Inhibitory Effects of Shikonin Dispersion, an Extract of Lithospermum erythrorhizon Encapsulated in β-1,3-1,6 Glucan, on Streptococcus mutans and Non-Mutans Streptococci
Abstract
:1. Introduction
2. Results
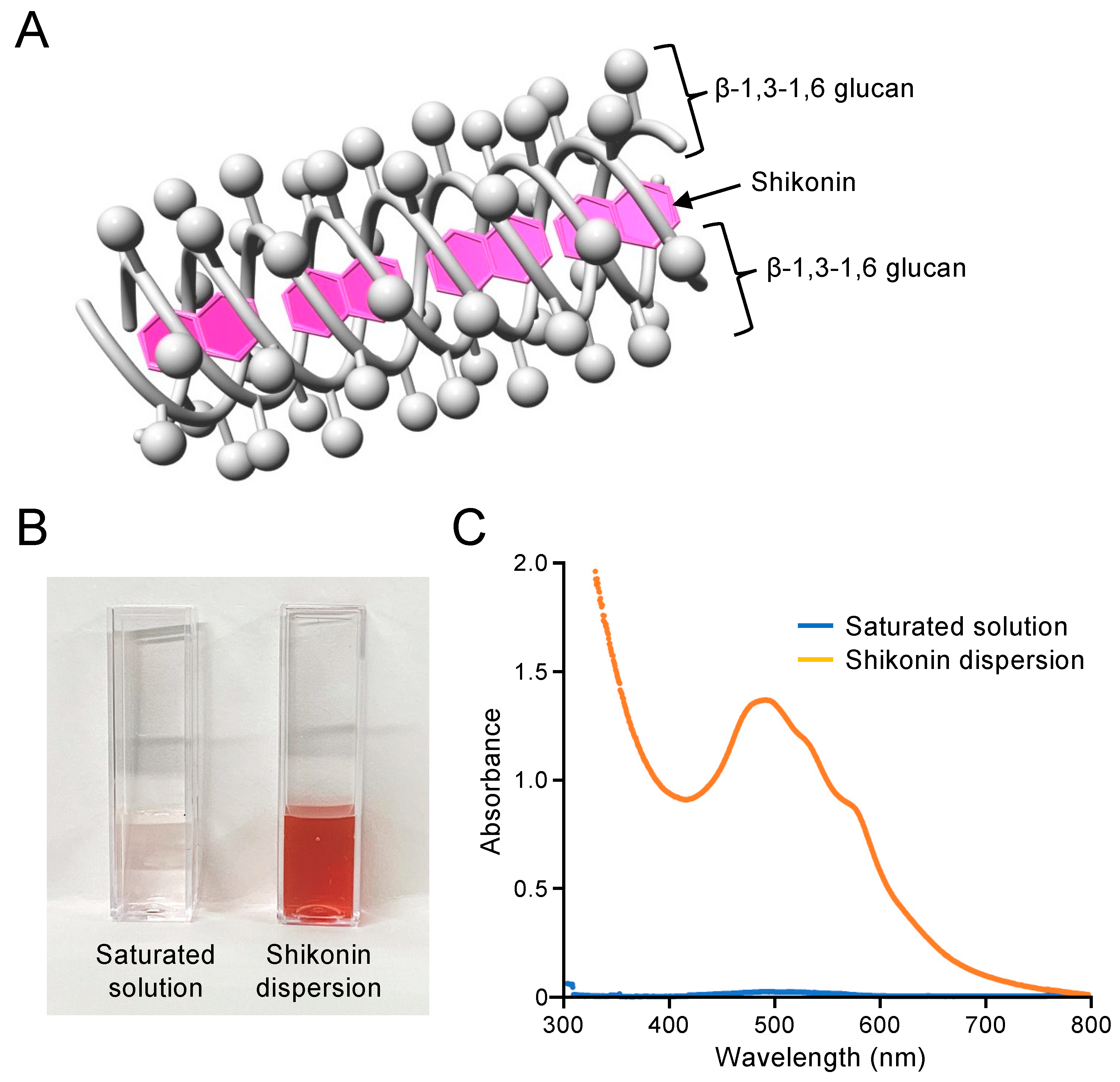
2.1. Preparation and Concentration Determination of Shikonin Dispersion
2.2. Inhibitory Effects of Shikonin Dispersion on S. mutans
2.3. Inhibitory Effects of Shikonin Dispersion on the Growth of S. mutans
2.4. Inhibitory Effects of Shikonin Dispersion on Biofilm Formation by S. mutans
2.5. Inhibitory Effects of Shikonin Dispersion on Non-Mutans Streptococci
2.6. Effect of Shikonin Dispersion-Containing Toothpaste on the Number of S. mutans in the Oral Cavity
3. Discussion
4. Materials and Methods
4.1. Preparation of Shikonin Dispersion
4.2. Bacterial Strains and Growth Conditions
4.3. Antimicrobial Activity
4.4. Bacterial Growth Assay
4.5. Microscopic Observation of In Vitro Biofilms
4.6. Biofilm Assay
4.7. Design for Human Study
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baty, J.J.; Stoner, S.N.; Scoffield, J.A. Oral Commensal Streptococci: Gatekeepers of the Oral Cavity. J. Bacteriol. 2022, 204, e0025722. [Google Scholar] [CrossRef]
- Simón-Soro, A.; Tomás, I.; Cabrera-Rubio, R.; Catalan, M.D.; Nyvad, B.; Mira, A. Microbial geography of the oral cavity. J. Dent. Res. 2013, 92, 616–621. [Google Scholar] [CrossRef]
- Marsh, P.D. Microbial ecology of dental plaque and its significance in health and disease. Adv. Dent. Res. 1994, 8, 263–271. [Google Scholar] [CrossRef]
- Takahashi, N.; Nyvad, B. Caries ecology revisited: Microbial dynamics and the caries process. Caries Res. 2008, 42, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Nyvad, B. Ecological Hypothesis of Dentin and Root Caries. Caries Res. 2016, 50, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Momeni, S.S.; Ghazal, T.; Grenett, H.; Whiddon, J.; Moser, S.A.; Childers, N.K. Streptococcus mutans serotypes and collagen-binding proteins Cnm/Cbm in children with caries analysed by PCR. Mol. Oral Microbiol. 2019, 34, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Abranches, J.; Miller, J.H.; Martinez, A.R.; Simpson-Haidaris, P.J.; Burne, R.A.; Lemos, J.A. The collagen-binding protein Cnm is required for Streptococcus mutans adherence to and intracellular invasion of human coronary artery endothelial cells. Infect. Immun. 2011, 79, 2277–2284. [Google Scholar] [CrossRef]
