Analysis of Cytokine and Chemokine Level in Tear Film in Keratoconus Patients before and after Corneal Cross-Linking (CXL) Treatment
Abstract
:1. Introduction
2. Results
2.1. Values of Topographic Measurements
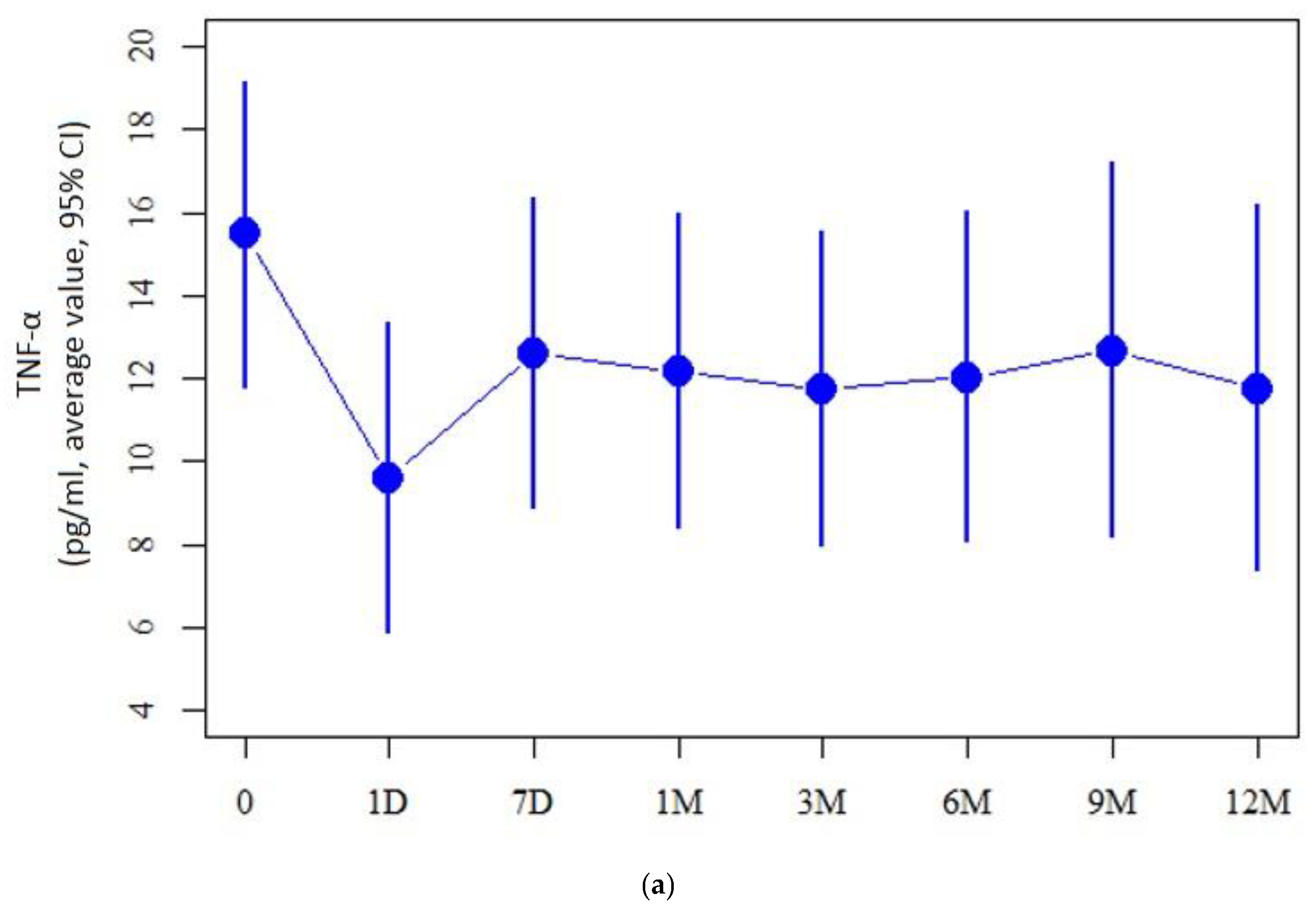
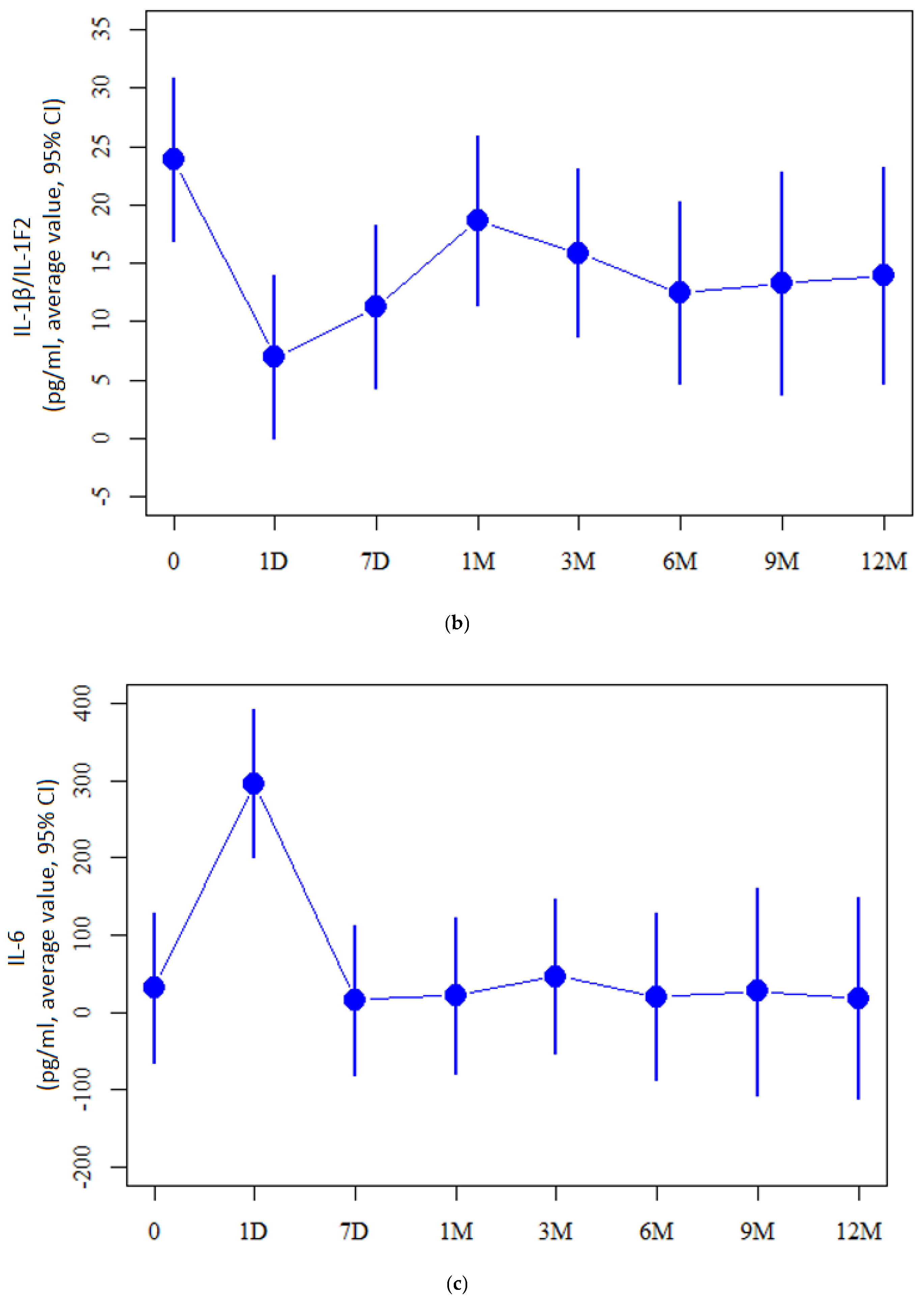
2.2. Cytokine Measurement Values
2.3. Correlation of Cytokines with Cornea Topographic Parameters
3. Discussion
4. Materials and Methods
4.1. Study Population and Measurements
4.2. Cross-Linking Treatment
4.3. Collection and Analysis of Tears
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Godefrooij, D.A.; de Wit, G.A.; Uiterwaal, C.S.; Imhof, S.M.; Wisse, R.P. Age-specific Incidence and Prevalence of Keratoconus: A Nationwide Registration Study. Am. J. Ophthalmol. 2017, 175, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.A.; Tan, D.; Rapuano, C.J.; Belin, M.W.; Ambrósio, R., Jr.; Guell, J.L.; Malecaze, F.; Nishida, K.; Sangwan, V.S.; Group of Panelists for the Global Delphi Panel of Keratoconus and Ectatic Diseases. Global Consensus on Keratoconus and Ectatic Diseases. Cornea 2015, 34, 359–369. [Google Scholar] [CrossRef]
- Pedrotti, E.; Chierego, C.; Bonacci, E.; De Gregorio, A.; De Rossi, A.; Zuliani, A.; Fasolo, A.; Marchini, G. New treatments for keratoconus. Int. Ophthalmol. 2020, 40, 1619–1623. [Google Scholar] [CrossRef] [PubMed]
- Loh, I.P.; Sherwin, T. Is keratoconus an inflammatory disease? The implication of inflammatory pathways. Ocul. Immunol. Inflamm. 2022, 30, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Sawaguchi, S.; Twining, S.S.; Sugar, J.; Feder, R.S.; Yue, B.Y. Expression of degradative enzymes and protease inhibitors in corneas with keratoconus. Investig. Ophthalmol. Vis. Sci. 1998, 39, 1117–1124. [Google Scholar]
- Collier, S.A.; Madigan, M.C.; Penfold, P.L. Expression of membrane-type 1 matrix metalloproteinase (MT1-MMP) and MMP-2 in normal and keratoconus corneas. Curr. Eye Res. 2000, 21, 662–668. [Google Scholar] [CrossRef]
- Jun, A.S.; Cope, L.; Speck, C.; Feng, X.; Lee, S.; Meng, H. Subnormal cytokine profile in the tear fluid of keratoconus patients. PLoS ONE 2011, 6, e16437. Available online: http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0016437 (accessed on 27 January 2011). [CrossRef]
- Lema, I.; Durán, J.A. Inflammatory molecules in the tears of patients with keratoconus. Ophthalmology 2005, 112, 654–659. [Google Scholar] [CrossRef]
- Galvis, V.; Sherwin, T.; Tello, A.; Merayo, J.; Barrera, R.; Acera, A. Keratoconus: An inflammatory disorder? Eye 2015, 29, 843–859. [Google Scholar] [CrossRef]
