The Neurovascular Unit as a Locus of Injury in Low-Level Blast-Induced Neurotrauma
Abstract
:1. Blast-Induced Neurotrauma in the Military
2. A Rat Model of Low-Level Blast Injury with Chronic PTSD-Related Behavioral Traits
3. The Neurovascular Unit
4. The Vasculature as a Locus of Injury in Low-Level BINT
5. Comparisons to Other Models of Low-Level BINT
6. Is Direct Damage to Endothelial Cells by BINT a Thoracic Effect?
7. Blast Damage to the Endothelial Glycocalyx
8. Damage to Smooth Muscle Layers by BINT
9. Low-Level Blast Induces a Gliovascular and Neurovascular Disconnection
10. Functional Consequences of Lost Gliovascular and Neurovascular Connections on Blood–Brain Barrier Function and Neurovascular Coupling
11. BINT and Glymphatic Function
12. Perivascular Tau following BINT
13. BINT Damage to Pericytes and the Extracellular Matrix
14. Vascular-Associated Neuroinflammation following BINT
15. Linking Neurovascular and Chronic Neurobehavioral Effects That Follow BINT
16. Future Studies
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elder, G.A.; Ehrlich, M.E.; Gandy, S. Relationship of traumatic brain injury to chronic mental health problems and dementia in military veterans. Neurosci. Lett. 2019, 707, 134294. [Google Scholar] [CrossRef]
- Hoge, C.W.; McGurk, D.; Thomas, J.L.; Cox, A.L.; Engel, C.C.; Castro, C.A. Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N. Engl. J. Med. 2008, 358, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Tanielian, T.; Jaycox, L.H. Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery; Rand Corporation: Santa Monica, CA, USA, 2008. [Google Scholar]
- DePalma, R.G. Combat TBI: History, Epidemiology and Injury Modes. In Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects; Kobeissy, F., Ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 5–14. [Google Scholar]
- Elder, G.A. Update on TBI and Cognitive Impairment in Military Veterans. Curr. Neurol. Neurosci. Rep. 2015, 15, 68. [Google Scholar] [CrossRef] [PubMed]
- Warden, D.L.; Ryan, L.; Helmick, K.; Schwab, K.; French, L.; Lu, W.; Lux, W.; Ling, G.; Ecklund, J. War neurotrauma: The defense and veterans brain injury center (DVBIC) experience at the Walter Reed Army Medical Center. J. Neurotrauma 2005, 22, 1178. [Google Scholar]
- Bell, R.S.; Vo, A.H.; Neal, C.J.; Tigno, J.; Roberts, R.; Mossop, C.; Dunne, J.R.; Armonda, R.A. Military traumatic brain and spinal column injury: A 5-year study of the impact blast and other military grade weaponry on the central nervous system. J. Trauma. 2009, 66 (Suppl. 4), S104–S111. [Google Scholar] [CrossRef] [PubMed]
- Engel, C.; Hoch, E.; Simmons, M. The Neurological Effects of Repeated Exposure to Military Occupational Blast: Implications for Prevention and Health, Proceedings of the Findings, and Expert Recommendations from the Seventh Department of Defense State-of-the-Science Meeting, Arlington, VA, USA, 12–14 March 2018; Rand Corporation: Arlington VA USA, 2019. [Google Scholar]
- Tschiffely, A.E.; Statz, J.K.; Edwards, K.A.; Goforth, C.; Ahlers, S.T.; Carr, W.S.; Gill, J.M. Assessing a Blast-Related Biomarker in an Operational Community: Glial Fibrillary Acidic Protein in Experienced Breachers. J. Neurotrauma 2020, 37, 1091–1096. [Google Scholar] [CrossRef]
- Wang, Z.; Wilson, C.M.; Mendelev, N.; Ge, Y.; Galfalvy, H.; Elder, G.; Ahlers, S.; Yarnell, A.M.; LoPresti, M.L.; Kamimori, G.H.; et al. Acute and Chronic Molecular Signatures and Associated Symptoms of Blast Exposure in Military Breachers. J. Neurotrauma 2020, 37, 1221–1232. [Google Scholar] [CrossRef]
- Pagulayan, K.F.; Rau, H.; Madathil, R.; Werhane, M.; Millard, S.P.; Petrie, E.C.; Parmenter, B.; Peterson, S.; Sorg, S.; Hendrickson, R.; et al. Retrospective and Prospective Memory Among OEF/OIF/OND Veterans With a Self-Reported History of Blast-Related mTBI. J. Int. Neuropsychol. Soc. 2018, 24, 324–334. [Google Scholar] [CrossRef]
- Bailie, J.; Lippa, S.; Hungerford, L.; French, L.M.; Brickell, T.A.; Lange, R.T. Cumulative Blast Exposure During a Military Career Negatively Impacts Recovery from Traumatic Brain Injury. J. Neurotrauma 2023. [Google Scholar] [CrossRef]
- Lange, R.T.; French, L.M.; Lippa, S.M.; Gillow, K.; Tippett, C.E.; Barnhart, E.A.; Glazer, M.E.; Bailie, J.M.; Hungerford, L.; Brickell, T.A. High Lifetime Blast Exposure Using the Blast Exposure Threshold Survey Is Associated With Worse Warfighter Brain Health Following Mild Traumatic Brain Injury. J. Neurotrauma 2024, 41, 186–198. [Google Scholar] [CrossRef]
- Belding, J.N.; Englert, R.; Bonkowski, J.; Thomsen, C.J. Occupational Risk of Low-Level Blast Exposure and TBI-Related Medical Diagnoses: A Population-Based Epidemiological Investigation (2005–2015). Int. J. Environ. Res. Public. Health 2021, 18, 12925. [Google Scholar] [CrossRef] [PubMed]
- Belding, J.N.; Fitzmaurice, S.; Englert, R.M.; Lee, I.; Kowitz, B.; Highfill-McRoy, R.M.; Thomsen, C.J.; da Silva, U. Blast Exposure and Risk of Recurrent Occupational Overpressure Exposure Predict Deployment TBIs. Mil. Med. 2020, 185, e538–e544. [Google Scholar] [CrossRef]
- Stone, J.R.; Avants, B.B.; Tustison, N.J.; Wassermann, E.M.; Gill, J.; Polejaeva, E.; Dell, K.C.; Carr, W.; Yarnell, A.M.; LoPresti, M.L.; et al. Functional and Structural Neuroimaging Correlates of Repetitive Low-Level Blast Exposure in Career Breachers. J. Neurotrauma 2020, 37, 2468–2481. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.U.S. Troops Still Train on Weapons With Known Risk of Brain Injury. New York Times, 26 November 2023. [Google Scholar]
- Brenner, L.A.; Homaifar, B.Y.; Olson-Madden, J.H.; Nagamoto, H.T.; Huggins, J.; Schneider, A.L.; Forster, J.E.; Matarazzo, B.; Corrigan, J.D. Prevalence and screening of traumatic brain injury among veterans seeking mental health services. J. Head. Trauma. Rehabil. 2013, 28, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Jorge, R.E.; Arciniegas, D.B. Mood disorders after TBI. Psychiatr. Clin. North. Am. 2014, 37, 13–29. [Google Scholar] [CrossRef]
- Dreer, L.E.; Tang, X.; Nakase-Richardson, R.; Pugh, M.J.; Cox, M.K.; Bailey, E.K.; Finn, J.A.; Zafonte, R.; Brenner, L.A. Suicide and traumatic brain injury: A review by clinical researchers from the National Institute for Disability and Independent Living Rehabilitation Research (NIDILRR) and Veterans Health Administration Traumatic Brain Injury Model Systems. Curr. Opin. Psychol. 2018, 22, 73–78. [Google Scholar] [CrossRef]
- Elder, G.A.; Stone, J.R.; Ahlers, S.T. Effects of Low-Level Blast Exposure on the Nervous System: Is There Really a Controversy? Front. Neurol. 2014, 5, 269. [Google Scholar] [CrossRef]
- Jones, E.; Fear, N.T.; Wessely, S. Shell shock and mild traumatic brain injury: A historical review. Am. J. Psychiatry 2007, 164, 1641–1645. [Google Scholar] [CrossRef]
- Mac Donald, C.L.; Barber, J.; Jordan, M.; Johnson, A.M.; Dikmen, S.; Fann, J.R.; Temkin, N. Early Clinical Predictors of 5-Year Outcome After Concussive Blast Traumatic Brain Injury. JAMA Neurol. 2017, 74, 821–829. [Google Scholar] [CrossRef]
- Elder, G.A.; Dorr, N.P.; De Gasperi, R.; Gama Sosa, M.A.; Shaughness, M.C.; Maudlin-Jeronimo, E.; Hall, A.A.; McCarron, R.M.; Ahlers, S.T. Blast exposure induces post-traumatic stress disorder-related traits in a rat model of mild traumatic brain injury. J. Neurotrauma 2012, 29, 2564–2575. [Google Scholar] [CrossRef]
- Perez-Garcia, G.; Gama Sosa, M.A.; De Gasperi, R.; Lashof-Sullivan, M.; Maudlin-Jeronimo, E.; Stone, J.R.; Haghighi, F.; Ahlers, S.T.; Elder, G.A. Chronic post-traumatic stress disorder-related traits in a rat model of low-level blast exposure. Behav. Brain Res. 2018, 340, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Perez-Garcia, G.; Gama Sosa, M.A.; De Gasperi, R.; Tschiffely, A.E.; McCarron, R.M.; Hof, P.R.; Gandy, S.; Ahlers, S.T.; Elder, G.A. Blast-induced “PTSD”: Evidence from an animal model. Neuropharmacology 2019, 145 Pt B, 220–229. [Google Scholar] [CrossRef]
- Perez Garcia, G.; Perez, G.M.; De Gasperi, R.; Gama Sosa, M.A.; Otero-Pagan, A.; Pryor, D.; Abutarboush, R.; Kawoos, U.; Hof, P.R.; Cook, D.G.; et al. Progressive Cognitive and Post-Traumatic Stress Disorder-Related Behavioral Traits in Rats Exposed to Repetitive Low-Level Blast. J. Neurotrauma 2021, 38, 2030–2045. [Google Scholar] [CrossRef] [PubMed]
- Perez-Garcia, G.; De Gasperi, R.; Gama Sosa, M.A.; Perez, G.M.; Otero-Pagan, A.; Tschiffely, A.; McCarron, R.M.; Ahlers, S.T.; Elder, G.A.; Gandy, S. PTSD-Related Behavioral Traits in a Rat Model of Blast-Induced mTBI Are Reversed by the mGluR2/3 Receptor Antagonist BCI-838. eNeuro 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- Perez-Garcia, G.; Gama Sosa, M.A.; De Gasperi, R.; Lashof-Sullivan, M.; Maudlin-Jeronimo, E.; Stone, J.R.; Haghighi, F.; Ahlers, S.T.; Elder, G.A. Exposure to a Predator Scent Induces Chronic Behavioral Changes in Rats Previously Exposed to Low-level Blast: Implications for the Relationship of Blast-Related TBI to PTSD. Front. Neurol. 2016, 7, 176. [Google Scholar] [CrossRef] [PubMed]
- Kobeissy, F.; Mondello, S.; Tumer, N.; Toklu, H.Z.; Whidden, M.A.; Kirichenko, N.; Zhang, Z.; Prima, V.; Yassin, W.; Anagli, J.; et al. Assessing Neuro-Systemic & Behavioral Components in the Pathophysiology of Blast-Related Brain Injury. Front. Neurol. 2013, 4, 186. [Google Scholar]
- Elder, G.A.; Gama Sosa, M.A.; De Gasperi, R.; Stone, J.R.; Dickstein, D.L.; Haghighi, F.; Hof, P.R.; Ahlers, S.T. Vascular and inflammatory factors in the pathophysiology of blast-induced brain injury. Front. Neurol. 2015, 6, 48. [Google Scholar] [CrossRef]
- De Gasperi, R.; Gama Sosa, M.A.; Kim, S.H.; Steele, J.W.; Shaughness, M.C.; Maudlin-Jeronimo, E.; Hall, A.A.; Dekosky, S.T.; McCarron, R.M.; Nambiar, M.P.; et al. Acute blast injury reduces brain abeta in two rodent species. Front. Neurol. 2012, 3, 177. [Google Scholar] [CrossRef]
- Dickstein, D.L.; De Gasperi, R.; Gama Sosa, M.A.; Perez-Garcia, G.; Short, J.A.; Sosa, H.; Perez, G.M.; Tschiffely, A.E.; Dams-O’Connor, K.; Pullman, M.Y.; et al. Brain and blood biomarkers of tauopathy and neuronal injury in humans and rats with neurobehavioral syndromes following blast exposure. Mol. Psychiatry 2021, 26, 5940–5954. [Google Scholar] [CrossRef]
- Gama Sosa, M.A.; De Gasperi, R.; Janssen, P.L.; Yuk, F.J.; Anazodo, P.C.; Pricop, P.E.; Paulino, A.J.; Wicinski, B.; Shaughness, M.C.; Maudlin-Jeronimo, E.; et al. Selective vulnerability of the cerebral vasculature to blast injury in a rat model of mild traumatic brain injury. Acta Neuropathol. Commun. 2014, 2, 67. [Google Scholar] [CrossRef]
- Gama Sosa, M.A.; De Gasperi, R.; Perez Garcia, G.S.; Perez, G.M.; Searcy, C.; Vargas, D.; Spencer, A.; Janssen, P.L.; Tschiffely, A.E.; McCarron, R.M.; et al. Low-level blast exposure disrupts gliovascular and neurovascular connections and induces a chronic vascular pathology in rat brain. Acta Neuropathol. Commun. 2019, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Gama Sosa, M.A.; De Gasperi, R.; Perez Garcia, G.S.; Sosa, H.; Searcy, C.; Vargas, D.; Janssen, P.L.; Perez, G.M.; Tschiffely, A.E.; Janssen, W.G.; et al. Lack of chronic neuroinflammation in the absence of focal hemorrhage in a rat model of low-energy blast-induced TBI. Acta Neuropathol. Commun. 2017, 5, 80. [Google Scholar] [CrossRef]
