. Front. Psychiatry 2020, 11, 832. [Google Scholar] [CrossRef]
- Spencer, K.M.; Nakhnikian, A.; Hirano, Y.; Levin, M. The Contribution of Gamma Bursting to Spontaneous Gamma Activity in Schizophrenia. Front. Hum. Neurosci. 2023, 17, 1130897. [Google Scholar] [CrossRef]
- Moghaddam, B.; Javitt, D. From Revolution to Evolution: The Glutamate Hypothesis of Schizophrenia and Its Implication for Treatment. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2012, 37, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Sivarao, D.V.; Chen, P.; Senapati, A.; Yang, Y.; Fernandes, A.; Benitex, Y.; Whiterock, V.; Li, Y.-W.; Ahlijanian, M.K. 40 Hz Auditory Steady-State Response Is a Pharmacodynamic Biomarker for Cortical NMDA Receptors. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2016, 41, 2232–2240. [Google Scholar] [CrossRef]
- Koshiyama, D.; Miyakoshi, M.; Thomas, M.L.; Joshi, Y.B.; Molina, J.L.; Tanaka-Koshiyama, K.; Sprock, J.; Braff, D.L.; Swerdlow, N.R.; Light, G.A. Unique Contributions of Sensory Discrimination and Gamma Synchronization Deficits to Cognitive, Clinical, and Psychosocial Functional Impairments in Schizophrenia. Schizophr. Res. 2021, 228, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hu, R.; Yan, H.; Chu, L.; Qiu, Y.; Gao, Y.; Li, M.; Li, J. 40-Hz Auditory Steady-State Response Deficits Are Correlated with the Severity of Persistent Auditory Verbal Hallucination in Patients with Schizophrenia. Psychiatry Res. Neuroimaging 2023, 336, 111748. [Google Scholar] [CrossRef]
- Zhou, T.-H.; Mueller, N.E.; Spencer, K.M.; Mallya, S.G.; Lewandowski, K.E.; Norris, L.A.; Levy, D.L.; Cohen, B.M.; Öngür, D.; Hall, M.-H. Auditory Steady State Response Deficits Are Associated with Symptom Severity and Poor Functioning in Patients with Psychotic Disorder. Schizophr. Res. 2018, 201, 278–286. [Google Scholar] [CrossRef]
- Yizhar, O.; Fenno, L.E.; Prigge, M.; Schneider, F.; Davidson, T.J.; O’Shea, D.J.; Sohal, V.S.; Goshen, I.; Finkelstein, J.; Paz, J.T.; et al. Neocortical Excitation/Inhibition Balance in Information Processing and Social Dysfunction. Nature 2011, 477, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Adraoui, F.W.; Douw, L.; Martens, G.J.M.; Maas, D.A. Connecting Neurobiological Features with Interregional Dysconnectivity in Social-Cognitive Impairments of Schizophrenia. Int. J. Mol. Sci. 2023, 24, 7680. [Google Scholar] [CrossRef] [PubMed]
- Baune, B.T. Aripiprazole 2-Month Ready-to-Use 960 Mg (Ari 2MRTU): Review of Its Possible Role in Schizophrenia Therapy. Curr. Med. Res. Opin. 2023, 40, 87–96. [Google Scholar] [CrossRef]
- Oh, S.; Lee, T.Y.; Kim, M.; Kim, S.H.; Lee, S.; Cho, S.; Kim, J.H.; Kwon, J.S. Effectiveness of Antipsychotic Drugs in Schizophrenia: A 10-Year Retrospective Study in a Korean Tertiary Hospital. NPJ Schizophr. 2020, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Brasso, C.; Colli, G.; Sgro, R.; Bellino, S.; Bozzatello, P.; Montemagni, C.; Villari, V.; Rocca, P. Efficacy of Serotonin and Dopamine Activity Modulators in the Treatment of Negative Symptoms in Schizophrenia: A Rapid Review. Biomedicines 2023, 11, 921. [Google Scholar] [CrossRef] [PubMed]
