Unveiling Novel ERCC1–XPF Complex Inhibitors: Bridging the Gap from In Silico Exploration to Experimental Design
Abstract
:1. Introduction
2. Results and Discussion
2.1. Computational Studies on the ERCC1–XPF Protein Complex
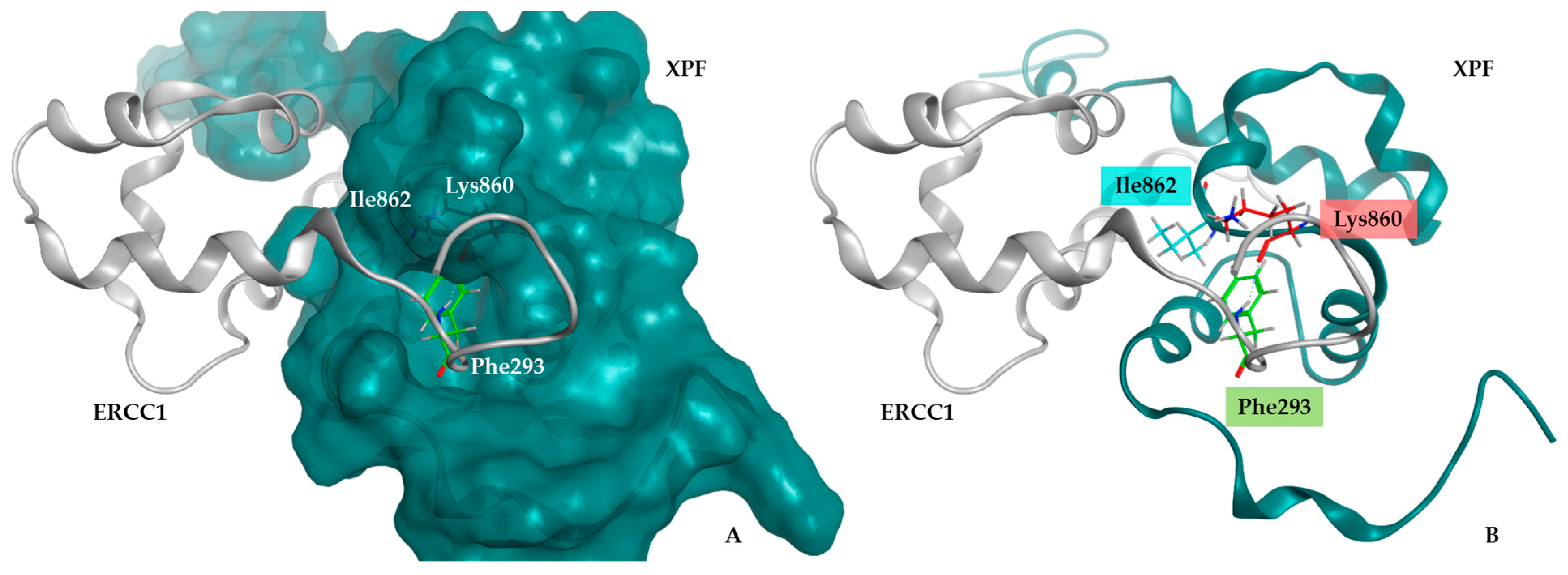
2.1.1. Structural Analysis of the Protein Complex
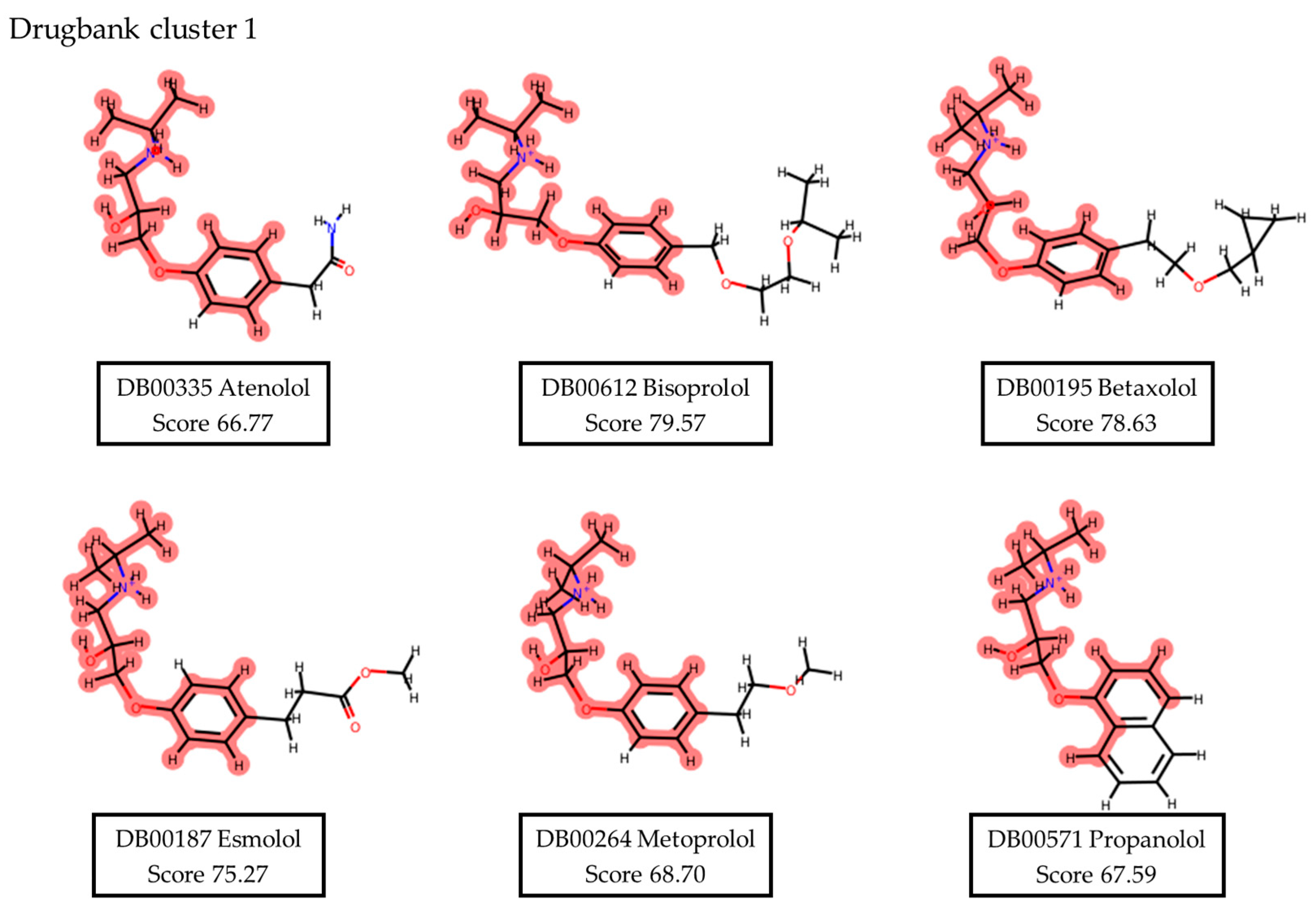
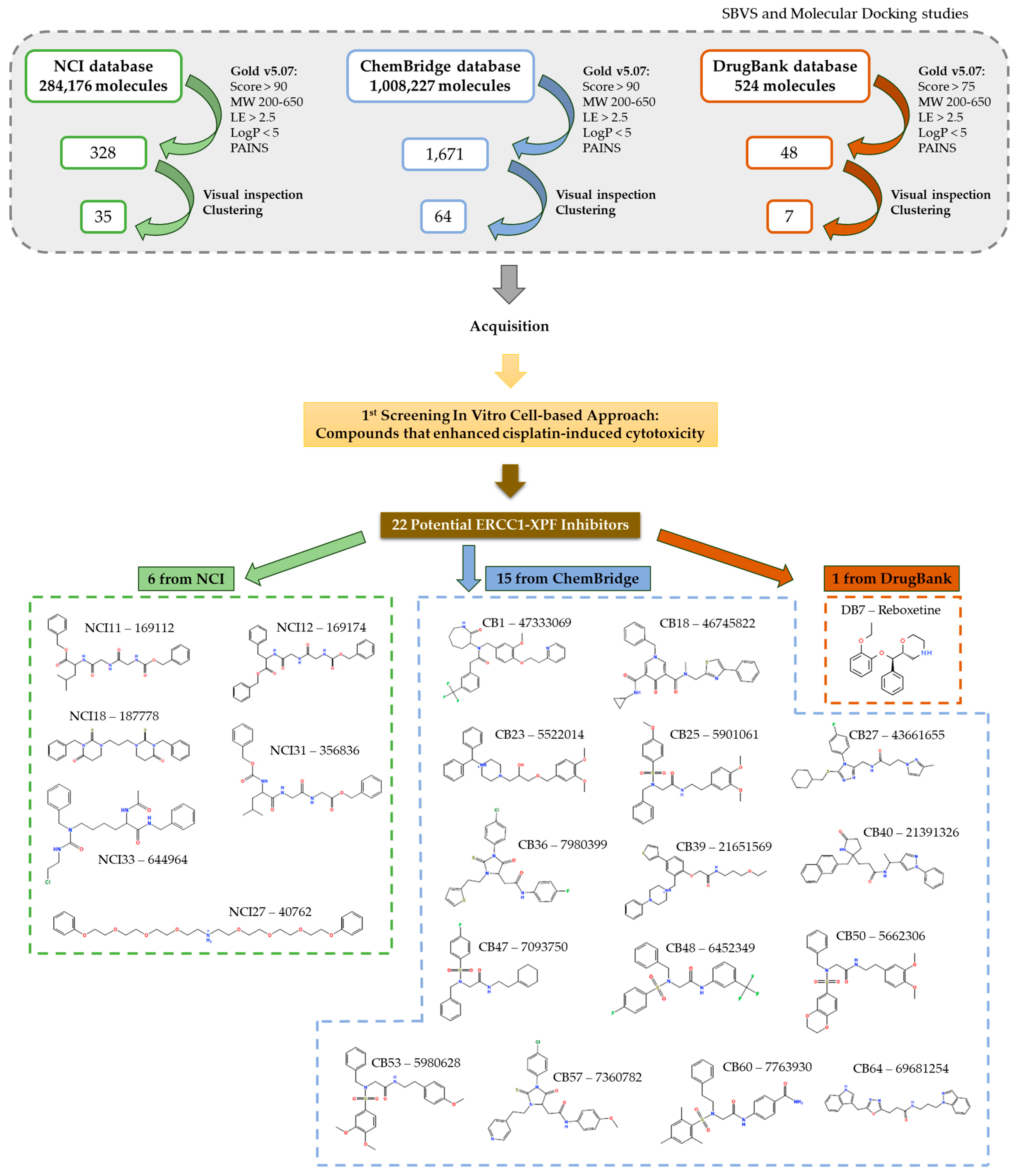
2.1.2. Assembly of an ERCC1–XPF Small Molecule Inhibitors Set
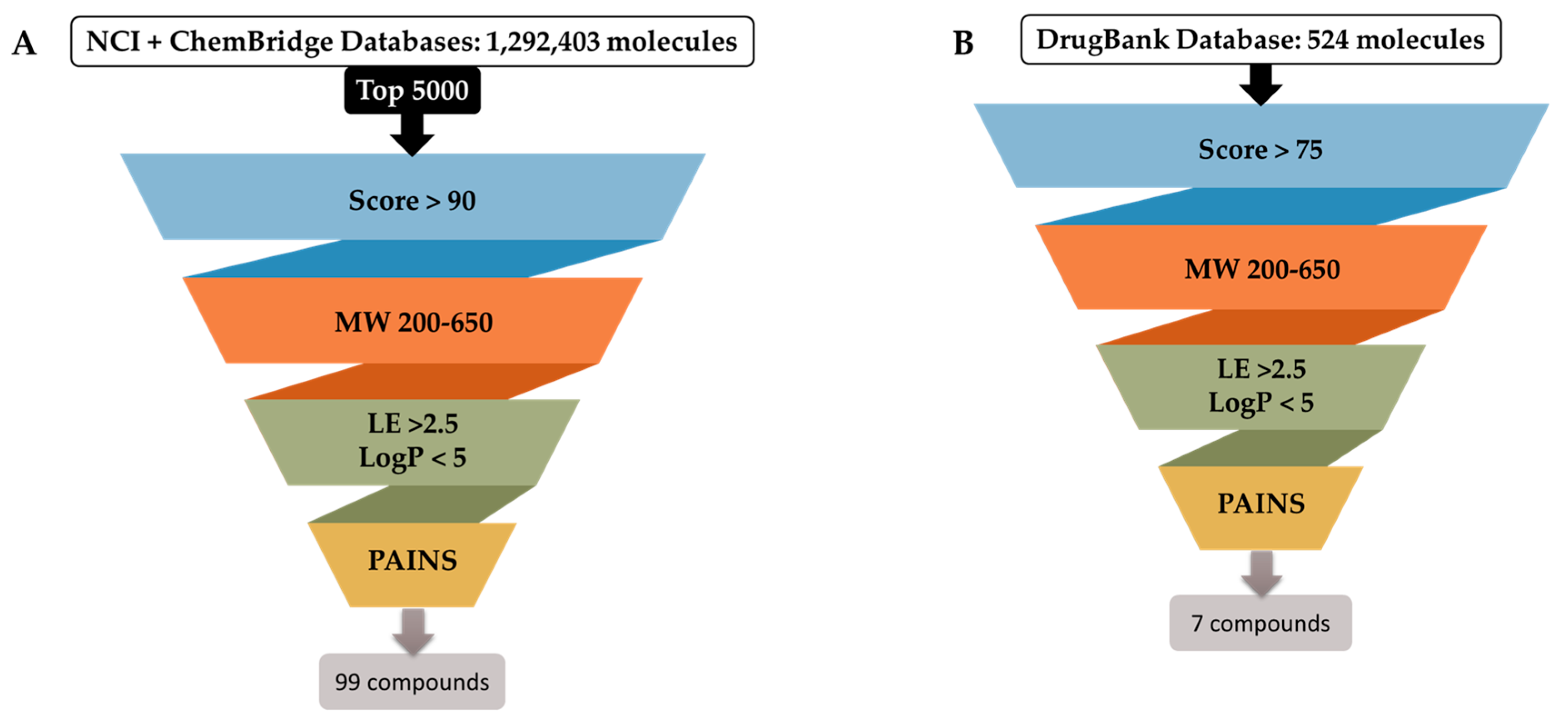
2.1.3. Optimization of Docking Protocol and Virtual Screening Campaign
2.2. Characterization of NSCLC Cell Lines
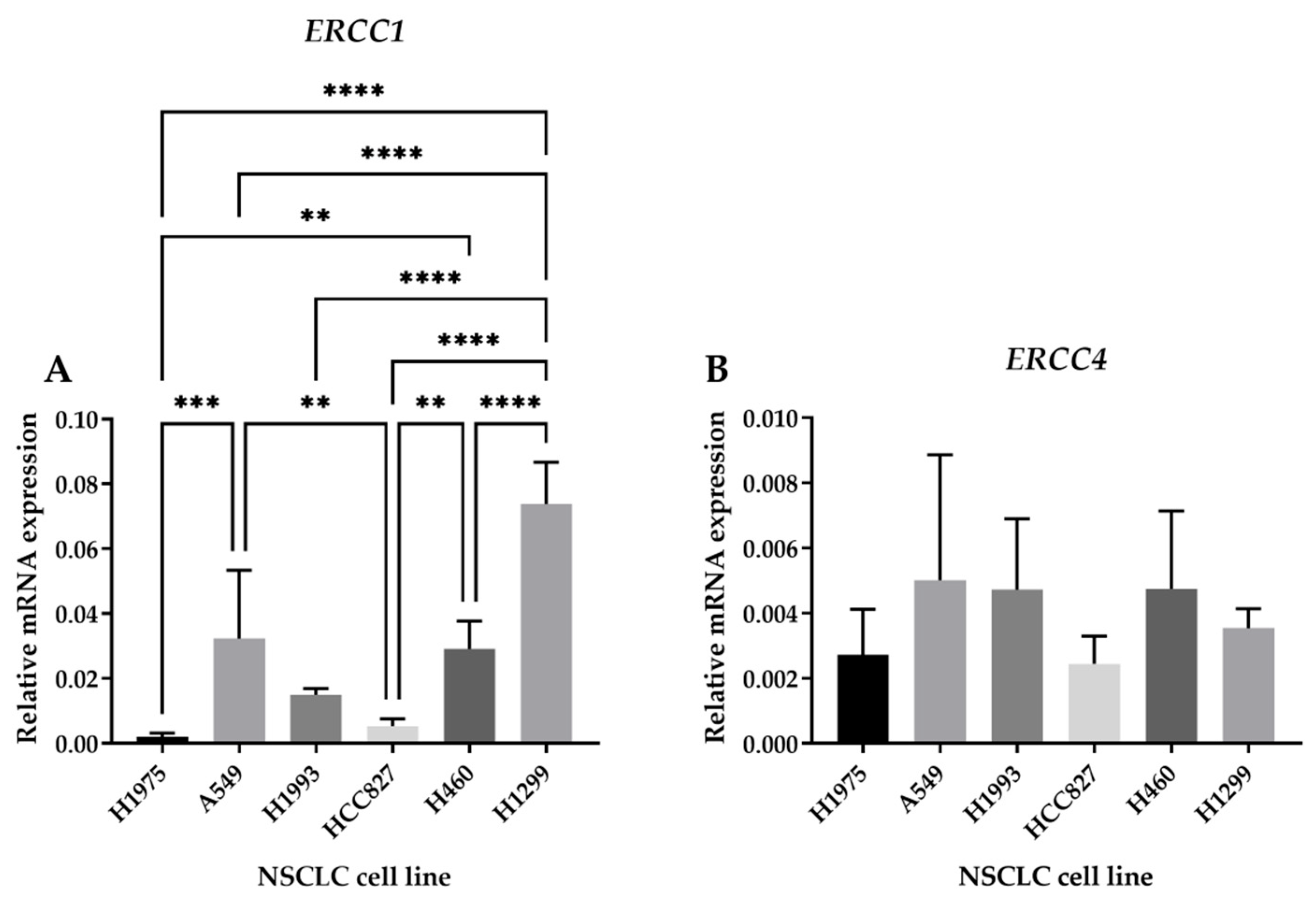
2.2.1. ERCC1 and ERCC4 Gene Expression in NSCLC Cell Lines
2.2.2. Cisplatin Cytotoxicity Profile in H1299 Cells
2.3. Validation of the Putative ERCC1–XPF Inhibitors in In Vitro Cell-Based Assays
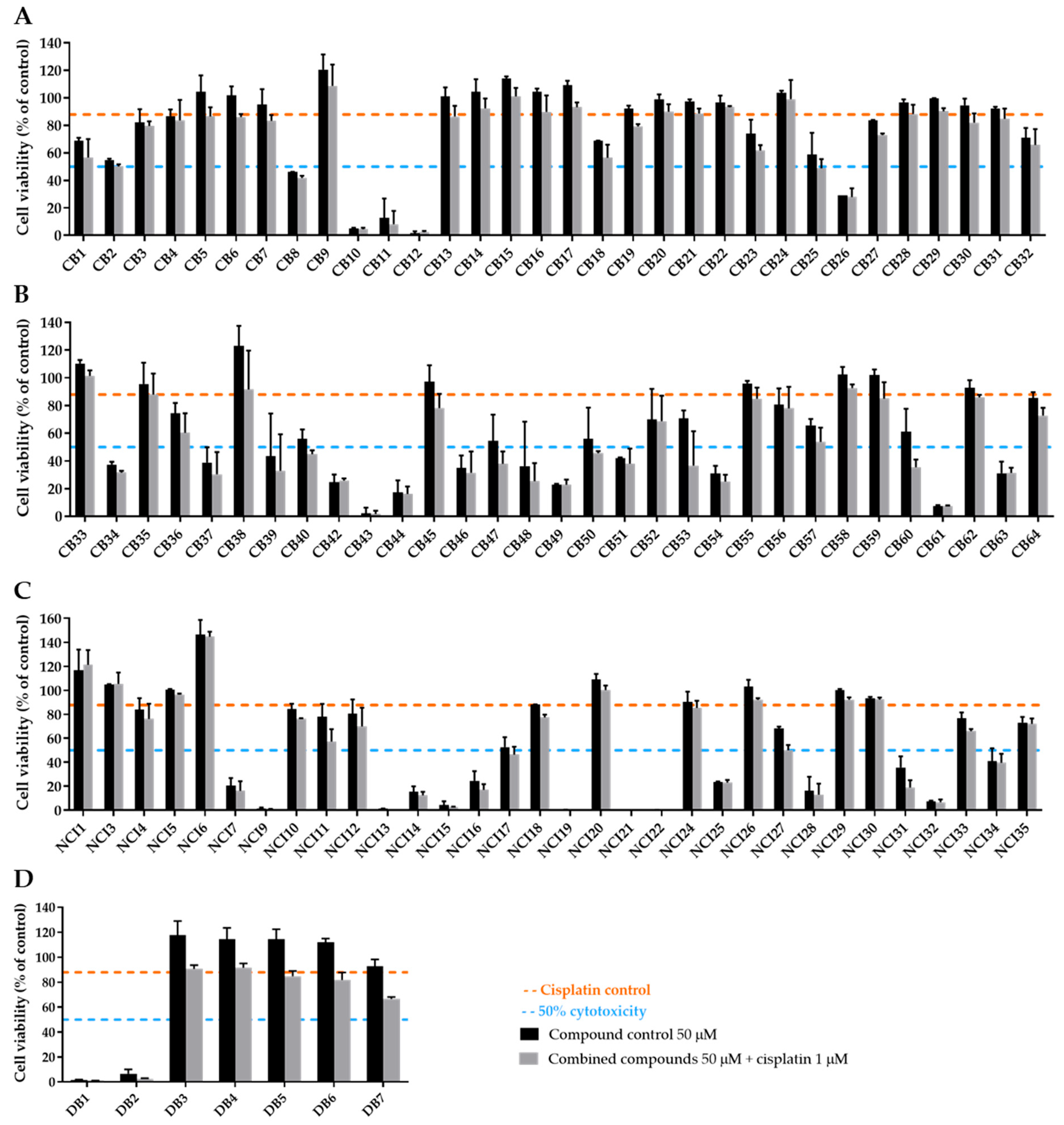
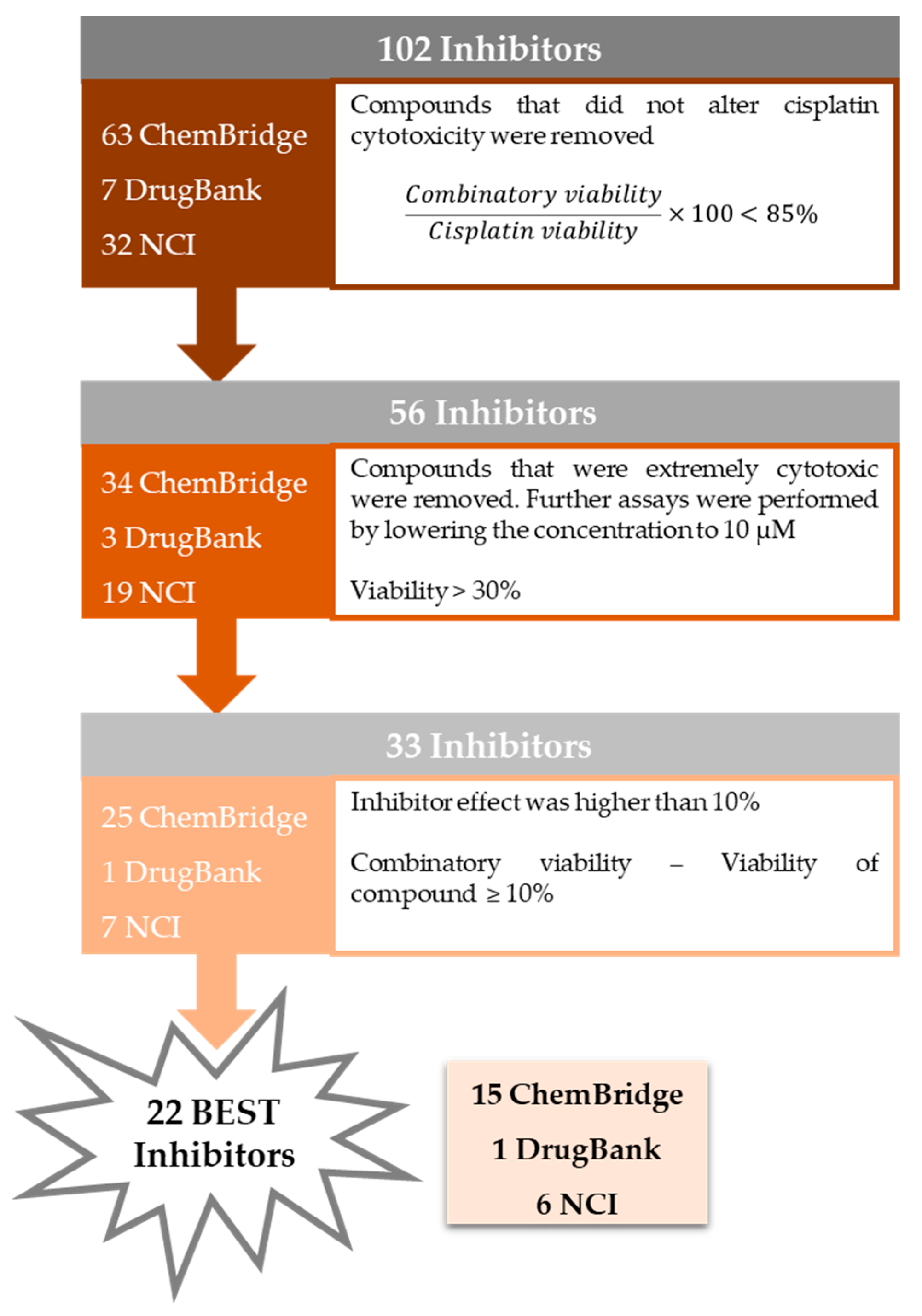
2.3.1. Screening of the Putative ERCC1–XPF Inhibitors in Combination with Cisplatin on H1299 Cells
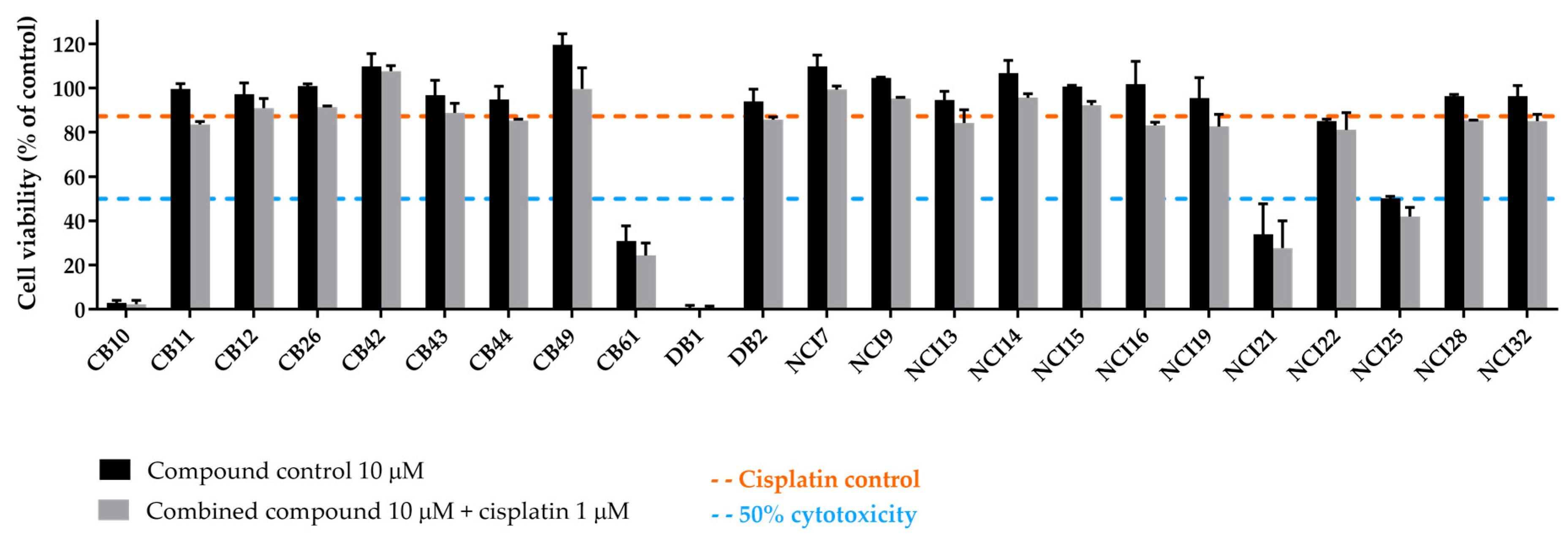
2.3.2. Extremely Cytotoxic Compounds in H1299 Cells
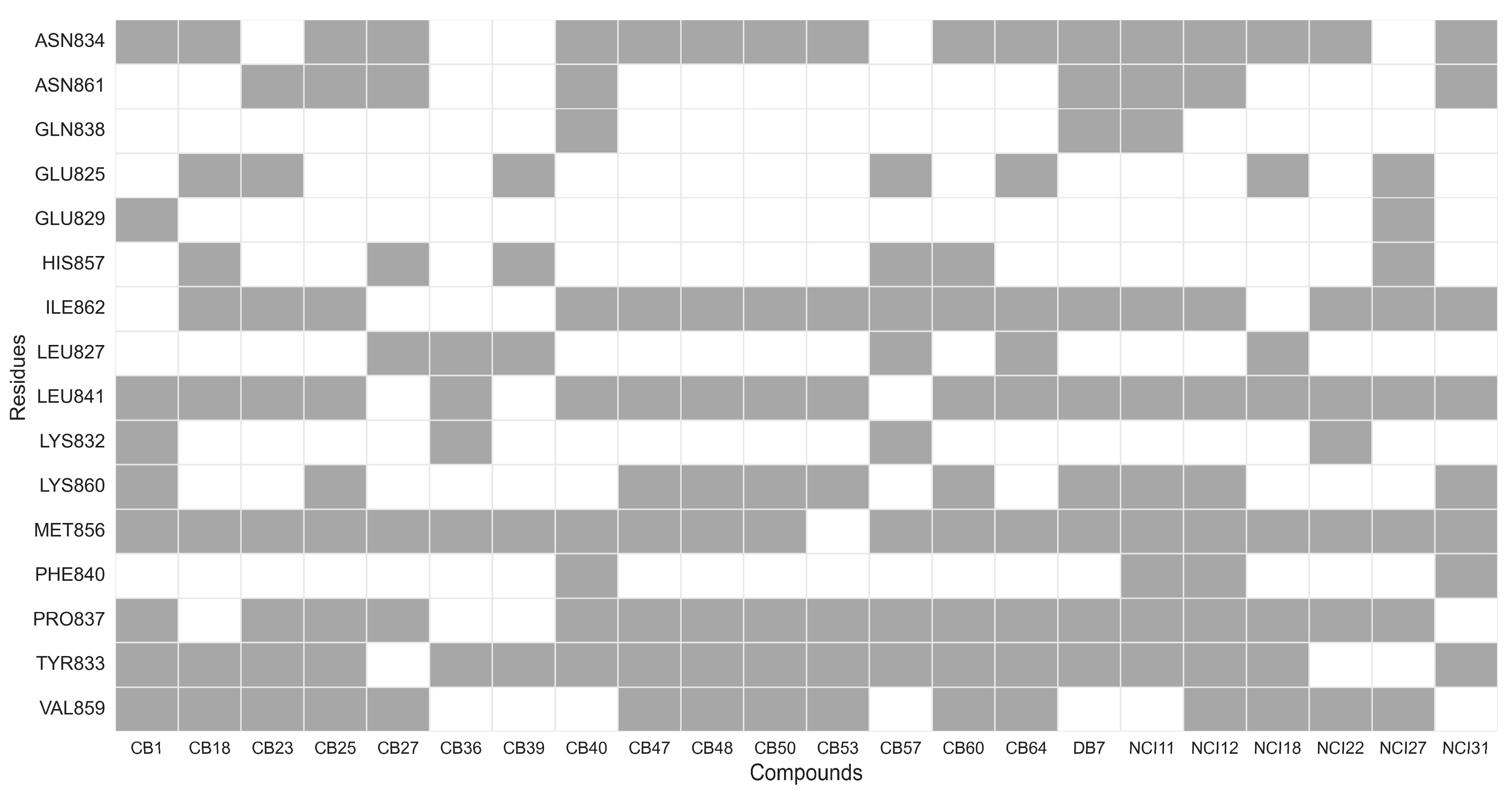
2.4. Interaction Analysis of Inhibitors with XPF Protein
3. Materials and Methods
3.1. Computational Simulations
3.1.1. Selection and Preparation of Protein Structures
3.1.2. Selection and Preparation of the Ligands
3.1.3. Validation of Docking and Virtual Screening Approaches
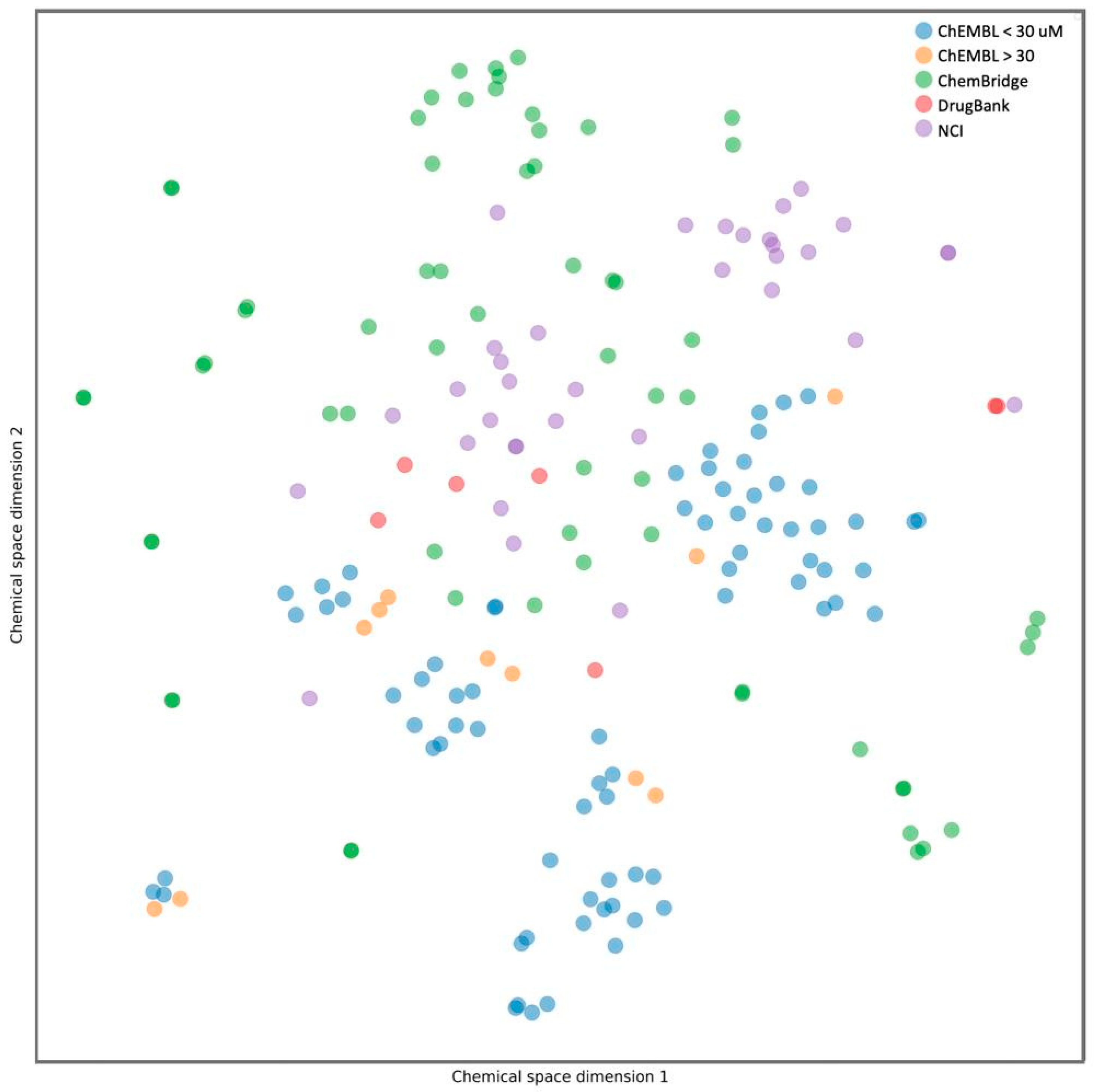
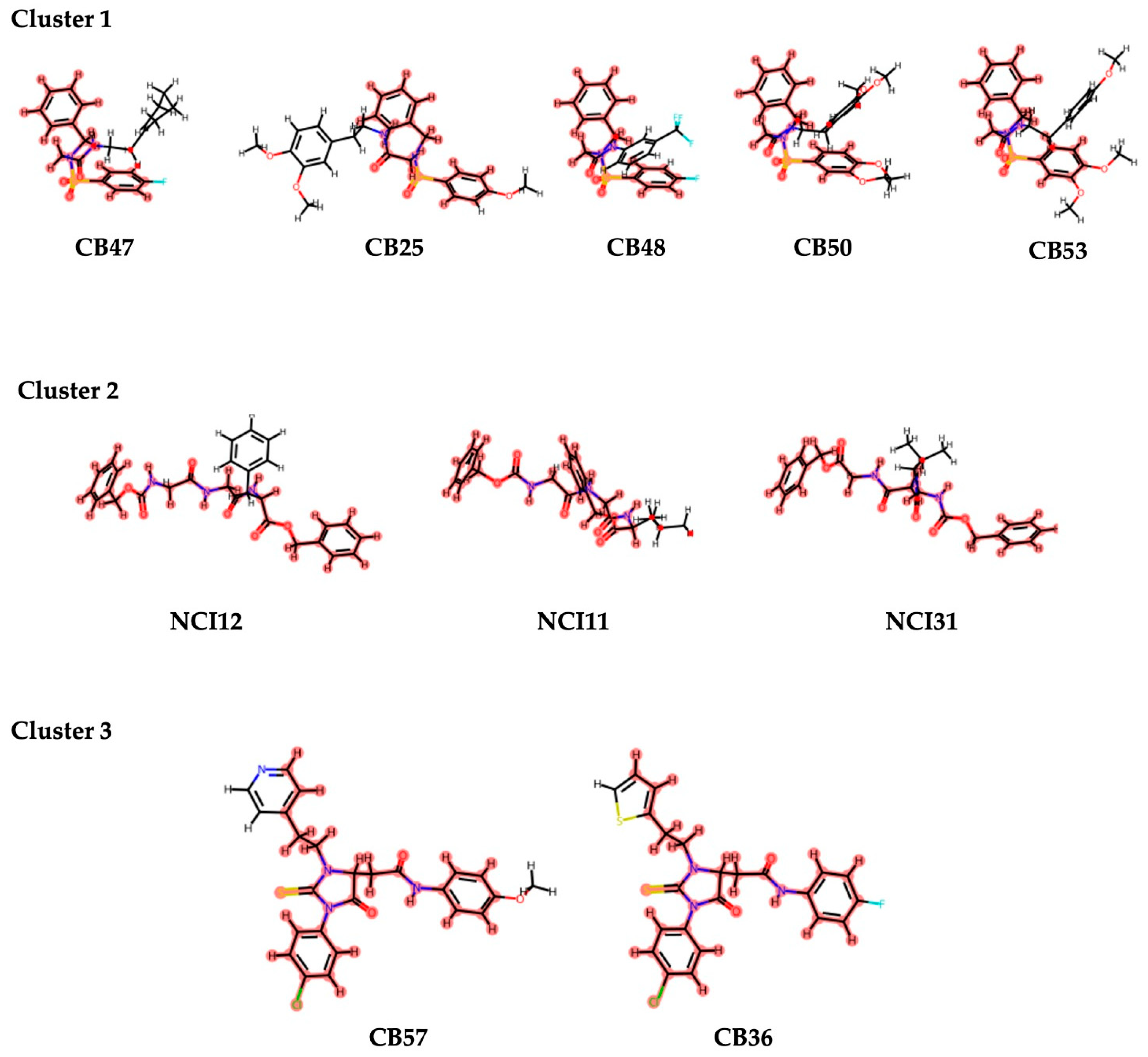
3.1.4. Determination of Key interactions, Clustering Structurally Similar Scaffolds, and Chemical Space
3.2. Cell-Based Assays
3.2.1. Chemicals
3.2.2. Cell Culture
3.2.3. Gene Expression
3.2.4. Crystal Violet Staining Assay
3.2.5. MTS Reduction Assay
3.2.6. First Screening Approach of the ERCC1–XPF Inhibitors in an NSCLC Cell Line
3.2.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xiang, D.-B.; Yang, X.-Q.; Chen, L.-S.; Li, M.-X.; Zhong, Z.-Y.; Zhang, Y.-S. APE1 overexpression is associated with cisplatin resistance in non-small cell lung cancer and targeted inhibition of APE1 enhances the activity of cisplatin in A549 cells. Lung Cancer 2009, 66, 298–304. [Google Scholar] [CrossRef]
- Zappa, C.; Mousa, S.A. Non-small cell lung cancer: Current treatment and future advances. Transl. Lung Cancer Res. 2016, 5, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, N.; Walker, G.C. Mechanisms of DNA damage, repair and mutagenesis. Environ. Mol. Mutagen. 2017, 58, 235–263. [Google Scholar] [CrossRef]
- De Bont, R.; van Larebeke, N. Endogenous DNA damage in humans: A review of quantitative data. Mutagenesis 2004, 19, 169–185. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, A.D. DNA Repair Pathways and Human Cancer. In The Molecular Basis of Cancer, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 47–66.e2. [Google Scholar]
