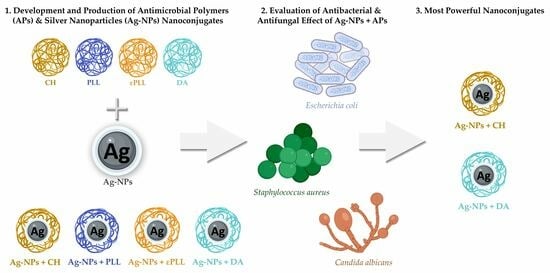
Silver and Antimicrobial Polymer Nanocomplexes to Enhance Biocidal Effects
Abstract
:1. Introduction
2. Results and Discussion
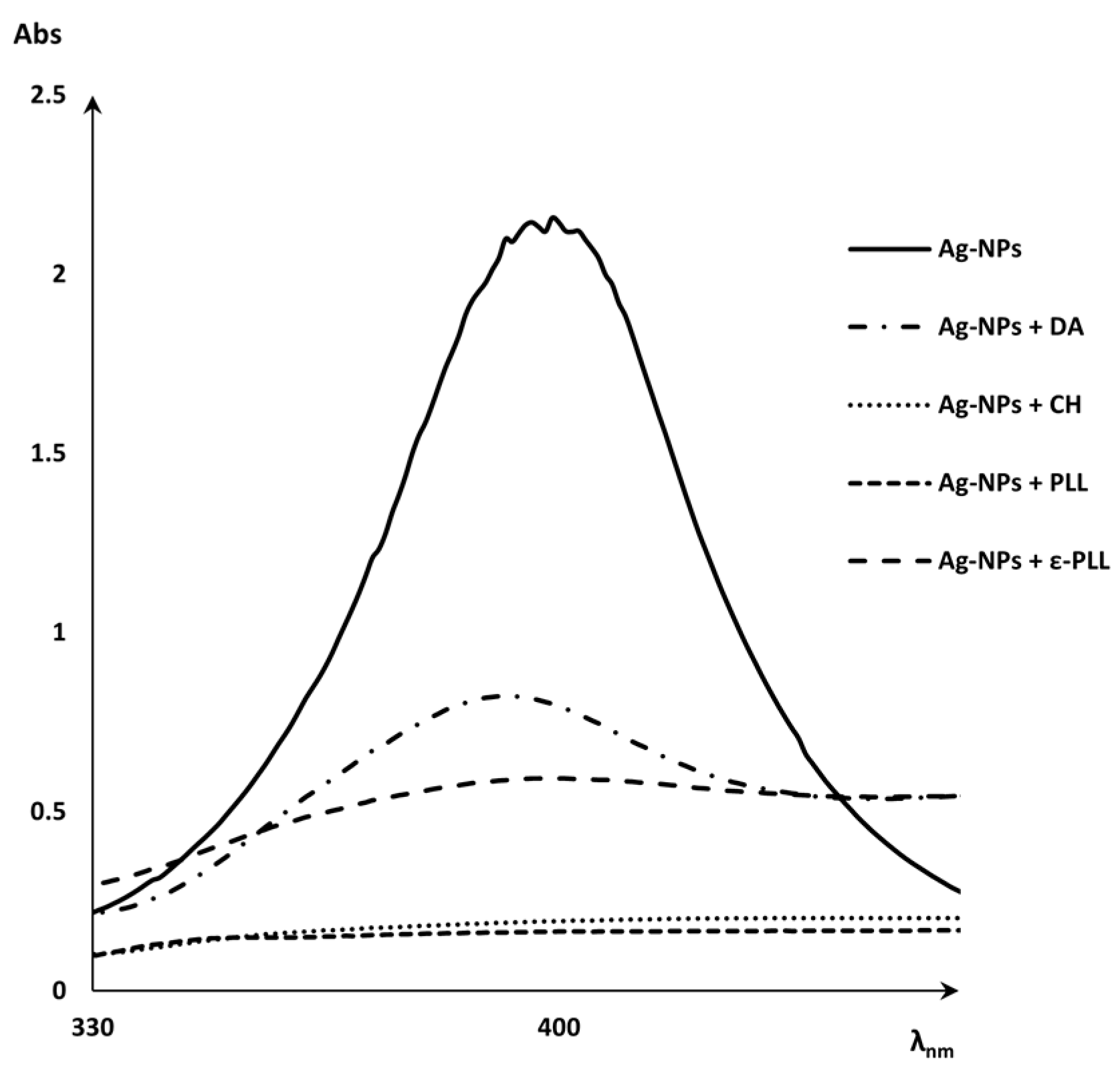
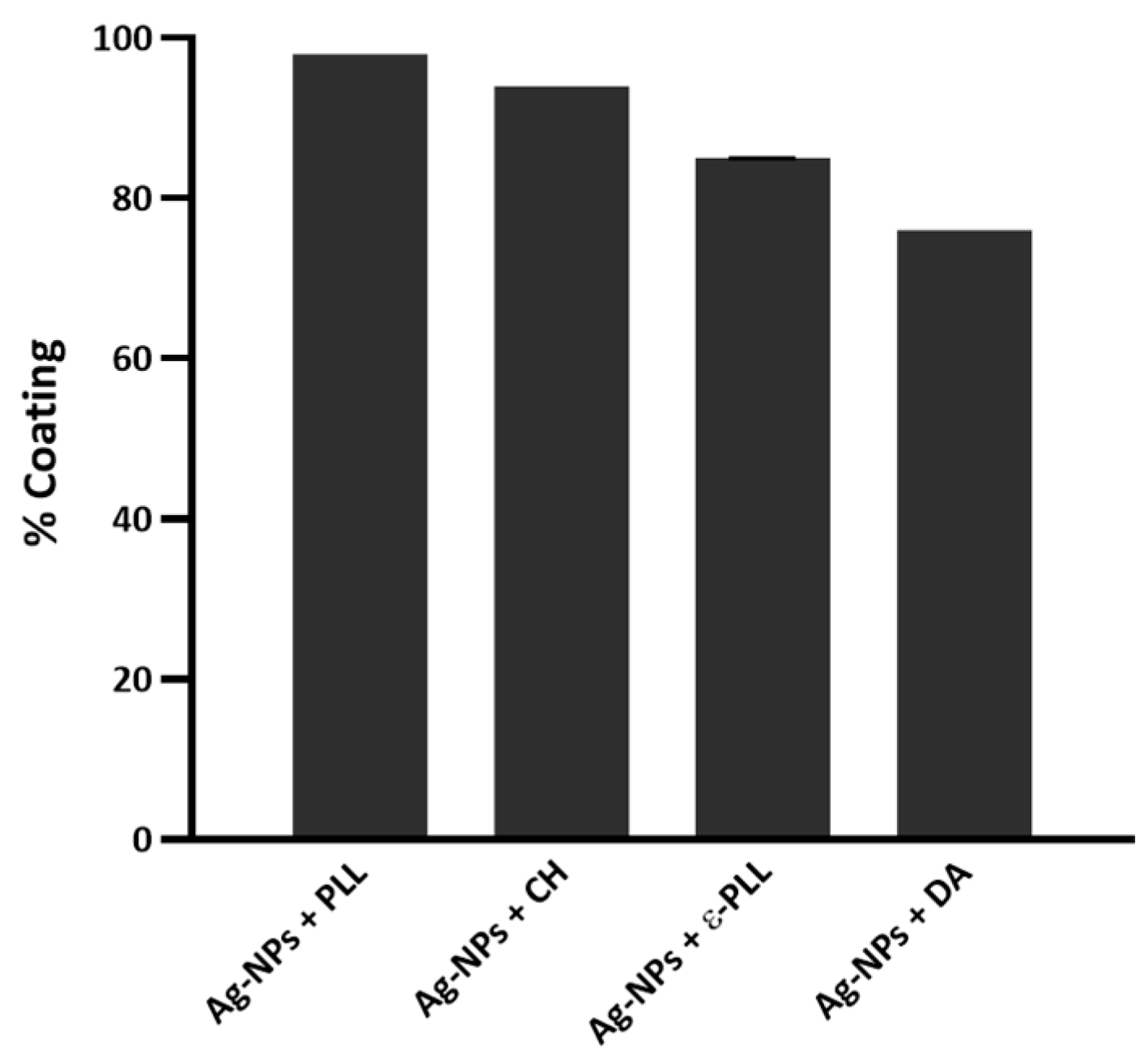
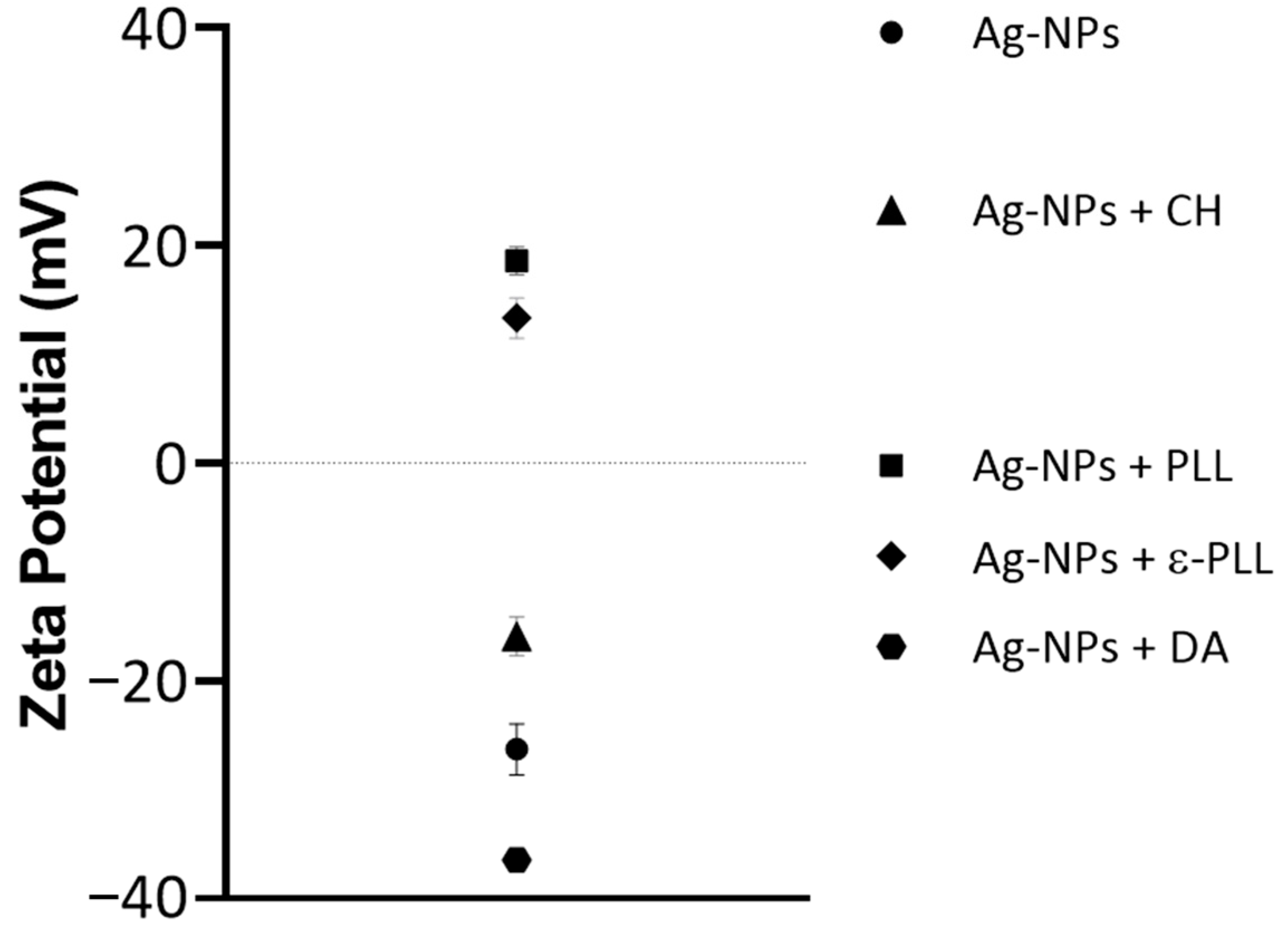
2.1. Characterization of Ag-NP + AP Coatings Using UV-Vis Spectra Measurements
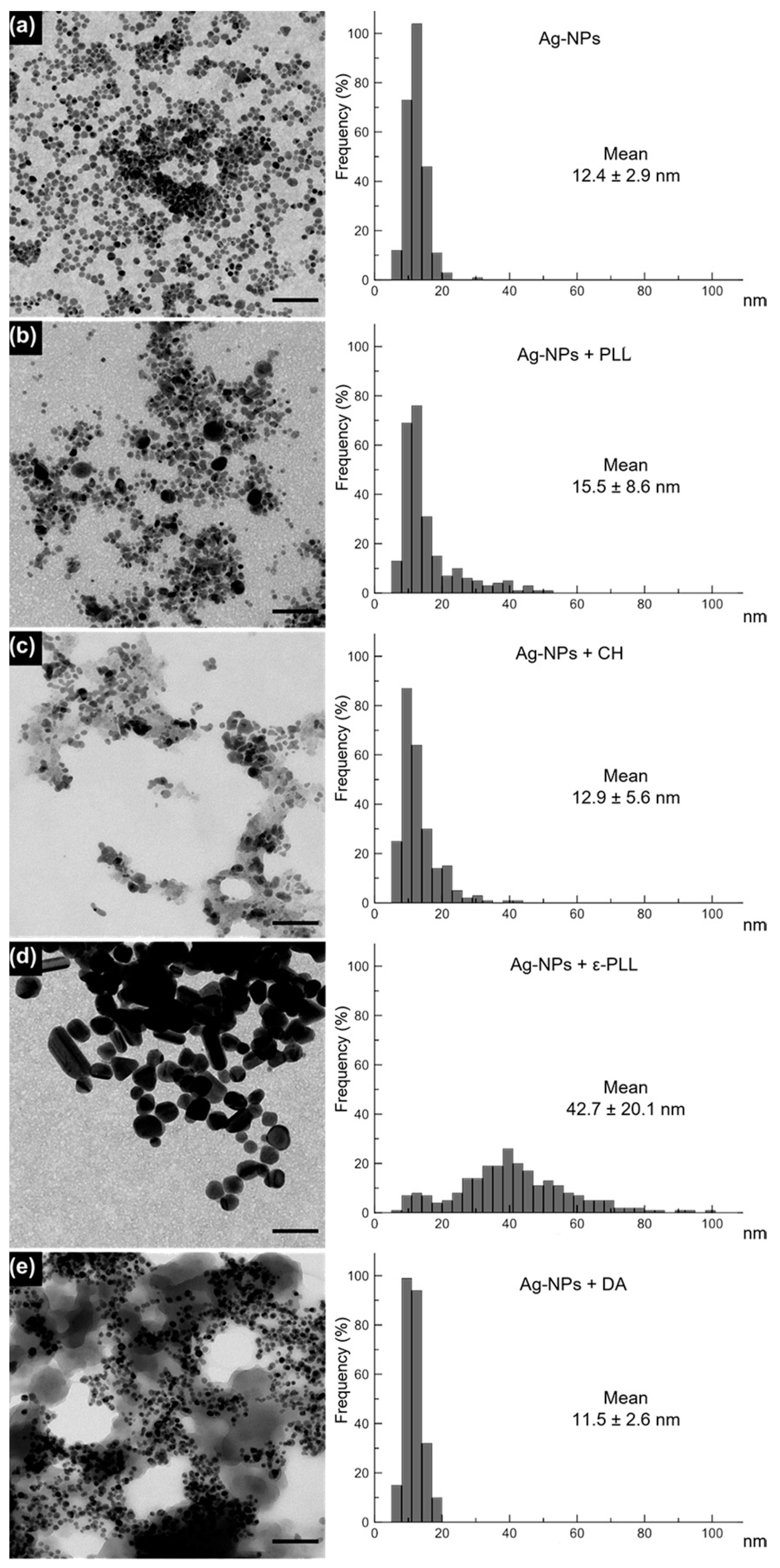
2.2. Morphological and Chemical Analysis of the Ag-NP + AP Nanocomplexes
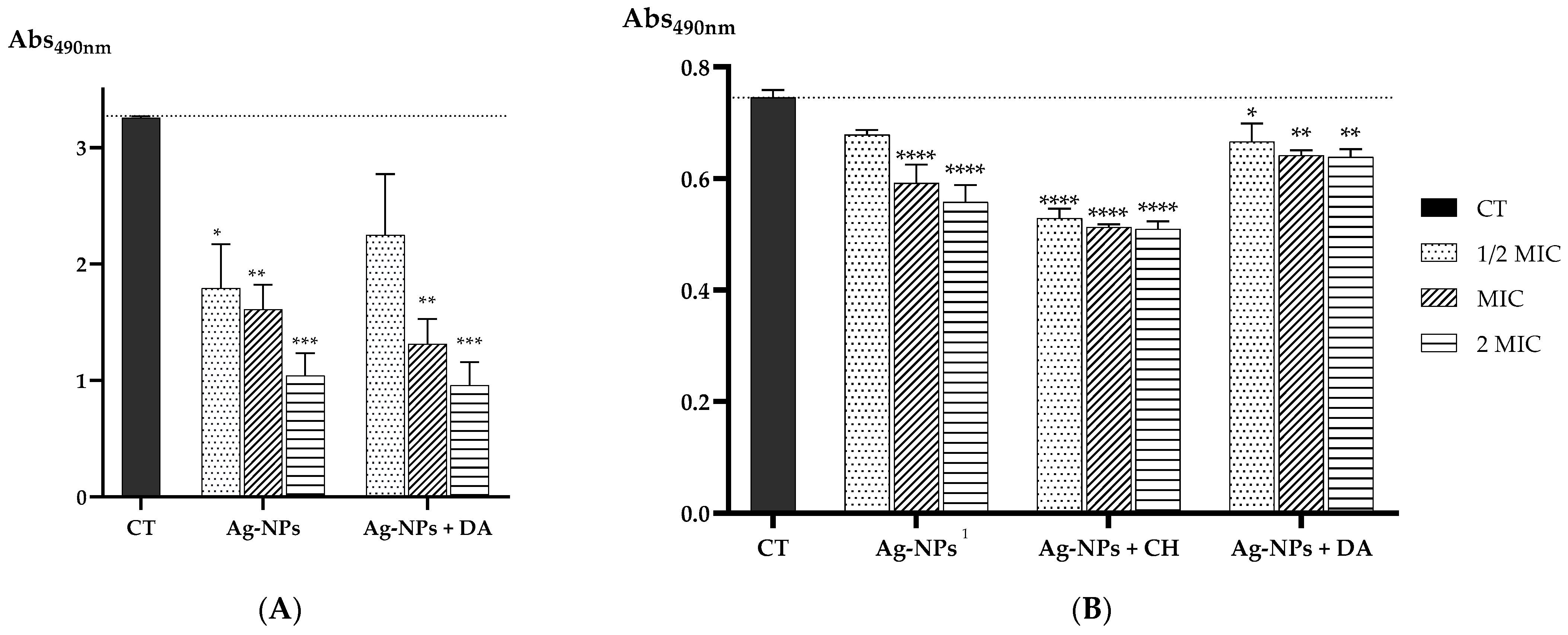
2.3. Antimicrobial Activity of Ag-NPs and the Developed Nanoconjugates
Evaluation of the Biofilm Eradication Ability
3. Materials and Methods
3.1. Ag-NPs Coating with APs
3.2. Morphological and Chemical Characterization
3.3. Antimicrobial Activity
3.3.1. Microorganisms and Culture Media
3.3.2. Determination of the Minimum Inhibitory Concentration (MIC) and Minimum Lethal Concentration (MLC)
3.3.3. Antibiofilm Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Christaki, E.; Marcou, M.; Tofarides, A. Antimicrobial Resistance in Bacteria: Mechanisms, Evolution, and Persistence. J. Mol. Evol. 2020, 88, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.; Carreira, T.S.; Alves, N.; Sousa, Â.; Valente, J.F.A. Metallic Structures: Effective Agents to Fight Pathogenic Microorganisms. Int. J. Mol. Sci. 2022, 23, 1165. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.W.K.; Millar, B.C.; Moore, J.E. Antimicrobial Resistance (AMR). Br. J. Biomed. Sci. 2023, 80, 11387. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report 2022; World Health Organization: Geneva, Switzerland, 2022; ISBN 9789240062702.
