Temperature-Induced Sex Differentiation in River Prawn (Macrobrachium nipponense): Mechanisms and Effects
Abstract
1. Introduction
2. Results
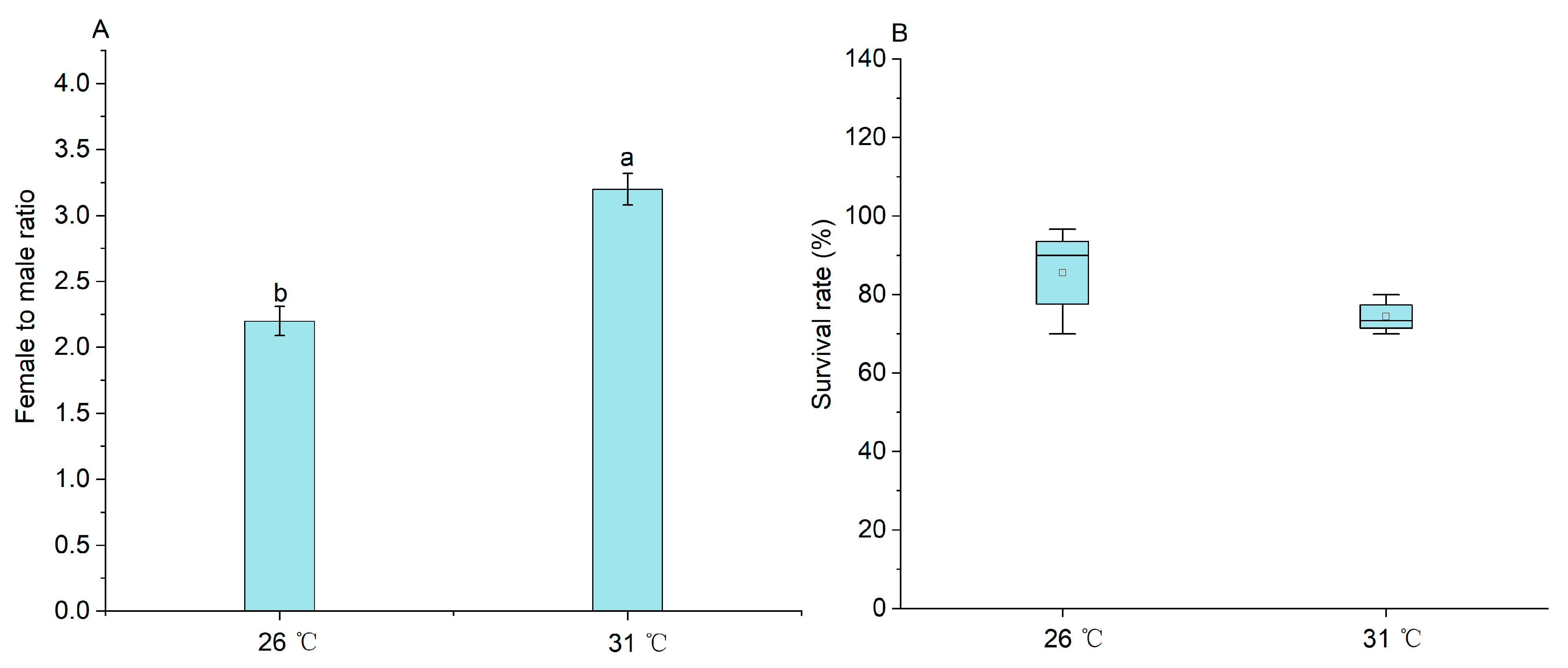
2.1. The Effect of Temperature on the Sex Ratio of M. nipponense
2.2. Temperature-Sensitive Sex Differentiation Phase of M. nipponense
2.3. Methylation Levels on M. nipponense from Two Temperature Treatments
2.4. DEGs in Male and Female Prawns from Two Temperature Treatments
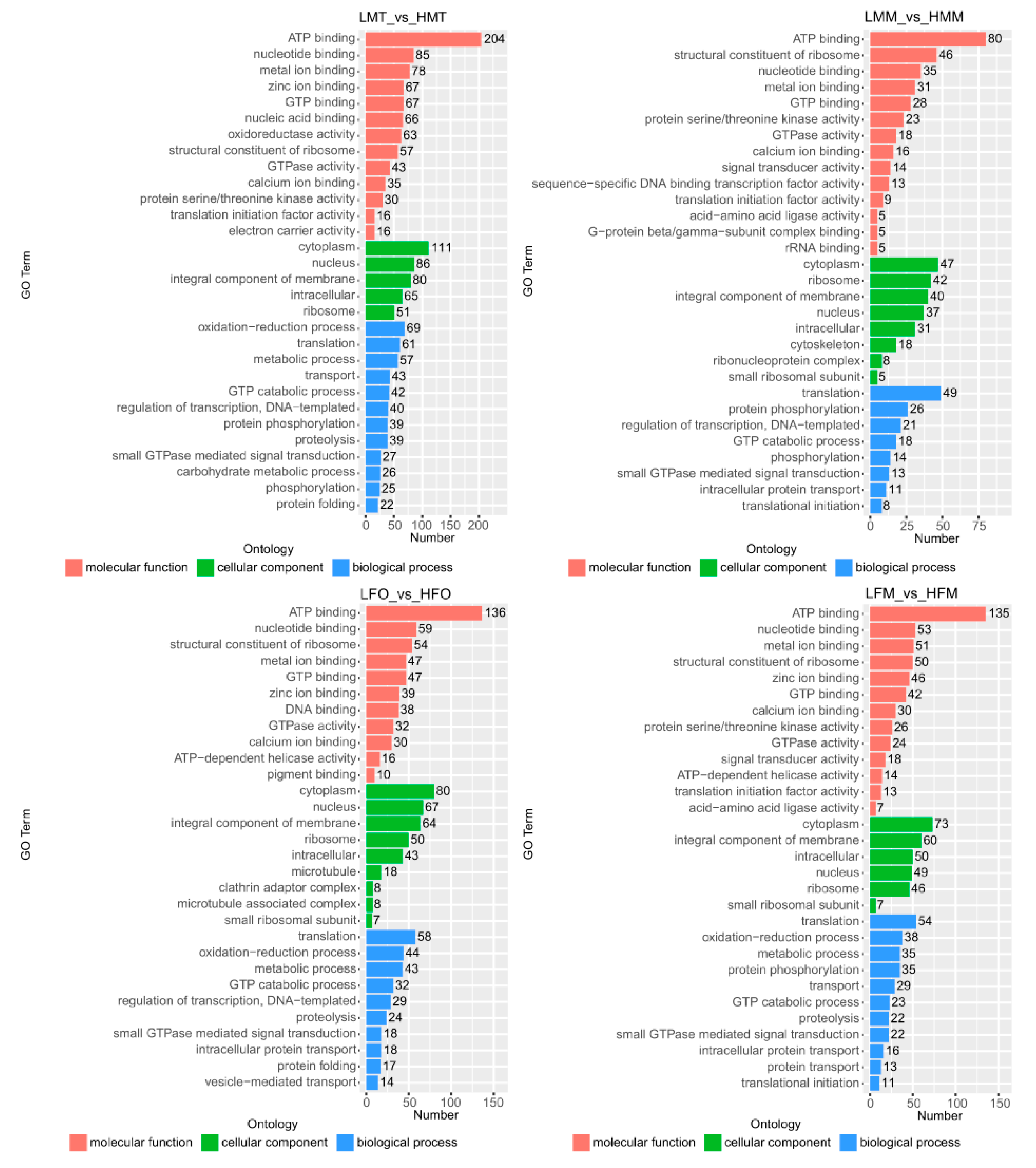
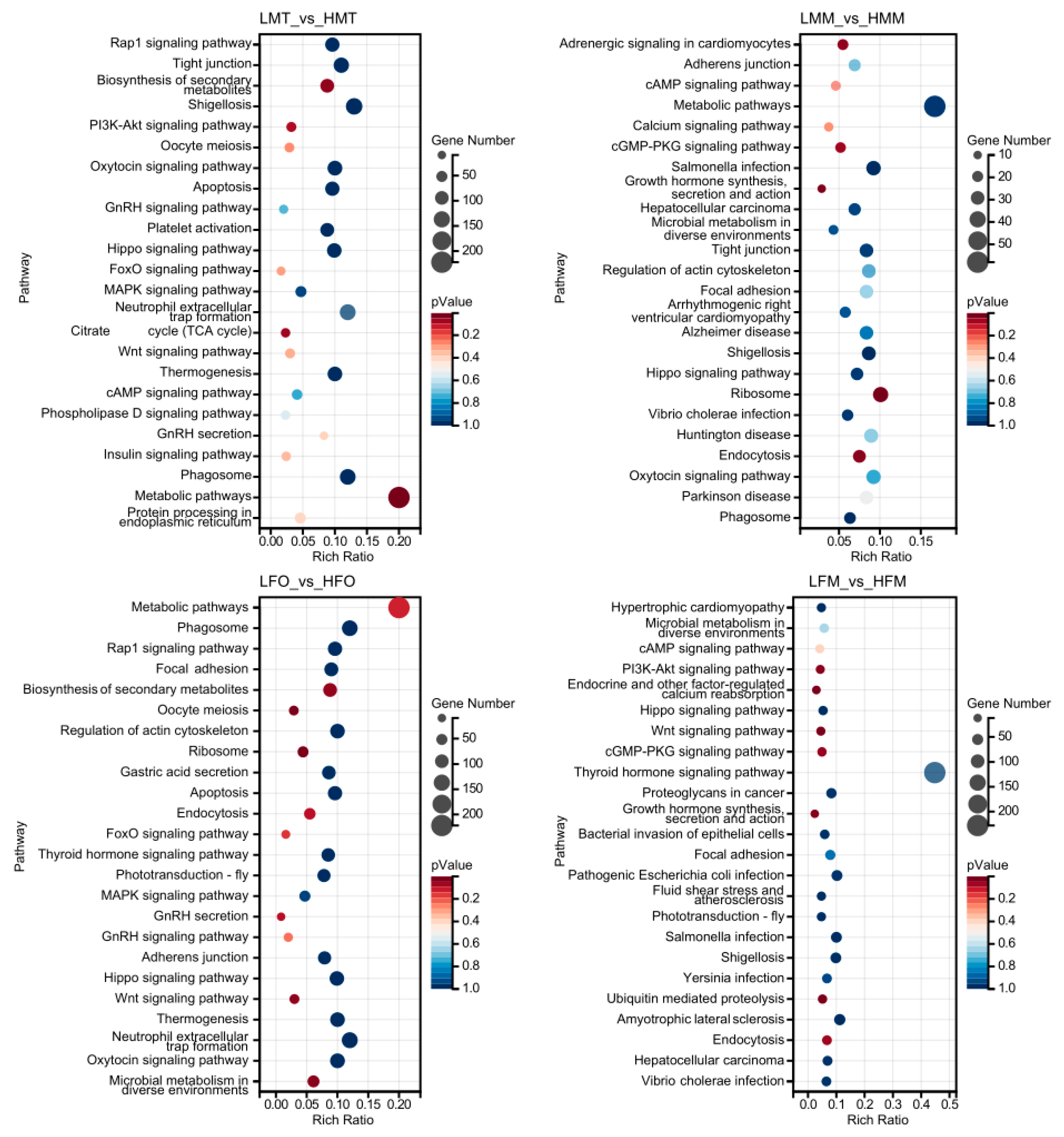
2.5. DEG Functional Enrichment Analysis with GO and KEGG Databases
2.6. Candidates of Temperature-Regulated Sex-Differentiation Genes
2.7. mRNA Expression Profiling of Potential Temperature-Regulated Sex-Differentiation Genes
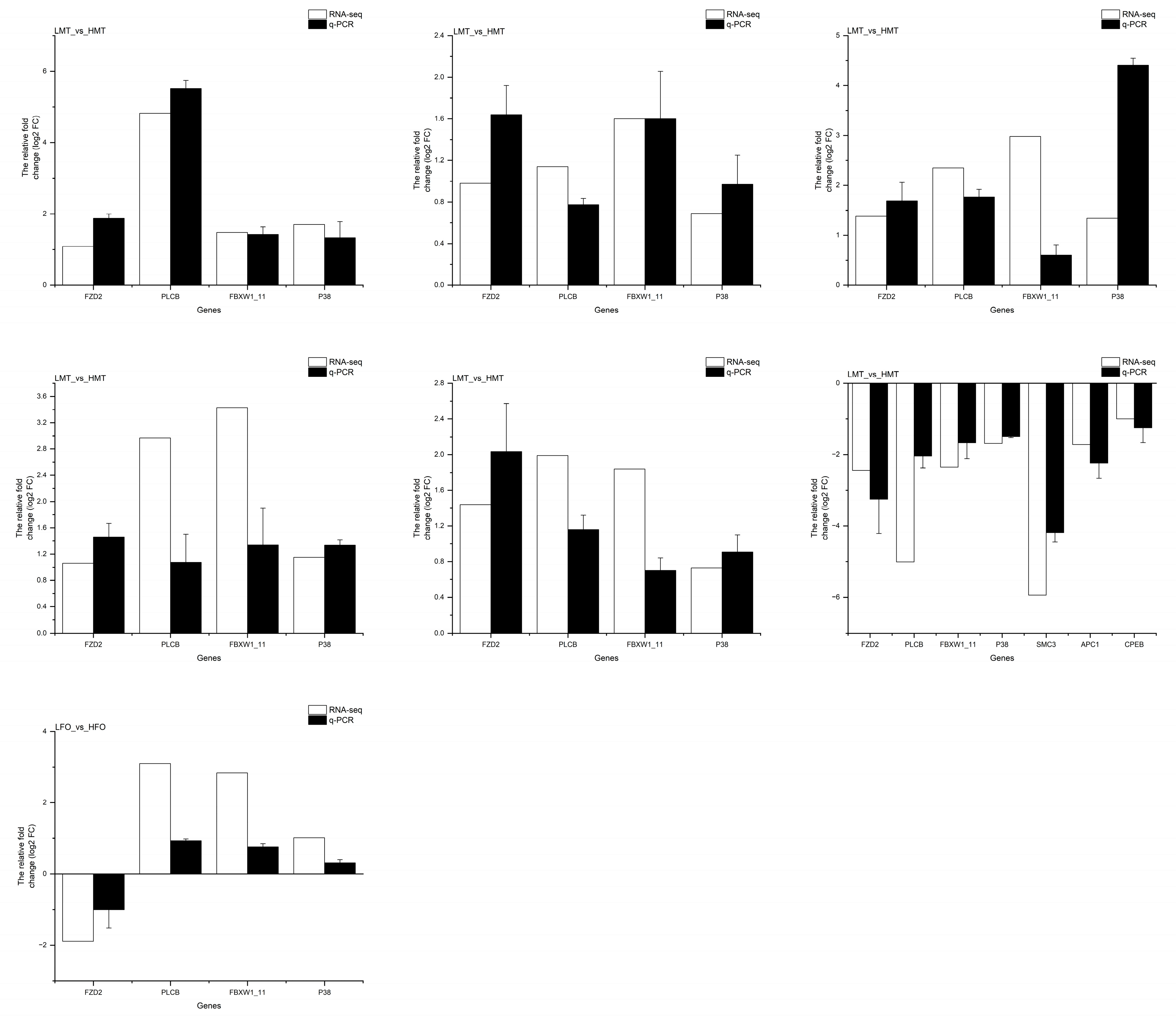
2.8. Quantitative Polymerase Chain Reaction (qPCR) Analysis
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Sex Differentiation under Different Temperatures
4.3. Temperature-Sensitive Sex-Differentiation Phase
4.4. Whole-Genome Bisulfite Sequencing (WGBS) Libraries
4.5. Transcriptomic Analysis Using RNA-Sequencing
4.6. Quantitative Real-Time PCR (qRT-PCR) Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, K.; Feng, J.; Lin, J.; Li, J. The complete mitochondrial genome of Macrobrachium nipponense. Gene 2011, 487, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Cui, L.F.; Li, S.M. Bureau of Fisheries, Ministry of Agriculture, P.R.C. Fisheries Economic Statistics. China Fishery Yearbook; Beijing China Agricultural Press: Beijing, China, 2020; Volume 2020, p. 24. [Google Scholar]
