Recent Progress in Systemic Therapy for Advanced Hepatocellular Carcinoma
Abstract
:1. Introduction
2. Evolution of First-Line Treatment
3. Second-Line and Beyond
4. Completed Phase III Trials
5. Future Directions
5.1. Three-Drug Regimens
5.2. Two-Drug Regimens
5.3. Chimeric Antigen Receptor-Modified T Cells (CAR T-Cells)
5.4. Bispecific Antibodies
5.5. Cytokine-Induced Killer (CIK) Cells and NK Cells
5.6. Targeted Agents
5.7. Vaccines
6. Drug Resistance
7. Discussion
Funding
Acknowledgments
Conflicts of Interest
References
- Nagahama, H.; Okada, S.; Okusaka, T.; Ishii, H.; Ikeda, M.; Nakasuka, H.; Yoshimori, M. Predictive factors for tumor response to systemic chemotherapy in patients with hepatocellular carcinoma. Jpn. J. Clin. Oncol. 1997, 27, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Chernyak, V.; Fowler, K.J.; Kamaya, A.; Kielar, A.Z.; Elsayes, K.M.; Bashir, M.R.; Kono, Y.; Do, R.K.; Mitchell, D.G.; Singal, A.G.; et al. Liver Imaging Reporting and Data System (LI-RADS) Version 2018: Imaging of Hepatocellular Carcinoma in At-Risk Patients. Radiology 2018, 289, 816–830. [Google Scholar] [CrossRef] [PubMed]
- Vietti Violi, N.; Lewis, S.; Hectors, S.; Said, D.; Taouli, B. Radiological Diagnosis and Characterization of HCC. In Hepatocellular Carcinoma: Translational Precision Medicine Approaches, 1st ed.; Hoshida, Y., Ed.; Humana Press: Totowa, NJ, USA, 2019; Chapter 4. Available online: https://www.ncbi.nlm.nih.gov/books/NBK553760/ (accessed on 23 August 2023).
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef] [PubMed]
- NCCN Clinical Practice Guideline in Oncology (NCCN Guidelines) Hepatocellular Carcinoma Version 2. 2023. Available online: https://www.nccn.org/professionals/physician_gls/pdf/hcc.pdf (accessed on 1 November 2023).
- Bishayee, A. The role of inflammation and liver cancer. Adv. Exp. Med. Biol. 2014, 816, 401–435. [Google Scholar] [PubMed]
- Akinyemiju, T.; Abera, S.; Ahmed, M.; Alam, N.; Alemayohu, M.A.; Allen, C.; Al-Raddadi, R.; Alvis-Guzman, N.; Amoako, Y.; Artaman, A. The Burden of Primary Liver Cancer and Underlying Etiologies from 1990 to 2015 at the Global, Regional, and National Level: Results from the Global Burden of Disease Study 2015. JAMA Oncol. 2017, 3, 1683–1691. [Google Scholar]
- International Agency on Research on Cancer: Liver. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/11-Liver-fact-sheet.pdf (accessed on 25 August 2023).
