Exploring Immune Cell Diversity in the Lacrimal Glands of Healthy Mice: A Single-Cell RNA-Sequencing Atlas
Abstract
1. Introduction
2. Results
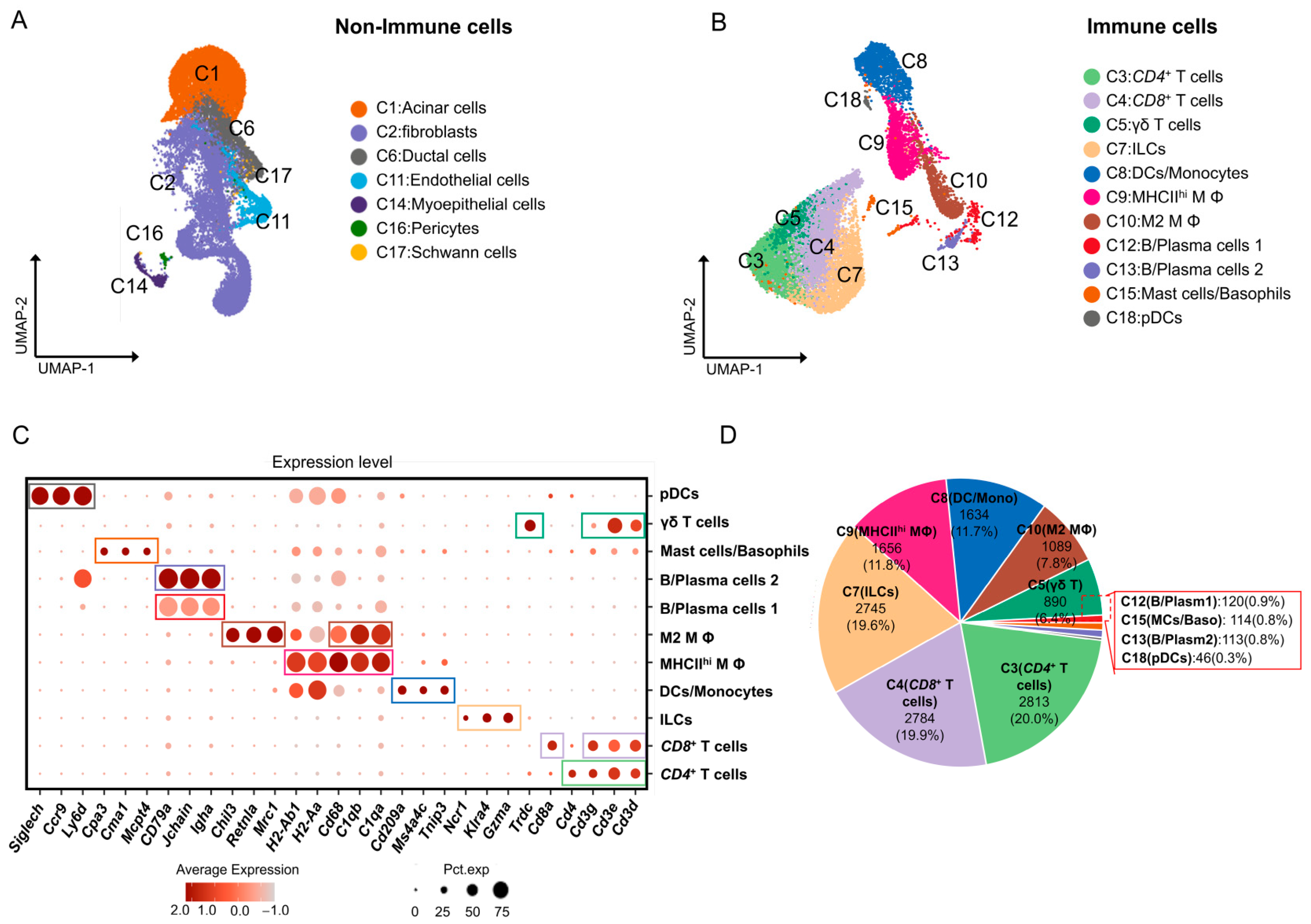
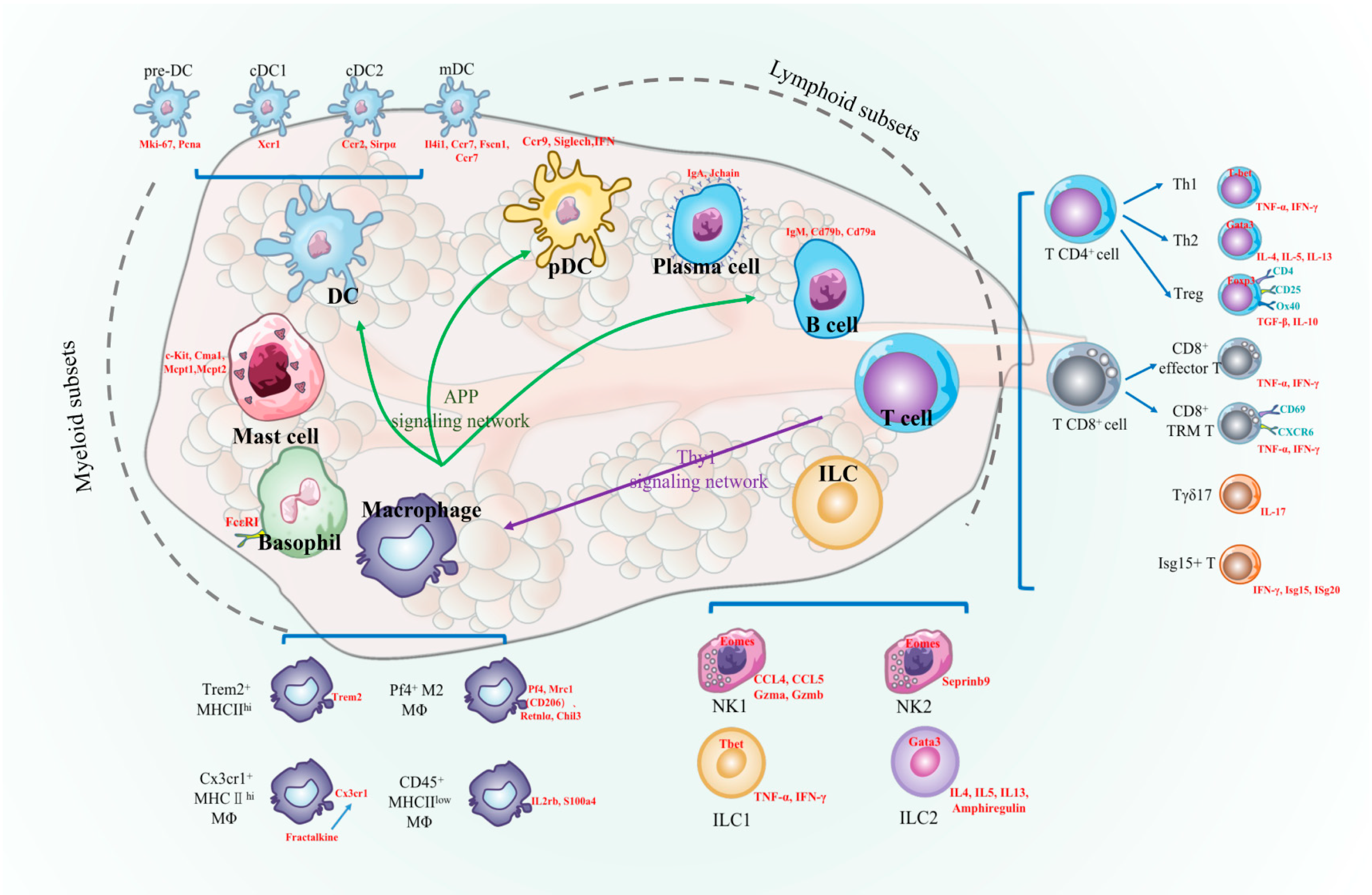
2.1. Single-Cell Transcription of Immune Cell Subpopulations in Mouse ELGs
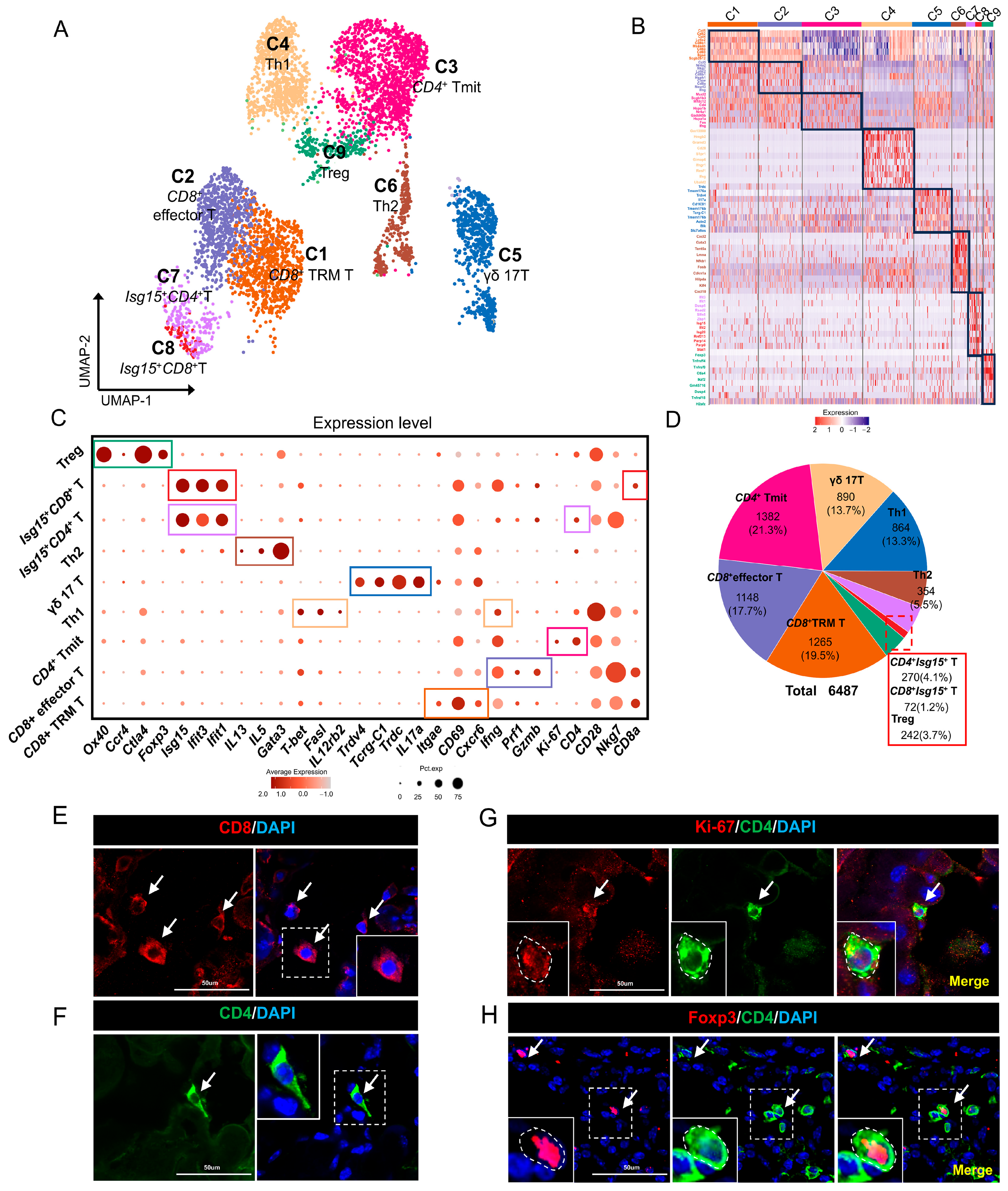
2.2. T Cells in Mouse ELGs
2.3. ILCs in Mouse ELGs
2.4. MΦs in Mouse ELGs
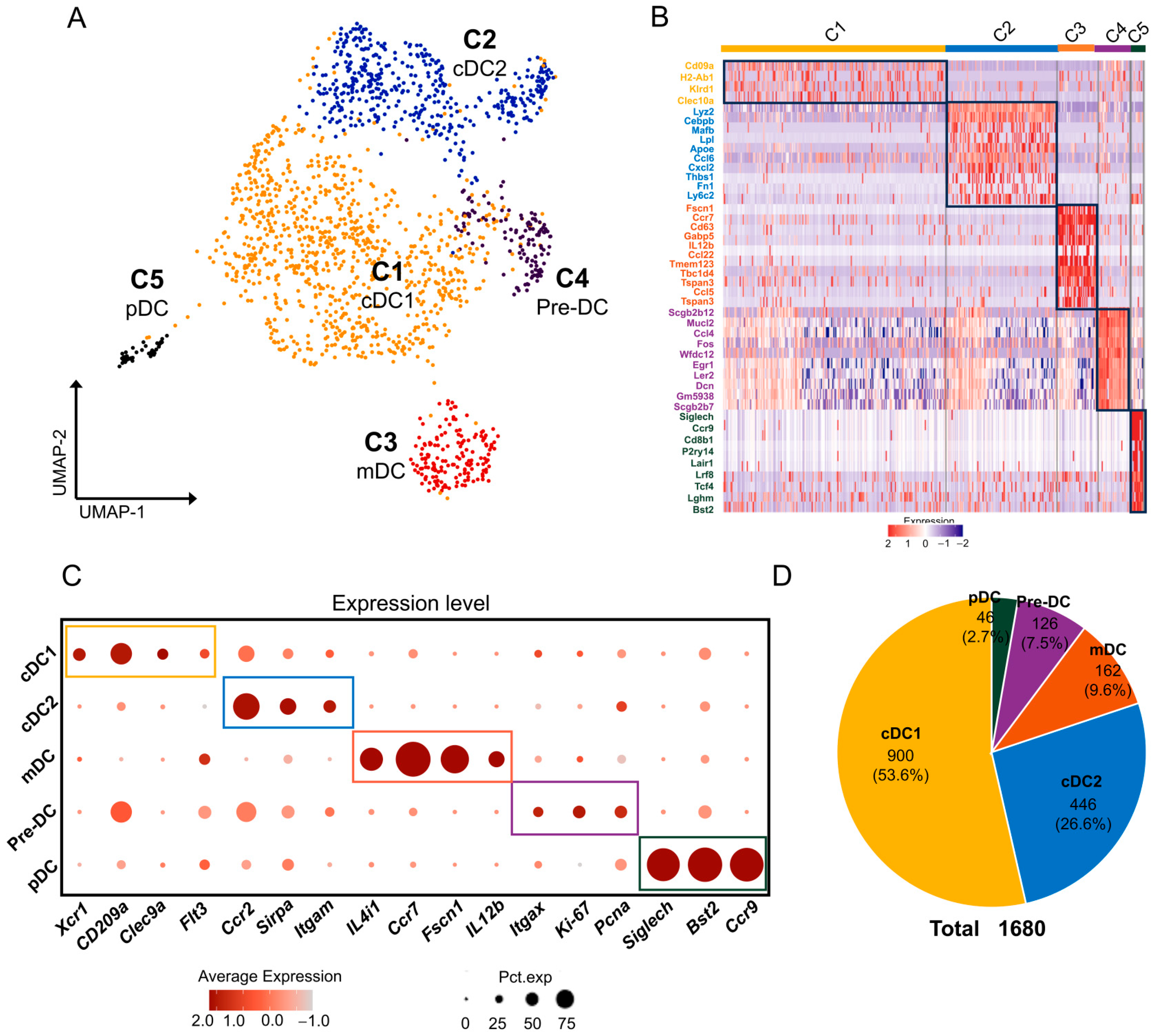
2.5. DCs/pDCs in Mouse ELGs
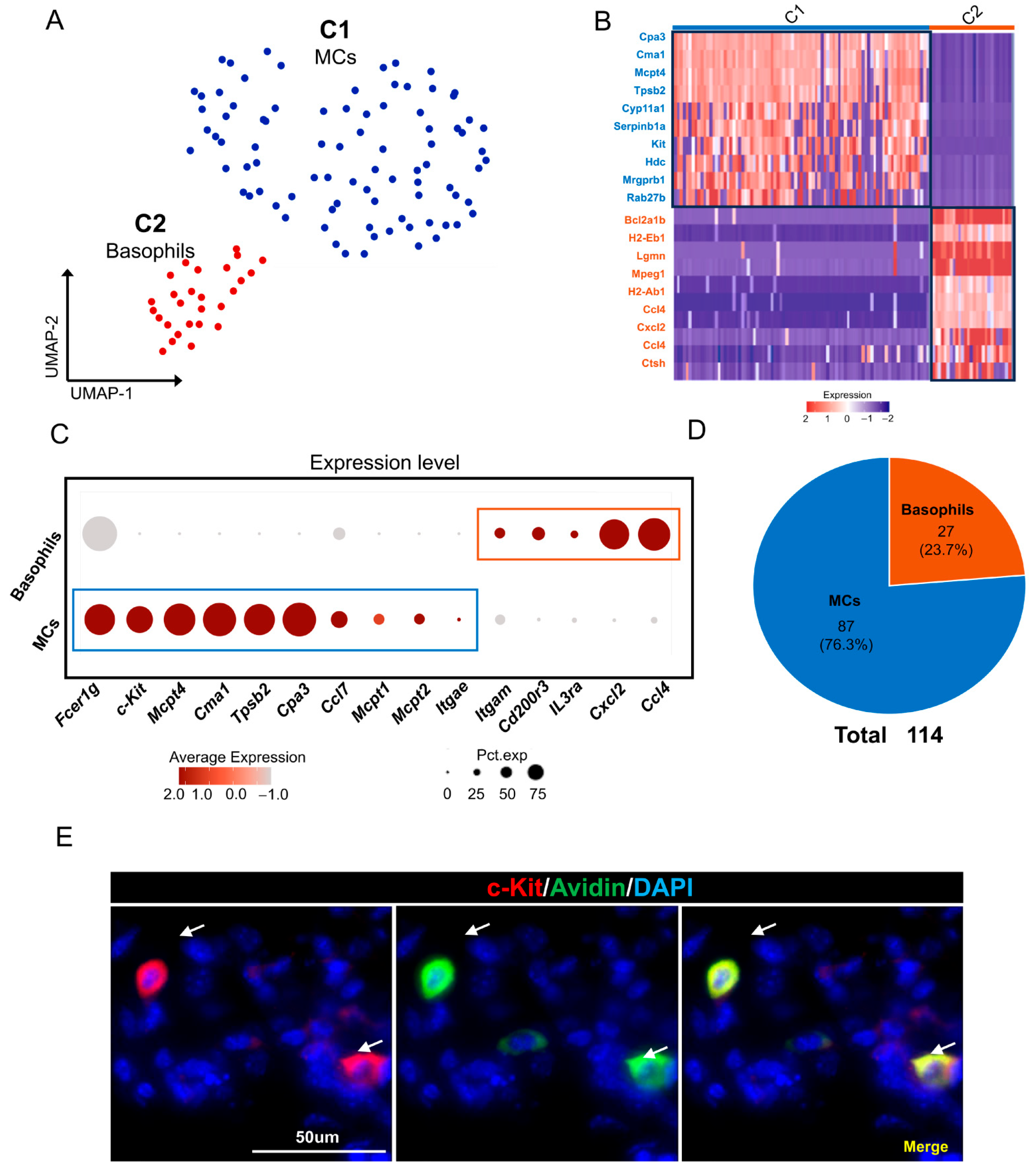
2.6. MC/Basophils in Mouse ELGs
2.7. B Cells/Plasma Cells in Mouse ELGs
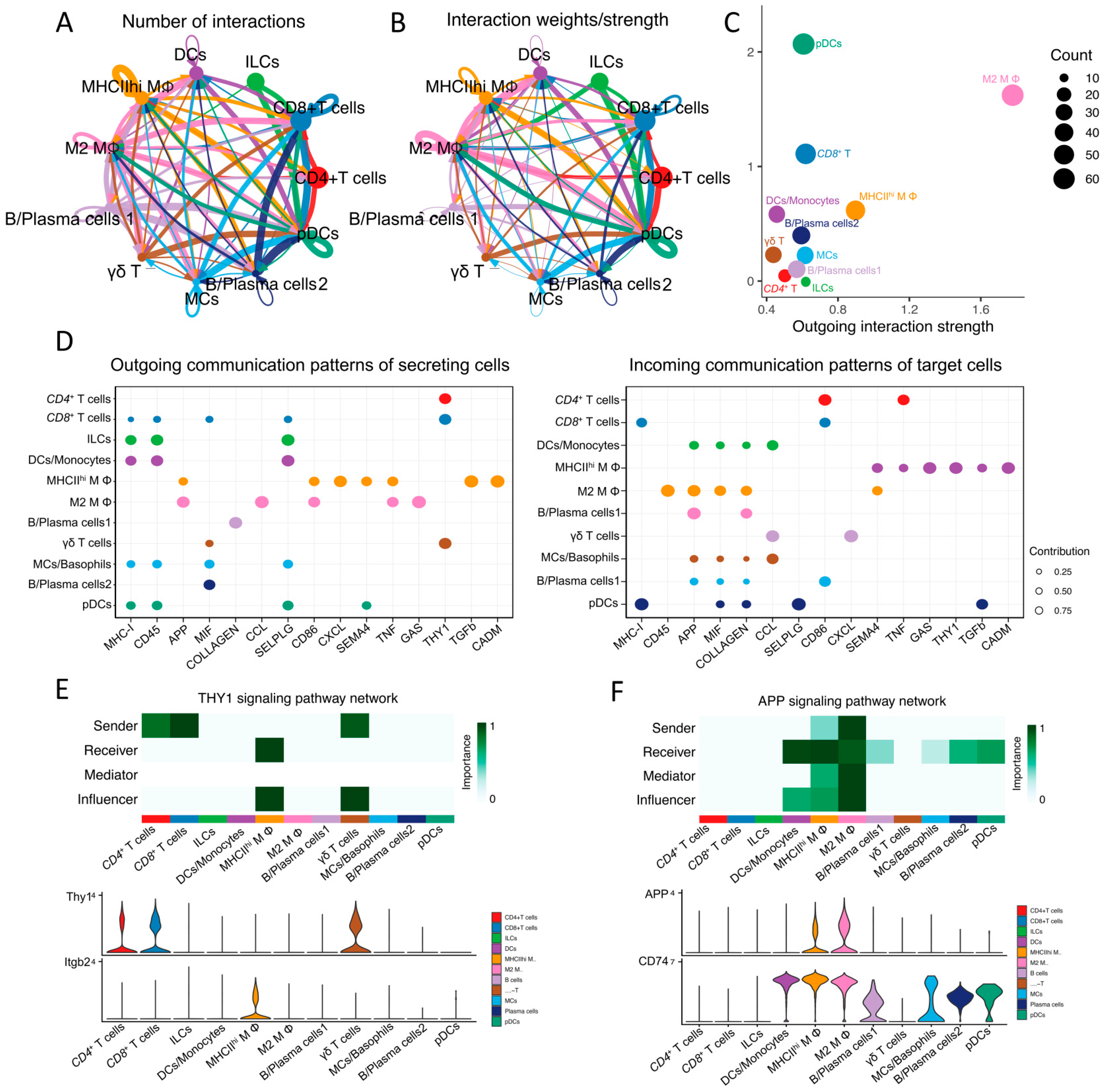
2.8. Analysis of Cell-Cell Communication between Immune Cell Populations in Mouse Lacrimal Glands
3. Discussion
3.1. T Cells in Mouse Lacrimal Glands
3.2. ILCs in Mouse Lacrimal Glands
3.3. MΦs in Mouse Lacrimal Glands
3.4. DC/pDCs in Mouse Lacrimal Glands
3.5. Mast Cells and Eosinophils in Mouse Lacrimal Glands
3.6. B Cells/Plasma Cells in Mouse Lacrimal Glands
3.7. Cell–Cell Signaling among Immune Cells
3.8. Study Limitations
3.9. Future Research Directions
4. Materials and Methods
4.1. Experimental Animals
4.2. Tissue Collection and Single-Cell Sample Preparation
4.3. Single-Cell RNA Sequencing
4.4. Identification of Cell Types
4.5. Analysis of Cell-Cell Interactions and Communication
4.6. Functional Signaling Pathway Enrichment Analysis
4.7. Immunohistological Staining of Mouse ELGs
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APP | amyloid precursor protein |
| BP | biological process |
| cDC | conventional dendritic cell |
| CCL | CC-chemokine ligand |
| CCR | CC-chemokine receptor |
| CXCL | chemokine (C-X-C motif) ligand |
| CXCR | chemokine (C-X-C motif) receptor |
| DCs | dendritic cells |
| ELG | extraorbital lacrimal gland |
| GO | gene ontology |
| IFN | interferon |
| IgA | immunoglobulin A |
| ILCs | innate lymphoid cells |
| Jak-STAT | the Janus kinase/signal transducers and activators of transcription |
| MCs | mast cells |
| mDCs | migratory dendritic cells |
| NKs | natural killers |
| pDCs | plasmacytoid dendritic cells |
| Thy1 | thymus cell antigen 1 |
| Tregs | regulatory T cells |
| TRM | tissue-resident memory |
| UMAP | uniform manifold approximation and projection |
References
- Stevenson, W.; Pugazhendhi, S.; Wang, M. Is the main lacrimal gland indispensable? Contributions of the corneal and conjunctival epithelia. Surv. Ophthalmol. 2016, 61, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Pflugfelder, S.C.; Stern, M.E. Biological functions of tear film. Exp. Eye Res. 2020, 197, 108115. [Google Scholar] [CrossRef] [PubMed]
- McDermott, A.M. Antimicrobial compounds in tears. Exp. Eye Res. 2013, 117, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Klenkler, B.; Sheardown, H.; Jones, L. Growth factors in the tear film: Role in tissue maintenance, wound healing, and ocular pathology. Ocul. Surf. 2007, 5, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Bylsma, L.M.; Gracanin, A.; Vingerhoets, A. The neurobiology of human crying. Clin. Auton. Res. 2019, 29, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, R.; Jakobiec, F.A.; Sacks, E.H.; Knowles, D.M. The immunoarchitecture of the normal human lacrimal gland. Relevancy for understanding pathologic conditions. Ophthalmology 1988, 95, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Si, H.; Huang, D.; Lu, D.; Zou, S.; Qi, D.; Pei, X.; Huang, S.; Li, Z. Mechanisms of Extraorbital Lacrimal Gland Aging in Mice: An Integrative Analysis of the Temporal Transcriptome. Investig. Ophthalmol. Vis. Sci. 2023, 64, 18. [Google Scholar] [CrossRef]
- Saitoh-Inagawa, W.; Hiroi, T.; Yanagita, M.; Iijima, H.; Uchio, E.; Ohno, S.; Aoki, K.; Kiyono, H. Unique characteristics of lacrimal glands as a part of mucosal immune network: High frequency of IgA-committed B-1 cells and NK1.1+ alphabeta T cells. Investig. Ophthalmol. Vis. Sci. 2000, 41, 138–144. [Google Scholar]
- Schechter, J.E.; Warren, D.W.; Mircheff, A.K. A lacrimal gland is a lacrimal gland, but rodent’s and rabbit’s are not human. Ocul. Surf. 2010, 8, 111–134. [Google Scholar] [CrossRef]
- Pappo, J.; Ebersole, J.L.; Taubman, M.A. Phenotype of mononuclear leucocytes resident in rat major salivary and lacrimal glands. Immunology 1988, 64, 295–300. [Google Scholar]
- Ortiz, G.; Chao, C.; Jamali, A.; Seyed-Razavi, Y.; Kenyon, B.; Harris, D.L.; Zoukhri, D.; Hamrah, P. Effect of Dry Eye Disease on the Kinetics of Lacrimal Gland Dendritic Cells as Visualized by Intravital Multi-Photon Microscopy. Front. Immunol. 2020, 11, 1713. [Google Scholar] [CrossRef]
- Jamali, A.; Kenyon, B.; Ortiz, G.; Abou-Slaybi, A.; Sendra, V.G.; Harris, D.L.; Hamrah, P. Plasmacytoid dendritic cells in the eye. Prog. Retin. Eye Res. 2021, 80, 100877. [Google Scholar] [CrossRef]
- Farmer, D.T.; Nathan, S.; Finley, J.K.; Shengyang Yu, K.; Emmerson, E.; Byrnes, L.E.; Sneddon, J.B.; McManus, M.T.; Tward, A.D.; Knox, S.M. Defining epithelial cell dynamics and lineage relationships in the developing lacrimal gland. Development 2017, 144, 2517–2528. [Google Scholar] [CrossRef]
- Bannier-Helaouet, M.; Post, Y.; Korving, J.; Trani Bustos, M.; Gehart, H.; Begthel, H.; Bar-Ephraim, Y.E.; van der Vaart, J.; Kalmann, R.; Imhoff, S.M.; et al. Exploring the human lacrimal gland using organoids and single-cell sequencing. Cell Stem Cell 2021, 28, 1221–1232. [Google Scholar] [CrossRef]
