The Role of Sensory Nerves in Dental Pulp Homeostasis: Histological Changes and Cellular Consequences after Sensory Denervation
Abstract
:1. Introduction
2. Results
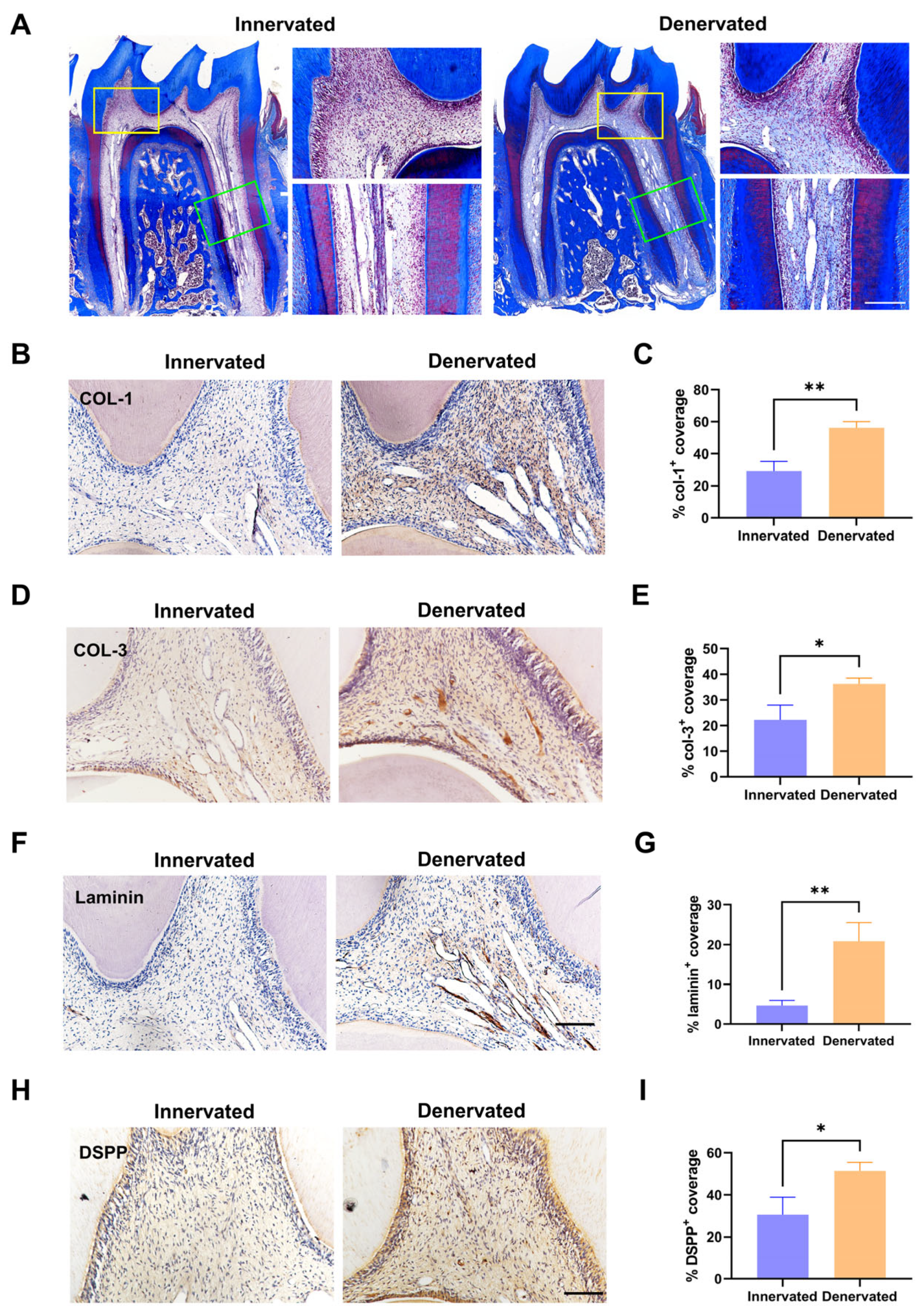
2.1. Sensory Denervation Accelerates Pulp Obliteration
2.2. Sensory Denervation Induces Premature Pulp Aging and Pulp Degeneration
2.3. Sensory Denervation Promotes the Senescence of Dental Pulp Cells
2.4. Denervated DPCs Show Cell Cycle Arrest and Imbalance in Synthesis and Secretion
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Inferior Alveolar Nerve Transection
4.3. Isolation and Culture of Rat DPCs
4.4. Hematoxylin and Eosin (HE) Staining
4.5. Scanning Electron Microscopy (SEM)
4.6. Masson Staining
4.7. Lipofuscin Staining
4.8. Immunohistochemistry Staining
4.9. Immunofluorescence Staining
4.10. Senescence-Associated β-Galactosidase Staining
4.11. RNA Isolation
4.12. Real-Time Quantitative PCR
4.13. Cell Proliferation Assay
4.14. Cell Cycle Analysis
4.15. Alkaline Phosphatase (ALP) Activity Assay and Alizarin Red S Staining
4.16. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodrigues, N.S.; França, C.M.; Tahayeri, A.; Ren, Z.; Saboia, V.P.A.; Smith, A.J.; Ferracane, J.L.; Koo, H.; Bertassoni, L.E. Biomaterial and Biofilm Interactions with the Pulp-Dentin Complex-on-a-Chip. J. Dent. Res. 2021, 100, 1136–1143. [Google Scholar] [CrossRef]
- da Rosa, W.L.O.; Piva, E.; da Silva, A.F. Disclosing the physiology of pulp tissue for vital pulp therapy. Int. Endod. J. 2018, 51, 829–846. [Google Scholar] [CrossRef] [PubMed]
- Franca, C.M.; Riggers, R.; Muschler, J.L.; Widbiller, M.; Lococo, P.M.; Diogenes, A.; Bertassoni, L.E. 3D-Imaging of Whole Neuronal and Vascular Networks of the Human Dental Pulp via CLARITY and Light Sheet Microscopy. Sci. Rep. 2019, 9, 10860. [Google Scholar] [CrossRef]
- Zhan, C.; Huang, M.; Yang, X.; Hou, J. Dental nerves: A neglected mediator of pulpitis. Int. Endod. J. 2021, 54, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.D.; Li, S.Y.; Zhang, S.J.; Gupta, A.; Zhang, C.P.; Wang, L. The neural system regulates bone homeostasis via mesenchymal stem cells: A translational approach. Theranostics 2020, 10, 4839–4850. [Google Scholar] [CrossRef]
- Xiao, Y.; Han, C.H.; Wang, Y.H.; Zhang, X.S.; Bao, R.; Li, Y.E.; Chen, H.J.; Hu, B.; Liu, S. Interoceptive regulation of skeletal tissue homeostasis and repair. Bone Res. 2023, 11, 48. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.M.; Aragona, M. Regulation of tissue architecture and stem cell dynamics to sustain homeostasis and repair in the skin epidermis. Semin. Cell Dev. Biol. 2022, 130, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.L.; Hill, L.J.; Downie, L.E.; Chinnery, H.R. Neuroimmune crosstalk in the cornea: The role of immune cells in corneal nerve maintenance during homeostasis and inflammation. Prog. Retin. Eye Res. 2022, 91, 101105. [Google Scholar] [CrossRef]