- Neill, J.C.; Barnes, S.; Cook, S.; Grayson, B.; Idris, N.F.; McLean, S.L.; Snigdha, S.; Rajagopal, L.; Harte, M.K. Animal Models of Cognitive Dysfunction and Negative Symptoms of Schizophrenia: Focus on NMDA Receptor Antagonism. Pharmacol. Ther. 2010, 128, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Ogren, S.O.; Goldstein, M. Phencyclidine- and Dizocilpine-Induced Hyperlocomotion Are Differentially Mediated. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 1994, 11, 167–177. [Google Scholar] [CrossRef]
- Del Arco, A.; Mora, F.; Mohammed, A.H.; Fuxe, K. Stimulation of D2 Receptors in the Prefrontal Cortex Reduces PCP-Induced Hyperactivity, Acetylcholine Release and Dopamine Metabolism in the Nucleus Accumbens. J. Neural Transm. 2007, 114, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, T.L.; Sachdeva, S.; Stahl, S.M. Glutamate Neurocircuitry: Theoretical Underpinnings in Schizophrenia. Front. Pharmacol. 2012, 3, 195. [Google Scholar] [CrossRef] [PubMed]
- Homberg, J.R. Measuring Behaviour in Rodents: Towards Translational Neuropsychiatric Research. Behav. Brain Res. 2013, 236, 295–306. [Google Scholar] [CrossRef]
- Leishman, E.; O’Donnell, B.F.; Millward, J.B.; Vohs, J.L.; Rass, O.; Krishnan, G.P.; Bolbecker, A.R.; Morzorati, S.L. Phencyclidine Disrupts the Auditory Steady State Response in Rats. PLoS ONE 2015, 10, e0134979. [Google Scholar] [CrossRef]
- Tanaka, M.; Kunugi, A.; Suzuki, A.; Suzuki, N.; Suzuki, M.; Kimura, H. Preclinical Characterization of AMPA Receptor Potentiator TAK-137 as a Therapeutic Drug for Schizophrenia. Pharmacol. Res. Perspect. 2019, 7, e00479. [Google Scholar] [CrossRef]
- Wilson, C.A.; Koenig, J.I. Social Interaction and Social Withdrawal in Rodents as Readouts for Investigating the Negative Symptoms of Schizophrenia. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2014, 24, 759–773. [Google Scholar] [CrossRef]
- Ishii, D.; Matsuzawa, D.; Kanahara, N.; Matsuda, S.; Sutoh, C.; Ohtsuka, H.; Nakazawa, K.; Kohno, M.; Hashimoto, K.; Iyo, M.; et al. Effects of Aripiprazole on MK-801-Induced Prepulse Inhibition Deficits and Mitogen-Activated Protein Kinase Signal Transduction Pathway. Neurosci. Lett. 2010, 471, 53–57. [Google Scholar] [CrossRef]
- Deiana, S.; Watanabe, A.; Yamasaki, Y.; Amada, N.; Kikuchi, T.; Stott, C.; Riedel, G. MK-801-Induced Deficits in Social Recognition in Rats: Reversal by Aripiprazole, but Not Olanzapine, Risperidone, or Cannabidiol. Behav. Pharmacol. 2015, 26, 748–765. [Google Scholar] [CrossRef] [PubMed]
- Sonnenschein, S.F.; Gill, K.M.; Grace, A.A. State-Dependent Effects of the D2 Partial Agonist Aripiprazole on Dopamine Neuron Activity in the MAM Neurodevelopmental Model of Schizophrenia. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2019, 44, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Lum, J.S.; Pan, B.; Deng, C.; Huang, X.-F.; Ooi, L.; Newell, K.A. Effects of Short- and Long-Term Aripiprazole Treatment on Group I MGluRs in the Nucleus Accumbens: Comparison with Haloperidol. Psychiatry Res. 2018, 260, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Lian, J.; Huang, X.-F.; Deng, C. Aripiprazole Increases the PKA Signalling and Expression of the GABAA Receptor and CREB1 in the Nucleus Accumbens of Rats. J. Mol. Neurosci. MN 2016, 59, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Emsley, R. Drugs in Development for the Treatment of Schizophrenia. Expert Opin. Investig. Drugs 2009, 18, 1103–1118. [Google Scholar] [CrossRef]