- Huang, R.; Zhou, P.-K. DNA Damage Repair: Historical Perspectives, Mechanistic Pathways and Clinical Translation for Targeted Cancer Therapy. Signal Transduction and Targeted Therapy. 2021, 6, 254. [Google Scholar] [CrossRef]
- Hakem, R. DNA-damage repair; the good, the bad, and the ugly. EMBO J. 2008, 27, 589–605. [Google Scholar] [CrossRef]
- Rodrigues, A.; Gomes, B.C.; Martins, C.; Gromicho, M.; Oliveira, N.G.; Guerreirp, P.S.; Rueff, J. DNA Repair and Resistance to Cancer Therapy. In New Research Directions in DNA Repair; InTech: London, UK, 2013; Chapter 18; pp. 489–528. [Google Scholar]
- Doherty, R.; Madhusudan, S. DNA repair endonucleases: Physiological roles and potential as drug targets. J. Biomol. Screen. 2015, 20, 829–841. [Google Scholar] [CrossRef]
- Allingham-Hawkins, D.; Lea, A.; Levine, S. ERCC1 expression analysis to guide therapy in non-small cell lung cancer. PLoS Curr. 2010, 2, RRN1202. [Google Scholar] [CrossRef]
- Shah, F.; Logsdon, D.; Messmann, R.A.; Fehrenbacher, J.C.; Fishel, M.L.; Kelley, M.R. Exploiting the Ref-1-APE1 node in cancer signaling and other diseases: From bench to clinic. NPJ Precis. Oncol. 2017, 1, 19. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.L.; He, F.; Ye, J.Z.; Wu, H.N.; Zhang, J.Y.; Liu, Z.H.; Li, Y.Q.; Luo, X.L.; Lin, Y.; Liang, R. APE1 overexpression is associated with poor survival in patients with solid tumors: A meta-analysis. Oncotarget 2017, 8, 59720–59728. [Google Scholar] [CrossRef] [PubMed]
- Rosell, R.; Lord, R.V.N.; Taron, M.; Reguart, N. DNA repair and cisplatin resistance in non-small-cell lung cancer. Lung Cancer 2002, 38, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Rosell, R.; Taron, M.; Barnadas, A.; Scagliotti, G.; Sarries, C.; Roig, B. Nucleotide excision repair pathways involved in cisplatin resistance in non-small-cell lung cancer. Cancer Control 2003, 10, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Rocha, C.R.R.; Silva, M.M.; Quinet, A.; Cabral-Neto, J.B.; Menck, C.F.M. DNA repair pathways and cisplatin resistance: An intimate relationship. Clinics 2018, 73, e478s. [Google Scholar] [CrossRef] [PubMed]
- Wynne, P.; Newton, C.; Ledermann, J.A.; Olaitan, A.; Mould, T.A.; Hartley, J.A. Enhanced repair of DNA interstrand crosslinking in ovarian cancer cells from patients following treatment with platinum-based chemotherapy. Br. J. Cancer 2007, 97, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Reed, E.; Li, Q.Q. Molecular basis of cellular response to cisplatin chemotherapy in non-small cell lung cancer (Review). Oncol. Rep. 2004, 12, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Liang, X.; Zhou, X.; Huang, R.; Chu, Z.; Zhan, Q. ERCC1 expression as a prognostic and predictive factor in patients with non-small cell lung cancer: A meta-analysis. Mol. Biol. Rep. 2012, 39, 6933–6942. [Google Scholar] [CrossRef]