- Stern, M.E.; Beuerman, R.W.; Fox, R.I.; Gao, J.; Mircheff, A.K.; Pflugfelder, S.C. The pathology of dry eye: The interaction between the ocular surface and lacrimal glands. Cornea 1998, 17, 584–589. [Google Scholar] [CrossRef]
- Perumal, N.; Funke, S.; Pfeiffer, N.; Grus, F.H. Proteomics analysis of human tears from aqueous-deficient and evaporative dry eye patients. Sci. Rep. 2016, 6, 29629. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Sheng, M.; Li, J.; Yan, G.; Lin, A.; Li, M.; Wang, W.; Chen, Y. Tear proteomic analysis of Sjögren syndrome patients with dry eye syndrome by two-dimensional-nano-liquid chromatography coupled with tandem mass spectrometry. Sci. Rep. 2014, 4, 5772. [Google Scholar] [CrossRef] [PubMed]
- Von Thun Und Hohenstein-Blaul, N.; Funke, S.; Grus, F.H. Tears as a source of biomarkers for ocular and systemic diseases. Exp. Eye Res. 2013, 117, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Soria, J.; Durán, J.A.; Etxebarria, J.; Merayo, J.; González, N.; Reigada, R.; García, I.; Acera, A.; Suárez, T. Tear proteome and protein network analyses reveal a novel pentamarker panel for tear film characterization in dry eye and meibomian gland dysfunction. J. Proteom. 2013, 78, 94–112. [Google Scholar] [CrossRef]
- Acera, A.; Vecino, E.; Rodriguez-Agirretxe, I.; Aloria, K.; Arizmendi, J.M.; Morales, C.; Duran, J.A. Changes in tear protein profile in keratoconus disease. Eye 2011, 25, 1225–1233. [Google Scholar] [CrossRef]
- García, B.; García-Suárez, O.; Merayo-Lloves, J.; Ferrara, G.; Alcalde, I.; González, J.; Lisa, C.; Alfonso, J.F.; Vazquez, F.; Quirós, L.M. Heparanase Overexpresses in Keratoconic Cornea and Tears Depending on the Pathologic Grade. Dis. Markers 2017, 2017, 3502386. [Google Scholar] [CrossRef]
- Nishtala, K.; Pahuja, N.; Shetty, R.; Nuijts, R.M.; Ghosh, A. Tear biomarkers for keratoconus. Eye Vis. 2016, 3, 19. [Google Scholar] [CrossRef]
- Karamichos, D.; Zieske, J.D.; Sejersen, H.; Sarker-Nag, A.; Asara, J.M.; Hjortdal, J. Tear metabolite changes in keratoconus. Exp. Eye Res. 2015, 132, 1–8. [Google Scholar] [CrossRef]
- Kolozsvári, B.L.; Berta, A.; Petrovski, G.; Miháltz, K.; Gogolák, P.; Rajnavölgyi, E.; Hassan, Z.; Széles, P.; Fodor, M. Alterations of tear mediators in patients with keratoconus after corneal crosslinking associate with corneal changes. PLoS ONE 2013, 8, e76333. [Google Scholar] [CrossRef]
