Abstract
Liver transplantation is the most effective treatment for end-stage liver disease. Transplant indications have been progressively increasing, with a huge discrepancy between the supply and demand of optimal organs. In this context, the use of extended criteria donor grafts has gained importance, even though these grafts are more susceptible to ischemic reperfusion injury (IRI). Hepatic IRI is an inherent and inevitable consequence of all liver transplants; it involves ischemia-mediated cellular damage exacerbated upon reperfusion and its severity directly affects graft function and post-transplant complications. Strategies for organ preservation have been constantly improving since they first emerged. The current gold standard for preservation is perfusion solutions and static cold storage. However, novel approaches that allow extended preservation times, organ evaluation, and their treatment, which could increase the number of viable organs for transplantation, are currently under investigation. This review discusses the mechanisms associated with IRI, describes existing strategies for liver preservation, and emphasizes novel developments and challenges for effective organ preservation and optimization.
1. Introduction
Liver transplantation stands as a life-saving therapeutic avenue for individuals afflicted with end-stage liver disease, acute liver failure, and a subset of patients grappling with primary and secondary hepatic malignancies [1]. The expanding spectrum of indications for liver transplantation has engendered a surge in the number of patients awaiting this procedure. Consequently, there exists an imperative to augment the pool of potential liver donors. This situation has necessitated the broadening of acceptance criteria for liver grafts, encompassing the incorporation of increasingly extended criteria donor grafts, colloquially referred to as marginal livers. These marginal livers comprise donations from older individuals, those with hepatic steatosis, grafts subjected to prolonged cold ischemia times (CITs), and donations procured after circulatory death (DCD) [2]. The liver is an organ with many synthetic and secretion functions that receives a dual blood supply, which makes it more susceptible to ischemia and hypoxia [3]. This type of damage can be caused by liver surgery, liver transplantation, and systemic shock. In the case of transplantation, allograft ischemia–reperfusion injury is an inevitable process that contributes to organ shortage and may lead to primary nonfunction or early allograft dysfunction, making livers from marginal donors more susceptible [4,5].
IRI manifests as a biphasic process, with the initial phase (ischemia) commencing when hepatocytes face oxygen deprivation following an abrupt cessation of blood flow. Cellular hypoxia disrupts electron flow within the respiratory chain, leading to the depletion of adenosine triphosphate (ATP) and the accumulation of detrimental metabolites and ions, including Na+, K+, and Ca++, due to the failure of ATP-dependent ion channels. This sequence eventually triggers cellular injury, particularly marked by cellular edema during prolonged ischemia, which activates proteases and initiates the apoptotic cascade [6].
A pivotal facet during hypoxia is the accrual of Krebs cycle metabolites, notably succinate, which precipitates the immediate release of reactive oxygen species (ROS) upon the reintroduction of oxygen [7,8].
The subsequent phase (reperfusion) constitutes an exacerbation of the initial insult and is initiated when liver blood flow, oxygen levels, and pH are restored. This restoration unleashes a cascade of injurious components, including proinflammatory cytokines, ROS, and later, aseptic inflammation, which amplifies the damage initiated during ischemia due to metabolic disturbances and the induction of a proinflammatory immune response, expediting cellular degeneration and hastening the decline in liver function [9]. Regrettably, this scenario is further exacerbated when employing marginal grafts sourced from extended criteria donors. Mitochondria are critical players in energy and glucose metabolism, as well as in the regulation of several signaling pathways [10]. Tissue ischemia, resulting from interruption of oxygen supply, severely compromises mitochondrial energy production, and depending on the duration of the ischemic damage, organ dysfunction or failure can be set [11].
Mitochondria have been demonstrated to play a pivotal role in the initiation and modulation of IRI. However, the precise underlying mechanisms of this remain incompletely elucidated, and effective therapeutic interventions are currently lacking.
Given the absence of established treatments for IRI prevention, the primary strategy for mitigating IRI revolves around minimizing the cold preservation time and optimizing graft rewarming. While ex vivo machine perfusion (MP) preservation presents a promising avenue and is gaining recognition, static cold storage (SCS) remains the gold standard for liver preservation. Ex vivo MP holds the capacity to sustain microcirculation, supply requisite substrates and oxygen for cellular metabolism, facilitate the administration of pharmacological or cytoprotective agents directly to the liver, and enable real-time monitoring of their therapeutic efficacy and organ viability during perfusion [12].
A more comprehensive explanation centered on the mechanical and molecular underpinnings of liver IRI would facilitate the identification of novel biomarkers and the discovery of new therapeutic targets. This, in turn, could lead to the establishment of more precise selection criteria and the formulation of innovative strategies aimed at enhancing the surgical outcomes of liver transplantation.
The aim of this review is to provide an overview of the underlying mechanisms of liver IRI and address different protective strategies used during ex vivo liver machine preservation, focusing on current clinical applications and future perspectives in liver transplantation.
2. Graft Vulnerability before Preservation: Pre Preservation Injury
2.1. Liver Steatosis
Hepatic steatosis is closely linked to conditions such as obesity, alcohol abuse, diabetes, and metabolic disorders. Currently, steatotic livers constitute the majority of extended criteria donor (ECD) grafts, contributing to diminished donor quality and consequently reduced organ utilization [13].
Hepatic steatosis induces alterations in liver grafts at the mitochondrial, Kupffer cell, and sinusoid levels, exacerbating cellular damage during cold ischemia and intensifying ischemia–reperfusion injury [14,15]. The critical challenge lies in discerning the type and extent of macrosteatosis when evaluating the suitability of liver grafts for transplantation [16]. Mild steatosis (<30%) exerts minimal influence on post-transplant outcomes, provided that the cold ischemia time remains brief [15]. Conversely, elevated levels of steatosis are associated with liver failure, graft dysfunction, renal impairment, and biliary complications [17,18]. An analysis of the Scientific Registry of Transplant Recipients revealed that liver allografts with macrovesicular steatosis exceeding 30% independently predicted reduced one-year graft survival [19].
2.2. Aged Liver
Liver transplantation has experienced a notable impact due to the utilization of older donor organs, which currently constitute a substantial and increasing segment of the donor pool. Aged livers exhibit significant deregulation of the sinusoidal architecture, thereby enhancing the susceptibility of their sinusoids to acute injuries [20]. Moreover, the synthetic, excretory, and metabolic functions of the liver are susceptible to age-related alterations [21].
Remarkably, outcomes following liver transplantation using organs from older donors have been reported to be on par with those from younger donors, as evidenced by several published studies. These studies demonstrate favorable outcomes with livers procured from donors aged 60, 70, or even 80 years [17].
It is important to note that older donors possess a reduced physiologic reserve and often present with comorbidities that can potentially impact organ procurement. Characteristic features of older liver allografts encompass reduced size, steatosis, capsular fibrosis, and arterial atherosclerosis [21].
2.3. Injury Associated with Cardiopulmonary Arrest or Hypotension before Donation
Following cardiac arrest, the cessation of blood flow initiates a progressive depletion of the energy reserves within the donor organ. This depletion leads to the failure of transmembrane ionic pumps and the subsequent development of edema. Furthermore, the absence of perfusion in the affected tissue can result in erythrocyte aggregation and microthrombus formation, which, when combined with the flushing of preservation solution, may further disrupt microcirculation during ischemic transport, potentially compromising tissue viability [22].
Within the literature, research data highlight the divergent impacts of ischemia on liver IRI, contingent on the duration of the ischemic event. It has been documented that a 60 min episode of warm ischemia leads to reversible cellular injury, as liver oxygen consumption returns to control levels upon reperfusion with oxygen. However, reperfusion following longer periods of warm ischemia (120–180 min) results in irreversible cellular damage. These findings align with previous research on hepatic IRI, revealing that hepatocytes reach a point of no return following 90 min of ischemia [23].
Experimental models of partial liver IRI, characterized by ischemic periods of 30–45 min, have indicated a limited role for inducible nitric oxide synthase in injury. However, with ischemia exceeding 60 min, alterations in the expression of inducible nitric oxide synthase become consequential in liver IRI injury [24].
Donors who experience delayed cardiac resuscitation are prone to severe IRI, which can exert a detrimental impact on the initial graft function, particularly when involving extended criteria donor organs.
2.4. Injury during Organ Procurement
Injury during the process of organ procurement can arise because of intraoperative hypotension and uncontrolled transient ischemia occurring during donor hepatectomy. This form of injury is predominantly ascribed to the hemodynamic instability of the donor during the procurement procedure, rather than any technical mishaps [5]. Studies have illustrated the adverse correlations between the duration of donor hepatectomy and early allograft dysfunction, ischemic cholangiopathy, as well as graft and patient survival following liver transplantation [25].
2.5. Warm Ischemia Injury in Donation after Cardiac Death
In contrast to organ donation after brain death (DBD), the liver in DCD incurs damage prior to preservation, occurring following the withdrawal of life support. The origin of this damage can be traced to warm ischemia initiated by cardiac arrest prior to organ procurement. Extubation marks the commencement of warm ischemia, characterized by inadequate perfusion and tissue oxygenation. There is substantiated evidence indicating that a shorter interval between donor extubation and cardiac arrest (referred to as time after extubation) is associated with a reduced risk of graft failure. Should the cumulative duration of warm ischemia exceed 30 min, numerous transplant centers opt not to accept the liver for transplantation [26,27].
Uncontrolled DCD procedures involve extended periods of warm ischemia, intentionally amplifying cellular damage through inflammatory responses, oxidative stress, and apoptotic processes [28].
3. Graft Injury Prevention during Organ Preservation
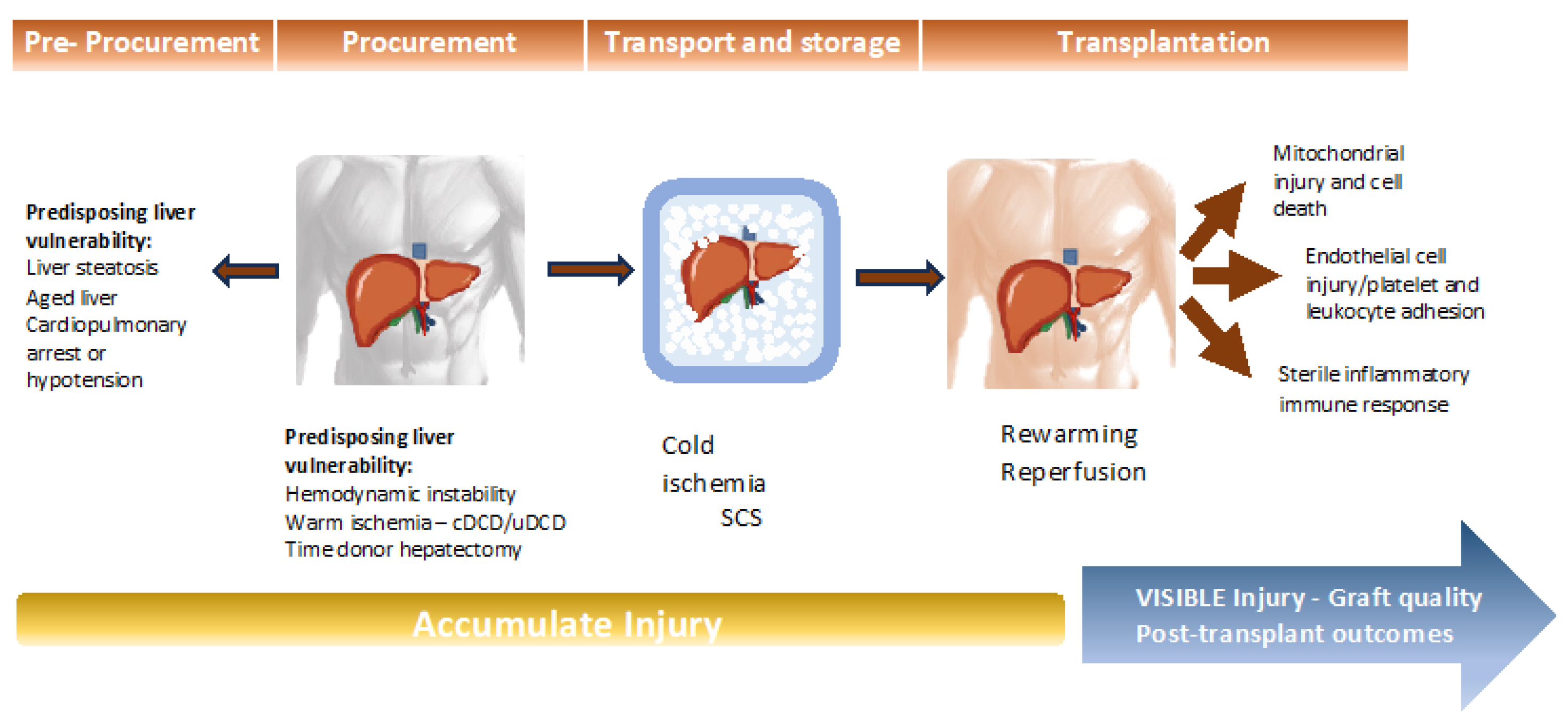
Liver preservation stands as a critical determinant in the realm of liver transplantation, exerting a profound influence on post-transplant outcomes [29]. Preservation injury encompasses a series of events that unfold before, during, and after liver preservation. In brain-dead donors, this injury stems from four sources: (1) pre-storage injury, (2) cold injury, (3) rewarming injury, and (4) reperfusion injury (Figure 1). Allografts procured after circulatory death are particularly susceptible to heat-related injury.
Figure 1.
Process of injury throughout the pre-procurement, procurement, preservation, and transplantation periods.
Organ preservation, defined as “the process of supplying organs for transplantation” [30], plays a pivotal role in ensuring the successful transport and distribution of grafts from distant locations to hospitals where liver transplantation procedures are performed. Over time, organ preservation techniques have evolved significantly. The conventional method, SCS, has served as the gold standard [30]. SCS is often favored for its simplicity, efficient preservation, and ease of organ transfer between donor organ recovery centers and recipient centers. Nevertheless, a major drawback of SCS lies in its limited preservation duration, which inevitably leads to IRI and associated complications, often resulting in graft dysfunction.
3.1. Static Cold Graft Storage Injury (SCSI)
Static cold preservation serves as the prevailing method for organ preservation, commencing with the induction of hypothermia. This hypothermic state is initiated during organ storage by perfusing cryopreservation fluid through the artery and portal vein. Therefore, the liver’s temperature rapidly plummets to approximately 16 °C, facilitated by the simultaneous application of ice to the organ’s surface and the abdominal cavity. During cold storage, the intraparenchymal temperature stabilizes at 0–4 °C over nearly one hour [31,32]. The hepatic core temperature reaches a state of equilibrium near 0 °C during static cold storage [32].
Crucially, low temperature stands as the linchpin in maintaining anaerobic conditions during preservation. The advantages of cold preservation are underpinned by the principle of decelerating metabolism during periods when the body is devoid of oxygen and nutrients. This is chiefly attributable to the fact that a mere 10 °C reduction in temperature results in a twofold reduction in most enzymatic reactions. However, studies have illuminated that even when metabolic activity decreases from 0 °C to 4 °C, the liver still necessitates oxygen for metabolic processes [33].
3.2. Organ Preservation Solutions for Preventing SCSI
Advancements in hepatic preservation, aimed at safeguarding allografts from IRI, have centered on the development of static cold preservation solutions boasting diverse compositions. These preservation solutions can be categorized based on their potassium levels, the type of oncotic agents employed, the presence of impermeant substances, and specific features tailored to mitigate IRI [34]. Variations in the composition of the most utilized solutions are delineated in Table 1.

Table 1.
Characteristics and compositions of current solutions for static and dynamic perfusion.