- Jorge, P.; Magalhães, A.P.; Grainha, T.; Alves, D.; Sousa, A.M.; Lopes, S.P.; Pereira, M.O. Antimicrobial Resistance Three Ways: Healthcare Crisis, Major Concepts and the Relevance of Biofilms. FEMS Microbiol. Ecol. 2019, 95, fiz115. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations the Review on Antimicrobial Resistance Chaired by Jim O’neill; Government of the United Kingdom: London, UK, 2016. [Google Scholar]
- Uruén, C.; Chopo-Escuin, G.; Tommassen, J.; Mainar-Jaime, R.C.; Arenas, J. Antibiotics Biofilms as Promoters of Bacterial Antibiotic Resistance and Tolerance. Antibiotics 2020, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Nadar, S.; Khan, T.; Patching, S.G.; Omri, A. Development of Antibiofilm Therapeutics Strategies to Overcome Antimicrobial Drug Resistance. Microorganisms 2022, 10, 303. [Google Scholar] [CrossRef]
- Rather, M.A.; Gupta, K.; Bardhan, P.; Borah, M.; Sarkar, A.; Eldiehy, K.S.H.; Bhuyan, S.; Mandal, M. Microbial Biofilm: A Matter of Grave Concern for Human Health and Food Industry. J. Basic Microbiol. 2021, 61, 380–395. [Google Scholar] [CrossRef]
- Haque, M.; Sartelli, M.; McKimm, J.; Bakar, M.A. Health Care-Associated Infections—An Overview. Infect. Drug Resist. 2018, 11, 2321–2333. [Google Scholar] [CrossRef]
- Dutta, P.; Wang, B. Zeolite-Supported Silver as Antimicrobial Agents. Coord. Chem. Rev. 2019, 383, 1–29. [Google Scholar] [CrossRef]
- Lansdown, A.B.G. Silver in Health Care: Antimicrobial Effects and Safety in Use Interactions between Skin and Biofunctional Metals. Biofunctional Text. Ski. 2006, 33, 17–34. [Google Scholar]
- Frei, A.; Verderosa, A.D.; Elliott, A.G.; Zuegg, J.; Blaskovich, M.A.T. Metals to Combat Antimicrobial Resistance. Nat. Rev. Chem. 2023, 7, 202–224. [Google Scholar] [CrossRef] [PubMed]
- Hamad, A.; Khashan, K.S.; Hadi, A. Silver Nanoparticles and Silver Ions as Potential Antibacterial Agents. J. Inorg. Organomet. Polym. Mater. 2020, 30, 4811–4828. [Google Scholar] [CrossRef]
- Bamal, D.; Singh, A.; Chaudhary, G.; Kumar, M.; Singh, M.; Rani, N.; Mundlia, P.; Sehrawat, A.R. Silver Nanoparticles Biosynthesis, Characterization, Antimicrobial Activities, Applications, Cytotoxicity and Safety Issues: An Updated Review. Nanomaterials 2021, 11, 2086. [Google Scholar] [CrossRef]
- Freire, P.L.L.; Albuquerque, A.J.R.; Farias, I.A.P.; da Silva, T.G.; Aguiar, J.S.; Galembeck, A.; Flores, M.A.P.; Sampaio, F.C.; Stamford, T.C.M.; Rosenblatt, A. Antimicrobial and Cytotoxicity Evaluation of Colloidal Chitosan—Silver Nanoparticles—Fluoride Nanocomposites. Int. J. Biol. Macromol. 2016, 93, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Kamaruzzaman, N.F.; Tan, L.P.; Hamdan, R.H.; Choong, S.S.; Wong, W.K.; Gibson, A.J.; Chivu, A.; De Fatima Pina, M. Antimicrobial Polymers: The Potential Replacement of Existing Antibiotics? Int. J. Mol. Sci. 2019, 20, 2747. [Google Scholar] [CrossRef] [PubMed]
- Inamudin; Ahamed, M.I.; Prasad, R. Advanced Antimicrobial Materials and Applications; Springer: Singapore, 2021; ISBN 9789811570971. [Google Scholar]
- Ke, C.L.; Deng, F.S.; Chuang, C.Y.; Lin, C.H. Antimicrobial Actions and Applications of Chitosan. Polymers 2021, 13, 904. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Abd El-Hakim, Y.M.; Al-Sagheer, A.A. Antimicrobial and Antioxidant Properties of Chitosan and Its Derivatives and Their Applications: A Review. Int. J. Biol. Macromol. 2020, 164, 2726–2744. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.; Huang, Z.; Luo, Z.; Qian, C.; Li, Y.; Duan, Y. Preparation of Low Molecular Chitosan by Microwave-Induced Plasma Desorption/Ionization Technology. Int. J. Biol. Macromol. 2021, 187, 441–450. [Google Scholar] [CrossRef]
- Confederat, L.G.; Tuchilus, C.G.; Dragan, M.; Sha’at, M.; Dragostin, O.M. Preparation and Antimicrobial Activity of Chitosan and Its Derivatives: A Concise Review. Molecules 2021, 26, 3694. [Google Scholar] [CrossRef]
- Lim, C.; Hwang, D.S.; Lee, D.W. Intermolecular Interactions of Chitosan: Degree of Acetylation and Molecular Weight. Carbohydr. Polym. 2021, 259, 117782. [Google Scholar] [CrossRef] [PubMed]
- Kašparová, P.; Zmuda, M.; Vaňková, E.; Maťátková, O.; Masák, J. Low-Molecular Weight Chitosan Enhances Antibacterial Effect of Antibiotics and Permeabilizes Cytoplasmic Membrane of Staphylococcus Epidermidis Biofilm Cells. Folia Microbiol. 2021, 66, 983–996. [Google Scholar] [CrossRef]
- Kuralay, F.; Dükar, N.; Bayramlı, Y. Poly-L-Lysine Coated Surfaces for Ultrasensitive Nucleic Acid Detection. Electroanalysis 2018, 30, 1556–1565. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Huang, S.; Li, Y.; Zhou, C. Recent Advances in Epsilon-Poly-L-Lysine and L-Lysine-Based Dendrimer Synthesis, Modification, and Biomedical Applications. Front. Chem. 2021, 9, 659304. [Google Scholar] [CrossRef] [PubMed]
- Hasanzadeh, A.; Mofazzal Jahromi, M.A.; Abdoli, A.; Mohammad-Beigi, H.; Fatahi, Y.; Nourizadeh, H.; Zare, H.; Kiani, J.; Radmanesh, F.; Rabiee, N.; et al. Photoluminescent Carbon Quantum Dot/Poly-L-Lysine Core-Shell Nanoparticles: A Novel Candidate for Gene Delivery. J. Drug Deliv. Sci. Technol. 2021, 61, 102118. [Google Scholar] [CrossRef]
