Serum Amyloid A as a Potential Biomarker in Inflammatory Bowel Diseases, Especially in Patients with Low C-Reactive Protein
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
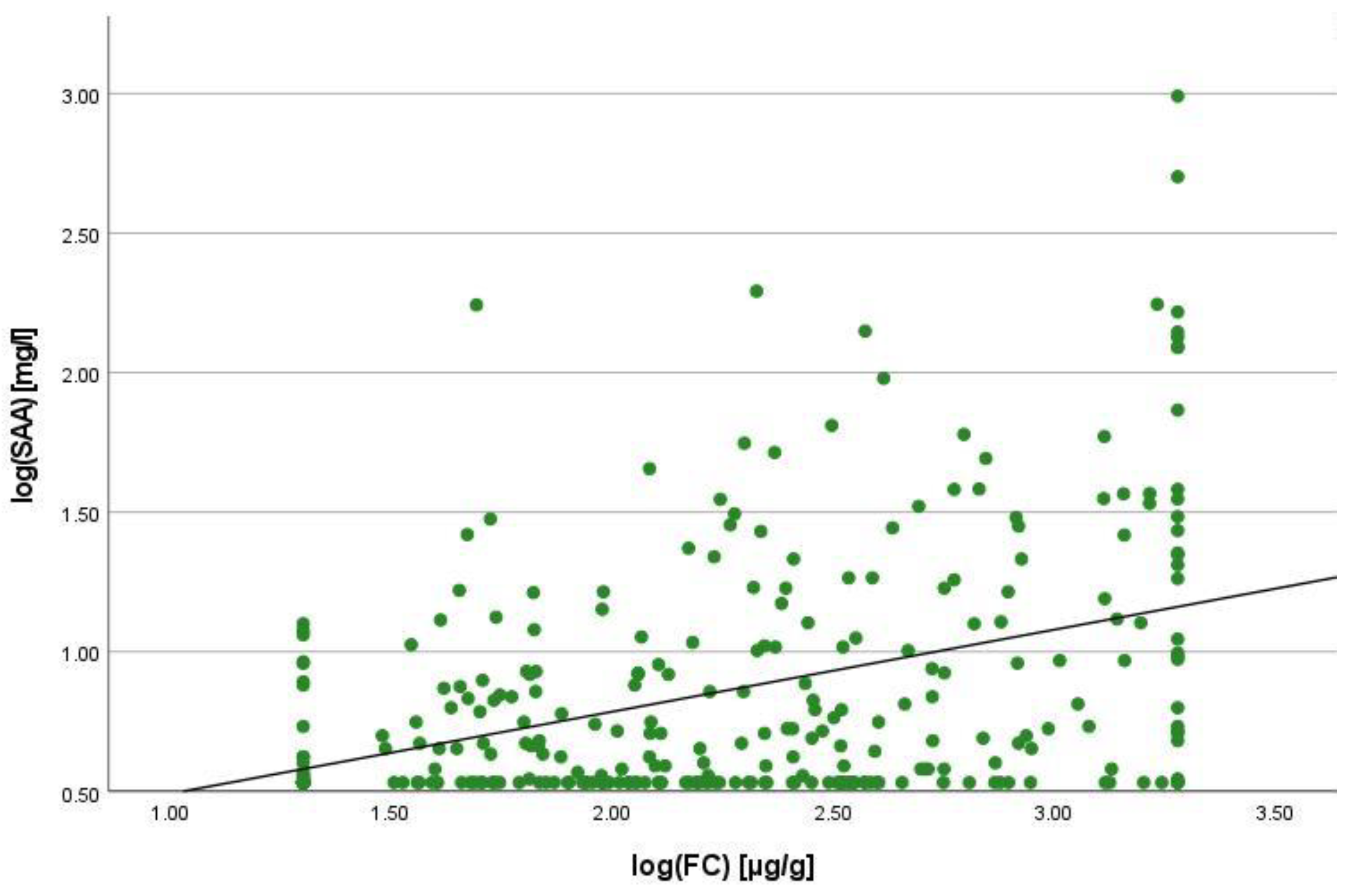
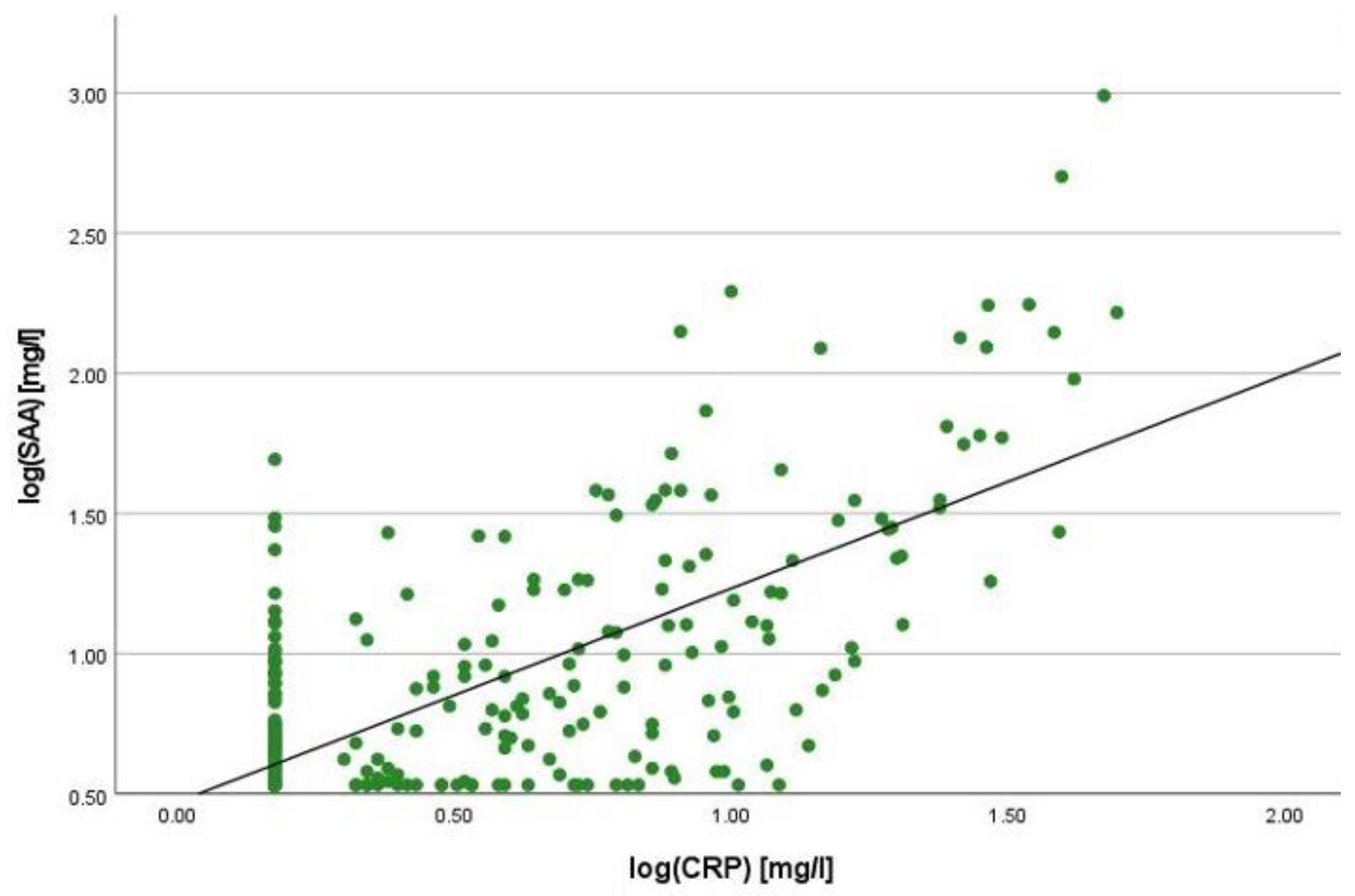
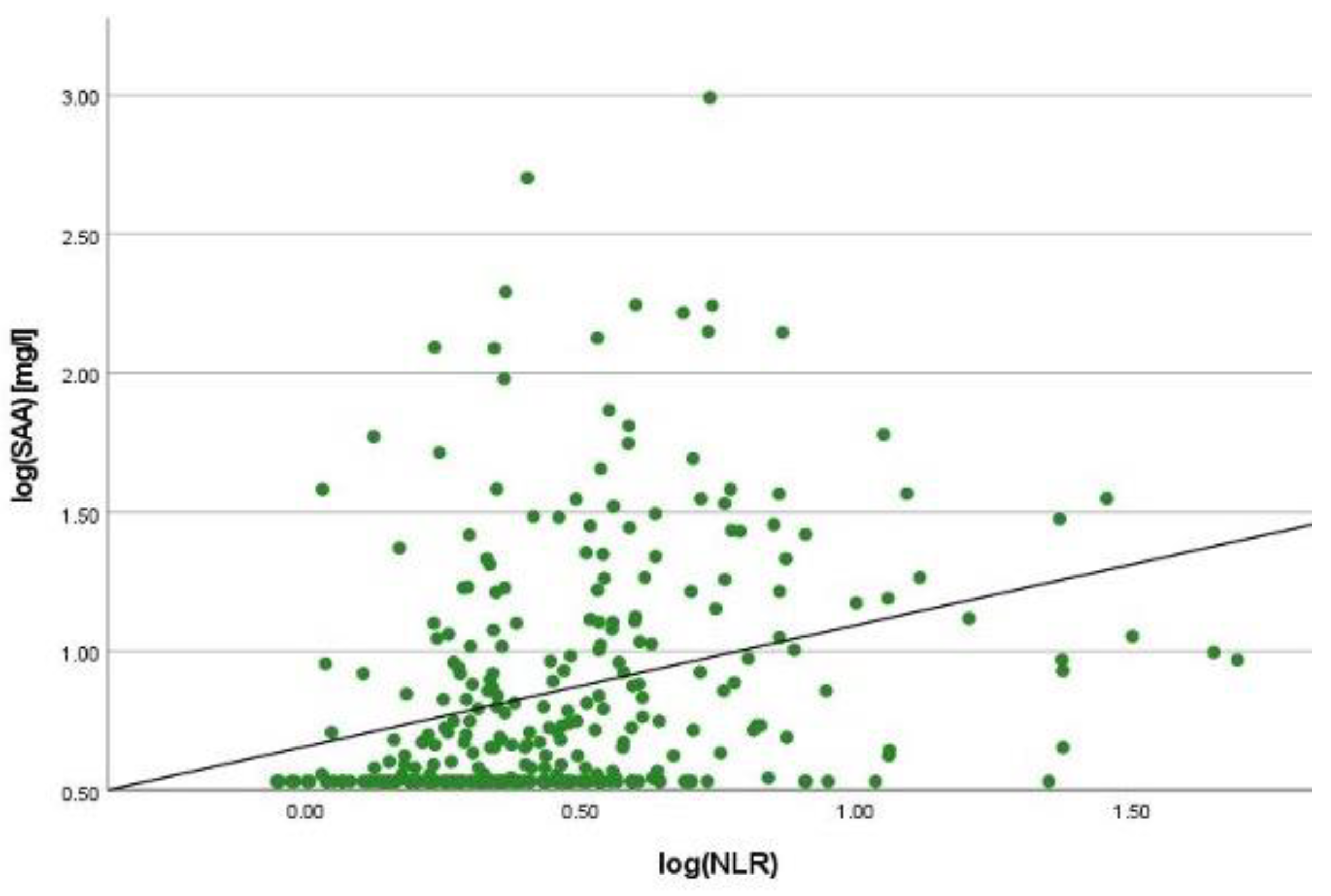
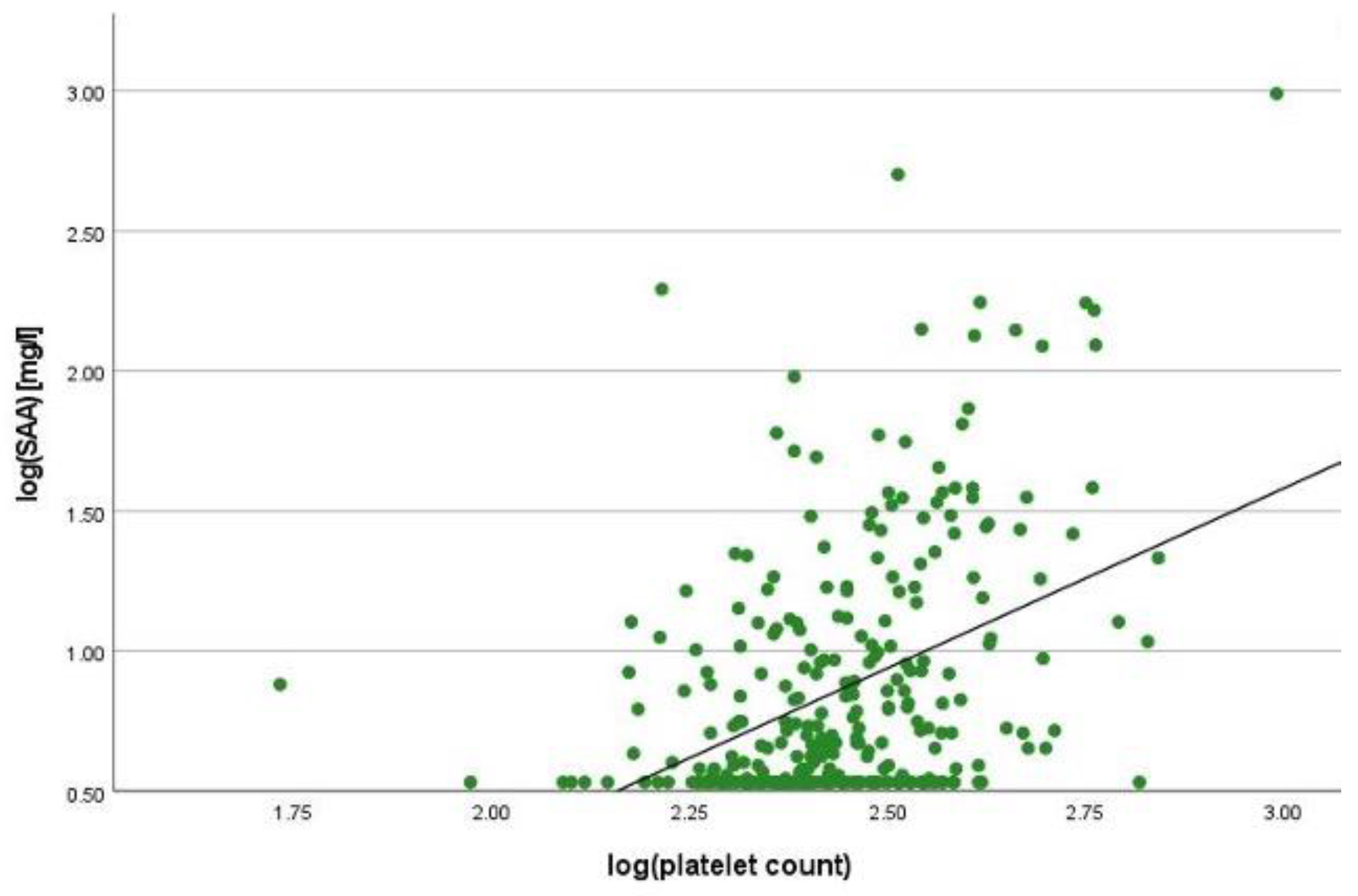
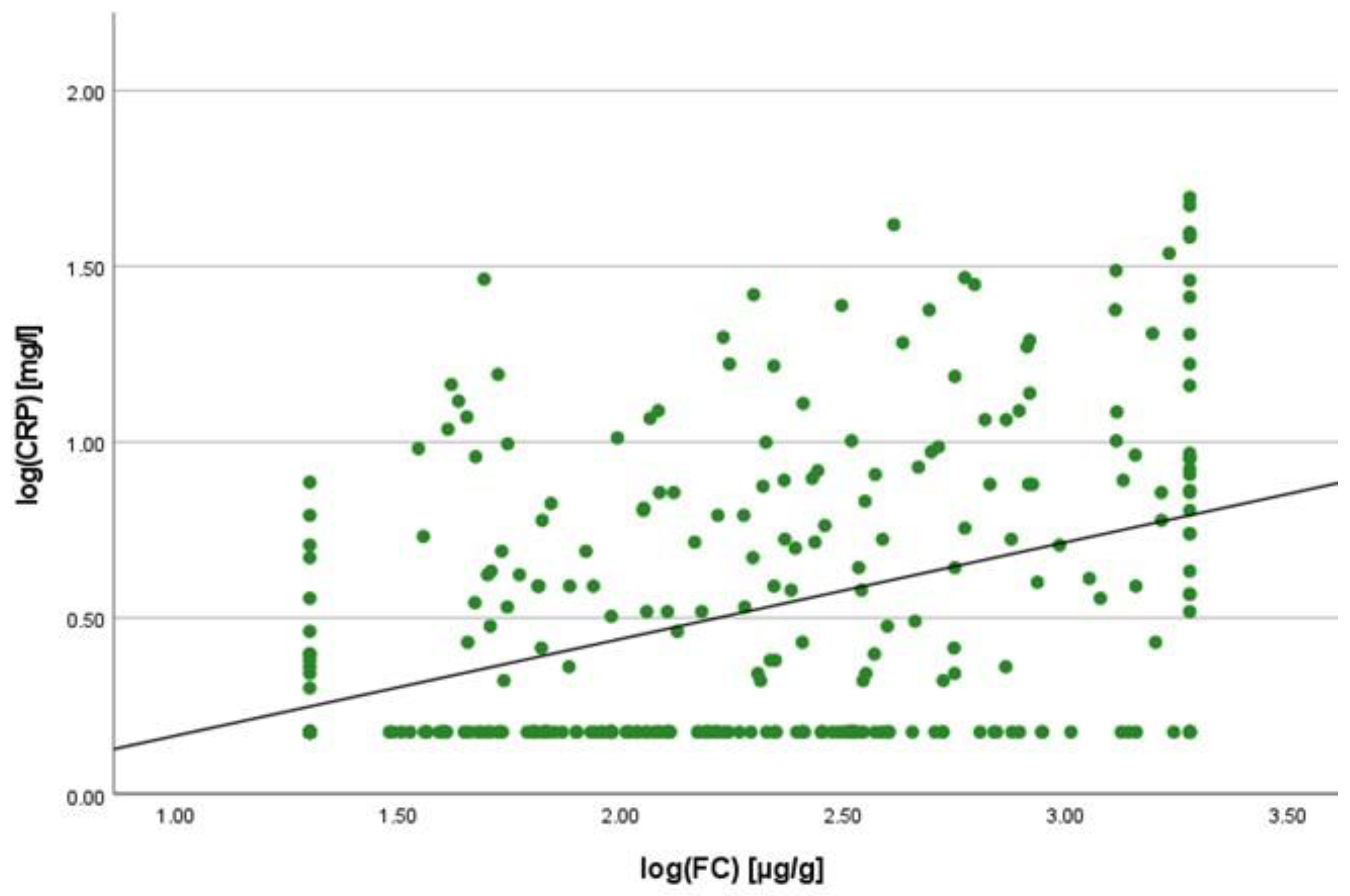
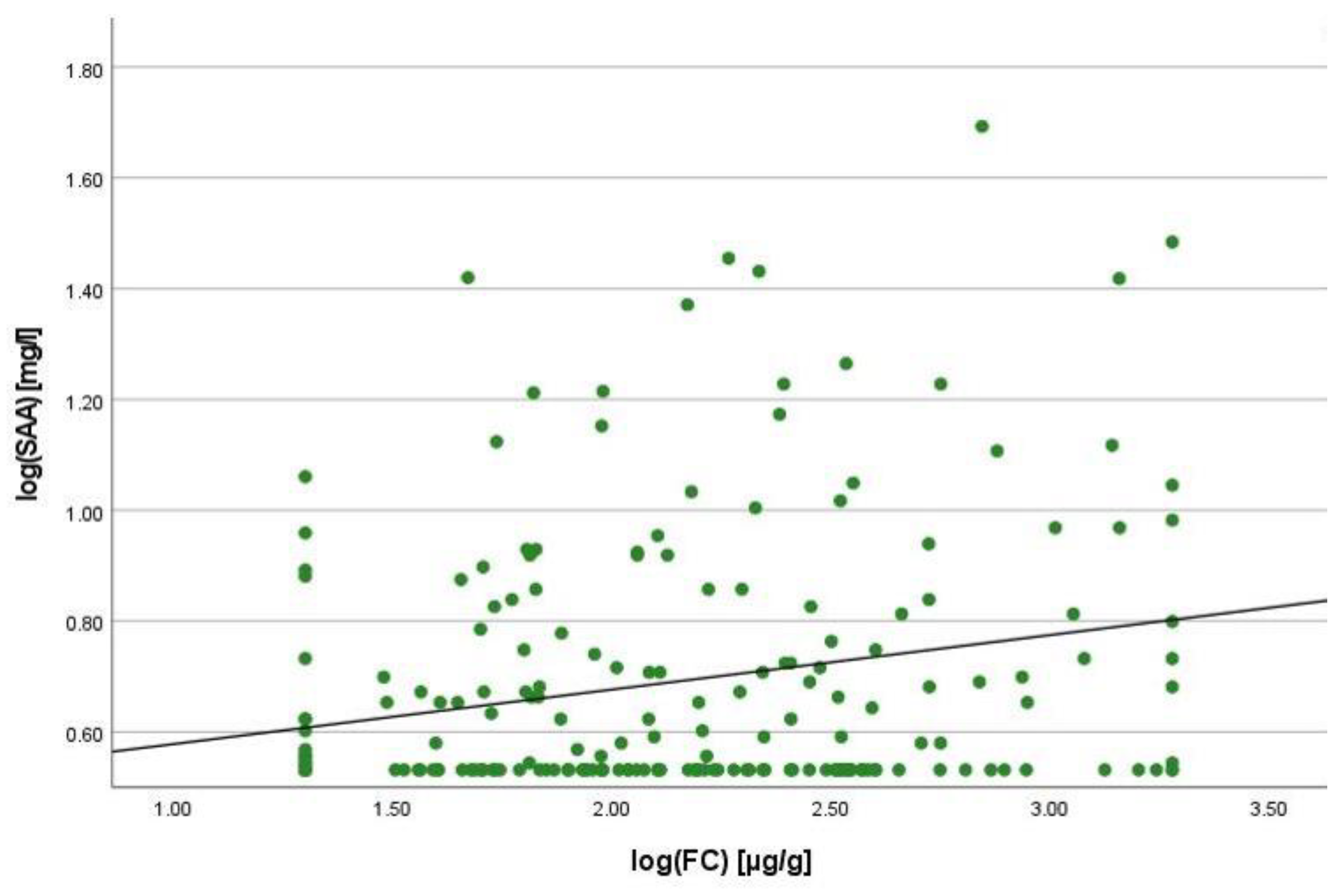
2.2. Correlation Analyses
2.3. Linear Regression Analyses
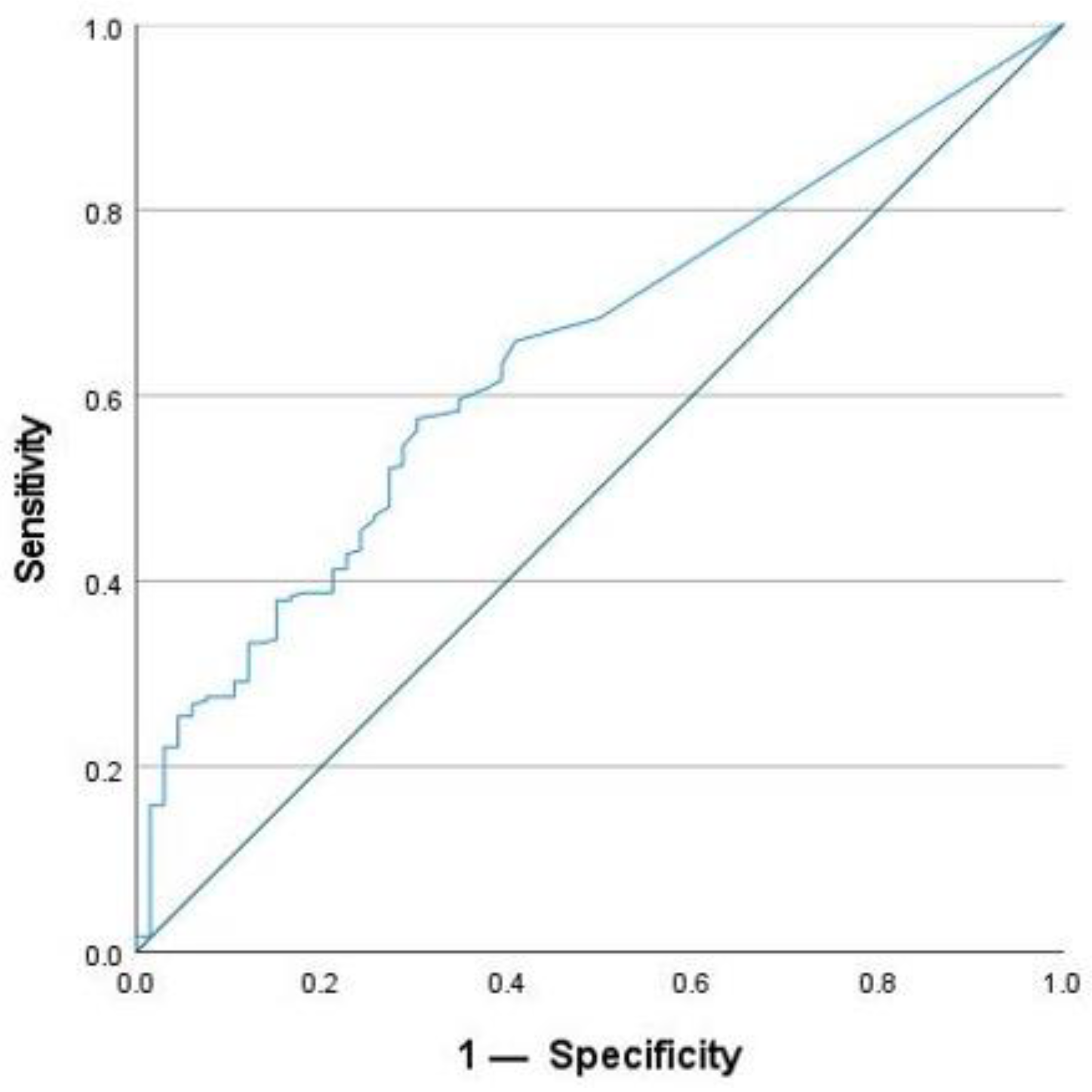
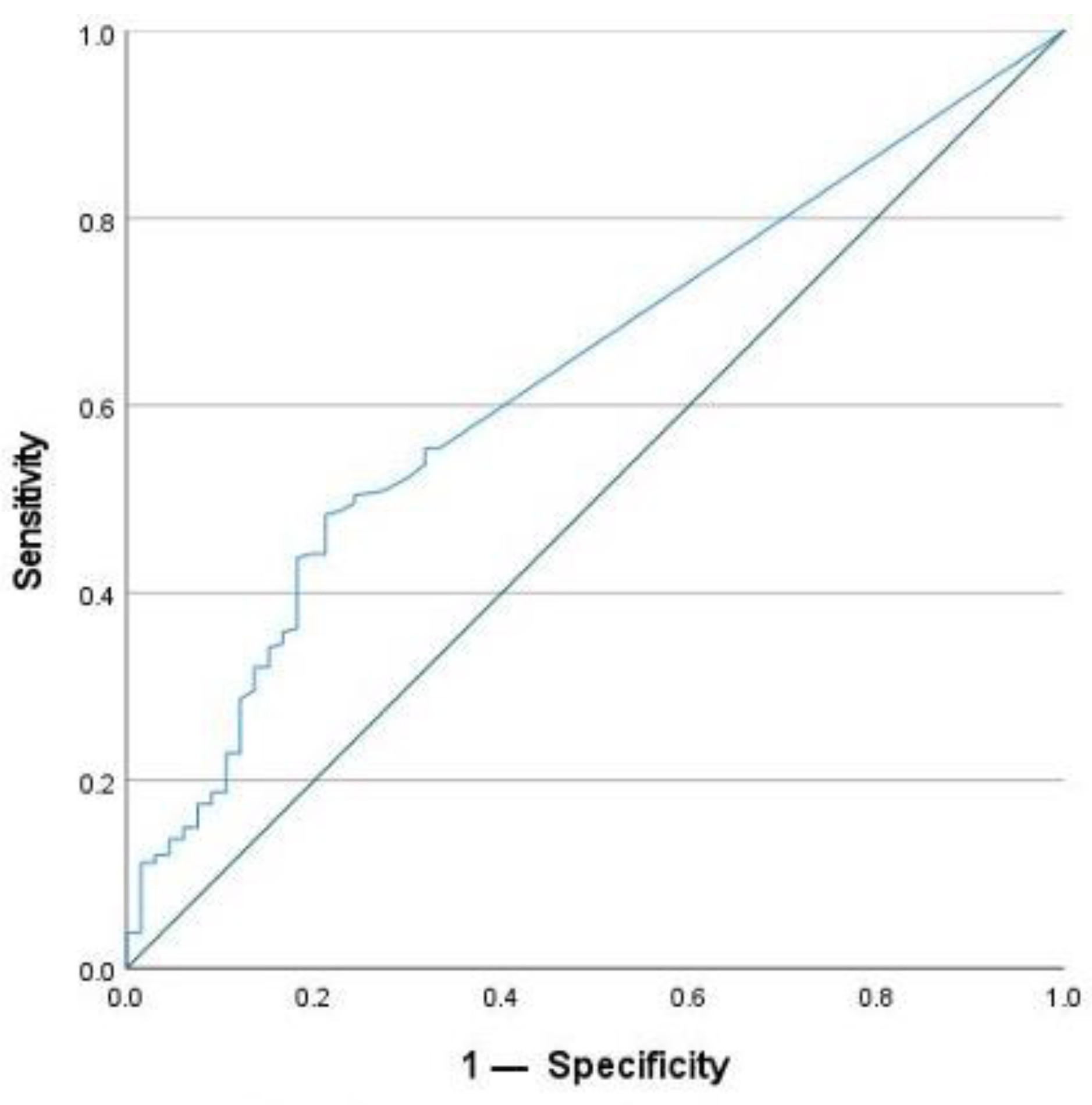
2.4. Cut-Off Value for SAA Concentration
2.5. Patients with CRP < 5 mg/L
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Measurement of SAA and CRP Concentrations
4.3. Measurement of FC Concentrations
4.4. Assessment of Clinical Disease Activity
4.5. Montreal Classification
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wakai, M.; Hayashi, R.; Tanaka, S.; Naito, T.; Kumada, J.; Nomura, M.; Takigawa, H.; Oka, S.; Ueno, Y.; Ito, M.; et al. Serum Amyloid A Is a Better Predictive Biomarker of Mucosal Healing than C-Reactive Protein in Ulcerative Colitis in Clinical Remission. BMC Gastroenterol. 2020, 20, 85. [Google Scholar] [CrossRef]
- Peyrin-Biroulet, L.; Ferrante, M.; Magro, F.; Campbell, S.; Franchimont, D.; Fidder, H.; Strid, H.; Ardizzone, S.; Veereman-Wauters, G.; Chevaux, J.-B.; et al. Results from the 2nd Scientific Workshop of the ECCO. I: Impact of Mucosal Healing on the Course of Inflammatory Bowel Disease. J. Crohn’s Colitis 2011, 5, 477–483. [Google Scholar] [CrossRef]