- Helleday, T.; Petermann, E.; Lundin, C.; Hodgson, B.; Sharma, R.A. DNA repair pathways as targets for cancer therapy. Nat. Rev. Cancer 2008, 8, 193–204. [Google Scholar] [CrossRef]
- Gavande, N.S.; Vandervere-Carozza, P.S.; Hinshaw, H.D.; Jalal, S.I.; Sears, C.R.; Pawelczak, K.S.; Turchi, J.J. DNA repair targeted therapy: The past or future of cancer treatment? Pharmacol. Ther. 2016, 160, 65–83. [Google Scholar] [CrossRef]
- Rehman, F.L.; Lord, C.J.; Ashworth, A. Synthetic lethal approaches to breast cancer therapy. Nat. Rev. Clin. Oncol. 2010, 7, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, K.A.; Alabdullah, M.; Griffin, M.; Toss, M.S.; Fatah, T.M.A.A.; Alblihy, A.; Moseley, P.; Chan, S.Y.T.; Rakha, E.A.; Madhusudan, S. ERCC1-XPF deficiency is a predictor of olaparib induced synthetic lethality and platinum sensitivity in epithelial ovarian cancers. Gynecol. Oncol. 2019, 153, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Mohni, K.N.; Kavanaugh, G.M.; Cortez, D. ATR Pathway Inhibition Is Synthetically Lethal in Cancer Cells with ERCC1 Deficiency. Cancer Res. 2014, 74, 2835–2845. [Google Scholar] [CrossRef] [PubMed]
- Topatana, W.; Juengpanich, S.; Li, S.; Cao, J.; Hu, J.; Lee, J.; Suliyanto, K.; Ma, D.; Zhang, B.; Chen, M.; et al. Advances in synthetic lethality for cancer therapy: Cellular mechanism and clinical translation. J. Hematol. Oncol. 2020, 13, 118. [Google Scholar] [CrossRef] [PubMed]
- Ohmoto, A.; Yachida, S. Current status of poly(ADP-ribose) polymerase inhibitors and future directions. OncoTargets Ther. 2017, 10, 5195–5208. [Google Scholar] [CrossRef] [PubMed]
- Lord, R.V.N.; Brabender, J.; Gandara, D.; Alberola, V.; Camps, C.; Domine, M.; Cardenal, F.; Sánchez, J.M.; Gumerlock, P.H.; Tarón, M.; et al. Low ERRC1 expression correlates with prolonged survival after cisplatin plus gemcitabine chemotherapy in non-small cell lung cancer. Clin. Cancer Res. 2002, 8, 2286–2291. [Google Scholar] [PubMed]
- Simon, G.R.; Sharma, S.; Cantor, A.; Smith, P.; Bepler, G. ERCC1 expression is a predictor of survival in resected patients with non-small cell lung cancer. Chest 2005, 127, 978–983. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, K.; Melton, D.W. Multiple Roles of the ERCC1-XPF Endonuclease in DNA Repair and Resistance to Anticancer Drugs. Anticancer Res. 2010, 30, 3223–3232. [Google Scholar]
- McNeil, E.M.; Melton, D.W. DNA repair endonuclease ERCC1-XPF as a novel therapeutic target to overcome chemoresistance in cancer therapy. Nucleic Acids Res. 2012, 40, 9990–10004. [Google Scholar] [CrossRef]