- Pavlenko, T.A.; Chesnokova, N.B.; Davydova, H.G.; Okhotsimskaia, T.D.; Beznos, O.V.; Grigor’ev, A.V. Level of tear endothelin-1 and plasminogen in patients with glaucoma and proliferative diabetic retinopathy. Vestn. Oftalmol. 2013, 129, 20–23. [Google Scholar]
- Recalde, J.I.; Duran, J.A.; Rodriguez-Agirretxe, I.; Soria, J.; Sanchez-Tena, M.A.; Pereiro, X.; Suarez, T.; Acera, A. Changes in tear biomarker levels in keratoconus after corneal collagen crosslinking. Mol. Vis. 2019, 25, 12–21. [Google Scholar] [PubMed]
- Tu, H.; Li, Y.L. Inflammation balance in skeletal muscle damage and repair. Front. Immunol. 2023, 14, 1133355. [Google Scholar] [CrossRef] [PubMed]
- Bonecchi, R.; Galliera, E.; Borroni, E.M.; Corsi, M.M.; Locati, M.; Mantovani, A. Chemokines and chemokine receptors: An overview. Front. Biosci. 2009, 14, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Carreño, E.; Enríquez-de-Salamanca, A.; Tesón, M.; García-Vázquez, C.; Stern, M.E.; Whitcup, S.M.; Calonge, M. Cytokine and chemokine levels in tears from healthy subjects. Acta Ophthalmol. 2010, 88, e250-8. [Google Scholar] [CrossRef]
- Pinheiro-Costa, J.; Lima Fontes, M.; Luís, C.; Martins, S.; Soares, R.; Madeira, D.; Falcão-Reis, F.; Carneiro, Â. Serum inflammatory biomarkers are associated with increased choroidal thickness in keratoconus. Sci. Rep. 2023, 13, 10862. [Google Scholar] [CrossRef]
- Elbeyli, A.; Kurtul, B.E. Systemic immune-inflammation index, neutrophil-to-lymphocyte ratio, and platelet-to-lymphocyte ratio levels are associated with keratoconus. Indian. J. Ophthalmol. 2021, 69, 1725–1729. [Google Scholar]
- Dou, S.; Wang, Q.; Zhang, B.; Wei, C.; Wang, H.; Liu, T.; Duan, H.; Jiang, H.; Liu, M.; Qi, X.; et al. Single-cell atlas of keratoconus corneas revealed aberrant transcriptional signatures and implicated mechanical stretch as a trigger for keratoconus pathogenesis. Cell Discov. 2022, 8, 66. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, S.; He, Y.; Zhang, Y. The research progress on the molecular mechanism of corneal cross-linking in keratoconus treatment. Cont. Lens Anterior Eye 2023, 46, 101795. [Google Scholar] [CrossRef]
- Acar Eser, N.; Dikmetas, O.; Kocabeyoglu, S.; Tan, C.; Irkec, M. Evaluation of Keratoconus Disease with Tear Cytokine and Chemokine Levels Before and After Corneal Cross-Linking Treatment. Ocul. Immunol. Inflamm. 2023, 1–7. [Google Scholar] [CrossRef]
- Balasubramanian, S.A.; Mohan, S.; Pye, D.C.; Willcox, M.D. Proteases, proteolysis and inflammatory molecules in the tears of people with keratoconus. Acta Ophthalmol. 2012, 90, e303–e309. [Google Scholar] [CrossRef]