- Wang, Y.; Jin, S.; Fu, H.; Qiao, H.; Sun, S.; Zhang, W.; Jiang, S.; Gong, Y.; Xiong, Y.; Wu, Y. Identification and Characterization of the DMRT11E Gene in the Oriental River Prawn Macrobrachium nipponense. Int. J. Mol. Sci. 2019, 20, 1734. [Google Scholar] [CrossRef] [PubMed]
- Mork, L.; Czerwinski, M.; Capel, B. Predetermination of sexual fate in a turtle with temperature-dependent sex determination. Dev. Biol. 2014, 386, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Zarkower, D. Establishing sexual dimorphism: Conservation amidst diversity? Nat. Rev. Genet. 2001, 2, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Kobayashi, K.; Watanabe, H.; Iguchi, T. Environmental Sex Determination in the Branchiopod Crustacean Daphnia magna: Deep Conservation of a Doublesex Gene in the Sex-Determining Pathway. PLoS Genet. 2011, 7, e1001345. [Google Scholar] [CrossRef]
- Bachtrog, D.; Mank, J.E.; Peichel, C.L.; Kirkpatrick, M.; Otto, S.P.; Ashman, T.L.; Hahn, M.W.; Kitano, J. Sex Determination: Why So Many Ways of Doing It? PLoS Biol. 2014, 12, e1001899. [Google Scholar] [CrossRef] [PubMed]
- Baroiller, J.F.; D’Cotta, H. The Reversible Sex of Gonochoristic Fish: Insights and Consequences. Sex. Dev. 2016, 10, 242–266. [Google Scholar] [CrossRef]
- Literman, R.; Burrett, A.; Bista, B.; Valenzuela, N. Putative Independent Evolutionary Reversals from Genotypic to Temperature-Dependent Sex Determination are Associated with Accelerated Evolution of Sex-Determining Genes in Turtles. J. Mol. Evol. 2018, 86, 11–26. [Google Scholar] [CrossRef]
- Bulmer, M.G.; Bull, J.J. Models of polygenic sex determination and sex ratio control. Evol. Int. J. Org. Evol. 1982, 36, 13–26. [Google Scholar] [CrossRef]
