Molecular Mechanisms and Regulatory Pathways Underlying Drought Stress Response in Rice
Abstract
1. Introduction
2. Morphological, Physiological, and Biochemical Changes in Rice in Response to Drought Stress
3. Genes Associated with Drought Stress Response and Their Biological Functions in Rice
3.1. Maintenance of Water Homeostasis
3.2. Osmotic Regulation
3.3. Maintenance of ROS Homeostasis
3.4. Regulation of Hormone Content
3.4.1. ABA
3.4.2. Other Plant Hormones
3.5. Regulation of Cuticular Wax Deposition
3.6. Regulating Stomatal Density and Stomatal Opening and Closing
3.7. Improvement in Root Architecture
4. Molecular Regulatory Pathways of Genes Associated with Drought Stress Responses in Rice
4.1. Regulation at the Transcriptional and Post-Transcriptional Levels
4.1.1. Transcriptional Regulation (TFs)
AP2/EREBP
bHLH
bZIP
MYB
NAC
WRKY
Zinc Finger and Zinc Finger-Like TF
Other TFs Associated with Drought Response
4.1.2. Post-Transcriptional Regulation (microRNAs)
4.2. Post-Translational Regulation
4.2.1. Ubiquitination and SUMOylation Modification
4.2.2. Phosphorylation and Dephosphorylation
4.3. Epigenetic Regulation
5. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Verma, A.; Deepti, S. Abiotic stress and crop improvement: Current scenario. Adv. Plants Agric. Res. 2016, 4, 00149. [Google Scholar] [CrossRef][Green Version]
- The World Health Organization Official Website. Available online: https://www.who.int/health-topics/drought#tab=tab_1 (accessed on 20 December 2023).
- Zhang, H.; Li, Y.Y.; Zhu, J.K. Developing naturally stress-resistant crops for a sustainable agriculture. Nat. Plants 2018, 4, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.M.; Rashid, M.A.R.; Siddiqui, M.A.; Khan, M.T.; Farhat, F.; Yasmeen, S.; Khan, I.A.; Raja, S.; Rasool, F.; Sial, M.A.; et al. Recent insights into signaling responses to cope drought stress in rice. Rice Sci. 2022, 29, 105–117. [Google Scholar] [CrossRef]
- Muthayya, S.; Sugimoto, J.D.; Montgomery, S.; Maberly, G.F. An overview of global rice production, supply, trade, and consumption. Ann. N. Y. Acad. Sci. 2014, 1324, 7–14. [Google Scholar] [CrossRef]
- Kadam, N.N.; Tamilselvan, A.; Lawas, L.M.F.; Quinones, C.; Bahuguna, R.N.; Thomson, M.J.; Dingkuhn, M.; Muthurajan, R.; Struik, P.C.; Yin, X.Y.; et al. Genetic control of plasticity in root morphology and anatomy of rice in response to water deficit. Plant Physiol. 2017, 174, 2302–2315. [Google Scholar] [CrossRef]
- Elliott, J.; Deryng, D.; Müller, C.; Frieler, K.; Konzmann, M.; Gerten, D.; Glotter, M.; Flörke, M.; Wada, Y.; Best, N.; et al. Constraints and potentials of future irrigation water availability on agricultural production under climate change. Proc. Natl. Acad. Sci. USA 2014, 111, 3239–3244. [Google Scholar] [CrossRef]
- Serraj, R.; Mcnally, K.L.; Slamet-Loedin, I.; Kohli, A.; Haefele, S.M.; Atlin, G.; Kumar, A. Drought resistance improvement in rice: An integrated genetic and resource management strategy. Plant Prod Sci. 2011, 14, 1–14. [Google Scholar] [CrossRef]
- Manickavelu, A.; Nadarajan, N.; Ganesh, S.; Gnanamalar, R.; Ranganathan, C. Drought tolerance in rice: Morphological and molecular genetic consideration. Plant Growth Regul. 2006, 50, 121–138. [Google Scholar] [CrossRef]
- Todaka, D.; Zhao, Y.; Yoshida, T.; Kudo, M.; Kidokoro, S.; Mizoi, J.; Kodaira, K.S.; Takebayashi, Y.; Kojima, M.; Sakakibara, H.; et al. Temporal and spatial changes in gene expression, metabolite accumulation and phytohormone content in rice seedlings grown under drought stress conditions. Plant J. 2017, 90, 61–78. [Google Scholar] [CrossRef]
- Acua, T.L.B.; Lafitte, H.R.; Wade, L.J. Genotype × environment interactions for grain yield of upland rice backcross lines in diverse hydrological environments. Field Crop Res. 2008, 108, 117–125. [Google Scholar] [CrossRef]
- Sharma, P.; Dubey, R.S. Drought induces oxidative stress and enhances the activities of antioxidant enzymes in growing rice seedlings. Plant Growth Regul. 2005, 46, 209–221. [Google Scholar] [CrossRef]
- Selote, D.S.; Khanna-Chopra, R. Drought-induced spikelet sterility is associated with an inefficient antioxidant defence in rice panicles. Physiol. Plant. 2004, 121, 462–471. [Google Scholar] [CrossRef]
- Upadhyaya, H.; Panda, S.K. Chapter 9-Drought stress responses and its management in rice. In Advances in Rice Research for Abiotic Stress Tolerance; Woodhead Publishing: Cambridge, UK, 2019; pp. 177–200. [Google Scholar] [CrossRef]
- Mishra, S.S.; Panda, D. Leaf traits and antioxidant defense for drought tolerance during early growth stage in some popular traditional rice landraces from Koraput, India. Rice Sci. 2017, 24, 207–217. [Google Scholar] [CrossRef]
- Vibhuti; Shahi, C.; Bargali, K.; Bargali, S. Seed germination and seedling growth parameters of rice (Oryza sativa L.) varieties as affected by salt and water stress. Indian J. Agric. Sci. 2015, 85, 102–108. [Google Scholar] [CrossRef]
- Lum, M.S.; Hanafi, M.M.; Rafii, Y.M.; Akmar, A.S.N. Effect of drought stress on growth, proline and antioxidant enzyme activities of upland rice. J. Anim. Plant Sci. 2014, 24, 1487–1493. [Google Scholar]
- Salleh, M.S.; Nordin, M.S.; Puteh, A.B. Germination performance and biochemical changes under drought stress of primed rice seeds. Seed Sci. Technol. 2020, 48, 333–343. [Google Scholar] [CrossRef]
- Vijayaraghavareddy, P.; Akula, N.N.; Vemanna, R.S.; Math, R.G.H.; Shinde, D.D.; Yin, X.Y.; Struik, P.C.; Makarla, U.; Sreeman, S. Metabolome profiling reveals impact of water limitation on grain filling in contrasting rice genotypes. Plant Physiol. Biochem. 2021, 162, 690–698. [Google Scholar] [CrossRef]
- Dash, P.K.; Rai, R.; Rai, V.; Pasupalak, S. Drought induced signaling in rice: Delineating canonical and non-canonical pathways. Front. Chem. 2018, 6, 264. [Google Scholar] [CrossRef]
- Singh, P.K.; Indoliya, Y.; Agrawal, L.; Awasthi, S.; Deeba, F.; Dwivedi, S.; Chakrabarty, D.; Shirke, P.; Pandey, V.; Singh, N.; et al. Genomic and proteomic responses to drought stress and biotechnological interventions for enhanced drought tolerance in plants. Curr. Plant Biol. 2022, 29, 100239. [Google Scholar] [CrossRef]
- Nasrin, S.; Saha, S.; Begum, H.H.; Samad, R. Impacts of drought stress on growth, protein, proline, pigment content and antioxidant enzyme activities in rice (Oryza sativa L. var. BRRI dhan-24). Dhaka Univ. J. Biol. Sci. 2020, 29, 117–123. [Google Scholar] [CrossRef]
- Yadav, C.; Bahuguna, R.N.; Dhankher, O.P.; Singla-Pareek, S.L.; Pareek, A. Physiological and molecular signatures reveal differential response of rice genotypes to drought and drought combination with heat and salinity stress. Physiol. Mol. Biol. Plants 2022, 28, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.F.; Yang, X.L.; Chen, L.; Jiang, Y.Y.; Bu, H.Y.; Jiang, Y.; Li, P.; Cao, C.G. Physiological mechanism of drought-resistant rice coping with drought stress. J. Plant Growth Regul. 2022, 41, 2638–2651. [Google Scholar] [CrossRef]
- Jiang, Y.; Ling, L.; Zhang, L.L.; Wang, K.X.; Li, X.X.; Cai, M.L.; Zhan, M.; Li, C.F.; Wang, J.P.; Cao, C.G. Comparison of transgenic Bt rice and their non-Bt counterpart in yield and physiological response to drought stress. Field Crop Res. 2018, 217, 45–52. [Google Scholar] [CrossRef]
- Ji, K.X.; Wang, Y.Y.; Sun, W.N.; Lou, Q.J.; Mei, H.W.; Shen, S.H.; Chen, H. Drought-responsive mechanisms in rice genotypes with contrasting drought tolerance during reproductive stage. J. Plant Physiol. 2012, 169, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Henry, A.; Cal, A.J.; Batoto, T.C.; Torres, R.O.; Serraj, R. Root attributes affecting water uptake of rice (Oryza sativa) under drought. J. Exp.Bot. 2012, 63, 4751–4763. [Google Scholar] [CrossRef]
- Mostajeran, A.; Rahimi-Eichi, V. Effects of drought stress on growth and yield of rice (Oryza sativa L.) cultivars and accumulation of proline and soluble sugars in sheath and blades of their different ages leaves. Am.-Euras. J. Agric. Environ. Sci. 2009, 5, 264–272. [Google Scholar]
- Ashfaq, M.; Haider, M.S.; Khan, A.S.; Allah, S.U. Breeding potential of the basmati rice germplasm under water stress condition. Afr. J. Biotechnol. 2012, 11, 6647–6657. [Google Scholar]
- Kumar, S.; Dwivedi, S.; Singh, S.; Bhatt, B.; Mehta, P.; Rajamanickam, E.; Singh, V.; Singh, O. Morpho-physiological traits associated with reproductive stage drought tolerance of rice (Oryza sativa L.) genotypes under rain-fed condition of eastern Indo-Gangetic Plain. Indian J. Plant Physiol. 2014, 19, 87–93. [Google Scholar] [CrossRef]
- Rao, Y.C.; Dai, Z.J.; Zhu, Y.T.; Jiang, J.J.; Ma, R.Y.; Wang, Y.Y.; Wang, Y.X. Advances in research of drought resistance in rice. J. Zhejiang Norm. Univ. (Nat. Sci.) 2020, 43, 417–429. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Lee, D.J.; Ito, O.; Siddique, K.H.M. Advances in drought resistance of rice. Crit. Rev. Plant Sci. 2009, 28, 199–217. [Google Scholar] [CrossRef]
- Yang, S.J.; Vanderbeld, B.; Wan, J.X.; Huang, Y.F. Narrowing down the targets: Towards successful genetic engineering of drought-tolerant crops. Mol. Plant 2010, 3, 469–490. [Google Scholar] [CrossRef] [PubMed]
- Maurel, C.; Boursiac, Y.; Luu, D.T.; Santoni, V.; Shahzad, Z.; Verdoucq, L. Aquaporins in plants. Physiol. Rev. 2015, 95, 1321–1358. [Google Scholar] [CrossRef]
- Grondin, A.; Mauleon, R.; Vadez, V.; Henry, A. Root aquaporins contribute to whole plant water fluxes under drought stress in rice (Oryza sativa L.). Plant Cell Environ. 2016, 39, 347–365. [Google Scholar] [CrossRef] [PubMed]
- Lian, H.L.; Yu, X.; Ye, Q.; Ding, X.D.; Kitagawa, Y.; Kwak, S.S.; Su, W.A.; Tang, Z.C. The role of aquaporin RWC3 in drought avoidance in rice. Plant Cell Physiol. 2004, 45, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.J.; Xu, K.; Kong, D.Y.; Wu, L.Y.; Chen, Q.; Ma, X.S.; Ma, S.Q.; Li, T.F.; Xie, Q.; Liu, H.Y.; et al. Ubiquitin ligase OsRINGzf1 regulates drought resistance by controlling the turnover of OsPIP2;1. Plant Biotechnol. J. 2022, 20, 1743–1755. [Google Scholar] [CrossRef]
- Nguyen, M.X.; Moon, S.; Jung, K.H. Genome-wide expression analysis of rice aquaporin genes and development of a functional gene network mediated by aquaporin expression in roots. Planta 2013, 238, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Malz, S.; Sauter, M. Expression of two PIP genes in rapidly growing internodes of rice is not primarily controlled by meristem activity or cell expansion. Plant Mol. Biol. 1999, 40, 985–995. [Google Scholar] [CrossRef]
- Guo, L.; Wang, Z.Y.; Lin, H.; Cui, W.E.; Chen, J.; Liu, M.H.; Chen, Z.L.; Qu, L.J.; Gu, H.Y. Expression and functional analysis of the rice plasma-membrane intrinsic protein gene family. Cell Res. 2006, 16, 277–286. [Google Scholar] [CrossRef]
- Bai, J.Q.; Wang, X.; Yao, X.H.; Chen, X.C.; Lu, K.; Hu, Y.Q.; Wang, Z.D.; Mu, Y.J.; Zhang, L.Y.; Dong, H.S. Rice aquaporin OsPIP2;2 is a water-transporting facilitator in relevance to drought-tolerant responses. Plant Direct. 2021, 5, e338. [Google Scholar] [CrossRef]