- Mace, S.; Taylor, D. Aripiprazole: Dose-Response Relationship in Schizophrenia and Schizoaffective Disorder. CNS Drugs 2009, 23, 773–780. [Google Scholar] [CrossRef]
- Tuplin, E.W.; Stocco, M.R.; Holahan, M.R. Attenuation of MK-801-Induced Behavioral Perseveration by Typical and Atypical Antipsychotic Pretreatment in Rats. Behav. Neurosci. 2015, 129, 399–411. [Google Scholar] [CrossRef]
- Nordquist, R.E.; Risterucci, C.; Moreau, J.L.; von Kienlin, M.; Künnecke, B.; Maco, M.; Freichel, C.; Riemer, C.; Spooren, W. Effects of Aripiprazole/OPC-14597 on Motor Activity, Pharmacological Models of Psychosis, and Brain Activity in Rats. Neuropharmacology 2008, 54, 405–416. [Google Scholar] [CrossRef]
- Breier, A.; Buchanan, R.W.; Kirkpatrick, B.; Davis, O.R.; Irish, D.; Summerfelt, A.; Carpenter, W.T. Effects of Clozapine on Positive and Negative Symptoms in Outpatients with Schizophrenia. Am. J. Psychiatry 1994, 151, 20–26. [Google Scholar] [CrossRef]
- Zorumski, C.F.; Izumi, Y.; Mennerick, S. Ketamine: NMDA Receptors and Beyond. J. Neurosci. Off. J. Soc. Neurosci. 2016, 36, 11158–11164. [Google Scholar] [CrossRef] [PubMed]
- Lodge, D.; Mercier, M.S. Ketamine and Phencyclidine: The Good, the Bad and the Unexpected. Br. J. Pharmacol. 2015, 172, 4254–4276. [Google Scholar] [CrossRef] [PubMed]
- Hiramatsu, M.; Cho, A.K.; Nabeshima, T. Comparison of the Behavioral and Biochemical Effects of the NMDA Receptor Antagonists, MK-801 and Phencyclidine. Eur. J. Pharmacol. 1989, 166, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Light, G.A.; Hsu, J.L.; Hsieh, M.H.; Meyer-Gomes, K.; Sprock, J.; Swerdlow, N.R.; Braff, D.L. Gamma Band Oscillations Reveal Neural Network Cortical Coherence Dysfunction in Schizophrenia Patients. Biol. Psychiatry 2006, 60, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Spencer, K.M.; Niznikiewicz, M.A.; Nestor, P.G.; Shenton, M.E.; McCarley, R.W. Left Auditory Cortex Gamma Synchronization and Auditory Hallucination Symptoms in Schizophrenia. BMC Neurosci. 2009, 10, 85. [Google Scholar] [CrossRef]
- Alegre, M.; Molero, P.; Valencia, M.; Mayner, G.; Ortuño, F.; Artieda, J. Atypical Antipsychotics Normalize Low-Gamma Evoked Oscillations in Patients with Schizophrenia. Psychiatry Res. 2017, 247, 214–221. [Google Scholar] [CrossRef]
- Hong, L.E.; Summerfelt, A.; McMahon, R.; Adami, H.; Francis, G.; Elliott, A.; Buchanan, R.W.; Thaker, G.K. Evoked Gamma Band Synchronization and the Liability for Schizophrenia. Schizophr. Res. 2004, 70, 293–302. [Google Scholar] [CrossRef]
- Kikuchi, M.; Koenig, T.; Wada, Y.; Higashima, M.; Koshino, Y.; Strik, W.; Dierks, T. Native EEG and Treatment Effects in Neuroleptic-Naïve Schizophrenic Patients: Time and Frequency Domain Approaches. Schizophr. Res. 2007, 97, 163–172. [Google Scholar] [CrossRef]