- Alkekhia, D.; Shukla, A. Influence of Poly-l-Lysine Molecular Weight on Antibacterial Efficacy in Polymer Multilayer Films. J. Biomed. Mater. Res. A 2019, 107, 1324–1339. [Google Scholar] [CrossRef] [PubMed]
- Mayandi, V.; Xi, Q.; Leng Goh, E.T.; Koh, S.K.; Jie Toh, T.Y.; Barathi, V.A.; Urf Turabe Fazil, M.H.; Somaraju Chalasani, M.L.; Varadarajan, J.; Jeng Ting, D.S.; et al. Rational Substitution of ϵ-Lysine for α-Lysine Enhances the Cell and Membrane Selectivity of Pore-Forming Melittin. J. Med. Chem. 2020, 63, 3522–3537. [Google Scholar] [CrossRef] [PubMed]
- Hamano, Y. Occurrence, Biosynthesis, Biodegradation, and Industrial and Medical Applications of a Naturally Occurringε-Poly-L-Lysine. Biosci. Biotechnol. Biochem. 2011, 75, 1226–1233. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Vad, B.S.; Stenvang, M.; Otzen, D.E.; Meyer, R.L. The Antimicrobial Mechanism of Action of Epsilon-Poly-L-Lysine. Appl. Environ. Microbiol. 2014, 80, 7758–7770. [Google Scholar] [CrossRef]
- Jain, A.; Duvvuri, L.S.; Farah, S.; Beyth, N.; Domb, A.J.; Khan, W. Antimicrobial Polymers. Adv. Healthc. Mater. 2014, 3, 1969–1985. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, C.; Zhang, J.; Rao, Z.; Xu, X.; Mao, Z.; Chen, X. Epsilon-Poly-L-Lysine: Recent Advances in Biomanufacturing and Applications. Front. Bioeng. Biotechnol. 2021, 9, 748976. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, S.F.C.; Ferreira, C.A.M.; Valente, J.F.A.; Patrício, T.M.F.; Alves, N.M.F.; Dias, J.R. Electrospun-Based Membranes as a Key Tool to Prevent Respiratory Infections. Polymers 2022, 14, 3787. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Guan, Q.; Walsh, J.P.; Boswell, J.S.; Winter, T.W.; Winter, E.S.; Boyd, S.S.; Li, C.; Savage, P.B. Correlation of the Antibacterial Activities of Cationic Peptide Antibiotics and Cationic Steroid Antibiotics. J. Med. Chem. 2002, 45, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, M.A.A.; Almasri, I.; Khayal, G. Spectrophotometric Determination of Dopamine in Bulk and Dosage Forms Using 2,4-Dinitrophenylhydrazine. Turk. J. Pharm. Sci. 2020, 17, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Shumbula, N.P.; Nkabinde, S.S.; Ndala, Z.B.; Mpelane, S.; Shumbula, M.P.; Mdluli, P.S.; Njengele-Tetyana, Z.; Tetyana, P.; Hlatshwayo, T.; Mlambo, M.; et al. Evaluating the Antimicrobial Activity and Cytotoxicity of Polydopamine Capped Silver and Silver/Polydopamine Core-Shell Nanocomposites. Arab. J. Chem. 2022, 15, 103798. [Google Scholar] [CrossRef]
- Zhang, G.; Hu, J.; Ren, T.; Zhu, P. Microstructural and Tribological Properties of a Dopamine Hydrochloride and Graphene Oxide Coating Applied to Multifilament Surgical Sutures. Polymers 2020, 12, 1630. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhao, W.; Wu, X.; Gan, J.; Zhang, H.; Cai, Y. A Superhydrophobic Moso Bamboo Cellulose Nano-Fibril Film Modified by Dopamine Hydrochloride. Front. Bioeng. Biotechnol. 2021, 9, 756839. [Google Scholar] [CrossRef]
- Niyonshuti, I.I.; Krishnamurthi, V.R.; Okyere, D.; Song, L.; Benamara, M.; Tong, X.; Wang, Y.; Chen, J. Polydopamine Surface Coating Synergizes the Antimicrobial Activity of Silver Nanoparticles. ACS Appl. Mater. Interfaces 2020, 12, 40067–40077. [Google Scholar] [CrossRef]
- Singh, I.; Dhawan, G.; Gupta, S.; Kumar, P. Recent Advances in a Polydopamine-Mediated Antimicrobial Adhesion System. Front. Microbiol. 2021, 11, 607099. [Google Scholar] [CrossRef]
- Thakur, A.; Ranote, S.; Kumar, D.; Bhardwaj, K.K.; Gupta, R.; Chauhan, G.S. Synthesis of a PEGylated Dopamine Ester with Enhanced Antibacterial and Antifungal Activity. ACS Omega 2018, 3, 7925–7933. [Google Scholar] [CrossRef]
- Su, L.; Yu, Y.; Zhao, Y.; Liang, F.; Zhang, X. Strong Antibacterial Polydopamine Coatings Prepared by a Shaking-Assisted Method. Sci. Rep. 2016, 6, 24420. [Google Scholar] [CrossRef]
- Mori, Y.; Ono, T.; Miyahira, Y.; Nguyen, V.Q.; Matsui, T.; Ishihara, M. Antiviral Activity of Silver Nanoparticle/Chitosan Composites against H1N1 Influenza A Virus. Nanoscale Res. Lett. 2013, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Chandrasekaran, N.; Raichur, A.M.; Mukherjee, A. Antibacterial Applications of Silver Nanoparticles Synthesized by Aqueous Extract of Azadirachta Indica (Neem) Leaves. J. Biomed. Nanotechnol. 2009, 5, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Shinde, S.; Folliero, V.; Chianese, A.; Zannella, C.; De Filippis, A.; Rosati, L.; Prisco, M.; Falanga, A.; Mali, A.; Galdiero, M.; et al. Synthesis of Chitosan-coated Silver Nanoparticle Bioconjugates and Their Antimicrobial Activity against Multidrug-resistant Bacteria. Appl. Sci. 2021, 11, 9340. [Google Scholar] [CrossRef]
- Cunningham, D.; Littleford, R.E.; Smith, W.E.; Lundahl, P.J.; Khan, I.; McComb, D.W.; Graham, D.; Laforest, N. Practical Control of SERRS Enhancement. Faraday Discuss. 2006, 132, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Xi, Y.; Bai, J.; Jiang, Z.; Wang, S.; Zhang, H.; Dai, W.; Chen, C.; Gou, Z.; Yang, G.; et al. Covalent Grafting of Hyperbranched Poly-L-Lysine on Ti-Based Implants Achieves Dual Functions of Antibacteria and Promoted Osteointegration in Vivo. Biomaterials 2021, 269, 120534. [Google Scholar] [CrossRef] [PubMed]