- Liu, S.Y.; Fukumoto, T.; Gena, P.; Feng, P.; Sun, Q.; Li, Q.; Matsumoto, T.; Kaneko, T.; Zhang, H.; Zhang, Y.; et al. Ectopic expression of a rice plasma membrane intrinsic protein (OsPIP1;3) promotes plant growth and water uptake. Plant J. 2020, 102, 779–796. [Google Scholar] [CrossRef]
- Ding, L.; Uehlein, N.; Kaldenhoff, R.; Guo, S.W.; Zhu, Y.Y.; Kai, L. Aquaporin PIP2;1 affects water transport and root growth in rice (Oryza sativa L.). Plant Physiol. Biochem. 2019, 139, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Nada, R.M.; Abogadallah, G.M. Contrasting root traits and native regulation of aquaporin differentially determine the outcome of overexpressing a single aquaporin (OsPIP2;4) in two rice cultivars. Protoplasma 2020, 257, 583–595. [Google Scholar] [CrossRef]
- Xu, K.; Zhou, L.G.; Yu, S.W.; Chen, S.J.; Ma, X.S.; Lou, Q.J.; Liu, H.Y.; Liao, Z.G.; Xia, H.; Liu, Z.C.; et al. Physiological and molecular regulation mechanism of response to drought stress in cultivated rice. Acta Agric. Shanghai 2022, 38, 56–65. [Google Scholar] [CrossRef]
- Kuchenbuch, R.; Claassen, N.; Jungk, A. Potassium availability in relation to soil moisture. Plant Soil 1986, 95, 221–231. [Google Scholar]
- Tanguilig, V.C.; Yambao, E.B.; O’toole, J.C.; Datta, S.K.D. Water stress effects on leaf elongation, leaf water potential, transpiration, and nutrient uptake of rice, maize, and soybean. Plant Soil 1987, 103, 155–168. [Google Scholar]
- Johnson, R.; Vishwakarma, K.; Hossen, M.S.; Kumar, V.; Shackira, A.M.; Puthur, J.T.; Abdi, G.; Sarraf, M.; Hasanuzzaman, M. Potassium in plants: Growth regulation, signaling, and environmental stress tolerance. Plant Physiol. Biochem. 2022, 172, 56–69. [Google Scholar] [CrossRef]
- Wang, S.; Wan, C.; Wang, Y.; Chen, H.; Zhou, Z.; Fu, H.; Sosebee, R.E. The characteristics of Na+, K+ and free proline distribution in several drought–resistant plants of the Alxa Desert, China. J. Arid. Environ. 2004, 56, 525–539. [Google Scholar] [CrossRef]
- Mahouachi, J.; Socorro, A.R.; Talon, M. Responses of papaya seedlings (Carica papaya L.) to water stress and re–hydration: Growth, photosynthesis and mineral nutrient imbalance. Plant Soil 2006, 281, 137–146. [Google Scholar] [CrossRef]
- Chen, G.; Liu, C.L.; Gao, Z.Y.; Zhang, Y.; Zhu, L.; Hu, J.; Ren, D.Y.; Xu, G.H.; Qian, Q. Driving the expression of RAA1 with a drought-responsive promoter enhances root growth in rice, its accumulation of potassium and its tolerance to moisture stress. Environ. Exp. Bot. 2018, 147, 147–156. [Google Scholar] [CrossRef]
- Chen, G.; Liu, C.L.; Gao, Z.Y.; Zhang, Y.; Jiang, H.Z.; Zhu, L.; Ren, D.Y.; Yu, L.; Xu, G.H.; Qian, Q. OsHAK1, a high-affinity potassium transporter, positively regulates responses to drought stress in rice. Front. Plant Sci. 2017, 8, 1885. [Google Scholar] [CrossRef]
- Ahmad, I.; Devonshire, J.; Mohamed, R.; Schultze, M.; Maathuis, F.J.M. Overexpression of the potassium channel TPKb in small vacuoles confers osmotic and drought tolerance to rice. New Phytol. 2016, 209, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.; Sahoo, K.K.; Singh, A.K.; Anwar, K.; Pundir, P.; Gautam, R.K.; Krishnamurthy, S.L.; Sopory, S.K.; Pareek, A.; Singla-Pareek, S.L. Enhancing trehalose biosynthesis improves yield potential in marker-free transgenic rice under drought, saline, and sodic conditions. J. Exp. Bot. 2020, 71, 653–668. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, M.G.; Ishizaki, T.; Valencia, M.; Ogawa, S.; Dedicova, B.; Ogata, T.; Yoshiwara, K.; Maruyama, K.; Kusano, M.; Saito, K.; et al. Overexpression of an Arabidopsis thaliana galactinol synthase gene improves drought tolerance in transgenic rice and increased grain yield in the field. Plant Biotechnol. J. 2017, 15, 1465–1477. [Google Scholar] [CrossRef]
- Yan, J.P.; Ninkuu, V.; Fu, Z.C.; Yang, T.F.; Ren, J.; Li, G.Y.; Yang, X.F.; Zeng, H.M. OsOLP1 contributes to drought tolerance in rice by regulating ABA biosynthesis and lignin accumulation. Front. Plant Sci. 2023, 14, 1163939. [Google Scholar] [CrossRef]
- Cui, Y.C.; Li, M.J.; Yin, X.M.; Song, S.F.; Xu, G.Y.; Wang, M.L.; Li, C.Y.; Peng, C.; Xia, X.J. OsDSSR1, a novel small peptide, enhances drought tolerance in transgenic rice. Plant Sci. 2018, 270, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Huang, Y.M.; Xiong, L.Z. Characterization of stress-responsive CIPK genes in rice for stress tolerance improvement. Plant Physiol. 2007, 144, 1416–1428. [Google Scholar] [CrossRef]
- Li, H.W.; Zang, B.S.; Deng, X.W.; Wang, X.P. Overexpression of the trehalose-6-phosphate synthase gene OsTPS1 enhances abiotic stress tolerance in rice. Planta 2011, 234, 1007–1018. [Google Scholar] [CrossRef]
- Wei, S.Y.; Hu, W.; Deng, X.M.; Zhang, Y.Y.; Liu, X.D.; Zhao, X.D.; Luo, Q.C.; Jin, Z.Y.; Li, Y.; Zhou, S.Y.; et al. A rice calcium-dependent protein kinase OsCPK9 positively regulates drought stress tolerance and spikelet fertility. BMC Plant Biol. 2014, 14, 133. [Google Scholar] [CrossRef]
- Zhu, B.C.; Su, J.; Chang, M.C.; Verma, D.P.S.; Fan, Y.L.; Wu, R. Overexpression of a Δ1-pyrroline-5-carboxylate synthetase gene and analysis of tolerance to water- and salt-stress in transgenic rice. Plant Sci. 1998, 139, 41–48. [Google Scholar] [CrossRef]
- Zou, J.; Liu, C.F.; Liu, A.L.; Zou, D.; Chen, X.B. Overexpression of OsHsp17. 0 and OsHsp23. 7 enhances drought and salt tolerance in rice. J. Plant Physiol. 2012, 69, 628–635. [Google Scholar] [CrossRef]
- Li, J.M.; Sun, J.; Hang, M.H.; Leng, Y.; Sun, Q.; Zhao, H.W.; Zou, D.T. Cloning of OsAMTR310 and functional analysis under drought stress in rice. Acta Agric. Boreali-Sin. 2018, 33, 44–49. [Google Scholar] [CrossRef]
- Liu, W.Y.; Wang, M.M.; Huang, J.; Tang, H.J.; Lan, H.X.; Zhang, H.S. The OsDHODH1 gene is involved in salt and drought tolerance in rice. J. Integr. Plant Biol. 2009, 51, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Capell, T.; Bassie, L.; Christou, P. Modulation of the polyamine biosynthetic pathway in transgenic rice confers tolerance to drought stress. Proc. Natl. Acad. Sci. USA 2004, 101, 9909–9914. [Google Scholar] [CrossRef]
- Nadarajah, K.K. ROS homeostasis in abiotic stress tolerance in plants. Int. J. Mol. Sci. 2020, 21, 5208. [Google Scholar] [CrossRef]
- Joo, J.; Lee, Y.H.; Song, S.I. Rice CatA, CatB, and CatC are involved in environmental stress response, root growth, and photorespiration, respectively. J. Plant Biol. 2014, 57, 375–382. [Google Scholar] [CrossRef]
- Sousa, R.H.V.; Carvalho, F.E.L.; Ribeiro, C.W.; Passaia, G.; Cunha, J.R.; Lima-Melo, Y.; Margis-Pinheiro, M.; Silveira, J.A.G. Peroxisomal APX knockdown triggers antioxidant mechanisms favourable for coping with high photorespiratory H2O2 induced by CAT deficiency in rice. Plant Cell Environ. 2015, 38, 499–513. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.G.; Zhang, Q.A.; Wu, J.X.; Zheng, X.; Zheng, S.; Sun, X.H.; Qiu, Q.S.; Lu, T.G. Gene knockout study reveals that cytosolic Ascorbate Peroxidase 2(OsAPX2) plays a critical role in growth and reproduction in rice under drought, salt and cold stresses. PLoS ONE 2013, 8, e57472. [Google Scholar] [CrossRef]
- Zhu, M.H.; He, Y.G.; Zhu, M.Q.; Ahmad, A.; Xu, S.; He, Z.J.; Jiang, S.; Huang, J.Q.; Li, Z.H.; Liu, S.J.; et al. ipa1 improves rice drought tolerance at seedling stage mainly through activating abscisic acid pathway. Plant Cell Rep. 2022, 41, 221–232. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, Y.H.; Zhou, Z.M.; Zhang, Y.F.; Yang, Y.M.; Zan, X.F.; Li, X.H.; Wan, J.L.; Gao, X.L.; Chen, R.J.; et al. OsSCL30 overexpression reduces the tolerance of rice seedlings to low temperature, drought and salt. Sci. Rep. 2022, 12, 8385. [Google Scholar] [CrossRef]
- Jing, X.Q.; Li, W.Q.; Zhou, M.R.; Shi, P.T.; Zhang, R.; Shalmani, A.; Muhammad, I.; Wang, G.F.; Liu, W.T.; Chen, K.M. Rice carbohydrate-binding malectin-like protein, OsCBM1, contributes to drought-stress tolerance by participating in NADPH oxidase-mediated ROS production. Rice 2021, 14, 100. [Google Scholar] [CrossRef]
- Shi, Y.; Chang, Y.L.; Wu, H.T.; Shalmani, A.; Liu, W.T.; Li, W.Q.; Xu, J.W.; Chen, K.M. OsRbohB-mediated ROS production plays a crucial role in drought stress tolerance of rice. Plant Cell Rep. 2020, 39, 1767–1784. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Xie, K.B.; Yao, J.L.; Qi, Z.Y.; Xiong, L.Z. A homolog of human ski-interacting protein in rice positively regulates cell viability and stress tolerance. Proc. Natl. Acad. Sci. USA 2009, 106, 6410–6415. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Wang, Y.X.; Huang, L.Y.; Du, F.P.; Zhao, X.Q.; Li, Z.K.; Wang, W.S.; Fu, B.Y. A U-box E3 ubiquitin ligase OsPUB67 is positively involved in drought tolerance in rice. Plant Mol. Biol. 2020, 102, 89–107. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.Y.; Yu, J.P.; Miao, J.L.; Li, J.J.; Zhang, H.L.; Wang, X.; Liu, P.L.; Zhao, Y.; Jiang, C.H.; Yin, Z.G.; et al. Natural variation in OsLG3 increases drought tolerance in rice by inducing ROS scavenging. Plant Physiol. 2018, 178, 451–467. [Google Scholar] [CrossRef]
- Gu, X.Y.; Gao, S.X.; Li, J.; Song, P.Y.; Zhang, Q.; Guo, J.F.; Wang, X.Y.; Han, X.Y.; Wang, X.J.; Zhu, Y.; et al. The bHLH transcription factor regulated gene OsWIH2 is a positive regulator of drought tolerance in rice. Plant Physiol. Biochem. 2021, 169, 269–279. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, Y.R.; Li, Y.; Ling, H.Q.; Chu, C.C. OsMT1a, a type 1 metallothionein, plays the pivotal role in zinc homeostasis and drought tolerance in rice. Plant Mol. Biol. 2009, 70, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wu, Q.Y.; Peng, Z.; Sprague, S.A.; Wang, W.; Park, J.; Akhunov, E.; Jagadish, K.S.V.; Nakata, P.A.; Cheng, N.H.; et al. Silencing of OsGRXS17 in rice improves drought stress tolerance by modulating ROS accumulation and stomatal closure. Sci. Rep. 2017, 7, 15950. [Google Scholar] [CrossRef]
- Duan, J.Z.; Zhang, M.H.; Zhang, H.L.; Xiong, H.Y.; Liu, P.L.; Ali, J.; Li, J.J.; Li, Z.C. OsMIOX, a myo-inositol oxygenase gene, improves drought tolerance through scavenging of reactive oxygen species in rice (Oryza sativa L.). Plant Sci. 2012, 196, 143–151. [Google Scholar] [CrossRef]
- Pan, J.W.; Li, Z.; Wang, Q.G.; Yang, L.Q.; Yao, F.Y.; Liu, W. An S-domain receptor-like kinase, OsESG1, regulates early crown root development and drought resistance in rice. Plant Sci. 2020, 290, 110318. [Google Scholar] [CrossRef]
- Ning, J.; Li, X.; Hicks, L.M.; Xiong, L. A Raf-like MAPKKK gene DSM1 mediates drought resistance through reactive oxygen species scavenging in rice. Plant Physiol. 2010, 152, 876–890. [Google Scholar] [CrossRef]
- Ning, Y.; Jantasuriyarat, C.; Zhao, Q.Z.; Zhang, H.W.; Chen, S.B.; Liu, J.L.; Liu, L.J.; Tang, S.Y.; Park, C.H.; Wang, X.J.; et al. The SINA E3 ligase OsDIS1 negatively regulates drought response in rice. Plant Physiol. 2011, 157, 242–255. [Google Scholar] [CrossRef]