- Wedekind, C. Demographic and genetic consequences of disturbed sex determination. Philos. Trans. R. Soc. B-Biol. Sci. 2017, 372, 20160326. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Crews, D. Molecular mechanisms of temperature-dependent sex determination in the context of ecological developmental biology. Mol. Cell. Endocrinol. 2012, 354, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.; Ye, J.; Weber, C.; Sun, W.; Zhang, H.; Zhou, Y.; Cai, C.; Qian, G.; Capel, B. The histone demethylase KDM6B regulates temperature-dependent sex determination in a turtle species. Science 2018, 360, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Fisher, L.R.; Godfrey, M.H.; Owens, D.W. Incubation Temperature Effects on Hatchling Performance in the Loggerhead Sea Turtle (Caretta caretta). PLoS ONE 2014, 9, e114880. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.F.; Feng, W.; Jin, S.B.; Bai, H.K.; Sun, S.M.; Fu, H.T. Molecular characterization of insulin-like androgenic gland hormone-binding protein gene from the oriental river prawn Macrobrachium nipponense and investigation of its transcriptional relationship with the insulin-like androgenic gland hormone gene. Gen. Comp. Endocrinol. 2015, 216, 152–160. [Google Scholar] [CrossRef]
- Jin, S.; Hu, Y.; Fu, H.; Jiang, S.; Xiong, Y.; Qiao, H.; Zhang, W.; Gong, Y.; Wu, Y. Potential Functions of Gem-Associated Protein 2-Like Isoform X1 in the Oriental River Prawn Macrobrachium nipponense: Cloning, qPCR, In Situ Hybridization, and RNAi Analysis. Int. J. Mol. Sci. 2019, 20, 3995. [Google Scholar] [CrossRef]
- Jin, S.; Fu, Y.; Hu, Y.; Fu, H.; Jiang, S.; Xiong, Y.; Qiao, H.; Zhang, W.; Gong, Y.; Wu, Y. Identification of candidate genes from androgenic gland in Macrobrachium nipponense regulated by eyestalk ablation. Sci. Rep. 2021, 11, 19855. [Google Scholar] [CrossRef]