- Chen, H.; Hu, B.; Lv, X.; Zhu, S.; Zhen, G.; Wan, M.; Jain, A.; Gao, B.; Chai, Y.; Yang, M.; et al. Prostaglandin E2 mediates sensory nerve regulation of bone homeostasis. Nat. Commun. 2019, 10, 181. [Google Scholar] [CrossRef]
- Zhao, L.; Lv, G.; Jiang, S.; Yan, Z.; Sun, J.; Wang, L.; Jiang, D. Morphological differences in skeletal muscle atrophy of rats with motor nerve and/or sensory nerve injury. Neural Regen. Res. 2012, 7, 2507–2515. [Google Scholar] [PubMed]
- Hu, B.; Lv, X.; Wei, L.X.; Wang, Y.H.; Zheng, G.J.; Yang, C.; Zang, F.Z.; Wang, J.X.; Li, J.; Wu, X.D.; et al. Sensory Nerve Maintains Intervertebral Disc Extracellular Matrix Homeostasis Via CGRP/CHSY1 Axis. Adv. Sci. 2022, 9, 2202620. [Google Scholar] [CrossRef] [PubMed]
- McCabe, P.S.; Dummer, P.M. Pulp canal obliteration: An endodontic diagnosis and treatment challenge. Int. Endod. J. 2012, 45, 177–197. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zeng, J.; Zhang, W.; Sun, X.; Ling, J. Evaluation of the interaction between calcifying nanoparticles and human dental pulp cells: A preliminary investigation. Int. J. Nanomed. 2010, 6, 13–18. [Google Scholar] [CrossRef]
- Couve, E.; Lovera, M.; Suzuki, K.; Schmachtenberg, O. Schwann Cell Phenotype Changes in Aging Human Dental Pulp. J. Dent. Res. 2018, 97, 347–355. [Google Scholar] [CrossRef]
- Carvalho, T.S.; Lussi, A. Age-related morphological, histological and functional changes in teeth. J. Oral Rehabil. 2017, 44, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Bernick, S. Effect of aging on the nerve supply to human teeth. J. Dent. Res. 1967, 46, 694–699. [Google Scholar] [CrossRef]
- Alvarez-Vasquez, J.L.; Castaneda-Alvarado, C.P. Dental Pulp Fibroblast: A Star Cell. J. Endod. 2022, 48, 1005–1019. [Google Scholar] [CrossRef] [PubMed]
- Balic, A.; Perver, D.; Pagella, P.; Rehrauer, H.; Stadlinger, B.; Moor, A.E.; Vogel, V.; Mitsiadis, T.A. Extracellular matrix remodelling in dental pulp tissue of carious human teeth through the prism of single-cell RNA sequencing. Int. J. Oral Sci. 2023, 15, 30. [Google Scholar] [CrossRef]
- Martinez, E.F.; Araújo, V.C. Immunoexpression of extracellular matrix proteins in dental pulpal and gingival human fibroblasts. Int. Endod. J. 2004, 37, 749–755. [Google Scholar] [CrossRef]