- Chang, S.; Malter, L.; Hudesman, D. Disease Monitoring in Inflammatory Bowel Disease. World J. Gastroenterol. 2015, 21, 11246–11259. [Google Scholar] [CrossRef]
- Plevris, N.; Lees, C.W. Disease Monitoring in Inflammatory Bowel Disease: Evolving Principles and Possibilities. Gastroenterology 2022, 162, 1456–1475.e1. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zhou, G.; Lin, J.; Li, L.; Zeng, Z.; Chen, M.; Zhang, S. Serum Biomarkers for Inflammatory Bowel Disease. Front. Med. 2020, 7, 123. [Google Scholar] [CrossRef]
- Sands, B.E. Biomarkers of Inflammation in Inflammatory Bowel Disease. Gastroenterology 2015, 149, 1275–1285.e2. [Google Scholar] [CrossRef] [PubMed]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef]
- Yarur, A.J.; Quintero, M.A.; Jain, A.; Czul, F.; Barkin, J.S.; Abreu, M.T. Serum Amyloid A as a Surrogate Marker for Mucosal and Histologic Inflammation in Patients with Crohn’s Disease. Inflamm. Bowel Dis. 2017, 23, 158–164. [Google Scholar] [CrossRef]
- Turner, D.; Ricciuto, A.; Lewis, A.; D’Amico, F.; Dhaliwal, J.; Griffiths, A.M.; Bettenworth, D.; Sandborn, W.J.; Sands, B.E.; Reinisch, W.; et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target Strategies in IBD. Gastroenterology 2021, 160, 1570–1583. [Google Scholar] [CrossRef]