- Harris, P.S.; Hansen, R.M.; Gray, M.E.; Massoud, O.I.; McGuire, B.M.; Shoreibah, M.G. Hepatocellular carcinoma surveillance: An evidence-based approach. World J. Gastroenterol. 2019, 25, 1550–1559. [Google Scholar] [CrossRef]
- Nies, A.T.; König, J.; Pfannschmidt, M.; Klar, E.; Hofmann, W.J.; Keppler, D. Expression of the multidrug resistance proteins MRP2 and MRP3 in human hepatocellular carcinoma. Int. J. Cancer 2001, 94, 492–499. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Cheng, A.L.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Lim, H.Y.; Kudo, M.; Breder, V.; Merle, P.; et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J. Hepatol. 2022, 76, 862–873. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Lau, G.; Kudo, M.; Chan, S.L.; Kelley, R.K.; Furuse, J.; Sukeepaisarnjaroen, W.; Kang, Y.K.; Dao, T.V.; De Toni, E.N.; et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid. 2022, 1, 8. [Google Scholar] [CrossRef]
- Verset, G.; Borbath, I.; Karwal, M.; Verslype, C.; Van Vlierberghe, H.; Kardosh, A.; Zagonel, V.; Stal, P.; Sarker, D.; Palmer, D.H.; et al. Pembrolizumab Monotherapy for Previously Untreated Advanced Hepatocellular Carcinoma: Data from the Open-Label, Phase II KEYNOTE-224 Trial. Clin. Cancer Res. 2022, 28, 2547–2554. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Kudo, M.; Merle, P.; Meyer, T.; Qin, S.; Ikeda, M.; Xu, R.; Edeline, J.; Ryoo, B.Y.; Ren, Z.; et al. LBA34—Primary results from the phase III LEAP-002 study: Lenvatinib plus pembrolizumab versus lenvatinib as first-line (1L) therapy for advanced hepatocellular carcinoma (aHCC). Ann. Oncol. 2022, 33, S808–S869. [Google Scholar] [CrossRef]
- Yau, T.; Park, J.W.; Finn, R.S.; Cheng, A.L.; Mathurin, P.; Edeline, J.; Kudo, M.; Harding, J.J.; Merle, P.; Rosmorduc, O.; et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022, 23, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Matilla, A.; Santoro, A.; Melero, I.; Gracián, A.C.; Acosta-Rivera, M.; Choo, S.P.; El-Khoueiry, A.B.; Kuromatsu, R.; El-Rayes, B.; et al. CheckMate 040 cohort 5: A phase I/II study of nivolumab in patients with advanced hepatocellular carcinoma and Child-Pugh B cirrhosis. J. Hepatol. 2021, 75, 600–609. [Google Scholar] [CrossRef]
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 389, 56–66. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Meyer, T.; Cheng, A.L.; El-Khoueiry, A.B.; Rimassa, L.; Ryoo, B.Y.; Cicin, I.; Merle, P.; Chen, Y.; Park, J.W.; et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N. Engl. J. Med. 2018, 379, 54–63. [Google Scholar] [CrossRef]
- Zhu, A.X.; Kang, Y.K.; Yen, C.J.; Finn, R.S.; Galle, P.R.; Llovet, J.M.; Assenat, E.; Brandi, G.; Pracht, M.; Lim, H.Y.; et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 282–296. [Google Scholar] [CrossRef]
- Finn, R.S.; Ryoo, B.Y.; Merle, P.; Kudo, M.; Bouattour, M.; Lim, H.Y.; Breder, V.; Edeline, J.; Chao, Y.; Ogasawara, S.; et al. Pembrolizumab As Second-Line Therapy in Patients with Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J. Clin. Oncol. 2020, 38, 193–202. [Google Scholar] [CrossRef]
- Qin, S.; Chen, Z.; Fang, W.; Ren, Z.; Xu, R.; Ryoo, B.Y.; Meng, Z.; Bai, Y.; Chen, X.; Liu, X.; et al. Pembrolizumab plus best supportive care versus placebo plus best supportive care as second-line therapy in patients in Asia with advanced hepatocellular carcinoma (HCC): Phase 3 KEYNOTE-394 study. J. Clin. Oncol. 2022, 40, 383. [Google Scholar] [CrossRef]