- Nishikawa, H.; Ueno, A.; Nishikawa, S.; Kido, J.; Ohishi, M.; Inoue, H.; Nagata, T. Sulfated glycosaminoglycan synthesis and its regulation by transforming growth factor-beta in rat clonal dental pulp cells. J. Endod. 2000, 26, 169–171. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kim, G.E.; Cho, H.J.; Yu, M.K.; Bhattarai, G.; Lee, N.H.; Yi, H.K. Aging of in vitro pulp illustrates change of inflammation and dentinogenesis. J. Endod. 2013, 39, 340–345. [Google Scholar] [CrossRef]
- Zhang, L.; Li, M.C.; Li, X.C.; Liao, T.Y.; Ma, Z.Y.; Zhang, L.; Xing, R.L.; Wang, P.M.; Mao, J. Characteristics of sensory innervation in synovium of rats within different knee osteoarthritis models and the correlation between synovial fibrosis and hyperalgesia. J. Adv. Res. 2022, 35, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Salmonowicz, H.; Passos, J.F. Detecting senescence: A new method for an old pigment. Aging Cell 2017, 16, 432–434. [Google Scholar] [CrossRef] [PubMed]
- Herranz, N.; Gil, J. Mechanisms and functions of cellular senescence. J. Clin. Investig. 2018, 128, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Pitcher, L.E.; Yousefzadeh, M.J.; Niedernhofer, L.J.; Robbins, P.D.; Zhu, Y. Cellular senescence: A key therapeutic target in aging and diseases. J. Clin. Investig. 2022, 132, e158450. [Google Scholar] [CrossRef]
- Glück, S.; Guey, B.; Gulen, M.F.; Wolter, K.; Kang, T.W.; Schmacke, N.A.; Bridgeman, A.; Rehwinkel, J.; Zender, L.; Ablasser, A. Innate immune sensing of cytosolic chromatin fragments through cGAS promotes senescence. Nat. Cell Biol. 2017, 19, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Tonnessen-Murray, C.A.; Lozano, G.; Jackson, J.G. The Regulation of Cellular Functions by the p53 Protein: Cellular Senescence. Cold Spring Harb. Perspect. Med. 2017, 7, a026112. [Google Scholar] [CrossRef] [PubMed]
- Connert, T.; Weiger, R.; Krastl, G. Present status and future directions: Guided endodontics. Int. Endod. J. 2022, 55 (Suppl. S4), 995–1002. [Google Scholar] [CrossRef]
- El-Fiqi, A.; Mandakhbayar, N.; Jo, S.B.; Knowles, J.C.; Lee, J.H.; Kim, H.W. Nanotherapeutics for regeneration of degenerated tissue infected by bacteria through the multiple delivery of bioactive ions and growth factor with antibacterial/angiogenic and osteogenic/odontogenic capacity. Bioact. Mater. 2021, 6, 123–136. [Google Scholar] [CrossRef]
- Hayano, S.; Fukui, Y.; Kawanabe, N.; Kono, K.; Nakamura, M.; Ishihara, Y.; Kamioka, H. Role of the Inferior Alveolar Nerve in Rodent Lower Incisor Stem Cells. J. Dent. Res. 2018, 97, 954–961. [Google Scholar] [CrossRef]
- Duan, Y.; Liang, Y.; Yang, F.; Ma, Y. Neural Regulations in Tooth Development and Tooth-Periodontium Complex Homeostasis: A Literature Review. Int. J. Mol. Sci. 2022, 23, 14150. [Google Scholar] [CrossRef] [PubMed]