- Ishihara, S.; Tada, Y.; Kawashima, K.; Kataoka, M.; Sonoyama, H.; Yamashita, N.; Oka, A.; Kusunoki, R.; Fukuba, N.; Mishima, Y.; et al. Serum Amyloid A Level Correlated with Endoscopic Findings in Patients with Crohn’s Disease-Possible Biomarker for Evaluating Mucosal Healing. Dig. Liver Dis. 2018, 50, 553–558. [Google Scholar] [CrossRef]
- Singh, S.; Ananthakrishnan, A.N.; Nguyen, N.H.; Cohen, B.L.; Velayos, F.S.; Weiss, J.M.; Sultan, S.; Siddique, S.M.; Adler, J.; Chachu, K.A.; et al. Electronic address: Clinicalpractice@gastro.org. AGA Clinical Practice Guideline on the Role of Biomarkers for the Management of Ulcerative Colitis. Gastroenterology 2023, 164, 344–372. [Google Scholar] [CrossRef]
- den Hartigh, L.J.; May, K.S.; Zhang, X.-S.; Chait, A.; Blaser, M.J. Serum Amyloid A and Metabolic Disease: Evidence for a Critical Role in Chronic Inflammatory Conditions. Front. Cardiovasc. Med. 2023, 10, 1197432. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; von Martels, J.Z.H.; de Vos, P.; Faber, K.N.; Dijkstra, G. Increased Fecal Calprotectin Levels in Crohn’s Disease Correlate with Elevated Serum Th1- and Th17-Associated Cytokines. PLoS ONE 2018, 13, e0193202. [Google Scholar] [CrossRef] [PubMed]
- Bourgonje, A.R.; von Martels, J.Z.H.; Gabriëls, R.Y.; Blokzijl, T.; Buist-Homan, M.; Heegsma, J.; Jansen, B.H.; van Dullemen, H.M.; Festen, E.A.M.; Ter Steege, R.W.F.; et al. A Combined Set of Four Serum Inflammatory Biomarkers Reliably Predicts Endoscopic Disease Activity in Inflammatory Bowel Disease. Front. Med. 2019, 6, 251. [Google Scholar] [CrossRef] [PubMed]
