The Impact of Obesity on Diabetes Onset and Neovascularization in Mouse Models of Metabolic Stress
Abstract
1. Introduction
2. Results
2.1. The Body Weight Gain, Hyperglycemia, and Insulin Resistance in Metabolic Models in Male Mice
2.2. Lipid Panels and Hepatic Steatosis in Metabolic Models in Male Mice
2.3. Female Mice Are Resistant to Metabolic Models
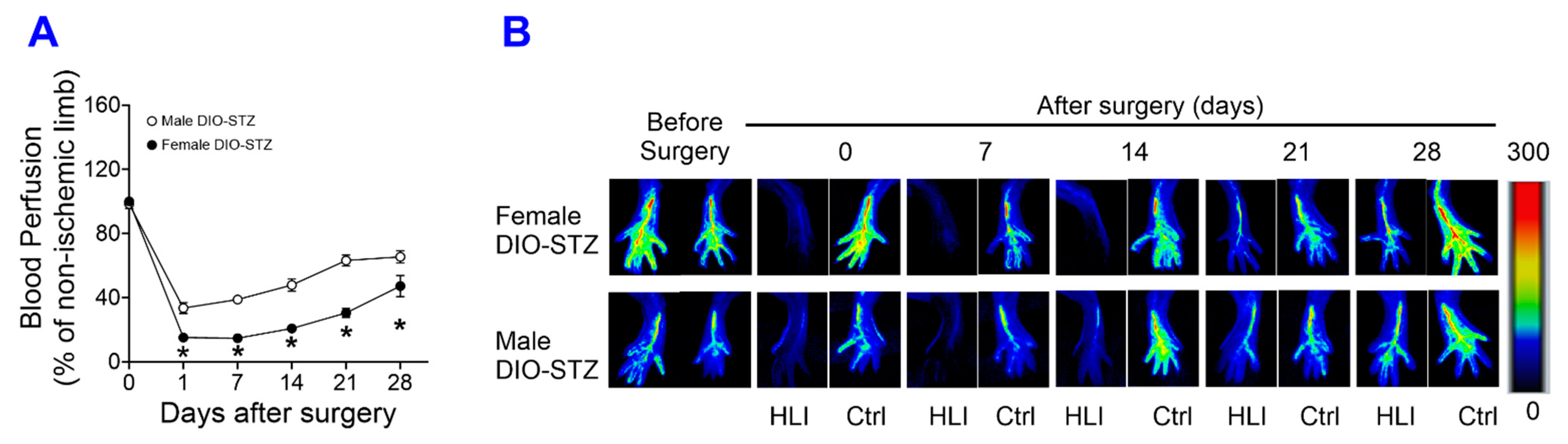
2.4. Neovascularization in Mouse Models of Metabolic Stress in Both Male and Female Mice
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Streptozotocin (STZ)-Induced Chronic Hyperglycemic Model
4.3. Diet-Induced Obesity (DIO) Model
4.4. DIO-STZ-Induced T2D Model
4.5. Glucose Tolerance Test
4.6. Insulin Tolerance Test
4.7. Triglycerides Assay
4.8. Cholesterol Assay
4.9. High-Density Lipoprotein (HDL)-Cholesterol and Low-Density Lipoprotein (LDL)-Cholesterol Assay
4.10. Oil Red O Staining
4.11. Picro-Sirius Staining
4.12. Hindlimb Ischemia Surgery
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sasidharan, S.R.; Joseph, J.A.; Anandakumar, S.; Venkatesan, V.; Ariyattu Madhavan, C.N.; Agarwal, A. An experimental approach for selecting appropriate rodent diets for research studies on metabolic disorders. BioMed Res. Int. 2013, 2013, 752870. [Google Scholar] [CrossRef] [PubMed]
- Della Vedova, M.C.; Muñoz, M.D.; Santillan, L.D.; Plateo-Pignatari, M.G.; Germanó, M.J.; Rinaldi Tosi, M.E.; Garcia, S.; Gomez, N.N.; Fornes, M.W.; Gomez Mejiba, S.E.; et al. A Mouse Model of Diet-Induced Obesity Resembling Most Features of Human Metabolic Syndrome. Nutr. Metab. Insights 2016, 9, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Bastias-Perez, M.; Serra, D.; Herrero, L. Dietary Options for Rodents in the Study of Obesity. Nutrients 2020, 12, 3234. [Google Scholar] [CrossRef]
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. Pharmacol. 2015, 70, 5–47. [Google Scholar] [CrossRef] [PubMed]
- Watts, L.M.; Manchem, V.P.; Leedom, T.A.; Rivard, A.L.; McKay, R.A.; Bao, D.; Neroladakis, T.; Monia, B.P.; Bodenmiller, D.M.; Cao, J.X.; et al. Reduction of hepatic and adipose tissue glucocorticoid receptor expression with antisense oligonucleotides improves hyperglycemia and hyperlipidemia in diabetic rodents without causing systemic glucocorticoid antagonism. Diabetes 2005, 54, 1846–1853. [Google Scholar] [CrossRef]
- Wang, C.Y.; Liao, J.K. A mouse model of diet-induced obesity and insulin resistance. Methods Mol. Biol. 2012, 821, 421–433. [Google Scholar]
- Rask-Madsen, C.; King, G.L. Vascular complications of diabetes: Mechanisms of injury and protective factors. Cell Metab. 2013, 17, 20–33. [Google Scholar] [CrossRef]
- Almeida-Suhett, C.P.; Scott, J.M.; Graham, A.; Chen, Y.; Deuster, P.A. Control diet in a high-fat diet study in mice: Regular chow and purified low-fat diet have similar effects on phenotypic, metabolic, and behavioral outcomes. Nutr. Neurosci. 2019, 22, 19–28. [Google Scholar] [CrossRef]