- Ma, K.Y.; Liu, Z.Q.; Lin, J.Y.; Li, J.L.; Qiu, G.F. Molecular characterization of a novel ovary-specific gene fem-1 homolog from the oriental river prawn, Macrobrachium nipponense. Gene 2016, 575, 244–252. [Google Scholar] [CrossRef]
- Sun, R.; Li, Y.H. A sex-reversing factor: Insulin-like androgenic gland hormone in decapods. Rev. Aquac. 2021, 13, 1352–1366. [Google Scholar] [CrossRef]
- Farhadi, A.; Cui, W.X.; Zheng, H.P.; Li, S.K.; Zhang, Y.L.; Ikhwanuddin, M.; Ma, H.Y. The regulatory mechanism of sexual development in decapod crustaceans. Front. Mar. Sci. 2021, 8, 679687. [Google Scholar] [CrossRef]
- Farhadi, A. Sex determination and developmental mechanism of crustaceans and shellfish-volume II. Front. Endocrinol. 2023, 14, 1155209. [Google Scholar] [CrossRef]
- Nagahama, Y.; Yoshikuni, M.; Yamashita, M.; Tanaka, M. 13 Regulation of Oocyte Maturation in Fish. Fish Physiol. 1994, 13, 393–439. [Google Scholar] [CrossRef]
- He, M.N.; Zhang, T.; Yang, Y.; Wang, C. Mechanisms of Oocyte Maturation and Related Epigenetic Regulation. Front. Cell Dev. Biol. 2021, 9, 654028. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.A.; Wagle, M.; Tran, K.; Zhan, X.M.; Dixon, M.A.; Liu, S.C.; Gros, D.; Korver, W.; Yonkovich, S.; Tomasevic, N. R-Spondin family members regulate the Wnt pathway by a common mechanism. Mol. Biol. Cell 2008, 19, 2588–2596. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, A.; Wu, S. Estrogen Receptor-beta in the Gonadotropin-Releasing Hormone Neuron. Semin. Reprod. Med. 2012, 30, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Chebbi, M.A.; Becking, T.; Moumen, B.; Giraud, I.; Gilbert, C.; Peccoud, J.; Cordaux, R. The