- Delcroix, V.; Mauduit, O.; Lee, H.S.; Ivanova, A.; Umazume, T.; Knox, S.M.; de Paiva, C.S.; Dartt, D.A.; Makarenkova, H.P. The First Transcriptomic Atlas of the Adult Lacrimal Gland Reveals Epithelial Complexity and Identifies Novel Progenitor Cells in Mice. Cells 2023, 12, 1435. [Google Scholar] [CrossRef] [PubMed]
- Basova, L.; Parfitt, G.J.; Richardson, A.; Delcroix, V.; Umazume, T.; Pelaez, D.; Tse, D.T.; Kalajzic, I.; Di Girolamo, N.; Jester, J.V.; et al. Origin and Lineage Plasticity of Endogenous Lacrimal Gland Epithelial Stem/Progenitor Cells. iScience 2020, 23, 101230. [Google Scholar] [CrossRef]
- Song, E.C.; Che, M.; Osinski, J.; Smalley, K.; Horeth, E.; Sinha, S.; Romano, R.A. DeltaNp63 maintains the fidelity of the myoepithelial cell lineage and directs cell differentiation programs in the murine salivary gland. Cell Death Differ. 2023, 30, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, A.B.; Roco, C.M.; Muscat, R.A.; Kuchina, A.; Sample, P.; Yao, Z.; Graybuck, L.T.; Peeler, D.J.; Mukherjee, S.; Chen, W.; et al. Single-cell profiling of the developing mouse brain and spinal cord with split-pool barcoding. Science 2018, 360, 176–182. [Google Scholar] [CrossRef]
- Kan, H.; Zhang, K.; Mao, A.; Geng, L.; Gao, M.; Feng, L.; You, Q.; Ma, X. Single-cell transcriptome analysis reveals cellular heterogeneity in the ascending aortas of normal and high-fat diet-fed mice. Exp. Mol. Med. 2021, 53, 1379–1389. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Vanlandewijck, M.; Mae, M.A.; Andrae, J.; Ando, K.; Del Gaudio, F.; Nahar, K.; Lebouvier, T.; Lavina, B.; Gouveia, L.; et al. Single-cell RNA sequencing of mouse brain and lung vascular and vessel-associated cell types. Sci. Data 2018, 5, 180160. [Google Scholar] [CrossRef]
- He, D.; Mao, A.; Zheng, C.B.; Kan, H.; Zhang, K.; Zhang, Z.; Feng, L.; Ma, X. Aortic heterogeneity across segments and under high fat/salt/glucose conditions at the single-cell level. Natl. Sci. Rev. 2020, 7, 881–896. [Google Scholar] [CrossRef]
- Rattner, A.; Heng, J.S.; Winer, B.L.; Goff, L.A.; Nathans, J. Normal and Sjogren’s syndrome models of the murine lacrimal gland studied at single-cell resolution. Proc. Natl. Acad. Sci. USA 2023, 120, e2311983120. [Google Scholar] [CrossRef]
- Saliba, A.E.; Li, L.; Westermann, A.J.; Appenzeller, S.; Stapels, D.A.; Schulte, L.N.; Helaine, S.; Vogel, J. Single-cell RNA-seq ties macrophage polarization to growth rate of intracellular Salmonella. Nat. Microbiol. 2016, 2, 16206. [Google Scholar] [CrossRef]
- Li, Z.; Yang, Q.; Tang, X.; Chen, Y.; Wang, S.; Qi, X.; Zhang, Y.; Liu, Z.; Luo, J.; Liu, H.; et al. Single-cell RNA-seq and chromatin accessibility profiling decipher the heterogeneity of mouse γδ T cells. Sci. Bull. 2022, 67, 408–426. [Google Scholar] [CrossRef]
- Alam, J.; Yazdanpanah, G.; Ratnapriya, R.; Borcherding, N.; de Paiva, C.S.; Li, D.; Pflugfelder, S.C. Single-cell transcriptional profiling of murine conjunctival immune cells reveals distinct populations expressing homeostatic and regulatory genes. Mucosal Immunol. 2022, 15, 620–628. [Google Scholar] [CrossRef]
- Lai, W.; Wang, X.; Liu, L.; Xu, L.; Mao, L.; Tan, J.; Zha, X.; Zhan, H.; Lei, W.; Lan, Y.; et al. Single-cell profiling of T cells uncovers a tissue-resident memory-like T-cell subset associated with bidirectional prognosis for B-cell acute lymphoblastic leukemia. Front. Immunol. 2022, 13, 957436. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, J.; Chen, S.; Yang, H.; Dong, Z. Synergized regulation of NK cell education by NKG2A and specific Ly49 family members. Nat. Commun. 2019, 10, 5010. [Google Scholar] [CrossRef]
- Fehniger, T.A.; Cai, S.F.; Cao, X.; Bredemeyer, A.J.; Presti, R.M.; French, A.R.; Ley, T.J. Acquisition of murine NK cell cytotoxicity requires the translation of a pre-existing pool of granzyme B and perforin mRNAs. Immunity 2007, 26, 798–811. [Google Scholar] [CrossRef]
- Jelenčić, V.; Šestan, M.; Kavazović, I.; Lenartić, M.; Marinović, S.; Holmes, T.D.; Prchal-Murphy, M.; Lisnić, B.; Sexl, V.; Bryceson, Y.T.; et al. NK cell receptor NKG2D sets activation threshold for the NCR1 receptor early in NK cell development. Nat. Immunol. 2018, 19, 1083–1092. [Google Scholar] [CrossRef]
- Locati, M.; Curtale, G.; Mantovani, A. Diversity, Mechanisms, and Significance of Macrophage Plasticity. Annu. Rev. Pathol. 2020, 15, 123–147. [Google Scholar] [CrossRef]
- Mauduit, O.; Delcroix, V.; Umazume, T.; de Paiva, C.S.; Dartt, D.A.; Makarenkova, H.P. Spatial transcriptomics of the lacrimal gland features macrophage activity and epithelium metabolism as key alterations during chronic inflammation. Front. Immunol. 2022, 13, 1011125. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yang, S.; Ma, J.; Chen, Z.; Song, G.; Rao, D.; Cheng, Y.; Huang, S.; Liu, Y.; Jiang, S.; et al. Spatiotemporal Immune Landscape of Colorectal Cancer Liver Metastasis at Single-Cell Level. Cancer Discov. 2022, 12, 134–153. [Google Scholar] [CrossRef] [PubMed]
- Flores, A.M.; Hosseini-Nassab, N.; Jarr, K.U.; Ye, J.; Zhu, X.; Wirka, R.; Koh, A.L.; Tsantilas, P.; Wang, Y.; Nanda, V.; et al. Pro-efferocytic nanoparticles are specifically taken up by lesional macrophages and prevent atherosclerosis. Nat. Nanotechnol. 2020, 15, 154–161. [Google Scholar] [CrossRef]
- Schaum, N.; Karkanias, J.; Neff, N.F.; May, A.P.; Quake, S.R.; Wyss-Coray, T.; Darmanis, S.; Batson, J.; Botvinnik, O.; Chen, M.B. Single-cell transcriptomics of 20 mouse organs creates a Tabula Muris. Nature 2018, 562, 367–372. [Google Scholar] [CrossRef]