- Meda Venkata, S.P.; Li, H.; Xu, L.; Koh, J.Y.; Nguyen, H.; Minjares, M.; Li, C.; Kowluru, A.; Milligan, G.; Wang, J.M. Inhibition of GPR39 restores defects in endothelial cell-mediated neovascularization under the duress of chronic hyperglycemia: Evidence for regulatory roles of the sonic hedgehog signaling axis. Proc. Natl. Acad. Sci. USA 2023, 120, e2208541120. [Google Scholar] [CrossRef]
- Nguyen, H.; Koh, J.Y.; Li, H.; Islas-Robles, A.; Meda Venkata, S.P.; Wang, J.M.; Monks, T.J. A novel imidazolinone metformin-methylglyoxal metabolite promotes endothelial cell angiogenesis via the eNOS/HIF-1α pathway. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2021, 35, e21645. [Google Scholar] [CrossRef]
- Benjamini, Y.; Krieger, A.M.; Yekutieli, D. Adaptive linear step-up procedures that control the false discovery rate. Biometrika 2006, 93, 491–507. [Google Scholar] [CrossRef]
- Zhou, Z.; Ribas, V.; Rajbhandari, P.; Drew, B.G.; Moore, T.M.; Fluitt, A.H.; Reddish, B.R.; Whitney, K.A.; Georgia, S.; Vergnes, L.; et al. Estrogen receptor alpha protects pancreatic beta-cells from apoptosis by preserving mitochondrial function and suppressing endoplasmic reticulum stress. J. Biol. Chem. 2018, 293, 4735–4751. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhang, C.; Zhang, K. Measurement of ER stress response and inflammation in the mouse model of nonalcoholic fatty liver disease. Methods Enzym. 2011, 489, 329–348. [Google Scholar]
- Pipitone, R.M.; Ciccioli, C.; Infantino, G.; La Mantia, C.; Parisi, S.; Tulone, A.; Pennisi, G.; Grimaudo, S.; Petta, S. MAFLD: A multisystem disease. Ther. Adv. Endocrinol. Metab. 2023, 14, 20420188221145549. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Adejumo, A.C.; Yoo, E.R.; Iqbal, U.; Li, A.A.; Pham, E.A.; Cholankeril, G.; Glenn, J.S.; Ahmed, A. Trends in Mortality From Extrahepatic Complications in Patients With Chronic Liver Disease, From 2007 Through 2017. Gastroenterology 2019, 157, 1055–1066 e11. [Google Scholar] [CrossRef]
- Mantovani, A.; Byrne, C.D.; Bonora, E.; Targher, G. Nonalcoholic Fatty Liver Disease and Risk of Incident Type 2 Diabetes: A Meta-analysis. Diabetes Care 2018, 41, 372–382. [Google Scholar] [CrossRef]
- Pais, R.; Giral, P.; Khan, J.F.; Rosenbaum, D.; Housset, C.; Poynard, T.; Ratziu, V.; Group, L.S. Fatty liver is an independent predictor of early carotid atherosclerosis. J. Hepatol. 2016, 65, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Csermely, A.; Petracca, G.; Beatrice, G.; Corey, K.E.; Simon, T.G.; Byrne, C.D.; Targher, G. Nonalcoholic fatty liver disease and risk of fatal and non-fatal cardiovascular events: An updated systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2021, 6, 903–913. [Google Scholar] [CrossRef]
- Kim, B.; Park, E.S.; Lee, J.S.; Suh, J.G. Outbred Mice with Streptozotocin-Induced Diabetes Show Sex Differences in Glucose Metabolism. Int. J. Mol. Sci. 2023, 24, 5210. [Google Scholar] [CrossRef]
- Leiter, E.H. Multiple low-dose streptozotocin-induced hyperglycemia and insulitis in C57BL mice: Influence of inbred background, sex, and thymus. Proc. Natl. Acad. Sci. USA 1982, 79, 630–634. [Google Scholar] [CrossRef]