- McNeil, E.M.; Astell, K.R.; Ritchie, A.M.; Shave, S.; Houston, D.R.; Bakrania, P.; Jones, H.M.; Khurana, P.; Wallace, C.; Chapman, T.; et al. Inhibition of the ERCC1-XPF structure-specific endonuclease to overcome cancer chemoresistance. DNA Repair 2015, 31, 19–28. [Google Scholar] [CrossRef]
- Gentile, F.; Tuszynski, J.A.; Barakat, K.H. New design of nucleotide excision repair (NER) inhibitors for combination cancer therapy. J. Mol. Graph. Model. 2016, 65, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Barakat, K.; Tuszynski, J. Nucleotide Excision Repair Inhibitors: Still a Long Way to Go. In New Research Directions in DNA Repair; Intech: London, UK, 2012; Chapter 20; pp. 559–577. [Google Scholar]
- Orelli, B.; McClendon, T.B.; Tsodikov, O.V.; Ellenberger, T.; Niedernhofer, L.J.; Schärer, O.D. The XPA-binding domain of ERCC1 is required for nucleotide excision repair but not other DNA repair pathways. J. Biol. Chem. 2010, 285, 3705–3712. [Google Scholar] [CrossRef]
- Elmenoufy, A.H.; Gentile, F.; Jay, D.; Karimi-Busheri, F.; Yang, X.; Soueidan, O.M.; Mani, R.S.; Ciniero, G.; Tuszynski, J.A.; Weinfeld, M.; et al. Design, synthesis and in vitro cell-free/cell-based biological evaluations of novel ERCC1-XPF inhibitors targeting DNA repair pathway. Eur. J. Med. Chem. 2020, 204, 112658. [Google Scholar] [CrossRef] [PubMed]
- Elmenoufy, A.H.; Gentile, F.; Jay, D.; Karimi-Busheri, F.; Yang, X.; Soueidan, O.M.; Weilbeer, C.; Mani, R.S.; Barakat, K.H.; Tuszynski, J.A.; et al. Targeting DNA Repair in Tumor Cells via Inhibition of ERCC1-XPF. J. Med. Chem. 2019, 62, 7684–7696. [Google Scholar] [CrossRef]
- Gentile, F.; Elmenoufy, A.H.; Ciniero, G.; Jay, D.; Karimi-Busheri, F.; Barakat, K.H.; Weinfeld, M.; West, F.G.; Tuszynski, J.A. Computer-aided drug design of small molecule inhibitors of the ERCC1-XPF protein–protein interaction. Chem. Biol. Drug Des. 2020, 95, 460–471. [Google Scholar] [CrossRef] [PubMed]
- Heyza, J.R.; Arora, S.; Zhang, H.; Conner, K.L.; Lei, W.; Floyd, A.M.; Deshmukh, R.R.; Sarver, J.; Trabbic, C.J.; Erhardt, P.; et al. Targeting the DNA repair endonuclease ERCC1-XPF with green tea polyphenol epigallocatechin-3-gallate (EGCG) and its prodrug to enhance cisplatin efficacy in human cancer cells. Nutrients 2018, 10, 1644. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Heyza, J.; Zhang, H.; Kalman-Maltese, V.; Tillison, K.; Floyd, A.M.; Chalfin, E.M.; Bepler, G.; Patrick, S.M. Identification of small molecule inhibitors of ERCC1-XPF that inhibit DNA repair and potentiate cisplatin efficacy in cancer cells. Oncotarget 2016, 7, 75104–75117. [Google Scholar] [CrossRef] [PubMed]