- Yau, T.; Kang, Y.; Kim, T.; El-Khoueiry, A.B.; Santoro, A.; Sangro, B.; Melero, I.; Kudo, M.; Hou, M.M.; Matilla, A.; et al. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients with Advanced Hepatocellular Carcinoma Previously Treated with Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 2020, 6, e204564. [Google Scholar] [CrossRef] [PubMed]
- Bourhis, M.; Palle, J.; Galy-Fauroux, I.; Terme, M. Direct and Indirect Modulation of T Cells by VEGF-A Counteracted by Anti-Angiogenic Treatment. Front. Immunol. 2021, 12, 616837. [Google Scholar] [CrossRef]
- Qin, S.; Chan, S.L.; Gu, S.; Bai, Y.; Ren, Z.; Lin, X.; Chen, Z.; Jia, W.; Jin, Y.; Guo, Y.; et al. Camrelizumab plus rivoceranib versus sorafenib as first-line therapy for unresectable hepatocellular carcinoma (CARES-310): A randomised, open-label, international phase 3 study. Lancet 2023, 402, 1133–1146. [Google Scholar] [CrossRef] [PubMed]
- Kelley, R.K.; Rimassa, L.; Cheng, A.L.; Kaseb, A.; Qin, S.; Zhu, A.X.; Chan, S.L.; Melkadze, T.; Sukeepaisarnjaroen, W.; Breder, V.; et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2022, 23, 995–1008. [Google Scholar] [CrossRef]
- Ren, Z.; Xu, J.; Bai, Y.; Xu, A.; Cang, S.; Du, C.; Li, Q.; Lu, Y.; Chen, Y.; Guo, Y.; et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): A randomised, open-label, phase 2-3 study. Lancet Oncol. 2021, 22, 977–990. [Google Scholar] [CrossRef]
- Qin, S.; Kudo, M.; Meyer, T.; Finn, R.S.; Vogel, A.; Bai, Y.; Guo, Y.; Meng, Z.; Zhang, T.; Satoh, T.; et al. LBA36 Final analysis of RATIONALE-301: Randomized, phase III study of tislelizumab versus sorafenib as first-line treatment for unresectable hepatocellular carcinoma. Ann. Oncol. 2022, 33, S1402–S1403. [Google Scholar] [CrossRef]
- Tislelizumab. Available online: https://www.beigene.com/our-science-and-medicines/tislelizumab/ (accessed on 23 August 2023).
- Abou-Alfa, G.K.; Qin, S.; Ryoo, B.Y.; Lu, S.N.; Yen, C.J.; Feng, Y.H.; Lim, H.Y.; Izzo, F.; Colombo, M.; Sarker, D.; et al. Phase III randomized study of second line ADI-PEG 20 plus best supportive care versus placebo plus best supportive care in patients with advanced hepatocellular carcinoma. Ann. Oncol. 2018, 29, 1402–1408. [Google Scholar] [CrossRef]
- Study of ADI-PEG 20 versus Placebo in Subjects with High Arginine Level and Unresectable Hepatocellular Carcinoma. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05317819 (accessed on 24 August 2023).
- Qin, S.; Xu, L.; Yi, M.; Yu, S.; Wu, K.; Luo, S. Novel immune checkpoint targets: Moving beyond PD-1 and CTLA-4. Mol. Cancer 2019, 18, 155. [Google Scholar] [CrossRef]
- Zheng, M.; Tian, Z. Liver-Mediated Adaptive Immune Tolerance. Front. Immunol. 2019, 10, 2525. [Google Scholar] [CrossRef]
- Yau, T.; Zagonel, V.; Santoro, A.; Acosta-Rivera, M.; Choo, S.P.; Matilla, A.; He, A.W.; Gracian, A.C.; El-Khoueiry, A.B.; Sangro, B.; et al. Nivolumab Plus Cabozantinib with or without Ipilimumab for Advanced Hepatocellular Carcinoma: Results from Cohort 6 of the CheckMate 040 Trial. J. Clin. Oncol. 2023, 41, 1747–1757. [Google Scholar] [CrossRef] [PubMed]
- Cabozantinib Combined with Ipilimumab/Nivolumab and TACE in Patients with Hepatocellular Carcinoma. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04472767 (accessed on 23 August 2023).
- Safety and Efficacy of Coformulated Pembrolizumab/Quavonlimab (MK-1308A) in Combination with Lenvatinib (E7080/MK-7902) in Advanced Hepatocellular Carcinoma (MK-1308A-004). Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04740307 (accessed on 23 August 2023).