Genome of Armadillidium vulgare (Crustacea, Isopoda) Provides Insights into Sex Chromosome Evolution in the Context of Cytoplasmic Sex Determination. Mol. Biol. Evol. 2019, 36, 727–741. [Google Scholar] [CrossRef]
- Zhang, A.; Huang, R.; Chen, L.; Xiong, L.; He, L.; Li, Y.; Liao, L.; Zhu, Z.; Wang, Y. Computational identification of Y-linked markers and genes in the grass carp genome by using a pool-and-sequence method. Sci. Rep. 2017, 7, 8213. [Google Scholar] [CrossRef]
- Thompson-Davis, D.C. The Effect of Temperature on Sex Determination in the Domestic Chicken (Gallus domesticus); The University of Manchester: Manchester, UK, 1995. [Google Scholar]
- Porter, E.; Booth, D.T.; Limpus, C.J.; Staines, M.N.; Smith, C.E. Influence of short-term temperature drops on sex-determination in sea turtles. J. Exp. Zool. Part A-Ecol. Integr. Physiol. 2021, 335, 649–658. [Google Scholar] [CrossRef]
- Saillant, E.; Fostier, A.; Haffray, P.; Menu, B.; Thimonier, J.; Chatain, B. Temperature effects and genotype-temperature interactions on sex determination in the European sea bass (Dicentrarchus labrax L.). J. Exp. Zool. 2002, 292, 494–505. [Google Scholar] [CrossRef]
- Epper, F.; Bryant, P.J. Sex-specific control of growth and differentiation in the Drosophila genital disc, studied using a temperature-sensitive transformer-2 mutation. Dev. Biol. 1983, 100, 294–307. [Google Scholar] [CrossRef]
- Raynaud, A.; Pieau, C. Embryonic development of the genital system. Biol. Reptil. 1985, 1, 149–300. Available online: https://www.researchgate.net/publication/312916852_Embryonic_development_of_the_genital_system (accessed on 20 May 2023).