- Gama Sosa, M.A.; De Gasperi, R.; Pryor, D.; Perez Garcia, G.S.; Perez, G.M.; Abutarboush, R.; Kawoos, U.; Hogg, S.; Ache, B.; Janssen, W.G.; et al. Low-level blast exposure induces chronic vascular remodeling, perivascular astrocytic degeneration and vascular-associated neuroinflammation. Acta Neuropathol. Commun. 2021, 9, 167. [Google Scholar] [CrossRef]
- Gama Sosa, M.A.; De Gasperi, R.; Pryor, D.; Perez Garcia, G.S.; Perez, G.M.; Abutarboush, R.; Kawoos, U.; Hogg, S.; Ache, B.; Sowa, A.; et al. Late chronic local inflammation, synaptic alterations, vascular remodeling and arteriovenous malformations in the brains of male rats exposed to repetitive low-level blast overpressures. Acta Neuropathol. Commun. 2023, 11, 81. [Google Scholar] [CrossRef] [PubMed]
- Garcia, G.P.; Perez, G.M.; Gasperi, R.; Sosa, M.A.G.; Otero-Pagan, A.; Abutarboush, R.; Kawoos, U.; Statz, J.K.; Patterson, J.; Zhu, C.W.; et al. (2R,6R)-Hydroxynorketamine Treatment of Rats Exposed to Repetitive Low-Level Blast Injury. Neurotrauma Rep. 2023, 4, 197–217. [Google Scholar] [CrossRef] [PubMed]
- De Gasperi, R.; Gama Sosa, M.A.; Perez Garcia, G.S.; Perez, G.M.; Abutarboush, R.; Kawoos, U.; Statz, J.K.; Patterson, J.; Hof, P.R.; Katsel, P.; et al. Progressive Transcriptional Changes in the Amygdala Implicate Neuroinflammation in the Effects of Repetitive Low-Level Blast Exposure in Male Rats. J. Neurotrauma 2023, 40, 561–577. [Google Scholar] [CrossRef]
- Haghighi, F.; Ge, Y.; Chen, S.; Xin, Y.; Umali, M.U.; De Gasperi, R.; Gama Sosa, M.A.; Ahlers, S.T.; Elder, G.A. Neuronal DNA Methylation Profiling of Blast-Related Traumatic Brain Injury. J. Neurotrauma 2015, 32, 1200–1209. [Google Scholar] [CrossRef]
- Gama Sosa, M.A.; De Gasperi, R.; Paulino, A.J.; Pricop, P.E.; Shaughness, M.C.; Maudlin-Jeronimo, E.; Hall, A.A.; Janssen, W.G.; Yuk, F.J.; Dorr, N.P.; et al. Blast overpressure induces shear-related injuries in the brain of rats exposed to a mild traumatic brain injury. Acta Neuropathol. Commun. 2013, 1, 51. [Google Scholar] [CrossRef]
- Ahlers, S.T.; Vasserman-Stokes, E.; Shaughness, M.C.; Hall, A.A.; Shear, D.A.; Chavko, M.; McCarron, R.M.; Stone, J.R. Assessment of the effects of acute and repeated exposure to blast overpressure in rodents: Toward a greater understanding of blast and the potential ramifications for injury in humans exposed to blast. Front. Neurol. 2012, 3, 32. [Google Scholar] [CrossRef]
- Chavko, M.; Koller, W.A.; Prusaczyk, W.K.; McCarron, R.M. Measurement of blast wave by a miniature fiber optic pressure transducer in the rat brain. J. Neurosci. Methods 2007, 159, 277–281. [Google Scholar] [CrossRef]
- Cernak, I.; Stein, D.G.; Elder, G.A.; Ahlers, S.; Curley, K.; DePalma, R.G.; Duda, J.; Ikonomovic, M.; Iverson, G.L.; Kobeissy, F.; et al. Preclinical modelling of militarily relevant traumatic brain injuries: Challenges and recommendations for future directions. Brain Inj. 2017, 31, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Cui, J.; Simonyi, A.; Johnson, C.E.; Hubler, G.K.; DePalma, R.G.; Gu, Z. Linking blast physics to biological outcomes in mild traumatic brain injury: Narrative review and preliminary report of an open-field blast model. Behav. Brain Res. 2018, 340, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Elder, G.A.; Mitsis, E.M.; Ahlers, S.T.; Cristian, A. Blast-induced mild traumatic brain injury. Psychiatr. Clin. North. Am. 2010, 33, 757–781. [Google Scholar] [CrossRef]
- Lange, R.T.; Brickell, T.A.; Ivins, B.; Vanderploeg, R.D.; French, L.M. Variable, not always persistent, postconcussion symptoms after mild TBI in U.S. military service members: A five-year cross-sectional outcome study. J. Neurotrauma 2013, 30, 958–969. [Google Scholar] [CrossRef]
- Mac Donald, C.L.; Barber, J.; Patterson, J.; Johnson, A.M.; Dikmen, S.; Fann, J.R.; Temkin, N. Association Between 5-Year Clinical Outcome in Patients With Nonmedically Evacuated Mild Blast Traumatic Brain Injury and Clinical Measures Collected Within 7 Days Postinjury in Combat. JAMA Netw. Open 2019, 2, e186676. [Google Scholar] [CrossRef] [PubMed]
- Xing, C.Y.; Tarumi, T.; Liu, J.; Zhang, Y.; Turner, M.; Riley, J.; Tinajero, C.D.; Yuan, L.J.; Zhang, R. Distribution of cardiac output to the brain across the adult lifespan. J. Cereb. Blood Flow. Metab. 2017, 37, 2848–2856. [Google Scholar] [CrossRef]
- Siegenthaler, J.A.; Sohet, F.; Daneman, R. ‘Sealing off the CNS’: Cellular and molecular regulation of blood-brain barriergenesis. Curr. Opin. Neurobiol. 2013, 23, 1057–1064. [Google Scholar] [CrossRef]
- Iadecola, C. The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron 2017, 96, 17–42. [Google Scholar] [CrossRef]
- Del Zoppo, G.J.; Milner, R.; Mabuchi, T.; Hung, S.; Wang, X.; Koziol, J.A. Vascular matrix adhesion and the blood-brain barrier. Biochem. Soc. Trans. 2006, 34, 1261–1266. [Google Scholar] [CrossRef]
- Sorokin, L. The impact of the extracellular matrix on inflammation. Nat. Rev. Immunol. 2010, 10, 712–723. [Google Scholar] [CrossRef]
- Ando, Y.; Okada, H.; Takemura, G.; Suzuki, K.; Takada, C.; Tomita, H.; Zaikokuji, R.; Hotta, Y.; Miyazaki, N.; Yano, H.; et al. Brain-Specific Ultrastructure of Capillary Endothelial Glycocalyx and Its Possible Contribution for Blood Brain Barrier. Sci. Rep. 2018, 8, 17523. [Google Scholar] [CrossRef] [PubMed]
- Strazielle, N.; Ghersi-Egea, J.F. Physiology of blood-brain interfaces in relation to brain disposition of small compounds and macromolecules. Mol. Pharm. 2013, 10, 1473–1491. [Google Scholar] [CrossRef] [PubMed]
- Daneman, R.; Prat, A. The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A. From blood-brain barrier to blood-brain interface: New opportunities for CNS drug delivery. Nat. Rev. Drug Discov. 2016, 15, 275–292. [Google Scholar] [CrossRef] [PubMed]
- Yurchenco, P.D.; Patton, B.L. Developmental and pathogenic mechanisms of basement membrane assembly. Curr. Pharm. Des. 2009, 15, 1277–1294. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Castro, B.; Robel, S.; Mishra, A. Astrocyte Endfeet in Brain Function and Pathology: Open Questions. Annu. Rev. Neurosci. 2023, 46, 101–121. [Google Scholar] [CrossRef]
- Coelho-Santos, V.; Berthiaume, A.A.; Ornelas, S.; Stuhlmann, H.; Shih, A.Y. Imaging the construction of capillary networks in the neonatal mouse brain. Proc. Natl. Acad. Sci. USA 2021, 118, e2100866118. [Google Scholar] [CrossRef]
- Bushong, E.A.; Martone, M.E.; Ellisman, M.H. Maturation of astrocyte morphology and the establishment of astrocyte domains during postnatal hippocampal development. Int. J. Dev. Neurosci. 2004, 22, 73–86. [Google Scholar] [CrossRef]
- Voutsinos-Porche, B.; Bonvento, G.; Tanaka, K.; Steiner, P.; Welker, E.; Chatton, J.Y.; Magistretti, P.J.; Pellerin, L. Glial glutamate transporters mediate a functional metabolic crosstalk between neurons and astrocytes in the mouse developing cortex. Neuron 2003, 37, 275–286. [Google Scholar] [CrossRef]
- Nedergaard, M.; Ransom, B.; Goldman, S.A. New roles for astrocytes: Redefining the functional architecture of the brain. Trends Neurosci. 2003, 26, 523–530. [Google Scholar] [CrossRef]
- Jessen, N.A.; Munk, A.S.; Lundgaard, I.; Nedergaard, M. The Glymphatic System: A Beginner’s Guide. Neurochem. Res. 2015, 40, 2583–2599. [Google Scholar] [CrossRef]
- Brown, L.S.; Foster, C.G.; Courtney, J.M.; King, N.E.; Howells, D.W.; Sutherland, B.A. Pericytes and Neurovascular Function in the Healthy and Diseased Brain. Front. Cell Neurosci. 2019, 13, 282. [Google Scholar] [CrossRef] [PubMed]
- Haruwaka, K.; Ikegami, A.; Tachibana, Y.; Ohno, N.; Konishi, H.; Hashimoto, A.; Matsumoto, M.; Kato, D.; Ono, R.; Kiyama, H.; et al. Dual microglia effects on blood brain barrier permeability induced by systemic inflammation. Nat. Commun. 2019, 10, 5816. [Google Scholar] [CrossRef]
- Ikegami, A.; Haruwaka, K.; Wake, H. Microglia: Lifelong modulator of neural circuits. Neuropathology 2019, 39, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Gullotta, G.S.; Costantino, G.; Sortino, M.A.; Spampinato, S.F. Microglia and the Blood-Brain Barrier: An External Player in Acute and Chronic Neuroinflammatory Conditions. Int. J. Mol. Sci. 2023, 24, 9144. [Google Scholar] [CrossRef]
- Sorokin, V.; Vickneson, K.; Kofidis, T.; Woo, C.C.; Lin, X.Y.; Foo, R.; Shanahan, C.M. Role of Vascular Smooth Muscle Cell Plasticity and Interactions in Vessel Wall Inflammation. Front. Immunol. 2020, 11, 599415. [Google Scholar] [CrossRef] [PubMed]
- Aldea, R.; Weller, R.O.; Wilcock, D.M.; Carare, R.O.; Richardson, G. Cerebrovascular Smooth Muscle Cells as the Drivers of Intramural Periarterial Drainage of the Brain. Front. Aging Neurosci. 2019, 11, 1. [Google Scholar] [CrossRef]
- Cheng, J.; Gu, J.; Ma, Y.; Yang, T.; Kuang, Y.; Li, B.; Kang, J. Development of a rat model for studying blast-induced traumatic brain injury. J. Neurol. Sci. 2010, 294, 23–28. [Google Scholar] [CrossRef]
- Reneer, D.V.; Hisel, R.D.; Hoffman, J.M.; Kryscio, R.J.; Lusk, B.T.; Geddes, J.W. A multi-mode shock tube for investigation of blast-induced traumatic brain injury. J. Neurotrauma 2011, 28, 95–104. [Google Scholar] [CrossRef]
- Rafaels, K.A.; Bass, C.R.; Panzer, M.B.; Salzar, R.S.; Woods, W.A.; Feldman, S.H.; Walilko, T.; Kent, R.W.; Capehart, B.P.; Foster, J.B.; et al. Brain injury risk from primary blast. J. Trauma. Acute Care Surg. 2012, 73, 895–901. [Google Scholar] [CrossRef]
- Li, B.C.; Li, Y.; Xu, C.; Wang, J.; Chen, Z.; Li, G.; Zhang, J.; Hu, S.; Wang, L.; Feng, H. Blast-induced traumatic brain injury of goats in confined space. Neurol. Res. 2014, 36, 974–982. [Google Scholar] [CrossRef]
- Kuehn, R.; Simard, P.F.; Driscoll, I.; Keledjian, K.; Ivanova, S.; Tosun, C.; Williams, A.; Bochicchio, G.; Gerzanich, V.; Simard, J.M. Rodent model of direct cranial blast injury. J. Neurotrauma 2011, 28, 2155–2169. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, Y.; Oguntayo, S.; Wilkins, W.; Arun, P.; Valiyaveettil, M.; Song, J.; Long, J.B.; Nambiar, M.P. Tightly coupled repetitive blast-induced traumatic brain injury: Development and characterization in mice. J. Neurotrauma 2011, 28, 2171–2183. [Google Scholar] [CrossRef] [PubMed]
- Cernak, I. Blast-induced neurotrauma models and their requirements. Front. Neurol. 2014, 5, 128. [Google Scholar] [CrossRef]
- Josey, T.; Ouellet, S.; Bieler, D.; Cernak, I.; Franke, A.; Gupta, R.; Kirkman, E.; Leggieri, M.J., Jr.; Orru, H.; Philippens, M.; et al. Guidelines for reproducing blast exposures in the laboratory. J. R. Army Med. Corps 2019, 165, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Watts, S.; Kirkman, E.; Bieler, D.; Bjarnason, S.; Franke, A.; Gupta, R.; Leggieri, M.J., Jr.; Orru, H.; Ouellet, S.; Philippens, M.; et al. Guidelines for using animal models in blast injury research. J. R. Army Med. Corps 2019, 165, 38–40. [Google Scholar] [CrossRef] [PubMed]
