Enhanced Sensitivity of A549 Cells to Doxorubicin with WS2 and WSe2 Nanosheets via the Induction of Autophagy
Abstract
1. Introduction
2. Results and Discussion
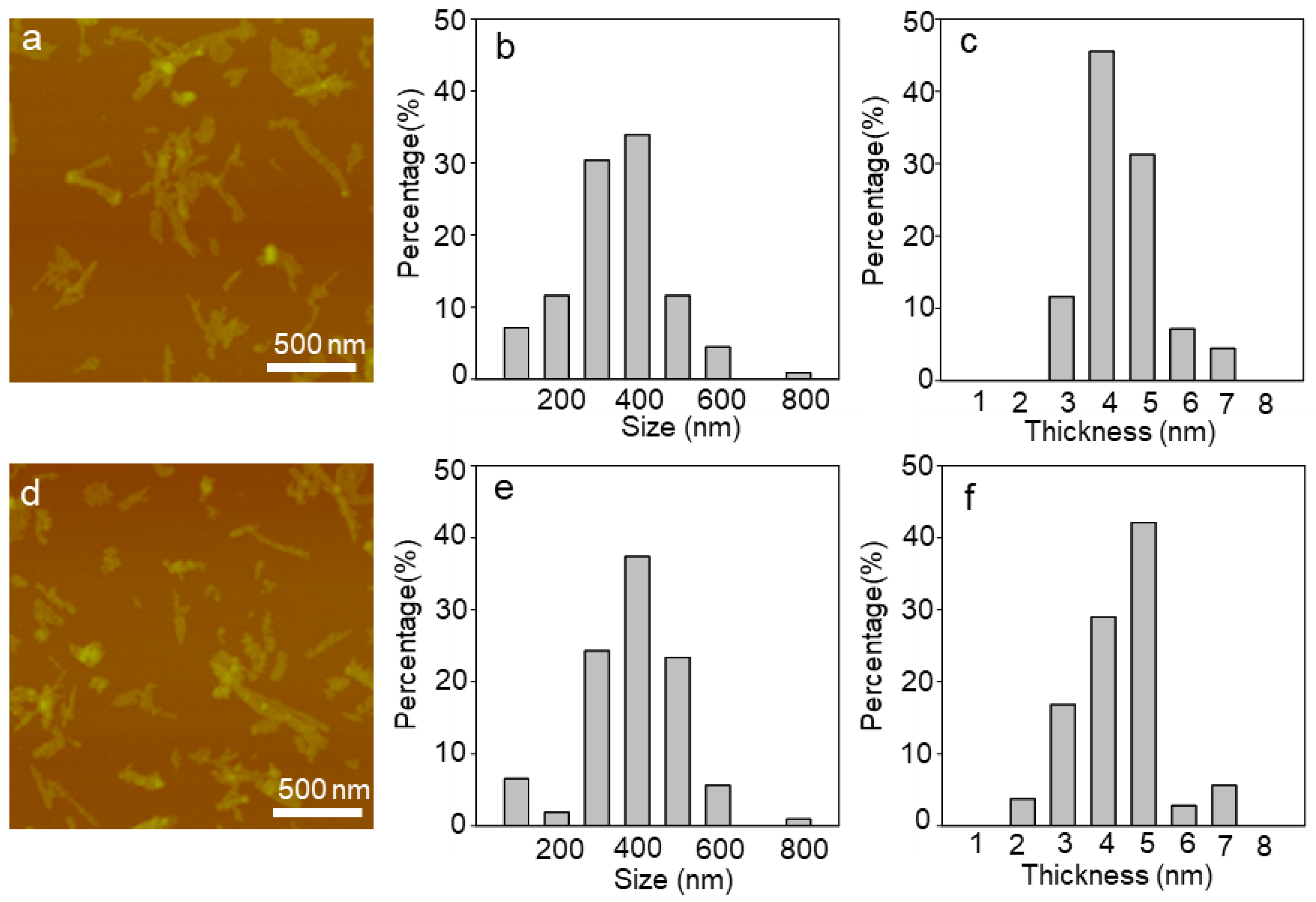
2.1. Characterization of WS2 and WSe2 Nanosheets
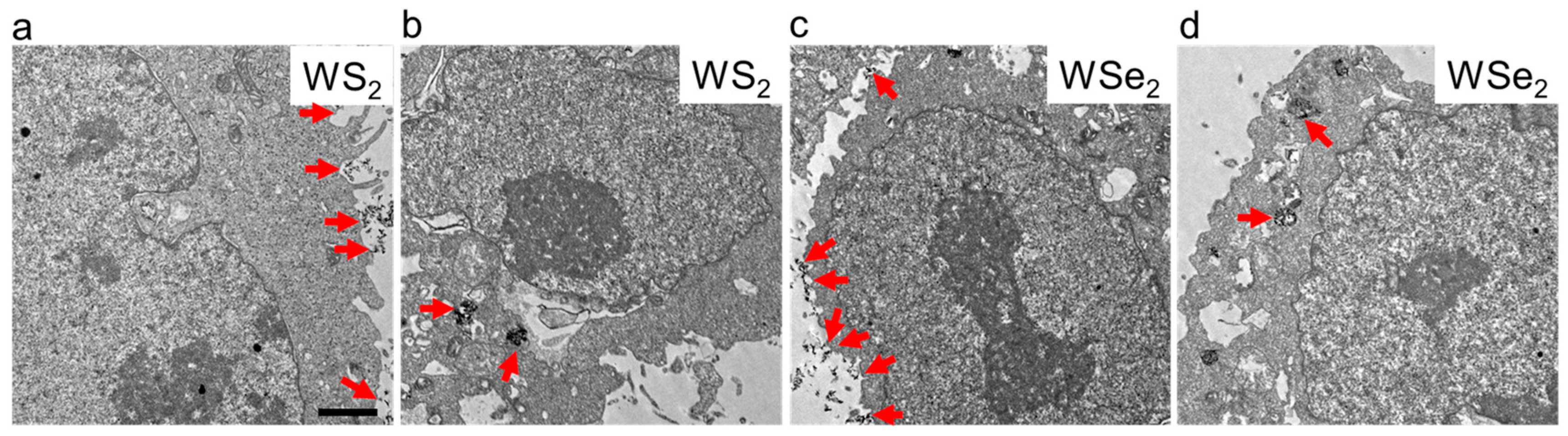
2.2. Cellular Localization of WS2 and WSe2
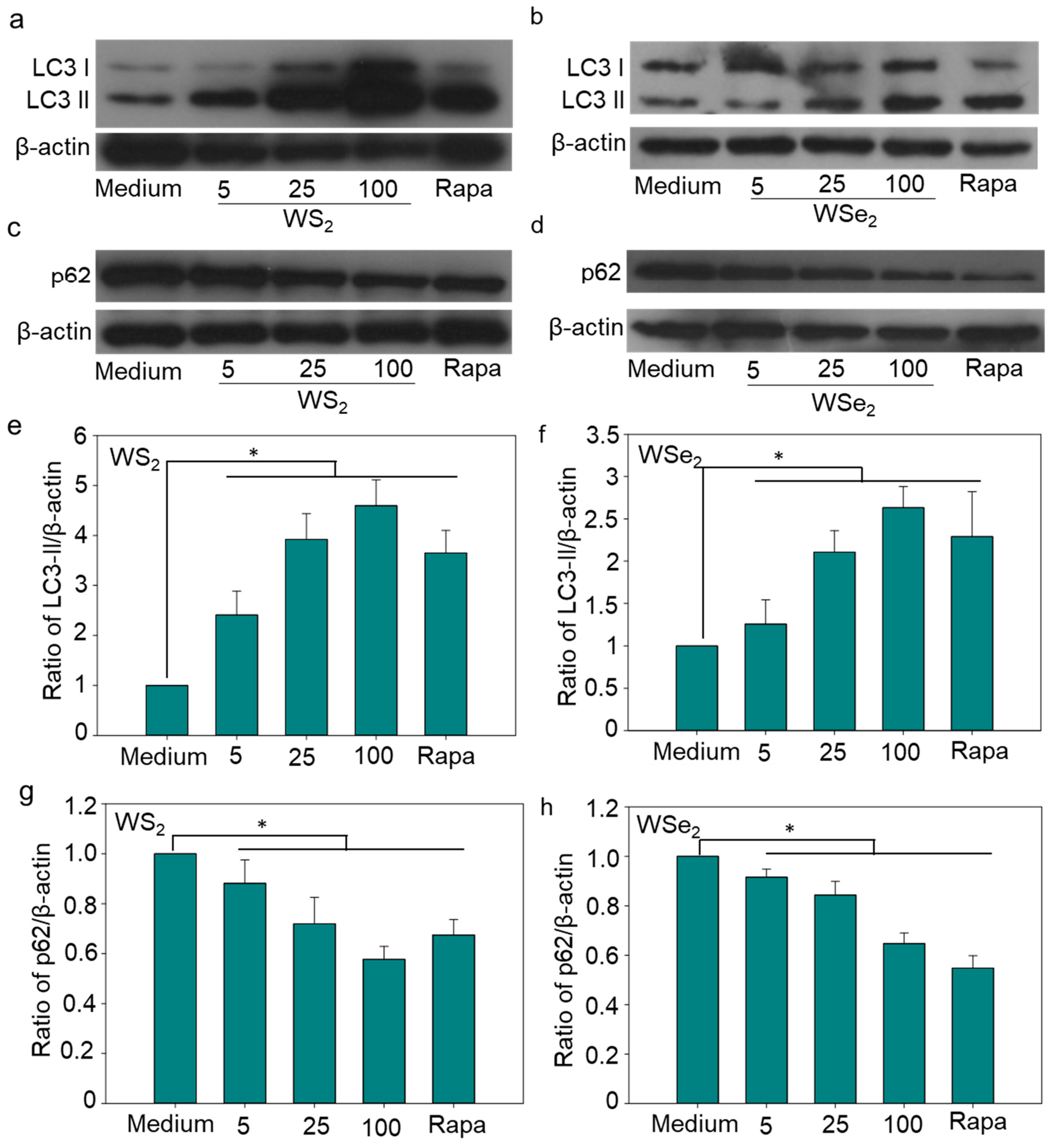
2.3. Autophagy Induction by WS2 and WSe2
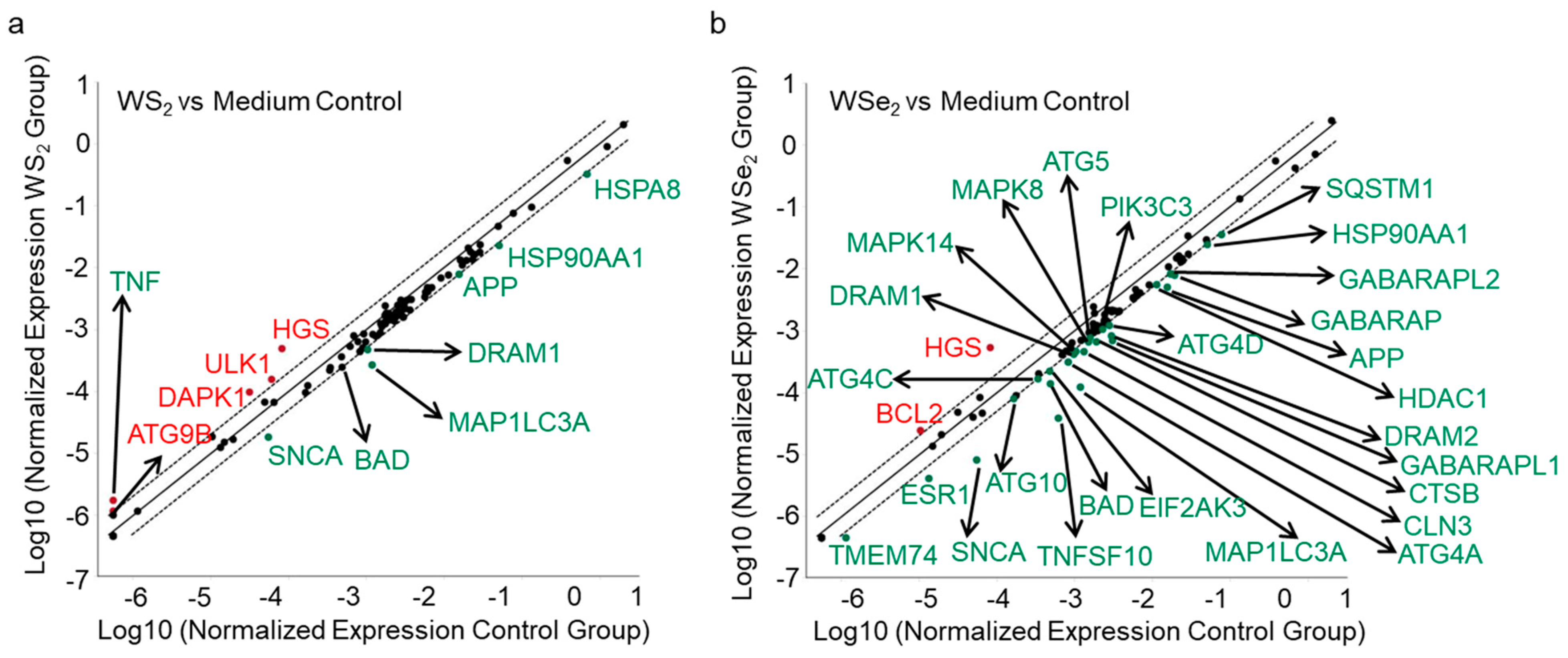
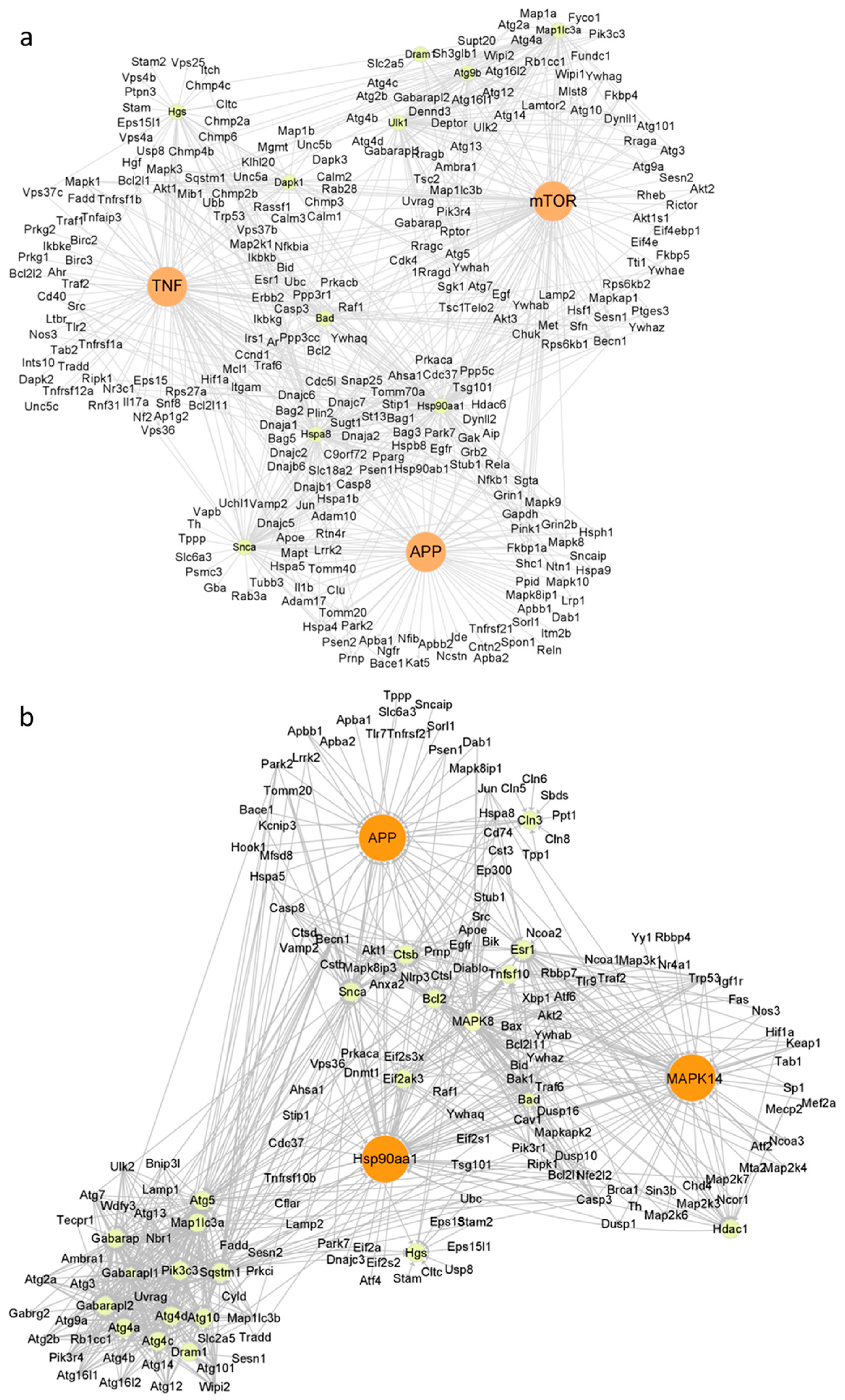
2.4. Autophagy Induction by WS2 or WSe2 through Perturbation of Different Autophagy-Related Genes
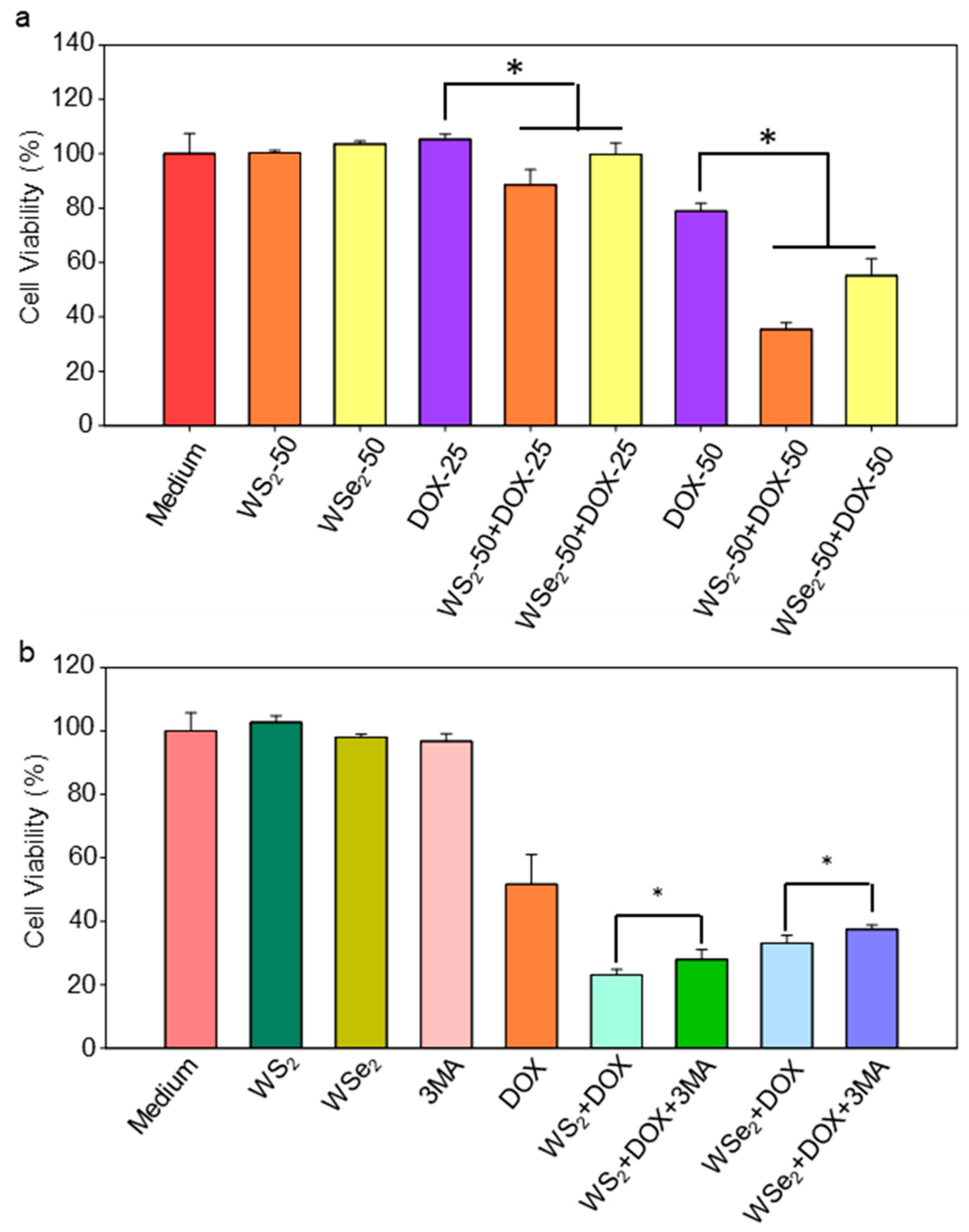
2.5. Enhanced Sensitivity of A549 Cells to DOX by WS2 and WSe2 Nanosheets
2.6. WS2 and WSe2 Sensitized A549 Cells to DOX by Triggering Autophagy
3. Materials and Methods
3.1. Reagents and Antibodies
3.2. Cell Culture
3.3. Cellular Localization of WS2 and WSe2 Determined through Transmission Electron Microscopy
3.4. Cell Viability Assay
3.5. Western Blot Analysis
3.6. The Effect of the Supernatant of WS2 and WSe2 Suspensions on Autophagy
3.7. PCR Array Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thai, A.A.; Solomon, B.J.; Sequist, L.V.; Gainor, J.F.; Heist, R.S. Lung cancer. Lancet 2021, 398, 535–554. [Google Scholar] [CrossRef] [PubMed]
- Relli, V.; Trerotola, M.; Guerra, E.; Alberti, S. Abandoning the Notion of Non-Small Cell Lung Cancer. Trends Mol. Med. 2019, 25, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Doroshow, D.B.; Sanmamed, M.F.; Hastings, K.; Politi, K.; Rimm, D.L.; Chen, L.P.; Melero, I.; Schalper, K.A.; Herbst, R.S. Immunotherapy in Non-Small Cell Lung Cancer: Facts and Hopes. Clin. Cancer Res. 2019, 25, 4592–4602. [Google Scholar] [CrossRef]
- Mundekkad, D.; Cho WL, C. Nanoparticles in Clinical Translation for Cancer Therapy. Int. J. Mol. Sci. 2022, 23, 29. [Google Scholar] [CrossRef] [PubMed]
- Bajalovic Meivita, M.P.; Loke Chan, S.S.Y.; Go, S.X.; Lee, D.N.S.; Bajalovic, N.; Loke, D.K. WS2/Polyethylene Glycol Nanostructures for Ultra-Efficient MCF-7 Cancer Cell Ablation and Electrothermal Therapy. ACS Omega 2022, 7, 23075–23082. [Google Scholar] [CrossRef] [PubMed]