- Pei, F.; Ma, L.; Jing, J.; Feng, J.; Yuan, Y.; Guo, T.; Han, X.; Ho, T.V.; Lei, J.; He, J.; et al. Sensory nerve niche regulates mesenchymal stem cell homeostasis via FGF/mTOR/autophagy axis. Nat. Commun. 2023, 14, 344. [Google Scholar] [CrossRef]
- Lee, K.; Lee, B.M.; Park, C.K.; Kim, Y.H.; Chung, G. Ion Channels Involved in Tooth Pain. Int. J. Mol. Sci. 2019, 20, 2266. [Google Scholar] [CrossRef] [PubMed]
- Marco, B.; Alessandro, R.; Philippe, F.; Fabio, B.; Paolo, R.; Giulio, F. The Effect of Aging on Nerve Morphology and Substance P Expression in Mouse and Human Corneas. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5329–5335. [Google Scholar] [CrossRef]
- Tomlinson, R.E.; Christiansen, B.A.; Giannone, A.A.; Genetos, D.C. The Role of Nerves in Skeletal Development, Adaptation, and Aging. Front. Endocrinol. 2020, 11, 646. [Google Scholar] [CrossRef]
- Verzar, F. Changes of thermoelastic contraction of skin and nerve in aging animals. Experientia 1955, 11, 230–232. [Google Scholar]
- Kaariainen, M.; Kauhanen, S. Skeletal muscle injury and repair: The effect of disuse and denervation on muscle and clinical relevance in pedicled and free muscle flaps. J. Reconstr. Microsurg. 2012, 28, 581–587. [Google Scholar] [CrossRef]
- Sener, S.; Cobankara, F.K.; Akgünlü, F. Calcifications of the pulp chamber: Prevalence and implicated factors. Clin. Oral Investig. 2009, 13, 209–215. [Google Scholar] [CrossRef]
- Yao, L.; Li, F.; Yu, C.; Wang, H.; Wang, Y.; Ye, L.; Yu, F. Chronological and Replicative Aging of CD51(+)/PDGFR-alpha(+) Pulp Stromal Cells. J. Dent. Res. 2023, 102, 929–937. [Google Scholar] [CrossRef]
- Childs, B.G.; Gluscevic, M.; Baker, D.J.; Laberge, R.M.; Marquess, D.; Dananberg, J.; van Deursen, J.M. Senescent cells: An emerging target for diseases of ageing. Nat. Rev. Drug Discov. 2017, 16, 718–735. [Google Scholar] [CrossRef]
- Cristofalo, V.J.; Lorenzini, A.; Allen, R.G.; Torres, C.; Tresini, M. Replicative senescence: A critical review. Mech. Ageing Dev. 2004, 125, 827–848. [Google Scholar] [CrossRef]
- Morsczeck, C. Cellular senescence in dental pulp stem cells. Arch. Oral Biol. 2019, 99, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Y.; Xia, Q.; Xia, Q.; Wang, B.; Yang, C.; Liang, J.; Liu, X. Potential roles of telomeres and telomerase in neurodegenerative diseases. Int. J. Biol. Macromol. 2020, 163, 1060–1078. [Google Scholar] [CrossRef]
- Liu, J.; Wang, L.; Wang, Z.; Liu, J.P. Roles of Telomere Biology in Cell Senescence, Replicative and Chronological Ageing. Cells 2019, 8, 54. [Google Scholar] [CrossRef] [PubMed]