- Kawa, L.; Arborelius, U.; Yoshitake, T.; Kehr, J.; Hokfelt, T.; Risling, M.; Agoston, D.V. Neurotransmitter systems in a mild blast traumatic brain injury model: Catecholamines and serotonin. J. Neurotrauma 2015, 32, 1190–1199. [Google Scholar] [CrossRef]
- Goldstein, L.E.; Fisher, A.M.; Tagge, C.A.; Zhang, X.L.; Velisek, L.; Sullivan, J.A.; Upreti, C.; Kracht, J.M.; Ericsson, M.; Wojnarowicz, M.W.; et al. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci. Transl. Med. 2012, 4, 134ra60. [Google Scholar]
- Rubovitch, V.; Ten-Bosch, M.; Zohar, O.; Harrison, C.R.; Tempel-Brami, C.; Stein, E.; Hoffer, B.J.; Balaban, C.D.; Schreiber, S.; Chiu, W.T.; et al. A mouse model of blast-induced mild traumatic brain injury. Exp. Neurol. 2011, 232, 280–289. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Z.; Liu, B.; Valdez, C.; Chavko, M.; Cancio, L.C. Low-Level Primary Blast Induces Neuroinflammation and Neurodegeneration in Rats. Mil. Med. 2019, 184 (Suppl. 1), 265–272. [Google Scholar] [CrossRef] [PubMed]
- Ravula, A.R.; Rodriguez, J.; Younger, D.; Perumal, V.; Shao, N.; Rama Rao, K.V.; Pfister, B.; Chandra, N. Animal model of repeated low-level blast traumatic brain injury displays acute and chronic neurobehavioral and neuropathological changes. Exp. Neurol. 2022, 349, 113938. [Google Scholar] [CrossRef]
- Li, C.; Chen, S.; Siedhoff, H.R.; Grant, D.; Liu, P.; Balderrama, A.; Jackson, M.; Zuckerman, A.; Greenlief, C.M.; Kobeissy, F.; et al. Low-intensity open-field blast exposure effects on neurovascular unit ultrastructure in mice. Acta Neuropathol. Commun. 2023, 11, 144. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Song, H.; Cui, J.; Johnson, C.E.; Hubler, G.K.; DePalma, R.G.; Gu, Z.; Xia, W. Proteomic Profiling of Mouse Brains Exposed to Blast-Induced Mild Traumatic Brain Injury Reveals Changes in Axonal Proteins and Phosphorylated Tau. J. Alzheimers Dis. 2018, 66, 751–773. [Google Scholar] [CrossRef] [PubMed]
- Konan, L.M.; Song, H.; Pentecost, G.; Fogwe, D.; Ndam, T.; Cui, J.; Johnson, C.E.; Grant, D.; White, T.; Chen, M.; et al. Multi-Focal Neuronal Ultrastructural Abnormalities and Synaptic Alterations in Mice after Low-Intensity Blast Exposure. J. Neurotrauma 2019, 36, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Konan, L.M.; Cui, J.; Johnson, C.E.; Langenderfer, M.; Grant, D.; Ndam, T.; Simonyi, A.; White, T.; Demirci, U.; et al. Ultrastructural brain abnormalities and associated behavioral changes in mice after low-intensity blast exposure. Behav. Brain Res. 2018, 347, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Nishii, K.; Satoh, Y.; Higashi, T.; Matsui, T.; Ishizuka, T.; Kashitani, M.; Saitoh, D.; Kobayashi, Y. Evans Blue and Fluorescein Isothiocyanate-Dextran Double Labeling Reveals Precise Sequence of Vascular Leakage and Glial Responses After Exposure to Mild-Level Blast-Associated Shock Waves. J. Neurotrauma 2023, 40, 1228–1242. [Google Scholar] [CrossRef]
- Satoh, Y.; Araki, Y.; Kashitani, M.; Nishii, K.; Kobayashi, Y.; Fujita, M.; Suzuki, S.; Morimoto, Y.; Tokuno, S.; Tsumatori, G.; et al. Molecular Hydrogen Prevents Social Deficits and Depression-Like Behaviors Induced by Low-Intensity Blast in Mice. J. Neuropathol. Exp. Neurol. 2018, 77, 827–836. [Google Scholar] [CrossRef]
- Hubbard, W.B.; Velmurugan, G.V.; Brown, E.P.; Sullivan, P.G. Resilience of females to acute blood-brain barrier damage and anxiety behavior following mild blast traumatic brain injury. Acta Neuropathol. Commun. 2022, 10, 93. [Google Scholar] [CrossRef]
- Hubbard, W.B.; Vekaria, H.J.; Velmurugan, G.V.; Kalimon, O.J.; Prajapati, P.; Brown, E.; Geisler, J.G.; Sullivan, P.G. Mitochondrial Dysfunction after Repeated Mild Blast Traumatic Brain Injury Is Attenuated by a Mild Mitochondrial Uncoupling Prodrug. J. Neurotrauma 2023, 40, 2396–2409. [Google Scholar] [CrossRef]
- Heyburn, L.; Batuure, A.; Wilder, D.; Long, J.; Sajja, V.S. Neuroinflammation Profiling of Brain Cytokines Following Repeated Blast Exposure. Int. J. Mol. Sci. 2023, 24, 12564. [Google Scholar] [CrossRef]
- Courtney, A.C.; Courtney, M.W. A thoracic mechanism of mild traumatic brain injury due to blast pressure waves. Med. Hypotheses 2009, 72, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Cernak, I. The importance of systemic response in the pathobiology of blast-induced neurotrauma. Front. Neurol. 2010, 1, 151. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, Y. Neuroscience. Shell shock revisited: Solving the puzzle of blast trauma. Science 2008, 319, 406–408. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, W.; Constantini, S. Concepts and strategies for clinical management of blast-induced traumatic brain injury and posttraumatic stress disorder. J. Neuropsychiatry Clin. Neurosci. 2013, 25, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Rubio, J.E.; Skotak, M.; Alay, E.; Sundaramurthy, A.; Subramaniam, D.R.; Kote, V.B.; Yeoh, S.; Monson, K.; Chandra, N.; Unnikrishnan, G.; et al. Does Blast Exposure to the Torso Cause a Blood Surge to the Brain? Front. Bioeng. Biotechnol. 2020, 8, 573647. [Google Scholar] [CrossRef] [PubMed]
- Long, J.B.; Bentley, T.L.; Wessner, K.A.; Cerone, C.; Sweeney, S.; Bauman, R.A. Blast overpressure in rats: Recreating a battlefield injury in the laboratory. J. Neurotrauma 2009, 26, 827–840. [Google Scholar] [CrossRef] [PubMed]
- Koliatsos, V.E.; Cernak, I.; Xu, L.; Song, Y.; Savonenko, A.; Crain, B.J.; Eberhart, C.G.; Frangakis, C.E.; Melnikova, T.; Kim, H.; et al. A mouse model of blast injury to brain: Initial pathological, neuropathological, and behavioral characterization. J. Neuropathol. Exp. Neurol. 2011, 70, 399–416. [Google Scholar] [CrossRef]
- Simard, J.M.; Pampori, A.; Keledjian, K.; Tosun, C.; Schwartzbauer, G.; Ivanova, S.; Gerzanich, V. Exposure of the thorax to a sublethal blast wave causes a hydrodynamic pulse that leads to perivenular inflammation in the brain. J. Neurotrauma 2014, 31, 1292–1304. [Google Scholar] [CrossRef]
- Yuan, W.; Barber Foss, K.D.; Dudley, J.; Thomas, S.; Galloway, R.; DiCesare, C.; Leach, J.; Scheifele, P.; Farina, M.; Valencia, G.; et al. Impact of Low-Level Blast Exposure on Brain Function after a One-Day Tactile Training and the Ameliorating Effect of a Jugular Vein Compression Neck Collar Device. J. Neurotrauma 2019, 36, 721–734. [Google Scholar] [CrossRef]
- Reitsma, S.; Slaaf, D.W.; Vink, H.; van Zandvoort, M.A.; oude Egbrink, M.G. The endothelial glycocalyx: Composition, functions, and visualization. Pflugers Arch. 2007, 454, 345–359. [Google Scholar] [CrossRef]
- Zhu, J.; Li, X.; Yin, J.; Hu, Y.; Gu, Y.; Pan, S. Glycocalyx degradation leads to blood-brain barrier dysfunction and brain edema after asphyxia cardiac arrest in rats. J. Cereb. Blood Flow. Metab. 2018, 38, 1979–1992. [Google Scholar] [CrossRef]
- Curry, F.E.; Adamson, R.H. Endothelial glycocalyx: Permeability barrier and mechanosensor. Ann. Biomed. Eng. 2012, 40, 828–839. [Google Scholar] [CrossRef] [PubMed]
- Florian, J.A.; Kosky, J.R.; Ainslie, K.; Pang, Z.; Dull, R.O.; Tarbell, J.M. Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circ. Res. 2003, 93, e136–e142. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.P.; Yang, Y.; Janssen, W.J.; Gandjeva, A.; Perez, M.J.; Barthel, L.; Zemans, R.L.; Bowman, J.C.; Koyanagi, D.E.; Yunt, Z.X.; et al. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat. Med. 2012, 18, 1217–1223. [Google Scholar] [CrossRef]
- Hall, A.A.; Mendoza, M.I.; Zhou, H.; Shaughness, M.; Maudlin-Jeronimo, E.; McCarron, R.M.; Ahlers, S.T. Repeated Low Intensity Blast Exposure Is Associated with Damaged Endothelial Glycocalyx and Downstream Behavioral Deficits. Front. Behav. Neurosci. 2017, 11, 104. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.A.; Mendoza, M.I.; Zhou, H.; Shaughness, M.; McCarron, R.M.; Ahlers, S.T. Corrigendum: Repeated Low Intensity Blast Exposure Is Associated With Damaged Endothelial Glycocalyx and Downstream Behavioral Deficits. Front. Behav. Neurosci. 2019, 13, 251. [Google Scholar] [CrossRef] [PubMed]
- Armonda, R.A.; Bell, R.S.; Vo, A.H.; Ling, G.; DeGraba, T.J.; Crandall, B.; Ecklund, J.; Campbell, W.W. Wartime traumatic cerebral vasospasm: Recent review of combat casualties. Neurosurgery 2006, 59, 1215–1225. [Google Scholar] [CrossRef] [PubMed]
- Bauman, R.A.; Ling, G.; Tong, L.; Januszkiewicz, A.; Agoston, D.; Delanerolle, N.; Kim, Y.; Ritzel, D.; Bell, R.; Ecklund, J.; et al. An introductory characterization of a combat-casualty-care relevant swine model of closed head injury resulting from exposure to explosive blast. J. Neurotrauma 2009, 26, 841–860. [Google Scholar] [CrossRef]
- Bir, C.; Vandevord, P.; Shen, Y.; Raza, W.; Haacke, E.M. Effects of variable blast pressures on blood flow and oxygen saturation in rat brain as evidenced using MRI. Magn. Reson. Imaging 2012, 30, 527–534. [Google Scholar] [CrossRef]
- Rodriguez, U.A.; Zeng, Y.; Deyo, D.; Parsley, M.A.; Hawkins, B.E.; Prough, D.S.; DeWitt, D.S. Effects of Mild Blast Traumatic Brain Injury on Cerebral Vascular, Histopathological, and Behavioral Outcomes in Rats. J. Neurotrauma 2018, 35, 375–392. [Google Scholar] [CrossRef]
- Toklu, H.Z.; Yang, Z.; Oktay, S.; Sakarya, Y.; Kirichenko, N.; Matheny, M.K.; Muller-Delp, J.; Strang, K.; Scarpace, P.J.; Wang, K.K.W.; et al. Overpressure blast injury-induced oxidative stress and neuroinflammation response in rat frontal cortex and cerebellum. Behav. Brain Res. 2018, 340, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Abutarboush, R.; Gu, M.; Kawoos, U.; Mullah, S.H.; Chen, Y.; Goodrich, S.Y.; Lashof-Sullivan, M.; McCarron, R.M.; Statz, J.K.; Bell, R.S.; et al. Exposure to Blast Overpressure Impairs Cerebral Microvascular Responses and Alters Vascular and Astrocytic Structure. J. Neurotrauma 2019, 36, 3138–3157. [Google Scholar] [CrossRef]
- Alford, P.W.; Dabiri, B.E.; Goss, J.A.; Hemphill, M.A.; Brigham, M.D.; Parker, K.K. Blast-induced phenotypic switching in cerebral vasospasm. Proc. Natl. Acad. Sci. USA 2011, 108, 12705–12710. [Google Scholar] [CrossRef]
- Hald, E.S.; Alford, P.W. Smooth muscle phenotype switching in blast traumatic brain injury-induced cerebral vasospasm. Transl. Stroke Res. 2014, 5, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Boulay, A.C.; Saubamea, B.; Decleves, X.; Cohen-Salmon, M. Purification of Mouse Brain Vessels. J. Vis. Exp. 2015, 105, e53208. [Google Scholar]
- Chun, H.B.; Scott, M.; Niessen, S.; Hoover, H.; Baird, A.; Yates, J., 3rd; Torbett, B.E.; Eliceiri, B.P. The proteome of mouse brain microvessel membranes and basal lamina. J. Cereb. Blood Flow. Metab. 2011, 31, 2267–2281. [Google Scholar] [CrossRef]
- Searcy, J.L.; Le Bihan, T.; Salvadores, N.; McCulloch, J.; Horsburgh, K. Impact of age on the cerebrovascular proteomes of wild-type and Tg-SwDI mice. PLoS ONE 2014, 9, e89970. [Google Scholar] [CrossRef]
- Logsdon, A.F.; Meabon, J.S.; Cline, M.M.; Bullock, K.M.; Raskind, M.A.; Peskind, E.R.; Banks, W.A.; Cook, D.G. Blast exposure elicits blood-brain barrier disruption and repair mediated by tight junction integrity and nitric oxide dependent processes. Sci. Rep. 2018, 8, 11344. [Google Scholar] [CrossRef]