- Finn, R.S.; Ryoo, B.Y.; Hsu, C.H.; Li, D.; Burgoyne, A.; Cotter, C.; Badhrinarayanan, S.; Wang, Y.; Yin, A.; Edubilli, T.A.; et al. Results from the MORPHEUS-liver study: Phase Ib/II randomized evaluation of tiragolumab (tira) in combination with atezolizumab (atezo) and bevacizumab (bev) in patients with unresectable, locally advanced or metastatic hepatocellular carcinoma (uHCC). J. Clin. Oncol. 2023, 41, 4010. [Google Scholar] [CrossRef]
- Chauvin, J.; Zarour, H.M. TIGIT in cancer immunotherapy. J. Immunother. Cancer 2020, 8, e000957. [Google Scholar] [CrossRef] [PubMed]
- A Study Evaluating Atezolizumab and Bevacizumab, with or without Tiragolumab, in Participants with Untreated Locally Advanced or Metastatic Hepatocellular Carcinoma (IMbrave152) (SKYSCRAPER-14). Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05904886 (accessed on 23 August 2023).
- Trial of Atezolizumab and Bevacizumab with SRF388 or Placebo in Patients with Hepatocellular Carcinoma. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05359861 (accessed on 23 August 2023).
- Study Evaluating the Benefit of Adding Ipilimumab to the Combination of Atezolizumab and Bevacizumab in Patients with Hepatocellular Carcinoma Receiving First-Line Systemic Therapy (TRIPLET). Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05665348 (accessed on 23 August 2023).
- Sangro, B.; Yau, T.; Harding, J.J.; Rivera, M.A.; Kazushi, N.; El-Khoueiry, A.B.; Cruz-Correa, M.; Perez-Callejo, D.; McLean, S.; Sparks, J.; et al. RELATIVITY-106: A phase 1/2 trial of nivolumab (NIVO) + relatlimab (RELA) in combination with bevacizumab (BEV) in first-line (1L) hepatocellular carcinoma (HCC). J. Clin. Oncol. 2023, 4, TPS636. [Google Scholar] [CrossRef]
- A Study of Nivolumab and Relatlimab in Combination with Bevacizumab in Advanced Liver Cancer (RELATIVITY-106). Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05337137 (accessed on 23 August 2023).
- A Clinical Study to Observe the Effectiveness and Safety of IBI310, Bevacizumab Combined with Sintilimab in the Treatment of Advanced Hepatocellular Carcinoma. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05363722 (accessed on 23 August 2023).
- A Study Investigating the Efficacy and Safety of Ociperlimab and Tislelizumab and BAT1706 Combinations in Patients with Advanced HCC. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04948697 (accessed on 23 August 2023).
- Li, H.; Qin, S.; Liu, Y.; Chen, Z.; Ren, Z.; Xiong, J.; Meng, Z.; Zhang, X.; Wang, L.; Zhang, X.; et al. Camrelizumab Combined with FOLFOX4 Regimen as First-Line Therapy for Advanced Hepatocellular Carcinomas: A Sub-Cohort of a Multicenter Phase Ib/II Study. Drug Des. Devel. Ther. 2021, 15, 1873–1882. [Google Scholar] [CrossRef] [PubMed]
- A Trial of SHR-1210 (an Anti-PD-1 Inhibitor) in Combination with FOLFOX4 in Subjects with Advanced HCC Who Have Never Received Prior Systemic Treatment. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT03605706 (accessed on 23 August 2023).
- A Study of Nofazinlimab (CS1003) in Subjects with Advanced Hepatocellular Carcinoma. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04194775 (accessed on 24 August 2023).
- A Phase III Clinical Trial of AK105 Injection Combined with Anlotinib Hydrochloride Capsules versus Sorafenib in Subjects with Advanced Hepatocellular Carcinoma (HCC). Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04344158 (accessed on 24 August 2023).
- Preliminary Antitumor Activity, Safety and Tolerability of Tislelizumab in Combination with Lenvatinib for Hepatocellular Carcinoma. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04401800 (accessed on 24 August 2023).
- A Study to Compare the Effectiveness and Safety of IBI310 Combined with Sintilimab versus Sorafenib in the First-line Treatment of Advanced HCC. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04720716 (accessed on 24 August 2023).