- Nichani, P.A.; Solomon, B.; Trinh, T.; Mimouni, M.; Rootman, D.; Singal, N.; Chan, C.C. Investigating the role of inflammation in keratoconus: A retrospective analysis of 551 eyes. Eur. J. Ophthalmol. 2023, 33, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Goñi, N.; Martínez-Soroa, I.; Ibarrondo, O.; Azkargorta, M.; Elortza, F.; Galarreta, D.J.; Acera, A. Tear proteome profile in eyes with keratoconus after intracorneal ring segment implantation or corneal crosslinking. Front. Med. 2022, 9, 944504. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.T.; Lee, Y.J.; Lee, J.W.; Lu, S.; Tucci, M.A.; Dai, X.; Ojeda, N.B.; Lee, H.J.; Fan, L.W.; Tien, L.T. Interleukin-1 receptor antagonist ameliorates the pain hypersensitivity, spinal inflammation and oxidative stress induced by systemic lipopolysaccharide in neonatal rats. Neurochem. Int. 2020, 135, 104686. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Che, M.; Xin, J.; Zheng, Z.; Li, J.; Zhang, S. The role of IL-1β and TNF-α in intervertebral disc degeneration. Biomed. Pharmacother. 2020, 131, 110660. [Google Scholar] [CrossRef]
- Kumar, N.R.; Praveen, M.; Narasimhan, R.; Khamar, P.; D’Souza, S.; Sinha-Roy, A.; Sethu, S.; Shetty, R.; Ghosh, A. Tear biomarkers in dry eye disease: Progress in the last decade. Indian J. Ophthalmol. 2023, 71, 1190–1202. [Google Scholar] [CrossRef]
- Greenstein, S.A.; Fry, K.L.; Hersh, P.S. Corneal topography indices after corneal collagen crosslinking for keratoconus and corneal ectasia: One-year results. J. Cataract Refract. Surg. 2011, 37, 1282–1290. [Google Scholar] [CrossRef]
- Wollensak, G.; Iomdina, E.; Dittert, D.D.; Herbst, H. Wound healing in the rabbit cornea after corneal collagen cross-linking with riboflavin and UVA. Cornea 2007, 26, 600–605. [Google Scholar] [CrossRef]
- Wollensak, G.; Iomdina, E.; Dittert, D.-D.; Herbst, H. Corneal healing after riboflavin ultraviolet-A collagen crosslinking determined by confocal laser scanning microscopy in vivo: Early and late modifications. Am. J. Ophthalmol. 2008, 146, 527–533. [Google Scholar]
- Mazzotta, C.; Traversi, C.; Baiocchi, S.; Caporossi, O.; Bovone, C.; Sparano, M.C.; Balestrazzi, A.; Caporossi, A. Corneal collagen crosslinking in progressive keratoconus: Multicenter results from the French National Reference Center for Keratoconus. J. Cataract Refract. Surg. 2011, 37, 2137–2143. [Google Scholar]