- Girondot, M.; Monsinjon, J.; Guillon, J.M. Delimitation of the embryonic thermosensitive period for sex determination using an embryo growth model reveals a potential bias for sex ratio prediction in turtles. J. Therm. Biol. 2018, 73, 32–40. [Google Scholar] [CrossRef]
- Rougeot, C.; Prignon, C.; Kengne, C.V.N.; Melard, C. Effect of high temperature during embryogenesis on the sex differentiation process in the Nile tilapia, Oreochromis niloticus. Aquaculture 2008, 276, 205–208. [Google Scholar] [CrossRef]
- Hui, S.; Ying, W.S.; Mei, P.K.; He, Y.X. Histological studies on gonadiai differentiation and development of Varicorhinus macrolepis. J. Fish. Sci. China 2006, 13, 723–731. [Google Scholar] [CrossRef]
- Jin, S.B.; Zhang, W.Y.; Xiong, Y.W.; Fu, H.T. Recent progress of male sexual differentiation and development in the oriental river prawn (Macrobrachium nipponense): A review. Rev. Aquac. 2023, 15, 305–317. [Google Scholar] [CrossRef]
- Ceballos-Vázquez, B.P.; Palacios, E.; Aguilar-Villavicencio, J.; Racotta, I.S. Gonadal development in male and female domesticated whiteleg shrimp, Litopenaeus vannamei, in relation to age and weight. Aquaculture 2010, 308, 116–123. [Google Scholar] [CrossRef]
- Valdivieso, A.; Ribas, L.; Monleon-Getino, A.; Orban, L.; Piferrer, F. Exposure of zebrafish to elevated temperature induces sex ratio shifts and alterations in the testicular epigenome of unexposed offspring. Environ. Res. 2020, 186, 109601. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Buemio, A.; Chu, R.; Vafaee, M.; Crews, D. Epigenetic Control of Gonadal Aromatase (cyp19a1) in Temperature-Dependent Sex Determination of Red-Eared Slider Turtles. PLoS ONE 2013, 8, e63599. [Google Scholar] [CrossRef]
- Navarro-Martin, L.; Vinas, J.; Ribas, L.; Diaz, N.; Gutierrez, A.; Di Croce, L.; Piferrer, F. DNA Methylation of the Gonadal Aromatase (cyp19a) Promoter Is Involved in Temperature-Dependent Sex Ratio Shifts in the European Sea Bass. PLoS Genet. 2011, 7, e1002447. [Google Scholar] [CrossRef]
- Monga, R.; Ghai, S.; Datta, T.K.; Singh, D. Tissue-specific promoter methylation and histone modification regulate CYP19 gene expression during folliculogenesis and luteinization in buffalo ovary. Gen. Comp. Endocrinol. 2011, 173, 205–215. [Google Scholar] [CrossRef]
- Angelopoulou, R.; Lavranos, G.; Manolakou, P. Sex determination strategies in 2012: Towards a common regulatory model? Reprod. Biol. Endocrinol. 2012, 10, 13. [Google Scholar] [CrossRef]
- Munger, S.C.; Capel, B. Sex and the circuitry: Progress toward a systems-level understanding of vertebrate sex determination. Wiley Interdiscip. Rev. -Syst. Biol. Med. 2012, 4, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, N.; Neuwald, J.L.; Literman, R. Transcriptional evolution underlying vertebrate sexual development. Dev. Dyn. 2013, 242, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Cutting, A.; Chue, J.; Smith, C.A. Just how conserved is vertebrate sex determination? Dev. Dyn. 2013, 242, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Navara, K.J. Hormone-Mediated Adjustment of Sex Ratio in Vertebrates. Integr. Comp. Biol. 2013, 53, 877–887. [Google Scholar] [CrossRef]
- Uengwetwanit, T.; Ponza, P.; Sangsrakru, D.; Wichadakul, D.; Ingsriswang, S.; Leelatanawit, R.; Klinbunga, S.; Tangphatsornruang, S.; Karoonuthaisiri, N. Transcriptome-based discovery of pathways and genes related to reproduction of the black tiger shrimp (Penaeus monodon). Mar. Genom. 2018, 37, 69–73. [Google Scholar] [CrossRef]
- Du, Y.X.; Ma, K.Y.; Qiu, G.F. Discovery of the genes in putative GnRH signaling pathway with focus on characterization of GnRH-like receptor transcripts in the brain and ovary of the oriental river prawn Macrobrachium nipponense. Aquaculture 2015, 442, 1–11. [Google Scholar] [CrossRef]
- Dang, A.K.; Murtazina, D.A.; Magee, C.; Navratil, A.M.; Clay, C.M.; Amberg, G.C. GnRH Evokes Localized Subplasmalemmal Calcium Signaling in Gonadotropes. Mol. Endocrinol. 2014, 28, 2049–2059. [Google Scholar] [CrossRef]
- Harrison, G.S.; Wierman, M.E.; Nett, T.M.; Glode, L.M. Gonadotropin-releasing hormone and its receptor in normal and malignant cells. Endocr. -Relat. Cancer 2004, 11, 725–748. [Google Scholar] [CrossRef]
- Mayer, S.I.; Willars, G.B.; Nishida, E.; Thiel, G. Elk-1, CREB, and MKP-1 Regulate Egr-1 Expression in Gonadotropin-Releasing Hormone Stimulated Gonadotrophs. J. Cell. Biochem. 2008, 105, 1267–1278. [Google Scholar] [CrossRef]