- A Study of Atezolizumab with Lenvatinib or Sorafenib versus Lenvatinib or Sorafenib Alone in Hepatocellular Carcinoma Previously Treated with Atezolizumab and Bevacizumab (IMbrave251). Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04770896 (accessed on 24 August 2023).
- Sorafenib and Nivolumab in Treating Participants with Unresectable, Locally Advanced or Metastatic Liver Cancer. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT03439891 (accessed on 24 August 2023).
- Sorafenib Tosylate and Pembrolizumab in Treating Patients with Advanced or Metastatic Liver Cancer. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT03211416 (accessed on 24 August 2023).
- A Study of Nivolumab in Combination with Ipilimumab in Participants with Advanced Hepatocellular Carcinoma (CheckMate 9DW). Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04039607 (accessed on 24 August 2023).
- Radomised Phase II Study of MTL-CEBPA Plus Sorafenib or Sorafenib Alone (OUTREACH2). Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04710641 (accessed on 24 August 2023).
- Sarker, D.; Plummer, R.; Meyer, T.; Sodergren, M.H.; Basu, B.; Chee, C.E.; Huang, K.W.; Palmer, D.H.; Ma, Y.T.; Evans, T.R.J.; et al. MTL-CEBPA, a Small Activating RNA Therapeutic Upregulating C/EBP-α, in Patients with Advanced Liver Cancer: A First-in-Human, Multicenter, Open-Label, Phase I Trial. Clin. Cancer Res. 2020, 26, 3936–3946. [Google Scholar] [CrossRef]
- Moehler, M.; Heo, J.; Lee, H.C.; Tak, W.Y.; Chao, Y.; Paik, S.W.; Yim, H.J.; Byun, K.S.; Baron, A.; Ungerechts, G.; et al. Vaccinia-based oncolytic immunotherapy Pexastimogene Devacirepvec in patients with advanced hepatocellular carcinoma after sorafenib failure: A randomized multicenter Phase IIb trial (TRAVERSE). Oncoimmunology 2019, 8, e1615817. [Google Scholar] [CrossRef]
- Hepatocellular Carcinoma Study Comparing Vaccinia Virus Based Immunotherapy Plus Sorafenib vs. Sorafenib Alone (PHOCUS). Available online: https://classic.clinicaltrials.gov/ct2/show/NCT02562755 (accessed on 24 August 2023).
- Grupp, S.A.; Kalos, M.; Barrett, D.; Aplenc, R.; Porter, D.L.; Rheingold, S.R.; Teachey, D.T.; Chew, A.; Hauck, B.; Wright, J.F.; et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 2013, 368, 1509–1518. [Google Scholar] [CrossRef]
- Dargel, C.; Bassani-Sternberg, M.; Hasreiter, J.; Zani, F.; Bockmann, J.H.; Thiele, F.; Bohne, F.; Wisskirchen, K.; Wilde, S.; Sprinzl, M.F.; et al. T Cells Engineered to Express a T-Cell Receptor Specific for Glypican-3 to Recognize and Kill Hepatoma Cells In Vitro and in Mice. Gastroenterology 2015, 149, 1042–1052. [Google Scholar] [CrossRef]
- Jiang, Z.; Jiang, X.; Chen, S.; Lai, Y.; Wei, X.; Li, B.; Lin, S.; Wang, S.; Wu, Q.; Liang, Q.; et al. Anti-GPC3-CAR T Cells Suppress the Growth of Tumor Cells in Patient-Derived Xenografts of Hepatocellular Carcinoma. Front. Immunol. 2017, 7, 690. [Google Scholar] [CrossRef]