The formulation and additional components of these solutions are meticulously designed to avert issues that may arise during the cooling process. As per the criteria set forth by Belzer and Southard [30], the optimal attributes of these solutions encompass (1) the prevention of cell swelling and interstitial edema induced by hypothermia, (2) the mitigation of intracellular acidosis, (3) the prevention of electrolyte imbalances, (4) the reduction of oxidative damage, especially during reperfusion, and (5) the provision of substrates relevant to cellular energy metabolism. In recent times, preservation solutions have also found utility in dynamic preservation, with their composition encompassing many of the fundamental elements utilized in static cold storage. Notably, the Belzer Machine Perfusion Solution (Belzer-MPS) serves as the primary solution employed in Hypothermic Machine Perfusion for hepatic and renal applications within the clinical domain [35,36,37].
4. Supercooling Strategies for Organ Preservation
Presently, SCS at +4 °C represents the benchmark for liver preservation before transplantation. This approach hinges on the principle that lower temperatures lead to a reduction in metabolic activity, thereby extending the duration of ex vivo viability. SCS at +4 °C can uphold liver viability for up to 12 h, although this timeframe may prove inadequate for certain transplantation scenarios or for long-distance transportation. Lowering the temperature further could potentially prolong storage time; however, this endeavor is hampered by the fact that tissues freeze at temperatures below 0 °C.
The University of Wisconsin (UW) preservation solution has garnered acclaim in the field of liver transplantation. Nonetheless, to enable supercooling, enriching the solution becomes imperative to mitigate cold-induced membrane damage.
Numerous publications detail the preservation of whole human livers at subfreezing temperatures without ice formation. A cryoprotective perfusion cocktail primarily comprising PEG-35 (polyethylene), 3-OMG (a glucose molecule), trehalose, and glycerol plays a pivotal role in this endeavor. The addition of 5% PEG 35 kDa to the storage medium prevents cold-induced lipid peroxidation, preserving hepatocyte viability and functionality during storage. MP is employed to alleviate the consequences of prolonged ischemia, which has been demonstrated to mitigate hypothermic endothelial damage, reactivate metabolic processes, replenish ATP levels, and mechanically prepare the vasculature for reperfusion [38,39,40,41].
When it comes to individual cells, they are often preserved in UW solution at temperatures ranging from 0 to 4 °C, akin to the conditions employed for whole organ preservation. Research has indicated that it is possible to extend the viable storage period of rat hepatocytes by incorporating 5% 35 kDa PEG into the storage medium and reducing the typical storage temperature from 4 °C to −4.4 °C, all while preventing ice formation [42]. These findings suggest that the combination of PEG supplementation and supercooling may offer an enhanced approach for the preservation of both cells and organs.
5. Pathophysiological Mechanisms of IRI
5.1. Pathophysiology of the Ischemic Cascade
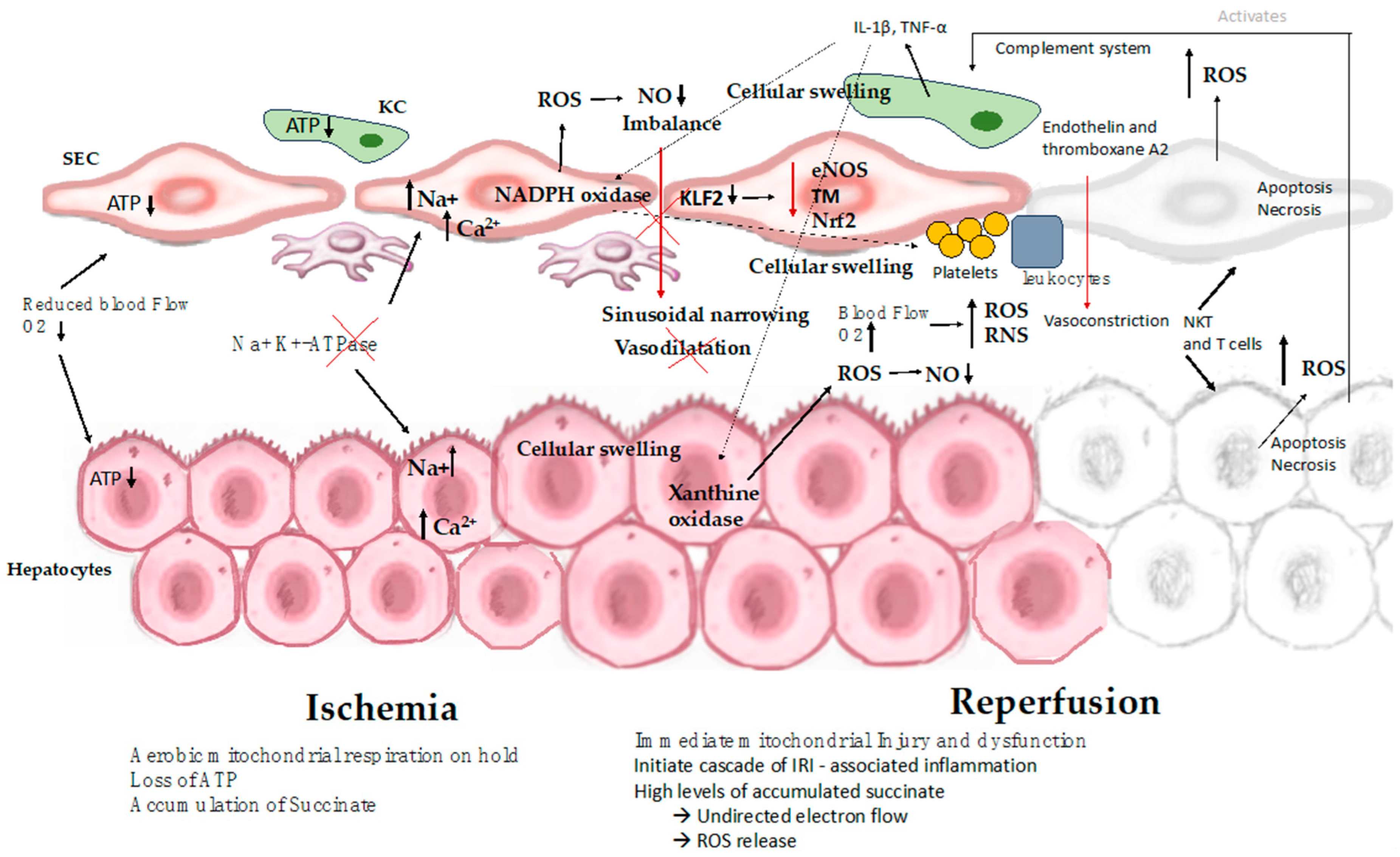
While organ cooling reduces the metabolic demands placed on the tissue, certain metabolic processes persist even as the temperature decreases. During cold ischemia, hepatocytes undergo a transition from self-limiting aerobic metabolism to anaerobic metabolism, which leads to inadequate ATP production. In this context, anaerobic glycolysis becomes the sole process capable of generating ATP. Nevertheless, the rate of ATP consumption exceeds its production. Additionally, ischemia prompts the conversion of xanthine hydrogenase into xanthine oxidase, which catalyzes the degradation of hypoxanthine under aerobic conditions [43]. This enzymatic activity gives rise to superoxide radicals, a subset of ROS. The accumulation of specific metabolites, such as succinate, during the ischemic phase, has been associated with mitochondrial dysfunction in various tissues. When the organ is cooled, this metabolic reaction proceeds at a sluggish pace due to the absence of oxygen. However, upon reoxygenation of the organelle, this activity leads to an elevated generation of ROS. ROS are highly toxic as they can oxidize organic molecules and inflict damage on cell membranes through lipid peroxidation [6,9,12] (Figure 2).
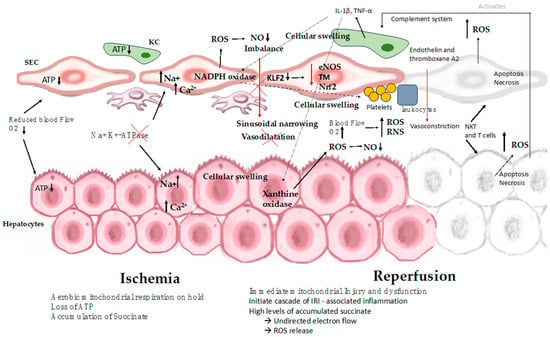
Figure 2.
Mechanisms involved in hepatic ischemia-reperfusion injury. The process of IRI involves a series of complex events, such as mitochondrial deenergization, metabolic acidosis, ATP depletion, Kupffer cell activation, calcium overload, oxidative stress, and the upregulation of pro-inflammatory cytokine signal transduction, resulting in cellular swelling, apoptosis, and necrosis. ATP: adenosine triphosphate; SEC: sinusoidal endothelial cell; KC: Kupffer cell; IL-1β: interleukin-1β; NKT: natural killer T cell; NO: nitric oxide; ROS: reactive oxygen species; T cell: CD4+ T lymphocyte; TNF: tumor necrosis factor; eNOS: endothelial nitric oxide synthase; TM: thrombomodulin; Nrf2: nuclear factor erythroid 2-related factor 2.
5.1.1. Hypothermia-Induced Cell Swelling
The temperature during cold storage impacts enzyme activity, and the inadequate ATP supply during cold ischemia leads to a near shutdown of Na+, K+-ATPase function. Consequently, the pump’s failure leads to the establishment of a sodium and potassium osmotic imbalance between the intracellular and extracellular compartments, allowing sodium to infiltrate the cell. Furthermore, intracellular proteins with negative charges (anions) amplify the influx of sodium ions. The resultant intracellular hyperosmolarity eventually triggers water uptake, culminating in cellular swelling, the formation of bulging sacs, and fragmentation. In hypothermic conditions, the membrane-bound Na+, K+-ATPase pump, responsible for maintaining osmotic equilibrium between the extracellular and intracellular spaces, becomes disrupted, further exacerbating cell swelling [43].
5.1.2. Sinusoidal Endothelial Cell Injury
The primary mechanism underlying damage during cold storage involves injury to the sinusoidal endothelial cells (SECs). These cells play a pivotal role in maintaining vascular homeostasis and immune function, making them a central component of cold ischemia, whereas warm ischemia predominantly affects hepatocytes. The transcription factor Krüppel-like factor 2 (KLF2), typically expressed by liver sinusoidal endothelial cells (LSECs), is involved in vasodilation, and exerts anti-thrombotic and anti-inflammatory effects. However, during cold ischemia, KLF2, along with endothelial nitric oxide synthase, thrombomodulin, and nuclear factor erythroid 2-related factor 2, experiences down-regulation. In rat livers subjected to cold storage, the restoration of these genes helps prevent liver damage [44]. Furthermore, SECs release damage-associated molecular patterns (DAMPs) that contribute to the initiation of an immune response and express adhesive molecules that facilitate neutrophil binding [45].
Additional effects observed during cold ischemia include the development of intracellular acidosis and elevated calcium levels within the cells. Intracellular acidosis primarily results from anaerobic glycolysis, as lactate represents the final metabolic pathway byproduct. The accumulation of intracellular protons inhibits crucial glycolytic enzymes such as phosphofructokinase and further depletes ATP, thereby impeding the remaining anaerobic energy production. Additionally, intracellular acidosis activates lipoprotein lipase and lysosomal hydrolases, culminating in membrane damage and heightened permeability. Elevated intracellular calcium levels serve as a signaling factor for numerous ischemic mechanisms that ultimately lead to cell death [45].
5.1.3. The Role of Mitochondria in IRI
Mitochondria, double-membrane structures located within the cytoplasm of eukaryotic cells, play multifaceted roles in ensuring cell survival and adaptation. These roles encompass the production of ATP, which provides energy for fundamental cellular processes through oxidative phosphorylation. Additionally, mitochondria are involved in numerous essential functions, including lipid, amino acid, and nucleotide metabolism, ROS signaling, calcium homeostasis, apoptosis, and safeguarding cells against oxidative stress [46]. Moreover, mitochondria have evolved signaling functions to communicate with other cells in response to environmental threats. Notably, biological responses are not initiated until mitochondrial regulatory input is integrated. For instance, mitochondrial capacity influences the selection of metabolic processes based on the cell’s energy requirements. In the physiological response to hypoxia, mitochondria serve as oxygen sensors. The production of mitochondrial ROS activates the transcription nuclear factor erythroid 2-related factor 2 (NRF2), leading to the expression of antioxidant and protective proteins. Therefore, the release of mitochondrial second messengers elicits protective antioxidant responses [47].
Dysfunctional mitochondria release components that trigger stress responses, activating various mitochondrial quality control mechanisms. These mechanisms encompass mitochondrial biogenesis, mitochondrial dynamics, and mitophagy, all aimed at optimizing mitochondrial function and preserving cellular homeostasis [48].
During the ischemic period, when nutrients and oxygen are scarce, cellular respiration is disrupted, affecting ATP synthesis. Initially, there is a reduction in ATP production, prompting the initiation of anaerobic respiration and lactate accumulation. This results in a loss of energy (ATP) and the buildup of succinat and dihydronicotinamide adenine dinucleotide (NADH). Succinate accumulation during ischemia/hypoxia becomes crucial for modulating reperfusion injury [49]. It is important to highlight that the graft responds to a lack of oxygen during cold ischemia via activating AMP kinases to avoid the intense reduction in ATP and to prevent graft injury during cold preservation [50].
Furthermore, hydrogen ions (H+) accumulate, leading to acidosis. Elevated intracellular H+ concentrations disrupt the sodium–hydrogen (Na+/H+) exchanger, causing intracellular sodium (Na+) accumulation. Additionally, reduced activity of the sodium–potassium adenosine triphosphatase (Na+/K+ATPase) pump exacerbates Na+ buildup, resulting in cell swelling and death. Widespread ischemia also disrupts sodium–calcium (Na+/Ca++) exchanger homeostasis, leading to elevated intracellular calcium levels. The increased levels of intracellular Na+, calcium (Ca+), and H+ ions contribute to mitochondrial deterioration, leading to the formation of mitochondrial permeability pores. Followed by concurrent apoptosis and necrosis, causing extensive damage [9].
Paradoxically, reoxygenation exacerbates mitochondrial dysfunction. The rapid restoration of efficient electron flow and subsequent ATP recovery come at the cost of reverse electron transfer (RET), an uncontrolled electron flow that generates ROS at mitochondrial complex-I. Furthermore, decreased activity of endogenous antioxidants like heme oxygenase 1 and superoxide dismutase (SOD) contributes to increased ROS production. Elevated ROS levels and mitochondrial membrane permeability both contribute to mitochondrial dysfunction and are significant contributors to hepatocyte death and IRI [12,51,52].
Mitochondria are pivotal components of all cells, such as the mitochondrial aldehyde dehydrogenase 2 (ALDH2) enzyme. ALDH2 plays a vital role in balancing damage and restoring the ability to protect cells from stress induced by hypoxia, and contributes to modulating lipoperoxidation and the generation of toxic species such as 4-hydroxy-2-nonenal (4-HNE) in IRI [53].
Mitochondrial uncoupling protein 2 (UCP2), a member of the mitochondrial anion carrier family, plays an important role in IRI, regulating processes such as cellular homeostasis, oxidative stress, and cell survival. UCP2 can serve as an oxidative stress sensor, though it is not directly involved in antioxidant defense. Additionally, UCP2 stimulates mitophagy caused by IRI, and UCP2 cardioprotective benefits in IRI are eliminated by inhibiting mitophagy [54].
Finally, the interaction of all these processes is essential for the survival of cells and organs during the processes of donation, preservation, and transplantation. It has been demonstrated that mitochondria have a pivotal role in these events and should therefore be a primary area of focus for future treatment approaches. This observation demonstrates that IRI is a multifaceted and multifactorial phenomenon that is not yet fully comprehended but plays a critical role in influencing the development of primary graft dysfunction.
5.2. Rewarming Injury
Following the removal of the liver and the placement of the allograft, the creation of vascular anastomosis typically takes between 30 and 60 min. During this interval, the liver gradually warms up, although reperfusion has not yet commenced. In a hypoxic environment, elevated tissue temperature leads to a heightened loss of ATP. After 30, 40, and 50 min, the liver’s temperature incrementally rises from approximately 0 °C to 12 °C, 17 °C, and finally 20 °C. During this phase, hepatocytes are more susceptible to warm ischemia when compared to SECs, since they require greater energy reserves at 20 °C [48].