- Steenstrup, T.; Hjelmborg, J.V.; Kark, J.D.; Christensen, K.; Aviv, A. The telomere lengthening conundrum—Artifact or biology? Nucleic Acids Res. 2013, 41, e131. [Google Scholar] [CrossRef]
- Di Micco, R.; Krizhanovsky, V.; Baker, D.; d’Adda di Fagagna, F. Cellular senescence in ageing: From mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol. 2021, 22, 75–95. [Google Scholar] [CrossRef]
- Kapetanos, K.; Asimakopoulos, D.; Christodoulou, N.; Vogt, A.; Khan, W. Chronological Age Affects MSC Senescence In Vitro—A Systematic Review. Int. J. Mol. Sci. 2021, 22, 7945. [Google Scholar] [CrossRef]
- Ma, D.D.; Ma, Z.F.; Zhang, X.J.; Wang, W.H.; Yang, Z.H.; Zhang, M.; Wu, G.; Lu, W.; Deng, Z.H.; Jin, Y. Effect of Age and Extrinsic Microenvironment on the Proliferation and Osteogenic Differentiation of Rat Dental Pulp Stem Cells. J. Endod. 2009, 35, 1546–1553. [Google Scholar] [CrossRef]
- Feng, X.M.; Xing, J.; Feng, G.J.; Huang, D.; Lu, X.H.; Liu, S.Z.; Tan, W.; Li, L.R.; Gu, Z.F. p16 mediates age-related changes in mesenchymal stem cells derived from human dental pulp through the DNA damage and stress response. Mech. Ageing Dev. 2014, 141, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Uxa, S.; Bernhart, S.H.; Mages, C.F.S.; Fischer, M.; Kohler, R.; Hoffmann, S.; Stadler, P.F.; Engeland, K.; Muller, G.A. DREAM and RB cooperate to induce gene repression and cell-cycle arrest in response to p53 activation. Nucleic Acids Res. 2019, 47, 9087–9103. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; Yang, F.; Liu, X.; Zhan, P.; Wu, J.; Wang, X.; Wang, Z.; Tang, W.; Sun, Y.; et al. HDAC9-mediated epithelial cell cycle arrest in G2/M contributes to kidney fibrosis in male mice. Nat. Commun. 2023, 14, 3007. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Tchkonia, T.; Ding, H.S.; Ogrodnik, M.; Lubbers, E.R.; Pirtskhalava, T.; White, T.A.; Johnson, K.O.; Stout, M.B.; Mezera, V.; et al. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc. Natl. Acad. Sci. USA 2015, 112, E6301–E6310. [Google Scholar] [CrossRef]
- Birch, J.; Gil, J. Senescence and the SASP: Many therapeutic avenues. Genes Dev. 2020, 34, 1565–1576. [Google Scholar] [CrossRef] [PubMed]
- Midha, A.; Pan, H.; Abarca, C.; Andle, J.; Carapeto, P.; Bonner-Weir, S.; Aguayo-Mazzucato, C. Unique Human and Mouse β-Cell Senescence-Associated Secretory Phenotype (SASP) Reveal Conserved Signaling Pathways and Heterogeneous Factors. Diabetes 2021, 70, 1098–1116. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.X.; Luo, Z.W.; Li, F.X.Z.; Cao, J.; Rao, S.S.; Liu, Y.W.; Wang, Y.Y.; Zhu, G.Q.; Gong, J.S.; Zou, J.T.; et al. Aged bone matrix-derived extracellular vesicles as a messenger for calcification paradox. Nat. Commun. 2022, 13, 1453. [Google Scholar] [CrossRef]