- Tourtellotte, W.G.; Nagarajan, R.; Auyeng, A.; Mueller, C.; Milbrandt, J. Infertility associated with incomplete spermatogenic arrest and oligozoospermia in Egr4-deficient mice. Development 1999, 126, 5061–5071. [Google Scholar] [CrossRef]
- Jeays-Ward, K. Endothelial and steroidogenic cell migration are regulated by WNT4 in the developing mammalian gonad. Development 2003, 130, 3663. [Google Scholar] [CrossRef]
- Vanio, S.; Heikkila, M.; Kispert, A.; Chin, N.; McMahon, A. Female Development in Mammals is Regulated by Wnt4Signaling. Endocrinol. 1999, 9, 323. [Google Scholar] [CrossRef]
- Chassot, A.A.; Ranc, F.; Gregoire, E.P.; Roepers-Gajadien, H.L.; Taketo, M.M.; Camerino, G.; De Rooij, D.G.; Schedl, A.; Chaboissier, M.C. Activation of beta-catenin signaling by Rspo1 controls differentiation of the mammalian ovary. Hum. Mol. Genet. 2008, 17, 1264–1277. [Google Scholar] [CrossRef] [PubMed]
- The significant sex-biased expression pattern of Sp-Wnt4 provides novel insights into the ovarian development of mud crab (Scylla paramamosain). Int. J. Biol. Macromol. 2021, 183, 490–501. [CrossRef] [PubMed]
- Rhen, T.; Even, Z.; Brenner, A.; Lodewykm, A.; Das, D.; Singh, S.; Simmons, R. Evolutionary Turnover in Wnt Gene Expression but Conservation of Wnt Signaling during Ovary Determination in a TSD Reptile. Sex. Dev. 2021, 15, 47–68. [Google Scholar] [CrossRef]
- Janda, C.Y.; Waghray, D.; Levin, A.M.; Thomas, C.; Garcia, K.C. Structural Basis of Wnt Recognition by Frizzled. Science 2012, 337, 59–64. [Google Scholar] [CrossRef]
- Guan, Y.; Leu, N.A.; Ma, J.; Chmatal, L.; Ruthel, G.; Bloom, J.C.; Lampson, M.A.; Schimenti, J.C.; Luo, M.; Wang, P.J. SKP1 drives the prophase I to metaphase I transition during male meiosis. Sci. Adv. 2020, 6, eaaz2129. [Google Scholar] [CrossRef]
- Risha, M.A.; Ali, A.; Siengdee, P.; Trakooljul, N.; Haack, F.; Dannenberger, D.; Wimmers, K.; Ponsuksili, S. Wnt signaling related transcripts and their relationship to energy metabolism in C2C12 myoblasts under temperature stress. PeerJ 2021, 9, e11625. [Google Scholar] [CrossRef]
- Jin, S.B.; Fu, H.T.; Sun, S.M.; Jiang, S.F.; Xiong, Y.W.; Gong, Y.S.; Qiao, H.; Zhang, W.Y.; Wu, Y. Integrated analysis of microRNA and mRNA expression profiles during the sex-differentiation sensitive period in oriental river prawn, Macrobrachium nipponense. Sci. Rep. 2017, 7, 12011. [Google Scholar] [CrossRef]
- Luo, D.D.; He, Z.; Yu, C.X.; Guan, Q.B. Role of p38 MAPK Signalling in Testis Development and Male Fertility. Oxid. Med. Cell. Longev. 2022, 2022, 6891897. [Google Scholar] [CrossRef]
- Johansen, T.; Lamark, T. Selective Autophagy: ATG8 Family Proteins, LIR Motifs and Cargo Receptors. J. Mol. Biol. 2020, 432, 80–103. [Google Scholar] [CrossRef]
- Pankiv, S.; Lamark, T.; Bruun, J.A.; Overvatn, A.; Bjorkoy, G.; Johansen, T. Nucleocytoplasmic Shuttling of p62/SQSTM1 and Its Role in Recruitment of Nuclear Polyubiquitinated Proteins to Promyelocytic Leukemia Bodies. J. Biol. Chem. 2010, 285, 5941–5953. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J.; Emr, S.D. Autophagy as a Regulated Pathway of Cellular Degradation. Science 2000, 290, 1717–1721. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Levine, B.; Cuervo, A.M.; Klionsky, D.J. Autophagy fights disease through cellular self-digestion. Nature 2008, 451, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Li, G.; Liu, C.; Gao, H.; Wang, H.; Liu, W.; Chen, M.; Shang, Y.; Wang, L.; Shi, J. Autophagy regulates testosterone synthesis by facilitating cholesterol uptake in Leydig cells. J. Cell Biol. 2018, 217, 2103–2119. [Google Scholar] [CrossRef]
- Pennell, T.M.; Morrow, E.H. Two sexes, one genome: The evolutionary dynamics of intralocus sexual conflict. Ecol. Evol. 2013, 3, 1819–1834. [Google Scholar] [CrossRef] [PubMed]
- Van Doorn, G.S.; Kirkpatrick, M. Turnover of sex chromosomes induced by sexual conflict. Nature 2007, 449, 909–912. [Google Scholar] [CrossRef]
- Werren, J.H.; Beukeboom, L.W. Sex determination, sex ratios, and genetic conflict. Annu. Rev. Ecol. Syst. 1998, 29, 233–261. [Google Scholar] [CrossRef]
- Bull, J.J. Sex determining mechanisms: An evolutionary perspective. Cell. Mol. Life Sci. 1985, 41, 1285–1296. [Google Scholar] [CrossRef]
- Janzen, F.J. Climate change and temperature-dependent sex determination in reptiles. Proc. Natl. Acad. Sci. USA 1994, 91, 7487–7490. [Google Scholar] [CrossRef]
- Jiang, G.; Zhu, Y.; Xue, Y.; Lu, Y.; Liu, Z.; Huang, X. Screening and Analyzing of Genes and Signaling Pathways Associated with Size Differentiation of Adult Male Prawn Macrobrachium nipponense. Aquac. Res. 2023, 2023, 3134932. [Google Scholar] [CrossRef]