- Shi, D.; Shi, Y.; Kaseb, A.O.; Qi, X.; Zhang, Y.; Chi, J.; Lu, Q.; Gao, H.; Jiang, H.; Wang, H.; et al. Chimeric Antigen Receptor-Glypican-3 T-Cell Therapy for Advanced Hepatocellular Carcinoma: Results of Phase I Trials. Clin. Cancer Res. 2020, 26, 3979–3989. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, Y.; Xiang, J.; Long, L.; Green, S.; Yang, Z.; Zimdahl, B.; Lu, J.; Cheng, N.; Horan, L.H.; et al. Targeting Alpha-Fetoprotein (AFP)-MHC Complex with CAR T-Cell Therapy for Liver Cancer. Clin. Cancer Res. 2017, 23, 478–488. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, Y.; Gong, J.; Rao, L.; Wu, Z.; Nie, T.; Shi, D.; Zhang, L. High expression of MAGE-A9 contributes to stemness and malignancy of human hepatocellular carcinoma. Int. J. Oncol. 2018, 52, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Nouso, K.; Noguchi, Y.; Higashi, T.; Ono, T.; Jungbluth, A.; Chen, Y.T.; Old, L.J.; Nakayama, E.; Shiratori, Y. Expression and immunogenicity of NY-ESO-1 in hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2006, 21, 1281–1285. [Google Scholar] [CrossRef] [PubMed]
- Miura, N.; Maeda, Y.; Kanbe, T.; Yazama, H.; Takeda, Y.; Sato, R.; Tsukamoto, T.; Sato, E.; Marumoto, A.; Harada, T.; et al. Serum human telomerase reverse transcriptase messenger RNA as a novel tumor marker for hepatocellular carcinoma. Clin. Cancer Res. 2005, 11, 3205–3209. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Yang, D.; Dai, H.; Liu, X.; Jia, R.; Cui, X.; Li, W.; Cai, C.; Xu, J.; Zhao, X. Eradication of Hepatocellular Carcinoma by NKG2D-Based CAR-T Cells. Cancer Immunol. Res. 2019, 7, 1813–1823. [Google Scholar] [CrossRef]
- Sung, J.J.; Noh, S.J.; Bae, J.S.; Park, H.S.; Jang, K.Y.; Chung, M.J.; Moon, W.S. Immunohistochemical Expression and Clinical Significance of Suggested Stem Cell Markers in Hepatocellular Carcinoma. J. Pathol. Transl. Med. 2016, 50, 52–57. [Google Scholar] [CrossRef]
- GPC3 Targeted CAR-T Cell Therapy in Advanced GPC3 Expressing Hepatocellular Carcinoma (HCC). Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05003895 (accessed on 24 August 2023).
- ECT204 T-Cell Therapy in Adults with Advanced HCC (ARYA3). Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04864054 (accessed on 24 August 2023).
- Study of ET140203 T Cells in Adults with Advanced Hepatocellular Carcinoma (ARYA-1). Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04502082 (accessed on 24 August 2023).
- AFPc332T in Advanced HCC. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT03132792 (accessed on 24 August 2023).
- GPC3-Directed CAR-T in the Treatment Amongst Subjects with Advanced Hepatocellular Carcinoma. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05926726 (accessed on 24 August 2023).
- Study of GPC-3 CAR-T Cells in Treating with Hepatocellular Carcinoma. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05620706 (accessed on 24 August 2023).
- To Evaluate the Safety, Tolerability and Preliminary Efficacy of EU307. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05783570 (accessed on 24 August 2023).
- BOXR1030 T Cells in Subjects with Advanced GPC3-Positive Solid Tumors. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05120271 (accessed on 24 August 2023).
- GPC3-CAR-T Cells for Immunotherapy of Cancer with GPC3 Expression. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT03198546 (accessed on 24 August 2023).
- Clinical Study of Intratumoral Injection of CAR-T Cells in the Treatment of Advanced Liver Tumors. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04951141 (accessed on 24 August 2023).
- NKG2D-Based CAR T-Cells Immunotherapy for Patient with r/r NKG2DL+ Solid Tumors. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05131763 (accessed on 24 August 2023).
- IMC001 for Clinical Research on Advanced Digestive System Malignancie. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05028933 (accessed on 24 August 2023).