- Ebihara, N.; Matsuda, A.; Nakamura, S.; Matsuda, H.; Murakami, A. Role of the IL-6 classic- and trans-signaling pathways in corneal sterile inflammation and wound healing. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8549–8557. [Google Scholar] [CrossRef]
- Pásztor, D.; Kolozsvári, B.L.; Csutak, A.; Berta, A.; Hassan, Z.; Kettesy, B.A.; Gogolák, P.; Fodor, M. Scheimpflug Imaging Parameters Associated with Tear Mediators and Bronchial Asthma in Keratoconus. J. Ophthalmol. 2016, 2016, 9392640. [Google Scholar] [CrossRef]
- Ionescu, I.C.; Corbu, C.G.; Tanase, C.; Ionita, G.; Nicula, C.; Coviltir, V.; Potop, V.; Constantin, M.; Codrici, E.; Mihai, S.; et al. Overexpression of Tear Inflammatory Cytokines as Additional Finding in Keratoconus Patients and Their First Degree Family Members. Mediat. Inflamm. 2018, 2018, 4285268. [Google Scholar] [CrossRef] [PubMed]
- Fodor, M.; Vitályos, G.; Losonczy, G.; Hassan, Z.; Pásztor, D.; Gogolák, P.; Kolozsvári, B.L. Tear Mediators NGF along with IL-13 Predict Keratoconus Progression. Ocul. Immunol. Inflamm. 2021, 29, 1090–1101. [Google Scholar] [CrossRef] [PubMed]
- Vinciguerra, P.; Albè, E.; Trazza, S.; Seiler, T.; Epstein, D. Intraoperative and postoperative effects of corneal collagen crosslinking on progressive keratoconus. Arch. Ophthalmol. 2009, 127, 1258–1265. [Google Scholar] [CrossRef] [PubMed]
- Greenstein, S.A.; Fry, K.L.; Hersh, M.J.; Hersh, P.S. Higher-order aberrations after corneal collagen crosslinking for keratoconus and corneal ectasia. J. Cataract Refract. Surg. 2012, 38, 292–302. [Google Scholar] [CrossRef]
- Hersh, P.S.; Greenstein, S.A.; Fry, K.L. Corneal collagen crosslinking for keratoconus and corneal ectasia: One-year results. J. Cataract Refract. Surg. 2011, 37, 149–160. [Google Scholar] [CrossRef]
- Kránitz, K.; Kovács, I.; Miháltz, K.; Sándor, G.L.; Knorz, M.C.; Németh, J.; Nagy, Z.Z. Corneal changes in progressive keratoconus after cross-linking assessed by Scheimpflug camera. J. Refract. Surg. 2012, 28, 645–649. [Google Scholar] [CrossRef]
- Zaheer, N.; Khan, W.A.; Khan, S.; Khan, M.A.M. Comparison of Changes in Central Corneal Thickness During Corneal Collagen Cross-Linking, Using Isotonic Riboflavin Solutions With and Without Dextran, in the Treatment of Progressive Keratoconus. Cornea 2018, 37, 340–346. [Google Scholar] [CrossRef]
- Kobashi, H.; Hieda, O.; Itoi, M.; Kamiya, K.; Kato, N.; Shimazaki, J.; Tsubota, K. The Keratoconus Study Group Of Japan. Corneal Cross-Linking for Paediatric Keratoconus: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 2626. [Google Scholar] [CrossRef]