5.3. Reperfusion Injury
5.3.1. Events during Reperfusion
Tissue damage manifests within mere seconds to minutes immediately following reperfusion. This cascade of injury during reperfusion represents a response to the pre-reperfusion damage sustained by the allograft (Figure 2). Blood reperfusion, rather than promptly restoring normal conditions, exacerbates the situation. A pivotal event during this vulnerable phase is the excessive generation of ROS, including superoxide anions, hydrogen peroxide, and hydroxyl radicals. This ROS overproduction instigates abnormal cell signaling, inflicts damage on biomolecules, incites inflammation, and ultimately leads to cell death, culminating in a decline in organ function. Damage to biomolecules and ROS-mediated signaling also stimulates innate immune responses, ultimately resulting in fibrosis and the progressive deterioration of organ function. In this context, two distinct stages of reperfusion injury emerge: (1) immediate injury upon reperfusion and (2) injury accompanied by sterile inflammation, serving as an immune response to the initial injury [12,55].
5.3.2. Mitochondrial Injury and Cell Death
In the immediate aftermath of reperfusion, parenchymal cells, previously injured by both cold and warm ischemia, undergo additional stress due to reoxygenation. Among the various metabolic processes, the restoration of energy production in the form of ATP is arguably the most critical determinant of cell survival during the initial stages of reperfusion. However, the damage inflicted upon mitochondria during cold ischemia and early reperfusion provides an explanation for why cells may fail to generate adequate ATP levels following reoxygenation [12]. The reintroduction of oxygen prompts a substantial surge in oxygen consumption, leading to a mitochondrial burst of ROS and reactive nitrogen species (RNS). These ROS and RNS arise from the excessive leakage of electrons from the mitochondrial electron transport chain [48,51].
5.3.3. Endothelial Cell Injury Associated with Platelet and Leukocyte Adhesion
The activation of the surface of SECs plays a pivotal role in SEC injury, particularly concerning the adhesion of platelets and leukocytes during the early reperfusion phase. This activation process sets the stage for a proinflammatory environment and serves as a trigger for the inflammatory response. Upon reperfusion, LSECs express an array of cytokines along with DAMPs. Furthermore, ROS and RNS generated during reperfusion have the capacity to degrade proteoglycans, thus posing a threat to the glycocalyx. Disruption of the glycocalyx leads to the overexposure and activation of the endothelial cell surface, which, in turn, affects the adhesion of platelets and leukocytes, exerting a detrimental influence on microcirculation [52].
5.3.4. Injury Caused by Sterile Inflammatory Immune Response
Ischemia/reperfusion injury is associated with an activated innate immune response involving both local and circulating immune cells [55]. The release of DAMPs initiates the activation of the first wave of resident Kupffer cells and adhesive neutrophils by binding to pattern recognition receptors on their cell surfaces [56]. One of the most prevalent pattern recognition receptors, Toll-like receptor 4 (TLR4), is present on virtually all innate immune cells. The activation of adaptive immunity can further exacerbate liver graft injury, rendering the graft more vulnerable to allograft rejection. NKT cells and T cells have been implicated as primary contributors to both ischemia/reperfusion injury and allograft rejection, with CD4+ T cells playing a central role [55]. Additionally, the complement system is activated and contributes to graft injury post liver transplantation. The deposition of the membrane attack complex, assembled by C5–9, on cellular membranes contributes to parenchymal injury. The role of the complement system in hepatic ischemia/reperfusion injury is an area of ongoing investigation [12,57].
6. Pharmacological Strategies for IRI Modulation
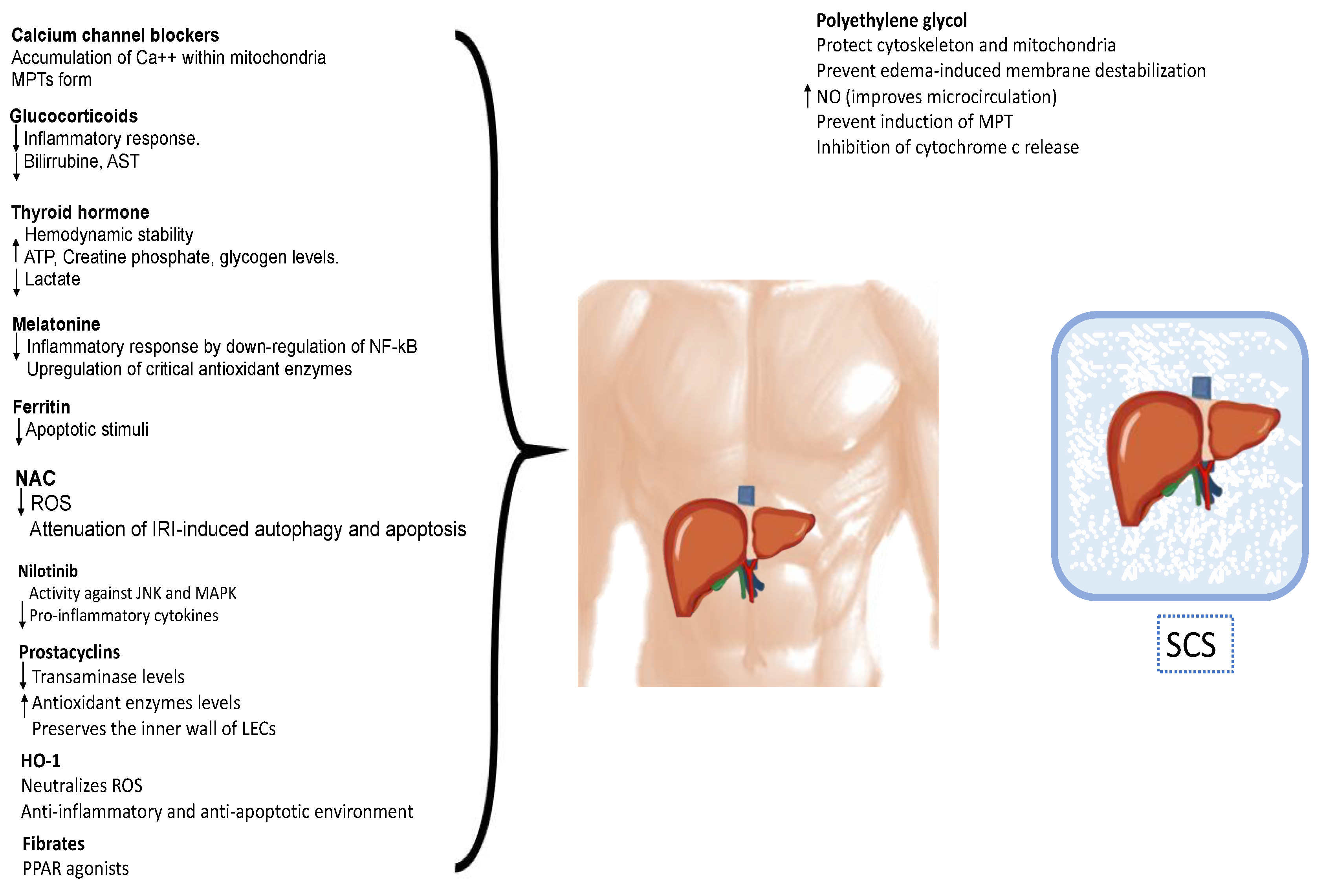
The utilization of pharmacological agents has become a significant component of organ preservation (Figure 3). Various interventions aimed at mitigating ischemia/reperfusion injury following liver transplantation have been assessed in preclinical and clinical models. These interventions encompass antioxidants, metabolism modulation, anti-adhesion strategies, anti-inflammatory agents, immunosuppression, vasodilation, and inflow modulation.
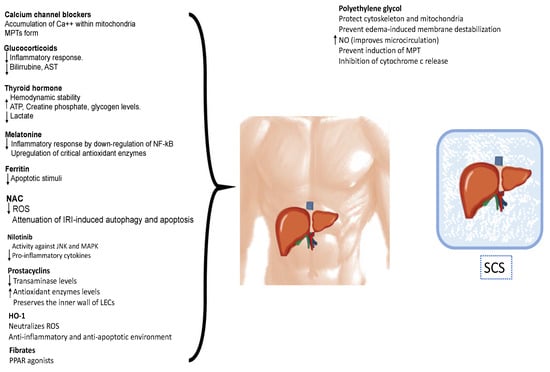
Figure 3.
Pharmacological strategies for protect the liver against ischemia–reperfusion injury. These interventions encompass antioxidants, metabolism modulation, anti-adhesion strategies, anti-inflammatory agents, immunosuppression, vasodilation, and inflow modulation, reducing the inflammatory response, inhibiting the apoptosis of hepatocytes, and promoting the regeneration of damaged liver tissue. MPT: mitochondrial permeability transition pores; ATP: adenosine triphosphate; NF-κB: nuclear factor-κB; ROS: reactive oxygen species; IRI: ischemic reperfusion injury; JNK: c-Jun N-terminal kinase; MAPK: mitogen-activated protein kinase; LECs: sinusoidal endothelial cells; HO-1: Heme oxugenase-1; PPAR: peroxisome proliferator-activated receptor; NO: nitric oxide; MPTPs: mitochondrial permeability transition pores; SCS: static cold storage.
Given that hepatic ischemia/reperfusion injury involves multiple targets and mechanisms, the literature reports several therapeutic approaches. Numerous experimental studies have identified several potential natural and synthetic drugs with promise in this regard. However, the main challenge lies in identifying the most effective drugs, appropriate dosages, and optimal combinations. Despite extensive evaluations, a definitive integrative therapy for hepatic IRI injury has yet to be established.
6.1. Calcium Channel Blockers
The initiation of cell death is initiated by mitochondrial permeability transition pores (MPTPs), which allow the leakage of cytochrome c from the inner mitochondrial membrane, subsequently activating caspases and inducing apoptosis. This phenomenon is triggered by an elevation in intracellular calcium levels. Increased cytosolic Ca++ levels activate Ca++ uniporters in the mitochondrial membrane, leading to an accumulation of Ca++ within the mitochondria. When Ca++ homeostasis is disrupted, MPTPs form, resulting in cell injury through either apoptosis or necrosis.
Studies have indicated that pretreatment with calcium channel blockers like amlodipine can reduce the likelihood of injury by restoring balance to the cellular environment and preventing mitochondrial disturbances caused by hepatic ischemia/reperfusion [58].
6.2. Hormones
Corticosteroids have been employed to mitigate acute inflammation and reduce postoperative complications resulting from IRI. Their protective effects have been observed in myocardial IRI by attenuating the inflammatory response after cardiopulmonary bypass and have shown benefits in liver trauma. However, the precise role of corticosteroids in hepatic IRI remains less clear.
In a study utilizing a rodent model, groups treated with intravenous methylprednisolone before inducing ischemia demonstrated a significant reduction in their inflammatory responses. Histological analysis, serum interleukin-6 (IL-6) levels, and aspartate aminotransferase (AST) release indicated that methylprednisolone treatment provided substantial protection in normal livers compared to ischemic controls [59].
A systematic review and meta-analysis investigating the effects of perioperative steroids on IRI revealed that perioperative steroid administration is associated with favorable postoperative outcomes following hepatectomy. The treated group exhibited a significant increase in early postoperative serum interleukin-10 (IL-10) levels, a decrease in blood bilirubin levels, and reduced inflammation markers postoperatively (IL-6 and C-reactive protein). Moreover, patients receiving intravenous glucocorticoids had a 24% lower postoperative morbidity compared to the control group, and there was no evidence to support a higher risk of infectious complications or impaired wound healing [60].
Additionally, corticosteroids have been included as a component of the perfusate in MP particularly during extended perfusion, to promote graft survival [61].
Thyroid hormones play an important role in intracellular calcium homeostasis and mitochondrial function. There is extensive evidence of a sharp decrease in serum triiodothyronine (T3) levels following brain death (BD). Reduced thyroid hormone concentrations following BD have been proposed to induce hemodynamic instability, leading to a decrease in myocardial energy storage and a shift in metabolism from aerobic to anaerobic. After administration of T3 hormone to DBD, creatine phosphate and adenosine phosphate levels returned to normal levels, lactate decreased, and glycogen levels improved [62]. In a rodent model, administration of T3 prior to the induction of BD has been shown to reduce apoptosis and injury to liver cells [63]. In a retrospective study that involved 66,629 donors, it was found that T3/T4 therapy increased the number of organs that could be transplanted, but it did not have any negative effect on graft survival after transplantation [64].
6.3. Antioxidants
Melatonin, an endogenous antioxidant synthesized in the pineal gland, exhibits significant potential in mitigating liver IRI. Melatonin’s multifaceted role encompasses the upregulation of critical antioxidant enzymes, including super-oxide dismutase, glutathione peroxidase, and glutathione reductase, thereby enhancing cellular defense mechanisms. Moreover, melatonin has demonstrated the capacity to dampen key inflammatory pathways, such as the Toll-like receptor 4 (TLR-4)–nuclear factor kappa B (NFκB)–NOD-like receptor protein 3 (NLRP-3) axis, in murine liver models of IRI [65]. Recent murine studies have further illuminated melatonin’s role in downregulating the activity of the NFκB signaling pathway during both the early and late phases of hepatic IRI. This attenuation of NFκB activity not only ameliorates the inflammatory response but also safeguards liver function, ultimately culminating in enhanced perioperative survival rates [66]. Melatonin has also been used as additive in organ preservation solutions [67].
Ferritin, with its inherent antioxidant properties, contributes to the reduction of apoptotic stimuli in the context of IRI [68]. Mangafodipir trisodium, when administered to the donor prior to organ harvesting, has also demonstrated its efficacy in preventing IRI [69].
In the realm of natural antioxidants, ubiquinol, also known as coenzyme Q10, plays a pivotal role as a component of the respiratory chain [70]. Notably, MitoQ, a specialized formulation of ubiquinol, leverages its antioxidant potential to mitigate IRI. MitoQ’s mechanism of action involves cycling between the ubiquinol and ubiquinone forms, facilitated by complex-II, thereby perpetuating its antioxidant effects, and intervening in the IRI cascade [71].
Furthermore, N-acetyl cysteine (NAC), a precursor to glutathione, emerges as a valuable agent in protecting the liver against IRI in animal models [72]. It is used as additive in organ preservation solutions (Custodiol). Through the process of replenishing glutathione reserves and enhancing cellular homeostasis, NAC promotes overall survival and reduces the likelihood of graft dysfunction. The protective mechanisms of this additive against liver IRI have been suggested to involve the regulation of the ROS/JNK/Bcl-2 pathway, leading to the attenuation of IRI-induced autophagy and apoptosis [73].
These multifaceted interventions collectively contribute to the preservation of liver function in the face of IRI.
6.4. Polyethylene Glycol
Polyethylene glycol has demonstrated its ability to protect cellular structures, especially the cytoskeleton and mitochondria. It also exhibits antioxidant properties that help prevent edema-induced membrane destabilization by inhibiting cellular reactions. PEG35 also enhances nitric oxide (NO) generation, which is important in vasodilation. NO generation improves microcirculation within the graft, reducing reperfusion damage, especially interesting for fatty livers [74]. Furthermore, certain PEG molecules could cross cell membranes and prevent the induction of MPT. As a result, cell inflammation is reduced, and the membrane potential is preserved. This preservation occurs through the direct inhibition of cytochrome c release and the prevention of apoptosis [75]. PEG, at a concentration of 1 g/L, is already present in the Institute-George-Lopez-1 (IGL-1) protective solution used in SCS [76]. Institute-George-Lopez-2 (IGL-2), a modified version of IGL-1 with a PEG35 concentration of 5 g/L, was developed to be suitable for SCS and dynamic preservation, such as hypothermic oxygenated perfusion (HOPE) [77].
This new preservation solution has a higher antioxidant capacity compared to Belzer-MPS, the perfusion solution normally used for HOPE, due to its higher concentration of glutathione. Additionally, IGL2 has a lower viscosity than Belzer-MPS, facilitating graft rinsing and HOPE procedures [77]. This lower viscosity also aids in glycocalyx preservation, which enhances graft microcirculation, as well as vasodilation via nitric oxide generation [78]. The use of IGL-2 as the unique perfusion solution for static and dynamic preservation strategies would simplify the logistics of using different perfusates.