- Colombel, J.-F.; Panaccione, R.; Bossuyt, P.; Lukas, M.; Baert, F.; Vaňásek, T.; Danalioglu, A.; Novacek, G.; Armuzzi, A.; Hébuterne, X.; et al. Effect of Tight Control Management on Crohn’s Disease (CALM): A Multicentre, Randomised, Controlled Phase 3 Trial. Lancet 2017, 390, 2779–2789. [Google Scholar] [CrossRef]
- Roblin, X.; Serone, A.; Yoon, O.K.; Zhuo, L.; Grant, E.; Woo, J.; Liu, J.; Galien, R.; D’Haens, G. Effects of JAK1-Preferential Inhibitor Filgotinib on Circulating Biomarkers and Whole Blood Genes/Pathways of Patients with Moderately to Severely Active Crohn’s Disease. Inflamm. Bowel Dis. 2022, 28, 1207–1218. [Google Scholar] [CrossRef]
- Lobatón, T.; López-García, A.; Rodríguez-Moranta, F.; Ruiz, A.; Rodríguez, L.; Guardiola, J. A New Rapid Test for Fecal Calprotectin Predicts Endoscopic Remission and Postoperative Recurrence in Crohn’s Disease. J. Crohn’s Colitis 2013, 7, e641–e651. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Liu, Y.; Zhu, L.; Xu, L.; Shen, H. Evaluation of Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio as Potential Markers for Ulcerative Colitis: A Retrospective Study. BMC Gastroenterol. 2022, 22, 485. [Google Scholar] [CrossRef]
- Cherfane, C.E.; Gessel, L.; Cirillo, D.; Zimmerman, M.B.; Polyak, S. Monocytosis and a Low Lymphocyte to Monocyte Ratio Are Effective Biomarkers of Ulcerative Colitis Disease Activity. Inflamm. Bowel Dis. 2015, 21, 1769–1775. [Google Scholar] [CrossRef] [PubMed]