- Huda, K.M.K.; Banu, M.S.A.; Garg, B.; Tula, S.; Tuteja, R.; Tuteja, N. OsACA6, a P-type IIB Ca2+ ATPase promotes salinity and drought stress tolerance in tobacco by ROS scavenging and enhancing the expression of stress-responsive genes. Plant J. 2013, 76, 997–1015. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhai, N.; Ma, X.; Zhou, H.N.; Cui, Y.C.; Wang, C.; Xu, G.Y. Overexpression of OsRLCK241 confers enhanced salt and drought tolerance in transgenic rice (Oryza sativa L.). Gene 2021, 768, 145278. [Google Scholar] [CrossRef]
- Fang, Y.J.; Xiong, L.Z. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell Mol. Life Sci. 2015, 72, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Sah, S.K.; Reddy, K.R.; Li, J.X. Abscisic acid and abiotic stress tolerance in crop plants. Front. Plant Sci. 2016, 7, 571. [Google Scholar] [CrossRef] [PubMed]
- Oliver, S.N.; Dennis, E.S.; Dolferus, R. ABA regulates apoplastic sugar transport and is a potential signal for cold-induced pollen sterility in rice. Plant Cell Physiol. 2007, 48, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Welsch, R.; Wüst, F.; Bär, C.; Al-Babili, S.; Beyer, P. A third phytoene synthase is devoted to abiotic stress-induced abscisic acid formation in rice and defines functional diversification of phytoene synthase genes. Plant Physiol. 2008, 147, 367–380. [Google Scholar] [CrossRef]
- Zhu, G.H.; Ye, N.H.; Zhang, J.H. Glucose induced delay of seed germination in rice is mediated by the suppression of ABA catabolism rather than an enhancement of ABA biosynthesis. Plant Cell Physiol. 2009, 50, 644–651. [Google Scholar] [CrossRef]
- Huang, L.Y.; Bao, Y.C.; Qin, S.W.; Ning, M.; Lyu, J.; Zhang, S.L.; Huang, G.F.; Zhang, J.; Wang, W.S.; Fu, B.Y.; et al. An ABA synthesis enzyme allele OsNCED2 promotes the aerobic adaption in upland rice. bioRxiv 2020, 11, 146092. [Google Scholar] [CrossRef]
- Hwang, S.G.; Chen, H.C.; Huang, W.Y.; Chu, Y.C.; Shii, C.T.; Cheng, W.H. Ectopic expression of rice OsNCED3 in Arabidopsis increases ABA level and alters leaf morphology. Plant Sci. 2010, 178, 12–22. [Google Scholar] [CrossRef]
- Hwang, S.G.; Lee, C.Y.; Tseng, C.S. Heterologous expression of rice 9-cis-epoxycarotenoid dioxygenase 4 (OsNCED4) in Arabidopsis confers sugar oversensitivity and drought tolerance. Bot. Stud. 2018, 59, 2. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Jiao, Y.; Xie, N.K.; Guo, Y.M.; Zhang, F.; Xiang, Z.P.; Wang, R.; Wang, F.; Gao, Q.M.; Tian, L.F.; et al. OsNCED5, a 9-cis-epoxycarotenoid dioxygenase gene, regulates salt and water stress tolerance and leaf senescence in rice. Plant Sci. 2019, 287, 110188. [Google Scholar] [CrossRef]
- Kumar, V.V.S.; Yadav, S.K.; Verma, R.K.; Shrivastava, S.; Ghimire, O.; Pushkar, S.; Rao, M.V.; Kumar, T.S.; Chinnusamy, V. The abscisic acid receptor OsPYL6 confers drought tolerance to indica rice through dehydration avoidance and tolerance mechanisms. J. Exp. Bot. 2021, 72, 1411–1431. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, K.; Hwang, H.; Bhatnagar, N.; Kim, D.Y.; Yoon, I.S.; Byun, M.O.; Kim, S.T.; Jung, K.H.; Kim, B.G. Overexpression of PYL5 in rice enhances drought tolerance, inhibits growth, and modulates gene expression. J. Exp. Bot. 2014, 65, 453–464. [Google Scholar] [CrossRef]
- Zhang, D.P.; Zhou, Y.; Yin, J.F.; Yan, X.J.; Lin, S.; Xu, W.F.; Baluška, F.; Wang, Y.P.; Xia, Y.J.; Liang, G.H.; et al. Rice G-protein subunits qPE9-1 and RGB1 play distinct roles in abscisic acid responses and drought adaptation. J. Exp. Bot. 2015, 66, 6371–6384. [Google Scholar] [CrossRef]
- Luo, C.K.; Guo, C.M.; Wang, W.J.; Wang, L.J.; Chen, L. Overexpression of a new stress-repressive gene OsDSR2 encoding a protein with a DUF966 domain increases salt and simulated drought stress sensitivities and reduces ABA sensitivity in rice. Plant Cell Rep. 2014, 33, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.L.; Jiang, G.B.; Ye, N.H.; Chu, Z.Z.; Xu, X.Z.; Zhang, J.H.; Zhu, G.H. A key ABA catabolic gene, OsABA8ox3, is involved in drought stress resistance in rice. PLoS ONE 2015, 10, e0116646. [Google Scholar] [CrossRef]
- Li, J.J.; Li, Y.; Yin, Z.G.; Jiang, J.H.; Zhang, M.H.; Guo, X.; Ye, Z.J.; Zhao, Y.; Xiong, H.Y.; Zhang, Z.Y.; et al. OsASR5 enhances drought tolerance through a stomatal closure pathway associated with ABA and H2O2 signalling in rice. Plant Biotechnol. J. 2017, 15, 183–196. [Google Scholar] [CrossRef]
- Li, X.M.; Han, H.P.; Chen, M.; Yang, W.; Liu, L.; Li, N.; Ding, X.H.; Chu, Z.H. Overexpression of OsDT11, which encodes a novel cysteine-rich peptide, enhances drought tolerance and increases ABA concentration in rice. Plant Mol. Biol. 2017, 93, 21–34. [Google Scholar] [CrossRef]
- Ma, B.; Chen, S.Y.; Zhang, J.S. Ethylene signaling in rice. Chin. Sci. Bull. 2010, 55, 2204–2210. [Google Scholar] [CrossRef]
- Yu, Y.W.; Yang, D.X.; Zhou, S.R.; Gu, J.T.; Wang, F.R.; Dong, J.G.; Huang, R.F. The ethylene response factor OsERF109 negatively affects ethylene biosynthesis and drought tolerance in rice. Protoplasma 2017, 254, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Colebrook, E.H.; Thomas, S.G.; Phillips, A.L.; Hedden, P. The role of gibberellin signalling in plant responses to abiotic stress. J. Exp. Biol. 2014, 217, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Lo, S.F.; Ho, T.H.D.; Liu, Y.L.; Jiang, M.J.; Hsieh, K.T.; Chen, K.T.; Yu, L.C.; Lee, M.H.; Chen, C.Y.; Huang, T.P.; et al. Ectopic expression of specific GA2 oxidase mutants promotes yield and stress tolerance in rice. Plant Biotechnol. J. 2017, 15, 850–864. [Google Scholar] [CrossRef]
- Maimaiti, Y.S.J.; Xiong, Y.H.; Mijiti, M.H.M.T.J.; Sehroon, K.; Androw, C.; Zeng, H.M.; Qiu, D.W. Studies on drought tolerance of IPT transgenic rice. J. Agric. Sci. Technol. 2012, 14, 30–35. [Google Scholar] [CrossRef]
- Sharma, L.; Dalal, M.; Verma, R.K.; Kumar, S.V.V.; Yadav, S.K.; Pushkar, S.; Kushwaha, S.R.; Bhowmik, A.; Chinnusamy, V. Auxin protects spikelet fertility and grain yield under drought and heat stresses in rice. Environ. Exp. Bot. 2018, 150, 9–24. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, J.J.; Zhang, W.J.; Yan, S.N.; Wang, R.; Zhao, J.F.; Li, Y.J.; Qi, Z.G.; Sun, Z.X.; Zhu, Z.G. The putative auxin efflux carrier OsPIN3t is involved in the drought stress response and drought tolerance. Plant J. 2012, 72, 805–816. [Google Scholar] [CrossRef]
- Jung, H.; Lee, D.K.; Choi, Y.D.; Kim, J.K. OsIAA6, a member of the rice Aux/IAA gene family, is involved in drought tolerance and tiller outgrowth. Plant Sci. 2015, 236, 304–312. [Google Scholar] [CrossRef]
- Wang, F.B.; Niu, H.F.; Xin, D.Q.; Long, Y.; Wang, G.P.; Liu, Z.M.; Li, G.; Zhang, F.; Qi, M.Y.; Ye, Y.X.; et al. OsIAA18, an Aux/IAA transcription factor gene, is involved in salt and drought tolerance in rice. Front. Plant Sci. 2021, 12, 738660. [Google Scholar] [CrossRef]
- Zhang, A.Y.; Yang, X.; Lu, J.; Song, F.Y.; Sun, J.H.; Wang, C.; Lian, J.; Zhao, L.L.; Zhao, B.C. OsIAA20, an Aux/IAA protein, mediates abiotic stress tolerance in rice through an ABA pathway. Plant Sci. 2021, 308, 110903. [Google Scholar] [CrossRef]
- Creelman, R.A.; Mullet, J.E. Jasmonic acid distribution and action in plants: Regulation during development and response to biotic and abiotic stress. Proc. Natl. Acad. Sci. USA 1995, 92, 4114–4119. [Google Scholar] [CrossRef]
- Fu, J.; Wu, H.; Ma, S.Q.; Xiang, D.H.; Liu, R.Y.; Xiong, L.Z. OsJAZ1 attenuates drought resistance by regulating JA and ABA signaling in rice. Front. Plant Sci. 2017, 8, 2108. [Google Scholar] [CrossRef]
- Singh, A.P.; Mani, B.; Giri, J. OsJAZ9 is involved in water-deficit stress tolerance by regulating leaf width and stomatal density in rice. Plant Physiol. Biochem. 2021, 162, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, D.; Verma, G.; Chawda, K.; Chauhan, A.S.; Pande, V.; Chakrabarty, D. Overexpression of Asr6, abscisic acid stress-ripening protein, enhances drought tolerance and modulates gene expression in rice (Oryza sativa L.). Environ. Exp. Bot. 2022, 202, 105005. [Google Scholar] [CrossRef]
- Qin, B.X.; Tang, D.; Huang, J.; Li, M.; Wu, X.R.; Lu, L.L.; Wang, K.J.; Yu, H.X.; Chen, J.M.; Gu, M.H.; et al. Rice OsGL1-1 is involved in leaf cuticular wax and cuticle membrane. Mol. Plant 2011, 4, 985–995. [Google Scholar] [CrossRef]
- Islam, M.A.; Du, H.; Ning, J.; Ye, H.Y.; Xiong, L.Z. Characterization of Glossy1-homologous genes in rice involved in leaf wax accumulation and drought resistance. Plant Mol. Biol. 2009, 70, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.Y.; Li, L.Z.; Xiang, J.H.; Gao, G.F.; Xu, F.X.; Liu, A.L.; Zhang, X.W.; Peng, Y.; Chen, X.B.; Wan, X.Y. OsGL1-3 is involved in cuticular wax biosynthesis and tolerance to water deficit in rice. PLoS ONE 2015, 10, e116676. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.Y.; Ni, E.; Yang, J.W.; Zhou, H.; Liang, H.; Li, J.; Jiang, D.G.; Wang, Z.H.; Liu, Z.L.; Zhuang, C.X. Rice OsGL1-6 is involved in leaf cuticular wax accumulation and drought resistance. PLoS ONE 2013, 8, e65139. [Google Scholar] [CrossRef]
- Wang, Y.H.; Wan, L.Y.; Zhang, L.X.; Zhang, Z.J.; Zhang, H.W.; Quan, R.D.; Zhou, S.R.; Huang, R.F. An ethylene response factor OsWR1 responsive to drought stress transcriptionally activates wax synthesis related genes and increases wax production in rice. Plant Mol. Biol. 2012, 78, 275–288. [Google Scholar] [CrossRef]
- Yu, D.M.; Ranathunge, K.; Huang, H.S.; Pei, Z.Y.; Franke, R.; Schreiber, L.; He, C.Z. Wax Crystal-Sparse Leaf1 encodes a β–ketoacyl CoA synthase involved in biosynthesis of cuticular waxes on rice leaf. Planta 2008, 228, 675–685. [Google Scholar] [CrossRef]
- Mao, B.G.; Cheng, Z.J.; Lei, C.L.; Xu, F.H.; Gao, S.W.; Ren, Y.L.; Wang, J.L.; Zhang, X.; Wang, J.; Wu, F.Q.; et al. Wax crystal-sparse leaf2, a rice homologue of WAX2/GL1, is involved in synthesis of leaf cuticular wax. Planta 2012, 235, 39–52. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, H.F.; Wang, X.C.; Qiu, Y.J.; Tian, L.H.; Qi, X.Q.; Qu, L.Q. Cytochrome P450 family member CYP96B5 hydroxylates alkanes to primary alcohols and is involved in rice leaf cuticular wax synthesis. New Phytol. 2020, 225, 2094–2107. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.L.; Xia, D.N.; Hu, N.; Zhang, H.B.; Wu, H.Q.; Jiang, Q.Q.; Li, X.; Sun, Y.K.; Wang, Y.; Wang, Z.H. OsFAR1 is involved in primary fatty alcohol biosynthesis and promotes drought tolerance in rice. Planta 2023, 258, 24. [Google Scholar] [CrossRef]
- Shim, Y.; Seong, G.; Choi, Y.; Lim, C.; Baek, S.A.; Park, Y.J.; Kim, J.K.; An, G.; Kang, K.; Paek, N.C. Suppression of cuticular wax biosynthesis mediated by rice LOV KELCH REPEAT PROTEIN 2 supports a negative role in drought stress tolerance. Plant Cell Environ. 2023, 46, 1504–1520. [Google Scholar] [CrossRef]