- Cole, T.B.A.W.; Geo, P.; Ali, M. Transcript assembly and abundance estimation from RNA-Seq reveals thousands of new transcripts and switching among isoforms. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef]
- Robinson, M.D.; Mccarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139. [Google Scholar] [CrossRef] [PubMed]
- Kwong, K.S.; Holland, B.; Cheung, S.H. A modified Benjamini-Hochberg multiple comparisons procedure for controlling the false discovery rate. J. Stat. Plan. Inference 2002, 104, 351–362. [Google Scholar] [CrossRef]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3-new capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef]





| Category | LFM | HFM | LMM | HMM | LFO | HFO | LMT | HMT |
|---|---|---|---|---|---|---|---|---|
| mCG | 91.97 ± 0.18 a | 92.66 ± 0.18 a | 92.09 ± 0.11 a | 89.78 ± 1.01 a | 87.23 ± 1.11 a | 90.49 ± 0.38 a | 89.66 ± 0.41 a | 92.25 ± 0.15 a |
| mCHG | 1.92 ± 0.04 c | 1.93 ± 0.04 c | 2.04 ± 0.03 c | 2.47 ± 0.18 c | 2.56 ± 0.23 c | 2.19 ± 0.07 c | 2.36 ± 0.08 c | 1.77 ± 0.03 c |
| mCHH | 6.29 ± 0.14 b | 5.41 ± 0.14 b | 5.87 ± 0.08 b | 7.75 ± 0.83 b | 8.48 ± 0.88 b | 7.32 ± 0.31 b | 7.99 ± 0.34 b | 5.98 ± 0.12 b |
| Signal Pathway | DEGs | Gene Annotation | Tissues | |
|---|---|---|---|---|
| LMT_vs_ HMT | LFO_vs_ HFO | |||
| CACNA1C | voltage-dependent calcium channel L type alpha-1C | Up | No | |
| GnRH signaling pathway | GNAS | guanine nucleotide-binding protein G(s) subunit alpha | Up | No |
| CAMKII | calcium/calmodulin-dependent protein kinase (CaM kinase) II | Up | Down | |
| ADCY1 | adenylate cyclase 1 | Up | Down | |
| P38 | p38 MAP kinase | Up | No | |
| EGR1 | early growth response protein 1 | Up | No | |
| CALM | calmodulin | Up | Down | |
| PLD1_2 | phospholipase D1/2 | Up | Down | |
| GnRH Secretion | ITPR1 | inositol 1,4,5-triphosphate receptor type 1 | Up | Down |
| KCNN1 | potassium intermediate/small conductance calcium-activated channel subfamily N member 1 | Up | No | |
| CACNA1G | voltage-dependent calcium channel T type alpha-1G | Up | No | |
| PLCB | phosphatidylinositol phospholipase C, beta | Down | No | |
| RAF1 | RAF proto-oncogene serine/threonine-protein kinase | Up | Down | |
| CACNA1S | voltage-dependent calcium channel L type alpha-1S | Up | No | |
| GNAQ | guanine nucleotide-binding protein G(q) subunit alpha | Up | Down | |
| FOXO signaling pathway | FOXG | forkhead box protein G | Up | No |
| P38 | p38 MAP kinase | Up | No | |
| GABARAP | GABA(A) receptor-associated protein | Up | No | |
| Wnt signaling pathway | Wnt | wingless-type MMTV integration site family, member 1 | Down | No |
| Wnt5 | wingless-type MMTV integration site family, member 5 | Up | Down | |
| Wnt11 | wingless-type MMTV integration site family, member 11 | Down | No | |
| DVL | segment polarity protein dishevelled | Down | Up | |
| FZD2 | Frizzled 2 | Down | Up | |
| PLCB | phosphatidylinositol phospholipase C, beta | No | Down | |
| RAC1 | Ras-related C3 botulinum toxin substrate 1 | No | Up | |
| SKP1 | S-phase kinase-associated protein 1 | Down | Up | |
| CSNK1A | casein kinase 1, alpha | Down | Up | |
| Oocyte meiosis | FBXW1/11 | F-box and WD-40 domain protein 1/11 | Down | Up |
| CPEB | cytoplasmic polyadenylation element-binding protein | Up | No | |
| APC1 | anaphase-promoting complex subunit 1 | Up | Down | |
| CDC20 | cell division cycle 20, cofactor of APC complex | Down | Up | |
| SMC3 | structural maintenance of chromosome 3 (chondroitin sulfate proteoglycan 6) | Up | No | |
| PPP2R1 | serine/threonine-protein phosphatase 2A regulatory subunit A | Down | No | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, G.; Xue, Y.; Huang, X. Temperature-Induced Sex Differentiation in River Prawn (Macrobrachium nipponense): Mechanisms and Effects. Int. J. Mol. Sci. 2024, 25, 1207. https://doi.org/10.3390/ijms25021207
Jiang G, Xue Y, Huang X. Temperature-Induced Sex Differentiation in River Prawn (Macrobrachium nipponense): Mechanisms and Effects. International Journal of Molecular Sciences. 2024; 25(2):1207. https://doi.org/10.3390/ijms25021207
Chicago/Turabian StyleJiang, Gang, Yucai Xue, and Xuxiong Huang. 2024. "Temperature-Induced Sex Differentiation in River Prawn (Macrobrachium nipponense): Mechanisms and Effects" International Journal of Molecular Sciences 25, no. 2: 1207. https://doi.org/10.3390/ijms25021207
APA StyleJiang, G., Xue, Y., & Huang, X. (2024). Temperature-Induced Sex Differentiation in River Prawn (Macrobrachium nipponense): Mechanisms and Effects. International Journal of Molecular Sciences, 25(2), 1207. https://doi.org/10.3390/ijms25021207