- Zhang, J.; Raper, A.; Sugita, N.; Hingorani, R.; Salio, M.; Palmowski, M.J.; Cerundolo, V.; Crocker, P.R. Characterization of Siglec-H as a novel endocytic receptor expressed on murine plasmacytoid dendritic cell precursors. Blood 2006, 107, 3600–3608. [Google Scholar] [CrossRef]
- Lutz, K.; Musumeci, A.; Sie, C.; Dursun, E.; Winheim, E.; Bagnoli, J.; Ziegenhain, C.; Rausch, L.; Bergen, V.; Luecken, M.D.; et al. Ly6D(+)Siglec-H(+) precursors contribute to conventional dendritic cells via a Zbtb46(+)Ly6D(+) intermediary stage. Nat. Commun. 2022, 13, 3456. [Google Scholar] [CrossRef]
- Hadeiba, H.; Sato, T.; Habtezion, A.; Oderup, C.; Pan, J.; Butcher, E.C. CCR9 expression defines tolerogenic plasmacytoid dendritic cells able to suppress acute graft-versus-host disease. Nat. Immunol. 2008, 9, 1253–1260. [Google Scholar] [CrossRef]
- Davis, R.E.; Ngo, V.N.; Lenz, G.; Tolar, P.; Young, R.M.; Romesser, P.B.; Kohlhammer, H.; Lamy, L.; Zhao, H.; Yang, Y.; et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature 2010, 463, 88–92. [Google Scholar] [CrossRef]
- Ma, X.; Deng, J.; Han, L.; Song, Y.; Miao, Y.; Du, X.; Dang, G.; Yang, D.; Zhong, B.; Jiang, C.; et al. Single-cell RNA sequencing reveals B cell-T cell interactions in vascular adventitia of hyperhomocysteinemia-accelerated atherosclerosis. Protein Cell 2022, 13, 540–547. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, J.; Lindholt, J.S.; Sukhova, G.K.; Liu, J.; He, A.; Abrink, M.; Pejler, G.; Stevens, R.L.; Thompson, R.W.; et al. Critical role of mast cell chymase in mouse abdominal aortic aneurysm formation. Circulation 2009, 120, 973–982. [Google Scholar] [CrossRef]
- Duque-Wilckens, N.; Teis, R.; Sarno, E.; Stoelting, F.; Khalid, S.; Dairi, Z.; Douma, A.; Maradiaga, N.; Hench, S.; Dharshika, C.D.; et al. Early life adversity drives sex-specific anhedonia and meningeal immune gene expression through mast cell activation. Brain Behav. Immun. 2022, 103, 73–84. [Google Scholar] [CrossRef]
- Zheng, M.Z.M.; Wakim, L.M. Tissue resident memory T cells in the respiratory tract. Mucosal Immunol. 2022, 15, 379–388. [Google Scholar] [CrossRef]
- Tiberti, S.; Catozzi, C.; Croci, O.; Ballerini, M.; Cagnina, D.; Soriani, C.; Scirgolea, C.; Gong, Z.; He, J.; Macandog, A.D.; et al. GZMK(high) CD8(+) T effector memory cells are associated with CD15(high) neutrophil abundance in non-metastatic colorectal tumors and predict poor clinical outcome. Nat. Commun. 2022, 13, 6752. [Google Scholar] [CrossRef]
- Brunet, J.F.; Denizot, F.; Golstein, P. A differential molecular biology search for genes preferentially expressed in functional T lymphocytes: The CTLA genes. Immunol. Rev. 1988, 103, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.S.; De Labastida Rivera, F.; Yan, J.; Corvino, D.; Das, I.; Zhang, P.; Kuns, R.; Chauhan, S.B.; Hou, J.; Li, X.Y.; et al. The NK cell granule protein NKG7 regulates cytotoxic granule exocytosis and inflammation. Nat. Immunol. 2020, 21, 1205–1218. [Google Scholar] [CrossRef]
- Luo, Y.; Xu, C.; Wang, B.; Niu, Q.; Su, X.; Bai, Y.; Zhu, S.; Zhao, C.; Sun, Y.; Wang, J.; et al. Single-cell transcriptomic analysis reveals disparate effector differentiation pathways in human T(reg) compartment. Nat. Commun. 2021, 12, 3913. [Google Scholar] [CrossRef] [PubMed]
- Cencioni, M.T.; Santini, S.; Ruocco, G.; Borsellino, G.; De Bardi, M.; Grasso, M.G.; Ruggieri, S.; Gasperini, C.; Centonze, D.; Barila, D.; et al. FAS-ligand regulates differential activation-induced cell death of human T-helper 1 and 17 cells in healthy donors and multiple sclerosis patients. Cell Death Dis. 2015, 6, e1741. [Google Scholar] [CrossRef]
- Zhang, X.; Brunner, T.; Carter, L.; Dutton, R.W.; Rogers, P.; Bradley, L.; Sato, T.; Reed, J.C.; Green, D.; Swain, S.L. Unequal death in T helper cell (Th)1 and Th2 effectors: Th1, but not Th2, effectors undergo rapid Fas/FasL-mediated apoptosis. J. Exp. Med. 1997, 185, 1837–1849. [Google Scholar] [CrossRef]
- Dzialo-Hatton, R.; Milbrandt, J.; Hockett, R.D., Jr.; Weaver, C.T. Differential expression of Fas ligand in Th1 and Th2 cells is regulated by early growth response gene and NF-AT family members. J. Immunol. 2001, 166, 4534–4542. [Google Scholar] [CrossRef] [PubMed]
- Papotto, P.H.; Ribot, J.C.; Silva-Santos, B. IL-17(+) gammadelta T cells as kick-starters of inflammation. Nat. Immunol. 2017, 18, 604–611. [Google Scholar] [CrossRef]
- Castillo-Gonzalez, R.; Cibrian, D.; Sanchez-Madrid, F. Dissecting the complexity of gammadelta T-cell subsets in skin homeostasis, inflammation, and malignancy. J. Allergy Clin. Immunol. 2021, 147, 2030–2042. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; Cui, K.; Hu, G.; Gurram, R.K.; Zhong, C.; Oler, A.J.; Yagi, R.; Zhao, M.; Sharma, S.; Liu, P.; et al. Bcl11b, a novel GATA3-interacting protein, suppresses Th1 while limiting Th2 cell differentiation. J. Exp. Med. 2018, 215, 1449–1462. [Google Scholar] [CrossRef]
- Hosokawa, H.; Tanaka, T.; Endo, Y.; Kato, M.; Shinoda, K.; Suzuki, A.; Motohashi, S.; Matsumoto, M.; Nakayama, K.I.; Nakayama, T. Akt1-mediated Gata3 phosphorylation controls the repression of IFNgamma in memory-type Th2 cells. Nat. Commun. 2016, 7, 11289. [Google Scholar] [CrossRef] [PubMed]
- Maggi, L.; Mazzoni, A.; Capone, M.; Liotta, F.; Annunziato, F.; Cosmi, L. The dual function of ILC2: From host protection to pathogenic players in type 2 asthma. Mol. Asp. Med. 2021, 80, 100981. [Google Scholar] [CrossRef]