- Kaur, C.; Singh, J.; Lim, M.K.; Ng, B.L.; Ling, E.A. Macrophages/microglia as ‘sensors’ of injury in the pineal gland of rats following a non-penetrative blast. Neurosci. Res. 1997, 27, 317–322. [Google Scholar] [CrossRef]
- Lu, J.; Ng, K.C.; Ling, G.; Wu, J.; Poon, D.J.; Kan, E.M.; Tan, M.H.; Wu, Y.J.; Li, P.; Moochhala, S.; et al. Effect of blast exposure on the brain structure and cognition in Macaca fascicularis. J. Neurotrauma 2012, 29, 1434–1454. [Google Scholar] [CrossRef]
- Agoston, D.V.; McCullough, J.; Aniceto, R.; Lin, I.H.; Kamnaksh, A.; Eklund, M.; Graves, W.M., 3rd; Dunbar, C.; Engall, J.; Schneider, E.B.; et al. Blood-Based Biomarkers of Repetitive, Subconcussive Blast Overpressure Exposure in the Training Environment: A Pilot Study. Neurotrauma Rep. 2022, 3, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Shively, S.B.; Horkayne-Szakaly, I.; Jones, R.V.; Kelly, J.P.; Armstrong, R.C.; Perl, D.P. Characterisation of interface astroglial scarring in the human brain after blast exposure: A post-mortem case series. Lancet Neurol. 2016, 15, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Janzer, R.C.; Raff, M.C. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature 1987, 325, 253–257. [Google Scholar] [CrossRef]
- Obermeier, B.; Verma, A.; Ransohoff, R.M. The blood-brain barrier. Handb. Clin. Neurol. 2016, 133, 39–59. [Google Scholar]
- Spampinato, S.F.; Bortolotto, V.; Canonico, P.L.; Sortino, M.A.; Grilli, M. Astrocyte-Derived Paracrine Signals: Relevance for Neurogenic Niche Regulation and Blood-Brain Barrier Integrity. Front. Pharmacol. 2019, 10, 1346. [Google Scholar] [CrossRef] [PubMed]
- Lecuyer, M.A.; Kebir, H.; Prat, A. Glial influences on BBB functions and molecular players in immune cell trafficking. Biochim. Biophys. Acta 2016, 1862, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef]
- Heithoff, B.P.; George, K.K.; Phares, A.N.; Zuidhoek, I.A.; Munoz-Ballester, C.; Robel, S. Astrocytes are necessary for blood-brain barrier maintenance in the adult mouse brain. Glia 2021, 69, 436–472. [Google Scholar] [CrossRef]
- Schreiner, B.; Romanelli, E.; Liberski, P.; Ingold-Heppner, B.; Sobottka-Brillout, B.; Hartwig, T.; Chandrasekar, V.; Johannssen, H.; Zeilhofer, H.U.; Aguzzi, A.; et al. Astrocyte Depletion Impairs Redox Homeostasis and Triggers Neuronal Loss in the Adult CNS. Cell Rep. 2015, 12, 1377–1384. [Google Scholar] [CrossRef]
- Tsai, H.H.; Li, H.; Fuentealba, L.C.; Molofsky, A.V.; Taveira-Marques, R.; Zhuang, H.; Tenney, A.; Murnen, A.T.; Fancy, S.P.; Merkle, F.; et al. Regional astrocyte allocation regulates CNS synaptogenesis and repair. Science 2012, 337, 358–362. [Google Scholar] [CrossRef]
- Kubotera, H.; Ikeshima-Kataoka, H.; Hatashita, Y.; Allegra Mascaro, A.L.; Pavone, F.S.; Inoue, T. Astrocytic endfeet re-cover blood vessels after removal by laser ablation. Sci. Rep. 2019, 9, 1263. [Google Scholar] [CrossRef]
- Mills, W.A., 3rd; Woo, A.M.; Jiang, S.; Martin, J.; Surendran, D.; Bergstresser, M.; Kimbrough, I.F.; Eyo, U.B.; Sofroniew, M.V.; Sontheimer, H. Astrocyte plasticity in mice ensures continued endfoot coverage of cerebral blood vessels following injury and declines with age. Nat. Commun. 2022, 13, 1794. [Google Scholar] [CrossRef]
- Hamel, E. Perivascular nerves and the regulation of cerebrovascular tone. J Appl Physiol 2006, 100, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Wardlaw, J.M.; Benveniste, H.; Nedergaard, M.; Zlokovic, B.V.; Mestre, H.; Lee, H.; Doubal, F.N.; Brown, R.; Ramirez, J.; MacIntosh, B.J.; et al. Perivascular spaces in the brain: Anatomy, physiology and pathology. Nat. Rev. Neurol. 2020, 16, 137–153. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, M.K.; Mestre, H.; Nedergaard, M. The glymphatic pathway in neurological disorders. Lancet Neurol. 2018, 17, 1016–1024. [Google Scholar] [CrossRef]
- Chakraborty, S.; ThimmaReddygari, J.; Selvaraj, D. G-lymphatic, vascular and immune pathways for Abeta clearance cascade and therapeutic targets for Alzheimer’s disease. Comb. Chem. High. Throughput Screen. 2020, 24, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Iliff, J.J.; Chen, M.J.; Plog, B.A.; Zeppenfeld, D.M.; Soltero, M.; Yang, L.; Singh, I.; Deane, R.; Nedergaard, M. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J. Neurosci. 2014, 34, 16180–16193. [Google Scholar] [CrossRef]
- Peng, W.; Achariyar, T.M.; Li, B.; Liao, Y.; Mestre, H.; Hitomi, E.; Regan, S.; Kasper, T.; Peng, S.; Ding, F.; et al. Suppression of glymphatic fluid transport in a mouse model of Alzheimer’s disease. Neurobiol. Dis. 2016, 93, 215–225. [Google Scholar] [CrossRef]
- Diem, A.K.; Carare, R.O.; Weller, R.O.; Bressloff, N.W. A control mechanism for intra-mural peri-arterial drainage via astrocytes: How neuronal activity could improve waste clearance from the brain. PLoS ONE 2018, 13, e0205276. [Google Scholar] [CrossRef]
- Gandy, S.; Ikonomovic, M.D.; Mitsis, E.; Elder, G.; Ahlers, S.T.; Barth, J.; Stone, J.R.; DeKosky, S.T. Chronic traumatic encephalopathy: Clinical-biomarker correlations and current concepts in pathogenesis. Mol. Neurodegener. 2014, 9, 37. [Google Scholar] [CrossRef]
- DeKosky, S.T.; Blennow, K.; Ikonomovic, M.D.; Gandy, S. Acute and chronic traumatic encephalopathies: Pathogenesis and biomarkers. Nat. Rev. Neurol. 2013, 9, 192–200. [Google Scholar] [CrossRef]
- DeKosky, S.T.; Ikonomovic, M.D.; Gandy, S. Traumatic brain injury: Football, warfare, and long-term effects. N. Engl. J. Med. 2010, 363, 1293–1296. [Google Scholar] [CrossRef] [PubMed]
- Uryu, K.; Chen, X.H.; Martinez, D.; Browne, K.D.; Johnson, V.E.; Graham, D.I.; Lee, V.M.; Trojanowski, J.Q.; Smith, D.H. Multiple proteins implicated in neurodegenerative diseases accumulate in axons after brain trauma in humans. Exp. Neurol. 2007, 208, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Johnson, V.E.; Stewart, W.; Smith, D.H. Traumatic brain injury and amyloid-beta pathology: A link to Alzheimer’s disease? Nat. Rev. Neurosci. 2010, 11, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Ikonomovic, M.D.; Uryu, K.; Abrahamson, E.E.; Ciallella, J.R.; Trojanowski, J.Q.; Lee, V.M.; Clark, R.S.; Marion, D.W.; Wisniewski, S.R.; DeKosky, S.T. Alzheimer’s pathology in human temporal cortex surgically excised after severe brain injury. Exp. Neurol. 2004, 190, 192–203. [Google Scholar] [CrossRef]
- DeKosky, S.T.; Abrahamson, E.E.; Ciallella, J.R.; Paljug, W.R.; Wisniewski, S.R.; Clark, R.S.; Ikonomovic, M.D. Association of increased cortical soluble abeta42 levels with diffuse plaques after severe brain injury in humans. Arch. Neurol. 2007, 64, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.G.; Laird, M.D.; Han, D.; Nguyen, K.; Scott, E.; Dong, Y.; Dhandapani, K.M.; Brann, D.W. Critical role of NADPH oxidase in neuronal oxidative damage and microglia activation following traumatic brain injury. PLoS ONE 2012, 7, e34504. [Google Scholar] [CrossRef]
- Loane, D.J.; Pocivavsek, A.; Moussa, C.E.; Thompson, R.; Matsuoka, Y.; Faden, A.I.; Rebeck, G.W.; Burns, M.P. Amyloid precursor protein secretases as therapeutic targets for traumatic brain injury. Nat. Med. 2009, 15, 377–379. [Google Scholar] [CrossRef]
- Loane, D.J.; Washington, P.M.; Vardanian, L.; Pocivavsek, A.; Hoe, H.S.; Duff, K.E.; Cernak, I.; Rebeck, G.W.; Faden, A.I.; Burns, M.P. Modulation of ABCA1 by an LXR agonist reduces beta-amyloid levels and improves outcome after traumatic brain injury. J. Neurotrauma 2011, 28, 225–236. [Google Scholar] [CrossRef]
- Yu, F.; Zhang, Y.; Chuang, D.M. Lithium reduces BACE1 overexpression, beta amyloid accumulation, and spatial learning deficits in mice with traumatic brain injury. J. Neurotrauma 2012, 29, 2342–2351. [Google Scholar] [CrossRef]
- Tian, L.; Guo, R.; Yue, X.; Lv, Q.; Ye, X.; Wang, Z.; Chen, Z.; Wu, B.; Xu, G.; Liu, X. Intranasal administration of nerve growth factor ameliorate beta-amyloid deposition after traumatic brain injury in rats. Brain Res. 2012, 1440, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.H.; Nakamura, M.; McIntosh, T.K.; Wang, J.; Rodriguez, A.; Chen, X.H.; Raghupathi, R.; Saatman, K.E.; Clemens, J.; Schmidt, M.L.; et al. Brain trauma induces massive hippocampal neuron death linked to a surge in beta-amyloid levels in mice overexpressing mutant amyloid precursor protein. Am. J. Pathol. 1998, 153, 1005–1010. [Google Scholar] [CrossRef] [PubMed]
- Tajiri, N.; Kellogg, S.L.; Shimizu, T.; Arendash, G.W.; Borlongan, C.V. Traumatic brain injury precipitates cognitive impairment and extracellular Abeta aggregation in Alzheimer’s disease transgenic mice. PLoS ONE 2013, 8, e78851. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.T.; LaFerla, F.M.; Holtzman, D.M.; Brody, D.L. Controlled cortical impact traumatic brain injury in 3xTg-AD mice causes acute intra-axonal amyloid-beta accumulation and independently accelerates the development of tau abnormalities. J. Neurosci. 2011, 31, 9513–9525. [Google Scholar] [CrossRef]
- Tran, H.T.; Sanchez, L.; Esparza, T.J.; Brody, D.L. Distinct temporal and anatomical distributions of amyloid-beta and tau abnormalities following controlled cortical impact in transgenic mice. PLoS ONE 2011, 6, e25475. [Google Scholar] [CrossRef] [PubMed]
- Uryu, K.; Laurer, H.; McIntosh, T.; Pratico, D.; Martinez, D.; Leight, S.; Lee, V.M.; Trojanowski, J.Q. Repetitive mild brain trauma accelerates Abeta deposition, lipid peroxidation, and cognitive impairment in a transgenic mouse model of Alzheimer amyloidosis. J. Neurosci. 2002, 22, 446–454. [Google Scholar] [CrossRef]
- Washington, P.M.; Morffy, N.; Parsadanian, M.; Zapple, D.N.; Burns, M.P. Experimental traumatic brain injury induces rapid aggregation and oligomerization of amyloid-beta in an Alzheimer’s disease mouse model. J. Neurotrauma 2014, 31, 125–134. [Google Scholar] [CrossRef]
- Webster, S.J.; Van Eldik, L.J.; Watterson, D.M.; Bachstetter, A.D. Closed head injury in an age-related Alzheimer mouse model leads to an altered neuroinflammatory response and persistent cognitive impairment. J. Neurosci. 2015, 35, 6554–6569. [Google Scholar] [CrossRef]
- Zohar, O.; Lavy, R.; Zi, X.; Nelson, T.J.; Hongpaisan, J.; Pick, C.G.; Alkon, D.L. PKC activator therapeutic for mild traumatic brain injury in mice. Neurobiol. Dis. 2011, 41, 329–337. [Google Scholar] [CrossRef]
- Blasko, I.; Beer, R.; Bigl, M.; Apelt, J.; Franz, G.; Rudzki, D.; Ransmayr, G.; Kampfl, A.; Schliebs, R. Experimental traumatic brain injury in rats stimulates the expression, production and activity of Alzheimer’s disease beta-secretase (BACE-1). J. Neural Transm. 2004, 111, 523–536. [Google Scholar] [CrossRef]
- Nadler, Y.; Alexandrovich, A.; Grigoriadis, N.; Hartmann, T.; Rao, K.S.; Shohami, E.; Stein, R. Increased expression of the gamma-secretase components presenilin-1 and nicastrin in activated astrocytes and microglia following traumatic brain injury. Glia 2008, 56, 552–567. [Google Scholar] [CrossRef]