- Carestia, A.; Godin, L.C.; Jenne, C.N. Step up to the Platelet: Role of Platelets in Inflammation and Infection. Thromb. Res. 2023, 231, 182–194. [Google Scholar] [CrossRef]
- Danese, S.; de la Motte, C.C.; Fiocchi, C. Platelets in Inflammatory Bowel Disease: Clinical, Pathogenic, and Therapeutic Implications. Am. J. Gastroenterol. 2004, 99, 938–945. [Google Scholar] [CrossRef]
- Chen, R.; Chen, Q.; Zheng, J.; Zeng, Z.; Chen, M.; Li, L.; Zhang, S. Serum Amyloid Protein A in Inflammatory Bowel Disease: From Bench to Bedside. Cell Death Discov. 2023, 9, 154. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.H.; Im, J.P.; Ye, B.D.; Cheon, J.H.; Jang, H.J.; Lee, K.M.; Kim, Y.S.; Kim, S.W.; Kim, Y.H.; Song, G.A.; et al. Disease Phenotype, Activity and Clinical Course Prediction Based on C-Reactive Protein Levels at Diagnosis in Patients with Crohn’s Disease: Results from the CONNECT Study. Gut Liver 2016, 10, 595–603. [Google Scholar] [CrossRef] [PubMed]
- MacGregor, A.J.; Gallimore, J.R.; Spector, T.D.; Pepys, M.B. Genetic Effects on Baseline Values of C-Reactive Protein and Serum Amyloid a Protein: A Comparison of Monozygotic and Dizygotic Twins. Clin. Chem. 2004, 50, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Sturm, A.; Maaser, C.; Calabrese, E.; Annese, V.; Fiorino, G.; Kucharzik, T.; Vavricka, S.R.; Verstockt, B.; van Rheenen, P.; Tolan, D.; et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 2: IBD Scores and General Principles and Technical Aspects. J. Crohns’s Colitis 2019, 13, 273–284. [Google Scholar] [CrossRef]