- Stagi, L.; de Forni, D.; Innocenzi, P. Blocking Viral Infections with Lysine-Based Polymeric Nanostructures: A Critical Review. Biomater. Sci. 2022, 10, 1904–1919. [Google Scholar] [CrossRef] [PubMed]
- Akmaz, S.; Dilaver Adgüzel, E.; Yasar, M.; Erguven, O. The Effect of Ag Content of the Chitosan-Silver Nanoparticle Composite Material on the Structure and Antibacterial Activity. Adv. Mater. Sci. Eng. 2013, 2013, 12–18. [Google Scholar] [CrossRef]
- Wulandari, I.O.; Pebriatin, B.E.; Valiana, V.; Hadisaputra, S.; Ananto, A.D.; Sabarudin, A. Green Synthesis of Silver Nanoparticles Coated by Water Soluble Chitosan and Its Potency as Non-Alcoholic Hand Sanitizer Formulation. Materials 2022, 15, 4641. [Google Scholar] [CrossRef]
- Hu, H.; Dyke, J.C.; Bowman, B.A.; Ko, C.C.; You, W. Investigation of Dopamine Analogues: Synthesis, Mechanistic Understanding, and Structure-Property Relationship. Langmuir 2016, 32, 9873–9882. [Google Scholar] [CrossRef]
- Radziuk, D.; Skirtach, A.; Sukhorukov, G.; Shchukin, D.; Möhwald, H. Stabilization of Silver Nanoparticles by Polyelectrolytes and Polyethylene Glycol. Macromol. Rapid Commun. 2007, 28, 848–855. [Google Scholar] [CrossRef]
- Shakeel, M.; Kiani, M.H.; Sarwar, H.S.; Akhtar, S.; Rauf, A.; Ibrahim, I.M.; Ajalli, N.; Shahnaz, G.; Rahdar, A.; Díez-Pascual, A.M. Emulgel-Loaded Mannosylated Thiolated Chitosan-Coated Silver Nanoparticles for the Treatment of Cutaneous Leishmaniasis. Int. J. Biol. Macromol. 2023, 227, 1293–1304. [Google Scholar] [CrossRef] [PubMed]
- Pem, B.; Ćurlin, M.; Jurašin, D.D.; Vrček, V.; Barbir, R.; Micek, V.; Fratila, R.M.; de la Fuente, J.M.; Vrček, I.V. Fate and Transformation of Silver Nanoparticles in Different Biological Conditions. Beilstein J. Nanotechnol. 2021, 12, 665–679. [Google Scholar] [CrossRef] [PubMed]
- Fereshteh, Z.; Rojaee, R.; Sharifnabi, A. Effect of Different Polymers on Morphology and Particle Size of Silver Nanoparticles Synthesized by Modified Polyol Method. Superlattices Microstruct. 2016, 98, 267–275. [Google Scholar] [CrossRef]
- Moccia, M.; Roviello, G.N.; Bucci, E.M.; Pedone, C.; Saviano, M. Synthesis of a L-Lysine-Based Alternate Alpha, Epsilon-Peptide: A Novel Linear Polycation with Nucleic Acids-Binding Ability. Int. J. Pharm. 2010, 397, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.N.; Chang, S.L.; Xu, P.W.; Tan, M.H.; Zhao, B.; Wang, X.D.; Zhao, Q.S. Structural Changes and Antibacterial Activity of Epsilon-Poly- l -Lysine in Response to PH and Phase Transition and Their Mechanisms. J. Agric. Food Chem. 2020, 68, 1101–1109. [Google Scholar] [CrossRef]
- Pang, C.; Zhang, P.; Mu, Y.; Ren, J.; Zhao, B. Transformation and Cytotoxicity of Surface-Modified Silver Nanoparticles Undergoing Long-Term Aging. Nanomaterials 2020, 10, 2255. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Singh, S.K.; Anil, S.; Kim, S.K.; Shim, M.S. Preparation, Characterization and Biological Applications of Biosynthesized Silver Nanoparticles with Chitosan-Fucoidan Coating. Molecules 2018, 23, 1429. [Google Scholar] [CrossRef]
- Athavale, R.; Sapre, N.; Rale, V.; Tongaonkar, S.; Manna, G.; Kulkarni, A.; Shirolkar, M.M. Tuning the Surface Charge Properties of Chitosan Nanoparticles. Mater. Lett. 2022, 308, 131114. [Google Scholar] [CrossRef]
- Abd Al-jabbar, S.; Atiroğlu, V.; Hameed, R.M.; Guney Eskiler, G.; Atiroğlu, A.; Deveci Ozkan, A.; Özacar, M. Fabrication of Dopamine Conjugated with Protein @metal Organic Framework for Targeted Drug Delivery: A Biocompatible PH-Responsive Nanocarrier for Gemcitabine Release on MCF-7 Human Breast Cancer Cells. Bioorg. Chem. 2022, 118, 105467. [Google Scholar] [CrossRef]
- Zheng, D.; Bai, B.; He, Y.; Hu, N.; Wang, H. Synthesis and Characterization of Dopamine-Modified Ca-Alginate/Poly(N-Isopropylacrylamide) Microspheres for Water Retention and Multi-Responsive Controlled Release of Agrochemicals. Int. J. Biol. Macromol. 2020, 160, 518–530. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, M.F.; Melo, K.R.T.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O. Does the Use of Chitosan Contribute to Oxalate Kidney Stone Formation? Mar. Drugs 2015, 13, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Chekuri, M.; Gangarudraiah, S.; Bharadwaj, L.R.; Kaphle, A.; Chalimeswamy, A. Green Synthesis of Stable Silver Nanoparticles Using Flower Extract of Rosa Damascena: Characterization, Antimicrobial and Anti- Oxidant Activity Study. Eur. Chem. Bull. 2015, 4, 454–459. [Google Scholar]
- Goudarzi, M.; Mir, N.; Mousavi-Kamazani, M.; Bagheri, S.; Salavati-Niasari, M. Biosynthesis and Characterization of Silver Nanoparticles Prepared from Two Novel Natural Precursors by Facile Thermal Decomposition Methods. Sci. Rep. 2016, 6, 32539. [Google Scholar] [CrossRef]