- Guo, T.T.; Wang, D.F.; Fang, J.J.; Zhao, J.F.; Yuan, S.J.; Xiao, L.T.; Li, X.Y. Mutations in the rice OsCHR4 gene, encoding a CHD3 family chromatin remodeler, induce narrow and rolled leaves with increased cuticular wax. Int. J. Mol. Sci. 2019, 20, 2567. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.N.T.; Lee, S.B.; Suh, M.C.; An, G.; Jung, K.H. OsABCG9 is an important ABC transporter of cuticular wax deposition in rice. Front. Plant Sci. 2018, 9, 960. [Google Scholar] [CrossRef]
- Xia, K.F.; Ou, X.J.; Gao, C.Z.; Tang, H.D.; Jia, Y.X.; Deng, R.F.; Xu, X.L.; Zhang, M.Y. OsWS1 involved in cuticular wax biosynthesis is regulated by osa-miR1848. Plant Cell Environ. 2015, 38, 2662–2673. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.Y.; Xiong, L.Z. Putative megaenzyme DWA1 plays essential roles in drought resistance by regulating stress-induced wax deposition in rice. Proc. Natl. Acad. Sci. USA 2013, 110, 17790–17795. [Google Scholar] [CrossRef] [PubMed]
- Pitaloka, M.K.; Caine, R.S.; Hepworth, C.; Harrison, E.L.; Sloan, J.; Chutteang, C.; Phunthong, C.; Nongngok, R.; Toojinda, T.; Ruengphayak, S.; et al. Induced genetic variations in stomatal density and size of rice strongly affects water use efficiency and responses to drought stresses. Front. Plant Sci. 2022, 13, 801706. [Google Scholar] [CrossRef]
- Phetluan, W.; Wanchana, S.; Aesomnuk, W.; Adams, J.; Pitaloka, M.K.; Ruanjaichon, V.; Vanavichit, A.; Toojinda, T.; Gray, J.E.; Arikit, S. Candidate genes affecting stomatal density in rice (Oryza sativa L.) identified by genome-wide association. Plant Sci. 2023, 330, 111624. [Google Scholar] [CrossRef]
- Caine, R.S.; Yin, X.J.; Sloan, J.; Harrison, E.L.; Mohammed, U.; Fulton, T.; Biswal, A.K.; Dionora, J.; Chater, C.C.; Coe, R.A.; et al. Rice with reduced stomatal density conserves water and has improved drought tolerance under future climate conditions. New Phytol. 2019, 221, 371–384. [Google Scholar] [CrossRef]
- Santosh-Kumar, V.V.; Verma, R.K.; Yadav, S.K.; Yadav, P.; Watts, A.; Rao, M.V.; Chinnusamy, V. CRISPR-Cas9 mediated genome editing of drought and salt tolerance (OsDST) gene in indica mega rice cultivar MTU1010. Physiol. Mol. Biol. Plants 2020, 26, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Karavolias, N.G.; Patel-Tupper, D.; Seong, K.; Tjahjadi, M.; Gueorguieva, G.A.; Tanaka, J.; Cruz, G.A.; Lieberman, S.; Litvak, L.; Dahlbeck, D.; et al. Paralog editing tunes rice stomatal density to maintain photosynthesis and improve drought tolerance. Plant Physiol. 2023, 192, 1168–1182. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Chen, L.; Zhou, W.Q.; An, Y.H.; Luo, T.X.; Wu, Z.L.; Wang, Y.Q.; Xi, Y.F.; Yan, L.F.; Hou, S.W. RSD1 is essential for stomatal patterning and files in rice. Front. Plant Sci. 2020, 11, 600021. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.N.; Essemine, J.; Xu, J.L.; Ablat, G.; Perveen, S.; Wang, H.R.; Chen, K.; Zhao, Y.; Chen, G.Y.; Chu, C.C.; et al. Alterations in stomatal response to fluctuating light increase biomass and yield of rice under drought conditions. Plant J. 2020, 104, 1334–1347. [Google Scholar] [CrossRef] [PubMed]
- Park, S.I.; Kim, J.J.; Shin, S.Y.; Kim, Y.S.; Yoon, H.S. ASR enhances environmental stress tolerance and improves grain yield by modulating stomatal closure in rice. Front. Plant Sci. 2020, 10, 1752. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.C.; Chen, L.; Wang, L.; Yang, Y.L.; Rao, Y.C.; Ren, D.Y.; Dai, L.P.; Gao, Y.H.; Zou, W.W.; Lu, X.L.; et al. A Nck-associated protein 1-like protein affects drought sensitivity by its involvement in leaf epidermal development and stomatal closure in rice. Plant J. 2019, 98, 884–897. [Google Scholar] [CrossRef]
- Cui, L.G.; Shan, J.X.; Shi, M.; Gao, J.P.; Lin, H.X. DCA1 Acts as a transcriptional co-activator of DST and contributes to drought and salt tolerance in rice. PLoS Genet. 2015, 11, e1005617. [Google Scholar] [CrossRef]
- Qin, T.Y.; Kazim, A.; Wang, Y.H.; Richard, D.; Yao, P.F.; Bi, Z.Z.; Liu, Y.H.; Sun, C.; Bai, J.P. Root-related genes in crops and their application under drought stress resistance-a review. Int. J. Mol. Sci. 2022, 23, 11477. [Google Scholar] [CrossRef]
- Xu, K.; Lou, Q.J.; Wang, D.; Li, T.M.; Chen, S.J.; Li, T.F.; Luo, L.J.; Chen, L. Overexpression of a novel small auxin-up RNA gene, OsSAUR11, enhances rice deep rootedness. BMC Plant Biol. 2023, 23, 319. [Google Scholar] [CrossRef]
- Gao, J.; Zhao, Y.; Zhao, Z.K.; Liu, W.; Jiang, C.H.; Li, J.J.; Zhang, Z.Y.; Zhang, H.L.; Zhang, Y.G.; Wang, X.N.; et al. RRS1 shapes robust root system to enhance drought resistance in rice. New Phytol. 2023, 238, 1146–1162. [Google Scholar] [CrossRef]
- Sharma, E.; Bhatnagar, A.; Bhaskar, A.; Majee, S.M.; Kieffer, M.; Kepinski, S.; Khurana, P.; Khurana, J.P. Stress-induced F-Box protein-coding gene OsFBX257 modulates drought stress adaptations and ABA responses in rice. Plant Cell Environ. 2023, 46, 1207–1231. [Google Scholar] [CrossRef]
- Yang, J.; Chang, Y.; Qin, Y.H.; Chen, D.J.; Zhu, T.; Peng, K.Q.; Wang, H.J.; Tang, N.; Li, X.K.; Wang, Y.S.; et al. A lamin-like protein OsNMCP1 regulates drought resistance and root growth through chromatin accessibility modulation by interacting with a chromatin remodeller OsSWI3C in rice. New Phytol. 2020, 227, 65–83. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, V.; Rahman, H.; Subramanian, S.; Nallathambi, J.; Kaliyaperumal, A.; Manickam, S.; Ranganathan, C.; Muthurajan, R. OsARD4 encoding an acireductone dioxygenase improves root architecture in rice by promoting development of secondary roots. Sci. Rep. 2018, 8, 15713. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.K.; Yoon, S.; Kim, Y.S.; Kim, J.K. Rice OsERF71-mediated root modification affects shoot drought tolerance. Plant Signal. Behav. 2017, 12, e1268311. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.F.; Zhou, D.X.; Zhao, Y. WUSCHEL-related homeobox gene WOX11 increases rice drought resistance by controlling root hair formation and root system development. Plant Signal. Behav. 2016, 11, e1130198. [Google Scholar] [CrossRef]
- Uga, Y.; Sugimoto, K.; Ogawa, S.; Rane, J.; Ishitani, M.; Hara, N.; Kitomi, Y.; Inukai, Y.; Ono, K.; Kanno, N.; et al. Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nat. Genet. 2013, 45, 1097–1102. [Google Scholar] [CrossRef]
- Reege, R.J.E.; Wheatley, M.; Yang, Y.; Brown, K.M. Targeted mutation of transcription factor genes alters metaxylem vessel size and number in rice roots. Plant Direct. 2021, 5, e00328. [Google Scholar] [CrossRef]
- Wang, Y.N.; Xu, T.; Wang, W.P.; Zhang, Q.Z.; Xie, L.N. Role of epigenetic modifications in the development of crops essential traits. Hereditas 2021, 43, 858–879. [Google Scholar] [CrossRef]
- Weidemüller, P.; Kholmatov, M.; Petsalaki, E.; Zaugg, J.B. Transcription factors: Bridge between cell signaling and gene regulation. Proteomics 2021, 21, e2000034. [Google Scholar] [CrossRef]
- Oh, S.J.; Kim, Y.S.; Kwon, C.W.; Park, H.K.; Jeong, J.S.; Kim, J.K. Overexpression of the transcription factor AP37 in rice improves grain yield under drought conditions. Plant Physiol. 2009, 150, 1368–1379. [Google Scholar] [CrossRef]
- Ramegowda, V.; Basu, S.; Krishnan, A.; Pereira, A. Rice GROWTH UNDER DROUGHT KINASE is required for drought tolerance and grain yield under normal and drought stress conditions. Plant Physiol. 2014, 166, 1634–1645. [Google Scholar] [CrossRef]
- Chakraborty, K.; Jena, P.; Mondal, S.; Dash, G.K.; Ray, S.; Baig, M.J.; Swain, P. Relative contribution of different members of OsDREB gene family to osmotic stress tolerance in indica and japonica ecotypes of rice. Plant Biol. 2022, 24, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.Y.; Guan, Y.C.; Wu, Y.R.; Chen, H.L.; Chen, F.; Chu, C.C. Overexpression of a rice OsDREB1F gene increases salt, drought, and low temperature tolerance in both Arabidopsis and rice. Plant Mol. Biol. 2008, 67, 589–602. [Google Scholar] [CrossRef]
- Chen, J.Q.; Meng, X.P.; Zhang, Y.; Xia, M.; Wang, X.P. Over-expression of OsDREB genes lead to enhanced drought tolerance in rice. Biotechnol. Lett. 2008, 30, 2191–2198. [Google Scholar] [CrossRef]
- Ke, Y.G.; Yang, Z.J.; Yu, S.W.; Li, T.F.; Wu, J.H.; Gao, H.; Fu, Y.P.; Luo, L.J. Characterization of OsDREB6 responsive to osmotic and cold stresses in rice. J. Plant Biol. 2014, 57, 150–161. [Google Scholar] [CrossRef]
- Zhao, L.F.; Hu, Y.B.; Chong, K.; Wang, T. ARAG1, an ABA-responsive DREB gene, plays a role in seed germination and drought tolerance of rice. Ann. Bot. 2010, 105, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.F.; Xue, Y.; Wang, R.; Xu, R.R.; Bian, L.; Zhu, B.; Han, H.J.; Peng, R.H.; Yao, Q.H. Transcription factor OsAP21 gene increases salt/drought tolerance in transgenic Arabidopsis thaliana. Mol. Biol. Rep. 2013, 40, 1743–1752. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.Y.; Wang, Y.X.; Wang, W.S.; Zhao, X.Q.; Qin, Q.; Sun, F.; Hu, F.Y.; Zhao, Y.; Li, Z.C.; Fu, B.Y.; et al. Characterization of transcription factor gene OsDRAP1 conferring drought tolerance in rice. Front. Plant Sci. 2018, 9, 94. [Google Scholar] [CrossRef]
- Li, J.J.; Guo, X.; Zhang, M.H.; Wang, X.; Zhao, Y.; Yin, Z.G.; Zhang, Z.Y.; Wang, Y.M.; Xiong, H.Y.; Zhang, H.L.; et al. OsERF71 confers drought tolerance via modulating ABA signaling and proline biosynthesis. Plant Sci. 2018, 270, 131–139. [Google Scholar] [CrossRef]
- Jin, Y.; Pan, W.Y.; Zheng, X.F.; Cheng, X.; Liu, M.M.; Ma, H.; Ge, X.C. OsERF101, an ERF family transcription factor, regulates drought stress response in reproductive tissues. Plant Mol. Biol. 2018, 98, 51–65. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Chen, S.J.; Ma, X.S.; Wei, H.B.; Chen, C.; Gao, N.N.; Zou, Y.Q.; Kong, D.Y.; Li, T.F.; et al. An APETALA2/ethylene responsive factor, OsEBP89 knockout enhances adaptation to direct-seeding on wet land and tolerance to drought stress in rice. Mol. Genet. Genom. 2020, 295, 941–956. [Google Scholar] [CrossRef]
- Ambavaram, M.M.; Basu, S.; Krishnan, A.; Ramegowda, V.; Batlang, U.; Rahman, L.; Baisakh, N.; Pereira, A. Coordinated regulation of photosynthesis in rice increases yield and tolerance to environmental stress. Nat. Commun. 2014, 5, 5302. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Z.; Shen, Y.T.; Cao, H.X.; He, K.; Chu, Z.H.; Li, N. OsbHLH057 targets the AATCA cis-element to regulate disease resistance and drought tolerance in rice. Plant Cell Rep. 2022, 41, 1285–1299. [Google Scholar] [CrossRef] [PubMed]
- Se, O.J.S.; Joo, J.; Kim, M.J.; Kim, Y.K.; Nahm, B.H.; Song, S.I.; Cheong, J.J.; Lee, J.S.; Kim, J.K.; Choi, Y.D. OsbHLH148, a basic helix-loop-helix protein, interacts with OsJAZ proteins in a jasmonate signaling pathway leading to drought tolerance in rice. Plant J. 2011, 65, 907–921. [Google Scholar] [CrossRef]
- Sahid, S.; Roy, C.; Shee, D.; Shee, R.; Datta, R.; Paul, S. ZFP37, C3H, NAC94, and bHLH148 transcription factors regulate cultivar-specific drought response by modulating r40C1 gene expression in rice. Environ. Exp. Bot. 2023, 214, 105480. [Google Scholar] [CrossRef]