6.5. Other Agents
Nilotinib, an oral receptor tyrosine kinase inhibitor, has demonstrated substantial in vitro activity against c-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinase (MAPK), both of which play pivotal roles in mediating liver IRI by modulating the expression of inflammatory factors and cell death processes. In a murine model of liver IRI, experimental studies have highlighted the noteworthy impact of nilotinib. It has been shown to effectively reduce the recruitment of inflammatory monocytes, lower the expression of pro-inflammatory cytokines, and ameliorate hepatocellular apoptosis [79].
Iloprost, an analog of prostacyclin, underwent testing in a rat model of IRI, where it displayed a reduction in hepatic enzyme levels and an upregulation in the expression of antioxidant enzymes. Notably, it also maintained the regular sinusoidal structures with normal morphology, devoid of congestion, in the IRI-afflicted rats that had been pretreated with iloprost [80]. Similarly, treprostinil, another prostacyclin analog, showcased analogous findings in a rat orthotopic liver transplantation model [81].
Heme oxygenase-1 (HO-1), a pivotal enzyme responsible for the degradation of heme molecules into biliverdin, carbon monoxide (CO), and ferrous ions, has emerged as a positive modulator of hepatic IRI prophylaxis. Biliverdin’s action in neutralizing ROS alleviates inflammatory processes, while CO, operating through the MAPK pathway, fosters an anti-inflammatory and anti-apoptotic environment crucial for maintaining microcirculation integrity. Furthermore, the regulatory influence of HO-1 on autophagy has been demonstrated to be dependent on the enzyme silent information regulator factor 2-related enzyme 1 (SIRT1). Experimental interventions involving adenovirus-mediated HO-1 gene transfer in mouse liver grafts have underscored the interplay between HO-1 and SIRT1 in controlling autophagy, offering novel insights into its multifaceted impact [82,83].
Fibrates, a class of anti-inflammatory drugs that act as direct activators of peroxisome proliferator-activated receptor alpha (PPARα), have exhibited efficacy in mitigating IRI in rat kidney models. Moreover, recent revelations have brought to light the antioxidant potential of PPARγ agonist pioglitazone, further solidifying the role of fibrates in combating IRI [84].
7. Dynamic Preservation
The landscape of organ preservation techniques in the field of transplantation has experienced significant evolution since the introduction of the University of Wisconsin solution in the early 1990s [85]. However, the conventional SCS method, which involves maintaining donor organs at low temperatures without continuous perfusion, has remained relatively unchanged. The limitations of SCS are apparent in its reliance on metabolic processes, particularly ATP depletion, the accumulation of metabolites, and the gradual loss of cellular membrane functions. These constraints impose a strict time limit on organ preservation using SCS.
Over the years, the criteria for organ transplantation have expanded to encompass a wider range of transplant indications. Many of the initial contraindications have been systematically addressed and overcome. This growth in transplant indications, coupled with a growing aging population with higher rates of obesity, has intensified the challenge of procuring an adequate supply of viable donor organs. Consequently, there has been a notable increase in the discard rate of donor livers, underscoring the pressing issue of organ scarcity in transplantation [1,2,5,13,17].
To address the scarcity of viable organs, efforts have been directed toward broadening the selection criteria for eligible organ donors. It is especially important to highlight that a graft will be more vulnerable to IRI the more extended criteria characteristics it has (such as steatosis, advanced age, and ischemia duration).
Additionally, ongoing investigations are focused on optimizing organ preservation techniques and devising strategies to salvage organs that were once considered marginal or unsuitable for transplantation.
A particularly promising area of innovation in organ preservation is the concept of dynamic perfusion, which has garnered renewed interest and has been the subject of numerous clinical studies [37,86,87,88,89,90]. While these technologies share the overarching goal of mitigating liver ischemia, they possess distinctive characteristics concerning the timing of their application, temperature settings, composition of the perfusate, and the types of perfusion devices employed.
Broadly, different strategies have emerged within the realm of dynamic perfusion: (1) Normothermic Regional Perfusion (NRP), (2) ex situ Normothermic Machine Perfusion (NMP), (3) Hypothermic Machine Perfusion (HMP) and (4) sequential HOPE-NMP. These approaches represent diverse methods for maintaining and revitalizing donor organs, ultimately enhancing their viability and potential for successful transplantation.
7.1. In Situ Normothermic Regional Perfusion
The initial approach, known as NRP, was pioneered in Spain within the context of unexpected cardiac arrest situations. In this innovative method, individuals who had the potential to become organ donors were initiated on extra corporeal membrane oxygenation (ECMO) through femoral vessel cannulation while cardiopulmonary resuscitation (CPR) was still in progress [91,92]. This pioneering technique was subsequently expanded to encompass DCD donors.
The core principle of in situ perfusion during NRP is to curtail the ischemic damage incurred by the donor organ during the warm ischemia period. It facilitates the recovery of organs before subjecting them to the cold organ flush during the organ procurement process. An additional advantage of NRP is the opportunity it offers for conducting a range of biochemical assays during perfusion. These assays can include measurements of lactate levels, transaminase activity, and pH levels, all of which enable the early assessment of the extent of IRI. This early assessment is pivotal in determining the viability of the organ.
Several comprehensive studies investigating NRP have reported superior outcomes and reduced complications, particularly in the context of DCD liver transplantation across various European countries. Building upon these favorable results, NRP has become an established standard of care, if not mandatory, for DCD donors in certain countries [91,93,94,95]. This approach represents a significant advancement in organ preservation techniques, offering enhanced outcomes and minimizing complications, including the occurrence of biliary complications.
Despite the advantage of maintaining the organ under more physiological conditions, its potential in the long-term preservation or treatment of ECD organs has not been explored. This can be explained as being mainly due to ethical and resource concerns. Furthermore, perfusion of the remaining abdominal organs implies the use of higher treatment doses than those used in the case of isolated organs. Research for specific biomarkers of organs or cell types would be necessary because multiple organs would be involved in the perfusion and interpreting the results would be challenging.
7.2. Ex Situ Normothermic Machine Perfusion
The second method, NMP, demonstrates the capability to extend the duration of successful organ preservation compared to conventional cold storage techniques. NMP strategies are designed to maintain the liver in a state that closely mimics physiological metabolism and synthetic liver function. This is achieved by providing the liver with oxygen and essential nutrients at a temperature of 37 °C through the utilization of a blood-based perfusate, thereby preserving physiological metabolism. When NMP is employed, the severity of hepatic IRI appears to be notably reduced [86].
NMP plays a pivotal role in maintaining the health of the liver’s endothelium and replenishing hepatic ATP reserves. Furthermore, NMP induces alterations in the expression of genes involved in liver regeneration and the regulation of inflammation. In clinical practices, the assessment of graft viability during NMP relies on various parameters, including hemodynamic stability according to the measurement of arterial and portal venous flows, resistances and pressures, bile production, perfusate lactate clearance, and the biochemical composition of the perfusate [86,87,96,97,98]. However, the quest for the most precise biomarkers for evaluating a liver’s transplantability remains ongoing [86,88,96,99]. The early initiation of NMP offers advantages since it mitigates the need for SCS, limiting IRI. Positive results have been reported in the literature regarding the successful implementation of “ischemia-free liver transplantation” (IFLT), which involves the complete avoidance of SCS [100]. However, this approach requires the transportation of NMP devices to the donation center, which complicates logistics and increases the costs of the procedure. On the other hand, the implementation of end ischemic NMP, while associated with simpler logistics and lower costs, may be somewhat less effective in preventing injuries related to ischemia [86,96,97].
NMP research initially focused on improving allograft selection and evaluation using graft viability testing, and, more recently, on rescuing rejected organs for transplantation [96], which will help ameliorate the shortage of livers for transplantation. New studies are focusing on prolonging the liver graft preservation time, which may provide more possibilities for liver transplantation, improve liver regeneration, and allow IRI modulation. However, long-term perfusions also bring new challenges, such as hemolysis, controlling oxygenation and glucose levels, removing waste products, and simulating diaphragm movements to prevent pressure necrosis. Clavien et al. [101] reported the longest preservation of a human liver, with subsequent transplantation after 68 h of NMP. Special devices are being developed to maintain perfusion for longer periods given that commercial devices only offer perfusions for up to 24 h [101,102].
7.3. Ex Situ Hypothermic Machine Perfusion
Perfusion strategies such as hypothermic oxygenated machine perfusion (HMP) have made significant inroads in clinical practices, particularly through techniques like hypothermic oxygenated perfusion via the portal vein (HOPE) or dual perfusion involving the portal vein and hepatic artery, known as dual HOPE (DHOPE). These methods have demonstrated considerable promise in mitigating hepatic IRI. The key to their effectiveness lies in oxygenating the perfusate during the perfusion. HMP has displayed the ability to forestall damage to mitochondria and the nucleus, as well as the activation of Kupffer cells and endothelial cells [103].
The rationale behind the success of HMP hinges on the restoration of aerobic metabolism. During ischemia, hypoxic conditions prevail within mitochondria, resulting in energy depletion and the accumulation of toxic metabolites. The reintroduction of oxygen, however, has contrasting effects. When oxygen is administered in normothermic conditions, it can trigger the production of ROS, which are the primary inflammatory mediators associated with IRI. Conversely, the reintroduction of oxygen under hypothermic conditions triggers various protective pathways within mitochondria and throughout the organs [104]. In the cold, oxygenated environment, aerobic metabolism is swiftly reinstated in mitochondria. This not only reduces the levels of detrimental metabolites like succinate and NADH, but also reestablishes ATP levels within a mere two hours. Consequently, HMP has been found to significantly mitigate IRI, inflammation, and post-transplant complications in various organs [88,90,105,106,107].
Compared to NMP, the perfusion approach employed in HMP is more cost-effective and less time-consuming. It employs a perfusate like that used in SCS. However, one of the primary challenges of HMP is the limited data available for assessing liver function. Efforts are underway to enhance the evaluation of allograft viability during HMP before transplantation. Among the most extensively studied viability markers is flavin mononucleotide (FMN). Under physiological conditions, FMN is closely associated with complex I of the electron transport chain. When the electron chain is disrupted, FMN dissociates from complex I and becomes readily detectable in acellular machine perfusates. The fluorescent properties of FMN permit the real-time measurement of this molecule in perfusates, and its levels correlate with liver function, damage, recipient complications, and graft survival following transplantation. Other parameters under evaluation include glucose, lactate, and nicotinamide adenine dinucleotide (NAD) [108,109].
7.4. Ex Situ Combined Hypothermic and Normothermic Dynamic Perfusion
A recently developed method involves sequential HOPE-controlled oxygenated rewarming NMP (COR), which induces a synergistic protective effect to attenuate the IRI cascade. There is increasing evidence that an abrupt temperature shift from hypothermic to normothermic perfusion may induce mitochondrial dysfunction [110,111].
In a prospective observational cohort study of 54 discarded/high-risk livers that underwent DHOPE-NMP, after functional assessment during the NMP, 34 livers (63% utilization) met the viability criteria and were transplanted. The one-year graft and patient survival rates were 94% and 100%, respectively. Post-transplant cholangiopathy occurred in one patient (3%) [111].
In this context, it is probably advisable to consider a COR strategy as an approach to rescue and assess the viability of donor livers initially rejected for transplantation or when NMP is used after a long period of static cold storage (“back-to-base” or end-ischemic).
8. Therapeutics Agents during Ex Vivo Machine Perfusion
Perfusion machines can be used to restore injured organs by adding therapeutic agents to the perfusate. Various treatments such as defatting agents, vasodilators, anti-inflammatory medications, gene therapy agents, and mesenchymal stem cells have been investigated in animal and human models (Table 2).

Table 2.
Therapeutic agents in models of ex vivo liver machine perfusion.
8.1. Defatting Cocktail
Steatotic livers, known for their heightened susceptibility to IRI, pose a substantial risk of postoperative morbidity and mortality following liver transplantation [123]. In both experimental and preclinical models, researchers have explored the application of perfusion devices to implement metabolic preconditioning of liver grafts prior to transplantation. Notably, independent studies have revealed that NMP in isolation can yield a significant reduction in steatosis in porcine models [124].
This innovative approach, which involves a “deffating cocktail (DC)”, was more recently introduced by Nagrath et al. [119] and subsequently validated in the context of mechanical perfusion in rat livers [120,122].
The DC comprises five key components: (1) PPARδ ligand GW501516 (GW5)—stimulating the transcription of genes involved in lipid oxidation and export. (2) Pregnane X receptor (PXR) ligand hypericin (HSC)—enhancing the transcription of cytochrome P450 (CYP)3A4 ammonooxygenase, promoting pro-β-oxidation. (3) Constitutive androstane receptor (CAR) ligand scoparone (SCO)—promoting the transcription of enzymes related to β-oxidation, including carnitine palmitoyltransferase. (4) Glucagon mimetic and cAMP activator forskolin (FOR)—instigating cyclic cAMP-driven β-oxidation and ketogenesis. (5) Insulin mimetic adipokine visfatin (VIS)—reducing triglyceride levels within the liver [119].
These promising findings have been corroborated in a study which involved ten discarded livers subjected to NMP. In this study, five livers were additionally treated with Nagrath’s deffating therapy, while five served as controls. The livers treated with the DC exhibited a substantial 38% reduction in their tissue triglyceride levels compared to a mere 7% decrease in the control group over a 6 h period, despite an increase in perfusate TG levels. This treatment was associated with increased tissue ketogenesis and ATP levels, as well as decreased levels of pro-inflammatory cytokines such as TNFα and IL1β, along with reduced lactate levels. Furthermore, the treated livers displayed a remarkable 40% reduction in macrovesicular steatosis upon histological examination [121].
8.2. Vasodilators
Effective microcirculatory support is paramount during ex vivo perfusion, particularly when dealing with severely injured livers. Microcirculation plays a pivotal role in exacerbating the damage induced by IRI and can significantly impact post-transplant outcomes. Liver sinusoidal endothelial cells are notably more susceptible to ischemic insults compared to hepatic parenchymal cells. This heightened vulnerability disrupts the normal barrier function, vascular tone regulation, and expression of adhesion molecules. Upon reperfusion, the decreased nitric oxide levels result in impaired vasodilation control. Moreover, the activation of inflammatory and coagulation cascades leads to capillary occlusion, further worsening the IRI [125].
To address these issues, vasodilators have been investigated to enhance microcirculatory integrity and subsequently improve graft viability during NMP. Among these vasodilators, prostacyclin (PGI2) exhibits the remarkable capacity to inhibit platelet aggregation, dampen leukocyte activation and chemotaxis, and curtail superoxide anion production. These actions collectively contribute to an anti-inflammatory effect while safeguarding endothelial cells from damage [118,126]. Its use has enabled successful transplantations of DCD porcine livers following NMP [117].
Another powerful vasodilator with additional antiplatelet and fibrinolytic properties is prostaglandin E1 (PGE1). Researchers have evaluated its potential to enhance microcirculation in various studies, offering a promising avenue for further investigation [115,116].
8.3. Other Therapeutics Agents
Incorporating anti-inflammatory agents into the perfusion process has emerged as a promising strategy [112]. Notably, the δ-opioid agonist enkephalin has garnered attention for its potential benefits in mitigating inflammation [113]. Additionally, recent reports have introduced a novel approach involving the selective NLRP3 inflammasome inhibitor mcc950, which is added to the perfusate during HMP. In a pig liver transplantation model, this innovative method showcased improved outcomes, particularly in the context of DCD organs, when compared to control groups [114].
8.4. Senolytics
Senescent cells are a natural consequence of the aging process and are associated with limited tissue regeneration capabilities [127]. The development of senescence involves intricate molecular processes, including chromatin modifications, the formation of senescence-associated heterochromatin foci, and the presence of senescence-associated β-galactosidase, often accompanied by the upregulation of p21CIP1 and/or p16INK4a, which contribute to senescent DNA fragmentation [128].