- Pettersson, U.S.; Walden, T.B.; Carlsson, P.O.; Jansson, L.; Phillipson, M. Female mice are protected against high-fat diet induced metabolic syndrome and increase the regulatory T cell population in adipose tissue. PLoS ONE 2012, 7, e46057. [Google Scholar] [CrossRef] [PubMed]
- Casimiro, I.; Stull, N.D.; Tersey, S.A.; Mirmira, R.G. Phenotypic sexual dimorphism in response to dietary fat manipulation in C57BL/6J mice. J. Diabetes Complicat. 2021, 35, 107795. [Google Scholar] [CrossRef] [PubMed]
- Saadane, A.; Lessieur, E.M.; Du, Y.; Liu, H.; Kern, T.S. Successful induction of diabetes in mice demonstrates no gender difference in development of early diabetic retinopathy. PLoS ONE 2020, 15, e0238727. [Google Scholar] [CrossRef] [PubMed]
- Pabon, M.; Cheng, S.; Altin, S.E.; Sethi, S.S.; Nelson, M.D.; Moreau, K.L.; Hamburg, N.; Hess, C.N. Sex Differences in Peripheral Artery Disease. Circ. Res. 2022, 130, 496–511. [Google Scholar] [CrossRef] [PubMed]
- Gluchowski, N.L.; Becuwe, M.; Walther, T.C.; Farese, R.V., Jr. Lipid droplets and liver disease: From basic biology to clinical implications. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 343–355. [Google Scholar] [CrossRef]
- Shi, X.; Li, J.; Zou, X.; Greggain, J.; Rodkaer, S.V.; Faergeman, N.J.; Liang, B.; Watts, J.L. Regulation of lipid droplet size and phospholipid composition by stearoyl-CoA desaturase. J. Lipid Res. 2013, 54, 2504–2514. [Google Scholar] [CrossRef] [PubMed]
- Verges, B. Dyslipidemia in Type 1 Diabetes: AMaskedDanger. Trends Endocrinol. Metab. 2020, 31, 422–434. [Google Scholar] [CrossRef]
- Reaven, P.; Merat, S.; Casanada, F.; Sutphin, M.; Palinski, W. Effect of streptozotocin-induced hyperglycemia on lipid profiles, formation of advanced glycation endproducts in lesions, and extent of atherosclerosis in LDL receptor-deficient mice. Arter. Thromb. Vasc. Biol. 1997, 17, 2250–2256. [Google Scholar] [CrossRef]
- Willecke, F.; Scerbo, D.; Nagareddy, P.; Obunike, J.C.; Barrett, T.J.; Abdillahi, M.L.; Trent, C.M.; Huggins, L.A.; Fisher, E.A.; Drosatos, K.; et al. Lipolysis, and not hepatic lipogenesis, is the primary modulator of triglyceride levels in streptozotocin-induced diabetic mice. Arter. Thromb. Vasc. Biol. 2015, 35, 102–110. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, W.; Yao, Y.; Li, X.; Huang, X.; Wang, Y.; Ding, M.; Huang, X. An RDH-Plin2 axis modulates lipid droplet size by antagonizing Bmm lipase. EMBO Rep. 2022, 23, e52669. [Google Scholar] [CrossRef]
- Schott, M.B.; Weller, S.G.; Schulze, R.J.; Krueger, E.W.; Drizyte-Miller, K.; Casey, C.A.; McNiven, M.A. Lipid droplet size directs lipolysis and lipophagy catabolism in hepatocytes. J. Cell Biol. 2019, 218, 3320–3335. [Google Scholar] [CrossRef] [PubMed]
- Aref, Z.; de Vries, M.R.; Quax, P.H.A. Variations in Surgical Procedures for Inducing Hind Limb Ischemia in Mice and the Impact of These Variations on Neovascularization Assessment. Int. J. Mol. Sci. 2019, 20, 3704. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Dardik, A. A Murine Model of Hind Limb Ischemia to Study Angiogenesis and Arteriogenesis. Methods Mol. Biol. 2018, 1717, 135–143. [Google Scholar] [PubMed]