- Jordheim, L.P.; Barakat, K.H.; Heinrich-Balard, L.; Matera, E.L.; Cros-Perrial, E.; Bouledrak, K.; Sabeh, R.E.; Perez-Pineiro, R.; Wishart, D.S.; Cohen, R.; et al. Small molecule inhibitors of ERCC1-XPF protein-protein interaction synergize alkylating agents in cancer cellss. Mol. Pharmacol. 2013, 84, 12–24. [Google Scholar] [CrossRef]
- Chapman, T.M.; Gillen, K.J.; Wallace, C.; Lee, M.T.; Bakrania, P.; Khurana, P.; Coombs, P.J.; Stennett, L.; Fox, S.; Bureau, E.A.; et al. Catechols and 3-hydroxypyridones as inhibitors of the DNA repair complex ERCC1-XPF. Bioorganic Med. Chem. Lett. 2015, 25, 4097–4103. [Google Scholar] [CrossRef]
- Chapman, T.M.; Wallace, C.; Gillen, K.J.; Bakrania, P.; Khurana, P.; Coombs, P.J.; Fox, S.; Bureau, E.A.; Brownlees, J.; Melton, D.W.; et al. N-Hydroxyimides and hydroxypyrimidinones as inhibitors of the DNA repair complex ERCC1-XPF. Bioorganic Med. Chem. Lett. 2015, 25, 4104–4108. [Google Scholar] [CrossRef]
- Ciniero, G.; Elmenoufy, A.H.; Gentile, F.; Weinfeld, M.; Deriu, M.A.; West, F.G.; Tuszynski, J.A.; Dumontet, C.; Cros-Perrial, E.; Jordheim, L.P. Enhancing the activity of platinum-based drugs by improved inhibitors of ERCC1–XPF-mediated DNA repair. Cancer Chemother. Pharmacol. 2021, 87, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Weilbeer, C.; Jay, D.; Donnelly, J.C.; Gentile, F.; Karimi-Busheri, F.; Yang, X.; Mani, R.S.; Yu, Y.; Elmenoufy, A.H.; Barakat, K.H.; et al. Modulation of ERCC1-XPF Heterodimerization Inhibition via Structural Modification of Small Molecule Inhibitor Side-Chains. Front. Oncol. 2022, 12, 819172. [Google Scholar] [CrossRef] [PubMed]
- Rose, P.W.; Prlic, A.; Altunkaya, A.; Bi, C.; Bradley, A.R.; Christie, C.H.; Costanzo, L.D.; Duarte, J.M.; Dutta, S.; Feng, Z.; et al. The RCSB protein data bank: Integrative view of protein, gene and 3D structural information. Nucleic Acids Res. 2017, 45, 271–281. [Google Scholar]
- Jones, M.; Beuron, F.; Borg, A.; Nans, A.; Earl, C.P.; Briggs, D.C.; Snijders, A.P.; Bowles, M.; Morris, E.P.; Linch, M.; et al. Cryo-EM structures of the XPF-ERCC1 endonuclease reveal how DNA-junction engagement disrupts an auto-inhibited conformation. Nat. Commun. 2020, 11, 1120. [Google Scholar] [CrossRef] [PubMed]
- Faridounnia, M.; Wienk, H.; Kovačič, L.; Folkers, G.E.; Jaspers, N.G.J.; Kaptein, R.; Hoeijmakers, J.H.J.; Boelens, R. The cerebro-oculo-facio-skeletal syndrome point mutation F231L in the ERCC1 DNA repair protein causes dissociation of the ERCC1-XPF complex. J. Biol. Chem. 2015, 290, 20541–20555. [Google Scholar] [CrossRef] [PubMed]