- Segura-Egea, J.J.; Gould, K.; Şen, B.H.; Jonasson, P.; Cotti, E.; Mazzoni, A.; Sunay, H.; Tjäderhane, L.; Dummer, P.M.H. Antibiotics in Endodontics: A review. Int. Endod. J. 2017, 50, 1169–1184. [Google Scholar] [CrossRef]
- Tzen, J.T.C. Strictinin: A Key Ingredient of Tea. Molecules 2023, 28, 3961. [Google Scholar] [CrossRef]
- Andújar, I.; Ríos, J.L.; Giner, R.M.; Recio, M.C. Pharmacological properties of shikonin—A review of literature since 2002. Planta Med. 2013, 79, 1685–1697. [Google Scholar] [CrossRef]
- Kadoyama, K.; Takenokuchi, M.; Matsuura, K.; Shichiri, H.; Watanabe, A.; Yamaguchi, H.; Takahashi, H.; Takano-Ohmuro, H.; Taniguchi, T. Therapeutic effects of shikonin on skin lesions in mouse models of allergic dermatitis and wound. Tradit. Kampo Med. 2019, 6, 62–70. [Google Scholar] [CrossRef]
- Haghbeen, K.; Pourmolaei, S.; Mareftjo, M.J.; Mousavi, A.; Akbari Noghabi, K.; Hosseini Shirazi, F.; Meshkat, A. Detailed investigations on the solid cell culture and antimicrobial activities of the Iranian Arnebia euchroma. J. Biomed. Biotechnol. 2011, 2011, 165852. [Google Scholar] [CrossRef] [PubMed]
- Albreht, A.; Vovk, I.; Simonovska, B. Addition of β-lactoglobulin produces water-soluble shikonin. J. Agric. Food Chem. 2012, 60, 10834–10843. [Google Scholar] [CrossRef]
- Dobšíková, R.; Blahová, J.; Mikulíková, I.; Modrá, H.; Prášková, E.; Svobodová, Z.; Skorič, M.; Jarkovský, J.; Siwicki, A.K. The effect of oyster mushroom β-1.3/1.6-D-glucan and oxytetracycline antibiotic on biometrical, haematological, biochemical, and immunological indices, and histopathological changes in common carp (Cyprinus carpio L.). Fish Shellfish. Immunol. 2013, 35, 1813–1823. [Google Scholar] [CrossRef] [PubMed]
- Bobadilla, F.; Rodriguez-Tirado, C.; Imarai, M.; Galotto, M.J.; Andersson, R. Soluble β-1,3/1,6-glucan in seaweed from the southern hemisphere and its immunomodulatory effect. Carbohydr. Polym. 2013, 92, 241–248. [Google Scholar] [CrossRef]
- Felix, L.; Mylonakis, E.; Fuchs, B.B. Thioredoxin Reductase Is a Valid Target for Antimicrobial Therapeutic Development Against Gram-Positive Bacteria. Front. Microbiol. 2021, 12, 663481. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, Y.; Wang, L.; Li, M.; Shi, D.; Li, D.; Wang, J. Shikonin alleviates the biotoxicity produced by pneumococcal pneumolysin. Life Sci. 2017, 177, 1–7. [Google Scholar] [CrossRef]