- Burchill, M.A.; Salomon, M.P.; Golden-Mason, L.; Wieland, A.; Maretti-Mira, A.C.; Gale, M., Jr.; Rosen, H.R. Single-cell transcriptomic analyses of T cells in chronic HCV-infected patients dominated by DAA-induced interferon signaling changes. PLoS Pathog. 2021, 17, e1009799. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Qin, S.; Si, W.; Wang, A.; Xing, B.; Gao, R.; Ren, X.; Wang, L.; Wu, X.; Zhang, J.; et al. Pan-cancer single-cell landscape of tumor-infiltrating T cells. Science 2021, 374, abe6474. [Google Scholar] [CrossRef]
- Fu, G.; Chen, T.; Wu, J.; Jiang, T.; Tang, D.; Bonaroti, J.; Conroy, J.; Scott, M.J.; Deng, M.; Billiar, T.R. Single-Cell Transcriptomics Reveals Compartment-Specific Differences in Immune Responses and Contributions for Complement Factor 3 in Hemorrhagic Shock Plus Tissue Trauma. Shock 2021, 56, 994–1008. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Nishikawa, H.; Wada, H.; Nagano, Y.; Sugiyama, D.; Atarashi, K.; Maeda, Y.; Hamaguchi, M.; Ohkura, N.; Sato, E.; et al. Two FOXP3(+)CD4(+) T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat. Med. 2016, 22, 679–684. [Google Scholar] [CrossRef]
- Ohkura, N.; Sakaguchi, S. Transcriptional and epigenetic basis of Treg cell development and function: Its genetic anomalies or variations in autoimmune diseases. Cell Res. 2020, 30, 465–474. [Google Scholar] [CrossRef]
- Feng, G.; Bajpai, G.; Ma, P.; Koenig, A.; Bredemeyer, A.; Lokshina, I.; Lai, L.; Förster, I.; Leuschner, F.; Kreisel, D.; et al. CCL17 Aggravates Myocardial Injury by Suppressing Recruitment of Regulatory T Cells. Circulation 2022, 145, 765–782. [Google Scholar] [CrossRef]
- Fu, Y.; Lin, Q.; Zhang, Z.; Zhang, L. Therapeutic strategies for the costimulatory molecule OX40 in T-cell-mediated immunity. Acta Pharm. Sinica. B 2020, 10, 414–433. [Google Scholar] [CrossRef] [PubMed]
- Crinier, A.; Milpied, P.; Escaliere, B.; Piperoglou, C.; Galluso, J.; Balsamo, A.; Spinelli, L.; Cervera-Marzal, I.; Ebbo, M.; Girard-Madoux, M.; et al. High-Dimensional Single-Cell Analysis Identifies Organ-Specific Signatures and Conserved NK Cell Subsets in Humans and Mice. Immunity 2018, 49, 971–986.e975. [Google Scholar] [CrossRef] [PubMed]
- McFarland, A.P.; Yalin, A.; Wang, S.Y.; Cortez, V.S.; Landsberger, T.; Sudan, R.; Peng, V.; Miller, H.L.; Ricci, B.; David, E.; et al. Multi-tissue single-cell analysis deconstructs the complex programs of mouse natural killer and type 1 innate lymphoid cells in tissues and circulation. Immunity 2021, 54, 1320–1337. [Google Scholar] [CrossRef] [PubMed]
- Zeis, P.; Lian, M.; Fan, X.; Herman, J.S.; Hernandez, D.C.; Gentek, R.; Elias, S.; Symowski, C.; Knöpper, K.; Peltokangas, N.; et al. In Situ Maturation and Tissue Adaptation of Type 2 Innate Lymphoid Cell Progenitors. Immunity 2020, 53, 775–792. [Google Scholar] [CrossRef] [PubMed]
- Cochain, C.; Vafadarnejad, E.; Arampatzi, P.; Pelisek, J.; Winkels, H.; Ley, K.; Wolf, D.; Saliba, A.E.; Zernecke, A. Single-Cell RNA-Seq Reveals the Transcriptional Landscape and Heterogeneity of Aortic Macrophages in Murine Atherosclerosis. Circ. Res. 2018, 122, 1661–1674. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Smyth, M.J. TREM2 marks tumor-associated macrophages. Signal Transduct. Target. Ther. 2020, 5, 233. [Google Scholar] [CrossRef]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef]
- Szanto, A.; Balint, B.L.; Nagy, Z.S.; Barta, E.; Dezso, B.; Pap, A.; Szeles, L.; Poliska, S.; Oros, M.; Evans, R.M.; et al. STAT6 transcription factor is a facilitator of the nuclear receptor PPARgamma-regulated gene expression in macrophages and dendritic cells. Immunity 2010, 33, 699–712. [Google Scholar] [CrossRef]
- Soldano, S.; Trombetta, A.C.; Contini, P.; Tomatis, V.; Ruaro, B.; Brizzolara, R.; Montagna, P.; Sulli, A.; Paolino, S.; Pizzorni, C.; et al. Increase in circulating cells coexpressing M1 and M2 macrophage surface markers in patients with systemic sclerosis. Ann. Rheum. Dis. 2018, 77, 1842–1845. [Google Scholar] [CrossRef]
- Mushenkova, N.V.; Nikiforov, N.G.; Melnichenko, A.A.; Kalmykov, V.; Shakhpazyan, N.K.; Orekhova, V.A.; Orekhov, A.N. Functional Phenotypes of Intraplaque Macrophages and Their Distinct Roles in Atherosclerosis Development and Atheroinflammation. Biomedicines 2022, 10, 452. [Google Scholar] [CrossRef]
- He, J.; Song, Y.; Li, G.; Xiao, P.; Liu, Y.; Xue, Y.; Cao, Q.; Tu, X.; Pan, T.; Jiang, Z.; et al. Fbxw7 increases CCL2/7 in CX3CR1hi macrophages to promote intestinal inflammation. J. Clin. Investig. 2019, 129, 3877–3893. [Google Scholar] [CrossRef] [PubMed]
- Shea-Donohue, T.; Zhao, A.; Antalis, T.M. SerpinB2 mediated regulation of macrophage function during enteric infection. Gut Microbes 2014, 5, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Zlatanova, I.; Pinto, C.; Bonnin, P.; Mathieu, J.R.R.; Bakker, W.; Vilar, J.; Lemitre, M.; Voehringer, D.; Vaulont, S.; Peyssonnaux, C.; et al. Iron Regulator Hepcidin Impairs Macrophage-Dependent Cardiac Repair After Injury. Circulation 2019, 139, 1530–1547. [Google Scholar] [CrossRef] [PubMed]
- Guilliams, M.; Dutertre, C.A.; Scott, C.L.; McGovern, N.; Sichien, D.; Chakarov, S.; Van Gassen, S.; Chen, J.; Poidinger, M.; De Prijck, S.; et al. Unsupervised High-Dimensional Analysis Aligns Dendritic Cells across Tissues and Species. Immunity 2016, 45, 669–684. [Google Scholar] [CrossRef]