- Chen, X.; Chen, Y.; Liang, R.; Xiang, L.; Li, J.; Zhu, Y.; He, H.; Huang, L.; Zuo, D.; Li, W.; et al. Combination Therapy of Hepatocellular Carcinoma by GPC3-Targeted Bispecific Antibody and Irinotecan is Potent in Suppressing Tumor Growth in Mice. Mol. Cancer Ther. 2022, 21, 149–158. [Google Scholar] [CrossRef]
- Safran, H.; Druta, M.; Morse, M.; Lynce, F.; Pintova, S.; Almhanna, K.; Weiss, D.; Gianella-Borradori, A.; Ogita, Y.; Morley, R.; et al. Results of a phase 1 dose escalation study of ERY974, an anti-glypican 3 (GPC3)/CD3 bispecific antibody, in patients with advanced solid tumors [abstract]. In Proceedings of the American Association for Cancer Research Annual Meeting 2021, Philadelphia, PA, USA, 10–15 April and 17–21 May 2021. [Google Scholar]
- Lee, J.H.; Lee, J.H.; Lim, Y.S.; Yeon, J.E.; Song, T.J.; Yu, S.J.; Gwak, G.Y.; Kim, K.M.; Kim, Y.J.; Lee, J.W.; et al. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology 2015, 148, 1383–1391. [Google Scholar] [CrossRef]
- Kim, J.M.; Cho, S.Y.; Rhu, J.; Jung, M.; Her, J.H.; Lim, O.; Choi, G.S.; Shin, E.C.; Hwang, Y.K.; Joh, J.W. Adjuvant therapy using ex vivo-expanded allogenic natural killer cells in hepatectomy patients with hepatitis B virus related solitary hepatocellular carcinoma: MG4101 study. Ann. Hepatobiliary Pancreat. Surg. 2021, 25, 206–214. [Google Scholar] [CrossRef]
- Single-Arm, Open-Label Clinical Study of SZ003 in the Treatment of Advanced Hepatocellular Carcinoma. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05845502 (accessed on 24 August 2023).
- CAR-pNK Cell Immunotherapy in MUC1 Positive Relapsed or Refractory Solid Tumor. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT02839954 (accessed on 24 August 2023).
- Safety and Efficacy of Allogeneic NK Cells Therapy in Patients with Advanced Hepatocellular Carcinoma. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04162158 (accessed on 24 August 2023).
- Camrelizumab in Combination with Apatinib Plus NK Cell for Advanced HCC. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05171309 (accessed on 24 August 2023).
- Clinical Efficacy and Safety of NKT Cell Infusion in Patients with Advanced Solid Tumor. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT02562963 (accessed on 24 August 2023).
- Natural Killer (NK) Cell Therapy in Locally Advanced HCC. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05040438 (accessed on 24 August 2023).
- Kim, R.D.; Sarker, D.; Meyer, T.; Yau, T.; Macarulla, T.; Park, J.W.; Choo, S.P.; Hollebecque, A.; Sung, M.W.; Lim, H.Y.; et al. First-in-Human Phase I Study of Fisogatinib (BLU-554) Validates Aberrant FGF19 Signaling as a Driver Event in Hepatocellular Carcinoma. Cancer Discov. 2019, 9, 1696–1707. [Google Scholar] [CrossRef] [PubMed]
- Stemmer, S.M.; Manojlovic, N.S.; Marinca, M.V.; Petrov, P.; Cherciu, N.; Ganea, D.; Ciuleanu, T.E.; Pusca, I.A.; Beg, M.S.; Purcell, W.T.; et al. Namodenoson in Advanced Hepatocellular Carcinoma and Child-Pugh B Cirrhosis: Randomized Placebo-Controlled Clinical Trial. Cancers 2021, 13, 187. [Google Scholar] [CrossRef] [PubMed]
- Namodenoson in the Treatment of Advanced Hepatocellular Carcinoma in Patients with Child-Pugh Class B7 Cirrhosis (LIVERATION). Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05201404 (accessed on 24 August 2023).