- Huang, X.Y.; Fu, H.L.; Tang, H.Q.; Yin, Z.Q.; Zhang, W.; Shu, G.; Yin, L.Z.; Zhao, L.; Yan, X.R.; Lin, J.C. Optimization Extraction of Shikonin Using Ultrasound-Assisted Response Surface Methodology and Antibacterial Studies. Evid. Based Complement. Altern. Med. 2020, 2020, 1208617. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Serrano, J.; Iniesta, M.; Santa Cruz, I.; Herrera, D. Antiplaque and antigingivitis toothpastes. Monogr. Oral. Sci. 2013, 23, 27–44. [Google Scholar] [CrossRef]
- Teng, F.; He, T.; Huang, S.; Bo, C.P.; Li, Z.; Chang, J.L.; Liu, J.Q.; Charbonneau, D.; Xu, J.; Li, R.; et al. Cetylpyridinium chloride mouth rinses alleviate experimental gingivitis by inhibiting dental plaque maturation. Int. J. Oral. Sci. 2016, 8, 182–190. [Google Scholar] [CrossRef]
- Rodricks, J.V.; Swenberg, J.A.; Borzelleca, J.F.; Maronpot, R.R.; Shipp, A.M. Triclosan: A critical review of the experimental data and development of margins of safety for consumer products. Crit. Rev. Toxicol. 2010, 40, 422–484. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.I.; Koustas, E.; Vesterinen, H.M.; Sutton, P.; Atchley, D.S.; Kim, A.N.; Campbell, M.; Donald, J.M.; Sen, S.; Bero, L.; et al. Application of the Navigation Guide systematic review methodology to the evidence for developmental and reproductive toxicity of triclosan. Environ. Int. 2016, 92–93, 716–728. [Google Scholar] [CrossRef]
- Datta, S.; He, G.; Tomilov, A.; Sahdeo, S.; Denison, M.S.; Cortopassi, G. In Vitro Evaluation of Mitochondrial Function and Estrogen Signaling in Cell Lines Exposed to the Antiseptic Cetylpyridinium Chloride. Environ. Health Perspect. 2017, 125, 087015. [Google Scholar] [CrossRef]
- Weatherly, L.M.; Gosse, J.A. Triclosan exposure, transformation, and human health effects. J. Toxicol. Environ. Health B Crit. Rev. 2017, 20, 447–469. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Yoo, J.; Lim, Y.M.; Kim, E.J.; Yoon, B.I.; Kim, P.; Yu, S.D.; Eom, I.C.; Shim, I. Comprehensive pulmonary toxicity assessment of cetylpyridinium chloride using A549 cells and Sprague-Dawley rats. J. Appl. Toxicol. 2021, 41, 470–482. [Google Scholar] [CrossRef]
- Su, L.; Liu, L.; Wang, Y.; Yan, G.; Zhang, Y. Long-term systemic toxicity of shikonin derivatives in Wistar rats. Pharm. Biol. 2014, 52, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Nomura, R.; Morita, Y.; Matayoshi, S.; Nakano, K. Inhibitory effect of surface pre-reacted glass-ionomer (S-PRG) eluate against adhesion and colonization by Streptococcus mutans. Sci. Rep. 2018, 8, 5056. [Google Scholar] [CrossRef]