- Mitra, S.; Nizamie, S.H.; Goyal, N.; Tikka, S.K. Evaluation of Resting State Gamma Power as a Response Marker in Schizophrenia. Psychiatry Clin. Neurosci. 2015, 69, 630–639. [Google Scholar] [CrossRef]
- Bowman, C.; Richter, U.; Jones, C.R.; Agerskov, C.; Herrik, K.F. Activity-State Dependent Reversal of Ketamine-Induced Resting State EEG Effects by Clozapine and Naltrexone in the Freely Moving Rat. Front. Psychiatry 2022, 13, 737295. [Google Scholar] [CrossRef]
- Jones, N.C.; Reddy, M.; Anderson, P.; Salzberg, M.R.; O’Brien, T.J.; Pinault, D. Acute Administration of Typical and Atypical Antipsychotics Reduces EEG γ Power, but Only the Preclinical Compound LY379268 Reduces the Ketamine-Induced Rise in γ Power. Int. J. Neuropsychopharmacol. 2012, 15, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Ahnaou, A.; Huysmans, H.; Van de Casteele, T.; Drinkenburg, W.H.I.M. Cortical High Gamma Network Oscillations and Connectivity: A Translational Index for Antipsychotics to Normalize Aberrant Neurophysiological Activity. Transl. Psychiatry 2017, 7, 1285. [Google Scholar] [CrossRef]
- Meier, M.A.; Lemercier, C.E.; Kulisch, C.; Kiss, B.; Lendvai, B.; Adham, N.; Gerevich, Z. The Novel Antipsychotic Cariprazine Stabilizes Gamma Oscillations in Rat Hippocampal Slices. Br. J. Pharmacol. 2020, 177, 1622–1634. [Google Scholar] [CrossRef] [PubMed]
- Rebollo, B.; Perez-Zabalza, M.; Ruiz-Mejias, M.; Perez-Mendez, L.; Sanchez-Vives, M.V. Beta and Gamma Oscillations in Prefrontal Cortex During NMDA Hypofunction: An In Vitro Model of Schizophrenia Features. Neuroscience 2018, 383, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Fusar-Poli, P.; Papanastasiou, E.; Stahl, D.; Rocchetti, M.; Carpenter, W.; Shergill, S.; McGuire, P. Treatments of Negative Symptoms in Schizophrenia: Meta-Analysis of 168 Randomized Placebo-Controlled Trials. Schizophr. Bull. 2015, 41, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Stabell, L.A.; Johnsen, E.; Kroken, R.A.; Løberg, E.M.; Blindheim, A.; Joa, I.; Reitan, S.K.; Rettenbacher, M.; Munk-Jørgensen, P.; Gjestad, R. Clinical Insight among Persons with Schizophrenia Spectrum Disorders Treated with Amisulpride, Aripiprazole or Olanzapine: A Semi-Randomised Trial. BMC Psychiatry 2023, 23, 482. [Google Scholar] [CrossRef] [PubMed]
- Remington, G.; Foussias, G.; Fervaha, G.; Agid, O.; Takeuchi, H.; Lee, J.; Hahn, M. Treating Negative Symptoms in Schizophrenia: An Update. Curr. Treat. Options Psychiatry 2016, 3, 133–150. [Google Scholar] [CrossRef]
- Leucht, S.; Corves, C.; Arbter, D.; Engel, R.R.; Li, C.; Davis, J.M. Second-Generation versus First-Generation Antipsychotic Drugs for Schizophrenia: A Meta-Analysis. Lancet Lond. Engl. 2009, 373, 31–41. [Google Scholar] [CrossRef]
- Hereta, M.; Kamińska, K.; Rogóż, Z. Co-Treatment with Antidepressants and Aripiprazole Reversed the MK-801-Induced Some Negative Symptoms of Schizophrenia in Rats. Pharmacol. Rep. PR 2019, 71, 768–773. [Google Scholar] [CrossRef]
- Białoń, M.; Chocyk, A.; Majcher-Maślanka, I.; Żarnowska, M.; Michalski, K.; Antkiewicz-Michaluk, L.; Wąsik, A. 1MeTIQ and Olanzapine, despite Their Neurochemical Impact, Did Not Ameliorate Performance in Fear Conditioning and Social Interaction Tests in an MK-801 Rat Model of Schizophrenia. Pharmacol. Rep. PR 2021, 73, 490–505. [Google Scholar] [CrossRef]