- Waring, O.J.; Skenteris, N.T.; Biessen, E.A.L.; Donners, M. Two-faced Janus: The dual role of macrophages in atherosclerotic calcification. Cardiovasc. Res. 2022, 118, 2768–2777. [Google Scholar] [CrossRef]
- Bakhshian Nik, A.; Kaiser, K.; Sun, P.; Khomtchouk, B.B.; Hutcheson, J.D. Altered Caveolin-1 Dynamics Result in Divergent Mineralization Responses in Bone and Vascular Calcification. Cell Mol. Bioeng. 2023, 16, 299–308. [Google Scholar] [CrossRef]
- Mandatori, D.; Pelusi, L.; Schiavone, V.; Pipino, C.; Di Pietro, N.; Pandolfi, A. The Dual Role of Vitamin K2 in “Bone-Vascular Crosstalk”: Opposite Effects on Bone Loss and Vascular Calcification. Nutrients 2021, 13, 1222. [Google Scholar] [CrossRef]
- Lv, X.T.; Chen, Q.Q.; Zhang, S.Y.; Gao, F.; Liu, Q.C. CGRP: A New Endogenous Cell Stemness Maintenance Molecule. Oxid. Med. Cell. Longev. 2022, 2022, 4107433. [Google Scholar] [CrossRef]
- Liu, L.; Dana, R.; Yin, J. Sensory neurons directly promote angiogenesis in response to inflammation via substance P signaling. FASEB J. 2020, 34, 6229–6243. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Feng, J.; Seidel, K.; Shi, S.; Klein, O.; Sharpe, P.; Chai, Y. Secretion of shh by a neurovascular bundle niche supports mesenchymal stem cell homeostasis in the adult mouse incisor. Cell Stem Cell 2014, 14, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Nel, S.; Durandt, C.; Murdoch, C.; Pepper, M.S. Determinants of Dental Pulp Stem Cell Heterogeneity. J. Endod. 2022, 48, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- Moqri, M.; Herzog, C.; Poganik, J.R.; Biomarkers of Aging, C.; Justice, J.; Belsky, D.W.; Higgins-Chen, A.; Moskalev, A.; Fuellen, G.; Cohen, A.A.; et al. Biomarkers of aging for the identification and evaluation of longevity interventions. Cell 2023, 186, 3758–3775. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.N.; Zhang, Y.; Sterling, K.; Song, W.H. Brain-derived neurotrophic factor in Alzheimer’s disease and its pharmaceutical potential. Transl Neurodegener 2022, 11, 1–34. [Google Scholar] [CrossRef]
- Deeks, E.D.; Lamb, Y.N. Cenegermin: A Review in Neurotrophic Keratitis. Drugs 2020, 80, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.Q.; Zhang, L.S.; Fei, D.D.; Guo, H.; Wu, M.L.; Liu, J.; He, X.N.; Zhang, Y.J.; Xuan, K.; Li, B. Sensory nerve-deficient microenvironment impairs tooth homeostasis by inducing apoptosis of dental pulp stem cells. Cell Prolif. 2020, 53, e12803. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, C.; Pang, L.; Pan, L.; Zhang, Q. Combination of resolvin E1 and lipoxin A4 promotes the resolution of pulpitis by inhibiting NF-kappaB activation through upregulating sirtuin 7 in dental pulp fibroblasts. Cell Prolif. 2022, 55, e13227. [Google Scholar] [CrossRef]