- Chen, X.H.; Siman, R.; Iwata, A.; Meaney, D.F.; Trojanowski, J.Q.; Smith, D.H. Long-term accumulation of amyloid-beta, beta-secretase, presenilin-1, and caspase-3 in damaged axons following brain trauma. Am. J. Pathol. 2004, 165, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Haass, C.; Hung, A.Y.; Schlossmacher, M.G.; Teplow, D.B.; Selkoe, D.J. Beta-Amyloid peptide and a 3-kDa fragment are derived by distinct cellular mechanisms. J. Biol. Chem. 1993, 268, 3021–3024. [Google Scholar] [CrossRef]
- Perez Garcia, G.; De Gasperi, R.; Tschiffely, A.E.; Gama Sosa, M.A.; Abutarboush, R.; Kawoos, U.; Statz, J.K.; Ciarlone, S.; Reed, E.; Jeyarajah, T.; et al. Repetitive Low-Level Blast Exposure Improves Behavioral Deficits and Chronically Lowers Abeta42 in an Alzheimer Disease Transgenic Mouse Model. J. Neurotrauma 2021, 38, 3146–3173. [Google Scholar] [CrossRef] [PubMed]
- Jankowsky, J.L.; Fadale, D.J.; Anderson, J.; Xu, G.M.; Gonzales, V.; Jenkins, N.A.; Copeland, N.G.; Lee, M.K.; Younkin, L.H.; Wagner, S.L.; et al. Mutant presenilins specifically elevate the levels of the 42 residue beta-amyloid peptide in vivo: Evidence for augmentation of a 42-specific gamma secretase. Hum. Mol. Genet. 2004, 13, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Abutarboush, R.; Reed, E.; Chen, Y.; Gu, M.; Cameron, W.; Kawoos, U.; Statz, J.; Tschiffely, A.; Ciarlone, S.; Perez-Garcia, G.; et al. Exposure to low-intensity blast increases clearance of brain Aβ. J. Neurotruama 2023, Published online. [CrossRef]
- Harper, M.M.; Hedberg-Buenz, A.; Herlein, J.; Abrahamson, E.E.; Anderson, M.G.; Kuehn, M.H.; Kardon, R.H.; Poolman, P.; Ikonomovic, M.D. Blast-Mediated Traumatic Brain Injury Exacerbates Retinal Damage and Amyloidosis in the APPswePSENd19e Mouse Model of Alzheimer’s Disease. Invest. Ophthalmol. Vis. Sci. 2019, 60, 2716–2725. [Google Scholar] [CrossRef]
- Edwards, K.A.; Leete, J.J.; Tschiffely, A.E.; Moore, C.Y.; Dell, K.C.; Statz, J.K.; Carr, W.; Walker, P.B.; LoPresti, M.L.; Ahlers, S.T.; et al. Blast exposure results in tau and neurofilament light chain changes in peripheral blood. Brain Inj. 2020, 34, 1213–1221. [Google Scholar] [CrossRef]
- Gill, J.; Cashion, A.; Osier, N.; Arcurio, L.; Motamedi, V.; Dell, K.C.; Carr, W.; Kim, H.S.; Yun, S.; Walker, P.; et al. Moderate blast exposure alters gene expression and levels of amyloid precursor protein. Neurol. Genet. 2017, 3, e186. [Google Scholar] [CrossRef]
- Thangavelu, B.; LaValle, C.R.; Egnoto, M.J.; Nemes, J.; Boutte, A.M.; Kamimori, G.H. Overpressure Exposure from.50-Caliber Rifle Training Is Associated with Increased Amyloid Beta Peptides in Serum. Front. Neurol. 2020, 11, 620. [Google Scholar] [CrossRef]
- Boutte, A.M.; Thangavelu, B.; Nemes, J.; LaValle, C.R.; Egnoto, M.; Carr, W.; Kamimori, G.H. Neurotrauma Biomarker Levels and Adverse Symptoms Among Military and Law Enforcement Personnel Exposed to Occupational Overpressure Without Diagnosed Traumatic Brain Injury. JAMA Netw. Open 2021, 4, e216445. [Google Scholar] [CrossRef] [PubMed]
- McKee, A.C.; Robinson, M.E. Military-related traumatic brain injury and neurodegeneration. Alzheimers Dement. 2014, 10 (Suppl. 3), S242–S253. [Google Scholar] [CrossRef] [PubMed]
- Omalu, B.; Hammers, J.L.; Bailes, J.; Hamilton, R.L.; Kamboh, M.I.; Webster, G.; Fitzsimmons, R.P. Chronic traumatic encephalopathy in an Iraqi war veteran with posttraumatic stress disorder who committed suicide. Neurosurg. Focus. 2011, 31, E3. [Google Scholar] [CrossRef] [PubMed]
- Olivera, A.; Lejbman, N.; Jeromin, A.; French, L.M.; Kim, H.S.; Cashion, A.; Mysliwiec, V.; Diaz-Arrastia, R.; Gill, J. Peripheral Total Tau in Military Personnel Who Sustain Traumatic Brain Injuries During Deployment. JAMA Neurol. 2015, 72, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Edwards, K.A.; Greer, K.; Leete, J.; Lai, C.; Devoto, C.; Qu, B.X.; Yarnell, A.M.; Polejaeva, E.; Dell, K.C.; LoPresti, M.L.; et al. Neuronally-derived tau is increased in experienced breachers and is associated with neurobehavioral symptoms. Sci. Rep. 2021, 11, 19527. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.Z.; Cumming, P.; Gotz, J.; Nasrallah, F.; Department of Defense Alzheimer’s Disease Neuroimaging Initiative. Tauopathy in veterans with long-term posttraumatic stress disorder and traumatic brain injury. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.E.; McKee, A.C.; Salat, D.H.; Rasmusson, A.M.; Radigan, L.J.; Catana, C.; Milberg, W.P.; McGlinchey, R.E. Positron emission tomography of tau in Iraq and Afghanistan Veterans with blast neurotrauma. Neuroimage Clin. 2019, 21, 101651. [Google Scholar] [CrossRef]
- Priemer, D.S.; Iacono, D.; Rhodes, C.H.; Olsen, C.H.; Perl, D.P. Chronic Traumatic Encephalopathy in the Brains of Military Personnel. N. Engl. J. Med. 2022, 386, 2169–2177. [Google Scholar] [CrossRef]
- Johnson, G.V.; Stoothoff, W.H. Tau phosphorylation in neuronal cell function and dysfunction. J. Cell Sci. 2004, 117 Pt 24, 5721–5729. [Google Scholar] [CrossRef]
- Wang, Y.; Mandelkow, E. Tau in physiology and pathology. Nat. Rev. Neurosci. 2016, 17, 5–21. [Google Scholar] [CrossRef]
- Goedert, M.; Jakes, R.; Crowther, R.A.; Cohen, P.; Vanmechelen, E.; Vandermeeren, M.; Cras, P. Epitope mapping of monoclonal antibodies to the paired helical filaments of Alzheimer’s disease: Identification of phosphorylation sites in tau protein. Biochem. J. 1994, 301 Pt 3, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Perez Garcia, G.; De Gasperi, R.; Gama Sosa, M.A.; Perez, G.M.; Otero-Pagan, A.; Pryor, D.; Abutarboush, R.; Kawoos, U.; Hof, P.R.; Dickstein, D.L.; et al. Laterality and region-specific tau phosphorylation correlate with PTSD-related behavioral traits in rats exposed to repetitive low-level blast. Acta Neuropathol. Commun. 2021, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Denenberg, V. Hemispheric laterality in animals and the effects of early experience. Behav. Brain Sci. 1981, 4, 1–49. [Google Scholar] [CrossRef]
- Klur, S.; Muller, C.; Pereira de Vasconcelos, A.; Ballard, T.; Lopez, J.; Galani, R.; Certa, U.; Cassel, J.C. Hippocampal-dependent spatial memory functions might be lateralized in rats: An approach combining gene expression profiling and reversible inactivation. Hippocampus 2009, 19, 800–816. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, Y.; Hosoya, A.; Yamasaki, N.; Ahmed, H.; Hattori, S.; Eguchi, M.; Yamaguchi, S.; Miyakawa, T.; Hirase, H.; Shigemoto, R. Right-hemispheric dominance of spatial memory in split-brain mice. Hippocampus 2012, 22, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Schindler, A.G.; Meabon, J.S.; Pagulayan, K.F.; Hendrickson, R.C.; Meeker, K.D.; Cline, M.; Li, G.; Sikkema, C.; Wilkinson, C.W.; Perl, D.P.; et al. Blast-related disinhibition and risk seeking in mice and combat Veterans: Potential role for dysfunctional phasic dopamine release. Neurobiol. Dis. 2017, 106, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Castellano, M.A.; Diaz-Palarea, M.D.; Rodriguez, M.; Barroso, J. Lateralization in male rats and dopaminergic system: Evidence of right-side population bias. Physiol. Behav. 1987, 40, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Berridge, C.; Espana, R.; Stalnaker, T. Stress and coping: Asymmetry of dopamine efferents within the prefrontal cortex. In The Asymmetrical Brain; Hugdahl, K., Davidison, R., Eds.; MIT Press: Cambridge, MA, USA, 2003; pp. 69–103. [Google Scholar]
- Sullivan, R.M.; Dufresne, M.M.; Siontas, D.; Chehab, S.; Townsend, J.; Laplante, F. Mesocortical dopamine depletion and anxiety-related behavior in the rat: Sex and hemisphere differences. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 54, 59–66. [Google Scholar] [CrossRef]
- Liberzon, I.; Sripada, C.S. The functional neuroanatomy of PTSD: A critical review. Prog. Brain Res. 2008, 167, 151–169. [Google Scholar]
- Mahan, A.L.; Ressler, K.J. Fear conditioning, synaptic plasticity and the amygdala: Implications for posttraumatic stress disorder. Trends Neurosci. 2012, 35, 24–35. [Google Scholar] [CrossRef]
- Liberzon, I.; Abelson, J.L. Context Processing and the Neurobiology of Post-Traumatic Stress Disorder. Neuron 2016, 92, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Mate De Gerando, A.; Welikovitch, L.A.; Khasnavis, A.; Commins, C.; Glynn, C.; Chun, J.E.; Perbet, R.; Hyman, B.T. Tau seeding and spreading in vivo is supported by both AD-derived fibrillar and oligomeric tau. Acta Neuropathol. 2023, 146, 191–210. [Google Scholar] [CrossRef] [PubMed]
- Stern, A.M.; Selkoe, D.J. Soluble oligomers or insoluble fibrils? Scientific commentary on “Tau seeding and spreading in vivo is supported by both AD-derived fibrillar and oligomeric tau”. Acta Neuropathol. 2023, 146, 861–862. [Google Scholar] [CrossRef]
- Mothes, T.; Portal, B.; Konstantinidis, E.; Eltom, K.; Libard, S.; Streubel-Gallasch, L.; Ingelsson, M.; Rostami, J.; Lindskog, M.; Erlandsson, A. Astrocytic uptake of neuronal corpses promotes cell-to-cell spreading of tau pathology. Acta Neuropathol. Commun. 2023, 11, 97. [Google Scholar] [CrossRef]
- Lopes, S.; Vaz-Silva, J.; Pinto, V.; Dalla, C.; Kokras, N.; Bedenk, B.; Mack, N.; Czisch, M.; Almeida, O.F.; Sousa, N.; et al. Tau protein is essential for stress-induced brain pathology. Proc. Natl. Acad. Sci. USA 2016, 113, E3755–E3763. [Google Scholar] [CrossRef]
- Lopes, S.; Teplytska, L.; Vaz-Silva, J.; Dioli, C.; Trindade, R.; Morais, M.; Webhofer, C.; Maccarrone, G.; Almeida, O.F.; Turck, C.W.; et al. Tau Deletion Prevents Stress-Induced Dendritic Atrophy in Prefrontal Cortex: Role of Synaptic Mitochondria. Cereb. Cortex 2016, 27, 2580–2591. [Google Scholar] [CrossRef]
- McKee, A.C. The Neuropathology of Chronic Traumatic Encephalopathy: The Status of the Literature. Semin. Neurol. 2020, 40, 359–369. [Google Scholar] [CrossRef]
- McKee, A.C.; Cairns, N.J.; Dickson, D.W.; Folkerth, R.D.; Keene, C.D.; Litvan, I.; Perl, D.P.; Stein, T.D.; Vonsattel, J.P.; Stewart, W.; et al. The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol. 2016, 131, 75–86. [Google Scholar] [CrossRef]
- Butler, M.; Dixon, E.; Stein, T.D.; Alvarez, V.E.; Huber, B.; Buckland, M.E.; McKee, A.C.; Cherry, J.D. Tau Pathology in Chronic Traumatic Encephalopathy is Primarily Neuronal. J. Neuropathol. Exp. Neurol. 2022, 81, 773–780. [Google Scholar] [CrossRef]
- Huber, B.R.; Meabon, J.S.; Martin, T.J.; Mourad, P.D.; Bennett, R.; Kraemer, B.C.; Cernak, I.; Petrie, E.C.; Emery, M.J.; Swenson, E.R.; et al. Blast exposure causes early and persistent aberrant phospho- and cleaved-tau expression in a murine model of mild blast-induced traumatic brain injury. J. Alzheimers Dis. 2013, 37, 309–323. [Google Scholar] [CrossRef]
- Huber, B.R.; Meabon, J.S.; Hoffer, Z.S.; Zhang, J.; Hoekstra, J.G.; Pagulayan, K.F.; McMillan, P.J.; Mayer, C.L.; Banks, W.A.; Kraemer, B.C.; et al. Blast exposure causes dynamic microglial/macrophage responses and microdomains of brain microvessel dysfunction. Neuroscience 2016, 319, 206–220. [Google Scholar] [CrossRef] [PubMed]