- Chegini, M.N.; Panahi, H.A.; Manoochehri, M.; Moniri, E.; Rafiee, A. Dendrimer-modified WS2 nanosheets as a pH and thermosensitive nanocarrier for capecitabine controlled delivery by near-infrared laser irradiation for breast cancer cell. J. Drug Deliv. Sci. Technol. 2022, 78, 10. [Google Scholar]
- Ge, H.Y.; Du, J.J.; Zheng, J.Z.; Xu, N.; Yao, Q.C.; Long, S.R.; Fan, J.L.; Peng, X.J. Effective treatment of cisplatin-resistant ovarian tumors with a MoS2-based sonosensitizer and nanoenzyme capable of reversing the resistant-microenvironment and enhancing ferroptosis and apoptosis. Chem. Eng. J. 2022, 446, 11. [Google Scholar] [CrossRef]
- Yu, G.; Klionsky, D.J. Life and Death Decisions—The Many Faces of Autophagy in Cell Survival and Cell Death. Biomolecules 2022, 12, 15. [Google Scholar] [CrossRef]
- Yu, X.; Munoz-Alarcon, A.; Ajayi, A.; Webling, K.E.; Steinhof, A.; Langel, U.; Strom, A.L. Inhibition of Autophagy via p53-Mediated Disruption of ULK1 in a SCA7 Polyglutamine Disease Model. J. Mol. Neurosci. 2013, 50, 586–599. [Google Scholar] [CrossRef]
- Shi, B.H.; Ma, M.Q.; Zheng, Y.T.; Pan, Y.Y.; Lin, X.H. mTOR and Beclin1: Two key autophagy-related molecules and their roles in myocardial ischemia/reperfusion injury. J. Cell. Physiol. 2019, 234, 12562–12568. [Google Scholar] [CrossRef]
- Zalckvar, E.; Berissi, H.; Mizrachy, L.; Idelchuk, Y.; Koren, I.; Eisenstein, M.; Sabanay, H.; Pinkas-Kramarski, R.; Kimchi, A. DAP-kinase-mediated phosphorylation on the BH3 domain of beclin 1 promotes dissociation of beclin 1 from Bcl-X-L and induction of autophagy. EMBO Rep. 2009, 10, 285–292. [Google Scholar] [CrossRef]
- Piktel, E.; Oscilowska, I.; Suprewicz, L.; Depciuch, J.; Marcinczyk, N.; Chabielska, E.; Wolak, P.; Wollny, T.; Janion, M.; Parlinska-Wojtan, M.; et al. ROS-Mediated Apoptosis Autophagy in Ovarian Cancer Cells Treated with Peanut-Shaped Gold Nanoparticles. Int. J. Nanomed. 2021, 16, 1993–2011. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.M.; Wu, X.R.; Ren, Z.; Li, Y.L.; Zou, W.L.; Chen, J.C.; Wang, H.Q. Overcoming cancer chemotherapy resistance by the induction of ferroptosis. Drug Resist. Update 2023, 66, 16. [Google Scholar]