- Chander, S.; Almeida, D.M.; Serra, T.S.; Jardim-Messeder, D.; Barros, P.M.; Lourenço, T.F.; Figueiredo, D.D.; Margis-Pinheiro, M.; Costa, J.M.; Oliveira, M.M.; et al. OsICE1 transcription factor improves photosynthetic performance and reduces grain losses in rice plants subjected to drought. Environ. Exp. Bot. 2018, 150, 88–98. [Google Scholar] [CrossRef]
- Amir-Hossain, M.; Lee, Y.; Cho, J.I.; Ahn, C.H.; Lee, S.K.; Jeon, J.S.; Kang, H.; Lee, C.H.; An, G.; Park, P.B. The bZIP transcription factor OsABF1 is an ABA responsive element binding factor that enhances abiotic stress signaling in rice. Plant Mol. Biol. 2010, 72, 557–566. [Google Scholar] [CrossRef]
- Joo, J.; Lee, Y.H.; Song, S.I. Overexpression of the rice basic leucine zipper transcription factor OsbZIP12 confers drought tolerance to rice and makes seedlings hypersensitive to ABA. Plant Biotechnol. Rep. 2014, 8, 431–441. [Google Scholar] [CrossRef]
- Chen, H.; Chen, W.; Zhou, J.L.; He, H.; Chen, L.B.; Chen, H.D.; Deng, X.W. Basic leucine zipper transcription factor OsbZIP16 positively regulates drought resistance in rice. Plant Sci. 2012, 193–194, 8–17. [Google Scholar] [CrossRef]
- Xiang, Y.; Tang, N.; Du, H.; Ye, H.Y.; Xiong, L.Z. Characterization of OsbZIP23 as a key player of the basic leucine zipper transcription factor family for conferring abscisic acid sensitivity and salinity and drought tolerance in rice. Plant Physiol. 2008, 148, 1938–1952. [Google Scholar] [CrossRef]
- Chen, H.; Dai, X.J.; Gu, Z.Y. OsbZIP33 is an ABA-dependent enhancer of drought tolerance in rice. Crop Sci. 2015, 55, 1673. [Google Scholar] [CrossRef]
- Joo, J.; Lee, Y.H.; Song, S.I. OsbZIP42 is a positive regulator of ABA signaling and confers drought tolerance to rice. Planta 2019, 249, 1521–1533. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Zhang, H.; Li, X.H.; Xiao, J.H.; Xiong, L.Z. Constitutive activation of transcription factor OsbZIP46 improves drought tolerance in rice. Plant Physiol. 2012, 158, 1755–1768. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.T.; Wu, Y.B.; Wang, X.P. bZIP transcription factor OsbZIP52/RISBZ5: A potential negative regulator of cold and drought stress response in rice. Planta 2012, 235, 1157–1169. [Google Scholar] [CrossRef]
- Yang, S.Q.; Xu, K.; Chen, S.J.; Li, T.F.; Xia, H.; Chen, L.; Liu, H.Y.; Luo, L.J. A stress-responsive bZIP transcription factor OsbZIP62 improves drought and oxidative tolerance in rice. BMC Plant Biol. 2019, 19, 260. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Lee, D.; Yu, I.; Kim, Y.; Choi, Y.; Kim, J.J. Overexpression of the OsbZIP66 transcription factor enhances drought tolerance of rice plants. Plant Biotechnol. Rep. 2017, 11, 53–62. [Google Scholar] [CrossRef]
- Liu, C.T.; Mao, B.G.; Ou, S.J.; Wang, W.; Liu, L.C.; Wu, Y.B.; Chu, C.C.; Wang, X.P. OsbZIP71, a bZIP transcription factor, confers salinity and drought tolerance in rice. Plant Mol. Biol. 2014, 84, 19–36. [Google Scholar] [CrossRef]
- Lu, G.J.; Gao, C.X.; Zheng, X.N.; Han, B. Identification of OsbZIP72 as a positive regulator of ABA response and drought tolerance in rice. Planta 2009, 229, 605–615. [Google Scholar] [CrossRef]
- Gao, W.W.; Li, M.K.; Yang, S.G.; Gao, C.Z.; Su, Y.; Zeng, X.; Jiao, Z.L.; Xu, W.J.; Zhang, M.Y.; Xia, K.F. miR2105 and the kinase OsSAPK10 co-regulate OsbZIP86 to mediate drought-induced ABA biosynthesis in rice. Plant Physiol. 2022, 189, 889–905. [Google Scholar] [CrossRef]
- Das, P.; Lakra, N.; Nutan, K.K.; Singla-Pareek, S.L.; Pareek, A. A unique bZIP transcription factor imparting multiple stress tolerance in rice. Rice 2019, 12, 58. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, M.X.; Zhang, H.J.; Huang, K.; Chen, M.J.; Chen, C.; Yang, X.; Li, Z.; Chen, H.Y.; Ma, Z.M.; et al. Identification and characterization of EDT1 conferring drought tolerance in rice. J. Plant Biol. 2019, 62, 39–47. [Google Scholar] [CrossRef]
- Yang, A.; Dai, X.Y.; Zhang, W.H. A R2R3-type MYB gene, OsMYB2, is involved in salt, cold, and dehydration tolerance in rice. J. Exp. Bot. 2012, 63, 2541–2556. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.Y.; Xu, Y.Y.; Ma, Q.B.; Xu, W.Y.; Wang, T.; Xue, Y.B.; Chong, K. Overexpression of an R1R2R3 MYB gene, OsMYB3R-2, increases tolerance to freezing, drought, and salt stress in transgenic Arabidopsis. Plant Physiol. 2007, 143, 1739–1751. [Google Scholar] [CrossRef]
- Tang, Y.H.; Bao, X.X.; Zhi, Y.L.; Wu, Q.; Guo, Y.R.; Yin, X.H.; Zeng, L.Q.; Li, J.; Zhang, J.; He, W.L.; et al. Overexpression of a MYB family gene, OsMYB6, increases drought and salinity stress tolerance in transgenic rice. Front. Plant Sci. 2019, 10, 168. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.Y.; Li, J.J.; Liu, P.L.; Duan, J.Z.; Zhao, Y.; Guo, X.; Li, Y.; Zhang, H.L.; Ali, J.; Li, Z.C. Overexpression of OsMYB48-1, a novel MYB-related transcription factor, enhances drought and salinity tolerance in rice. PLoS ONE 2014, 9, e92913. [Google Scholar] [CrossRef]
- Jian, L.; Kang, K.; Choi, Y.; Suh, M.C.; Paek, N.C. Mutation of OsMYB60 reduces rice resilience to drought stress by attenuating cuticular wax biosynthesis. Plant J. 2022, 112, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.X.; Zou, J.J.; Wang, J.X.; Yang, K.Z.; Wang, X.Q.; Le, J. A rice R2R3-Type MYB transcription factor OsFLP positively regulates drought stress response via OsNAC. Int. J. Mol. Sci. 2022, 23, 5873. [Google Scholar] [CrossRef]
- Guo, C.K.; Yao, L.Y.; You, C.J.; Wang, S.S.; Cui, J.; Ge, X.C.; Ma, H. MID1 plays an important role in response to drought stress during reproductive development. Plant J. 2016, 88, 280–293. [Google Scholar] [CrossRef]
- Hu, H.H.; Dai, M.Q.; Yao, J.L.; Xiao, B.Z.; Li, X.H.; Zhang, Q.F.; Xiong, L.Z. Overexpressing a NAM, ATAF, and CUC (NAC) transcription factor enhances drought resistance and salt tolerance in rice. Proc. Natl. Acad. Sci. USA 2006, 103, 12987–12992. [Google Scholar] [CrossRef]
- Lee, D.K.; Chung, P.J.; Jeong, J.S.; Jang, G.; Bang, S.W.; Jung, H.; Kim, Y.S.; Ha, S.H.; Choi, Y.D.; Kim, J.K. The rice OsNAC6 transcription factor orchestrates multiple molecular mechanisms involving root structural adaptions and nicotianamine biosynthesis for drought tolerance. Plant Biotechnol. J. 2017, 15, 754–764. [Google Scholar] [CrossRef]
- Nakashima, K.; Tran, L.S.; Van-Nguyen, D.; Fujita, M.; Maruyama, K.; Todaka, D.; Ito, Y.; Hayashi, N.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Functional analysis of a NAC-type transcription factor OsNAC6 involved in abiotic and biotic stress-responsive gene expression in rice. Plant J. 2007, 51, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.J.; Liao, K.F.; Du, H.; Xu, Y.; Song, H.Z.; Li, X.H.; Xiong, L.Z. A stress-responsive NAC transcription factor SNAC3 confers heat and drought tolerance through modulation of reactive oxygen species in rice. J. Exp. Bot. 2015, 66, 6803–6817. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.B.; Lv, B.; Luo, L.Q.; He, J.M.; Mao, C.J.; Xi, D.D.; Ming, M. The NAC-type transcription factor OsNAC2 regulates ABA-dependent genes and abiotic stress tolerance in rice. Sci. Rep. 2017, 7, 40641. [Google Scholar] [CrossRef]
- Takasaki, H.; Maruyama, K.; Kidokoro, S.; Ito, Y.; Fujita, Y.; Shinozaki, K.; Yamaguchi-Shinozaki, K.; Nakashima, K. The abiotic stress-responsive NAC-type transcription factor OsNAC5 regulates stress-inducible genes and stress tolerance in rice. Mol. Genet. Genom. 2010, 284, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Bang, S.W.; Choi, S.; Jin, X.J.; Jung, S.E.; Choi, J.W.; Seo, J.S.; Kim, J.K. Transcriptional activation of rice CINNAMOYL-CoA REDUCTASE 10 by OsNAC5, contributes to drought tolerance by modulating lignin accumulation in roots. Plant Biotechnol. J. 2022, 20, 736–747. [Google Scholar] [CrossRef]
- Jeong, J.S.; Kim, Y.S.; Redillas, M.C.; Jang, G.; Jung, H.; Bang, S.W.; Choi, Y.D.; Ha, S.H.; Reuzeau, C.; Kim, J.K. OsNAC5 overexpression enlarges root diameter in rice plants leading to enhanced drought tolerance and increased grain yield in the field. Plant Biotechnol. J. 2013, 11, 101–114. [Google Scholar] [CrossRef]
- Jeong, J.S.; Kim, Y.S.; Baek, K.H.; Jung, H.; Ha, S.H.; Do-Choi, Y.; Kim, M.; Reuzeau, C.; Kim, J.K. Root-specific expression of OsNAC10 improves drought tolerance and grain yield in rice under field drought conditions. Plant Physiol. 2010, 153, 185–197. [Google Scholar] [CrossRef]
- Shim, J.S.; Oh, N.; Chung, P.J.; Kim, Y.S.; Choi, Y.D.; Kim, J.K. Overexpression of OsNAC14 improves drought tolerance in rice. Front. Plant Sci. 2018, 9, 310. [Google Scholar] [CrossRef]
- Jung, S.E.; Kim, T.H.; Shim, J.S.; Bang, S.W.; Bin-Yoon, H.; Oh, S.H.; Kim, Y.S.; Oh, S.J.; Seo, J.S.; Kim, J.K. Rice NAC17 transcription factor enhances drought tolerance by modulating lignin accumulation. Plant Sci. 2022, 323, 111404. [Google Scholar] [CrossRef]
- Zheng, X.N.; Chen, B.; Lu, G.J.; Han, B. Overexpression of a NAC transcription factor enhances rice drought and salt tolerance. Biochem. Biophys. Res. Commun. 2009, 379, 985–989. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.F.; Lv, B.; Li, J.; Luo, L.Q.; Lu, S.C.; Zhang, X.; Ma, H.; Ming, F. The NAC family transcription factor OsNAP confers abiotic stress response through the ABA pathway. Plant Cell Physiol. 2014, 55, 604–619. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, Y.H.; Yu, W.C.; Wang, L.P.; Lan, Q.K.; Wang, Y.; Chen, C.B.; Zhang, Y. Knocking out the transcription factor OsNAC092 promoted rice drought tolerance. Biology 2022, 11, 1830. [Google Scholar] [CrossRef]
- Hong, Y.B.; Zhang, H.J.; Huang, L.; Li, D.Y.; Song, F.M. Overexpression of a stress-responsive NAC transcription factor gene ONAC022 improves drought and salt tolerance in rice. Front. Plant Sci. 2016, 7, 4. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, H.; Cai, J.T.; Bi, Y.; Li, D.Y.; Song, F.M. Rice NAC transcription factor ONAC066 functions as a positive regulator of drought and oxidative stress response. BMC Plant Biol. 2019, 19, 278. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Hong, Y.B.; Zhang, H.J.; Li, D.Y.; Song, F.M. Rice NAC transcription factor ONAC095 plays opposite roles in drought and cold stress tolerance. BMC Plant Biol. 2016, 16, 203. [Google Scholar] [CrossRef]
- Lim, C.; Kang, K.; Shim, Y.; Yoo, S.C.; Paek, N.C. Inactivating transcription factor OsWRKY5 enhances drought tolerance through abscisic acid signaling pathways. Plant Physiol. 2022, 188, 1900–1916. [Google Scholar] [CrossRef]
- Song, Y.; Jing, S.; Yu, D. Overexpression of the stress-induced OsWRKY08 improves osmotic stress tolerance in Arabidopsis. Chin. Sci. Bull. 2010, 54, 4671–4678. [Google Scholar] [CrossRef]
- Wu, X.; Shiroto, Y.; Kishitani, S.; Ito, Y.; Toriyama, K. Enhanced heat and drought tolerance in transgenic rice seedlings overexpressing OsWRKY11 under the control of HSP101 promoter. Plant Cell Rep. 2009, 28, 21–30. [Google Scholar] [CrossRef]
- Shen, H.S.; Liu, C.T.; Zhang, Y.; Meng, X.P.; Zhou, X.; Chu, C.C.; Wang, X.P. OsWRKY30 is activated by MAP kinases to confer drought tolerance in rice. Plant Mol. Biol. 2012, 80, 241–253. [Google Scholar] [CrossRef]