- Moore, E.R.; Michot, B.; Erdogan, O.; Ba, A.; Gibbs, J.L.; Yang, Y. CGRP and Shh Mediate the Dental Pulp Cell Response to Neuron Stimulation. J. Dent. Res. 2022, 101, 1119–1126. [Google Scholar] [CrossRef] [PubMed]




| Antibody | Species | Manufacturer | Catalog |
|---|---|---|---|
| Lamin B1 | Rabbit | Boster | BA1228 |
| COL-1 | Rabbit | Boster | PB0981 |
| COL-3 | Rabbit | Affinity | AF5457 |
| Laminin | Rabbit | Boster | PA1581 |
| DSPP | Mouse | Absin | abs12771 |
| α-tublin | Rabbit | Absin | abs619501 |
| p53 | Rabbit | Affinity | BF8013 |
| Target Gene | Primer Sequence (5′to3′Sequence) |
|---|---|
| p53-F | CTCCGACTATACCACTATCC |
| p53-R | GTCTTCCAGCGTGATGAT |
| p21-F | AGATGTGCCTATGGTCCTA |
| p21-R | TGTCTTGTCTTCGCTGAG |
| p16-F | CGATACAGGTGATGATGATG |
| p16-R | CTACCAGAGTGTCTAGGAAG |
| col-1-F | CGAGTATGGAAGCGAAGG |
| col-1-R | GCAGTGATAGGTGATGTTCT |
| col-3-F | CTACCTTGGTCAGTCCTATG |
| col-3-R | GCAGTCTAGTGGCTCATC |
| Laminin-F | AAGGTGTCTGGCTGGTTA |
| Laminin-R | GGTCGGTAGTGTCAATGTT |
| Fibronectin-F | GTAGCACAGAACTCAACCT |
| Fibronectin-R | CTCCTCCACAGCATAGATAG |
| MMP-1-F | GACCGACAACAGTGACAA |
| MMP-1-R | CATTAGTGCTCCTACATCTCT |
| MMP-2-F | ACAACCAACTACGATGATGA |
| MMP-2-R | TGGATAGTCGGAAGTTCTTG |
| MMP-13-F | GATGATGCTAACCAGACTATG |
| MMP-13-R | CTCTCACAATGCGATTACTC |
| TIMP1-F | TCTGGCATCCTCTTGTTG |
| TIMP1-R | GCTGGTATAAGGTGGTCTC |
| TGFb-F | CAACAACGCAATCTATGACA |
| TGFb-R | CAAGGTAACGCCAGGAAT |
| CTGF-F | TATGATGCGAGCCAACTG |
| CTGF-R | GCAGAAGGTATTGTCATTGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Liu, X.; Zhou, J.; Zhang, Q. The Role of Sensory Nerves in Dental Pulp Homeostasis: Histological Changes and Cellular Consequences after Sensory Denervation. Int. J. Mol. Sci. 2024, 25, 1126. https://doi.org/10.3390/ijms25021126
Wang C, Liu X, Zhou J, Zhang Q. The Role of Sensory Nerves in Dental Pulp Homeostasis: Histological Changes and Cellular Consequences after Sensory Denervation. International Journal of Molecular Sciences. 2024; 25(2):1126. https://doi.org/10.3390/ijms25021126
Chicago/Turabian StyleWang, Chunmeng, Xiaochen Liu, Jiani Zhou, and Qi Zhang. 2024. "The Role of Sensory Nerves in Dental Pulp Homeostasis: Histological Changes and Cellular Consequences after Sensory Denervation" International Journal of Molecular Sciences 25, no. 2: 1126. https://doi.org/10.3390/ijms25021126