- Wen, J.; Chen, H.N.; Ren, Z.Y.; Zhang, P.; Chen, J.J.; Jiang, S.L. Ultrasmall iron oxide nanoparticles induced ferroptosis via Beclin1/ATG5-dependent autophagy pathway. Nano Converg. 2021, 8, 10. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, H.R.; Dong, L.; Xu, M.R.; Zhang, L.; Ding, W.P.; Zhang, J.Q.; Lin, J.; Zhang, Y.J.; Qiu, B.S.; et al. Enhancing tumor chemotherapy and overcoming drug resistance through autophagy-mediated intracellular dissolution of zinc oxide nanoparticles. Nanoscale 2019, 11, 11789–11807. [Google Scholar] [CrossRef] [PubMed]
- He, G.P.; Ma, Y.L.; Zhu, Y.; Yong, L.; Liu, X.; Wang, P.; Liang, C.; Yang, C.L.; Zhao, Z.G.; Hai, B.; et al. Cross talk between autophagy and apoptosis contributes to ZnO nanoparticle-induced human osteosarcoma cell death. Adv. Healthc. Mater. 2018, 7, 1800332. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.C.; Yao, Y.S.; Wang, Z.H.; Huang, X.Q.; Wei, T.T.; Zou, L.Y.; Liu, N.; Zhang, T.; Tang, M. CdTe quantum dots trigger oxidative stress and endoplasmic reticulum stress-induced apoptosis and autophagy in rat Schwann cell line RSC96. J. Appl. Toxicol. 2022, 42, 1962–1977. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-Q.; Zhou, W.; Zhu, S.-S.; Lin, J.; Wei, P.-F.; Li, F.-F.; Jin, P.-P.; Yao, H.; Zhang, Y.-J.; Hu, Y.; et al. Persistency of Enlarged Autolysosomes Underscores Nanoparticle-Induced Autophagy in Hepatocytes. Small 2017, 13, 1602876. [Google Scholar] [CrossRef]
- Fedotcheva, T.A.; Fedotcheva, N.I.; Shimanovsky, N.L. Progestins as Anticancer Drugs and Chemosensitizers, New Targets and Applications. Pharmaceutics 2021, 13, 21. [Google Scholar] [CrossRef]
- Oei, A.L.; Kok, H.P.; Oei, S.B.; Horsman, M.R.; Stalpers, L.J.A.; Franken, N.A.P.; Crezee, J. Molecular and biological rationale of hyperthermia as radio- and chemosensitizer. Adv. Drug Deliv. Rev. 2020, 163, 84–97. [Google Scholar] [CrossRef]
- Khatoon, E.; Banik, K.; Harsha, C.; Sailo, B.L.; Thakur, K.K.; Khwairakpam, A.D.; Vikkurthi, R.; Devi, T.B.; Gupta, S.C.; Kunnumakkara, A.B. Phytochemicals in cancer cell chemosensitization: Current knowledge and future perspectives. Semin. Cancer Biol. 2022, 80, 306–339. [Google Scholar] [CrossRef]