- Kovesdi, E.; Gyorgy, A.B.; Kwon, S.K.; Wingo, D.L.; Kamnaksh, A.; Long, J.B.; Kasper, C.E.; Agoston, D.V. The effect of enriched environment on the outcome of traumatic brain injury; a behavioral, proteomics, and histological study. Front. Neurosci. 2011, 5, 42. [Google Scholar] [CrossRef]
- Arun, P.; Abu-Taleb, R.; Oguntayo, S.; Tanaka, M.; Wang, Y.; Valiyaveettil, M.; Long, J.B.; Zhang, Y.; Nambiar, M.P. Distinct patterns of expression of traumatic brain injury biomarkers after blast exposure: Role of compromised cell membrane integrity. Neurosci. Lett. 2013, 552, 87–91. [Google Scholar] [CrossRef]
- Arun, P.; Oguntayo, S.; Albert, S.V.; Gist, I.; Wang, Y.; Nambiar, M.P.; Long, J.B. Acute decrease in alkaline phosphatase after brain injury: A potential mechanism for tauopathy. Neurosci. Lett. 2015, 609, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Plantman, S.; Cernak, I.; Agoston, D.V. The Temporal Pattern of Changes in Serum Biomarker Levels Reveals Complex and Dynamically Changing Pathologies after Exposure to a Single Low-Intensity Blast in Mice. Front. Neurol. 2015, 6, 114. [Google Scholar] [CrossRef]
- Perez-Polo, J.R.; Rea, H.C.; Johnson, K.M.; Parsley, M.A.; Unabia, G.C.; Xu, G.Y.; Prough, D.; DeWitt, D.S.; Spratt, H.; Hulsebosch, C.E. A rodent model of mild traumatic brain blast injury. J. Neurosci. Res. 2015, 93, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Sajja, V.S.; Hubbard, W.B.; Hall, C.S.; Ghoddoussi, F.; Galloway, M.P.; VandeVord, P.J. Enduring deficits in memory and neuronal pathology after blast-induced traumatic brain injury. Sci. Rep. 2015, 5, 15075. [Google Scholar] [CrossRef]
- Liu, M.D.; Luo, P.; Wang, Z.J.; Fei, Z. Changes of serum Tau, GFAP, TNF-alpha and malonaldehyde after blast-related traumatic brain injury. Chin. J. Traumatol. 2014, 17, 317–322. [Google Scholar]
- Lucke-Wold, B.P.; Naser, Z.J.; Logsdon, A.F.; Turner, R.C.; Smith, K.E.; Robson, M.J.; Bailes, J.E.; Lee, J.M.; Rosen, C.L.; Huber, J.D. Amelioration of nicotinamide adenine dinucleotide phosphate-oxidase mediated stress reduces cell death after blast-induced traumatic brain injury. Transl. Res. 2015, 166, 509–528.e1. [Google Scholar] [CrossRef]
- Lucke-Wold, B.P.; Turner, R.C.; Logsdon, A.F.; Nguyen, L.; Bailes, J.E.; Lee, J.M.; Robson, M.J.; Omalu, B.I.; Huber, J.D.; Rosen, C.L. Endoplasmic reticulum stress implicated in chronic traumatic encephalopathy. J. Neurosurg. 2016, 124, 687–702. [Google Scholar] [CrossRef]
- Du, X.; West, M.B.; Cheng, W.; Ewert, D.L.; Li, W.; Saunders, D.; Towner, R.A.; Floyd, R.A.; Kopke, R.D. Ameliorative Effects of Antioxidants on the Hippocampal Accumulation of Pathologic Tau in a Rat Model of Blast-Induced Traumatic Brain Injury. Oxid. Med. Cell Longev. 2016, 2016, 4159357. [Google Scholar] [CrossRef] [PubMed]
- Meabon, J.S.; Huber, B.R.; Cross, D.J.; Richards, T.L.; Minoshima, S.; Pagulayan, K.F.; Li, G.; Meeker, K.D.; Kraemer, B.C.; Petrie, E.C.; et al. Repetitive blast exposure in mice and combat veterans causes persistent cerebellar dysfunction. Sci. Transl. Med. 2016, 8, 321ra6. [Google Scholar] [CrossRef]
- Gerson, J.; Castillo-Carranza, D.L.; Sengupta, U.; Bodani, R.; Prough, D.S.; DeWitt, D.S.; Hawkins, B.E.; Kayed, R. Tau Oligomers Derived from Traumatic Brain Injury Cause Cognitive Impairment and Accelerate Onset of Pathology in Htau Mice. J. Neurotrauma 2016, 33, 2034–2043. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Gaamouch, F.E.; Meabon, J.S.; Meeker, K.D.; Zhu, L.; Zhong, M.B.; Bendik, J.; Elder, G.; Jing, P.; Xia, J.; et al. ApoE4-associated phospholipid dysregulation contributes to development of Tau hyper-phosphorylation after traumatic brain injury. Sci. Rep. 2017, 7, 11372. [Google Scholar] [CrossRef] [PubMed]
- Logsdon, A.F.; Lucke-Wold, B.P.; Turner, R.C.; Li, X.; Adkins, C.E.; Mohammad, A.S.; Huber, J.D.; Rosen, C.L.; Lockman, P.R. A mouse Model of Focal Vascular Injury Induces Astrocyte Reactivity, Tau Oligomers, and Aberrant Behavior. Arch. Neurosci. 2017, 4, e44254. [Google Scholar] [CrossRef]
- Du, X.; West, M.B.; Cai, Q.; Cheng, W.; Ewert, D.L.; Li, W.; Floyd, R.A.; Kopke, R.D. Antioxidants reduce neurodegeneration and accumulation of pathologic Tau proteins in the auditory system after blast exposure. Free Radic. Biol. Med. 2017, 108, 627–643. [Google Scholar] [CrossRef] [PubMed]
- Lucke-Wold, B.P.; Logsdon, A.F.; Turner, R.C.; Huber, J.D.; Rosen, C.L. Endoplasmic Reticulum Stress Modulation as a Target for Ameliorating Effects of Blast Induced Traumatic Brain Injury. J. Neurotrauma 2017, 34 (Suppl. 1), S62–S70. [Google Scholar] [CrossRef]
- Mammadova, N.; Ghaisas, S.; Zenitsky, G.; Sakaguchi, D.S.; Kanthasamy, A.G.; Greenlee, J.J.; West Greenlee, M.H. Lasting Retinal Injury in a Mouse Model of Blast-Induced Trauma. Am. J. Pathol. 2017, 187, 1459–1472. [Google Scholar] [CrossRef]
- Shi, Q.X.; Chen, B.; Nie, C.; Zhao, Z.P.; Zhang, J.H.; Si, S.Y.; Cui, S.J.; Gu, J.W. A novel model of blast induced traumatic brain injury caused by compressed gas produced sustained cognitive deficits in rats: Involvement of phosphorylation of tau at the Thr205 epitope. Brain Res. Bull. 2020, 157, 149–161. [Google Scholar] [CrossRef]
- Bugay, V.; Bozdemir, E.; Vigil, F.A.; Chun, S.H.; Holstein, D.M.; Elliott, W.R.; Sprague, C.J.; Cavazos, J.E.; Zamora, D.O.; Rule, G.; et al. A Mouse Model of Repetitive Blast Traumatic Brain Injury Reveals Post-Trauma Seizures and Increased Neuronal Excitability. J. Neurotrauma 2020, 37, 248–261. [Google Scholar] [CrossRef]
- Murphy, E.K.; Iacono, D.; Pan, H.; Grimes, J.B.; Parks, S.; Raiciulescu, S.; Leonessa, F.; Perl, D.P. Explosive-driven double-blast exposure: Molecular, histopathological, and behavioral consequences. Sci. Rep. 2020, 10, 17446. [Google Scholar] [CrossRef] [PubMed]
- Kofuji, P.; Araque, A. Astrocytes and Behavior. Annu. Rev. Neurosci. 2021, 44, 49–67. [Google Scholar] [CrossRef]
- Biesecker, K.R.; Srienc, A.I.; Shimoda, A.M.; Agarwal, A.; Bergles, D.E.; Kofuji, P.; Newman, E.A. Glial Cell Calcium Signaling Mediates Capillary Regulation of Blood Flow in the Retina. J. Neurosci. 2016, 36, 9435–9445. [Google Scholar] [CrossRef]
- Mishra, A.; Reynolds, J.P.; Chen, Y.; Gourine, A.V.; Rusakov, D.A.; Attwell, D. Astrocytes mediate neurovascular signaling to capillary pericytes but not to arterioles. Nat. Neurosci. 2016, 19, 1619–1627. [Google Scholar] [CrossRef]
- Kisler, K.; Nelson, A.R.; Rege, S.V.; Ramanathan, A.; Wang, Y.; Ahuja, A.; Lazic, D.; Tsai, P.S.; Zhao, Z.; Zhou, Y.; et al. Pericyte degeneration leads to neurovascular uncoupling and limits oxygen supply to brain. Nat. Neurosci. 2017, 20, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Davis, G.E.; Senger, D.R. Endothelial extracellular matrix: Biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ. Res. 2005, 97, 1093–1107. [Google Scholar] [CrossRef]
- Kruegel, J.; Miosge, N. Basement membrane components are key players in specialized extracellular matrices. Cell Mol. Life Sci. 2010, 67, 2879–2895. [Google Scholar] [CrossRef] [PubMed]
- Franciosi, S.; De Gasperi, R.; Dickstein, D.L.; English, D.F.; Rocher, A.B.; Janssen, W.G.; Christoffel, D.; Sosa, M.A.; Hof, P.R.; Buxbaum, J.D.; et al. Pepsin pretreatment allows collagen IV immunostaining of blood vessels in adult mouse brain. J. Neurosci. Methods 2007, 163, 76–82. [Google Scholar] [CrossRef]
- Gama Sosa, M.A.; Gasperi, R.D.; Rocher, A.B.; Wang, A.C.; Janssen, W.G.; Flores, T.; Perez, G.M.; Schmeidler, J.; Dickstein, D.L.; Hof, P.R.; et al. Age-related vascular pathology in transgenic mice expressing presenilin 1-associated familial Alzheimer’s disease mutations. Am. J. Pathol. 2010, 176, 353–368. [Google Scholar] [CrossRef]
- Prima, V.; Serebruany, V.L.; Svetlov, A.; Hayes, R.L.; Svetlov, S.I. Impact of moderate blast exposures on thrombin biomarkers assessed by calibrated automated thrombography in rats. J. Neurotrauma 2013, 30, 1881–1887. [Google Scholar] [CrossRef]
- Inui, T.; Hoffer, M.; Balaban, C.D. Mild blast wave exposure produces intensity-dependent changes in MMP2 expression patches in rat brains—Findings from different blast severities. Brain Res. 2021, 1767, 147541. [Google Scholar] [CrossRef] [PubMed]
- Scrimgeour, A.G.; Carrigan, C.T.; Condlin, M.L.; Urso, M.L.; van den Berg, R.M.; van Helden, H.P.M.; Montain, S.J.; Joosen, M.J.A. Dietary Zinc Modulates Matrix Metalloproteinases in Traumatic Brain Injury. J. Neurotrauma 2018, 35, 2495–2506. [Google Scholar] [CrossRef]
- Szabo, A.; Kalman, M. Disappearance of the post-lesional laminin immunopositivity of brain vessels is parallel with the formation of gliovascular junctions and common basal lamina. A double-labelling immunohistochemical study. Neuropathol. Appl. Neurobiol. 2004, 30, 169–177. [Google Scholar] [CrossRef]
- Caley, D.W.; Maxwell, D.S. Development of the blood vessels and extracellular spaces during postnatal maturation of rat cerebral cortex. J. Comp. Neurol. 1970, 138, 31–47. [Google Scholar] [CrossRef]
- Abdul-Muneer, P.M.; Schuetz, H.; Wang, F.; Skotak, M.; Jones, J.; Gorantla, S.; Zimmerman, M.C.; Chandra, N.; Haorah, J. Induction of oxidative and nitrosative damage leads to cerebrovascular inflammation in an animal model of mild traumatic brain injury induced by primary blast. Free Radic. Biol. Med. 2013, 60, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Lucke-Wold, B.P.; Logsdon, A.F.; Smith, K.E.; Turner, R.C.; Alkon, D.L.; Tan, Z.; Naser, Z.J.; Knotts, C.M.; Huber, J.D.; Rosen, C.L. Bryostatin-1 Restores Blood Brain Barrier Integrity following Blast-Induced Traumatic Brain Injury. Mol. Neurobiol. 2015, 52, 1119–1134. [Google Scholar] [CrossRef]
- Willis, C.L.; Leach, L.; Clarke, G.J.; Nolan, C.C.; Ray, D.E. Reversible disruption of tight junction complexes in the rat blood-brain barrier, following transitory focal astrocyte loss. Glia 2004, 48, 1–13. [Google Scholar] [CrossRef]
- Hue, C.D.; Cao, S.; Bass, C.R.; Meaney, D.F.; Morrison, B., 3rd. Repeated Primary Blast Injury Causes Delayed Recovery, but not Additive Disruption, in an In Vitro Blood-Brain Barrier Model. J. Neurotrauma 2014, 31, 951–960. [Google Scholar] [CrossRef]
- Heyburn, L.; Abutarboush, R.; Goodrich, S.; Urioste, R.; Batuure, A.; Statz, J.; Wilder, D.; Ahlers, S.T.; Long, J.B.; Sajja, V. Repeated Low-Level Blast Overpressure Leads to Endovascular Disruption and Alterations in TDP-43 and Piezo2 in a Rat Model of Blast TBI. Front. Neurol. 2019, 10, 766. [Google Scholar] [CrossRef]
- De Luca, C.; Papa, M. Looking Inside the Matrix: Perineuronal Nets in Plasticity, Maladaptive Plasticity and Neurological Disorders. Neurochem. Res. 2016, 41, 1507–1515. [Google Scholar] [CrossRef]
- John, U.; Patro, N.; Patro, I. Perineuronal nets: Cruise from a honeycomb to the safety nets. Brain Res. Bull. 2022, 190, 179–194. [Google Scholar] [CrossRef]