- Maaser, C.; Sturm, A.; Vavricka, S.R.; Kucharzik, T.; Fiorino, G.; Annese, V.; Calabrese, E.; Baumgart, D.C.; Bettenworth, D.; Borralho Nunes, P.; et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial Diagnosis, Monitoring of Known IBD, Detection of Complications. J. Crohns Colitis 2019, 13, 144–164. [Google Scholar] [CrossRef]
- Agrawal, M.; Spencer, E.A.; Colombel, J.-F.; Ungaro, R.C. Approach to the Management of Recently Diagnosed Inflammatory Bowel Disease Patients: A User’s Guide for Adult and Pediatric Gastroenterologists. Gastroenterology 2021, 161, 47–65. [Google Scholar] [CrossRef]
- Harvey, R.F.; Bradshaw, J.M. A Simple Index of Crohn’s-Disease Activity. Lancet 1980, 1, 514. [Google Scholar] [CrossRef]
- Walmsley, R.S.; Ayres, R.C.; Pounder, R.E.; Allan, R.N. A Simple Clinical Colitis Activity Index. Gut 1998, 43, 29–32. [Google Scholar] [CrossRef]
- Satsangi, J.; Silverberg, M.S.; Vermeire, S.; Colombel, J.F. The Montreal Classification of Inflammatory Bowel Disease: Controversies, Consensus, and Implications. Gut 2006, 55, 749–753. [Google Scholar] [CrossRef]
| Parameter | Cases (306) |
|---|---|
| Age (years), median (range) | 44 (18–77) |
| Female, n (%) | 159 (52.0) |
| Male, n (%) | 147 (48.0) |
| Crohn’s disease, n (%) | 182 (59.5) |
| Ulcerative Colitis, n (%) | 116 (37.9) |
| IBD unclassified, n (%) | 8 (2.61) |
| Disease duration at serum sample collection (years), median (IQR) | 10 (16) |