- Brown, C.C.; Gudjonson, H.; Pritykin, Y.; Deep, D.; Lavallee, V.P.; Mendoza, A.; Fromme, R.; Mazutis, L.; Ariyan, C.; Leslie, C.; et al. Transcriptional Basis of Mouse and Human Dendritic Cell Heterogeneity. Cell 2019, 179, 846–863. [Google Scholar] [CrossRef]
- Villani, A.C.; Satija, R.; Reynolds, G.; Sarkizova, S.; Shekhar, K.; Fletcher, J.; Griesbeck, M.; Butler, A.; Zheng, S.; Lazo, S.; et al. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science 2017, 356, eaah4573. [Google Scholar] [CrossRef]
- Cabeza-Cabrerizo, M.; van Blijswijk, J.; Wienert, S.; Heim, D.; Jenkins, R.P.; Chakravarty, P.; Rogers, N.; Frederico, B.; Acton, S.; Beerling, E.; et al. Tissue clonality of dendritic cell subsets and emergency DCpoiesis revealed by multicolor fate mapping of DC progenitors. Sci. Immunol. 2019, 4, eaaw1941. [Google Scholar] [CrossRef] [PubMed]
- Czepielewski, R.S.; Randolph, G.J. Resident dendritic cell density in the lymph node paracortex is preDC-estined. Immunity 2023, 56, 1699–1701. [Google Scholar] [CrossRef]
- Reizis, B. Plasmacytoid Dendritic Cells: Development, Regulation, and Function. Immunity 2019, 50, 37–50. [Google Scholar] [CrossRef]
- Schlitzer, A.; Loschko, J.; Mair, K.; Vogelmann, R.; Henkel, L.; Einwachter, H.; Schiemann, M.; Niess, J.H.; Reindl, W.; Krug, A. Identification of CCR9- murine plasmacytoid DC precursors with plasticity to differentiate into conventional DCs. Blood 2011, 117, 6562–6570. [Google Scholar] [CrossRef]
- Blasius, A.L.; Giurisato, E.; Cella, M.; Schreiber, R.D.; Shaw, A.S.; Colonna, M. Bone marrow stromal cell antigen 2 is a specific marker of type I IFN-producing cells in the naive mouse, but a promiscuous cell surface antigen following IFN stimulation. J. Immunol. 2006, 177, 3260–3265. [Google Scholar] [CrossRef] [PubMed]
- St John, A.L.; Rathore, A.P.S.; Ginhoux, F. New perspectives on the origins and heterogeneity of mast cells. Nat. Rev. Immunol. 2023, 23, 55–68. [Google Scholar] [CrossRef]
- Kuo, C.H.; Collins, A.M.; Boettner, D.R.; Yang, Y.; Ono, S.J. Role of CCL7 in Type I Hypersensitivity Reactions in Murine Experimental Allergic Conjunctivitis. J. Immunol. 2017, 198, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Tai, W.-C.; Wang, S.-T.; Wu, C.-S.; Lin, T.-Y.; Wu, M.-T. Chemokine receptor CCR3 is important for migration of mast cells in neurofibroma. Dermatol. Sin. 2010, 28, 146–153. [Google Scholar] [CrossRef]
- Hennersdorf, F.; Florian, S.; Jakob, A.; Baumgärtner, K.; Sonneck, K.; Nordheim, A.; Biedermann, T.; Valent, P.; Bühring, H.J. Identification of CD13, CD107a, and CD164 as novel basophil-activation markers and dissection of two response patterns in time kinetics of IgE-dependent upregulation. Cell Res. 2005, 15, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.; Giladi, A.; Gorki, A.D.; Solodkin, D.G.; Zada, M.; Hladik, A.; Miklosi, A.; Salame, T.M.; Halpern, K.B.; David, E.; et al. Lung Single-Cell Signaling Interaction Map Reveals Basophil Role in Macrophage Imprinting. Cell 2018, 175, 1031–1044.e1018. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Di, G.; Cao, X.; Liu, Y.; Wang, Y.; Zhao, H.; Wang, D.; Chen, P. Lacrimal gland homeostasis is maintained by the AQP5 pathway by attenuating endoplasmic reticulum stress inflammation in the lacrimal gland of AQP5 knockout mice. Mol. Vis. 2021, 27, 679–690. [Google Scholar]
- Xu, A.Q.; Barbosa, R.R.; Calado, D.P. Genetic timestamping of plasma cells in vivo reveals tissue-specific homeostatic population turnover. eLife 2020, 9, e59850. [Google Scholar] [CrossRef]
- Isho, B.; Florescu, A.; Wang, A.A.; Gommerman, J.L. Fantastic IgA plasma cells and where to find them. Immunol. Rev. 2021, 303, 119–137. [Google Scholar] [CrossRef]
- Heidt, S.; Vergunst, M.; Anholts, J.D.H.; Swings, G.; Gielis, E.M.J.; Groeneweg, K.E.; Witkamp, M.J.; de Fijter, J.W.; Reinders, M.E.J.; Roelen, D.L.; et al. Presence of intragraft B cells during acute renal allograft rejection is accompanied by changes in peripheral blood B cell subsets. Clin. Exp. Immunol. 2019, 196, 403–414. [Google Scholar] [CrossRef]
- Weston-Bell, N.; Townsend, M.; Di Genova, G.; Forconi, F.; Sahota, S.S. Defining origins of malignant B cells: A new circulating normal human IgM(+)D(+) B-cell subset lacking CD27 expression and displaying somatically mutated IGHV genes as a relevant memory population. Leukemia 2009, 23, 2075–2080. [Google Scholar] [CrossRef] [PubMed]
- Enders, A.; Short, A.; Miosge, L.A.; Bergmann, H.; Sontani, Y.; Bertram, E.M.; Whittle, B.; Balakishnan, B.; Yoshida, K.; Sjollema, G.; et al. Zinc-finger protein ZFP318 is essential for expression of IgD, the alternatively spliced Igh product made by mature B lymphocytes. Proc. Natl. Acad. Sci. USA 2014, 111, 4513–4518. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ji, W.; Huang, J.; Zhang, Y.; Zhou, Y.; Zhang, J.; Dong, Y.; Yuan, T.; Yang, Q.; Ding, X.; et al. Characterization of the tumour microenvironment phenotypes in malignant tissues and pleural effusion from advanced osteoblastic osteosarcoma patients. Clin. Transl. Med. 2022, 12, e1072. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Guerrero-Juarez, C.F.; Zhang, L.; Chang, I.; Ramos, R.; Kuan, C.H.; Myung, P.; Plikus, M.V.; Nie, Q. Inference and analysis of cell-cell communication using CellChat. Nat. Commun. 2021, 12, 1088. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Si, H.; Liu, J.; Qi, D.; Pei, X.; Lu, D.; Zou, S.; Li, Z. Sleep Loss Causes Dysfunction in Murine Extraorbital Lacrimal Glands. Investig. Ophthalmol. Vis. Sci. 2022, 63, 19. [Google Scholar] [CrossRef]