- Tao, Z.; Kou, Y.J.; Liu, H.B.; Li, X.H.; Xiao, J.H.; Wang, S.P. OsWRKY45 alleles play different roles in abscisic acid signaling and salt stress tolerance but similar roles in drought and cold tolerance in rice. J. Exp. Bot. 2011, 62, 4863–4874. [Google Scholar] [CrossRef]
- Raineri, J.; Wang, S.; Peleg, Z.; Blumwald, E.; Chan, R.L. The rice transcription factor OsWRKY47 is a positive regulator of the response to water deficit stress. Plant Mol. Biol. 2015, 88, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Chen, L.G.; Zhang, L.P.; Yu, D.Q. Overexpression of OsWRKY72 gene interferes in the abscisic acid signal and auxin transport pathway of Arabidopsis. J. Biosci. 2010, 35, 459–471. [Google Scholar] [CrossRef]
- Zhang, M.X.; Zhao, R.R.; Huang, K.; Wei, Z.Q.; Guo, B.Y.; Huang, S.Z.; Li, Z.; Jiang, W.Z.; Wu, T.; Du, X.L. OsWRKY76 positively regulates drought stress via OsbHLH148-mediated jasmonate signaling in rice. Front. Plant Sci. 2023, 14, 1168723. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Son, S.; Lee, K.S.; Park, Y.J.; Suh, E.J.; Lee, S.I.; Park, S.R. OsWRKY114 negatively regulates drought tolerance by restricting stomatal closure in rice. Plants 2022, 11, 1938. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Sun, S.J.; Xu, D.Q.; Yang, X.; Bao, Y.M.; Wang, Z.F.; Tang, H.J.; Zhang, H.S. Increased tolerance of rice to cold, drought and oxidative stresses mediated by the overexpression of a gene that encodes the zinc finger protein ZFP245. Biochem. Biophys. Res. Commun. 2009, 389, 556–561. [Google Scholar] [CrossRef]
- Xu, D.Q.; Huang, J.; Guo, S.Q.; Yang, X.; Bao, Y.M.; Tang, H.J.; Zhang, H.S. Overexpression of a TFIIIA-type zinc finger protein gene ZFP252 enhances drought and salt tolerance in rice (Oryza sativa L.). FEBS Lett. 2008, 582, 1037–1043. [Google Scholar] [CrossRef]
- Huang, X.Y.; Chao, D.Y.; Gao, J.P.; Zhu, M.Z.; Shi, M.; Lin, H.X. A previously unknown zinc finger protein, DST, regulates drought and salt tolerance in rice via stomatal aperture control. Genes Dev. 2009, 23, 1805–1817. [Google Scholar] [CrossRef]
- Liu, K.M.; Wang, L.; Xu, Y.Y.; Chen, N.; Ma, Q.B.; Li, F.; Chong, K. Overexpression of OsCOIN, a putative cold inducible zinc finger protein, increased tolerance to chilling, salt and drought, and enhanced proline level in rice. Planta 2007, 226, 1007–1016. [Google Scholar] [CrossRef]
- Kothari, K.S.; Dansana, P.K.; Giri, J.; Tyagi, A.K. Rice stress associated protein 1 (OsSAP1) interacts with aminotransferase (OsAMTR1) and pathogenesis-related 1a protein (OsSCP) and regulates abiotic stress responses. Front. Plant Sci. 2016, 7, 1057. [Google Scholar] [CrossRef]
- Dansana, P.K.; Kothari, K.S.; Vij, S.; Tyagi, A.K. OsiSAP1 overexpression improves water-deficit stress tolerance in transgenic rice by affecting expression of endogenous stress-related genes. Plant Cell Rep. 2014, 33, 1425–1440. [Google Scholar] [CrossRef]
- Sharma, G.; Giri, J.; Tyagi, A.K. Rice OsiSAP7 negatively regulates ABA stress signalling and imparts sensitivity to water-deficit stress in Arabidopsis. Plant Sci. 2015, 237, 80–92. [Google Scholar] [CrossRef]
- Kanneganti, V.; Gupta, A.K. Overexpression of OsiSAP8, a member of stress associated protein (SAP) gene family of rice confers tolerance to salt, drought and cold stress in transgenic tobacco and rice. Plant Mol. Biol. 2008, 66, 445–462. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.Y.; Liu, B.H.; Xu, M.Y.; Jamil, M.; Wang, G.P. ABA-induced CCCH tandem zinc finger protein OsC3H47 decreases ABA sensitivity and promotes drought tolerance in Oryza sativa. Biochem. Biophys. Res. Commun. 2015, 464, 33–37. [Google Scholar] [CrossRef]
- Li, J.; Zhang, M.; Yang, L.; Mao, X.; Zou, D. OsADR3 increases drought stress tolerance by inducing antioxidant defense mechanisms and regulating OsGPX1 in rice (Oryza sativa L.). Crop J. 2021, 9, 1003–1017. [Google Scholar] [CrossRef]
- Xin, Z.; Zhang, B.; Li, M.; Yin, X.; Huang, L.; Cui, Y.; Wang, M.; Xia, X. OsMSR15 encoding a rice C2H2-type zinc finger protein confers enhanced drought tolerance in transgenic Arabidopsis. J. Plant Biol. 2016, 59, 271–281. [Google Scholar] [CrossRef]
- Yuan, X.; Huang, P.; Wang, R.Q.; Li, H.Y.; Lv, X.Q.; Duan, M.; Tang, H.J.; Zhang, H.S.; Huang, J. A zinc finger transcriptional repressor confers pleiotropic effects on rice growth and drought tolerance by down-regulating stress-responsive genes. Plant Cell Physiol. 2018, 59, 2129–2142. [Google Scholar] [CrossRef]
- Liu, H.Z.; Zhang, H.J.; Yang, Y.Y.; Li, G.J.; Yang, Y.X.; Wang, X.E.; Basnayake, B.M.V.S.; Li, D.Y.; Song, F.M. Functional analysis reveals pleiotropic effects of rice RING-H2 finger protein gene OsBIRF1 on regulation of growth and defense responses against abiotic and biotic stresses. Plant Mol. Biol. 2008, 68, 17–30. [Google Scholar] [CrossRef]
- Zeng, D.E.; Hou, P.; Xiao, F.; Liu, Y. Overexpressing a novel RING-H2 finger protein gene, OsRHP1, enhances drought and salt tolerance in rice (Oryza sativa L.). J. Plant Biol. 2014, 57, 357–365. [Google Scholar] [CrossRef]
- Xu, K.; Chen, S.J.; Li, T.F.; Ma, X.S.; Liang, X.H.; Ding, X.F.; Liu, H.Y.; Luo, L.J. OsGRAS23, a rice GRAS transcription factor gene, is involved in drought stress response through regulating expression of stress-responsive genes. BMC Plant Biol. 2015, 15, 141. [Google Scholar] [CrossRef]
- Bang, S.W.; Lee, D.K.; Jung, H.; Chung, P.J.; Kim, Y.S.; Choi, Y.D.; Suh, J.W.; Kim, J.K. Overexpression of OsTF1L, a rice HD-Zip transcription factor, promotes lignin biosynthesis and stomatal closure that improves drought tolerance. Plant Biotechnol. J. 2019, 17, 118–131. [Google Scholar] [CrossRef]
- Zhang, S.X.; Haider, I.; Kohlen, W.; Jiang, L.; Bouwmeester, H.; Meijer, A.H.; Schluepmann, H.; Liu, C.M.; Ouwerkerk, P.B. Function of the HD-Zip I gene Oshox22 in ABA-mediated drought and salt tolerances in rice. Plant Mol. Biol. 2012, 80, 571–585. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.L.; Zou, J.; Liu, C.F.; Zhou, X.Y.; Zhang, X.W.; Luo, G.Y.; Chen, X.B. Over-expression of OsHsfA7 enhanced salt and drought tolerance in transgenic rice. BMB Rep. 2013, 46, 31–36. [Google Scholar] [CrossRef]
- Li, X.X.; Yu, B.; Wu, Q.; Min, Q.; Zeng, R.F.; Xie, Z.Z.; Huang, J.L. OsMADS23 phosphorylated by SAPK9 confers drought and salt tolerance by regulating ABA biosynthesis in rice. PLoS Genet. 2021, 17, e1009699. [Google Scholar] [CrossRef] [PubMed]
- Khong, G.N.; Pati, P.K.; Richaud, F.; Parizot, B.; Bidzinski, P.; Mai, C.D.; Bès, M.; Bourrié, I.; Meynard, D.; Beeckman, T.; et al. OsMADS26 negatively regulates resistance to pathogens and drought tolerance in rice. Plant Physiol. 2015, 169, 2935–2949. [Google Scholar] [CrossRef]
- Lee, D.K.; Kim, H.I.; Jang, G.; Chung, P.J.; Jeong, J.S.; Kim, Y.S.; Bang, S.W.; Jung, H.; Choi, Y.D.; Kim, J.K. The NF-YA transcription factor OsNF-YA7 confers drought stress tolerance of rice in an abscisic acid independent manner. Plant Sci. 2015, 241, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.X.; Han, S.C.; Sun, X.M.; Khan, N.U.; Zhong, Q.; Zhang, Z.Y.; Zhang, H.L.; Ming, F.; Li, Z.C.; Li, J.J. Variations in OsSPL10 confer drought tolerance by directly regulating OsNAC2 expression and ROS production in rice. J. Integr. Plant Biol. 2023, 65, 918–933. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy, R.; Jiang, S.Y.; Kumar, N.; Venkatesh, P.N.; Ramachandran, S. A comprehensive transcriptional profiling of the WRKY gene family in rice under various abiotic and phytohormone treatments. Plant Cell Physiol. 2008, 49, 865–879. [Google Scholar] [CrossRef] [PubMed]
- Giri, J.; Parida, S.K.; Raghuvanshi, S.; Tyagi, A.K. Emerging molecular strategies for improving rice drought tolerance. Curr. Genom. 2021, 22, 16–25. [Google Scholar] [CrossRef]
- Zhou, L.G.; Liu, Y.H.; Liu, Z.C.; Kong, D.Y.; Duan, M.; Luo, L.J. Genome-wide identification and analysis of drought-responsive microRNAs in Oryza sativa. J. Exp. Bot. 2010, 61, 4157–4168. [Google Scholar] [CrossRef]
- Mutum, R.D.; Kumar, S.; Balyan, S.; Kansal, S.; Mathur, S.; Raghuvanshi, S. Identification of novel miRNAs from drought tolerant rice variety Nagina 22. Sci. Rep. 2016, 6, 30786. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.S.; Yan, J.; Gou, F.; Mao, Y.F.; Tang, G.L.; Botella, J.R.; Zhu, J.K. Short tandem target mimic rice lines uncover functions of miRNAs in regulating important agronomic traits. Proc. Natl. Acad. Sci. USA 2017, 114, 5277–5282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.W.; Long, Y.; Xue, M.D.; Xiao, X.G.; Pei, X.W. Identification of microRNAs in response to drought in common wild rice (Oryza rufipogon Griff.) shoots and roots. PLoS ONE 2017, 12, e0170330. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Tao, Y.; Zhu, C. Emerging roles of microRNAs in the mediation of drought stress response in plants. J. Exp. Bot. 2013, 64, 3077–3086. [Google Scholar] [CrossRef]
- Tian, C.J.; Zuo, Z.L.; Qiu, J.L. Identification and characterization of ABA-responsive MicroRNAs in rice. J. Genet Genom. 2015, 42, 393–402. [Google Scholar] [CrossRef]
- Um, T.; Choi, J.; Park, T.; Chung, P.J.; Jung, S.E.; Shim, J.S.; Kim, Y.S.; Choi, I.Y.; Park, S.C.; Oh, S.J.; et al. Rice microRNA171f/SCL6 module enhances drought tolerance by regulation of flavonoid biosynthesis genes. Plant Direct. 2022, 6, e374. [Google Scholar] [CrossRef] [PubMed]
- Balyan, S.; Kumar, M.; Mutum, R.D.; Raghuvanshi, U.; Agarwal, P.; Mathur, S.; Raghuvanshi, S. Identification of miRNA-mediated drought responsive multi-tiered regulatory network in drought tolerant rice, Nagina 22. Sci. Rep. 2017, 7, 15446. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Ramkumar, M.K.; Dutta, B.; Kumar, A.; Pandey, R.; Jain, P.K.; Gaikwad, K.; Mishra, D.C.; Chaturvedi, K.K.; Rai, A.; et al. Integration of miRNA dynamics and drought tolerant QTLs in rice reveals the role of miR2919 in drought stress response. BMC Genom. 2023, 24, 526. [Google Scholar] [CrossRef]
- Jiang, D.G.; Zhou, L.Y.; Chen, W.T.; Ye, N.H.; Xia, J.X.; Zhuang, C.X. Overexpression of a microRNA-targeted NAC transcription factor improves drought and salt tolerance in rice via ABA-mediated pathways. Rice 2019, 12, 76. [Google Scholar] [CrossRef]
- Lu, Y.Z.; Feng, Z.; Liu, X.Y.; Bian, L.Y.; Xie, H.; Zhang, C.L.; Mysore, K.S.; Liang, J.S. MiR393 and miR390 synergistically regulate lateral root growth in rice under different conditions. BMC Plant Biol. 2018, 18, 261. [Google Scholar] [CrossRef]
- Xia, K.F.; Wang, R.; Ou, X.J.; Fang, Z.M.; Tian, C.G.; Duan, J.; Wang, Y.Q.; Zhang, M.Y. OsTIR1 and OsAFB2 downregulation via OsmiR393 overexpression leads to more tillers, early flowering and less tolerance to salt and drought in rice. PLoS ONE 2012, 7, e30039. [Google Scholar] [CrossRef]