One hallmark of senescent cells is their release of a substantial quantity of proinflammatory factors, collectively referred to as the senescence-associated secretory phenotype (SASP) [129]. These factors exert a profound influence on tissue homeostasis and can significantly impact the functions of neighboring cells.
Emerging evidence suggests the potential utility of senolytic agents in targeting and eliminating senescent cells [130]. These agents aim to counteract the effects of senescence and hold promise in the field of research on anti-aging. Recent investigations have unveiled various cellular targets for anti-aging interventions, including members of the BCL family, HSP90, and PI3K/AKT inhibitors [131].
Senolytics have demonstrated their potential in preclinical studies by mitigating age-related dysfunction, inflammation, and multi-organ diseases. Their application may extend to addressing age-related issues in organ transplantation, particularly when dealing with organs from older donors. Administering senolytics before IRI has shown promise in reducing key markers such as cell-free mitochondrial DNA (cf-mt-DNA), Th17, and IFN+ T-cell values, ultimately diminishing the burden of senescent cells within the recipient’s body [132].
Remarkably, this therapeutic approach has been successfully integrated into perfusion machines, offering a novel method for organ preservation [133].
8.5. Gene Therapy
Genetic modulation represents a promising avenue for addressing the challenges inherent in solid organ transplantation, offering the potential to expand the donor pool (Table 3). Numerous gene therapy approaches have undergone extensive research, with their feasibility validated through in vivo animal studies. In allogeneic transplantation, gene therapy holds substantial promise for addressing a range of issues, including the treatment of genetic defects, congenital metabolic disorders, and coagulation abnormalities associated with donor organs [134]. Various biochemical techniques have been developed to introduce or modify genes, allowing for the precise control of gene expression. For instance, messenger RNA-based strategies employ antisense oligonucleotides (ASOs) or antagonists, while RNA interference (RNAi) leverages RNA molecules such as micro-RNA (miRNA), short hairpin RNA (shRNA), or small interfering RNA (siRNA).

Table 3.
Gene modulation approaches during ex vivo liver machine perfusion.
In a notable study, a mouse model was employed to investigate the role of high mobility group box 1 (HMGB-1), an early mediator of reperfusion-related damage. The administration of siRNA targeting HMGB-1 resulted in improved liver function following reperfusion [139]. Another study focused on the silencing of proapoptotic caspases 3 and 8 in a mouse model of IRI, wherein the mice treated with siRNA exhibited a significantly enhanced protective response. Specifically, 30% of animals received caspase-8 siRNA treatment, while 50% were treated with caspase-3 siRNA; remarkably, they survived beyond 30 days post the ischemic event, in stark contrast to the control group, which succumbed within 5 days [140].
Among the various miRNAs found in the liver, miR-122 stands out as the most abundant. Previous research has linked miRNAs to an array of diseases and dysfunctions [141]. Notably, in an ischemic porcine cardiogenic shock model, circulating levels of miR-122 surged by nearly 460,000-fold following cardiogenic shock, far surpassing the modest elevation observed in classic markers of hepatocellular necrosis, which increased by only about 3-fold. Importantly, miR-122 levels exhibited a significant decrease following therapeutic hypothermia [142]. Additionally, during warm hepatic IRI in rats, the level of miR-122 showed a correlational increase with serum hepatic enzymes and lactate dehydrogenase (LDH) levels [143].
In the context of organ preservation during mechanical perfusion, the application of gene modulation agents, such as ASOs and siRNA, holds significant potential due to its enhanced specificity compared to systemic gene modulation.
The first study that demonstrated the application of ASOs in suppressing the pathogenicity of hepatitis C virus (HCV) in a porcine model employed ASOs that specifically targeted miRNA-122, a crucial element for HCV replication. The inhibition of miRNA expression demonstrated a substantial reduction in HCV activity [135].
The pioneering application of siRNA during mechanical perfusion systems was also reported, revealing that the direct introduction of siRNA into the perfusion system facilitated its uptake by rat liver grafts, regardless of whether hypothermic or normothermic perfusion systems were employed [136].
This same group also employed siRNA targeting p53 to modulate apoptosis in a murine model [137].
Another recent study was conducted to examine the potential of inhibiting the apoptosis-associated gene FAS using siRNA to mitigate IRI in a rat liver transplantation model. The study resulted in inconclusive findings regarding the efficacy and feasibility of gene modulation during MP to enhance graft function. However, the study did demonstrate that absorption of liver siRNA occurs during HMP [138].
These results underscore the potential of siRNA-based approaches in the context of organ preservation and transplantation.
8.6. Mesenchymal Stem Cells
Mesenchymal stem cells (MSCs) have emerged as a promising avenue for improving organ transplantation outcomes (Table 4) by modulating the inflammatory response and reducing tissue damage [144]. MSCs, derived from various tissues, possess the capacity not only to replace damaged cells but also to exert beneficial effects through paracrine mechanisms [145]. These mechanisms include anti-inflammatory, antiapoptotic, antioxidant, antifibrotic, and proangiogenic properties mediated by the release of extracellular vesicles (EVs), cytokines, and growth factors [146]. The impact of MSCs and their EVs on the repair of target organs involves a range of mechanisms. These mechanisms encompass the mitigation of IRI, the stimulation of angiogenesis, the regulation of target cell proliferation, and the inhibition of the epithelial–mesenchymal transition [147]. One study underscored the potential of MSCs in enhancing graft survival rates when incorporated into the donor organ before transplantation [148].

Table 4.
Mesenchymal stem cells during ex vivo liver machine perfusion.
With the advent of perfusion machines capable of maintaining organs under physiological conditions outside the human body, there is now an opportunity to directly administer MSC therapies to the perfused graft. Pioneering work by Sasajima et al. [149] has demonstrated successful reconditioning of rat livers procured after DCD by administering MSCs during NMP. Swine adipose MSCs administered at the initiation of perfusion with blood-free perfusate exhibited increased bile production and preserved sinusoidal and hepatocellular morphology after 2 h of NMP.
Another study successfully demonstrated the internalization of extracellular vesicles derived from human liver stem cells during ex situ perfusion. Following this, the livers that were treated exhibited reduced histological damage and lower levels of liver injury markers following a 4 h perfusion period [150].
Building on these initial findings, several experiments have been conducted, yielding significant advances in in vitro liver preservation techniques, and optimizing MSC culture methods.
Nevertheless, it is important to note that the application of MSC therapy in this context remains in the early stages of clinical trials and laboratory research, signifying ongoing exploration and development.
9. Summary
The principal contributors to organ shortages lie in the diminishing quality of donor organs within the aging population and associated pathological conditions such as obesity. Within this context, the utilization of extended criteria donor (ECD) grafts has gained prominence [5,13,17].
IRI stands out as one of the foremost challenges in the field of liver transplantation, and its severity directly influences graft function and post-transplant outcomes. This is particularly pertinent in ECD grafts, which exhibit a heightened susceptibility to IRI, exacerbated by their absence of reliable biomarkers for pre-transplantation assessments of graft function [4,6,7,12].
The cumulative impact of graft IRI throughout liver transplantation procedures, encompassing organ recovery, static and dynamic graft preservation, graft implantation, and reperfusion, plays a pivotal role in determining the success of the transplantation outcome. In this context, our efforts have concentrated on minimizing IRI and, more recently, exploring its treatment to leverage marginal livers for expanding the donor pool.
Addressing the challenge of formulating a viable therapeutic strategy for preventing and treating IRI necessitates an exhaustive understanding of the mechanical and molecular foundations of hepatic IRI. In this light, a diverse range of pharmacological therapies and technical static and dynamic preservation strategies has been scrutinized for the prevention of multifactorial IRI, as succinctly outlined in this review.
Recent years have witnessed a significant shift in abdominal organ transplantation preservation towards an increased application of dynamic preservation techniques. In this scenario, a novel preservation solution, IGL-2, emerges with distinct advantages over common perfusion solutions. This innovative preservation solution holds the promise of providing a singular perfusion solution for static and dynamic preservation strategies, streamlining the logistics of using diverse perfusates [76,77,78].
Broadly, three primary strategies in dynamic preservation are presently applied to human organs before implantation: Normothermic Regional Perfusion (NRP), ex situ Normothermic Machine Perfusion (NMP), and Hypothermic Machine Perfusion (HMP).
A promising strategy has been developed via a sequential combination of HOPE-controlled oxygenated rewarming NMP (COR) [111].
Most of the existing research studies on therapeutic interventions have been conducted within the realm of NMP. This technique leverages the treatment of a metabolically active organ to modulate or potentially reverse a disease process in the donor organ, thereby optimizing it for transplantation.
The current challenges revolve around implementing standardized protocols and guidelines into daily clinical practice. These protocols and guidelines seek to assess the risks associated with specific donors, determine utilization rates, and establish criteria for using perfusion techniques to facilitate the standardized use of machine perfusion (MP) for ECD organs [156]. Such implementation would empower surgeons to accurately assess the ECD graft, determine the most appropriate perfusion strategy, and ultimately decide on its suitability for transplantation.
10. Concluding Remarks
Future endeavors in liver transplantation must concentrate on comprehending the processes linked with IRI, enhancing techniques for evaluating liver quality and function pre transplantation, improving the understanding of graft viability, and refining methods for reconditioning marginal grafts before implantation in the recipient. The ultimate goal in organ transplantation is to establish perfusion protocols that minimize and modulate IRI, achieve hepatic regeneration of ECD grafts, and provide reliable biomarkers for organ viability evaluation and prediction of post-transplant outcomes.
Author Contributions
Conceptualization, Y.F. and G.C.; methodology, Y.F.; validation, all authors., formal analysis, Y.F. and G.C.; investigation, Y.F., G.C. and A.P.-R.; resources, Y.F., G.C. and A.P.-R.; writing—original draft preparation, Y.F. and G.C.; writing—review and editing, Y.F., G.C. and A.P.-R.; supervision, all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Quaresima, S.; Melandro, F.; Giovanardi, F.; Shah, K.; De Peppo, V.; Mennini, G.; Ghinolfi, D.; Limkemann, A.; Pawlik, T.M.; Lai, Q. New Insights in the Setting of Transplant Oncology. Medicina 2023, 59, 568. [Google Scholar] [CrossRef] [PubMed]
- Vodkin, I.; Kuo, A. Extended Criteria Donors in Liver Transplantation. Clin. Liver Dis. 2017, 21, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.S.; Cassim, S.; Raymond, V.A.; Gottschalk, S.; Merlen, G.; Zwingmann, C.; Lapierre, P.; Darby, P.; Mazer, C.D.; Bilodeau, M. Upregulation of Krebs cycle and anaerobic glycolysis activity early after onset of liver ischemia. PLoS ONE 2018, 13, e0199177. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Naini, B.V.; Markovic, D.; Aziz, A.; Younan, S.; Lu, M.; Hirao, H.; Kadono, K.; Kojima, H.; DiNorcia, J.; et al. Ischemia-reperfusion injury and its relationship with early allograft dysfunction in liver transplant patients. Am. J. Transplant. 2021, 21, 614–625. [Google Scholar] [CrossRef] [PubMed]
- Busuttil, R.W.; Tanaka, K. The utility of marginal donors in liver transplantation. Liver Transpl. 2003, 9, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Machado, I.F.; Palmeira, C.M.; Rolo, A.P. Preservation of Mitochondrial Health in Liver Ischemia/Reperfusion Injury. Biomedicines 2023, 11, 948. [Google Scholar] [CrossRef]
- Liu, J.; Man, K. Mechanistic Insight and Clinical Implications of Ischemia/Reperfusion Injury Post Liver Transplantation. Cell. Mol. Gastroenterol. Hepatol. 2023, 15, 1463–1474. [Google Scholar] [CrossRef]
- Schlegel, A.; Muller, X.; Mueller, M.; Stepanova, A.; Kron, P.; de Rougemont, O.; Muiesan, P.; Clavien, P.A.; Galkin, A.; Meierhofer, D.; et al. Hypothermic oxygenated perfusion protects from mitochondrial injury before liver transplantation. EBioMedicine. 2020, 60, 103014. [Google Scholar] [CrossRef]