- Lieberman, S.M.; Kreiger, P.A.; Koretzky, G.A. Reversible lacrimal gland-protective regulatory T-cell dysfunction underlies male-specific autoimmune dacryoadenitis in the non-obese diabetic mouse model of Sjogren syndrome. Immunology 2015, 145, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Bantug, G.R.; Galluzzi, L.; Kroemer, G.; Hess, C. The spectrum of T cell metabolism in health and disease. Nat. Rev. Immunol. 2018, 18, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Crowl, J.T.; Heeg, M.; Ferry, A.; Milner, J.J.; Omilusik, K.D.; Toma, C.; He, Z.; Chang, J.T.; Goldrath, A.W. Tissue-resident memory CD8(+) T cells possess unique transcriptional, epigenetic and functional adaptations to different tissue environments. Nat. Immunol. 2022, 23, 1121–1131. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Y.; Jin, S.; Wang, M.; Jiao, Y.; Yang, B.; Lu, X.; Ji, X.; Fei, Y.; Yang, H.; et al. Single-cell sequencing of immune cells from anticitrullinated peptide antibody positive and negative rheumatoid arthritis. Nat. Commun. 2021, 12, 4977. [Google Scholar] [CrossRef]
- Carding, S.R.; Egan, P.J. Gammadelta T cells: Functional plasticity and heterogeneity. Nat. Rev. Immunol. 2002, 2, 336–345. [Google Scholar] [CrossRef]
- Fahl, S.P.; Coffey, F.; Wiest, D.L. Origins of gammadelta T cell effector subsets: A riddle wrapped in an enigma. J. Immunol. 2014, 193, 4289–4294. [Google Scholar] [CrossRef] [PubMed]
- Ribot, J.C.; Lopes, N.; Silva-Santos, B. gammadelta T cells in tissue physiology and surveillance. Nat. Rev. Immunol. 2021, 21, 221–232. [Google Scholar] [CrossRef]
- Ciofani, M.; Zuniga-Pflucker, J.C. Determining gammadelta versus alphass T cell development. Nat. Rev. Immunol. 2010, 10, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Narayan, K.; Sylvia, K.E.; Malhotra, N.; Yin, C.C.; Martens, G.; Vallerskog, T.; Kornfeld, H.; Xiong, N.; Cohen, N.R.; Brenner, M.B.; et al. Intrathymic programming of effector fates in three molecularly distinct gammadelta T cell subtypes. Nat. Immunol. 2012, 13, 511–518. [Google Scholar] [CrossRef]
- Liang, D.; Zuo, A.; Shao, H.; Born, W.K.; O’Brien, R.L.; Kaplan, H.J.; Sun, D. IL-23 receptor expression on gammadelta T cells correlates with their enhancing or suppressive effects on autoreactive T cells in experimental autoimmune uveitis. J. Immunol. 2013, 191, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Artis, D.; Spits, H. The biology of innate lymphoid cells. Nature 2015, 517, 293–301. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, C.; Wang, H.; Xue, Y.; Dong, D.; Lin, C.; Song, F.; Fu, T.; Wang, Z.; Chen, J.; et al. Local Group 2 Innate Lymphoid Cells Promote Corneal Regeneration after Epithelial Abrasion. Am. J. Pathol. 2017, 187, 1313–1326. [Google Scholar] [CrossRef]
- Bal, S.M.; Golebski, K.; Spits, H. Plasticity of innate lymphoid cell subsets. Nat. Rev. Immunol. 2020, 20, 552–565. [Google Scholar] [CrossRef]
- Tsymala, I.; Kuchler, K. Innate lymphoid cells-Underexplored guardians of immunity. PLoS Pathog. 2023, 19, e1011678. [Google Scholar] [CrossRef]
- Bird, C.H.; Christensen, M.E.; Mangan, M.S.; Prakash, M.D.; Sedelies, K.A.; Smyth, M.J.; Harper, I.; Waterhouse, N.J.; Bird, P.I. The granzyme B-Serpinb9 axis controls the fate of lymphocytes after lysosomal stress. Cell Death Differ. 2014, 21, 876–887. [Google Scholar] [CrossRef]
- Friedrich, C.; Taggenbrock, R.; Doucet-Ladeveze, R.; Golda, G.; Moenius, R.; Arampatzi, P.; Kragten, N.A.M.; Kreymborg, K.; Gomez de Aguero, M.; Kastenmuller, W.; et al. Effector differentiation downstream of lineage commitment in ILC1s is driven by Hobit across tissues. Nat. Immunol. 2021, 22, 1256–1267. [Google Scholar] [CrossRef] [PubMed]
- Weizman, O.E.; Adams, N.M.; Schuster, I.S.; Krishna, C.; Pritykin, Y.; Lau, C.; Degli-Esposti, M.A.; Leslie, C.S.; Sun, J.C.; O’Sullivan, T.E. ILC1 Confer Early Host Protection at Initial Sites of Viral Infection. Cell 2017, 171, 795–808. [Google Scholar] [CrossRef]
- Vivier, E.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.J.; Mebius, R.E.; et al. Innate Lymphoid Cells: 10 Years On. Cell 2018, 174, 1054–1066. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Li, Y.; Wang, X.; Liu, S.; Zhang, Y.; Liu, G.; Ye, S.; Zheng, Y.; Zhao, J.; Zhu, X.; et al. Dopamine inhibits group 2 innate lymphoid cell-driven allergic lung inflammation by dampening mitochondrial activity. Immunity 2023, 56, 320–335. [Google Scholar] [CrossRef] [PubMed]
- Tsou, A.M.; Yano, H.; Parkhurst, C.N.; Mahlakoiv, T.; Chu, C.; Zhang, W.; He, Z.; Jarick, K.J.; Zhong, C.; Putzel, G.G.; et al. Neuropeptide regulation of non-redundant ILC2 responses at barrier surfaces. Nature 2022, 611, 787–793. [Google Scholar] [CrossRef]
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Rochford, C.D.; Neumann, H. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J. Exp. Med. 2005, 201, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Binnewies, M.; Pollack, J.L.; Rudolph, J.; Dash, S.; Abushawish, M.; Lee, T.; Jahchan, N.S.; Canaday, P.; Lu, E.; Norng, M.; et al. Targeting TREM2 on tumor-associated macrophages enhances immunotherapy. Cell Rep. 2021, 37, 109844. [Google Scholar] [CrossRef]
- Park, M.D.; Reyes-Torres, I.; LeBerichel, J.; Hamon, P.; LaMarche, N.M.; Hegde, S.; Belabed, M.; Troncoso, L.; Grout, J.A.; Magen, A.; et al. TREM2 macrophages drive NK cell paucity and dysfunction in lung cancer. Nat. Immunol. 2023, 24, 792–801. [Google Scholar] [CrossRef]
