Theoretical and Experimental Study of Different Side Chains on 3,4-Ethylenedioxythiophene and Diketopyrrolopyrrole-Derived Polymers: Towards Organic Transistors
Abstract
:1. Introduction
2. Results
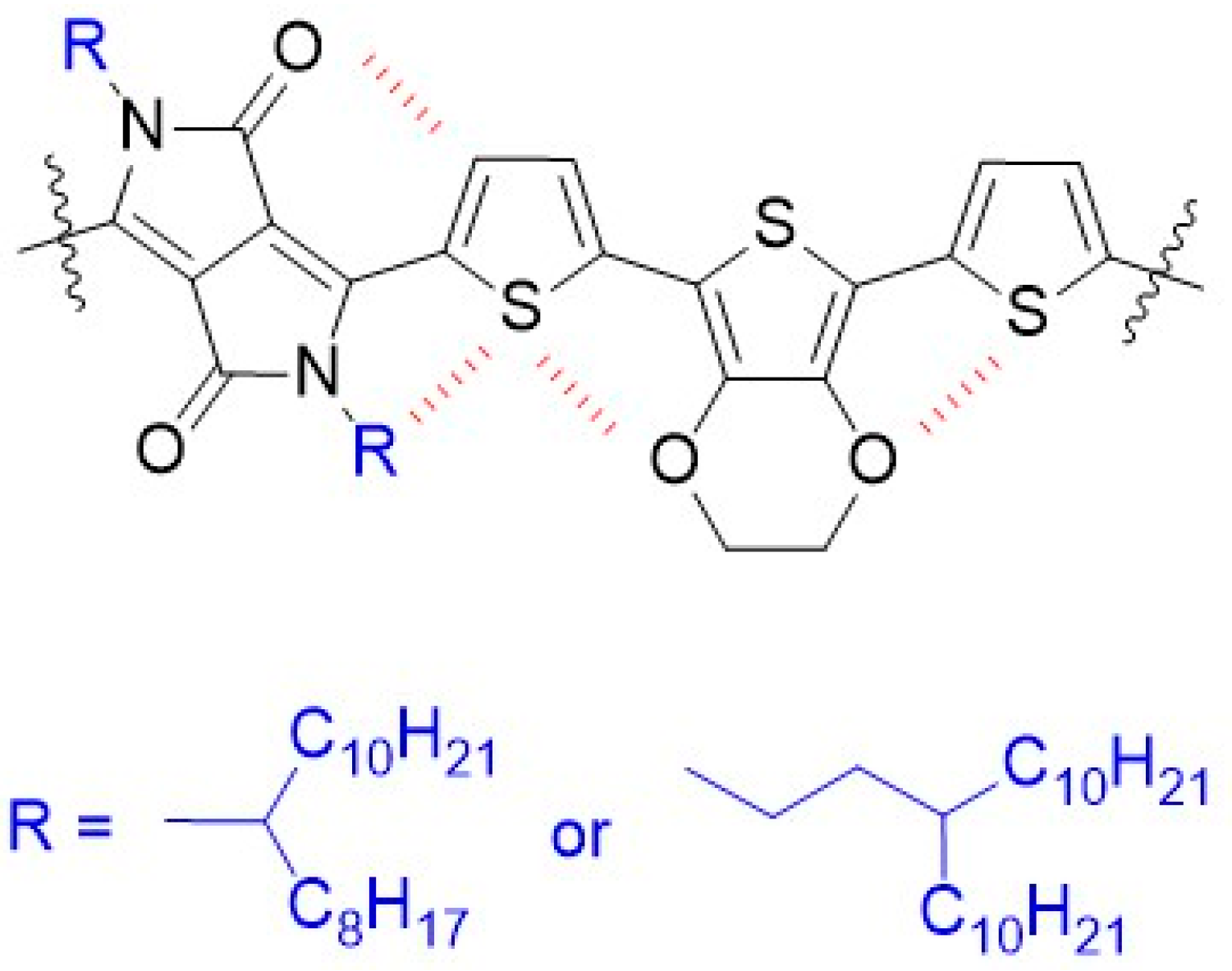
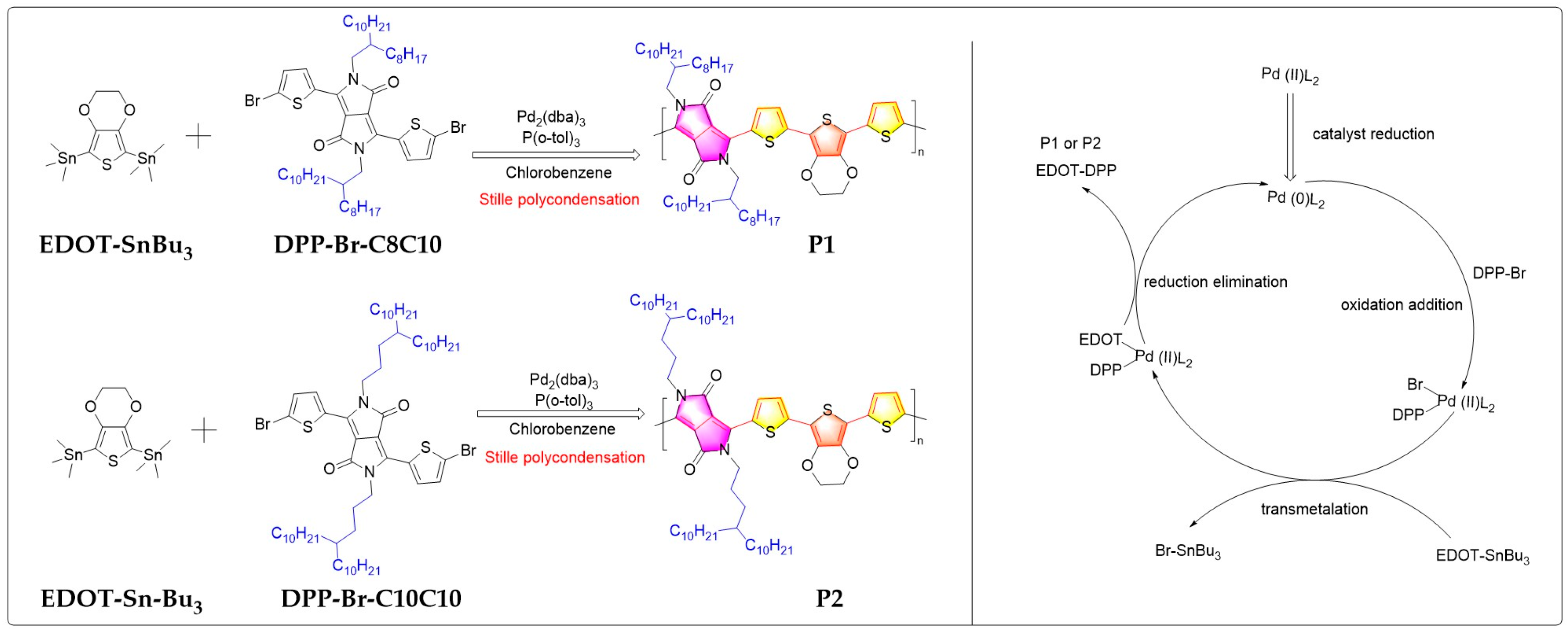
2.1. Synthesis Routes to Polymers P1 and P2 through Stille Coupling Polymerization
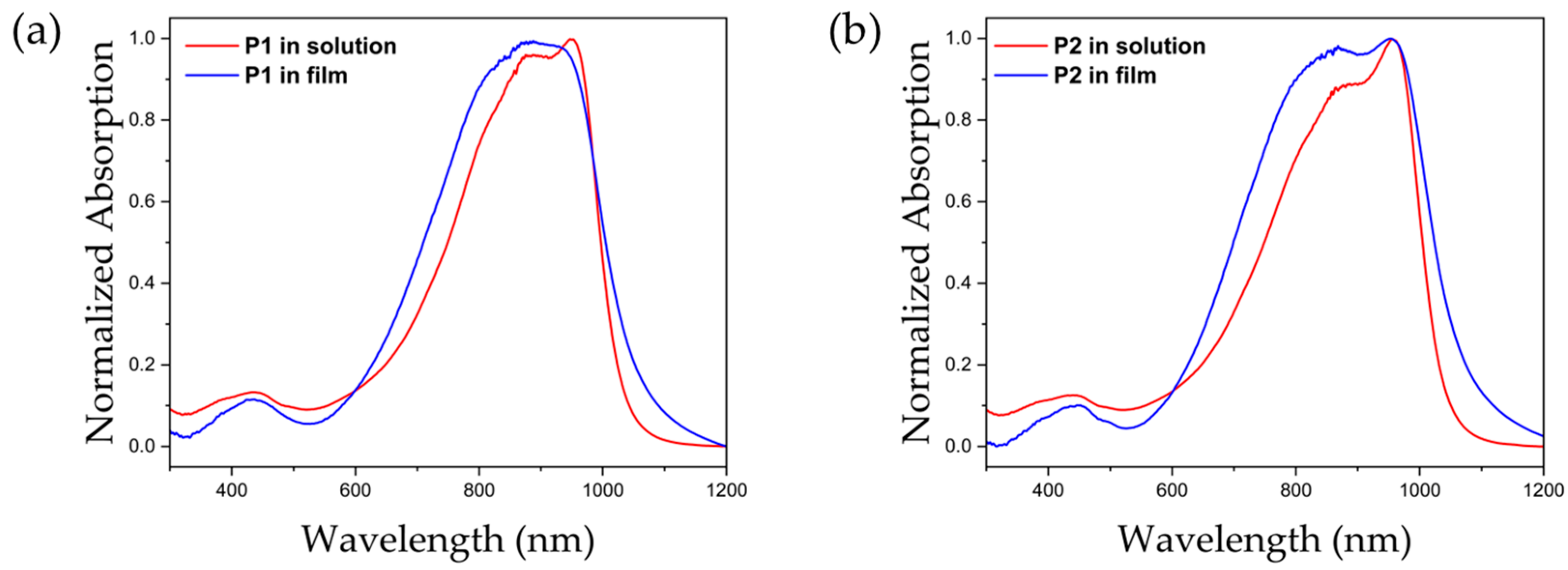
2.2. Photochemical Properties
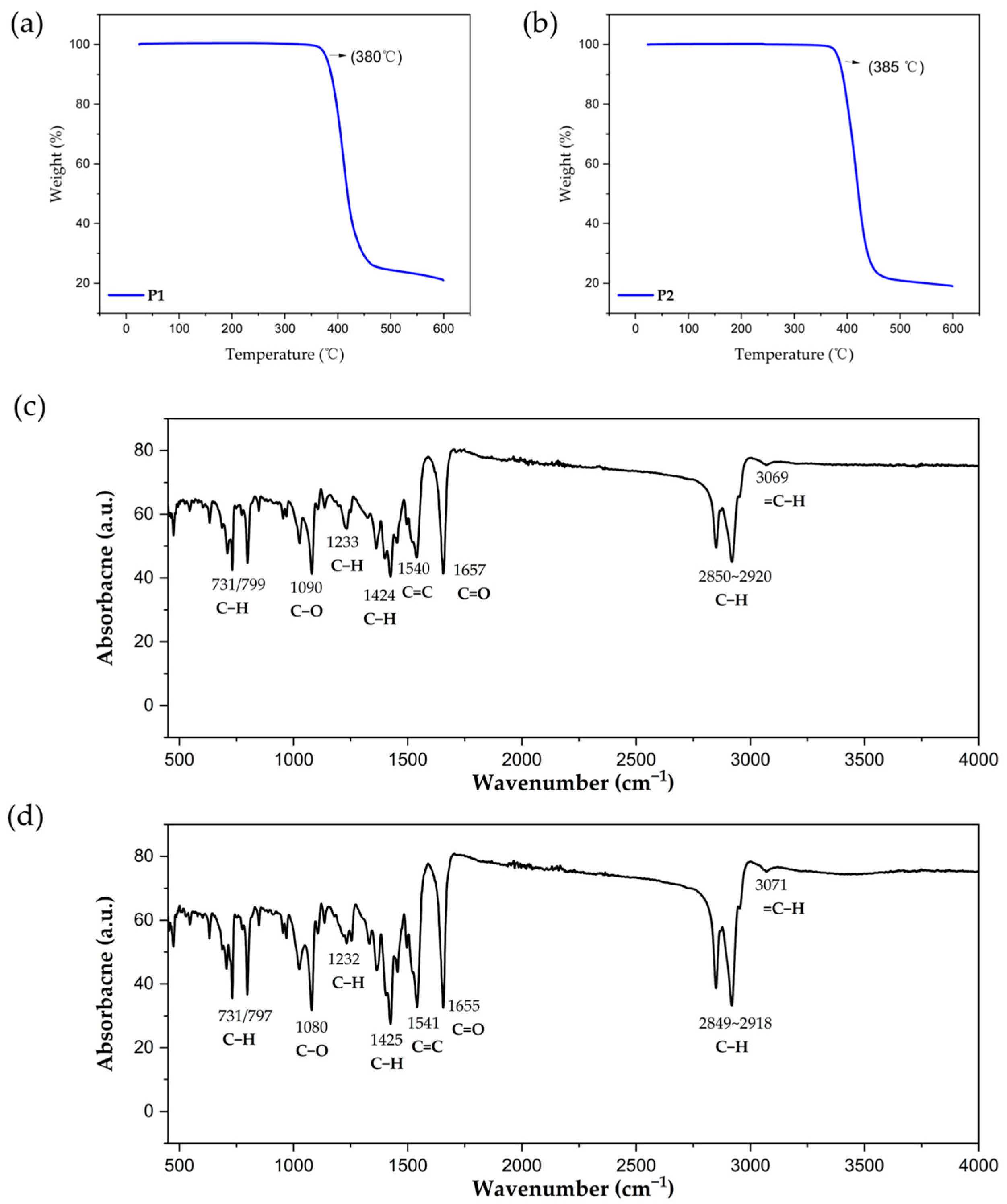
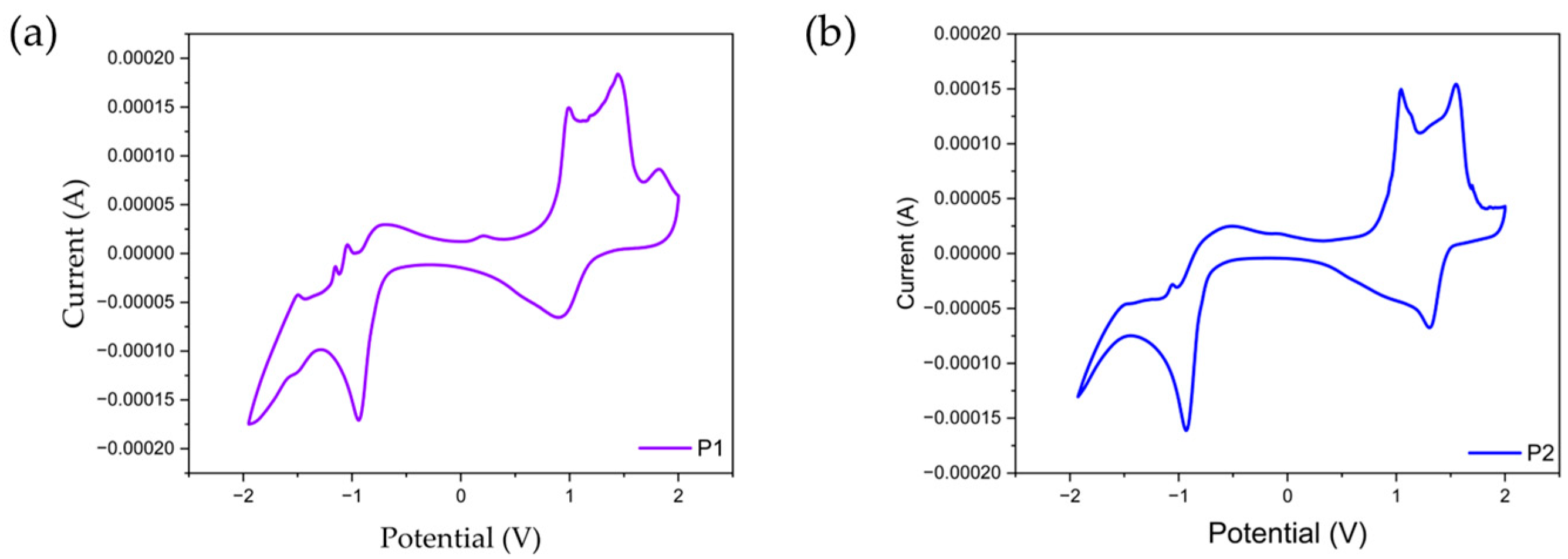
2.3. Electrochemical Properties
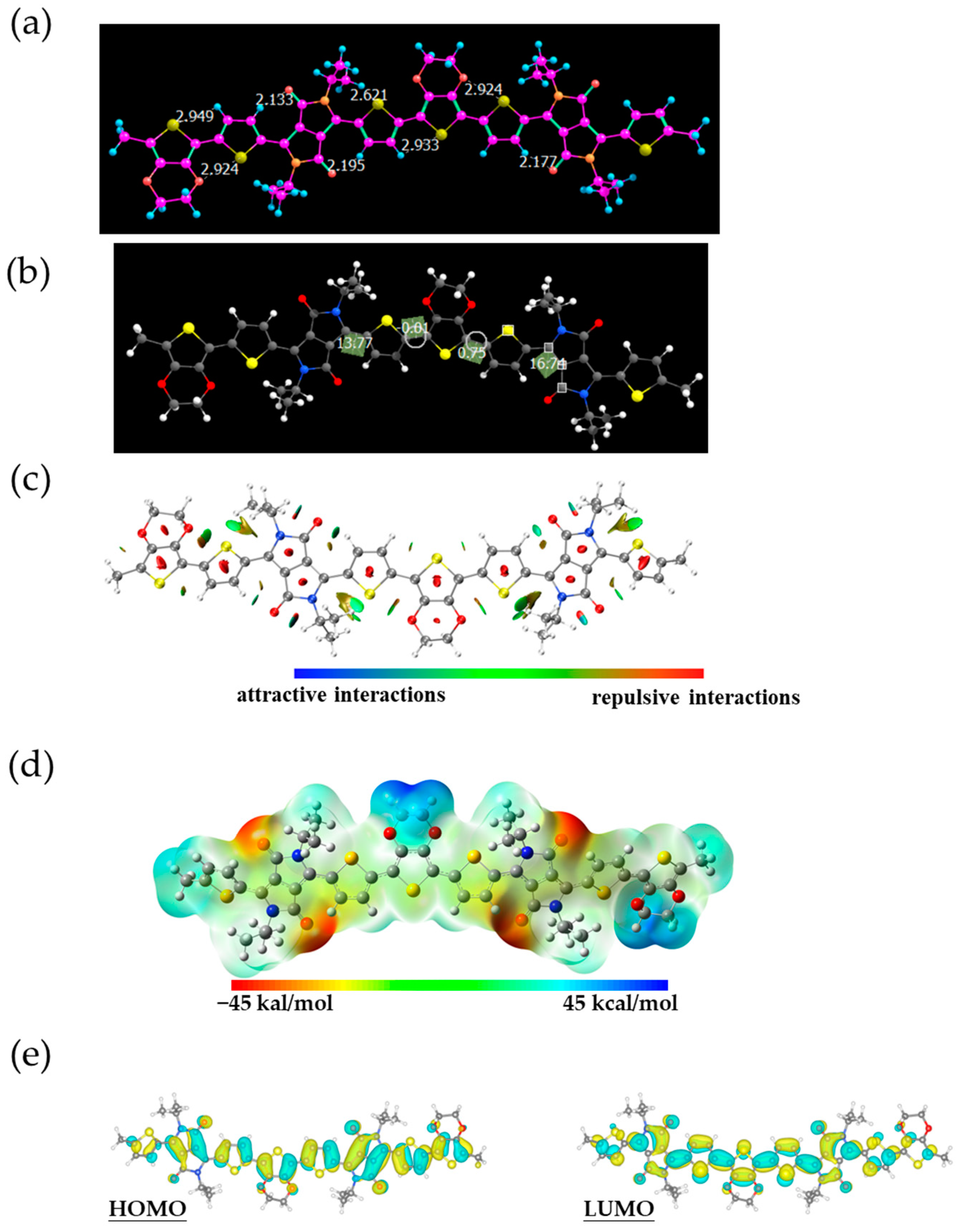
2.4. Density Functional Theory (DFT) Calculations
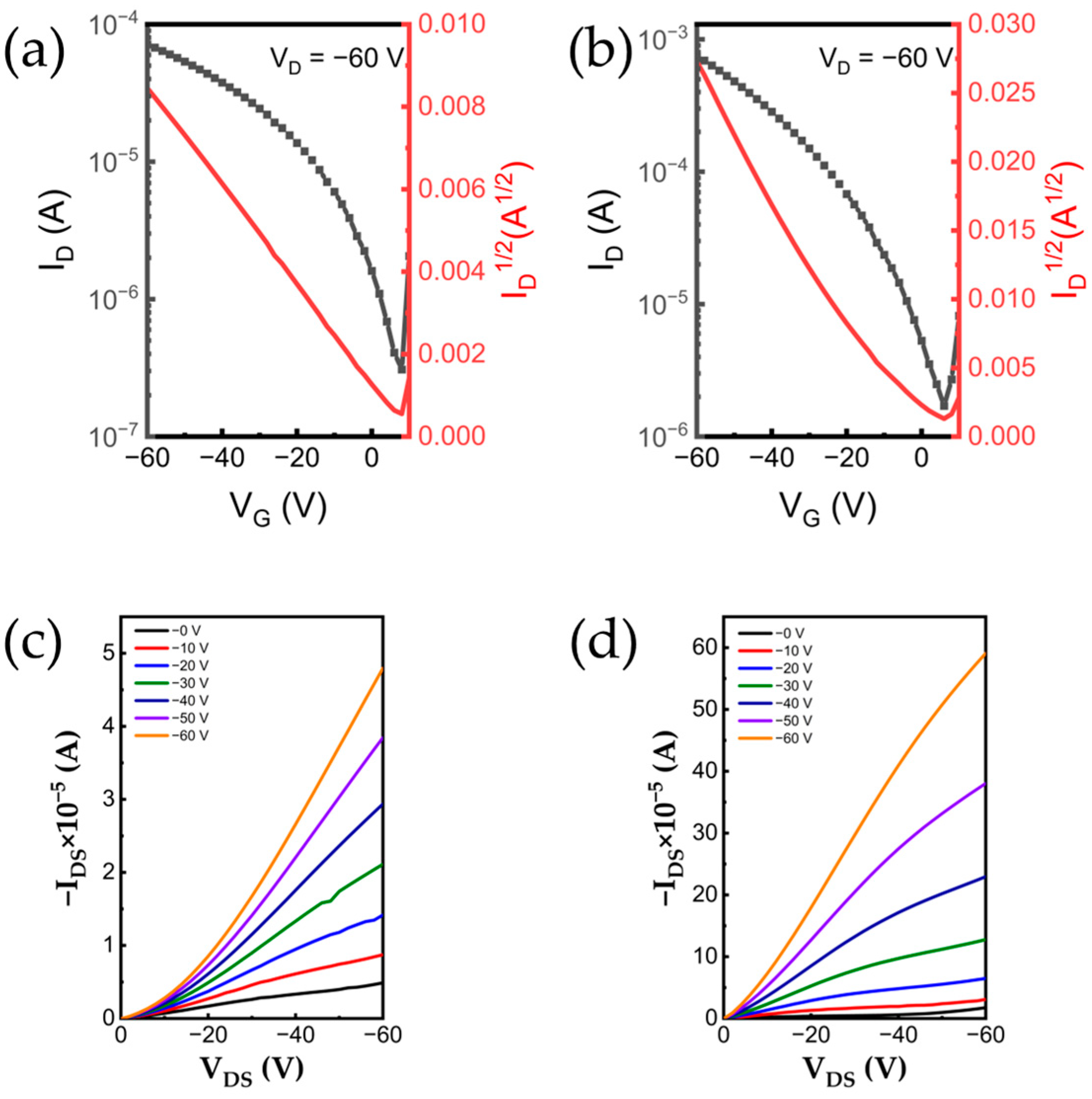
2.5. OFET Device Performance
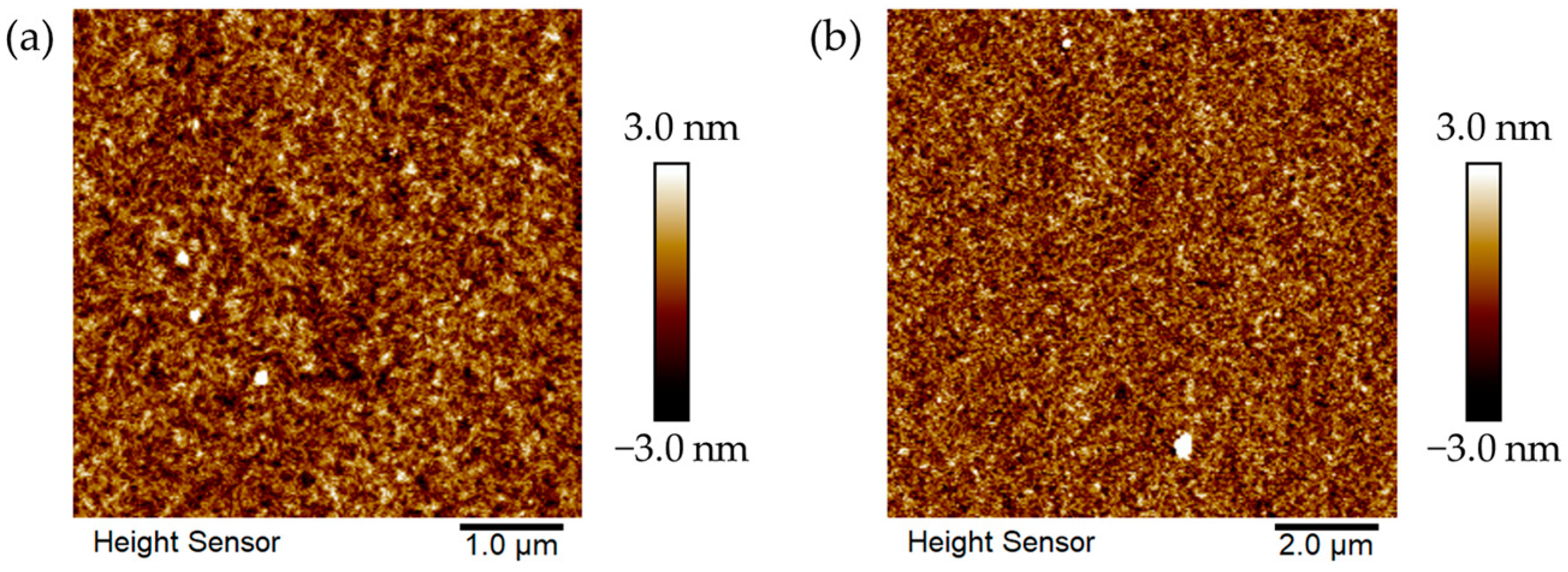
2.6. Microstructure and Morphological Analysis of Polymer P1 and P2 Films
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Griggs, S.; Marks, A.; Bristow, H.; McCulloch, I. n-Type organic semiconducting polymers: Stability limitations, design considerations and applications. J. Mater. Chem. C 2021, 9, 8099–8128. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Ryu, S.U.; Park, S.A.; Choi, K.; Kim, T.; Chung, D.; Park, T. Donor–Acceptor-Conjugated Polymer for High-Performance Organic Field-Effect Transistors: A Progress Report. Adv. Funct. Mater. 2019, 30, 1904545. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Gao, C.; Ni, Z.; Zhang, X.; Hu, W.; Dong, H. Recent advances in n-type and ambipolar organic semiconductors and their multi-functional applications. Chem. Soc. Rev. 2023, 52, 1331–1381. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, Y.; Liu, Y. 25th Anniversary Article: Recent Advances in n-Type and Ambipolar Organic Field-Effect Transistors. Adv. Mater. 