| Montreal Classification n (%) | |
| Age at diagnosis (MC and UC) (A1, A2, A3) | 25 (8.2), 212 (69.3), 69 (22.5) |
| Location (MC) (L1, L2, L3, L4) | 68 (37.4), 21 (11.5), 83 (45.6), 11 (6.0) |
| Behaviour (MC) (B1, B2, B3) | 69 (37.9), 64 (35.2), 63 (34.6) |
| Location (CU) (E1, E2, E3) | 12 (10.3), 44 (37.9), 64 (55.2) |
| History of resecting surgery for IBD, n (%) | 85 (27.8) |
| Extraintestinal manifestation(s) (EIM), n (%) * | 102 (33.3) |
| EIM: Peripheral arthritis, n (%) | 81 (26.5) |
| EIM: Axial arthritis, n (%) | 11 (3.59) |
| EIM: Skin, n (%) | 16 (5.23) |
| EIM: Eyes, n (%) | 7 (2.29) |
| EIM: Primary sclerosing cholangitis, n (%) | 3 (0.98) |
| HBI/SCCAI (Mean) (SD) | 3.2 (3.6)/2.9 (3.0) |
| Biologic therapy at the time of sample collection, n (%) | 152 (49.7) |
| Number of days between FC and SAA determination, mean (SD) | 3.7 (4.4) |
| N | Mean | Standard Deviation | Minimum | Maximum | Percentile | |||
|---|---|---|---|---|---|---|---|---|
| 25 | 50 (Median) | 75 | ||||||
| FC concentration [µg/g] | 306 | 446.1 | 591.1 | 20.0 | 1900.0 | 54.5 | 170.5 | 529.8 |
| SAA concentration [mg/L] | 306 | 18.7 | 67.6 | 3.40 | 980.0 | 3.40 | 4.70 | 10.9 |
| CRP concentration [mg/L] | 306 | 5.69 | 7.98 | 1.50 | 49.7 | 1.50 | 2.10 | 6.40 |
| NLR | 303 | 4.07 | 5.33 | 0.88 | 49.2 | 1.89 | 2.70 | 3.95 |
| Platelet count | 306 | 290.1 | 103.9 | 54.0 | 981.0 | 222.2 | 266.5 | 335.3 |
| Age at diagnosis | A1: <17 years A2: 17–40 years A3: >40 years |