- Park, I.; Goddard, M.E.; Cole, J.E.; Zanin, N.; Lyytikainen, L.P.; Lehtimaki, T.; Andreakos, E.; Feldmann, M.; Udalova, I.; Drozdov, I.; et al. C-type lectin receptor CLEC4A2 promotes tissue adaptation of macrophages and protects against atherosclerosis. Nat. Commun. 2022, 13, 215. [Google Scholar] [CrossRef]
- Yeo, L.; Adlard, N.; Biehl, M.; Juarez, M.; Smallie, T.; Snow, M.; Buckley, C.D.; Raza, K.; Filer, A.; Scheel-Toellner, D. Expression of chemokines CXCL4 and CXCL7 by synovial macrophages defines an early stage of rheumatoid arthritis. Ann. Rheum. Dis. 2016, 75, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Pitsilos, S.; Hunt, J.; Mohler, E.R.; Prabhakar, A.M.; Poncz, M.; Dawicki, J.; Khalapyan, T.Z.; Wolfe, M.L.; Fairman, R.; Mitchell, M.; et al. Platelet factor 4 localization in carotid atherosclerotic plaques: Correlation with clinical parameters. Thromb. Haemost. 2003, 90, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- Pollard, J.W. Trophic macrophages in development and disease. Nat. Rev. Immunol. 2009, 9, 259–270. [Google Scholar] [CrossRef]
- Li, L.; Huang, L.; Sung, S.S.; Vergis, A.L.; Rosin, D.L.; Rose, C.E., Jr.; Lobo, P.I.; Okusa, M.D. The chemokine receptors CCR2 and CX3CR1 mediate monocyte/macrophage trafficking in kidney ischemia-reperfusion injury. Kidney Int. 2008, 74, 1526–1537. [Google Scholar] [CrossRef]
- Ishida, Y.; Gao, J.L.; Murphy, P.M. Chemokine receptor CX3CR1 mediates skin wound healing by promoting macrophage and fibroblast accumulation and function. J. Immunol. 2008, 180, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Yang, M.; Shao, J.; Miao, Y.; Han, J.; Du, J. Chemokine receptor CX3CR1 contributes to macrophage survival in tumor metastasis. Mol. Cancer 2013, 12, 141. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Lee, Y.; Song, J.; Lee, J.; Chang, S.Y. Tissue-specific Role of CX(3)CR1 Expressing Immune Cells and Their Relationships with Human Disease. Immune Netw. 2018, 18, e5. [Google Scholar] [CrossRef] [PubMed]
- Zaid, A.; Tharmarajah, K.; Mostafavi, H.; Freitas, J.R.; Sheng, K.C.; Foo, S.S.; Chen, W.; Vider, J.; Liu, X.; West, N.P.; et al. Modulation of Monocyte-Driven Myositis in Alphavirus Infection Reveals a Role for CX(3)CR1(+) Macrophages in Tissue Repair. mBio 2020, 11, 10-1128. [Google Scholar] [CrossRef]
- Zhang, Z.; Gothe, F.; Pennamen, P.; James, J.R.; McDonald, D.; Mata, C.P.; Modis, Y.; Alazami, A.M.; Acres, M.; Haller, W.; et al. Human interleukin-2 receptor beta mutations associated with defects in immunity and peripheral tolerance. J. Exp. Med. 2019, 216, 1311–1327. [Google Scholar] [CrossRef]
- Chinen, T.; Kannan, A.K.; Levine, A.G.; Fan, X.; Klein, U.; Zheng, Y.; Gasteiger, G.; Feng, Y.; Fontenot, J.D.; Rudensky, A.Y. An essential role for the IL-2 receptor in T(reg) cell function. Nat. Immunol. 2016, 17, 1322–1333. [Google Scholar] [CrossRef]
- Abdelfattah, N.; Kumar, P.; Wang, C.; Leu, J.S.; Flynn, W.F.; Gao, R.; Baskin, D.S.; Pichumani, K.; Ijare, O.B.; Wood, S.L.; et al. Single-cell analysis of human glioma and immune cells identifies S100A4 as an immunotherapy target. Nat. Commun. 2022, 13, 767. [Google Scholar] [CrossRef] [PubMed]
- Marangoni, R.G.; Datta, P.; Paine, A.; Duemmel, S.; Nuzzo, M.; Sherwood, L.; Varga, J.; Ritchlin, C.; Korman, B.D. Thy-1 plays a pathogenic role and is a potential biomarker for skin fibrosis in scleroderma. JCI Insight 2022, 7, e149426. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.H.; Ryu, J.S.; Yoon, C.H.; Kim, M.K. Age-Dependent Distinct Distributions of Dendritic Cells in Autoimmune Dry Eye Murine Model. Cells 2021, 10, 1857. [Google Scholar] [CrossRef] [PubMed]
- See, P.; Dutertre, C.A.; Chen, J.; Gunther, P.; McGovern, N.; Irac, S.E.; Gunawan, M.; Beyer, M.; Handler, K.; Duan, K.; et al. Mapping the human DC lineage through the integration of high-dimensional techniques. Science 2017, 356, eaag3009. [Google Scholar] [CrossRef] [PubMed]
- Haniffa, M.; Shin, A.; Bigley, V.; McGovern, N.; Teo, P.; See, P.; Wasan, P.S.; Wang, X.N.; Malinarich, F.; Malleret, B.; et al. Human tissues contain CD141hi cross-presenting dendritic cells with functional homology to mouse CD103+ nonlymphoid dendritic cells. Immunity 2012, 37, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Bachem, A.; Guttler, S.; Hartung, E.; Ebstein, F.; Schaefer, M.; Tannert, A.; Salama, A.; Movassaghi, K.; Opitz, C.; Mages, H.W.; et al. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J. Exp. Med. 2010, 207, 1273–1281. [Google Scholar] [CrossRef]
- Dudziak, D.; Kamphorst, A.O.; Heidkamp, G.F.; Buchholz, V.R.; Trumpfheller, C.; Yamazaki, S.; Cheong, C.; Liu, K.; Lee, H.W.; Park, C.G.; et al. Differential antigen processing by dendritic cell subsets in vivo. Science 2007, 315, 107–111. [Google Scholar] [CrossRef]
- Soares, H.; Waechter, H.; Glaichenhaus, N.; Mougneau, E.; Yagita, H.; Mizenina, O.; Dudziak, D.; Nussenzweig, M.C.; Steinman, R.M. A subset of dendritic cells induces CD4+ T cells to produce IFN-gamma by an IL-12-independent but CD70-dependent mechanism in vivo. J. Exp. Med. 2007, 204, 1095–1106. [Google Scholar] [CrossRef]
- Tussiwand, R.; Everts, B.; Grajales-Reyes, G.E.; Kretzer, N.M.; Iwata, A.; Bagaitkar, J.; Wu, X.; Wong, R.; Anderson, D.A.; Murphy, T.L.; et al. Klf4 expression in conventional dendritic cells is required for T helper 2 cell responses. Immunity 2015, 42, 916–928. [Google Scholar] [CrossRef]