2013, 25, 5372–5391. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Yoo, H.; Lee, E.K. New Opportunities for Organic Semiconducting Polymers in Biomedical Applications. Polymers 2022, 14, 2960. [Google Scholar] [CrossRef] [PubMed]
- Koch, N. Organic electronic devices and their functional interfaces. Chemphyschem Eur. J. Chem. Phys. Phys. Chem. 2007, 8, 1438–1455. [Google Scholar] [CrossRef] [PubMed]
- Katz, H.E.; Huang, J. Thin-Film Organic Electronic Devices. Annu. Rev. Mater. Res. 2009, 39, 71–92. [Google Scholar] [CrossRef]
- Yan, Y.; Zhao, Y.; Liu, Y. Recent progress in organic field-effect transistor-based integrated circuits. J. Polym. Sci. 2021, 60, 311–327. [Google Scholar] [CrossRef]
- Bronstein, H.; Nielsen, C.B.; Schroeder, B.C.; McCulloch, I. The role of chemical design in the performance of organic semiconductors. Nat. Rev. Chem. 2020, 4, 66–77. [Google Scholar] [CrossRef]
- Henson, Z.B.; Müllen, K.; Bazan, G.C. Design strategies for organic semiconductors beyond the molecular formula. Nat. Chem. 2012, 4, 699–704. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y. Insight into conjugated polymers for organic electrochemical transistors. Trends Chem. 2023, 5, 279–294. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, X.; Zang, Y.; Di, C.-A.; Diao, Y.; Mei, J. Conjugation-Break Spacers in Semiconducting Polymers: Impact on Polymer Processability and Charge Transport Properties. Macromolecules 2015, 48, 2048–2053. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, K.; Li, J.; Yu, X.; Zhang, Q.; Han, Y. Microstructural Evolution of P(NDI2OD-T2) Films with Different Molecular Weight during Stretching Deformation. Macromol. Rapid Commun. 2023, 2300624. [Google Scholar] [CrossRef]