- Peyman, A.; Namgar, M.; Feizi, A.; Hakemi, M.G.; Nasab, F.H.; Pourazizi, M. Interleukin-6 and tumor necrosis factor-α levels in tear film of Keratoconus patients. J. Res. Med. Sci. 2021, 26, 75. [Google Scholar] [CrossRef]
- Dawidowicz, M.; Kula, A.; Mielcarska, S.; Kiczmer, P.; Skiba, H.; Krygier, M.; Chrabańska, M.; Piecuch, J.; Szrot, M.; Robotycka, J.; et al. B7H4 Expression Is More Frequent in MSS Status Colorectal Cancer and Is Negatively Associated with Tumour Infiltrating Lymphocytes. Cells 2023, 12, 861. [Google Scholar] [CrossRef] [PubMed]
- Bronikowska, J.; Kłósek, M.; Janeczko, T.; Kostrzewa-Susłow, E.; Czuba, Z.P. The modulating effect of methoxy-derivatives of 2’-hydroxychalcones on the release of IL-8, MIF, VCAM-1 and ICAM-1 by colon cancer cells. Biomed. Pharmacother. 2022, 145, 112428. [Google Scholar] [CrossRef] [PubMed]
| Parameter | 0 | 7D | 1M | 3M | 6M | 9M | 12M |
|---|---|---|---|---|---|---|---|
| Ks [D] | 49.8 (3.7) | 50.8 (4.1) | 50.3 (3.9) | 49.4 (3.9) | 49.7 (4.1) | 49.8 (4.5) | 48.5 (3.2) |
| Kf [D] | 46.3 (3.2) | 46.9 (3.6) | 46.6 (3.6) | 45.8 (3.4) | 46.3 (3.8) | 46.2 (4) | 45.3 (2.9) |
| CYL [D] | 3.5 (1.7) | 4 (1.5) | 3.8 (2) | 3.6 (1.7) | 3.4 (1.4) | 3.6 (1.6) | 3.2 (1.7) |
| PI-Apex [μm] | 483 (30.8) | 480.3 (59.9) | 465.3 (37.7) | 463.4 (49.3) | 452.6 (36.4) | 462 (39.9) | 460.9 (40.8) |
| PI-Thinnest [μm] | 451.4 (35.7) | 450.7 (55.9) | 436.6 (34.7) | 429.9 (38.3) | 421.1 (36.9) | 435.3 (42.9) | 429.2 (39) |
| (a) | |||||
| Ks | Kf | Cyl | PI-Apex | PI-Thinnest | |
| TNF-α | r = 0.35, p = 0.017 * | r = 0.4, p = 0.006 * | r = 0.133, p = 0.379 | r = −0.11, p = 0.467 | r = −0.215, p = 0.151 |
| IL-6 | r = 0.339, p = 0.021 * | r = 0.447, p = 0.002 * | r = −0.09, p = 0.554 | r = −0.13, p = 0.39 | r = −0.185, p = 0.22 |
| PDGF-BB | r = 0.227, p = 0.129 | r = 0.305, p = 0.039 * | r = 0.043, p = 0.775 | r = −0.199, p = 0.185 | r = −0.282, p = 0.058 |
| IL-8/CXCL-8 | r = 0.316, p = 0.032 * | r = 0.353, p = 0.016 * | r = 0.124, p = 0.412 | r = −0.142, p = 0.345 | r = −0.058, p = 0.702 |
| IL-7 | r = 0.002, p = 0.99 | r = −0.115, p = 0.448 | r = 0.166, p = 0.269 | r = −0.036, p = 0.813 | r = 0.117, p = 0.438 |
| CXCL-10/IP-10/CRG2 | r = 0.227, p = 0.129 | r = 0.286, p = 0.054 | r = −0.014, p = 0.926 | r = −0.229, p = 0.126 | r = −0.326, p = 0.027 * |
| IL-10 | r = 0.329, p = 0.026 * | r = 0.408, p = 0.005 * | r = 0.046, p = 0.76 | r = −0.248, p = 0.096 | r = −0.37, p = 0.011 * |