- Tsodikov, O.V.; Enzlin, J.H.; Schärer, O.D.; Ellenberger, T. Crystal structure and DNA binding functions of ERCC1, a subunit of the DNA structure-specific endonuclease XPF-ERCC1. Proc. Natl. Acad. Sci. USA 2005, 102, 11236–11241. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Folkers, G.E.; Van Dijk, M.; Jaspers, N.G.J.; Hoeijmakers, J.H.J.; Kaptein, R.; Boelens, R. The structure of the XPF-ssDNA complex underscores the distinct roles of the XPF and ERCC1 helix-hairpin-helix domains in ss/ds DNA recognition. Structure 2012, 20, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Tripsianes, K.; Jaspers, N.G.J.; Hoeijmakers, J.H.J.; Kaptei, R.; Boelens, R.; Folkers, G.E. The HhH domain of the human DNA repair protein XPF forms stable homodimers. Proteins 2007, 70, 1551–1563. [Google Scholar] [CrossRef]
- Tripsianes, K.; Folkers, G.; Ab, E.; Das, D.; Odijk, H.; Jaspers, N.G.J.; Hoeijmakers, J.H.J.; Kaptein, R.; Boelens, R. The structure of the human ERCC1/XPF interaction domains reveals a complementary role for the two proteins in nucleotide excision repair. Structure 2005, 13, 1849–1858. [Google Scholar] [CrossRef]
- Lucas, S.D.; Gonçalves, L.M.; Cardote, T.A.F.; Correia, H.F.; Moreira, R.; Guedes, R.C. Structure based virtual screening for discovery of novel human neutrophil elastase inhibitors. Medchemcomm 2012, 3, 1299–1304. [Google Scholar] [CrossRef]
- Ahmad, A.; Enzlin, J.H.; Bhagwat, N.R.; Wijgers, N.; Raams, A.; Appledoorn, E.; Theil, A.F.; Hoeijmakers, J.H.J.; Vermeulen, W.; Jaspers, N.G.J.; et al. Mislocalization of XPF-ERCC1 nuclease contributes to reduced DNA repair in XP-F patients. PLoS Genet. 2010, 6, e1000871. [Google Scholar] [CrossRef] [PubMed]
- Gentile, F.; Barakat, K.H.; Tuszynski, J.A. Computational characterization of small molecules binding to the human XPF active site and virtual screening to identify potential new DNA repair inhibitors targeting the ERCC1-XPF endonuclease. Int. J. Mol. Sci. 2018, 19, 1328. [Google Scholar] [CrossRef]
- Gaulton, A.; Hersey, A.; Nowotka, M.L.; Patricia Bento, A.; Chambers, J.; Mendez, D.; Mutowo, P.; Atkinson, F.; Bellis, L.J.; Cibrian-Uhalte, E.; et al. The ChEMBL database in 2017. Nucleic Acids Res. 2017, 45, D945–D954. [Google Scholar] [CrossRef] [PubMed]
- Lagorce, D.; Sperandio, O.; Baell, J.B.; Miteva, M.A.; Villoutreix, B.O. FAF-Drugs3: A web server for compound property calculation and chemical library design. Nucleic Acids Res. 2015, 43, W200–W207. [Google Scholar] [CrossRef]
- Bento, A.P.; Hersey, A.; Félix, E.; Landrum, G.; Gaulton, A.; Atkinson, F.; Bellis, L.J.; De Veij, M.; Leach, A.R. An open source chemical structure curation pipeline using RDKit. J. Cheminform. 2020, 12, 51. [Google Scholar] [CrossRef] [PubMed]
- van der Maaten, L.; Hinton, G. Viualizing data using t-SNE. J. Mach. Learn. Res. 2008, 9, 2579–2605. [Google Scholar]