- Wu, F.; Liu, Y.; Zhang, J.; Duan, S.; Ji, D.; Yang, H. Recent Advances in High-Mobility and High-Stretchability Organic Field-Effect Transistors: From Materials, Devices to Applications. Small Methods 2021, 5, 2100676. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Li, W.; Zhao, Y.; Liu, Y.; Wang, Y. An all-C–H-activation strategy to rapidly synthesize high-mobility well-balanced ambipolar semiconducting polymers. Matter 2022, 5, 1953–1968. [Google Scholar] [CrossRef]
- Yang, L.; Wu, Y.; Yan, Y.; Wang, Z.; Qiao, Y.; Chang, D.; Zhang, C.; Wang, Y.; Lu, X.; Liu, Y.; et al. Molecular Packing and Charge Transport Behaviors of Semiconducting Polymers Over a Wide Temperature Range. Adv. Funct. Mater. 2022, 32, 2202456. [Google Scholar] [CrossRef]
- Urquhart, S.G.; Martinson, M.; Eger, S.; Murcia, V.; Ade, H.; Collins, B.A. Connecting Molecular Conformation to Aggregation in P3HT Using Near Edge X-ray Absorption Fine Structure Spectroscopy. J. Phys. Chem. C 2017, 121, 21720–21728. [Google Scholar] [CrossRef]
- Mauer, R.; Kastler, M.; Laquai, F. The Impact of Polymer Regioregularity on Charge Transport and Efficiency of P3HT:PCBM Photovoltaic Devices. Adv. Funct. Mater. 2010, 20, 2085–2092. [Google Scholar] [CrossRef]
- Anthony, J.E.; Facchetti, A.; Heeney, M.; Marder, S.R.; Zhan, X. n-Type Organic Semiconductors in Organic Electronics. Adv. Mater. 2010, 22, 3876–3892. [Google Scholar] [CrossRef]
- Yang, X.; Yan, Y.; Zeng, W.; Song, Y.; Li, W.; Zhao, L.; Zhao, Y.; Chen, H.; Liu, Y. Bis-acenaphthoquinone diimides with high electron deficiency and good coplanar conformation. Chem. Commun. 2021, 57, 7822–7825. [Google Scholar] [CrossRef]