| Location | L1: ileal L2: colonic L3: ileocolonic L4: isolated upper disease |
| Behaviour | B1: non-stricturing, non-penetrating B2: stricturing B3: penetrating |
| Extent | E1: Ulcerative proctitis E2: Left sided UC (distal UC) E3: Extensive UC (pancolitis) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stute, M.; Kreysing, M.; Zorn, M.; Michl, P.; Gauss, A. Serum Amyloid A as a Potential Biomarker in Inflammatory Bowel Diseases, Especially in Patients with Low C-Reactive Protein. Int. J. Mol. Sci. 2024, 25, 1177. https://doi.org/10.3390/ijms25021177
Stute M, Kreysing M, Zorn M, Michl P, Gauss A. Serum Amyloid A as a Potential Biomarker in Inflammatory Bowel Diseases, Especially in Patients with Low C-Reactive Protein. International Journal of Molecular Sciences. 2024; 25(2):1177. https://doi.org/10.3390/ijms25021177
Chicago/Turabian StyleStute, Marie, Martin Kreysing, Markus Zorn, Patrick Michl, and Annika Gauss. 2024. "Serum Amyloid A as a Potential Biomarker in Inflammatory Bowel Diseases, Especially in Patients with Low C-Reactive Protein" International Journal of Molecular Sciences 25, no. 2: 1177. https://doi.org/10.3390/ijms25021177
APA StyleStute, M., Kreysing, M., Zorn, M., Michl, P., & Gauss, A. (2024). Serum Amyloid A as a Potential Biomarker in Inflammatory Bowel Diseases, Especially in Patients with Low C-Reactive Protein. International Journal of Molecular Sciences, 25(2), 1177. https://doi.org/10.3390/ijms25021177