- Dehkordi, N.G.; Mirzaei, S.A.; Elahian, F. Pharmacodynamic mechanisms of anti-inflammatory drugs on the chemosensitization of multidrug-resistant cancers and the pharmacogenetics effectiveness. Inflammopharmacology 2021, 29, 49–74. [Google Scholar] [CrossRef]
- Sung, Y.C.; Jin, P.R.; Chu, L.A.; Hsu, F.F.; Wang, M.R.; Chang, C.C.; Chiou, S.J.; Qiu, J.T.; Gao, D.Y.; Lin, C.C.; et al. Delivery of nitric oxide with a nanocarrier promotes tumour vessel normalization and potentiates anti-cancer therapies. Nat. Nanotechnol. 2019, 14, 1160–1169. [Google Scholar] [CrossRef]
- Liu, J.M.; Lu, K.D.; Gao, F.C.; Zhao, L.; Li, H.; Jiang, Y.Y. Multifunctional MoS2 composite nanomaterials for drug delivery and synergistic photothermal therapy in cancer treatment. Ceram. Int. 2022, 48, 22378–22386. [Google Scholar] [CrossRef]
- Zhou, X.F.; Jia, J.B.; Luo, Z.; Su, G.X.; Yue, T.T.; Yan, B. Remote Induction of Cell Autophagy by 2D MoS2 Nanosheets via Perturbing Cell Surface Receptors and mTOR Pathway from Outside of Cells. ACS Appl. Mater. Interfaces 2019, 11, 6829–6839. [Google Scholar] [CrossRef]
- Zhou, X.F.; Yan, B. Induction of mTOR-dependent autophagy by WS2 nanosheets from both inside and outside of human cells. Nanoscale 2019, 11, 10684–10694. [Google Scholar] [CrossRef]
- Ma, J.; Liu, R.; Wang, X.; Liu, Q.; Chen, Y.N.; Valle, R.P.; Zuo, Y.Y.; Xia, T.; Liu, S.J. Crucial Role of Lateral Size for Graphene Oxide in Activating Macrophages and Stimulating Pro-inflammatory Responses in Cells and Animals. ACS Nano 2015, 9, 10498–10515. [Google Scholar] [CrossRef]
- Zhou, X.F.; Jin, W.T.; Zhang, R.; Mao, X.; Jia, J.B.; Zhou, H.Y. Perturbation of autophagy pathways in murine alveolar macrophage by 2D TMDCs is chalcogen-dependent. J. Environ. Sci. 2024, 135, 97–107. [Google Scholar] [CrossRef]
- Luo, N.; Ni, D.; Yue, H.; Wei, W.; Ma, G.H. Surface-Engineered Graphene Navigate Divergent Biological Outcomes toward Macrophages. ACS Appl. Mater. Interfaces 2015, 7, 5239–5247. [Google Scholar] [CrossRef]