- Sampedro-Viana, D.; Cañete, T.; Sanna, F.; Oliveras, I.; Lavín, V.; Torrecilla, P.; Río-Álamos, C.; Tapias-Espinosa, C.; Sánchez-González, A.; Tobeña, A.; et al. Atypical Antipsychotics Attenuate MK801-Induced Social Withdrawal and Hyperlocomotion in the RHA Rat Model of Schizophrenia-Relevant Features. Psychopharmacology 2023, 240, 1931–1945. [Google Scholar] [CrossRef]
- Savolainen, K.; Ihalainen, J.; Jalkanen, A.J.; Forsberg, M.M. Selective Adrenergic Alpha2C Receptor Antagonist Ameliorates Acute Phencyclidine-Induced Schizophrenia-like Social Interaction Deficits in Rats. Psychopharmacology 2019, 236, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Senkowski, D.; Gallinat, J. Dysfunctional Prefrontal Gamma-Band Oscillations Reflect Working Memory and Other Cognitive Deficits in Schizophrenia. Biol. Psychiatry 2015, 77, 1010–1019. [Google Scholar] [CrossRef]
- Clifton, N.E.; Morisot, N.; Girardon, S.; Millan, M.J.; Loiseau, F. Enhancement of Social Novelty Discrimination by Positive Allosteric Modulators at Metabotropic Glutamate 5 Receptors: Adolescent Administration Prevents Adult-Onset Deficits Induced by Neonatal Treatment with Phencyclidine. Psychopharmacology 2013, 225, 579–594. [Google Scholar] [CrossRef] [PubMed]
- Dogra, S.; Conn, P.J. Metabotropic Glutamate Receptors As Emerging Targets for the Treatment of Schizophrenia. Mol. Pharmacol. 2022, 101, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Kantrowitz, J.T.; Woods, S.W.; Petkova, E.; Cornblatt, B.; Corcoran, C.M.; Chen, H.; Silipo, G.; Javitt, D.C. D-Serine for the Treatment of Negative Symptoms in Individuals at Clinical High Risk of Schizophrenia: A Pilot, Double-Blind, Placebo-Controlled, Randomised Parallel Group Mechanistic Proof-of-Concept Trial. Lancet Psychiatry 2015, 2, 403–412. [Google Scholar] [CrossRef]
- Pei, J.-C.; Luo, D.-Z.; Gau, S.-S.; Chang, C.-Y.; Lai, W.-S. Directly and Indirectly Targeting the Glycine Modulatory Site to Modulate NMDA Receptor Function to Address Unmet Medical Needs of Patients With Schizophrenia. Front. Psychiatry 2021, 12, 742058. [Google Scholar] [CrossRef]
- Rosenbrock, H.; Dorner-Ciossek, C.; Giovannini, R.; Schmid, B.; Schuelert, N. Effects of the Glycine Transporter-1 Inhibitor Iclepertin (BI 425809) on Sensory Processing, Neural Network Function, and Cognition in Animal Models Related to Schizophrenia. J. Pharmacol. Exp. Ther. 2022, 382, 223–232. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; Academic Press: Cambridge, MA, USA, 1998; ISBN 978-0-12-547619-5. [Google Scholar]





| Compound | Dose, Route and Volume of Administration | Time of Administration |
|---|---|---|
| PCP | 5 mg/kg, sc (2 mL/kg) | 15 min before the ASSR paradigm |
| MK-801 | 0.2 mg/kg, sc (2 mL/kg) | 15 min before the ASSR paradigm |
| Ketamine | 20 mg/kg, sc (2 mL/kg) | 15 min before the ASSR paradigm |
| Vehicle of PCP, MK-801 and ketamine (NaCl 0.9%) | sc (2 mL/kg) | 15 min before the ASSR paradigm |
| Aripiprazole | 3 mg/kg, ip (2 mL/kg) | 45 min before the ASSR paradigm |