- Yu, S.H.; Song, H.G.; Cho, J.; Kwon, S.-K.; Kim, Y.-H.; Chung, D.S. Synthetic Approach for Enhancing Semiconductor Properties of Water-Borne DPP-Copolymer. Chem. Mater. 2018, 30, 4808–4815. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, K.; Lai, J.; Zhou, Y.; Wei, X.; Che, Q.; Wei, J.; Wang, L.; Yu, G. Record-High Electron Mobility Exceeding 16 cm2 V−1 s−1 in Bisisoindigo-Based Polymer Semiconductor with a Fully Locked Conjugated Backbone. Adv. Mater. 2023, 35, 2300145. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Guo, Y.; Yu, G.; Zhao, Y.; Zhang, J.; Gao, D.; Liu, H.; Liu, Y. Highly π-Extended Copolymers with Diketopyrrolopyrrole Moieties for High-Performance Field-Effect Transistors. Adv. Mater. 2012, 24, 4618–4622. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Liu, Z.; Ma, J.; Zhao, Z.; Tan, L.; Lin, G.; Tian, J.; Zhang, X.; Zhang, G.; Zhang, D. Half-Fused Diketopyrrolopyrrole-Based Conjugated Donor–Acceptor Polymer for Ambipolar Field-Effect Transistors. Adv. Funct. Mater. 2020, 30, 1910235. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, Y.; Li, P.; Wen, J.; He, J.; Gao, X. Enhancement of the thermoelectric performance of DPP based polymers by introducing one 3,4-ethylenedioxythiophene electron-rich building block. J. Mater. Chem. C 2020, 8, 10859–10867. [Google Scholar] [CrossRef]
- Wang, N.; Xie, L.; Ling, H.; Piradi, V.; Li, L.; Wang, X.; Zhu, X.; Yan, F. Ethylenedioxythiophene incorporated diketopyrrolopyrrole conjugated polymers for high-performance organic electrochemical transistors. J. Mater. Chem. C 2021, 9, 4260–4266. [Google Scholar] [CrossRef]