- Zhang, Y.; Tekobo, S.; Tu, Y.; Zhou, Q.F.; Jin, X.L.; Dergunov, S.A.; Pinkhassik, E.; Yan, B. Permission to Enter Cell by Shape: Nanodisk vs Nanosphere. ACS Appl. Mater. Interfaces 2012, 4, 4099–4105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.N.; Pan, X.H.; Li, F.; Zhang, L.; Zhai, S.M.; Mu, Q.X.; Liu, J.F.; Qu, G.B.; Jiang, G.B.; Yan, B. Cell Rescue by Nanosequestration: Reduced Cytotoxicity of An Environmental Remediation Residue, Mg(OH)2 Nanoflake/Cr(VI) Adduct. Environ. Sci. Technol. 2014, 48, 1984–1992. [Google Scholar] [CrossRef]
- Wang, M.X.; Li, J.; Dong, S.N.; Cai, X.B.; Simaiti, A.; Yang, X.; Zhu, X.Q.; Luo, J.H.; Jiang, L.H.; Du, B.Y.; et al. Silica nanoparticles induce lung inflammation in mice via ROS/PARP/TRPM2 signaling-mediated lysosome impairment and autophagy dysfunction. Part. Fibre Toxicol. 2020, 17, 22. [Google Scholar] [CrossRef]
- Zhou, H.L.; Gong, X.Q.; Lin, H.Y.; Chen, H.M.; Huang, D.T.; Li, D.; Shan, H.; Gao, J.H. Gold nanoparticles impair autophagy flux through shape-dependent endocytosis and lysosomal dysfunction. J. Mat. Chem. B 2018, 6, 8127–8136. [Google Scholar] [CrossRef]
- Wu, X.Y.; Tan, Y.B.; Mao, H.; Zhang, M.M. Toxic effects of iron oxide nanoparticles on human umbilical vein endothelial cells. Int. J. Nanomed. 2010, 5, 385–399. [Google Scholar] [CrossRef]
- Kumar, A.V.; Mills, J.; Lapierre, L.R. Selective Autophagy Receptor p62/SQSTM1, a Pivotal Player in Stress and Aging. Front. Cell Dev. Biol. 2022, 10, 11. [Google Scholar] [CrossRef]
- Kong, N.; Ding, L.; Zeng, X.B.; Wang, J.Q.; Li, W.L.; Shi, S.J.; Gan, S.T.; Zhu, X.B.; Tao, W.; Ji, X.Y. Comprehensive insights into intracellular fate of WS2 nanosheets for enhanced photothermal therapeutic outcomes via exocytosis inhibition. Nanophotonics 2019, 8, 2331–2346. [Google Scholar] [CrossRef]
- Bao, H.; Zhang, Q.Y.; Liu, X.Y.; Song, Y.X.; Li, X.H.; Wang, Z.; Li, C.B.; Peng, A.; Gong, R.J. Lithium targeting of AMPK protects against cisplatin-induced acute kidney injury by enhancing autophagy in renal proximal tubular epithelial cells. Faseb J. 2019, 33, 14370–14381. [Google Scholar] [CrossRef]