- Bruckner, G.; Grosche, J.; Hartlage-Rubsamen, M.; Schmidt, S.; Schachner, M. Region and lamina-specific distribution of extracellular matrix proteoglycans, hyaluronan and tenascin-R in the mouse hippocampal formation. J. Chem. Neuroanat. 2003, 26, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Carstens, K.E.; Phillips, M.L.; Pozzo-Miller, L.; Weinberg, R.J.; Dudek, S.M. Perineuronal Nets Suppress Plasticity of Excitatory Synapses on CA2 Pyramidal Neurons. J. Neurosci. 2016, 36, 6312–6320. [Google Scholar] [CrossRef] [PubMed]
- Pizzorusso, T.; Medini, P.; Berardi, N.; Chierzi, S.; Fawcett, J.W.; Maffei, L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science 2002, 298, 1248–1251. [Google Scholar] [CrossRef]
- Callaghan, B.L.; Graham, B.M.; Li, S.; Richardson, R. From resilience to vulnerability: Mechanistic insights into the effects of stress on transitions in critical period plasticity. Front. Psychiatry 2013, 4, 90. [Google Scholar] [CrossRef]
- Gogolla, N.; Caroni, P.; Luthi, A.; Herry, C. Perineuronal nets protect fear memories from erasure. Science 2009, 325, 1258–1261. [Google Scholar] [CrossRef]
- Quirk, G.J.; Pare, D.; Richardson, R.; Herry, C.; Monfils, M.H.; Schiller, D.; Vicentic, A. Erasing fear memories with extinction training. J. Neurosci. 2010, 30, 14993–14997. [Google Scholar] [CrossRef] [PubMed]
- Hylin, M.J.; Orsi, S.A.; Moore, A.N.; Dash, P.K. Disruption of the perineuronal net in the hippocampus or medial prefrontal cortex impairs fear conditioning. Learn. Mem. 2013, 20, 267–273. [Google Scholar] [CrossRef]
- Kochlamazashvili, G.; Henneberger, C.; Bukalo, O.; Dvoretskova, E.; Senkov, O.; Lievens, P.M.; Westenbroek, R.; Engel, A.K.; Catterall, W.A.; Rusakov, D.A.; et al. The extracellular matrix molecule hyaluronic acid regulates hippocampal synaptic plasticity by modulating postsynaptic L-type Ca2+ channels. Neuron 2010, 67, 116–128. [Google Scholar] [CrossRef]
- Li, X.; Ren, D.; Luo, B.; Liu, Z.; Li, N.; Zhou, T.; Fei, E. Perineuronal Nets Alterations Contribute to Stress-Induced Anxiety-Like Behavior. Mol. Neurobiol. 2023, 1–12. [Google Scholar] [CrossRef]
- Prinz, M.; Masuda, T.; Wheeler, M.A.; Quintana, F.J. Microglia and Central Nervous System-Associated Macrophages-From Origin to Disease Modulation. Annu. Rev. Immunol. 2021, 39, 251–277. [Google Scholar] [CrossRef]
- Bisht, K.; Sharma, K.P.; Lecours, C.; Sanchez, M.G.; El Hajj, H.; Milior, G.; Olmos-Alonso, A.; Gomez-Nicola, D.; Luheshi, G.; Vallieres, L.; et al. Dark microglia: A new phenotype predominantly associated with pathological states. Glia 2016, 64, 826–839. [Google Scholar] [CrossRef]
- Halder, S.K.; Milner, R. A critical role for microglia in maintaining vascular integrity in the hypoxic spinal cord. Proc. Natl. Acad. Sci. USA 2019, 116, 26029–26037. [Google Scholar] [CrossRef]
- Kettenmann, H.; Hanisch, U.K.; Noda, M.; Verkhratsky, A. Physiology of microglia. Physiol. Rev. 2011, 91, 461–553. [Google Scholar] [CrossRef]
- Kreutzberg, G.W. Microglia: A sensor for pathological events in the CNS. Trends Neurosci. 1996, 19, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.G.; Mrak, R.E.; Griffin, W.S. Neuritic plaque evolution in Alzheimer’s disease is accompanied by transition of activated microglia from primed to enlarged to phagocytic forms. Acta Neuropathol. 1997, 94, 1–5. [Google Scholar] [CrossRef]
- Soltys, Z.; Ziaja, M.; Pawlinski, R.; Setkowicz, Z.; Janeczko, K. Morphology of reactive microglia in the injured cerebral cortex. Fractal analysis and complementary quantitative methods. J. Neurosci. Res. 2001, 63, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Stence, N.; Waite, M.; Dailey, M.E. Dynamics of microglial activation: A confocal time-lapse analysis in hippocampal slices. Glia 2001, 33, 256–266. [Google Scholar] [CrossRef]
- Torres-Platas, S.G.; Comeau, S.; Rachalski, A.; Bo, G.D.; Cruceanu, C.; Turecki, G.; Giros, B.; Mechawar, N. Morphometric characterization of microglial phenotypes in human cerebral cortex. J. Neuroinflammation 2014, 11, 12. [Google Scholar] [CrossRef]
- Varnum, M.M.; Ikezu, T. The classification of microglial activation phenotypes on neurodegeneration and regeneration in Alzheimer’s disease brain. Arch Immunol Ther Exp (Warsz) 2012, 60, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.S.; Tang, Y.; Illes, P.; Verkhratsky, A. The Safeguarding Microglia: Central Role for P2Y(12) Receptors. Front. Pharmacol. 2020, 11, 627760. [Google Scholar] [CrossRef]
- Crotti, A.; Ransohoff, R.M. Microglial Physiology and Pathophysiology: Insights from Genome-wide Transcriptional Profiling. Immunity 2016, 44, 505–515. [Google Scholar] [CrossRef]
- Readnower, R.D.; Chavko, M.; Adeeb, S.; Conroy, M.D.; Pauly, J.R.; McCarron, R.M.; Sullivan, P.G. Increase in blood-brain barrier permeability, oxidative stress, and activated microglia in a rat model of blast-induced traumatic brain injury. J. Neurosci. Res. 2010, 88, 3530–3539. [Google Scholar] [CrossRef]
- Turner, R.C.; Naser, Z.J.; Logsdon, A.F.; DiPasquale, K.H.; Jackson, G.J.; Robson, M.J.; Gettens, R.T.; Matsumoto, R.R.; Huber, J.D.; Rosen, C.L. Modeling clinically relevant blast parameters based on scaling principles produces functional & histological deficits in rats. Exp. Neurol. 2013, 248, 520–529. [Google Scholar]
- Cho, H.J.; Sajja, V.S.; Vandevord, P.J.; Lee, Y.W. Blast induces oxidative stress, inflammation, neuronal loss and subsequent short-term memory impairment in rats. Neuroscience 2013, 253, 9–20. [Google Scholar] [CrossRef]
- Sajja, V.S.; Ereifej, E.S.; VandeVord, P.J. Hippocampal vulnerability and subacute response following varied blast magnitudes. Neurosci. Lett. 2014, 570, 33–37. [Google Scholar] [CrossRef]
- Kamnaksh, A.; Ahmed, F.; Kovedsi, E.; Barry, E.; Grunberg, N.; Long, J.; Agoston, D. Molecular mechanisms of increased cerebral vulnerability after repeated mild blast-induced traumatic brain injury. Translational Proteomics 2014, 3, 22–37. [Google Scholar] [CrossRef]
- Valiyaveettil, M.; Alamneh, Y.A.; Miller, S.A.; Hammamieh, R.; Arun, P.; Wang, Y.; Wei, Y.; Oguntayo, S.; Long, J.B.; Nambiar, M.P. Modulation of cholinergic pathways and inflammatory mediators in blast-induced traumatic brain injury. Chem. Biol. Interact. 2013, 203, 371–375. [Google Scholar] [CrossRef]
- Stone, J.R.; Avants, B.B.; Tustison, N.; Gill, J.; Wilde, E.A.; Neumann, K.D.; Gladney, L.A.; Kilgore, M.O.; Bowling, F.; Wilson, C.M.; et al. Neurological effects of repeated blast exposure in Special Operations personnel. J. Neurotrauma 2024. [Google Scholar] [CrossRef]
- Dalle Lucca, J.J.; Chavko, M.; Dubick, M.A.; Adeeb, S.; Falabella, M.J.; Slack, J.L.; McCarron, R.; Li, Y. Blast-induced moderate neurotrauma (BINT) elicits early complement activation and tumor necrosis factor alpha (TNFalpha) release in a rat brain. J. Neurol. Sci. 2012, 318, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chavko, M.; Slack, J.L.; Liu, B.; McCarron, R.M.; Ross, J.D.; Dalle Lucca, J.J. Protective effects of decay-accelerating factor on blast-induced neurotrauma in rats. Acta Neuropathol. Commun. 2013, 1, 52. [Google Scholar] [CrossRef]
- Hamacher, J.; Hadizamani, Y.; Huwer, H.; Moehrlen, U.; Bally, L.; Stammberger, U.; Wendel, A.; Lucas, R. Characteristics of inflammatory response and repair after experimental blast lung injury in rats. PLoS ONE 2023, 18, e0281446. [Google Scholar] [CrossRef]
- Meng, X.Y.; Lu, Q.Y.; Zhang, J.F.; Li, J.F.; Shi, M.Y.; Huang, S.Y.; Yu, S.F.; Zhao, Y.M.; Fan, H.J. A novel animal model of primary blast lung injury and its pathological changes in mice. J. Trauma. Acute Care Surg. 2022, 93, 530–537. [Google Scholar] [CrossRef]
- Pierce, M.E.; Hayes, J.; Huber, B.R.; Jeromin, A.; Fortier, C.B.; Fonda, J.R.; Lasseter, H.; Chaby, L.; McGlinchey, R.; Milberg, W. Plasma biomarkers associated with deployment trauma and its consequences in post-9/11 era veterans: Initial findings from the TRACTS longitudinal cohort. Transl. Psychiatry 2022, 12, 80. [Google Scholar] [CrossRef]
- Takahashi, S.; Fukushima, H.; Yu, Z.; Tomita, H.; Kida, S. Tumor necrosis factor alpha negatively regulates the retrieval and reconsolidation of hippocampus-dependent memory. Brain Behav. Immun. 2021, 94, 79–88. [Google Scholar] [CrossRef]
- Parekh, S.V.; Paniccia, J.E.; Adams, L.O.; Lysle, D.T. Hippocampal TNF-alpha Signaling Mediates Heroin Withdrawal-Enhanced Fear Learning and Withdrawal-Induced Weight Loss. Mol. Neurobiol. 2021, 58, 2963–2973. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, Z.; Shen, F.; Xie, P.; Wang, J.; Zhu, A.S.; Zhu, G. Ginsenoside Rg1 Prevents PTSD-Like Behaviors in Mice Through Promoting Synaptic Proteins, Reducing Kir4.1 and TNF-alpha in the Hippocampus. Mol. Neurobiol. 2021, 58, 1550–1563. [Google Scholar] [CrossRef]
- Yu, Z.; Fukushima, H.; Ono, C.; Sakai, M.; Kasahara, Y.; Kikuchi, Y.; Gunawansa, N.; Takahashi, Y.; Matsuoka, H.; Kida, S.; et al. Microglial production of TNF-alpha is a key element of sustained fear memory. Brain Behav. Immun. 2017, 59, 313–321. [Google Scholar] [CrossRef]
- Zupan, B.; Liu, B.; Taki, F.; Toth, J.G.; Toth, M. Maternal Brain TNF-alpha Programs Innate Fear in the Offspring. Curr. Biol. 2017, 27, 3859–3863.e3. [Google Scholar] [CrossRef]
- Vorn, R.; Edwards, K.A.; Hentig, J.; Yun, S.; Kim, H.S.; Lai, C.; Devoto, C.; Yarnell, A.M.; Polejaeva, E.; Dell, K.C.; et al. A Pilot Study of Whole-Blood Transcriptomic Analysis to Identify Genes Associated with Repetitive Low-Level Blast Exposure in Career Breachers. Biomedicines 2022, 10, 690. [Google Scholar] [CrossRef]
- Edwards, K.A.; Leete, J.J.; Smith, E.G.; Quick, A.; Modica, C.M.; Wassermann, E.M.; Polejaeva, E.; Dell, K.C.; LoPresti, M.; Walker, P.; et al. Elevations in Tumor Necrosis Factor Alpha and Interleukin 6 From Neuronal-Derived Extracellular Vesicles in Repeated Low-Level Blast Exposed Personnel. Front. Neurol. 2022, 13, 723923. [Google Scholar] [CrossRef]
- Muth, K.N.; Rech, J.; Losch, F.O.; Hoerning, A. Reversing the Inflammatory Process-25 Years of Tumor Necrosis Factor-alpha Inhibitors. J. Clin. Med. 2023, 12, 5039. [Google Scholar] [CrossRef]
- Souza, R.F.; Caetano, M.A.F.; Magalhaes, H.I.R.; Castelucci, P. Study of tumor necrosis factor receptor in the inflammatory bowel disease. World J. Gastroenterol. 2023, 29, 2733–2746. [Google Scholar] [CrossRef]
- Tuttolomondo, A.; Pecoraro, R.; Pinto, A. Studies of selective TNF inhibitors in the treatment of brain injury from stroke and trauma: A review of the evidence to date. Drug Des. Devel Ther. 2014, 8, 2221–2238. [Google Scholar] [CrossRef]
- Gonzalez Caldito, N. Role of tumor necrosis factor-alpha in the central nervous system: A focus on autoimmune disorders. Front. Immunol. 2023, 14, 1213448. [Google Scholar] [CrossRef]
- Muller, N. Immunology of major depression. Neuroimmunomodulation 2014, 21, 123–130. [Google Scholar] [CrossRef]
- Wieck, A.; Grassi-Oliveira, R.; Hartmann do Prado, C.; Teixeira, A.L.; Bauer, M.E. Neuroimmunoendocrine interactions in post-traumatic stress disorder: Focus on long-term implications of childhood maltreatment. Neuroimmunomodulation 2014, 21, 145–151. [Google Scholar] [CrossRef]
- Lee, D.H.; Lee, J.Y.; Hong, D.Y.; Lee, E.C.; Park, S.W.; Lee, M.R.; Oh, J.S. Neuroinflammation in Post-Traumatic Stress Disorder. Biomedicines 2022, 10, 953. [Google Scholar] [CrossRef]