- Jiang, W.; Yu, X.; Li, C.; Zhang, X.; Zhang, G.; Liu, Z.; Zhang, D. Fluoro-substituted DPP-bisthiophene conjugated polymer with azides in the side chains as ambipolar semiconductor and photoresist. Sci. China Chem. 2022, 65, 1791–1797. [Google Scholar] [CrossRef]
- Yu, X.; Li, C.; Gao, C.; Zhang, X.; Zhang, G.; Zhang, D. Incorporation of hydrogen-bonding units into polymeric semiconductors toward boosting charge mobility, intrinsic stretchability, and self-healing ability. SmartMat 2021, 2, 347–366. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, Y.; Liu, Z.; Yuan, Y.; Qiu, L. Ultrathin Polythiophene Films Prepared by Vertical Phase Separation for Highly Stretchable Organic Field-Effect Transistors. Adv. Electron. Mater. 2021, 7, 2100591. [Google Scholar] [CrossRef]
- Hsu, L.-C.; Kobayashi, S.; Isono, T.; Chiang, Y.-C.; Ree, B.J.; Satoh, T.; Chen, W.-C. Highly Stretchable Semiconducting Polymers for Field-Effect Transistors through Branched Soft–Hard–Soft Type Triblock Copolymers. Macromolecules 2020, 53, 7496–7510. [Google Scholar] [CrossRef]
- Turbiez, M.; Frère, P.; Allain, M.; Videlot, C.; Ackermann, J.; Roncali, J. Design of Organic Semiconductors: Tuning the Electronic Properties of π-Conjugated Oligothiophenes with the 3,4-Ethylenedioxythiophene (EDOT) Building Block. Chem. Eur. J. 2005, 11, 3742–3752. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Dai, X.; Zou, Y.; Li, C.; Di, C.A.; Zhang, D.; Zhu, D.-P. Boosting the Thermoelectric Performance of the Doped DPP-EDOT Conjugated Polymer by Incorporating an Ionic Additive. Small 2023, 19, 2300231. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liu, T.; Zhou, Y. Recent Advances of Synthesis, Properties, Film Fabrication Methods, Modifications of Poly(3,4-ethylenedioxythiophene), and Applications in Solution-Processed Photovoltaics. Adv. Funct. Mater. 2020, 30, 2006213. [Google Scholar] [CrossRef]