- Chang, X.R.; Wang, X.J.; Li, J.Y.; Shang, M.T.; Niu, S.Y.; Zhang, W.L.; Li, Y.J.; Sun, Z.Y.; Gan, J.Y.; Li, W.H.; et al. Silver nanoparticles induced cytotoxicity in HT22 cells through autophagy and apoptosis via PI3K/AKT/mTOR signaling pathway. Ecotoxicol. Environ. Saf. 2021, 208, 111696. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, Y.; Zhang, C.K.; Cui, X.H.; Zhai, S.M.; Liu, Y.; Li, C.L.; Zhu, H.; Qu, G.B.; Jiang, G.B.; et al. Tuning Cell Autophagy by Diversifying Carbon Nanotube Surface Chemistry. ACS Nano 2014, 8, 2087–2099. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, B. Cell Cycle Regulation by Carboxylated Multiwalled Carbon Nanotubes through p53-Independent Induction of p21 under the Control of the BMP Signaling Pathway. Chem. Res. Toxicol. 2012, 25, 1212–1221. [Google Scholar] [CrossRef]
- Venezia, V.; Nizzari, M.; Carlo, P.; Corsaro, A.; Florio, T.; Russo, C. Amyloid precursor protein and presenilin involvement in cell signaling. Neurodegener. Dis. 2007, 4, 101–111. [Google Scholar] [CrossRef]
- Sobol, A.; Galluzzo, P.; Liang, S.; Rambo, B.; Skucha, S.; Weber, M.J.; Alani, S.; Bocchetta, M. Amyloid Precursor Protein (APP) Affects Global Protein Synthesis in Dividing Human Cells. J. Cell. Physiol. 2015, 230, 1064–1074. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef]
- Wu, W.X.; Wang, X.J.; Sun, Y.D.; Berleth, N.; Deitersen, J.; Schlutermann, D.; Stuhldreier, F.; Wallot-Hieke, N.; Jose, M.M.; Cox, J.; et al. TNF-induced necroptosis initiates early autophagy events via RIPK3-dependent AMPK activation, but inhibits late autophagy. Autophagy 2021, 17, 3992–4009. [Google Scholar] [CrossRef]
- Desideri, E.; Vegliante, R.; Cardaci, S.; Nepravishta, R.; Paci, M.; Ciriolo, M.R. MAPK14/p38 alpha-dependent modulation of glucose metabolism affects ROS levels and autophagy during starvation. Autophagy 2014, 10, 1652–1665. [Google Scholar] [CrossRef]
- Hu, B.L.; Zhang, Y.N.; Jia, L.; Wu, H.S.; Fan, C.F.; Sun, Y.T.; Ye, C.J.; Liao, M.; Zhou, J.Y. Binding of the pathogen receptor HSP90AA1 to avibirnavirus VP2 induces autophagy by inactivating the AKT-MTOR pathway. Autophagy 2015, 11, 503–515. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, J.; Fan, X.S.; Miao, R.J.; Wang, Y.W. HSP90AA1 promotes the inflammation in human gingival fibroblasts induced by Porphyromonas gingivalis lipopolysaccharide via regulating of autophagy. BMC Oral Health 2022, 22, 10. [Google Scholar] [CrossRef]