| CCL2/JE/MCP-1 | r = 0.098, p = 0.519 | r = 0.193, p = 0.199 | r = −0.084, p = 0.58 | r = −0.197, p = 0.19 | r = −0.232, p = 0.121 |
| NGF-β | r = 0.265, p = 0.075 | r = 0.315, p = 0.033 * | r = 0.08, p = 0.597 | r = −0.172, p = 0.254 | r = −0.222, p = 0.138 |
| IFN-γ | r = 0.244, p = 0.103 | r = 0.331, p = 0.025 * | r = 0.021, p = 0.891 | r = −0.22, p = 0.141 | r = −0.239, p = 0.11 |
| CCL-3/MIP-1a | r = 0.292, p = 0.049 * | r = 0.368, p = 0.012 * | r = 0.108, p = 0.474 | r = −0.206, p = 0.17 | r = −0.309, p = 0.037 * |
| CCL4/MIP-1b | r = 0.271, p = 0.068 | r = 0.331, p = 0.024 * | r = 0.119, p = 0.431 | r = −0.083, p = 0.586 | r = −0.152, p = 0.313 |
| FGFbasic/FGF2/bFGF | r = 0.316, p = 0.032 * | r = 0.354, p = 0.016 * | r = 0.059, p = 0.698 | r = −0.04, p = 0.792 | r = −0.232, p = 0.121 |
| IL-15 | r = 0.184, p = 0.221 | r = 0.325, p = 0.028 * | r = −0.101, p = 0.505 | r = −0.215, p = 0.151 | r = −0.313, p = 0.034 * |
| IL-1β/IL-1F2 | r = 0.309, p = 0.037 * | r = 0.315, p = 0.033 * | r = 0.149, p = 0.324 | r = −0.154, p = 0.306 | r = −0.253, p = 0.09 |
| IL-1RI | r = 0.172, p = 0.252 | r = 0.152, p = 0.314 | r = 0.112, p = 0.459 | r = −0.038, p = 0.8 | r = −0.034, p = 0.821 |
| IL-13 | r = 0.248, p = 0.096 | r = 0.325, p = 0.027 * | r = 0.04, p = 0.794 | r = −0.064, p = 0.672 | r = −0.172, p = 0.252 |
| IL-12/IL-23 p40 | r = 0.304, p = 0.04 * | r = 0.322, p = 0.029 * | r = 0.138, p = 0.361 | r = −0.183, p = 0.222 | r = −0.301, p = 0.042 * |
| (b) | |||||
| Ks | Kf | Cyl | PI-Apex | PI-Thinnest | |
| TNF-α | r = 0.583, p = 0.002 * | r = 0.593, p = 0.002 * | r = 0.165, p = 0.431 | r = −0.338, p = 0.099 | r = −0.274, p = 0186 |
| IL-6 | r = 0.455, p = 0.022 * | r = 0.423, p = 0.035 * | r = 0.162, p = 0.438 | r = −0.45, p = 0.024 * | r = −0.373, p = 0.066 |
| PDGF-BB | r = −0.081, p = 0.7 | r = −0.05, p = 0.812 | r = −0.082, p = 0.698 | r = −0.066, p = 0.755 | r = −0.021, p = 0.92 |
| IL-8/CXCL-8 | r = 0.201, p = 0.334 | r = 0.084, p = 0.689 | r = 0.187, p = 0.369 | r = −0.099, p = 0.638 | r = −0.029, p = 0.891 |
| IL-7 | r = −0.179, p = 0.39 | r = −0.127, p = 0.544 | r = −0.041, p = 0.847 | r = −0.1, p = 0.633 | r = 0.179, p = 0.391 |
| CXCL-10/IP-10/CRG2 | r = 0.232, p = 0.264 | r = 0.347, p = 0.09 | r = −0.237, p = 0.253 | r = −0.179, p = 0.391 | r = −0.294, p = 0.154 |
| IL-10 | r = 0.318, p = 0.122 | r = 0.391, p = 0.054 | r = −0.01, p = 0.963 | r = −0.229, p = 0.271 | r = −0.242, p = 0.244 |
| CCL2/JE/MCP-1 | r = 0.19, p = 0.361 | r = 0.118, p = 0.574 | r = 0.077, p = 0.714 | r = 0.168, p = 0.423 | r = 0.049, p = 0.815 |