- Schreyer, S.A.; Wilson, D.L.; LeBoeuf, R.C. C57BL/6 mice fed high fat diets as models for diabetes-accelerated atherosclerosis. Atherosclerosis 1998, 136, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Nascimento-Sales, M.; Fredo-da-Costa, I.; Borges Mendes, A.C.B.; Melo, S.; Ravache, T.T.; Gomez, T.G.B.; Gaisler-Silva, F.; Ribeiro, M.O.; Santos, A.R., Jr.; Carneiro-Ramos, M.S.; et al. Is the FVB/N mouse strain truly resistant to diet-induced obesity? Physiol. Rep. 2017, 5, e13271. [Google Scholar] [CrossRef]
- Devlin, M.J.; Robbins, A.; Cosman, M.N.; Moursi, C.A.; Cloutier, A.M.; Louis, L.; Van Vliet, M.; Conlon, C.; Bouxsein, M.L. Differential effects of high fat diet and diet-induced obesity on skeletal acquisition in female C57BL/6J vs. FVB/NJ Mice. Bone Rep. 2018, 8, 204–214. [Google Scholar] [CrossRef]
- Al-Shaer, A.E.; Pal, A.; Shi, Q.; Carson, M.S.; Regan, J.; Behee, M.; Buddenbaum, N.; Drawdy, C.; Davis, T.; Virk, R.; et al. Modeling human heterogeneity of obesity with diversity outbred mice reveals a fat mass-dependent therapeutic window for resolvin E1. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2022, 36, e22354. [Google Scholar] [CrossRef]







| Model | SD | STZ | DIO | DIO-STZ |
|---|---|---|---|---|
| Body weight (g) | 28.95 ± 1.86 | 27.11 ± 2.33 | 32.76 ± 1.84 | 41.44 ± 2.40 |
| Blood glucose (mg/dL) | 183.7 ± 14.01 | 288.8 ± 13.80 | 225.5 ± 17.87 | 316.0 ± 27.56 |
| Triglycerides (mg/dL) | 38.13 ± 11.90 | 63.01 ± 24.58 | 262.61 ± 36.79 | 211.56 ± 45.74 |
| Cholesterol (mg/dL) | 45.91 ± 4.37 | 44.54 ± 5.13 | 177.21 ± 18.38 | 142.53 ± 32.94 |
| LDL-cholesterol (mg/dL) | 56.54 ± 19.09 | 53.72 ± 12.39 | 107.42 ± 23.63 | 163.97 ± 27.69 |
| HDL-cholesterol (mg/dL) | 52.65 ± 19.32 | 26.27 ± 8.67 | 142.96 ± 17.23 | 131.20 ± 57.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meda Venkata, S.P.; Li, H.; Xu, L.; Wang, J.-M. The Impact of Obesity on Diabetes Onset and Neovascularization in Mouse Models of Metabolic Stress. Int. J. Mol. Sci. 2024, 25, 1214. https://doi.org/10.3390/ijms25021214
Meda Venkata SP, Li H, Xu L, Wang J-M. The Impact of Obesity on Diabetes Onset and Neovascularization in Mouse Models of Metabolic Stress. International Journal of Molecular Sciences. 2024; 25(2):1214. https://doi.org/10.3390/ijms25021214
Chicago/Turabian StyleMeda Venkata, Sai Pranathi, Hainan Li, Liping Xu, and Jie-Mei Wang. 2024. "The Impact of Obesity on Diabetes Onset and Neovascularization in Mouse Models of Metabolic Stress" International Journal of Molecular Sciences 25, no. 2: 1214. https://doi.org/10.3390/ijms25021214
APA StyleMeda Venkata, S. P., Li, H., Xu, L., & Wang, J.-M. (2024). The Impact of Obesity on Diabetes Onset and Neovascularization in Mouse Models of Metabolic Stress. International Journal of Molecular Sciences, 25(2), 1214. https://doi.org/10.3390/ijms25021214