- Schlitzer, A.; McGovern, N.; Teo, P.; Zelante, T.; Atarashi, K.; Low, D.; Ho, A.W.; See, P.; Shin, A.; Wasan, P.S.; et al. IRF4 transcription factor-dependent CD11b+ dendritic cells in human and mouse control mucosal IL-17 cytokine responses. Immunity 2013, 38, 970–983. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.; Liu, S.; Guo, X.; Cen, D.; Xu, J.; Li, H.; Li, K.; Zeng, C.; Lu, L.; et al. Scaffolding protein Gab1 regulates myeloid dendritic cell migration in allergic asthma. Cell Res. 2016, 26, 1226–1241. [Google Scholar] [CrossRef] [PubMed]
- Randolph, G.J.; Ochando, J.; Partida-Sanchez, S. Migration of dendritic cell subsets and their precursors. Annu. Rev. Immunol. 2008, 26, 293–316. [Google Scholar] [CrossRef] [PubMed]
- Swiecki, M.; Colonna, M. The multifaceted biology of plasmacytoid dendritic cells. Nat. Rev. Immunol. 2015, 15, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Martin-Gayo, E.; Sierra-Filardi, E.; Corbi, A.L.; Toribio, M.L. Plasmacytoid dendritic cells resident in human thymus drive natural Treg cell development. Blood 2010, 115, 5366–5375. [Google Scholar] [CrossRef]
- Lilla, J.N.; Werb, Z. Mast cells contribute to the stromal microenvironment in mammary gland branching morphogenesis. Dev. Biol. 2010, 337, 124–133. [Google Scholar] [CrossRef]
- Lilla, J.N.; Joshi, R.V.; Craik, C.S.; Werb, Z. Active plasma kallikrein localizes to mast cells and regulates epithelial cell apoptosis, adipocyte differentiation, and stromal remodeling during mammary gland involution. J. Biol. Chem. 2009, 284, 13792–13803. [Google Scholar] [CrossRef]
- Williams, R.M.; Singh, J.; Sharkey, K.A. Innervation and mast cells of the rat exorbital lacrimal gland: The effects of age. J. Auton. Nerv. Syst. 1994, 47, 95–108. [Google Scholar] [CrossRef]
- Franklin, R.M. The ocular secretory immune system: A review. Curr. Eye Res. 1989, 8, 599–606. [Google Scholar] [CrossRef]
- Wu, Y.F.; Chang, N.W.; Chu, L.A.; Liu, H.Y.; Zhou, Y.X.; Pai, Y.L.; Yu, Y.S.; Kuan, C.H.; Wu, Y.C.; Lin, S.J.; et al. Single-Cell Transcriptomics Reveals Cellular Heterogeneity and Complex Cell-Cell Communication Networks in the Mouse Cornea. Investig. Ophthalmol. Vis. Sci. 2023, 64, 5. [Google Scholar] [CrossRef]
- Van Setten, G.B.; Nilsson, L.; Hahne, S.; Johnston, J.A.; Kvanta, A.; Gandy, S.E.; Naslund, J.; Nordstedt, C. Beta-amyloid protein protein precursor expression in lacrimal glands and tear fluid. Investig. Ophthalmol. Vis. Sci. 1996, 37, 2585–2593. [Google Scholar]
- Semanjski, K.; Majdic, G.; Kozina, V.; Jezek, D. Sexual dimorphism of the extraorbital lacrimal glands in SF-1 knockout mice. Acta Histochem. 2021, 123, 151669. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Jiao, X.; Lu, D.; Pei, X.; Qi, D.; Li, Z. Light cycle phase advance as a model for jet lag reprograms the circadian rhythms of murine extraorbital lacrimal glands. Ocul. Surf. 2021, 20, 95–114. [Google Scholar] [CrossRef] [PubMed]
- El-Fadaly, A.B.; El-Shaarawy, E.A.; Rizk, A.A.; Nasralla, M.M.; Shuaib, D.M. Age-related alterations in the lacrimal gland of adult albino rat: A light and electron microscopic study. Ann. Anat. 2014, 196, 336–351. [Google Scholar] [CrossRef] [PubMed]
- Coursey, T.G.; Bian, F.; Zaheer, M.; Pflugfelder, S.C.; Volpe, E.A.; de Paiva, C.S. Age-related spontaneous lacrimal keratoconjunctivitis is accompanied by dysfunctional T regulatory cells. Mucosal Immunol. 2017, 10, 743–756. [Google Scholar] [CrossRef]
- Trujillo-Vargas, C.M.; Mauk, K.E.; Hernandez, H.; de Souza, R.G.; Yu, Z.; Galletti, J.G.; Dietrich, J.; Paulsen, F.; de Paiva, C.S. Immune phenotype of the CD4(+) T cells in the aged lymphoid organs and lacrimal glands. Geroscience 2022, 44, 2105–2128. [Google Scholar] [CrossRef]
- Psianou, K.; Panagoulias, I.; Papanastasiou, A.D.; de Lastic, A.L.; Rodi, M.; Spantidea, P.I.; Degn, S.E.; Georgiou, P.; Mouzaki, A. Clinical and immunological parameters of Sjogren’s syndrome. Autoimmun. Rev. 2018, 17, 1053–1064. [Google Scholar] [CrossRef]
- Zhou, D.; McNamara, N.A. Macrophages: Important players in primary Sjogren’s syndrome? Expert. Rev. Clin. Immunol. 2014, 10, 513–520. [Google Scholar] [CrossRef]
- Sequi-Sabater, J.M.; Beretta, L. Defining the Role of Monocytes in Sjogren’s Syndrome. Int. J. Mol. Sci. 2022, 23, 12765. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Q.; Yan, R.; Li, Y.; Lu, L.; Liu, J.; Li, S.; Fu, T.; Xue, Y.; Liu, J.; Li, Z. Exploring Immune Cell Diversity in the Lacrimal Glands of Healthy Mice: A Single-Cell RNA-Sequencing Atlas. Int. J. Mol. Sci. 2024, 25, 1208. https://doi.org/10.3390/ijms25021208
Fan Q, Yan R, Li Y, Lu L, Liu J, Li S, Fu T, Xue Y, Liu J, Li Z. Exploring Immune Cell Diversity in the Lacrimal Glands of Healthy Mice: A Single-Cell RNA-Sequencing Atlas. International Journal of Molecular Sciences. 2024; 25(2):1208. https://doi.org/10.3390/ijms25021208
Chicago/Turabian StyleFan, Qiwei, Ruyu Yan, Yan Li, Liyuan Lu, Jiangman Liu, Senmao Li, Ting Fu, Yunxia Xue, Jun Liu, and Zhijie Li. 2024. "Exploring Immune Cell Diversity in the Lacrimal Glands of Healthy Mice: A Single-Cell RNA-Sequencing Atlas" International Journal of Molecular Sciences 25, no. 2: 1208. https://doi.org/10.3390/ijms25021208
APA StyleFan, Q., Yan, R., Li, Y., Lu, L., Liu, J., Li, S., Fu, T., Xue, Y., Liu, J., & Li, Z. (2024). Exploring Immune Cell Diversity in the Lacrimal Glands of Healthy Mice: A Single-Cell RNA-Sequencing Atlas. International Journal of Molecular Sciences, 25(2), 1208. https://doi.org/10.3390/ijms25021208