- Nomura, R.; Inaba, H.; Matayoshi, S.; Yoshida, S.; Matsumi, Y.; Matsumoto-Nakano, M.; Nakano, K. Inhibitory effect of a mouth rinse formulated with chlorhexidine gluconate, ethanol, and green tea extract against major oral bacterial species. J. Oral. Sci. 2020, 62, 206–211. [Google Scholar] [CrossRef]
- Asao, Y.; Iwamoto, Y.; Chea, C.; Chher, T.; Mitsuhata, C.; Naito, M.; Kozai, K. The effect of improving oral health literacy among teachers on the oral health condition of primary schoolchildren in Cambodia. Eur. J. Paediatr. Dent. 2022, 23, 321–326. [Google Scholar] [CrossRef]
- Asao, Y.; Iwamoto, Y.; Mitsuhata, C.; Naito, M.; Kozai, K. Three-year survey of oral hygiene conditions of Cambodian public primary school children. J. Oral. Sci. 2022, 64, 208–211. [Google Scholar] [CrossRef]
- Ooshima, T.; Izumitani, A.; Sobue, S.; Hamada, S. Cariostatic effect of palatinose on experimental dental caries in rats. Jpn. J. Med. Sci. Biol. 1983, 36, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Wagenknecht, D.R.; Gregory, R.L. Analyses of the Effects of Arginine, Nicotine, Serotype and Collagen-Binding Proteins on Biofilm Development by 33 Strains of Streptococcus mutans. Front. Oral. Health 2021, 2, 764784. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, T.; Kawaguchi, M.; Shimizu, N.; Hoshino, N.; Ooshima, T.; Fujiwara, T. PCR detection and identification of oral streptococci in saliva samples using gtf genes. Diagn. Microbiol. Infect. Dis. 2004, 48, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Matsumoto, M.; Tanaka, T.; Maeda, M.; Nakai, M.; Hamada, S.; Ooshima, T. Antibacterial activity of polyphenol components in oolong tea extract against Streptococcus mutans. Caries Res. 2004, 38, 2–8. [Google Scholar] [CrossRef]
- Usuda, M.; Kametani, M.; Hamada, M.; Suehiro, Y.; Matayoshi, S.; Okawa, R.; Naka, S.; Matsumoto-Nakano, M.; Akitomo, T.; Mitsuhata, C.; et al. Inhibitory Effect of Adsorption of Streptococcus mutans onto Scallop-Derived Hydroxyapatite. Int. J. Mol. Sci. 2023, 24, 11371. [Google Scholar] [CrossRef]
- Kuboniwa, M.; Tribble, G.D.; James, C.E.; Kilic, A.O.; Tao, L.; Herzberg, M.C.; Shizukuishi, S.; Lamont, R.J. Streptococcus gordonii utilizes several distinct gene functions to recruit Porphyromonas gingivalis into a mixed community. Mol. Microbiol. 2006, 60, 121–139. [Google Scholar] [CrossRef]
- Mayumi, S.; Kuboniwa, M.; Sakanaka, A.; Hashino, E.; Ishikawa, A.; Ijima, Y.; Amano, A. Potential of Prebiotic D-Tagatose for Prevention of Oral Disease. Front. Cell Infect. Microbiol. 2021, 11, 767944. [Google Scholar] [CrossRef]