- Tara, A.; Dominic, J.L.; Patel, J.N.; Garg, I.; Yeon, J.; Memon, M.S.; Gergal Gopalkrishna Rao, S.R.; Bugazia, S.; Dhandapani, T.P.M.; Kannan, A.; et al. Mitochondrial Targeting Therapy Role in Liver Transplant Preservation Lines: Mechanism and Therapeutic Strategies. Cureus 2021, 13, e16599. [Google Scholar] [CrossRef]
- Wang, L.; Feng, Z.J.; Ma, X.; Li, K.; Li, X.Y.; Tang, Y.; Peng, C. Mitochondrial quality control in hepatic ischemia-reperfusion injury. Heliyon 2023, 9, e17702. [Google Scholar] [CrossRef]
- McCully, J.D.; Del Nido, P.J.; Emani, S.M. Mitochondrial transplantation for organ rescue. Mitochondrion 2022, 64, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Petrowsky, H.; Hong, J.C.; Busuttil, R.W.; Kupiec-Weglinski, J.W. Ischaemia-reperfusion injury in liver transplantation—From bench to bedside. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 79–89. [Google Scholar] [CrossRef]
- Nair, A.; Hashimoto, K. Extended criteria donors in liver transplantation-from marginality to mainstream. Hepatobiliary Surg. Nutr. 2018, 7, 386–388. [Google Scholar] [CrossRef]
- Berthiaume, F.; Barbe, L.; Mokuno, Y.; MacDonald, A.D.; Jindal, R.; Yarmush, M.L. Steatosis reversibly increases hepatocyte sensitivity to hypoxia-reoxygenation injury. J. Surg. Res. 2009, 152, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Fukumori, T.; Ohkohchi, N.; Tsukamoto, S.; Satomi, S. The mechanism of injury in a steatotic liver graft during cold preservation. Transplantation 1999, 67, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Tien, C.; Remulla, D.; Kwon, Y.; Emamaullee, J. Contemporary strategies to assess and manage liver donor steatosis: A review. Curr. Opin. Organ Transplant. 2021, 26, 474–481. [Google Scholar] [CrossRef]
- Durand, F.; Renz, J.F.; Alkofer, B.; Burra, P.; Clavien, P.A.; Porte, R.J.; Freeman, R.B.; Belghiti, J. Report of the Paris consensus meeting on expanded criteria donors in liver transplantation. Liver Transpl. 2008, 14, 1694–1707. [Google Scholar] [CrossRef]
- de Graaf, E.L.; Kench, J.; Dilworth, P.; Shackel, N.A.; Strasser, S.I.; Joseph, D.; Pleass, H.; Crawford, M.; McCaughan, G.W.; Verran, D.J. Grade of deceased donor liver macrovesicular steatosis impacts graft and recipient outcomes more than the Donor Risk Index. J. Gastroenterol. Hepatol. 2012, 27, 540–546. [Google Scholar] [CrossRef]
- Spitzer, A.L.; Lao, O.B.; Dick, A.A.; Bakthavatsalam, R.; Halldorson, J.B.; Yeh, M.M.; Upton, M.P.; Reyes, J.D.; Perkins, J. The biopsied donor liver: Incorporating macrosteatosis into high-risk donor assessment. Liver Transpl. 2010, 16, 874–884. [Google Scholar] [CrossRef]
- Maeso-Díaz, R.; Ortega-Ribera, M.; Fernández-Iglesias, A.; Hide, D.; Muñoz, L.; Hessheimer, A.J.; Vila, S.; Francés, R.; Fonde-vila, C.; Albillos, A.; et al. Effects of aging on liver microcirculatory function and sinusoidal phenotype. Aging Cell. 2018, 17, e12829. [Google Scholar] [CrossRef]
- Jiménez-Romero, C.; Caso Maestro, O.; Cambra Molero, F.; Justo Alonso, I.; Alegre Torrado, C.; Manrique Municio, A.; Calvo Pulido, J.; Loinaz Segurola, C.; Moreno González, E. Using old liver grafts for liver transplantation: Where are the limits? World J. Gastroenterol. 2014, 20, 10691–10702. [Google Scholar] [CrossRef] [PubMed]
- von Horn, C.; Zlatev, H.; Pletz, J.; Lüer, B.; Minor, T. Comparison of thermal variations in post-retrieval graft conditioning on rat livers. Artif. Organs. 2022, 46, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Castro, M.B.; Cornide-Petronio, M.E.; Gracia-Sancho, J.; Peralta, C. Inflammasome-Mediated Inflammation in Liver Ischemia-Reperfusion Injury. Cells 2019, 8, 1131. [Google Scholar] [CrossRef] [PubMed]
- Kuboki, S.; Shin, T.; Huber, N.; Eismann, T.; Galloway, E.; Schuster, R.; Blanchard, J.; Edwards, M.J.; Lentsch, A.B. Hepatocyte signaling through CXC chemokine receptor-2 is detrimental to liver recovery after ischemia/reperfusion in mice. Hepatology 2008, 48, 1213–1223. [Google Scholar] [CrossRef]
- Heylen, L.; Pirenne, J.; Naesens, M.; Sprangers, B.; Jochmans, I. “Time is tissue”-A minireview on the importance of donor nephrectomy, donor hepatectomy, and implantation times in kidney and liver transplantation. Am. J. Transplant. 2021, 21, 2653–2661. [Google Scholar] [CrossRef]
- de Vera, M.E.; Lopez-Solis, R.; Dvorchik, I.; Campos, S.; Morris, W.; Demetris, A.J.; Fontes, P.; Marsh, J.W. Liver transplantation using donation after cardiac death donors: Long-term follow-up from a single center. Am. J. Transplant. 2009, 9, 773–781. [Google Scholar] [CrossRef]
- Ho, K.J.; Owens, C.D.; Johnson, S.R.; Khwaja, K.; Curry, M.P.; Pavlakis, M.; Mandelbrot, D.; Pomposelli, J.J.; Shah, S.A.; Saidi, R.F.; et al. Donor postextubation hypotension and age correlate with outcome after donation after cardiac death transplantation. Transplantation 2008, 85, 1588–1594. [Google Scholar] [CrossRef]
- Balibrea, J.M.; Núñez-Peña, J.R.; García-Martín, M.C.; Olmedilla, Y.; Martín-Antona, E.; Berthuin, J.; Rancan, L.; Vara, E.; Balibrea, J.L. The differential tissue expression of inflammatory, oxidative stress, and apoptosis markers in human uncontrolled non-heart-beating donors. Transplantation 2013, 95, 1346–1353. [Google Scholar] [CrossRef]
- Southard, J.H.; Belzer, F.O. Organ preservation. Annu. Rev. Med. 1995, 46, 235–247. [Google Scholar] [CrossRef]
- Belzer, F.O.; Southard, J.H. Principles of solid-organ preservation by cold storage. Transplantation 1988, 45, 673–676. [Google Scholar] [CrossRef]
- Hertl, M.; Chartrand, P.B.; West, D.D.; Harvey, P.R.; Strasberg, S.M. The effects of hepatic preservation at 0 degrees C compared to 5 degrees C: Influence of antiproteases and periodic flushing. Cryobiology 1994, 31, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Villa, R.; Fondevila, C.; Erill, I.; Guimerà, A.; Bombuy, E.; Gómez-Suárez, C.; Sacristán, J.C.; García-Valdecasas, J.C. Real-time direct measurement of human liver allograft temperature from recovery to transplantation. Transplantation 2006, 81, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Fujita, S.; Hamamoto, I.; Nakamura, K.; Tanaka, K.; Ozawa, K. Isolated perfusion of rat livers: Effect of temperature on O2 consumption, enzyme release, energy store, and morphology. Nihon Geka Hokan 1993, 62, 58–70. [Google Scholar] [PubMed]
- Adam, R.; Delvart, V.; Karam, V.; Ducerf, C.; Navarro, F.; Letoublon, C.; Belghiti, J.; Pezet, D.; Castaing, D.; Le Treut, Y.P.; et al. Compared efficacy of preservation solutions in liver transplantation: A long-term graft outcome study from the European Liver Transplant Registry. Am. J. Transplant. 2015, 15, 395–406. [Google Scholar] [CrossRef]
- Kox, J.; Moers, C.; Monbaliu, D.; Strelniece, A.; Treckmann, J.; Jochmans, I.; Leuvenink, H.; Van Heurn, E.; Pirenne, J.; Paul, A.; et al. The Benefits of Hypothermic Machine Preservation and Short Cold Ischemia Times in Deceased Donor Kidneys. Transplantation 2018, 102, 1344–1350. [Google Scholar] [CrossRef] [PubMed]
- van Rijn, R.; Schurink, I.J.; de Vries, Y.; van den Berg, A.P.; Cortes Cerisuelo, M.; Darwish Murad, S.; Erdmann, J.I.; Gilbo, N.; de Haas, R.J.; Heaton, N.; et al. Hypothermic Machine Perfusion in Liver Transplantation—A Randomized Trial. N. Engl. J. Med. 2021, 384, 1391–1401. [Google Scholar] [CrossRef] [PubMed]
- Czigany, Z.; Pratschke, J.; Froněk, J.; Guba, M.; Schöning, W.; Raptis, D.A.; Andrassy, J.; Kramer, M.; Strnad, P.; Tolba, R.H.; et al. Hypothermic Oxygenated Machine Perfusion Reduces Early Allograft Injury and Improves Post-transplant Outcomes in Extended Criteria Donation Liver Transplantation from Donation After Brain Death: Results from a Multicenter Randomized Controlled Trial (HOPE ECD-DBD). Ann. Surg. 2021, 274, 705–712. [Google Scholar] [CrossRef]
- de Vries, R.J.; Tessier, S.N.; Banik, P.D.; Nagpal, S.; Cronin, S.E.J.; Ozer, S.; Hafiz, E.O.A.; van Gulik, T.M.; Yarmush, M.L.; Markmann, J.F.; et al. Supercooling extends preservation time of human livers. Nat. Biotechnol. 2019, 37, 1131–1136. [Google Scholar] [CrossRef]
- Berendsen, T.A.; Bruinsma, B.G.; Puts, C.F.; Saeidi, N.; Usta, O.B.; Uygun, B.E.; Izamis, M.L.; Toner, M.; Yarmush, M.L.; Uygun, K. Supercooling enables long-term transplantation survival following 4 days of liver preservation. Nat. Med. 2014, 20, 790–793. [Google Scholar] [CrossRef]
- Goutard, M.; de Vries, R.J.; Tawa, P.; Pendexter, C.A.; Rosales, I.A.; Tessier, S.N.; Burlage, L.C.; Lantieri, L.; Randolph, M.A.; Lellouch, A.G.; et al. Exceeding the Limits of Static Cold Storage in Limb Transplantation Using Subnormothermic Machine Perfusion. J. Reconstr. Microsurg. 2023, 39, 350–360. [Google Scholar] [CrossRef]
- de Vries, R.J.; Tessier, S.N.; Banik, P.D.; Nagpal, S.; Cronin, S.E.J.; Ozer, S.; Hafiz, E.O.A.; van Gulik, T.M.; Yarmush, M.L.; Markmann, J.F.; et al. Subzero non-frozen preservation of human livers in the supercooled state. Nat. Protoc. 2020, 15, 2024–2040. [Google Scholar] [CrossRef] [PubMed]
- Puts, C.F.; Berendsen, T.A.; Bruinsma, B.G.; Ozer, S.; Luitje, M.; Usta, O.B.; Yarmush, M.L.; Uygun, K. Polyethylene glycol protects primary hepatocytes during supercooling preservation. Cryobiology 2015, 71, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M. Biology of cell survival in the cold: The basis for biopreservation of tissue and organs. In Advances in Biopreservation; CRC Press: Boca Raton, FL, USA, 2007; pp. 15–62. [Google Scholar]
- Russo, L.; Gracia-Sancho, J.; García-Calderó, H.; Marrone, G.; García-Pagán, J.C.; García-Cardeña, G.; Bosch, J. Addition of simvastatin to cold storage solution prevents endothelial dysfunction in explanted rat livers. Hepatology 2012, 55, 921–930. [Google Scholar] [CrossRef]
- Peralta, C.; Jiménez-Castro, M.B.; Gracia-Sancho, J. Hepatic ischemia and reperfusion injury: Effects on the liver sinusoidal milieu. J. Hepatol. 2013, 59, 1094–1106. [Google Scholar] [CrossRef]
- Pfanner, N.; Warscheid, B.; Wiedemann, N. Mitochondrial proteins: From biogenesis to functional networks. Nat. Rev. Mol. Cell Biol. 2019, 20, 267–284, Correction in Nat. Rev. Mol. Cell Biol. 2021, 22, 367. [Google Scholar] [CrossRef]
- Oanh, N.T.K.; Lee, H.S.; Kim, Y.H.; Min, S.; Park, Y.J.; Heo, J.; Park, Y.Y.; Lim, W.C.; Cho, H. Regulation of nuclear DNA damage response by mitochondrial morphofunctional pathway. Nucleic Acids Res. 2022, 50, 9247–9259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yan, Q.; Wang, X.; Chen, X.; Chen, Y.; Du, J.; Chen, L. The Role of Mitochondria in Liver Ischemia-Reperfusion Injury: From Aspects of Mitochondrial Oxidative Stress, Mitochondrial Fission, Mitochondrial Membrane Permeable Transport Pore Formation, Mitophagy, and Mitochondria-Related Protective Measures. Oxid. Med. Cell Longev. 2021, 2021, 6670579. [Google Scholar] [CrossRef]
- Martin, J.L.; Costa, A.S.H.; Gruszczyk, A.V.; Beach, T.E.; Allen, F.M.; Prag, H.A.; Hinchy, E.C.; Mahbubani, K.; Hamed, M.; Tronci, L.; et al. Succinate accumulation drives ischaemia-reperfusion injury during organ transplantation. Nat. Metab. 2019, 1, 966–974. [Google Scholar] [CrossRef]
- Bouma, H.R.; Ketelaar, M.E.; Yard, B.A.; Ploeg, R.J.; Henning, R.H. AMP-activated protein kinase as a target for preconditioning in transplantation medicine. Transplantation 2010, 90, 353–358. [Google Scholar] [CrossRef]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef]
- van Golen, R.F.; van Gulik, T.M.; Heger, M. Mechanistic overview of reactive species-induced degradation of the endothelial glycocalyx during hepatic ischemia/reperfusion injury. Free Radic. Biol. Med. 2012, 52, 1382–1402. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Ferreira, J.C.; Gross, E.R.; Mochly-Rosen, D. Targeting aldehyde dehydrogenase 2: New therapeutic opportunities. Physiol. Rev. 2014, 94, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Forte, M.; Bianchi, F.; Cotugno, M.; Marchitti, S.; Stanzione, R.; Maglione, V.; Sciarretta, S.; Valenti, V.; Carnevale, R.; Versaci, F.; et al. An interplay between UCP2 and ROS protects cells from high-salt-induced injury through autophagy stimulation. Cell Death Dis. 2021, 12, 919. [Google Scholar] [CrossRef]
- Hirao, H.; Nakamura, K.; Kupiec-Weglinski, J.W. Liver ischaemia-reperfusion injury: A new understanding of the role of innate immunity. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 239–256. [Google Scholar] [CrossRef]
- Corbitt, N.; Kimura, S.; Isse, K.; Specht, S.; Chedwick, L.; Rosborough, B.R.; Lunz, J.G.; Murase, N.; Yokota, S.; Demetris, A.J.; et al. Gut bacteria drive Kupffer cell expansion via MAMP-mediated ICAM-1 induction on sinusoidal endothelium and influence preservation-reperfusion injury after orthotopic liver transplantation. Am. J. Pathol. 2013, 182, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Corbitt, N.; Kimura, S.; Isse, K.; Specht, S.; Chedwick, L.; Rosborough, B.R.; Lunz, J.G.; Murase, N.; Yokota, S.; Demetris, A.J. The membrane attack complex (C5b-9) in liver cold ischemia and reperfusion injury. Liver Transpl. 2008, 14, 1133–1141. [Google Scholar] [CrossRef]
- Trocha, M.; Szelag, A. The role of calcium and calcium channel blocking drugs in damage to the liver preserved for transplantation. Ann. Transplant. 2004, 9, 5–11. [Google Scholar]
- Saidi, R.F.; Chang, J.; Verb, S.; Brooks, S.; Nalbantoglu, I.; Adsay, V.; Jacobs, M.J. The effect of methylprednisolone on warm ischemia-reperfusion injury in the liver. Am. J. Surg. 2007, 193, 345–348. [Google Scholar] [CrossRef]
- Orci, L.A.; Toso, C.; Mentha, G.; Morel, P.; Majno, P.E. Systematic review and meta-analysis of the effect of perioperative steroids on ischaemia-reperfusion injury and surgical stress response in patients undergoing liver resection. Br. J. Surg. 2013, 100, 600–609. [Google Scholar] [CrossRef]