- Xiong, X.; Qu, S.X.; Liu, Y.M. Synthesis and Characterization of Dopamine Graft Compound N-Methacryloyl 3,4-Dihydroxyl-Phenylamine. J. Phys. Conf. Ser. 2013, 419, 12047. [Google Scholar] [CrossRef]
- Durgadas, C.V.; Sharma, C.P.; Sreenivasan, K. Fluorescent and Superparamagnetic Hybrid Quantum Clusters for Magnetic Separation and Imaging of Cancer Cells from Blood. Nanoscale 2011, 3, 4780–4787. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, D.R.; Miller, D.J.; Freeman, B.D.; Paul, D.R.; Bielawski, C.W. Elucidating the Structure of Poly(Dopamine). Langmuir 2012, 28, 6428–6435. [Google Scholar] [CrossRef]
- Le, N.M.N.; Steinbring, C.; Le-Vinh, B.; Jalil, A.; Matuszczak, B.; Bernkop-Schnürch, A. Polyphosphate Coatings: A Promising Strategy to Overcome the Polycation Dilemma. J. Colloid Interface Sci. 2021, 587, 279–289. [Google Scholar] [CrossRef]
- Lv, J.M.; Meng, Y.C.; Shi, Y.G.; Li, Y.H.; Chen, J.; Sheng, F. Properties of Epsilon-Polylysine·HCl/High-Methoxyl Pectin Polyelectrolyte Complexes and Their Commercial Application. J. Food Process. Preserv. 2020, 44, e14320. [Google Scholar] [CrossRef]
- Miao, Y.; Yang, R.; Deng, D.Y.B.; Zhang, L.M. Poly(l-Lysine) Modified Zein Nanofibrous Membranes as Efficient Scaffold for Adhesion, Proliferation, and Differentiation of Neural Stem Cells. RSC Adv. 2017, 7, 17711–17719. [Google Scholar] [CrossRef]
- Ansari, M.A.; Khan, H.M.; Khan, A.A.; Ahmad, M.K.; Mahdi, A.A.; Pal, R.; Cameotra, S.S. Interaction of Silver Nanoparticles with Escherichia Coli and Their Cell Envelope Biomolecules. J. Basic Microbiol. 2014, 54, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Prakash, A.; Radhakrishnan, E.K. Sunlight Mediated Rapid Synthesis of Small Size Range Silver Nanoparticles Using Zingiber Officinale Rhizome Extract and Its Antibacterial Activity Analysis. Inorg. Nano-Met. Chem. 2018, 48, 139–145. [Google Scholar] [CrossRef]
- Khatoon, N.; Mishra, A.; Alam, H.; Manzoor, N.; Sardar, M. Biosynthesis, Characterization, and Antifungal Activity of the Silver Nanoparticles Against Pathogenic Candida Species. Bionanoscience 2015, 5, 65–74. [Google Scholar] [CrossRef]
- Masoumizadeh, M.; Madani, M.; Fatahian, S.; Shakib, P. Effect of Silver Nanoparticles (AgNPs) on Candida Albicans, Candida Dubliniensis and Candida Guilliermondii. Curr. Drug Ther. 2022, 17, 50–55. [Google Scholar]
- de Carvalho Bernardo, W.L.; Boriollo, M.F.G.; Tonon, C.C.; da Silva, J.J.; Cruz, F.M.; Martins, A.L.; Höfling, J.F.; Spolidorio, D.M.P. Antimicrobial Effects of Silver Nanoparticles and Extracts of Syzygium Cumini Flowers and Seeds: Periodontal, Cariogenic and Opportunistic Pathogens. Arch. Oral Biol. 2021, 125, 105101. [Google Scholar] [CrossRef] [PubMed]
- Khatoon, N.; Sharma, Y.; Sardar, M.; Manzoor, N. Mode of Action and Anti-Candida Activity of Artemisia Annua Mediated-Synthesized Silver Nanoparticles. J. Mycol. Med. 2019, 29, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Bettauer, V.; Costa, A.C.B.P.; Omran, R.P.; Massahi, S.; Kirbizakis, E.; Simpson, S.; Dumeaux, V.; Law, C.; Whiteway, M.; Hallett, M.T. A Deep Learning Approach to Capture the Essence of Candida Albicans Morphologies. Microbiol. Spectr. 2022, 10, e01472-22. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.P. Candida Albicans: A Major Fungal Pathogen of Humans. Pathogens 2022, 11, 459. [Google Scholar] [CrossRef]
- Davoodbasha, M.A.; Lee, S.Y.; Kim, J.W. Solution Plasma Mediated Formation of Low Molecular Weight Chitosan and Its Application as a Biomaterial. Int. J. Biol. Macromol. 2018, 118, 1511–1517. [Google Scholar] [CrossRef]
- Kulikouskaya, V.; Hileuskaya, K.; Kraskouski, A.; Kozerozhets, I.; Stepanova, E.; Kuzminski, I.; You, L.; Agabekov, V. Chitosan-capped Silver Nanoparticles: A Comprehensive Study of Polymer Molecular Weight Effect on the Reaction Kinetic, Physicochemical Properties, and Synergetic Antibacterial Potential. SPE Polym. 2022, 3, 77–90. [Google Scholar] [CrossRef]
- Mumtaz, S.; Ali, S.; Mumtaz, S.; Mughal, T.A.; Tahir, H.M.; Shakir, H.A. Chitosan Conjugated Silver Nanoparticles: The Versatile Antibacterial Agents. Polym. Bull. 2022, 80, 4719–4736. [Google Scholar] [CrossRef]
- Sushomasri, M.; Himangshu, M.; Pranabesh, C.; Sujata, G.D. Potential of Dopamine Hydrochloride as a Novel Antimicrobial Agent. Int. J. Biomed. Pharm. Sci. 2010, 4, 70–75. [Google Scholar]
- Bandyopadhayaya, S.; Akimov, M.G.; Verma, R.; Sharma, A.; Sharma, D.; Kundu, G.C.; Gretskaya, N.M.; Bezuglov, V.v.; Mandal, C.C. N-Arachidonoyl Dopamine Inhibits Epithelial–Mesenchymal Transition of Breast Cancer Cells through ERK Signaling and Decreasing the Cellular Cholesterol. J. Biochem. Mol. Toxicol. 2021, 35, e22693. [Google Scholar] [CrossRef]
- Singh, U.; Singh, P.; Singh, A.K.; Laxmi; Kumar, D.; Tilak, R.; Shrivastava, S.K.; Asthana, R.K. Identification of Antifungal and Antibacterial Biomolecules from a Cyanobacterium, Arthrospira Platensis. Algal Res. 2021, 54, 102215. [Google Scholar] [CrossRef]