- Cai, Y.; Yan, X.; Zhang, G.; Zhao, W.; Jiao, S. The predictive value of ERCC1 and p53 for the effect of panobinostat and cisplatin combination treatment in NSCLC. Oncotarget 2015, 6, 18997–19005. [Google Scholar] [CrossRef]
- Cheng, H.; Zhang, Z.; Borczuk, A.; Powell, C.A.; Balajee, A.S.; Lieberman, H.B.; Halmos, B. PARP inhibition selectively increases sensitivity to cisplatin in ERCC1-low non-small cell lung cancer cells. Carcinogenesis 2013, 34, 739–749. [Google Scholar] [CrossRef]
- He, Y.; Chen, D.; Yi, Y.; Zeng, S.; Liu, S.; Li, P.; Xie, H.; Yu, P.; Jiang, G.; Liu, H. Histone Deacetylase Inhibitor Sensitizes ERCC1-High Non-small-Cell Lung Cancer Cells to Cisplatin via Regulating miR-149. Mol. Ther.-Oncolytics 2020, 17, 448–459. [Google Scholar] [CrossRef]
- Korb, O.; Stützle, T.; Exner, T.E. Empirical scoring functions for advanced Protein-Ligand docking with PLANTS. J. Chem. Inf. Model. 2009, 49, 84–96. [Google Scholar] [CrossRef]
- Gareth, J.; Willett, P.; Glen, R.C.; Leach, A.R.; Taylor, R. Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 1997, 267, 727–748. [Google Scholar]
- Verdonk, M.L.; Cole, J.C.; Hartshorn, M.J.; Murray, C.W.; Taylor, R.D. Improved protein-ligand docking using GOLD. Proteins Struct. Funct. Genet. 2003, 52, 609–623. [Google Scholar] [CrossRef] [PubMed]
- Mooij, W.T.M.; Verdonk, M.L. General and targeted statistical potentials for protein-ligand interactions. Proteins Struct. Funct. Genet. 2005, 61, 272–287. [Google Scholar] [CrossRef] [PubMed]
- NCI Database Download Page. Available online: https://cactus.nci.nih.gov/download/nci/ (accessed on 18 April 2022).
- ChemBridge. Available online: https://www.chembridge.com/ (accessed on 24 February 2022).
- Law, V.; Knox, C.; Djoumbou, Y.; Jewison, T.; Guo, A.C.; Liu, Y.; MacIejewski, A.; Arndt, D.; Wilson, M.; Neveu, V.; et al. DrugBank 4.0: Shedding new light on drug metabolism. Nucleic Acids Res. 2014, 42, D1091–D1097. [Google Scholar] [CrossRef] [PubMed]
- Salentin, S.; Schreiber, S.; Haupt, V.J.; Adasme, M.F.; Schroeder, M. PLIP: Fully automated protein-ligand interaction profiler. Nucleic Acids Res. 2015, 43, W443–W447. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.B.; Manguinhas, R.; Costa, J.G.; Saraiva, N.; Gil, N.; Rosell, R.; Camões, S.P.; Batinic-Haberle, I.; Spasojevic, I.; Castro, M.; et al. MnTnHex-2-PyP5+ Displays Anticancer Properties and Enhances Cisplatin Effects in Non-Small Cell Lung Cancer Cells. Antioxidants 2022, 11, 2198. [Google Scholar] [CrossRef]