- Grewal, R.K.; Saraf, S.; Deb, A.; Kundu, S. Differentially expressed MicroRNAs link cellular physiology to phenotypic changes in rice under stress conditions. Plant Cell Physiol. 2018, 59, 2143–2154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.S.; Zhang, H.; Srivastava, A.K.; Pan, Y.J.; Bai, J.J.; Fang, J.J.; Shi, H.Z.; Zhu, J.K. Knockdown of rice MicroRNA166 confers drought resistance by causing leaf rolling and altering stem xylem development. Plant Physiol. 2018, 176, 2082–2094. [Google Scholar] [CrossRef] [PubMed]
- Smalley, S.; Hellmann, H. Review: Exploring possible approaches using ubiquitylation and sumoylation pathways in modifying plant stress tolerance. Plant Sci. 2022, 319, 111275. [Google Scholar] [CrossRef] [PubMed]
- Park, G.G.; Park, J.J.; Yoon, J.; Yu, S.N.; An, G. A RING finger E3 ligase gene, Oryza sativa Delayed Seed Germination 1 (OsDSG1), controls seed germination and stress responses in rice. Plant Mol. Biol. 2010, 74, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.; Kim, S.K.; Cho, S.K.; Kang, B.G.; Kim, W.T. Overexpression of OsRDCP1, a rice RING domain-containing E3 ubiquitin ligase, increased tolerance to drought stress in rice (Oryza sativa L.). Plant Sci. 2011, 180, 775–782. [Google Scholar] [CrossRef]
- Gao, T.; Wu, Y.R.; Zhang, Y.Y.; Liu, L.J.; Ning, Y.S.; Wang, D.J.; Tong, H.N.; Chen, S.Y.; Chu, C.C.; Xie, Q. OsSDIR1 overexpression greatly improves drought tolerance in transgenic rice. Plant Mol Biol. 2011, 76, 145–156. [Google Scholar] [CrossRef]
- Kim, S.; Park, S.I.; Kwon, H.; Cho, M.H.; Kim, B.G.; Chung, J.H.; Nam, M.H.; Song, J.S.; Kim, K.H.; Yoon, I.S. The rice abscisic acid-responsive RING Finger E3 Ligase OsRF1 targets OsPP2C09 for degradation and confers drought and salinity tolerance in rice. Front. Plant Sci. 2022, 12, 797940. [Google Scholar] [CrossRef]
- Cui, L.H.; Min, H.J.; Byun, M.Y.; Oh, H.G.; Kim, W.T. OsDIRP1, a putative RING E3 Ligase, plays an opposite role in drought and cold stress responses as a negative and positive factor, respectively, in rice (Oryza sativa L.). Front. Plant Sci. 2018, 9, 1797. [Google Scholar] [CrossRef]
- Kim, J.H.; Lim, S.D.; Jang, C.S. Oryza sativa drought-, heat-, and salt-induced RING finger protein 1 (OsDHSRP1) negatively regulates abiotic stress-responsive gene expression. Plant Mol. Biol. 2020, 103, 235–252. [Google Scholar] [CrossRef]
- Lv, Q.L.; Li, X.X.; Jin, X.K.; Sun, Y.; Wu, Y.Y.; Wang, W.M.; Huang, J.L. Rice OsPUB16 modulates the ‘SAPK9-OsMADS23-OsAOC’ pathway to reduce plant water-deficit tolerance by repressing ABA and JA biosynthesis. PLoS Genet. 2022, 18, e1010520. [Google Scholar] [CrossRef]
- Seo, D.H.; Lee, A.; Yu, S.G.; Cui, L.H.; Min, H.J.; Lee, S.E.; Cho, N.H.; Kim, S.; Bae, H.; Kim, W.T. OsPUB41, a U-box E3 ubiquitin ligase, acts as a negative regulator of drought stress response in rice (Oryza sativa L.). Plant Mol. Biol. 2021, 106, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Kamynina, E.; Stover, P.J. The roles of SUMO in metabolic regulation. Adv. Exp. Med. Biol. 2017, 963, 143–168. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.; Choi, D.H.; Lee, Y.H.; Seo, H.S.; Song, S.I. The rice SUMO conjugating enzymes OsSCE1 and OsSCE3 have opposing effects on drought stress. J. Plant Physiol. 2019, 240, 152993. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.; Srivastava, A.P.; Esmaeili, N.; Hu, W.; Shen, G. Overexpression of the rice gene OsSIZ1 in Arabidopsis improves drought-, heat-, and salt-tolerance simultaneously. PLoS ONE 2018, 13, e0201716. [Google Scholar] [CrossRef]
- Mishra, N.; Sun, L.; Zhu, X.; Smith, J.; Prakash-Srivastava, A.; Yang, X.; Pehlivan, N.; Esmaeili, N.; Luo, H.; Shen, G.; et al. Overexpression of the rice SUMO E3 ligase gene OsSIZ1 in cotton enhances drought and heat tolerance, and substantially improves fiber yields in the field under reduced irrigation and rainfed conditions. Plant Cell Physiol. 2017, 58, 735–746. [Google Scholar] [CrossRef]
- Li, Z.G.; Hu, Q.; Zhou, M.; Vandenbrink, J.; Li, D.Y.; Menchyk, N.; Reighard, S.; Norris, A.; Liu, H.B.; Sun, D.F.; et al. Heterologous expression of OsSIZ1, a rice SUMO E3 ligase, enhances broad abiotic stress tolerance in transgenic creeping bentgrass. Plant Biotechnol. J. 2013, 11, 432–445. [Google Scholar] [CrossRef]
- Zhang, W.J.; Zhou, Y.W.; Zhang, Y.; Su, Y.H.; Xu, T.D. Protein phosphorylation: A molecular switch in plant signaling. Cell Rep. 2023, 42, 112729. [Google Scholar] [CrossRef]
- Ke, Y.Q.; Han, G.P.; He, H.Q.; Li, J.X. Differential regulation of proteins and phosphoproteins in rice under drought stress. Biochem. Biophys. Res. Commun. 2009, 379, 133–138. [Google Scholar] [CrossRef]
- Rohila, J.; Yang, Y. Rice mitogen-activated protein kinase gene family and its role in biotic and abiotic stress response. J. Integr. Plant Biol. 2007, 49, 751–759. [Google Scholar] [CrossRef]
- Xiong, L.Z.; Yang, Y.N. Disease resistance and abiotic stress tolerance in rice are inversely modulated by an abscisic acid inducible mitogen-activated protein kinase. Plant Cell 2003, 15, 745–759. [Google Scholar] [CrossRef]
- Zhang, G.; Shen, T.; Ren, N.; Jiang, M.Y. Phosphorylation of OsABA2 at Ser197 by OsMPK1 regulates abscisic acid biosynthesis in rice. Biochem. Biophys. Res. Commun. 2022, 586, 68–73. [Google Scholar] [CrossRef]
- Koo, S.C.; Moon, B.C.; Kim, J.K.; Kim, C.Y.; Sung, S.J.; Kim, M.C.; Cho, M.J.; Cheong, Y.H. OsBWMK1 mediates SA-dependent defense responses by activating the transcription factor OsWRKY33. Biochem. Biophys. Res. Commun. 2009, 387, 365–370. [Google Scholar] [CrossRef]
- Lou, D.J.; Lu, S.P.; Chen, Z.; Lin, Y.; Yu, D.Q.; Yang, X.Y. Molecular characterization reveals that OsSAPK3 improves drought tolerance and grain yield in rice. BMC Plant Biol. 2023, 23, 53. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, Y.F.; Xie, Z.Z.; Yu, B.; Sun, Y.; Huang, J.L. OsNAC016 regulates plant architecture and drought tolerance by interacting with the kinases GSK2 and SAPK8. Plant Physiol. 2022, 189, 1296–1313. [Google Scholar] [CrossRef]
- Dey, A.; Samanta, M.K.; Gayen, S.; Maiti, M.K. The sucrose non-fermenting 1-related kinase 2 gene SAPK9 improves drought tolerance and grain yield in rice by modulating cellular osmotic potential, stomatal closure and stress-responsive gene expression. BMC Plant Biol. 2016, 16, 158. [Google Scholar] [CrossRef]
- Saijo, Y.; Hata, S.; Kyozuka, J.; Shimamoto, K.; Izui, K. Over-expression of a single Ca2+-dependent protein kinase confers both cold and salt/drought tolerance on rice plants. Plant J. 2000, 23, 319–327. [Google Scholar] [CrossRef]
- Wang, L.L.; Yu, C.C.; Xu, S.L.; Zhu, Y.G.; Huang, W.C. OsDi19-4 acts downstream of OsCDPK14 to positively regulate ABA response in rice. Plant Cell Environ. 2016, 39, 2740–2753. [Google Scholar] [CrossRef]
- Du, C.Q.; Cai, W.G.; Lin, F.M.; Wang, K.; Li, S.; Chen, C.; Tian, H.R.; Wang, D.C.; Zhao, Q.Z. Leucine-rich repeat receptor-like kinase OsASLRK regulates abscisic acid and drought responses via cooperation with S-like RNase OsRNS4 in rice. Environ. Exp. Bot. 2022, 201, 104949. [Google Scholar] [CrossRef]
- Nagar, P.; Sharma, N.; Jain, M.; Sharma, G.; Prasad, M.; Mustafiz, A. OsPSKR15, a phytosulfokine receptor from rice enhances abscisic acid response and drought stress tolerance. Physiol. Plant. 2022, 174, e13569. [Google Scholar] [CrossRef]
- Wu, F.Q.; Sheng, P.K.; Tan, J.J.; Chen, X.L.; Lu, G.W.; Ma, W.W.; Heng, Y.Q.; Lin, Q.B.; Zhu, S.S.; Wang, J.L.; et al. Plasma membrane receptor-like kinase leaf panicle 2 acts downstream of the DROUGHT AND SALT TOLERANCE transcription factor to regulate drought sensitivity in rice. J. Exp. Bot. 2015, 66, 271–281. [Google Scholar] [CrossRef]
- Kang, J.F.; Li, J.M.; Gao, S.; Tian, C.; Zha, X.J. Overexpression of the leucine-rich receptor-like kinase gene LRK2 increases drought tolerance and tiller number in rice. Plant Biotechnol. J. 2017, 15, 1175–1185. [Google Scholar] [CrossRef]
- Ouyang, S.Q.; Liu, Y.F.; Liu, P.; Lei, G.; He, S.J.; Ma, B.; Zhang, W.K.; Zhang, J.S.; Chen, S.Y. Receptor-like kinase OsSIK1 improves drought and salt stress tolerance in rice (Oryza sativa) plants. Plant J. 2010, 62, 316–329. [Google Scholar] [CrossRef]
- Aubry, S.; Brown, N.J.; Hibberd, J.M. The role of proteins in C(3) plants prior to their recruitment into the C(4) pathway. J. Exp. Bot. 2011, 62, 3049–3059. [Google Scholar] [CrossRef]
- Liu, X.L.; Li, X.; Zhang, C.; Dai, C.C.; Zhou, J.Y.; Ren, C.G.; Zhang, J.F. Phosphoenolpyruvate carboxylase regulation in C4-PEPC-expressing transgenic rice during early responses to drought stress. Physiol. Plant 2017, 159, 178–200. [Google Scholar] [CrossRef]
- You, J.; Zong, W.; Hu, H.H.; Li, X.H.; Xiao, J.H.; Xiong, L.Z. A STRESS-RESPONSIVE NAC1-regulated protein phosphatase gene rice protein phosphatase18 modulates drought and oxidative stress tolerance through abscisic acid-independent reactive oxygen species scavenging in rice. Plant Physiol. 2014, 166, 2100–2114. [Google Scholar] [CrossRef]
- Li, C.X.; Shen, H.Y.; Wang, T.; Wang, X.L. ABA regulates subcellular redistribution of OsABI-LIKE2, a negative regulator in aba signaling, to control root architecture and drought resistance in Oryza sativa. Plant Cell Physiol. 2015, 56, 2396–2408. [Google Scholar] [CrossRef]
- Kim, H.J.; Triplett, B.A. Cotton fiber germin-like protein. I. Molecular cloning and gene expression. Planta 2004, 218, 516–524. [Google Scholar] [CrossRef]
- Gucciardo, S.; Wisniewski, J.P.; Brewin, N.J.; Bornemann, S. A germin-like protein with superoxide dismutase activity in pea nodules with high protein sequence identity to a putative rhicadhesin receptor. J. Exp. Bot. 2007, 58, 1161–1171. [Google Scholar] [CrossRef]
- Lu, Y.; Zhou, D.X.; Zhao, Y. Understanding epigenomics based on the rice model. Theor. Appl. Genet. 2020, 133, 1345–1363. [Google Scholar] [CrossRef]
- Gayacharan; Joel, A.J. Epigenetic responses to drought stress in rice (Oryza sativa L.). Physiol. Mol. Biol. Plants 2013, 19, 379–387. [Google Scholar] [CrossRef]
- Auler, P.A.; Nogueira do Amaral, M.; Bolacel-Braga, E.J.; Maserti, B. Drought stress memory in rice guard cells: Proteome changes and genomic stability of DNA. Plant Physiol. Biochem. 2021, 169, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.S.; Qin, Q.; Sun, F.; Wang, Y.X.; Xu, D.D.; Li, Z.K.; Fu, B.Y. Genome-wide differences in DNA methylation changes in two contrasting rice genotypes in response to drought conditions. Front. Plant Sci. 2016, 7, 1675. [Google Scholar] [CrossRef] [PubMed]
- Kou, S.; Gu, Q.; Duan, L.; Liu, G.; Yuan, P.; Li, H.; Wu, Z.; Liu, W.; Huang, P.; Liu, L. Genome-wide bisulphite sequencing uncovered the contribution of DNA methylation to rice short-term drought memory formation. J. Plant Growth Regul. 2021, 41, 2903–2917. [Google Scholar] [CrossRef]
- Ding, G.; Cao, L.; Zhou, J.; Li, Z.; Lai, Y.; Liu, K.; Luo, Y.; Bai, L.; Wang, X.; Wang, T. DNA methylation correlates with the expression of drought-responsive genes and drought resistance in rice. Agronomy 2022, 12, 1445. [Google Scholar] [CrossRef]