- Xu, Y.; Jia, Y.; Liu, P.; Jiang, Q.; Hu, D.; Ma, Y. Poly(3,4-ethylenedioxythiophene) (PEDOT) as promising thermoelectric materials and devices. Chem. Eng. J. 2021, 404, 126552. [Google Scholar] [CrossRef]
- Naik, M.A.; Patil, S. Diketopyrrolopyrrole-based conjugated polymers and small molecules for organic ambipolar transistors and solar cells. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 4241–4260. [Google Scholar] [CrossRef]
- Liu, Q.; Bottle, S.E.; Sonar, P. Developments of Diketopyrrolopyrrole-Dye-Based Organic Semiconductors for a Wide Range of Applications in Electronics. Adv. Mater. 2019, 32, 1903882. [Google Scholar] [CrossRef] [PubMed]
- Carsten, B.; He, F.; Son, H.J.; Xu, T.; Yu, L. Stille Polycondensation for Synthesis of Functional Materials. Chem. Rev. 2011, 111, 1493–1528. [Google Scholar] [CrossRef] [PubMed]
- Yiu, A.T.; Beaujuge, P.M.; Lee, O.P.; Woo, C.H.; Toney, M.F.; Fréchet, J.M.J. Side-Chain Tunability of Furan-Containing Low-Band-Gap Polymers Provides Control of Structural Order in Efficient Solar Cells. J. Am. Chem. Soc. 2012, 134, 2180–2185. [Google Scholar] [CrossRef]
- Pan, Y.; Huang, J.; Gao, D.; Chen, Z.; Zhang, W.; Yu, G. An insight into the role of side chains in the microstructure and carrier mobility of high-performance conjugated polymers. Polym. Chem. 2021, 12, 2471–2480. [Google Scholar] [CrossRef]
- Shi, Y.; Li, W.; Wang, X.; Tu, L.; Li, M.; Zhao, Y.; Wang, Y.; Liu, Y. Isomeric Acceptor–Acceptor Polymers: Enabling Electron Transport with Strikingly Different Semiconducting Properties in n-Channel Organic Thin-Film Transistors. Chem. Mater. 2022, 34, 2403–2413. [Google Scholar] [CrossRef]