- Wang, N.; Tan, H.Y.; Li, S.; Feng, Y.B. Atg9b Deficiency Suppresses Autophagy and Potentiates Endoplasmic Reticulum Stress-Associated Hepatocyte Apoptosis in Hepatocarcinogenesis. Theranostics 2017, 7, 2325–2338. [Google Scholar] [CrossRef]
- Xiao, Y.H.; Yuan, J.; Yang, C.S.; Xiong, J.R.; Deng, L.Y.; Liang, Q.H.; He, C.; Li, L.S.; He, F.T.; Huang, X.Q. I-125 Radioactive Particles Drive Protective Autophagy in Hepatocellular Carcinoma by Upregulating, ATG9B. J. Clin. Transl. Hepatol. 2023, 11, 360–368. [Google Scholar]
- Qu, Y.L.; Gao, Q.; Wu, S.; Xu, T.; Jiang, D.; Xu, G.X. MicroRNA-142-3p inhibits autophagy and promotes intracellular survival of Mycobacterium tuberculosis by targeting ATG16L1 and ATG4c. Int. Immunopharmacol. 2021, 101, 8. [Google Scholar] [CrossRef]
- Chu, P.F.; Zhu, Y.C.; Xu, L.Q.; Yao, X.Y.; Liang, Y.; Zhang, X.J. ATG4C positively facilitates autophagy activation and restricts GCRV replication in grass carp (Ctenopharyngodon idella). Aquaculture 2022, 549, 12. [Google Scholar] [CrossRef]
- Yorimitsu, T.; Klionsky, D.J. Autophagy: Molecular machinery for self-eating. Cell Death Differ. 2005, 12, 1542–1552. [Google Scholar] [CrossRef]
- Zhang, K.; Zhou, X.T.; Wang, J.Q.; Zhou, Y.J.; Qi, W.C.; Chen, H.H.; Nie, S.P.; Xie, M.Y. Dendrobium officinale polysaccharide triggers mitochondrial disorder to induce colon cancer cell death via ROS-AMPK-autophagy pathway. Carbohydr. Polym. 2021, 264, 12. [Google Scholar] [CrossRef]
- Kim, T.W. Cinnamaldehyde induces autophagy-mediated cell death through ER stress and epigenetic modification in gastric cancer cells. Acta Pharmacol. Sin. 2022, 43, 712–723. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Zhang, G.P.; Xiang, L.; Pang, H.W.; Xiong, K.; Lu, Y.; Li, J.M.; Dai, J.; Lin, S.; et al. Radiotherapy-induced enrichment of EGF-modified doxorubicin nanoparticles enhances the therapeutic outcome of lung cancer. Drug Deliv. 2022, 29, 588–599. [Google Scholar] [CrossRef]
- Shi, Y.F.; Tao, M.; Ma, X.Y.; Hu, Y.; Huang, G.S.; Qiu, A.D.; Zhuang, S.G.; Liu, N. Delayed treatment with an autophagy inhibitor 3-MA alleviates the progression of hyperuricemic nephropathy. Cell Death Dis. 2020, 11, 16. [Google Scholar] [CrossRef]