- Takemoto, S.; Nakamura, Y.; Gyoutoku, H.; Senju, H.; Ogawara, D.; Ikeda, T.; Yamaguchi, H.; Kitazaki, T.; Nakano, H.; Nakatomi, K.; et al. Phase II trial of a non-platinum triplet for patients with advanced non-small cell lung carcinoma (NSCLC) overexpressing ERCC1 messenger RNA. Thorac. Cancer 2019, 10, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Manguinhas, R.; Fernandes, A.S.; Costa, J.G.; Saraiva, N.; Camões, S.P.; Gil, N.; Rosell, R.; Castro, M.; Miranda, J.P.; Oliveira, N.G. Impact of the APE1 redox function inhibitor E3330 in non-small cell lung cancer cells exposed to cisplatin: Increased cytotoxicity and impairment of cell migration and invasion. Antioxidants 2020, 9, 550. [Google Scholar] [CrossRef]





| PDB ID | Res. (Å) | Year | Complex | Ref. |
|---|---|---|---|---|
| 6SXA | 3.60 | 2020 | ERCC1–XPF Cryo-EM structure, Apo-form | Jones et al., 2020 [47] |
| 6SXB | 7.90 | 2020 | ERCC1–XPF Cryo-EM structure, DNA-bound form | Jones et al., 2020 [47] |
| 2MUT | Solution | 2015 | Solution structure of the F231L mutant ERCC1–XPF dimerization region | Faridounnia et al., 2015 [48] |
| 2KN7 | Solution | 2010 | Structure of the XPF–single-strand DNA complex | Das et al., 2012 [50] |
| 2AQ0 | Solution | 2006 | Solution structure of the human homodimeric DNA repair protein XPF | Das et al., 2007 [51] |
| 2A1J | 2.70 | 2005 | Crystal structure of the complex between the C-terminal domains of human XPF and ERCC1 | Tsodikov et al., 2005 [49] |
| 2A1I | 1.90 | 2005 | Crystal structure of the central domain of human ERCC1 | Tsodikov et al., 2005 [49] |
| 1Z00 | Solution | 2005 | Solution structure of the C-terminal domain of ERCC1 complexed with the C-terminal domain of XPF | Tripsianes et al., 2005 [52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manguinhas, R.; Serra, P.A.; Soares, R.B.; Rosell, R.; Gil, N.; Oliveira, N.G.; Guedes, R.C. Unveiling Novel ERCC1–XPF Complex Inhibitors: Bridging the Gap from In Silico Exploration to Experimental Design. Int. J. Mol. Sci. 2024, 25, 1246. https://doi.org/10.3390/ijms25021246
Manguinhas R, Serra PA, Soares RB, Rosell R, Gil N, Oliveira NG, Guedes RC. Unveiling Novel ERCC1–XPF Complex Inhibitors: Bridging the Gap from In Silico Exploration to Experimental Design. International Journal of Molecular Sciences. 2024; 25(2):1246. https://doi.org/10.3390/ijms25021246
Chicago/Turabian StyleManguinhas, Rita, Patrícia A. Serra, Rita B. Soares, Rafael Rosell, Nuno Gil, Nuno G. Oliveira, and Rita C. Guedes. 2024. "Unveiling Novel ERCC1–XPF Complex Inhibitors: Bridging the Gap from In Silico Exploration to Experimental Design" International Journal of Molecular Sciences 25, no. 2: 1246. https://doi.org/10.3390/ijms25021246
APA StyleManguinhas, R., Serra, P. A., Soares, R. B., Rosell, R., Gil, N., Oliveira, N. G., & Guedes, R. C. (2024). Unveiling Novel ERCC1–XPF Complex Inhibitors: Bridging the Gap from In Silico Exploration to Experimental Design. International Journal of Molecular Sciences, 25(2), 1246. https://doi.org/10.3390/ijms25021246