| Aripiprazole’s vehicle | ip (2 mL/kg) | 45 min before the ASSR paradigm |
| Compound | Dose, Route and Volume of Administration | Time of Administration |
|---|---|---|
| PCP | 5 mg/kg, sc (2 mL/kg) | Just before actimetry |
| MK-801 | 0.2 mg/kg, sc (2 mL/kg) | Just before actimetry |
| Ketamine | 20 mg/kg, sc (2 mL/kg) | Just before actimetry |
| Vehicle of PCP, MK-801 and ketamine (NaCl 0.9%) | sc (2 mL/kg) | Just before actimetry |
| Aripiprazole | 1, 3, 10, 30 mg/kg, ip (2 mL/kg) | 30 min before actimetry |
| Aripiprazole’s vehicle | ip (2 mL/kg) | 30 min before actimetry |
| Compound | Dose, Route and Volume of Administration | Time of Administration |
|---|---|---|
| PCP | 5 mg/kg, sc (2 mL/kg) | 45 min before the social interaction test |
| MK-801 | 0.2 mg/kg, sc (2 mL/kg) | 240 min before the social interaction test |
| Ketamine | 20 mg/kg, sc (2 mL/kg) | 30 min before the social interaction test |
| Vehicle of PCP, MK-801 and ketamine (NaCl 0.9%, used in Section 2.2) | sc (2 mL/kg) | 30 min before the social interaction test |
| MK-801’s vehicle (used in Section 2.5) | sc (2 mL/kg) | 240 min before the social interaction test |
| Aripiprazole | 1, 3, 10 mg/kg, ip (2 mL/kg) | 30 min before the social interaction test |
| Aripiprazole’s vehicle | ip (2 mL/kg) | 30 min before the social interaction test |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adraoui, F.W.; Hettak, K.; Viardot, G.; Alix, M.; Guiffard, S.; Meot, B.; L’Hostis, P.; Maurin, A.; Delpy, E.; Drieu La Rochelle, C.; et al. Differential Effects of Aripiprazole on Electroencephalography-Recorded Gamma-Band Auditory Steady-State Response, Spontaneous Gamma Oscillations and Behavior in a Schizophrenia Rat Model. Int. J. Mol. Sci. 2024, 25, 1035. https://doi.org/10.3390/ijms25021035
Adraoui FW, Hettak K, Viardot G, Alix M, Guiffard S, Meot B, L’Hostis P, Maurin A, Delpy E, Drieu La Rochelle C, et al. Differential Effects of Aripiprazole on Electroencephalography-Recorded Gamma-Band Auditory Steady-State Response, Spontaneous Gamma Oscillations and Behavior in a Schizophrenia Rat Model. International Journal of Molecular Sciences. 2024; 25(2):1035. https://doi.org/10.3390/ijms25021035
Chicago/Turabian StyleAdraoui, Florian W., Kenza Hettak, Geoffrey Viardot, Magali Alix, Sabrina Guiffard, Benoît Meot, Philippe L’Hostis, Anne Maurin, Eric Delpy, Christophe Drieu La Rochelle, and et al. 2024. "Differential Effects of Aripiprazole on Electroencephalography-Recorded Gamma-Band Auditory Steady-State Response, Spontaneous Gamma Oscillations and Behavior in a Schizophrenia Rat Model" International Journal of Molecular Sciences 25, no. 2: 1035. https://doi.org/10.3390/ijms25021035
APA StyleAdraoui, F. W., Hettak, K., Viardot, G., Alix, M., Guiffard, S., Meot, B., L’Hostis, P., Maurin, A., Delpy, E., Drieu La Rochelle, C., & Carvalho, K. (2024). Differential Effects of Aripiprazole on Electroencephalography-Recorded Gamma-Band Auditory Steady-State Response, Spontaneous Gamma Oscillations and Behavior in a Schizophrenia Rat Model. International Journal of Molecular Sciences, 25(2), 1035. https://doi.org/10.3390/ijms25021035