- Sun, Y.; Qu, Y.; Zhu, J. The Relationship Between Inflammation and Post-traumatic Stress Disorder. Front. Psychiatry 2021, 12, 707543. [Google Scholar] [CrossRef]
- Hassamal, S. Chronic stress, neuroinflammation, and depression: An overview of pathophysiological mechanisms and emerging anti-inflammatories. Front. Psychiatry 2023, 14, 1130989. [Google Scholar] [CrossRef]
- Lee, C.H.; Giuliani, F. The Role of Inflammation in Depression and Fatigue. Front. Immunol. 2019, 10, 1696. [Google Scholar] [CrossRef] [PubMed]
- Haroon, E.; Miller, A.H.; Sanacora, G. Inflammation, Glutamate, and Glia: A Trio of Trouble in Mood Disorders. Neuropsychopharmacology 2017, 42, 193–215. [Google Scholar] [CrossRef]
- Chen, S.; Siedhoff, H.R.; Zhang, H.; Liu, P.; Balderrama, A.; Li, R.; Johnson, C.; Greenlief, C.M.; Koopmans, B.; Hoffman, T.; et al. Low-intensity blast induces acute glutamatergic hyperexcitability in mouse hippocampus leading to long-term learning deficits and altered expression of proteins involved in synaptic plasticity and serine protease inhibitors. Neurobiol. Dis. 2022, 165, 105634. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sawyer, T.W.; Tse, Y.C.; Fan, C.; Hennes, G.; Barnes, J.; Josey, T.; Weiss, T.; Nelson, P.; Wong, T.P. Primary Blast-Induced Changes in Akt and GSK3beta Phosphorylation in Rat Hippocampus. Front. Neurol. 2017, 8, 413. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.; Piehler, T.; Benjamin, R.; Farizatto, K.L.; Pait, M.C.; Almeida, M.F.; Ghukasyan, V.V.; Bahr, B.A. Blast waves from detonated military explosive reduce GluR1 and synaptophysin levels in hippocampal slice cultures. Exp. Neurol. 2016, 286, 107–115. [Google Scholar] [CrossRef]
- De Gasperi, R.; Gama Sosa, M.; Perez Garcia, G.; Perez, G.; Pryor, D.; Morrison, C.L.-A.; Lind, R.; Abutarboush, R.; Kawoos, U.; Statz, J.; et al. Metabotropic glutamate receptor 2 expression is chronically elevated in male rats with post-traumatic stress disorder related behavioral traits following repetitive low-level blast exposure. J. Neurotrauma 2023. Published online. [Google Scholar] [CrossRef]
- Highland, J.N.; Zanos, P.; Riggs, L.M.; Georgiou, P.; Clark, S.M.; Morris, P.J.; Moaddel, R.; Thomas, C.J.; Zarate, C.A., Jr.; Pereira, E.F.R.; et al. Hydroxynorketamines: Pharmacology and Potential Therapeutic Applications. Pharmacol. Rev. 2021, 73, 763–791. [Google Scholar] [CrossRef] [PubMed]
- Zanos, P.; Highland, J.N.; Stewart, B.W.; Georgiou, P.; Jenne, C.E.; Lovett, J.; Morris, P.J.; Thomas, C.J.; Moaddel, R.; Zarate, C.A., Jr.; et al. (2R,6R)-hydroxynorketamine exerts mGlu2 receptor-dependent antidepressant actions. Proc. Natl. Acad. Sci. USA 2019, 116, 6441–6450. [Google Scholar] [CrossRef]
- Batiuk, M.Y.; Martirosyan, A.; Wahis, J.; de Vin, F.; Marneffe, C.; Kusserow, C.; Koeppen, J.; Viana, J.F.; Oliveira, J.F.; Voet, T.; et al. Identification of region-specific astrocyte subtypes at single cell resolution. Nat. Commun. 2020, 11, 1220. [Google Scholar] [CrossRef]
- Sun, N.; Akay, L.A.; Murdock, M.H.; Park, Y.; Galiana-Melendez, F.; Bubnys, A.; Galani, K.; Mathys, H.; Jiang, X.; Ng, A.P.; et al. Single-nucleus multiregion transcriptomic analysis of brain vasculature in Alzheimer’s disease. Nat. Neurosci. 2023, 26, 970–982. [Google Scholar] [CrossRef]

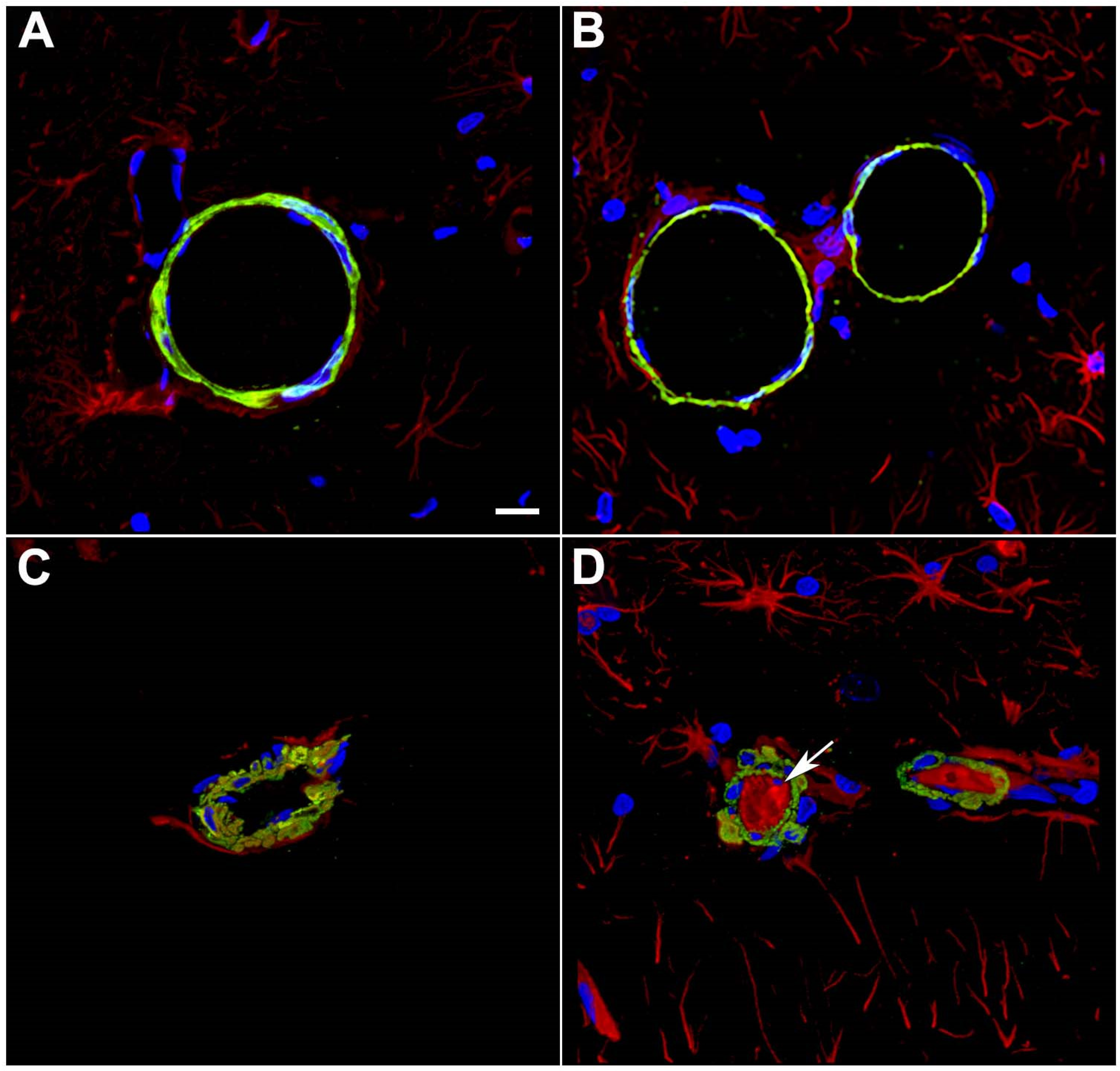
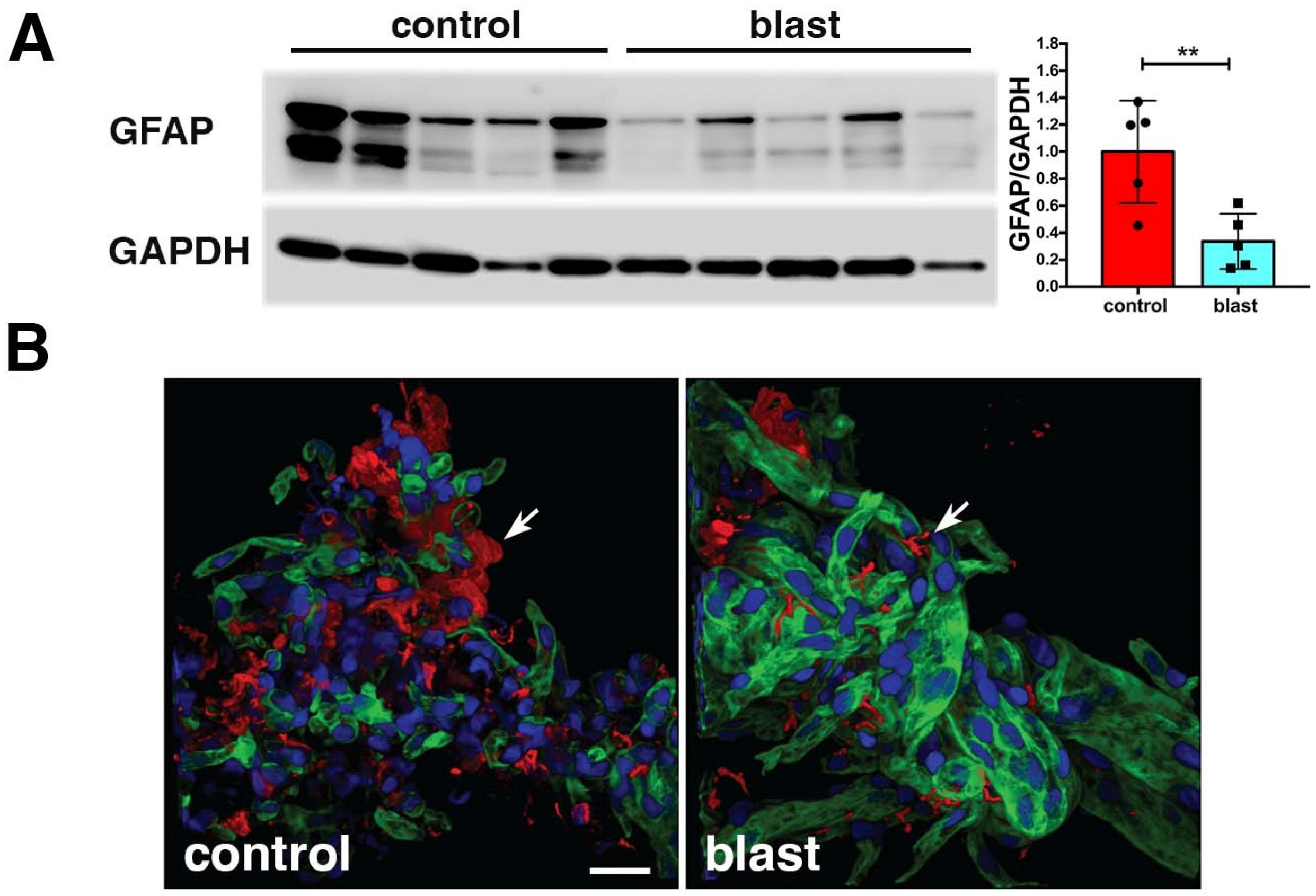

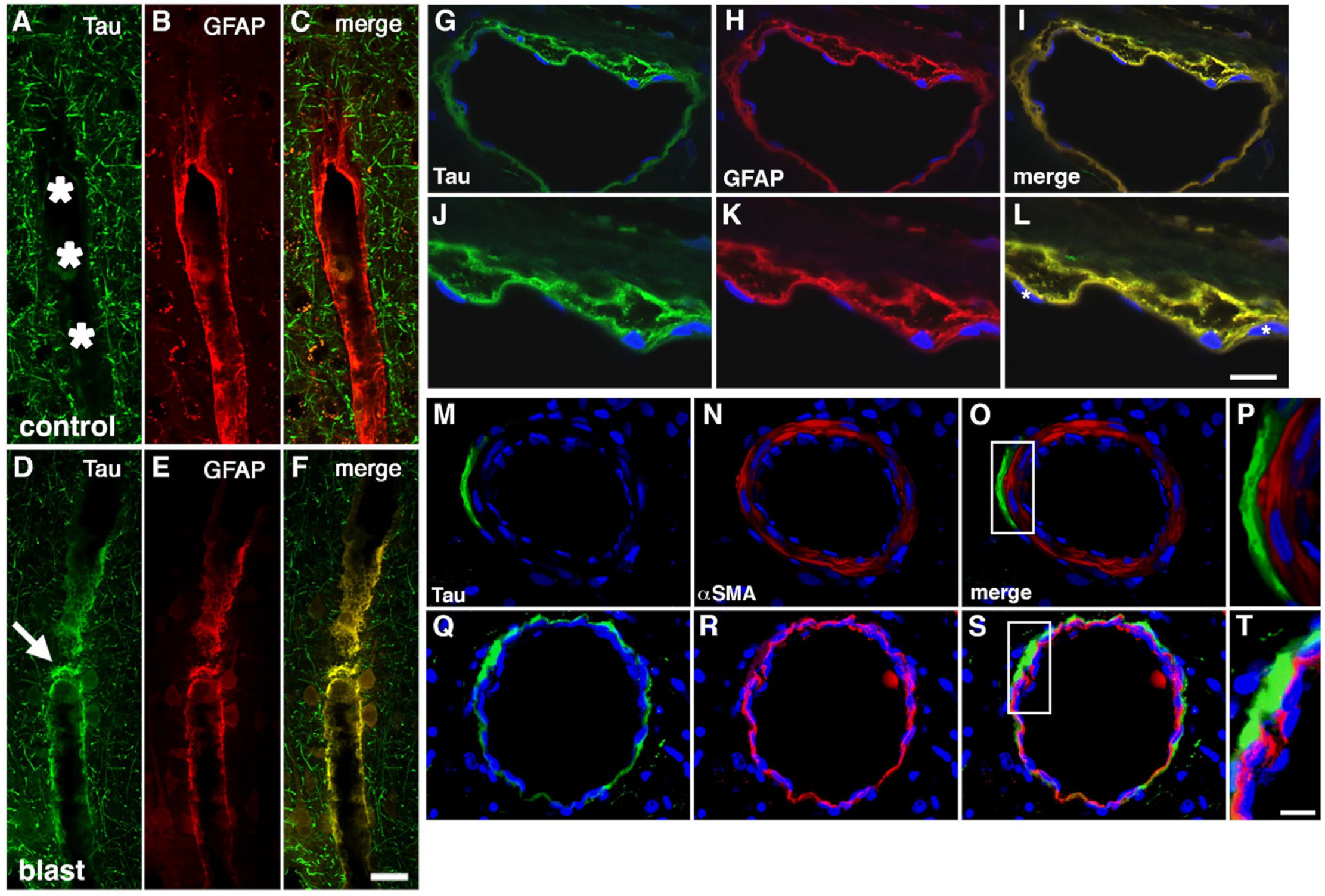
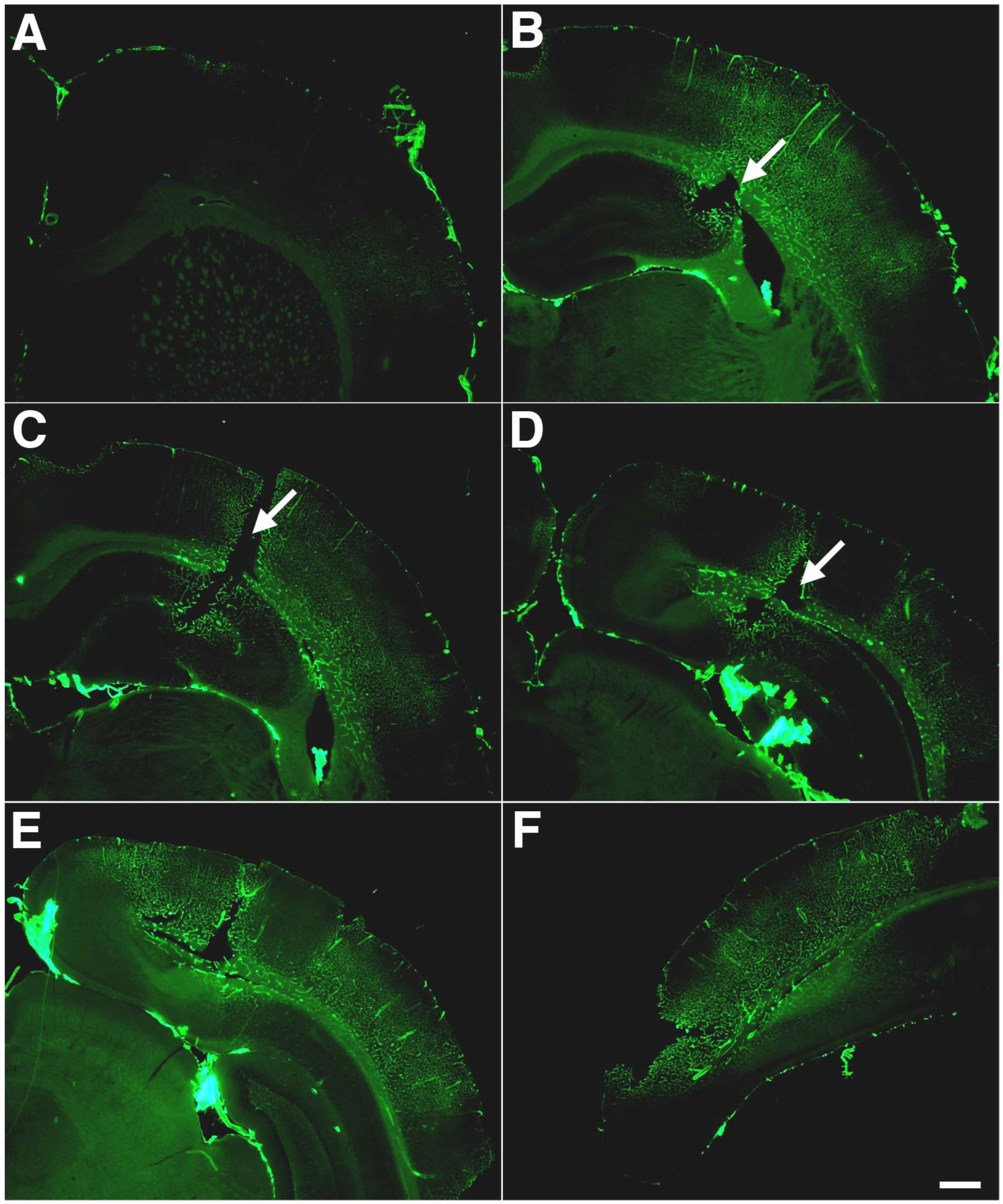
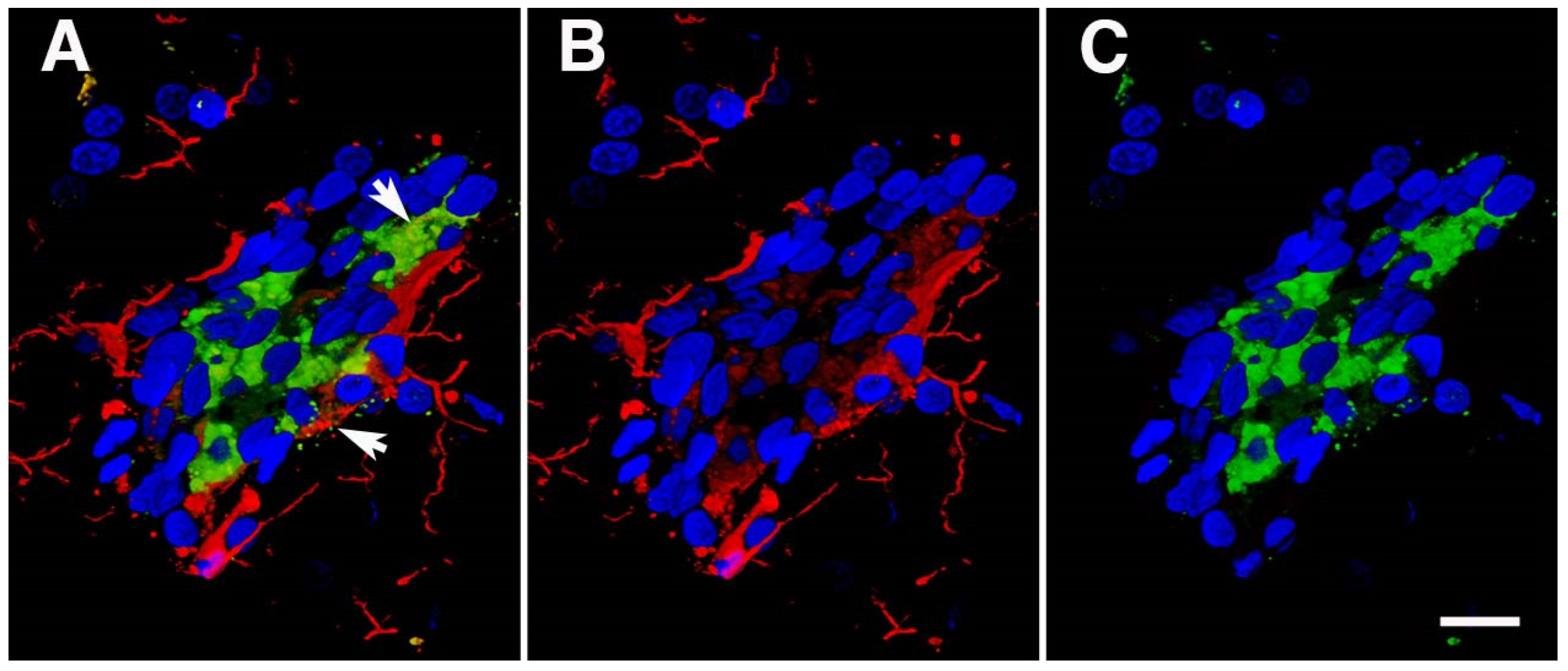

| Species | Blast Exposure | Body Shiel-Ding | Head Restraint | Vascular Pathology | Non-Vascular Pathology | References |
|---|---|---|---|---|---|---|
| Mouse | Live explosives; 17.2 to 37.9 kPa | None | None | BBB permeability increase at 30 days but not at 7 days in 37.9 kPa-exposed animals based on T1 MRI contrast enhancement. | Increased fractional anisotropy on diffusion tensor imaging in thalamus and hypothalamus. | [83] |
| Mouse | Shock tube; 77 kPa | None | Yes and no | Dysmorphic capillaries; swollen, perivascular astrocytic endfeet thickened and often containing amorphous material and damage to capillary basal lamina. | p-tau CTE-like pathology; light and EM hippocampal neuronal pathology; ultrastructural damage to myelinated axons; and acute behavioral change prevented by head restraint. | [82] |
| Rat | Shock tube; single 70 kPa | No | Yes | None described. | At 5 days post injury, neuronal degeneration and increased density of inflammatory cells in frontal and parietal cortex, hippocampus, and thalamus. | [84] |
| Rat | Shock tube; single or repeated (5 × 70 kPa) | None | None | None described. | Microglial activation and reactive astrocytosis with 5×, but not single blast exposure at 35 days after exposure. | [85] |
| C57BL/6 mice | Live explosives; single-exposure 46.6 kPa peak overpressure; subjects prone; front of head exposure | None | None, but report no detectible motion of head or torso | Ultrastructural changes, including alterations in endothelial cells, pericytes, and basement membranes as well as astrocytic endfeet swelling. | Acute axonal damage seen by silver staining; ultrastructural abnormalities in myelin sheaths, mitochondria, and synapses; and proteomic changes in axonal and synaptic proteins. | [86,87,88,89] |
| C57BL/6 mice | Shock tube; single-exposure 25 kPa peak overpressure; subject prone outside of tube; front of head exposure | None | None | Multifocal areas of BBB breakdown as judged by Evans Blue and fluorescein isothiocyanate dextran extravasation 3 h to 7 days after exposure; clusters of reactive astrocytes and microglia at sites of BBB breakdown. | None described. | [90,91] |
| Rat | Shock tube; repetitive exposure (2×); 75.8 kPa; left side of head exposed | Body shielded | Head motion restricted | Tight junction (TJ) proteins (zonula occludens-1 [ZO-1], occludin, and claudin-5), decreased at 6 h and 24 h in males, but not in females; females decreased GFAP at 6 h; males decreased GFAP at 24 h. At 24 h, vascular integrity and astrocytic endfoot coverage of blood vessels decreased in males; by 7 d, no significant differences in TJ or GFAP levels between groups in either sex. | Regional impairments in synaptic mitochondrial respiration but not in mitochondrial respiration in glia. | [92,93] |
| Rat | Shock tube; single or multiple (up to 30) 27.5 to 58.6 kPa exposures; head on or side exposures | None | Yes | 24 h, variable changes in occludin and claudin-5 depending on region and exposure. | Pathology not described. | [94] |
| Component | Observations | Potential Functional Consequences | Relationship to Chronic Behavioral Phenotype |
|---|---|---|---|
| Endothelial cell | Acute endothelial cell damage, at least in part mediated by a thoracic effect if the blast involves a full-body exposure. | Altered BBB properties as a result of direct endothelial cell damage as well as disrupted endothelial cell connections to other mural cells, the vascular basement membrane, and perivascular astrocytes. | Predates |
| Endothelial glycocalyx | Glycocalyx degradation acutely. | Altered BBB regulation; greater leukocyte adhesion to endothelial cells and sensitivity of the endothelial cells to injury; impaired sensing of luminal shear forces. | Predates and appears to be repaired by the time the behavioral phenotype emerges. |
| Vascular smooth muscle | Changes in the microarchitecture of the smooth muscle layer; acute, delayed, and prolonged alterations in cerebrovascular reactivity. | Altered long-term vascular reactivity in the NVU. | Predates |
| Gliovascular connections | Decreased GFAP and lost astrocytic endfeet in isolated vascular fractions; swollen and dysmorphic astrocytic endfeet in vivo. | Altered BBB function, neurovascular coupling, perivascular glutamate clearance and calcium regulation; and immune cell entry. | Predates |
| Neurovascular connections | Decreased neuronal intermediate filaments (NF-L, NF-M, NF-H, and α-internexin) in isolated vascular fractions. | Impaired cerebral autoregulation and activity-dependent neurovascular coupling. | Predates |
| Glymphatics | Decreased brain Aβ 42. | Could imply increased glymphatic transport, although recent studies suggest that increased transendothelial transport out of the brain may also be a factor. | Predates |
| Perivascular tau | Perivascular p-tau in astrocytes. | Early vascular damage; seed for spread of neuronal tau later. | Predates |
| Pericytes | Pericytes and their normal association with other components of the NVU disrupted acutely. | Altered BBB maintenance, regulation of immune cell entry into CNS and neuroinflammatory states; disrupted neurovascular coupling. | Predates |
| Extracellular matrix | Chronic degradation and remodeling of the extracellular matrix. | Altered regulation of BBB permeability; chronic effects on perineuronal nets. | Predates |
| Perivascular inflammation | Activation of TNFα-regulated genes and downregulation of P2ry12 between 6 weeks and 12 months after blast exposure; late perivascular inflammation with presence of activated microglial. | Perivascular neuroinflammatory environment leading to a hyperglutamatergic state early followed by a hypoglutamatergic one late; inflammation mediated neurodegeneration; and cytokine-mediated effects on behavioral function. | May correlate with appearance of the behavioral phenotype. |
| Neuronal mGlu2 | Elevation late; behavioral phenotype reversed by mGluR2/3 antagonists. | Hypoglutamatergic synapse. | Correlates with appearance of the behavioral phenotype. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elder, G.A.; Gama Sosa, M.A.; De Gasperi, R.; Perez Garcia, G.; Perez, G.M.; Abutarboush, R.; Kawoos, U.; Zhu, C.W.; Janssen, W.G.M.; Stone, J.R.; et al. The Neurovascular Unit as a Locus of Injury in Low-Level Blast-Induced Neurotrauma. Int. J. Mol. Sci. 2024, 25, 1150. https://doi.org/10.3390/ijms25021150
Elder GA, Gama Sosa MA, De Gasperi R, Perez Garcia G, Perez GM, Abutarboush R, Kawoos U, Zhu CW, Janssen WGM, Stone JR, et al. The Neurovascular Unit as a Locus of Injury in Low-Level Blast-Induced Neurotrauma. International Journal of Molecular Sciences. 2024; 25(2):1150. https://doi.org/10.3390/ijms25021150
Chicago/Turabian StyleElder, Gregory A., Miguel A. Gama Sosa, Rita De Gasperi, Georgina Perez Garcia, Gissel M. Perez, Rania Abutarboush, Usmah Kawoos, Carolyn W. Zhu, William G. M. Janssen, James R. Stone, and et al. 2024. "The Neurovascular Unit as a Locus of Injury in Low-Level Blast-Induced Neurotrauma" International Journal of Molecular Sciences 25, no. 2: 1150. https://doi.org/10.3390/ijms25021150
APA StyleElder, G. A., Gama Sosa, M. A., De Gasperi, R., Perez Garcia, G., Perez, G. M., Abutarboush, R., Kawoos, U., Zhu, C. W., Janssen, W. G. M., Stone, J. R., Hof, P. R., Cook, D. G., & Ahlers, S. T. (2024). The Neurovascular Unit as a Locus of Injury in Low-Level Blast-Induced Neurotrauma. International Journal of Molecular Sciences, 25(2), 1150. https://doi.org/10.3390/ijms25021150