- Fernandez, D.; Restrepo-Acevedo, A.; Rocha-Roa, C.; le Lagadec, R.; Abonia, R.; Zacchino, S.A.; Gómez Castaño, J.A.; Cuenú-Cabezas, F. Synthesis, Structural Characterization, and In Vitro and In Silico Antifungal Evaluation of Azo-Azomethine Pyrazoles (PhN2 (PhoH)CHN(C3N2(CH3)3)PhR, R = H or NO2). Molecules 2021, 26, 7435. [Google Scholar] [CrossRef]
- Niu, X.; Lin, L.; Liu, L.; Yu, Y.; Wang, H. Antifungal Activity and Molecular Mechanisms of Mulberrin Derivatives against Colletotrichum Gloeosporioides for Mango Storage. Int. J. Food Microbiol. 2022, 378, 109817. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Sae-Tia, S.; Fries, B.C. Candidiasis and Mechanisms of Antifungal Resistance. Antibiotics 2020, 9, 312. [Google Scholar] [CrossRef]
- Ferreira, C.A.M.; Guerreiro, S.F.C.; Valente, J.F.A.; Patrício, T.M.F.; Alves, N.; Mateus, A.; Dias, J.R. Advanced Face Mask Filters Based on PCL Electrospun Meshes Dopped with Antimicrobial MgO and CuO Nanoparticles. Polymers 2022, 14, 3329. [Google Scholar] [CrossRef]
- Lara, H.H.; Romero-Urbina, D.G.; Pierce, C.; Lopez-Ribot, J.L.; Arellano-Jiménez, M.J.; Jose-Yacaman, M. Effect of Silver Nanoparticles on Candida Albicans Biofilms: An Ultrastructural Study. J. Nanobiotechnol. 2015, 13, 91. [Google Scholar] [CrossRef]
- Farha, A.K.; Yang, Q.Q.; Kim, G.; Zhang, D.; Mavumengwana, V.; Habimana, O.; Li, H.B.; Corke, H.; Gan, R.Y. Inhibition of Multidrug-Resistant Foodborne Staphylococcus Aureus Biofilms by a Natural Terpenoid (+)-Nootkatone and Related Molecular Mechanism. Food Control 2020, 112, 107154. [Google Scholar] [CrossRef]
- Xu, J.; Li, Y.; Wang, H.; Zhu, M.; Feng, W.; Liang, G. Enhanced Antibacterial and Anti-Biofilm Activities of Antimicrobial Peptides Modified Silver Nanoparticles. Int. J. Nanomed. 2021, 16, 4831–4846. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Neupane, N.; Dahal, P.R.; Rahimi, S.; Cao, Z.; Pandit, S.; Mijakovic, I. Antibiotic-Loaded Boron Nitride Nanoconjugate with Strong Performance against Planktonic Bacteria and Biofilms. ACS Appl. Bio Mater. 2023, 6, 3131–3142. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Park, J.S.; Kim, M.-G. Strong Hyper-Rayleigh Scattering from Silver Nanoparticle Aggregates to Be Used for the Optical Bio-Sensing Assay. Nano-Bio Sens. Imaging Spectrosc. 2013, 8879, 887905. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, C.; Fan, S.; Gao, Z.; Tong, L.; Gao, H.; Zhou, Q.; Shao, H.; Liao, Y.; Li, Q.; et al. Engineering Polydopamine-Glued Sandwich-like Nanocomposites with Antifouling and Antibacterial Properties for the Development of Advanced Mixed Matrix Membranes. Sep. Purif. Technol. 2020, 237, 116326. [Google Scholar] [CrossRef]
- NCCLS Método de Referência Para Testes de Diluição Em Caldo Para a Determinação Da Sensibilidade de Leveduras à Terapia Antifúngica; Norma Aprovada—Segunda Edição; Norma M27-A2 do NCCLS; NCCLS: Wayne, PA, USA, 2002; ISBN 1-56238-469-4.
- NCCLS Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 6th ed.; NCCLS Document M7-A6; NCCLS: Wayne, PA, USA, 2003; ISBN 1-56238-486-4.
- Kong, F.; Wang, J.; Han, R.; Ji, S.; Yue, J.; Wang, Y.; Ma, L. Antifungal Activity of Magnesium Oxide Nanoparticles: Effect on the Growth and Key Virulence Factors of Candida Albicans. Mycopathologia 2020, 185, 485–494. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, W.; Zhao, C.; Zhang, W.; Yan, Z. Graphene Coated Ti-6Al-4V Exhibits Antibacterial and Antifungal Properties Against Oral Pathogens. J. Prosthodont. 2023, 32, 505–511. [Google Scholar] [CrossRef]

| Compound | MIC/MLC (µg/mL) | ||
|---|---|---|---|
| E. coli | S. aureus | C. albicans | |
| Ag-NPs | 20/20 | 40/>40 | >40/ND * |
| Ag-NPs + CH | 10/20 * | 20/40 * | 10/10 * |
| CH | >40/ND * | >40/ND * | >40/ND * |
| Ag-NPs + PLL | 40/40 | 40/40 * | 20/40 * |
| PLL | 40/40 | >40/ND | >40/ND * |
| Ag-NPs + ε-PLL | >40/ND * | >40/ND * | 20/40 * |
| ε-PLL | 1.25/1.25 | 2.5/2.5 | 0.625/5 |
| Ag-NPs + DA | 20/20 | 10/40 * | 5/10 * |
| DA | 40/40 | 10/10 | 40/>40 |
| Tet | 1/>4 | 0.5 >4 | - |
| Amp B | - | - | 0.25/0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, D.; Ferreira, S.; Ramírez-Rodríguez, G.B.; Alves, N.; Sousa, Â.; Valente, J.F.A. Silver and Antimicrobial Polymer Nanocomplexes to Enhance Biocidal Effects. Int. J. Mol. Sci. 2024, 25, 1256. https://doi.org/10.3390/ijms25021256
Pereira D, Ferreira S, Ramírez-Rodríguez GB, Alves N, Sousa Â, Valente JFA. Silver and Antimicrobial Polymer Nanocomplexes to Enhance Biocidal Effects. International Journal of Molecular Sciences. 2024; 25(2):1256. https://doi.org/10.3390/ijms25021256
Chicago/Turabian StylePereira, Diana, Susana Ferreira, Gloria Belén Ramírez-Rodríguez, Nuno Alves, Ângela Sousa, and Joana F. A. Valente. 2024. "Silver and Antimicrobial Polymer Nanocomplexes to Enhance Biocidal Effects" International Journal of Molecular Sciences 25, no. 2: 1256. https://doi.org/10.3390/ijms25021256
APA StylePereira, D., Ferreira, S., Ramírez-Rodríguez, G. B., Alves, N., Sousa, Â., & Valente, J. F. A. (2024). Silver and Antimicrobial Polymer Nanocomplexes to Enhance Biocidal Effects. International Journal of Molecular Sciences, 25(2), 1256. https://doi.org/10.3390/ijms25021256