- Saidi, R.F.; Chang, J.; Verb, S.; Brooks, S.; Nalbantoglu, I.; Adsay, V.; Jacobs, M.J. Long-term normothermic perfusion of human livers for longer than 12 days. Artif. Organs 2022, 46, 2504–2510. [Google Scholar] [CrossRef]
- Salim, A.; Vassiliu, P.; Velmahos, G.C.; Sava, J.; Murray, J.A.; Belzberg, H.; Asensio, J.A.; Demetriades, D. The role of thyroid hormone administration in potential organ donors. Arch. Surg. 2001, 136, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Rebolledo, R.A.; Van Erp, A.C.; Ottens, P.J.; Wiersema-Buist, J.; Leuvenink, H.G.; Romanque, P. Anti-Apoptotic Effects of 3,3’,5-Triiodo-L-Thyronine in the Liver of Brain-Dead Rats. PLoS ONE 2015, 10, e0138749. [Google Scholar] [CrossRef] [PubMed]
- Novitzky, D.; Mi, Z.; Sun, Q.; Collins, J.F.; Cooper, D.K. Thyroid hormone therapy in the management of 63,593 brain-dead organ donors: A retrospective analysis. Transplantation 2014, 98, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, Z.T.; Jin, L.; Lin, J.; Fan, Z.L.; Zeng, Z.; Huang, H.F. Melatonin attenuates hepatic ischemia-reperfusion injury in rats by inhibiting NF-κB signaling pathway. Hepatobiliary Pancreat. Dis. Int. 2021, 20, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Zhai, M.; Li, B.; Duan, W.; Jing, L.; Zhang, B.; Zhang, M.; Yu, L.; Liu, Z.; Yu, B.; Ren, K.; et al. Melatonin ameliorates myocardial ischemia reperfusion injury through SIRT3-dependent regulation of oxidative stress and apoptosis. J. Pineal Res. 2017, 63, 12419. [Google Scholar] [CrossRef]
- Coskun, A.; Yegen, C.; Arbak, S.; Attaallah, W.; Gunal, O.; Elmas, M.A.; Ucal, Y.; Can, O.; Baş, B.; Yildirim, Z.; et al. Melatonin in preservation solutions prevents ischemic injury in rat kidneys. PLoS ONE 2022, 17, e0273921. [Google Scholar] [CrossRef]
- Katwal, G.; Baral, D.; Fan, X.; Weiyang, H.; Zhang, X.; Ling, L.; Xiong, Y.; Ye, Q.; Wang, Y. SIRT3 a Major Player in Attenuation of Hepatic Ischemia-Reperfusion Injury by Reducing ROS via Its Downstream Mediators: SOD2, CYP-D, and HIF-1α. Oxid. Med. Cell. Longev. 2018, 2018, 2976957. [Google Scholar] [CrossRef]
- Ben Mosbah, I.; Mouchel, Y.; Pajaud, J.; Ribault, C.; Lucas, C.; Laurent, A.; Boudjema, K.; Morel, F.; Corlu, A.; Compagnon, P. Pretreatment with mangafodipir improves liver graft tolerance to ischemia/reperfusion injury in rat. PLoS ONE 2012, 7, e50235. [Google Scholar] [CrossRef]
- Dare, A.J.; Logan, A.; Prime, T.A.; Rogatti, S.; Goddard, M.; Bolton, E.M.; Bradley, J.A.; Pettigrew, G.J.; Murphy, M.P.; Saeb-Parsy, K. The mitochondria-targeted anti-oxidant MitoQ decreases ischemia-reperfusion injury in a murine syngeneic heart transplant model. J. Heart Lung Transplant. 2015, 34, 1471–1480. [Google Scholar] [CrossRef]
- James, A.M.; Sharpley, M.S.; Manas, A.R.; Frerman, F.E.; Hirst, J.; Smith, R.A.; Murphy, M.P. Interaction of the mitochondria-targeted antioxidant MitoQ with phospholipid bilayers and ubiquinone oxidoreductases. J. Biol. Chem. 2007, 282, 14708–14718. [Google Scholar] [CrossRef]
- Firuzi, O.; Miri, R.; Tavakkoli, M.; Saso, L. Antioxidant therapy: Current status and future prospects. Curr. Med. Chem. 2011, 18, 3871–3888. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, K.; Xia, Y.; Dai, W.; Wang, F.; Shen, M.; Cheng, P.; Wang, J.; Lu, J.; Zhang, Y.; et al. N-acetylcysteine attenuates ischemia-reperfusion-induced apoptosis and autophagy in mouse liver via regulation of the ROS/JNK/Bcl-2 pathway. PLoS ONE 2014, 9, e108855. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.; Panisello-Rosello, A.; Castro-Benitez, C.; Adam, R. Glycocalyx Preservation and NO Production in Fatty Livers-The Protective Role of High. Molecular Polyethylene Glycol in Cold Ischemia Injury. Int. J. Mol. Sci. 2018, 19, 2375. [Google Scholar] [CrossRef] [PubMed]
- Bardallo, R.G.; da Silva, R.T.; Carbonell, T.; Folch-Puy, E.; Palmeira, C.; Roselló-Catafau, J.; Pirenne, J.; Adam, R.; Panisello-Roselló, A. Role of PEG35, Mitochondrial ALDH2, and Glutathione in Cold Fatty Liver Graft Preservation: An IGL-2 Approach. Int. J. Mol. Sci. 2021, 22, 5332. [Google Scholar] [CrossRef]
- Asong-Fontem, N.; Panisello-Rosello, A.; Sebagh, M.; Gonin, M.; Rosello-Catafau, J.; Adam, R. The Role of IGL-2 Preservation Solution on Rat Livers during SCS and HOPE. Int. J. Mol. Sci. 2022, 23, 12615. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, R.T.; Bardallo, R.G.; Folch-Puy, E.; Carbonell, T.; Palmeira, C.M.; Fondevila, C.; Adam, R.; Roselló-Catafau, J.; Panisello-Roselló, A. IGL-2 as a Unique Solution for Cold Static Preservation and Machine Perfusion in Liver and Mitochondrial Protection. Transplant. Proc. 2022, 54, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Panisello Rosello, A.; Teixeira da Silva, R.; Castro, C.; G Bardallo, R.; Calvo, M.; Folch-Puy, E.; Carbonell, T.; Palmeira, C.; Roselló Catafau, J.; Adam, R. Polyethylene Glycol 35 as a Perfusate Additive for Mitochondrial and Glycocalyx Protection in HOPE Liver Preservation. Int. J. Mol. Sci. 2020, 21, 5703. [Google Scholar] [CrossRef]
- Ocuin, L.M.; Zeng, S.; Cavnar, M.J.; Sorenson, E.C.; Bamboat, Z.M.; Greer, J.B.; Kim, T.S.; Popow, R.; DeMatteo, R.P. Nilotinib protects the murine liver from ischemia/reperfusion injury. J. Hepatol. 2012, 57, 766–773. [Google Scholar] [CrossRef]
- Gedik, E.; Girgin, S.; Obay, B.D.; Ozturk, H.; Ozturk, H.; Buyukbayram, H. Iloprost, a prostacyclin (PGI2) analogue, reduces liver injury in hepatic ischemia-reperfusion in rats. Acta Cir. Bras. 2009, 24, 226–232. [Google Scholar] [CrossRef]
- Ghonem, N.; Yoshida, J.; Stolz, D.B.; Humar, A.; Starzl, T.E.; Murase, N.; Venkataramanan, R. Treprostinil, a prostacyclin analog, ameliorates ischemia-reperfusion injury in rat orthotopic liver transplantation. Am. J. Transplant. 2011, 11, 2508–2516. [Google Scholar] [CrossRef]
- Zhang, M.; Nakamura, K.; Kageyama, S.; Lawal, A.O.; Gong, K.W.; Bhetraratana, M.; Fujii, T.; Sulaiman, D.; Hirao, H.; Bolisetty, S.; et al. Myeloid HO-1 modulates macrophage polarization and protects against ischemia-reperfusion injury. JCI Insight. 2018, 3, e120596. [Google Scholar] [CrossRef]
- Hirao, H.; Dery, K.J.; Kageyama, S.; Nakamura, K.; Kupiec-Weglinski, J.W. Heme Oxygenase-1 in liver transplant ischemia-reperfusion injury: From bench-to-bedside. Free Radic. Biol. Med. 2020, 157, 75–82. [Google Scholar] [CrossRef]
- Kaur, J.; Kaur, T.; Sharma, A.K.; Kaur, J.; Yadav, H.N.; Pathak, D.; Singh, A.P. Fenofibrate attenuates ischemia reperfusion-induced acute kidney injury and associated liver dysfunction in rats. Drug Dev. Res. 2021, 82, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, N.V.; Johnston, P.S.; O’Grady, J.G.; Smith, M.F.; Friend, P.J.; Rolles, K.; Calne, R.Y. Clinical use of UW solution or a simplified liver preservation solution prior to transplantation in 179 human livers, December 1987–July 1989. Transplant Proc. 1990, 22, 2189–2190. [Google Scholar] [PubMed]
- Nasralla, D.; Coussios, C.C.; Mergental, H.; Akhtar, M.Z.; Butler, A.J.; Ceresa, C.D.L.; Chiocchia, V.; Dutton, S.J.; García-Valdecasas, J.C.; Heaton, N.; et al. A randomized trial of normothermic preservation in liver transplantation. Nature 2018, 557, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Markmann, J.F.; Abouljoud, M.S.; Ghobrial, R.M.; Bhati, C.S.; Pelletier, S.J.; Lu, A.D.; Ottmann, S.; Klair, T.; Eymard, C.; Roll, G.R.; et al. Impact of Portable Normothermic Blood-Based Machine Perfusion on Outcomes of Liver Transplant: The OCS Liver PROTECT Randomized Clinical Trial. JAMA Surg. 2022, 157, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.J.E.; Kosmoliaptsis, V.; Pley, C.; Randle, L.; Fear, C.; Crick, K.; Gimson, A.E.; Allison, M.; Upponi, S.; Brais, R.; et al. Observations on the ex situ perfusion of livers for transplantation. Am. J. Transplant. 2018, 18, 2005–2020. [Google Scholar] [CrossRef]
- Ravaioli, M.; Germinario, G.; Dajti, G.; Sessa, M.; Vasuri, F.; Siniscalchi, A.; Morelli, M.C.; Serenari, M.; Del Gaudio, M.; Zanfi, C.; et al. Hypothermic oxygenated perfusion in extended criteria donor liver transplantation-A randomized clinical trial. Am. J. Transplant. 2022, 22, 2401–2408. [Google Scholar] [CrossRef]
- Guarrera, J.V.; Henry, S.D.; Samstein, B.; Odeh-Ramadan, R.; Kinkhabwala, M.; Goldstein, M.J.; Ratner, L.E.; Renz, J.F.; Lee, H.T.; Brown, R.S., Jr.; et al. Hypothermic machine preservation in human liver transplantation: The first clinical series. Am. J. Transplant. 2010, 10, 372–381. [Google Scholar] [CrossRef]
- Hessheimer, A.J.; Coll, E.; Torres, F.; Ruíz, P.; Gastaca, M.; Rivas, J.I.; Gómez, M.; Sánchez, B.; Santoyo, J.; Ramírez, P.; et al. Normothermic regional perfusion vs. super-rapid recovery in controlled donation after circulatory death liver transplantation. J. Hepatol. 2019, 70, 658–665. [Google Scholar] [CrossRef]
- Fondevila, C.; Hessheimer, A.J.; Ruiz, A.; Calatayud, D.; Ferrer, J.; Charco, R.; Fuster, J.; Navasa, M.; Rimola, A.; Taurá, P.; et al. Liver transplant using donors after unexpected cardiac death: Novel preservation protocol and acceptance criteria. Am. J. Transplant. 2007, 7, 1849–1855. [Google Scholar] [CrossRef]
- Watson, C.J.E.; Hunt, F.; Messer, S.; Currie, I.; Large, S.; Sutherland, A.; Crick, K.; Wigmore, S.J.; Fear, C.; Cornateanu, S.; et al. In situ normothermic perfusion of livers in controlled circulatory death donation may prevent ischemic cholangiopathy and improve graft survival. Am. J. Transplant. 2019, 19, 1745–1758. [Google Scholar] [CrossRef] [PubMed]
- Gaurav, R.; Butler, A.J.; Kosmoliaptsis, V.; Mumford, L.; Fear, C.; Swift, L.; Fedotovs, A.; Upponi, S.; Khwaja, S.; Richards, J.; et al. Liver Transplantation Outcomes from Controlled Circulatory Death Donors: SCS vs in situ NRP vs ex situ NMP. Ann. Surg. 2022, 275, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- Oniscu, G.C.; Mehew, J.; Butler, A.J.; Sutherland, A.; Gaurav, R.; Hogg, R.; Currie, I.; Jones, M.; Watson, C.J.E.l. Improved Organ Utilization and Better Transplant Outcomes with In Situ Normothermic Regional Perfusion in Controlled Donation After Circulatory Death. Transplantation. 2023, 107, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Mergental, H.; Laing, R.W.; Kirkham, A.J.; Perera, M.T.P.R.; Boteon, Y.L.; Attard, J.; Barton, D.; Curbishley, S.; Wilkhu, M.; Neil, D.A.H.; et al. Transplantation of discarded livers following viability testing with normothermic machine perfusion. Nat. Commun. 2020, 11, 2939. [Google Scholar] [CrossRef] [PubMed]
- Bral, M.; Dajani, K.; Leon Izquierdo, D.; Bigam, D.; Kneteman, N.; Ceresa, C.D.L.; Friend, P.J.; Shapiro, A.M.J. A Back-to-Base Experience of Human. Normothermic Ex. Situ Liver Perfusion: Does the Chill Kill? Liver Transplant. 2019, 25, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Ghinolfi, D.; Rreka, E.; De Tata, V.; Franzini, M.; Pezzati, D.; Fierabracci, V.; Masini, M.; Cacciatoinsilla, A.; Bindi, M.L.; Marselli, L.; et al. Pilot, Open, Randomized, Prospective Trial for Normothermic Machine Perfusion Evaluation in Liver Transplantation From Older Donors. Liver Transplant. 2019, 25, 436–449. [Google Scholar] [CrossRef]
- Watson, C.J.E.; Gaurav, R.; Fear, C.; Swift, L.; Selves, L.; Ceresa, C.D.L.; Upponi, S.S.; Brais, R.; Allison, M.; Macdonald-Wallis, C.; et al. Predicting Early Allograft Function After Normothermic Machine Perfusion. Transplantation 2022, 106, 2391–2398. [Google Scholar] [CrossRef]
- Guo, Z.; Zhao, Q.; Huang, S.; Huang, C.; Wang, D.; Yang, L.; Zhang, J.; Chen, M.; Wu, L.; Zhang, Z.; et al. Ischaemia-free liver transplantation in humans: A first-in-human trial. Lancet Reg. Health West. Pac. 2021, 16, 100260. [Google Scholar] [CrossRef]
- Clavien, P.A.; Dutkowski, P.; Mueller, M.; Eshmuminov, D.; Bautista Borrego, L.; Weber, A.; Muellhaupt, B.; Sousa Da Silva, R.X.; Burg, B.R.; Rudolf von Rohr, P.; et al. Transplantation of a human liver following 3 days of ex situ normothermic preservation. Nat. Biotechnol. 2022, 40, 1610–1616. [Google Scholar] [CrossRef]
- Eshmuminov, D.; Becker, D.; Bautista Borrego, L.; Hefti, M.; Schuler, M.J.; Hagedorn, C.; Muller, X.; Mueller, M.; Onder, C.; Graf, R.; et al. An integrated perfusion machine preserves injured human livers for 1 week. Nat. Biotechnol. 2020, 38, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Parente, A.; Flores Carvalho, M.; Schlegel, A. Endothelial Cells and Mitochondria: Two Key Players in Liver Transplantation. Int. J. Mol. Sci. 2023, 24, 10091. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, A.; de Rougemont, O.; Graf, R.; Clavien, P.A.; Dutkowski, P. Protective mechanisms of end-ischemic cold machine perfusion in DCD liver grafts. J. Hepatol. 2013, 58, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Patrono, D.; Cussa, D.; Sciannameo, V.; Montanari, E.; Panconesi, R.; Berchialla, P.; Lepore, M.; Gambella, A.; Rizza, G.; Catalano, G.; et al. Outcome of liver transplantation with grafts from brain-dead donors treated with dual hypothermic oxygenated machine perfusion, with particular reference to elderly donors. Am. J. Transplant. 2022, 22, 1382–1395. [Google Scholar] [CrossRef]
- Jochmans, I.; Brat, A.; Davies, L.; Hofker, H.S.; van de Leemkolk, F.E.M.; Leuvenink, H.G.D.; Knight, S.R.; Pirenne, J.; Ploeg, R.J. Oxygenated versus standard cold perfusion preservation in kidney transplantation (COMPARE): A randomised, double-blind, paired, phase 3 trial. Lancet 2020, 396, 1653–1662, Correction in Lancet 2021, 396, 1978. [Google Scholar] [CrossRef]
- Wyss, R.K.; Méndez Carmona, N.; Arnold, M.; Segiser, A.; Mueller, M.; Dutkowski, P.; Carrel, T.P.; Longnus, S.L. Hypothermic, oxygenated perfusion (HOPE) provides cardioprotection via succinate oxidation prior to normothermic perfusion in a rat model of donation after circulatory death (DCD). Am. J. Transplant. 2021, 21, 1003–1011. [Google Scholar] [CrossRef]