- Lee, J.; Townsend, J.A.; Thompson, T.; Garitty, T.; De, A.; Yu, Q.; Peters, B.M.; Wen, Z.T. Analysis of the Cariogenic Potential of Various Almond Milk Beverages using a Streptococcus mutans Biofilm Model in vitro. Caries Res. 2018, 52, 51–57. [Google Scholar] [CrossRef]





| Species | Strain | Serotype | Origin | References |
|---|---|---|---|---|
| S. mutans | MT8148 | c | Oral cavity | [31] |
| S. mutans | NN2087 | e | Oral cavity | [28] |
| S. mutans | NN2072 | f | Oral cavity | [28] |
| S. mutans | SA31 | k | Oral cavity | [32] |
| S. sanguinis | ATCC 10556 | - | Oral cavity | [33] |
| S. oralis | ATCC 10557 | - | Oral cavity | [33] |
| S. gordonii | ATCC 10558 | - | Oral cavity | [33] |
| S. salivarius | HHT | - | Oral cavity | [33] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nomura, R.; Suehiro, Y.; Tojo, F.; Matayoshi, S.; Okawa, R.; Hamada, M.; Naka, S.; Matsumoto-Nakano, M.; Unesaki, R.; Koumoto, K.; et al. Inhibitory Effects of Shikonin Dispersion, an Extract of Lithospermum erythrorhizon Encapsulated in β-1,3-1,6 Glucan, on Streptococcus mutans and Non-Mutans Streptococci. Int. J. Mol. Sci. 2024, 25, 1075. https://doi.org/10.3390/ijms25021075
Nomura R, Suehiro Y, Tojo F, Matayoshi S, Okawa R, Hamada M, Naka S, Matsumoto-Nakano M, Unesaki R, Koumoto K, et al. Inhibitory Effects of Shikonin Dispersion, an Extract of Lithospermum erythrorhizon Encapsulated in β-1,3-1,6 Glucan, on Streptococcus mutans and Non-Mutans Streptococci. International Journal of Molecular Sciences. 2024; 25(2):1075. https://doi.org/10.3390/ijms25021075
Chicago/Turabian StyleNomura, Ryota, Yuto Suehiro, Fumikazu Tojo, Saaya Matayoshi, Rena Okawa, Masakazu Hamada, Shuhei Naka, Michiyo Matsumoto-Nakano, Rika Unesaki, Kazuya Koumoto, and et al. 2024. "Inhibitory Effects of Shikonin Dispersion, an Extract of Lithospermum erythrorhizon Encapsulated in β-1,3-1,6 Glucan, on Streptococcus mutans and Non-Mutans Streptococci" International Journal of Molecular Sciences 25, no. 2: 1075. https://doi.org/10.3390/ijms25021075
APA StyleNomura, R., Suehiro, Y., Tojo, F., Matayoshi, S., Okawa, R., Hamada, M., Naka, S., Matsumoto-Nakano, M., Unesaki, R., Koumoto, K., Kawauchi, K., Nishikata, T., Akitomo, T., Mitsuhata, C., Yagi, M., Mizoguchi, T., Fujikawa, K., Taniguchi, T., & Nakano, K. (2024). Inhibitory Effects of Shikonin Dispersion, an Extract of Lithospermum erythrorhizon Encapsulated in β-1,3-1,6 Glucan, on Streptococcus mutans and Non-Mutans Streptococci. International Journal of Molecular Sciences, 25(2), 1075. https://doi.org/10.3390/ijms25021075