- Nahid, M.M.; Matsidik, R.; Welford, A.; Gann, E.; Thomsen, L.; Sommer, M.; McNeill, C.R. Unconventional Molecular Weight Dependence of Charge Transport in the High Mobility n-type Semiconducting Polymer P(NDI2OD-T2). Adv. Funct. Mater. 2017, 27, 1604744. [Google Scholar] [CrossRef]
- Khatun, M.N.; Dey, A.; Meher, N.; Iyer, P.K. Long Alkyl Chain Induced OFET Characteristic with Low Threshold Voltage in an n-Type Perylene Monoimide Semiconductor. ACS Appl. Electron. Mater. 2021, 3, 3575–3587. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S. Density functional theory with London dispersion corrections. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2011, 1, 211–229. [Google Scholar] [CrossRef]
- Liu, Y.; Hao, W.; Yao, H.; Li, S.; Wu, Y.; Zhu, J.; Jiang, L. Solution Adsorption Formation of a π-Conjugated Polymer/Graphene Composite for High-Performance Field-Effect Transistors. Adv. Mater. 2017, 30, 1705377. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2011, 33, 580–592. [Google Scholar] [CrossRef]
- Hong, J.; Kim, J.; Li, Z.; Cong, C.; Rand, B.P.; Nam, S.Y.; Kim, S.H.; Kim, Y.H. Facile Direct Printing of DPP-Based Polymers for Organic Field-Effect Transistors and Logic Gates. ACS Appl. Electron. Mater. 2023, 5, 4114–4124. [Google Scholar] [CrossRef]
- Ren, S.; Ding, Y.; Zhang, W.; Wang, Z.; Wang, S.; Yi, Z. Rational Design of Novel Conjugated Terpolymers Based on Diketopyrrolopyrrole and Their Applications to Organic Thin-Film Transistors. Polymers 2023, 15, 3803. [Google Scholar] [CrossRef]
| Mn | Mw | Đ 1 | C | H | N | |
|---|---|---|---|---|---|---|
| (%) | (%) | (%) | ||||