- Flasz, B.; Dziewigcka, M.; Kgdziorski, A.; Tarnawska, M.; Augustyniak, J.; Augustyniak, M. Multigenerational selection towards longevity changes the protective role of vitamin C against graphene oxide-induced oxidative stress in house crickets. Environ. Pollut. 2021, 290, 117996. [Google Scholar] [CrossRef]
- Cerverò-Varona, A.; Canciello, A.; Peserico, A.; Montes, A.A.H.; Citeroni, M.R.; Mauro, A.; Russo, V.; Moffa, S.; Pilato, S.; Di Giacomo, S.; et al. Graphene oxide accelerates TGFβ-mediated epithelial-mesenchymal transition and stimulates pro-inflammatory immune response in amniotic epithelial cells. Mater. Today Bio 2023, 22, 100758. [Google Scholar] [CrossRef]
- Mariño, G.; Niso-Santano, M.; Baehrecke, E.H.; Kroemer, G. Self-consumption: The interplay of autophagy and apoptosis. Nat. Rev. Mol. Cell Bio. 2014, 15, 81–94. [Google Scholar] [CrossRef]
- D’Arcy, M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Wu, R.; Mei, J.; Hu, F.R.; Lei, C.J. Zinc oxide nanoparticles promotes liver cancer cell apoptosis through inducing autophagy and promoting p53. Eur. Rev. Med. Pharmaco. 2021, 25, 1557–1563. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, W.; Yang, T.; Jia, J.; Jia, J.; Zhou, X. Enhanced Sensitivity of A549 Cells to Doxorubicin with WS2 and WSe2 Nanosheets via the Induction of Autophagy. Int. J. Mol. Sci. 2024, 25, 1164. https://doi.org/10.3390/ijms25021164
Jin W, Yang T, Jia J, Jia J, Zhou X. Enhanced Sensitivity of A549 Cells to Doxorubicin with WS2 and WSe2 Nanosheets via the Induction of Autophagy. International Journal of Molecular Sciences. 2024; 25(2):1164. https://doi.org/10.3390/ijms25021164
Chicago/Turabian StyleJin, Weitao, Ting Yang, Jimei Jia, Jianbo Jia, and Xiaofei Zhou. 2024. "Enhanced Sensitivity of A549 Cells to Doxorubicin with WS2 and WSe2 Nanosheets via the Induction of Autophagy" International Journal of Molecular Sciences 25, no. 2: 1164. https://doi.org/10.3390/ijms25021164
APA StyleJin, W., Yang, T., Jia, J., Jia, J., & Zhou, X. (2024). Enhanced Sensitivity of A549 Cells to Doxorubicin with WS2 and WSe2 Nanosheets via the Induction of Autophagy. International Journal of Molecular Sciences, 25(2), 1164. https://doi.org/10.3390/ijms25021164