| NGF-β | r = 0.493, p = 0.012 * | r = 0.525, p = 0.007 * | r = 0.143, p = 0.495 | r = −0.201, p = 0.335 | r = −0.086, p = 0.681 |
| IFN-γ | r = 0.664, p < 0.001 * | r = 0.564, p = 0.003 * | r = 0.247, p = 0.235 | r = −0.367, p = 0.071 | r = −0.262, p = 0.206 |
| CCL-3/MIP-1a | r = 0.576, p = 0.003 * | r = 0.583, p = 0.002 * | r = 0.14, p = 0.505 | r = −0.441, p = 0.027 * | r = −0.404, p = 0.045 * |
| CCL4/MIP-1b | r = 0.432, p = 0.031 * | r = 0.448, p = 0.025 * | r = 0.094, p = 0.654 | r = −0.156, p = 0.457 | r = −0.043, p = 0.836 |
| FGFbasic/FGF2/bFGF | r = 0.445, p = 0.026 * | r = 0.504, p = 0.01 * | r = 0.01, p = 0.962 | r = −0.449, p = 0.024 * | r = −0.411, p = 0.041 * |
| IL-15 | r = 0.284, p = 0.17 | r = 0.414, p = 0.04 * | r = −0.14, p = 0.503 | r = −0.614, p = 0.001 * | r = −0.55, p = 0.004 * |
| IL-1β/IL-1F2 | r = 0.421, p = 0.036 * | r = 0.402, p = 0.046 * | r = 0.053, p = 0.801 | r = −0.054, p = 0.799 | r = −0.022, p = 0.916 |
| IL-1RI | r = 0.023, p = 0.914 | r = −0.073, p = 0.728 | r = 0.176, p = 0.398 | r = 0.283, p = 0.17 | r = 0.192, p = 0.359 |
| IL-13 | r = 0.569, p = 0.003 * | r = 0.564, p = 0.003 * | r = 0.228, p = 0.272 | r = −0.172, p = 0.41 | r = −0.199, p = 0.34 |
| IL-12/IL-23 p40 | r = 0.392, p = 0.052 | r = 0.459, p = 0.021 * | r = −0.025, p = 0.907 | r = −0.252, p = 0.224 | r = −0.325, p = 0.113 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krok, M.; Wróblewska-Czajka, E.; Łach-Wojnarowicz, O.; Bronikowska, J.; Czuba, Z.P.; Wylęgała, E.; Dobrowolski, D. Analysis of Cytokine and Chemokine Level in Tear Film in Keratoconus Patients before and after Corneal Cross-Linking (CXL) Treatment. Int. J. Mol. Sci. 2024, 25, 1052. https://doi.org/10.3390/ijms25021052
Krok M, Wróblewska-Czajka E, Łach-Wojnarowicz O, Bronikowska J, Czuba ZP, Wylęgała E, Dobrowolski D. Analysis of Cytokine and Chemokine Level in Tear Film in Keratoconus Patients before and after Corneal Cross-Linking (CXL) Treatment. International Journal of Molecular Sciences. 2024; 25(2):1052. https://doi.org/10.3390/ijms25021052
Chicago/Turabian StyleKrok, Magdalena, Ewa Wróblewska-Czajka, Olga Łach-Wojnarowicz, Joanna Bronikowska, Zenon P. Czuba, Edward Wylęgała, and Dariusz Dobrowolski. 2024. "Analysis of Cytokine and Chemokine Level in Tear Film in Keratoconus Patients before and after Corneal Cross-Linking (CXL) Treatment" International Journal of Molecular Sciences 25, no. 2: 1052. https://doi.org/10.3390/ijms25021052