| P1 | 15,419 | 54,417 | 3.53 | 72.94 | 9.98 | 1.22 |
| P2 | 8204 | 27,456 | 3.35 | 73.57 | 8.77 | 2.72 |
| repeating unit 2 | 1001 | 1001 | N/A | 71.95 | 9.26 | 2.80 |
| repeating unit 3 | 1113 | 1113 | N/A | 73.33 | 9.77 | 2.52 |
| Materials | λmax | λonset | Eg 1 |
|---|---|---|---|
| P1 in solution | 874, 952 | 1052 | 1.18 |
| P2 in solution | 873, 955 | 1049 | 1.18 |
| P1 in film | 887 | 1095 | 1.13 |
| P2 in film | 870, 955 | 1103 | 1.12 |
| Polymer Material | Coating Speed (rpm) | Annealing Temperature (°C) | Max Hole Mobilities 1 (cm2/(V s)) | Hole Mobilities 2 (cm2/(V s)) | Threshold Voltage (V) | Ion/Ioff |
|---|---|---|---|---|---|---|
| P1 | 2000 | 180 | 0.009 | 0.007 | 10 | 102 |
| P2 | 2000 | 180 | 0.15 | 0.12 | −10 | 103 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, S.; Zhang, W.; Chen, J.; Yassar, A. Theoretical and Experimental Study of Different Side Chains on 3,4-Ethylenedioxythiophene and Diketopyrrolopyrrole-Derived Polymers: Towards Organic Transistors. Int. J. Mol. Sci. 2024, 25, 1099. https://doi.org/10.3390/ijms25021099
Ren S, Zhang W, Chen J, Yassar A. Theoretical and Experimental Study of Different Side Chains on 3,4-Ethylenedioxythiophene and Diketopyrrolopyrrole-Derived Polymers: Towards Organic Transistors. International Journal of Molecular Sciences. 2024; 25(2):1099. https://doi.org/10.3390/ijms25021099
Chicago/Turabian StyleRen, Shiwei, Wenqing Zhang, Jinyang Chen, and Abderrahim Yassar. 2024. "Theoretical and Experimental Study of Different Side Chains on 3,4-Ethylenedioxythiophene and Diketopyrrolopyrrole-Derived Polymers: Towards Organic Transistors" International Journal of Molecular Sciences 25, no. 2: 1099. https://doi.org/10.3390/ijms25021099