- Muller, X.; Schlegel, A.; Kron, P.; Eshmuminov, D.; Würdinger, M.; Meierhofer, D.; Clavien, P.A.; Dutkowski, P. Novel Real-time Prediction of Liver Graft Function During Hypothermic Oxygenated Machine Perfusion Before Liver Transplantation. Ann. Surg. 2019, 270, 783–790. [Google Scholar] [CrossRef]
- Scholz, R.; Thurman, R.G.; Williamson, J.R.; Chance, B.; Bücher, T. Flavin and pyridine nucleotide oxidation-reduction changes in perfused rat liver. I. Anoxia and subcellular localization of fluorescent flavoproteins. J. Biol. Chem. 1969, 244, 2317–2324. [Google Scholar] [CrossRef]
- Minor, T.; Efferz, P.; Fox, M.; Wohlschlaeger, J.; Lüer, B. Controlled oxygenated rewarming of cold stored liver grafts by thermally graduated machine perfusion prior to reperfusion. Am. J. Transplant. 2013, 13, 1450–1460. [Google Scholar] [CrossRef]
- an Leeuwen, O.B.; Bodewes, S.B.; Lantinga, V.A.; Haring, M.P.D.; Thorne, A.M.; Brüggenwirth, I.M.A.; van den Berg, A.P.; de Boer, M.T.; de Jong, I.E.M.; de Kleine, R.H.J.; et al. Sequential hypothermic and normothermic machine perfusion enables safe transplantation of high-risk donor livers. Am. J. Transplant. 2022, 22, 1658–1670. [Google Scholar] [CrossRef]
- Goldaracena, N.; Echeverri, J.; Spetzler, V.N.; Kaths, J.M.; Barbas, A.S.; Louis, K.S.; Adeyi, O.A.; Grant, D.R.; Selzner, N.; Selzner, M. Anti-inflammatory signaling during ex vivo liver perfusion improves the preservation of pig liver grafts before transplantation. Liver Transplant. 2016, 22, 1573–1583. [Google Scholar] [CrossRef] [PubMed]
- Beal, E.W.; Kim, J.L.; Reader, B.F.; Akateh, C.; Maynard, K.; Washburn, W.K.; Zweier, J.L.; Whitson, B.A.; Black, S.M. [D-Ala2, D-Leu5] Enkephalin Improves Liver Preservation During Normothermic Ex Vivo Perfusion. J. Surg. Res. 2019, 241, 323–335. [Google Scholar] [CrossRef]
- Yu, Y.; Cheng, Y.; Pan, Q.; Zhang, Y.J.; Jia, D.G.; Liu, Y.F. Effect of the Selective NLRP3 Inflammasome Inhibitor mcc950 on Transplantation Outcome in a Pig Liver Transplantation Model With Organs From Donors After Circulatory Death Preserved by Hypothermic Machine Perfusion. Transplantation 2019, 103, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y.; Akamatsu, Y.; Maida, K.; Kashiwadate, T.; Kobayashi, Y.; Ohuchi, N.; Satomi, S. A new liver graft preparation method for uncontrolled non-heart-beating donors, combining short oxygenated warm perfusion and prostaglandin E1. J. Surg. Res. 2013, 184, 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- Maida, K.; Akamatsu, Y.; Hara, Y.; Tokodai, K.; Miyagi, S.; Kashiwadate, T.; Miyazawa, K.; Kawagishi, N.; Ohuchi, N. Short Oxygenated Warm Perfusion With Prostaglandin E1 Administration Before Cold Preservation as a Novel Resuscitation Method for Liver Grafts From Donors After Cardiac Death in a Rat In Vivo Model. Transplantation 2016, 100, 1052–1058. [Google Scholar] [CrossRef]
- Nassar, A.; Liu, Q.; Farias, K.; D’Amico, G.; Buccini, L.; Urcuyo, D.; Kelly, D.; Hashimoto, K.; Eghtesad, B.; Uso, T.D.; et al. Role of vasodilation during normothermic machine perfusion of DCD porcine livers. Int. J. Artif. Organs 2014, 37, 165–172. [Google Scholar] [CrossRef]
- Echeverri, J.; Goldaracena, N.; Kaths, J.M.; Linares, I.; Roizales, R.; Kollmann, D.; Hamar, M.; Urbanellis, P.; Ganesh, S.; Adeyi, O.A.; et al. Comparison of BQ123, Epoprostenol, and Verapamil as Vasodilators During Normothermic Ex Vivo Liver Machine Perfusion. Transplantation 2018, 102, 601–608. [Google Scholar] [CrossRef]
- Nagrath, D.; Xu, H.; Tanimura, Y.; Zuo, R.; Berthiaume, F.; Avila, M.; Yarmush, R.; Yarmush, M.L. Metabolic preconditioning of donor organs: Defatting fatty livers by normothermic perfusion ex vivo. Metab. Eng. 2009, 11, 274–283. [Google Scholar] [CrossRef]
- Liu, Q.; Berendsen, T.; Izamis, M.L.; Uygun, B.; Yarmush, M.L.; Uygun, K. Perfusion defatting at subnormothermic temperatures in steatotic rat livers. Transplant. Proc. 2013, 45, 3209–3213. [Google Scholar] [CrossRef]
- Boteon, Y.L.; Attard, J.; Boteon, A.P.C.S.; Wallace, L.; Reynolds, G.; Hubscher, S.; Mirza, D.F.; Mergental, H.; Bhogal, R.H.; Afford, S. Manipulation of Lipid Metabolism During Normothermic Machine Perfusion: Effect of Defatting Therapies on Donor Liver Functional Recovery. Liver Transplant. 2019, 25, 1007–1022. [Google Scholar] [CrossRef]
- Lin, F.; Zhen, F.; Yan, X.; Shaojun, Y.; Guizhu, P.; Yanfeng, W.; Qifa, Y. Hypothermic oxygenated perfusion with defatting cocktail further improves steatotic liver grafts in a transplantation rat model. Artif. Organs 2021, 45, E304–E316. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, H.; Kuroda, S.; Mikuriya, Y.; Ohdan, H. Ischemia–reperfusion injury in patients with fatty liver and the clinical impact of steatotic liver on hepatic surgery. Surg. Today 2014, 44, 1611–1625. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, R.W.; Zilvetti, M.; Roy, D.; Hughes, D.; Morovat, A.; Coussios, C.C.; Friend, P.J. Hepatic steatosis and normothermic perfusion-preliminary experiments in a porcine model. Transplantation 2011, 92, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Seal, J.B.; Gewertz, B.L. Vascular dysfunction in ischemia-reperfusion injury. Ann. Vasc. Surg. 2005, 19, 572–584. [Google Scholar] [CrossRef]
- Reddy, S.P.; Bhattacharjya, S.; Maniakin, N.; Greenwood, J.; Guerreiro, D.; Hughes, D.; Imber, C.J.; Pigott, D.W.; Fuggle, S.; Taylor, R.; et al. Preservation of porcine non-heart-beating donor livers by sequential cold storage and warm perfusion. Transplantation 2004, 77, 1328–1332. [Google Scholar] [CrossRef] [PubMed]
- Korolchuk, V.I.; Miwa, S.; Carroll, B.; von Zglinicki, T. Mitochondria in Cell Senescence: Is Mitophagy the Weakest Link? EBioMedicine 2017, 21, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Bernadotte, A.; Mikhelson, V.M.; Spivak, I.M. Markers of cellular senescence. Telomere shortening as a marker of cellular senescence. Aging 2016, 8, 3–11. [Google Scholar] [CrossRef]
- Gasek, N.S.; Kuchel, G.A.; Kirkland, J.L.; Xu, M. Strategies for Targeting Senescent Cells in Human Disease. Nat. Aging 2021, 1, 870–879. [Google Scholar] [CrossRef]
- Matsunaga, T.; Iske, J.; Schroeter, A.; Azuma, H.; Zhou, H.; Tullius, S.G. The potential of Senolytics in transplantation. Mech. Ageing Dev. 2021, 200, 111582. [Google Scholar] [CrossRef]
- Wu, Y.; Shen, S.; Shi, Y.; Tian, N.; Zhou, Y.; Zhang, X. Senolytics: Eliminating Senescent Cells and Alleviating Intervertebral Disc Degeneration. Front. Bioeng. Biotechnol. 2022, 10, 823945. [Google Scholar] [CrossRef]
- Kirkland, J.L.; Tchkonia, T. Senolytic drugs: From discovery to translation. J. Intern. Med. 2020, 288, 518–536. [Google Scholar] [CrossRef] [PubMed]
- Iske, J.; Seyda, M.; Heinbokel, T.; Maenosono, R.; Minami, K.; Nian, Y.; Quante, M.; Falk, C.S.; Azuma, H.; Martin, F.I.; et al. Senolytics prevent mt-DNA-induced inflammation and promote the survival of aged organs following transplantation. Nat. Commun. 2020, 11, 4289. [Google Scholar] [CrossRef]
- Thompson, W.S.; Mondal, G.; Vanlith, C.J.; Kaiser, R.A.; Lillegard, J.B. The future of gene-targeted therapy for hereditary tyrosinemia type 1 as a lead indication among the inborn errors of metabolism. Expert. Opin. Orphan Drugs. 2020, 8, 245–256. [Google Scholar] [CrossRef]
- Goldaracena, N.; Spetzler, V.N.; Echeverri, J.; Kaths, J.M.; Cherepanov, V.; Persson, R.; Hodges, M.R.; Janssen, H.L.; Selzner, N.; Grant, D.R.; et al. Inducing Hepatitis C Virus Resistance After Pig Liver Transplantation-A Proof of Concept of Liver Graft Modification Using Warm Ex Vivo Perfusion. Am. J. Transplant. 2017, 17, 970–978. [Google Scholar] [CrossRef]
- Gillooly, A.R.; Perry, J.; Martins, P.N. First Report of siRNA Uptake (for RNA Interference) During Ex Vivo Hypothermic and Normothermic Liver Machine Perfusion. Transplantation 2019, 103, e56–e57. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, M.F.; Brüggenwirth, I.M.A.; Gillooly, A.; Khvorova, A.; Kowalik, T.F.; Martins, P.N. Gene Silencing With siRNA (RNA Interference): A New Therapeutic Option During Ex Vivo Machine Liver Perfusion Preservation. Liver Transplant. 2019, 25, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorsi-Riani, E.; Gillooly, A.R.; Iesari, S.; Brüggenwirth, I.M.A.; Ferguson, C.M.; Komuta, M.; Xhema, D.; Daumerie, A.; Maistriaux, L.; Leuvenink, H.; et al. Delivering siRNA Compounds During HOPE to Modulate Organ Function: A Proof-of-concept Study in a Rat Liver Transplant Model. Transplantation 2022, 106, 1565–1576. [Google Scholar] [CrossRef]
- Zhao, G.; Fu, C.; Wang, L.; Zhu, L.; Yan, Y.; Xiang, Y.; Zheng, F.; Gong, F.; Chen, S.; Chen, G. Down-regulation of nuclear HMGB1 reduces ischemia-induced HMGB1 translocation and release and protects against liver ischemia-reperfusion injury. Sci. Rep. 2017, 7, 46272. [Google Scholar] [CrossRef]
- Contreras, J.L.; Vilatoba, M.; Eckstein, C.; Bilbao, G.; Anthony Thompson, J.; Eckhoff, D.E. Caspase-8 and caspase-3 small interfering RNA decreases ischemia/reperfusion injury to the liver in mice. Surgery 2004, 136, 390–400. [Google Scholar] [CrossRef]
- Wang, G.; Wan, L.; Zhang, L.; Yan, C.; Zhang, Y. MicroRNA-133a Regulates the Viability and Differentiation Fate of Bone Marrow Mesenchymal Stem Cells via MAPK/ERK Signaling Pathway by Targeting FGFR1. DNA Cell Biol. 2021, 40, 1112–1123. [Google Scholar] [CrossRef]
- Andersson, P.; Gidlöf, O.; Braun, O.O.; Götberg, M.; van der Pals, J.; Olde, B.; Erlinge, D. Plasma levels of liver-specific miR-122 is massively increased in a porcine cardiogenic shock model and attenuated by hypothermia. Shock 2012, 37, 234–238. [Google Scholar] [CrossRef]
- Van Caster, P.; Brandenburger, T.; Strahl, T.; Metzger, S.; Bauer, I.; Pannen, B.; Braun, S. Circulating microRNA-122, -21 and -223 as potential markers of liver injury following warm ischaemia and reperfusion in rats. Mol. Med. Rep. 2015, 12, 3146–3150. [Google Scholar] [CrossRef] [PubMed]
- Vandermeulen, M.; Erpicum, P.; Weekers, L.; Briquet, A.; Lechanteur, C.; Detry, O.; Beguin, Y.; Jouret, F. Mesenchymal Stromal Cells in Solid Organ Transplantation. Transplantation 2020, 104, 923–936. [Google Scholar] [CrossRef]
- Fu, X.; Liu, G.; Halim, A.; Ju, Y.; Luo, Q.; Song, A.G. Mesenchymal Stem Cell Migration and Tissue Repair. Cells 2019, 8, 784. [Google Scholar] [CrossRef]
- Gao, L.; Mei, S.; Zhang, S.; Qin, Q.; Li, H.; Liao, Y.; Fan, H.; Liu, Z.; Zhu, H. Cardio-renal Exosomes in Myocardial Infarction Serum Regulate Proangiogenic Paracrine Signaling in Adipose Mesenchymal Stem Cells. Theranostics 2020, 10, 1060–1073. [Google Scholar] [CrossRef] [PubMed]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Casiraghi, F.; Todeschini, M.; Azzollini, N.; Cravedi, P.; Cassis, P.; Solini, S.; Fiori, S.; Rota, C.; Karachi, A.; Carrara, C.; et al. Effect of Timing and Complement Receptor Antagonism on Intragraft Recruitment and Protolerogenic Effects of Mesenchymal Stromal Cells in Murine Kidney Transplantation. Transplantation 2019, 103, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Sasajima, H.; Miyagi, S.; Kakizaki, Y.; Kamei, T.; Unno, M.; Satomi, S.; Goto, M. Cytoprotective Effects of Mesenchymal Stem Cells During Liver Transplantation from Donors After Cardiac Death in Rats. Transplant. Proc. 2018, 50, 2815–2820. [Google Scholar] [CrossRef]
- Rigo, F.; De Stefano, N.; Navarro-Tableros, V.; David, E.; Rizza, G.; Catalano, G.; Gilbo, N.; Maione, F.; Gonella, F.; Roggio, D.; et al. Extracellular Vesicles from Human Liver Stem Cells Reduce Injury in an Ex Vivo Normothermic Hypoxic Rat Liver Perfusion Model. Transplantation 2018, 102, e205–e210. [Google Scholar] [CrossRef]
- Yang, L.; Cao, H.; Sun, D.; Hou, B.; Lin, L.; Shen, Z.Y.; Song, H.L. Bone marrow mesenchymal stem cells combine with normothermic machine perfusion to improve rat donor liver quality-the important role of hepatic microcirculation in donation after circulatory death. Cell Tissue Res. 2020, 381, 239–254. [Google Scholar] [CrossRef]
- Verstegen, M.M.A.; Mezzanotte, L.; Ridwan, R.Y.; Wang, K.; de Haan, J.; Schurink, I.J.; Sierra Parraga, J.M.; Hoogduijn, M.; Kessler, B.M.; Huang, H.; et al. First Report on Ex Vivo Delivery of Paracrine Active Human Mesenchymal Stromal Cells to Liver Grafts During Machine Perfusion. Transplantation 2020, 104, e5–e7. [Google Scholar] [CrossRef] [PubMed]
- Laing, R.W.; Stubblefield, S.; Wallace, L.; Roobrouck, V.D.; Bhogal, R.H.; Schlegel, A.; Boteon, Y.L.; Reynolds, G.M.; Ting, A.E.; Mirza, D.F.; et al. The Delivery of Multipotent Adult Progenitor Cells to Extended Criteria Human Donor Livers Using Normothermic Machine Perfusion. Front. Immunol. 2020, 11, 1226. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Yang, L.; Zheng, W.; Cao, H.; Wu, L.; Song, H. Protective Effects of Bone Marrow Mesenchymal Stem Cells (BMMSCS) Combined with Normothermic Machine Perfusion on Liver Grafts Donated After Circulatory Death via Reducing the Ferroptosis of Hepatocytes. Med. Sci. Monit. 2021, 27, e930258. [Google Scholar] [CrossRef]
- De Stefano, N.; Navarro-Tableros, V.; Roggio, D.; Calleri, A.; Rigo, F.; David, E.; Gambella, A.; Bassino, D.; Amoroso, A.; Patrono, D.; et al. Human liver stem cell-derived extracellular vesicles reduce injury in a model of normothermic machine perfusion of rat livers previously exposed to a prolonged warm ischemia. Transpl. Int. 2021, 34, 1607–1617. [Google Scholar] [CrossRef] [PubMed]
- Karangwa, S.A.; Dutkowski, P.; Fontes, P.; Friend, P.J.; Guarrera, J.V.; Markmann, J.F.; Mergental, H.; Minor, T.; Quintini, C.; Selzner, M.; et al. Machine Perfusion of Donor Livers for Transplantation: A Proposal for Standardized Nomenclature and Reporting Guidelines. Am. J. Transplant. 2016, 16, 2932–2942. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).