- Waseem, M.; Huang, F.; Wang, Q.; Aslam, M.M.; Abbas, F.; Ahmad, F.; Ashraf, U.; Hassan, W.; Fiaz, S.; Ye, X.; et al. Identification, methylation profiling, and expression analysis of stress-responsive cytochrome P450 genes in rice under abiotic and phytohormones stresses. GM Crop. Food 2021, 12, 551–563. [Google Scholar] [CrossRef]
- Wang, W.S.; Pan, Y.J.; Zhao, X.Q.; Dwivedi, D.; Zhu, L.H.; Ali, J.; Fu, B.Y.; Li, Z.K. Drought-induced site-specific DNA methylation and its association with drought tolerance in rice (Oryza sativa L.). J. Exp. Bot. 2011, 62, 1951–1960. [Google Scholar] [CrossRef]
- Zheng, X.G.; Chen, L.; Lou, Q.J.; Xia, H.; Li, M.S.; Luo, L.J. Changes in DNA methylation pattern at two seedling stages in water saving and drought-resistant rice variety after drought stress domestication. Rice Sci. 2014, 21, 262–270. [Google Scholar] [CrossRef]
- Zheng, X.G.; Chen, L.; Xia, H.; Wei, H.B.; Lou, Q.J.; Li, M.S.; Li, T.M.; Luo, L.J. Transgenerational epimutations induced by multi-generation drought imposition mediate rice plant’s adaptation to drought condition. Sci. Rep. 2017, 7, 39843. [Google Scholar] [CrossRef]
- Yuan, L.Y.; Liu, X.C.; Luo, M.; Yang, S.G.; Wu, K.Q. Involvement of histone modifications in plant abiotic stress responses. J. Integr. Plant Biol. 2013, 55, 892–901. [Google Scholar] [CrossRef]
- Zong, W.; Zhong, X.C.; You, J.; Xiong, L.Z. Genome-wide profiling of histone H3K4-tri-methylation and gene expression in rice under drought stress. Plant Mol. Biol. 2013, 81, 175–188. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, A.; Panda, D.; Modi, M.K.; Sen, P. Identification and characterization of drought responsive miRNAs from a drought tolerant rice genotype of Assam. Plant Gene 2020, 21, 100213. [Google Scholar] [CrossRef]
- Zong, W.; Yang, J.; Fu, J.; Xiong, L.Z. Synergistic regulation of drought-responsive genes by transcription factor OsbZIP23 and histone modification in rice. J. Integr. Plant Biol. 2020, 62, 723–729. [Google Scholar] [CrossRef]
- Song, T.; Zhang, Q.; Wang, H.Q.; Han, J.B.; Xu, Z.Q.; Yan, S.N.; Zhu, Z.G. OsJMJ703, a rice histone demethylase gene, plays key roles in plant development and responds to drought stress. Plant Physiol. Biochem. 2018, 132, 183–188. [Google Scholar] [CrossRef]
- Chen, K.; Du, K.X.; Shi, Y.C.; Yin, L.F.; Shen, W.H.; Yu, Y.; Liu, B.; Dong, A.W. H3K36 methyltransferase SDG708 enhances drought tolerance by promoting abscisic acid biosynthesis in rice. New Phytol. 2021, 230, 1967–1984. [Google Scholar] [CrossRef]
- Zhao, J.H.; Zhang, W.; da Silva, J.A.T.; Liu, X.C.; Duan, J. Rice histone deacetylase HDA704 positively regulates drought and salt tolerance by controlling stomatal aperture and density. Planta 2021, 254, 79. [Google Scholar] [CrossRef] [PubMed]
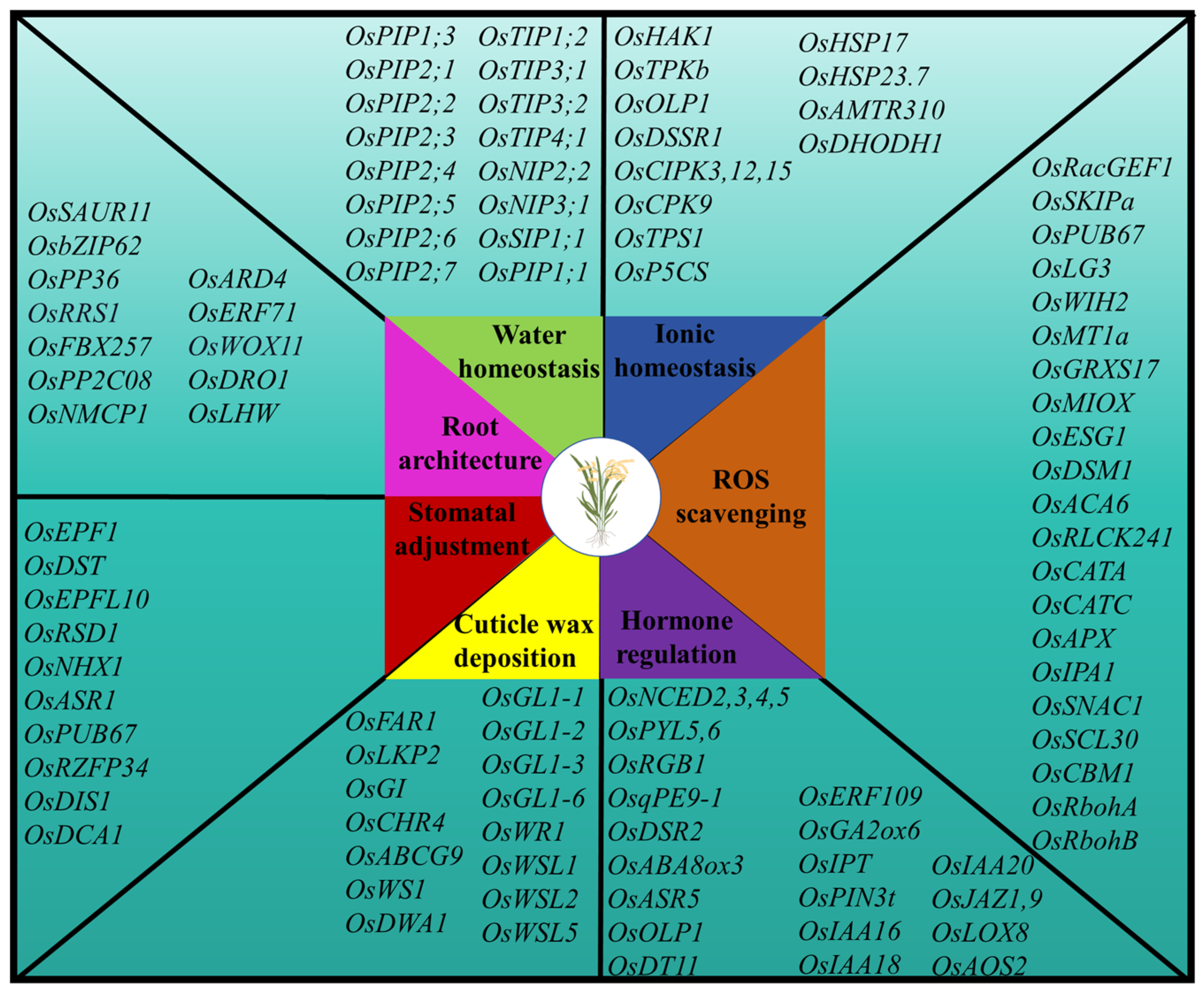
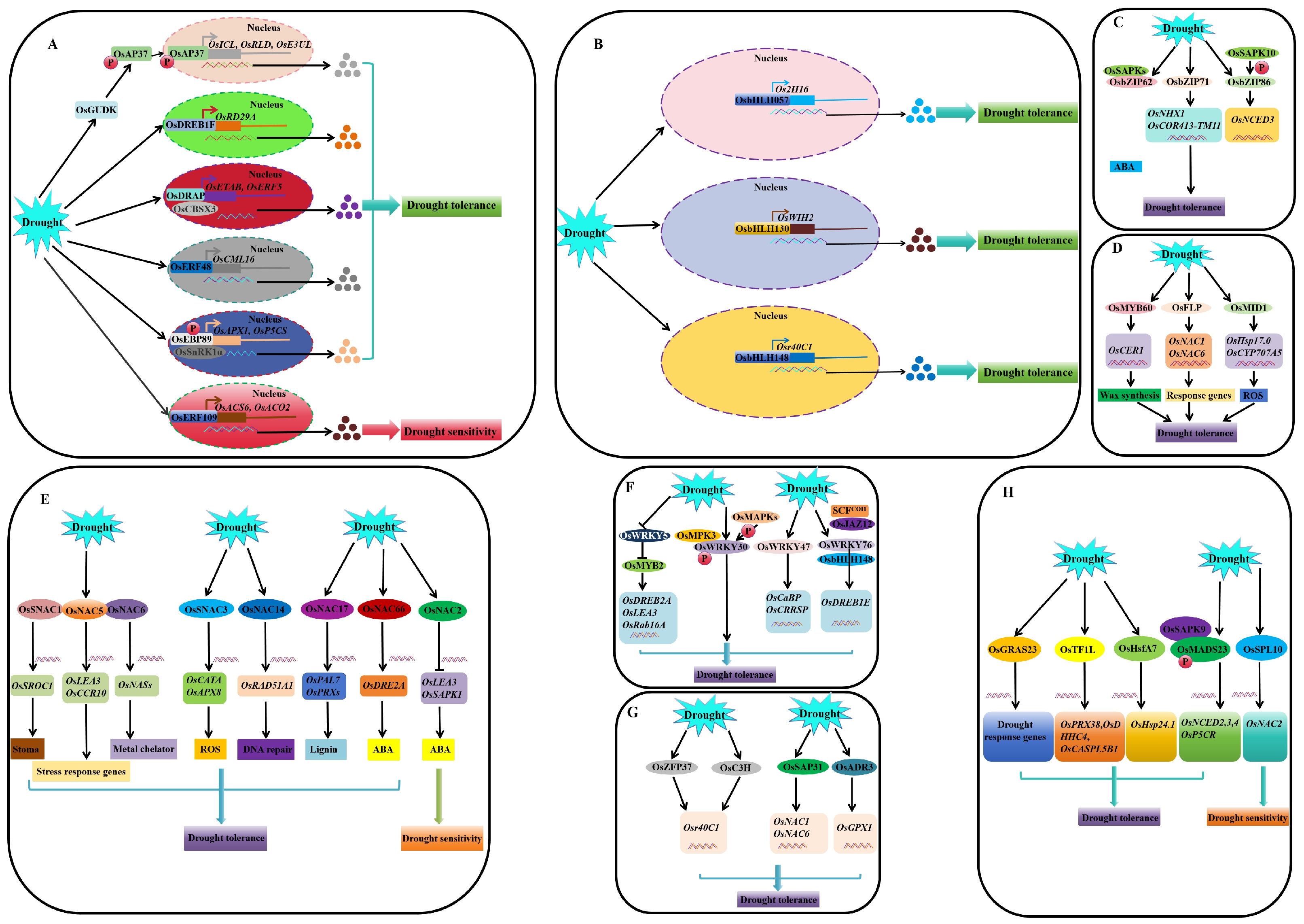


| Location (Organ) | Level | Influence | References |
|---|---|---|---|
| Seed germination (seedlings) | morphological | Buds wither, growth is slowed down, and seed root length and total seedling length are inhibited. | [6,15] |
| physiological and biochemical | Water balance is destroyed, membrane transport is damaged, ATP production is reduced, respiration is inhibited, seed germination is delayed and poor. Catalase (CAT), peroxidase (POD), and superoxide dismutase (SOD) activities are changed, and there is an accumulation of free proline. | [6,16,17,18] | |
| Leaves | morphological | Leaf length, width, area, and number, and cell growth and elongation are significantly reduced. The leaves curl, the stomata close, the leaf tip dries or even dies, and the leaf shape changes. There is a reduction in both fresh and dry leaf weight. | [15,19,20,21,22] |
| physiological and biochemical | Chlorophyll a, b, a/b, total chlorophyll content, carotenoid content, Fv/Fm, relative water content and membrane stability are significantly reduced, and photosynthesis is inhibited. The leaf water potential is reduced, gas exchange is disturbed, assimilate transport and phloem loading are damaged, relative electrolyte permeability increases, and the distribution of cytokinin and auxin (IAA) changes. There is an accumulation of proline and an increase in CAT, SOD, glutathione reductase (GR), monodehydroascorbate reductase (MDHAR), and dehydroascorbate reductase (DHAR) activity. The content of MDA and H2O2 increases, and notable changes in nitrogen metabolism indexes, such as nitrogen concentration, glutamine synthetase, and protein content, and carbohydrate metabolism indexes, such as soluble sugar and starch content. | [10,22,23,24,25] | |
| Roots | morphological | There is a decrease in fresh and dry weight, root diameter, xylem vessel diameter or vessel number, and aerenchyma formation. The roots shorten, leading to a decreased biomass. There is an increase in the lateral roots and sclerenchyma cell diameter. | [20,23,26,27] |
| physiological and biochemical | Root hydraulic conductivity decreases, xylem sap decreases, and there is a change in root activity, protein, proline, and pigment content. The activities of antioxidant enzymes such as GR, MDHAR, SOD, and DHAR increase. | [22,25,27] | |
| Flowers and grains | morphological | Florets formation is destroyed, resulting in slow grain filling, increased spikelet sterility, and decreased grain weight, size, 1000-grain weight, and seed setting rate. Maturity time is changed, and there is a decrease in biomass and yield. | [19,23,25] |
| physiological and biochemical | Starch and amino acid content changes, and there is sugar starvation and an increase in proline content. The activities of ascorbate peroxidase (APX), glutathione (GSH), and ascorbic acid (AsA) increase. | [28,29,30] |
| Gene Family | Gene | Gene ID | Positive (+)/Negative (−) Regulation | References |
|---|---|---|---|---|
| AP2/EREBP | OsAP37 | LOC_Os01g58420 | + | [152,153] |
| OsAP59 | LOC_Os02g43790 | + | [152] | |
| OsDREB1A | LOC_Os09g35030 | + | [154] | |
| OsDREB1B | LOC_Os09g35010 | + | [154] | |
| OsDREB1F | LOC_Os01g73770 | + | [155] | |
| OsDREB1G | LOC_Os02g45450 | + | [156] | |
| OsDREB2B | LOC_Os05g27930 | + | [156] | |
| OsDREB1E | LOC_Os04g48350 | + | [156] | |
| OsDREB6 | LOC_Os09g20350 | + | [157] | |
| OsARAG1 | LOC_Os02g43970 | + | [158] | |
| OsAP21 | LOC_Os01g10370 | + | [159] | |
| OsDRAP1 | LOC_Os08g31580 | + | [160] | |
| OsERF71 | LOC_Os06g09390 | + | [161] | |
| OsERF101 | LOC_Os04g32620 | + | [162] | |
| OsERF109 | LOC_Os09g13940 | − | [103] | |
| OsEBP89 | LOC_Os03g08460 | − | [163] | |
| OsLG3 | LOC_Os03g08470 | + | [76] | |
| OsHYR | LOC_Os03g02650 | + | [164] | |
| bHLH | OsbHLH057 | LOC_Os07g35870 | + | [165] |
| OsbHLH130 | LOC_Os09g31300 | + | [77] | |
| OsbHLH148 | LOC_Os03g53020 | + | [166,167] | |
| OsICE1 | LOC_Os11g32100 | + | [168] | |
| bZIP | OsbZIP10 | LOC_Os01g64000 | + | [169] |
| OsbZIP12 | LOC_Os01g64730 | + | [170] | |
| OsbZIP16 | LOC_Os02g09830 | + | [171] | |
| OsbZIP23 | LOC_Os02g52780 | + | [172] | |
| OsbZIP33 | LOC_Os03g58250 | + | [173] | |
| OsbZIP42 | LOC_Os05g41070 | + | [174] | |
| OsbZIP46 | LOC_Os06g10880 | + | [175] | |
| OsbZIP52 | LOC_Os06g45140 | − | [176] | |
| OsbZIP62 | LOC_Os07g48660 | + | [177] | |
| OsbZIP66 | LOC_Os08g36790 | + | [178] | |
| OsbZIP71 | LOC_Os09g13570 | + | [179] | |
| OsbZIP72 | LOC_Os09g28310 | + | [180] | |
| OsbZIP86 | LOC_Os12g13170 | + | [181] | |
| OsHBP1b | LOC_Os01g17260 | + | [182] | |
| OsEDT1 | LOC_Os05g36160 | + | [183] | |
| OsMYB2 | LOC_Os03g20090 | + | [184] | |
| OsMYB3R-2 | LOC_Os01g62410 | + | [185] | |
| OsMYB6 | LOC_Os04g58020 | + | [186] | |
| OsMYB48-1 | LOC_Os01g74410 | + | [187] | |
| OsMYB60 | LOC_Os12g03150 | + | [188] | |
| OsFLP | LOC_Os07g43420 | + | [189] | |
| OsMID1 | LOC_Os05g37060 | + | [190] | |
| NAC | OsSNAC1 | LOC_Os03g60080 | + | [191] |
| OsSNAC2 | LOC_Os01g66120 | + | [192,193] | |
| OsSNAC3 | LOC_Os01g09550 | + | [194] | |
| OsNAC2 | LOC_Os04g38720 | − | [195] | |
| OsNAC5 | LOC_Os11g08210 | + | [196,197,198] | |
| OsNAC6 | LOC_Os01g66120 | + | [197] | |
| OsNAC10 | LOC_Os11g03300 | + | [199] | |
| OsNAC14 | LOC_Os01g48446 | + | [200] | |
| OsNAC17 | LOC_Os03g21030 | + | [201] | |
| OsNAC45 | LOC_Os11g03370 | + | [202] | |
| OsNAC58 | LOC_Os03g21060 | + | [203] | |
| OsNAC092 | LOC_Os06g46270 | − | [204] | |
| ONAC022 | LOC_Os03g04070 | + | [205] | |
| ONAC066 | LOC_Os03g56580 | + | [206] | |
| ONAC095 | LOC_Os06g51070 | − | [207] | |
| WRKY | OsWRKY5 | LOC_Os05g04640 | + | [208] |
| OsWRKY8 | LOC_Os05g50610 | + | [209] | |
| OsWRKY11 | LOC_Os01g43650 | + | [210] | |
| OsWRKY30 | LOC_Os08g38990 | + | [211] | |
| OsWRKY45-1 | LOC_Os05g25770 | − | [212] | |
| OsWRKY45-2 | LOC_Os05g25770 | − | [212] | |
| OsWRKY47 | LOC_Os07g48260 | + | [213] | |
| OsWRKY72 | LOC_Os11g29870 | − | [214] | |
| OsWRKY76 | LOC_Os09g25060 | + | [215] | |
| OsWRKY114 | LOC_Os12g02400 | − | [216] | |
| ZFP | OsZFP37 | LOC_Os03g38870 | + | [77] |
| OsC3H | LOC_Os09g31482 | + | [77] | |
| OsZFP245 | LOC_Os07g39870 | + | [217] | |
| OsZFP252 | LOC_Os12g39400 | + | [218] | |
| OsDST | LOC_Os03g57240 | + | [219] | |
| OsCOIN | LOC_Os01g01420 | + | [220] | |
| OsSAP1 | LOC_Os09g31200 | + | [221,222] | |
| OsiSAP7 | LOC_Os03g57900 | − | [223] | |
| OsiSAP8 | LOC_Os06g41010 | + | [224] | |
| OsC3H47 | LOC_Os07g04580 | + | [225] | |
| OsADR3 | LOC_Os03g55540 | + | [226] | |
| OsMSR15 | LOC_Os03g41390 | + | [227] | |
| OsDRZ1 | LOC_Os03g32220 | + | [228] | |
| OsBIRF1 | LOC_Os02g50930 | + | [229] | |
| OsRHP1 | LOC_Os08g38460 | + | [230] | |
| Others | OsGRAS23 | LOC_Os04g50060 | + | [231] |
| OsTF1L | LOC_Os08g19590 | + | [232] | |
| Oshox22 | LOC_Os04g45810 | − | [233] | |
| OsHsfA7 | LOC_Os01g39020 | + | [234] | |
| OsMADS23 | LOC_Os08g33488 | + | [235] | |
| OsMADS26 | LOC_Os08g02070 | − | [236] | |
| OsNF-YA7 | LOC_Os08g09690 | + | [237] | |
| OsSPL10 | LOC_Os06g44860 | + | [238] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, A.; Lian, W.; Wang, Y.; Liu, M.; Zhang, Y.; Wang, X.; Chen, G. Molecular Mechanisms and Regulatory Pathways Underlying Drought Stress Response in Rice. Int. J. Mol. Sci. 2024, 25, 1185. https://doi.org/10.3390/ijms25021185
Geng A, Lian W, Wang Y, Liu M, Zhang Y, Wang X, Chen G. Molecular Mechanisms and Regulatory Pathways Underlying Drought Stress Response in Rice. International Journal of Molecular Sciences. 2024; 25(2):1185. https://doi.org/10.3390/ijms25021185
Chicago/Turabian StyleGeng, Anjing, Wenli Lian, Yihan Wang, Minghao Liu, Yue Zhang, Xu Wang, and Guang Chen. 2024. "Molecular Mechanisms and Regulatory Pathways Underlying Drought Stress Response in Rice" International Journal of Molecular Sciences 25, no. 2: 1185. https://doi.org/10.3390/ijms25021185
APA StyleGeng, A., Lian, W., Wang, Y., Liu, M., Zhang, Y., Wang, X., & Chen, G. (2024). Molecular Mechanisms and Regulatory Pathways Underlying Drought Stress Response in Rice. International Journal of Molecular Sciences, 25(2), 1185. https://doi.org/10.3390/ijms25021185