- Butterfield, L.H.; Ribas, A.; Dissette, V.B.; Lee, Y.; Yang, J.Q.; De la Rocha, P.; Duran, S.D.; Hernandez, J.; Seja, E.; Potter, D.M.; et al. A phase I/II trial testing immunization of hepatocellular carcinoma patients with dendritic cells pulsed with four alpha-fetoprotein peptides. Clin. Cancer Res. 2006, 12, 2817–2825. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Yoshikawa, T.; Ofuji, K.; Yoshimura, M.; Tsuchiya, N.; Takahashi, M.; Nobuoka, D.; Gotohda, N.; Takahashi, S.; Kato, Y.; et al. Phase II study of the GPC3-derived peptide vaccine as an adjuvant therapy for hepatocellular carcinoma patients. Oncoimmunology 2016, 5, e1129483. [Google Scholar] [CrossRef] [PubMed]
- Personalized Cancer Vaccine in Egyptian Cancer Patients (PROVE). Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05059821 (accessed on 24 August 2023).
- Neoantigen Dendritic Cell Vaccine and Nivolumab in HCC and Liver Metastases from CRC. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04912765 (accessed on 24 August 2023).
- A Clinical Study of mRNA Vaccine (ABOR2014/IPM511) in Patients with Advanced Hepatocellular Carcinoma. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05981066 (accessed on 24 August 2023).
- Application of mRNA Immunotherapy Technology in Hepatitis B Virus-related Refractory Hepatocellular Carcinoma. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05738447 (accessed on 24 August 2023).
- GNOS-PV02 Personalized Neoantigen Vaccine, INO-9012 and Pembrolizumab in Subjects with Advanced HCC. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04251117 (accessed on 24 August 2023).
- Akiyama, S.; Saku, N.; Miyata, S.; Ite, M.; Toyoda, M.; Kimura, T.; Kuroda, M.; Nakazawa, A.; Kasahara, M.; Nonaka, H.; et al. Drug metabolic activity is a critical cell-intrinsic determinant for selection of hepatocytes during long-term culture. Stem Cell Res. Ther. 2022, 13, 104. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Duan, B.; Guo, Y.; Zhou, R.; Sun, J.; Bie, B.; Yang, S.; Huang, C.; Yang, J.; Li, Z. Baicalein sensitizes hepatocellular carcinoma cells to 5-FU and Epirubicin by activating apoptosis and ameliorating P-glycoprotein activity. Biomed. Pharmacother. 2018, 98, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zheng, B.; Meng, S.; Xu, Y.; Guo, J.; Chen, L.J.; Xiao, J.; Zhang, W.; Tan, Z.R.; Tang, J.; et al. Increased expression of SLC46A3 to oppose the progression of hepatocellular carcinoma and its effect on sorafenib therapy. Biomed. Pharmacother. 2019, 114, 108864. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, J.; Ma, L.; Shan, J.; Shen, J.; Yang, Z.; Liu, L.; Luo, Y.; Yao, C.; Qian, C. MicroRNA-122 confers sorafenib resistance to hepatocellular carcinoma cells by targeting IGF-1R to regulate RAS/RAF/ERK signaling pathways. Cancer Lett. 2016, 371, 171–181. [Google Scholar] [CrossRef]
- De Lorenzo, S.; Tovoli, F.; Trevisani, F. Mechanisms of Primary and Acquired Resistance to Immune Checkpoint Inhibitors in Patients with Hepatocellular Carcinoma. Cancers 2022, 14, 4616. [Google Scholar] [CrossRef]
- Yang, Z.; Suda, G.; Maehara, O.; Ohara, M.; Yoda, T.; Sasaki, T.; Kohya, R.; Yoshida, S.; Hosoda, S.; Tokuchi, Y.; et al. Changes in Serum Growth Factors during Resistance to Atezolizumab Plus Bevacizumab Treatment in Patients with Unresectable Hepatocellular Carcinoma. Cancers 2023, 15, 593. [Google Scholar] [CrossRef]
- Ding, J.; Wen, Z. Survival improvement and prognosis for hepatocellular carcinoma: Analysis of the SEER database. BMC Cancer 2021, 21, 1157. [Google Scholar] [CrossRef] [PubMed]
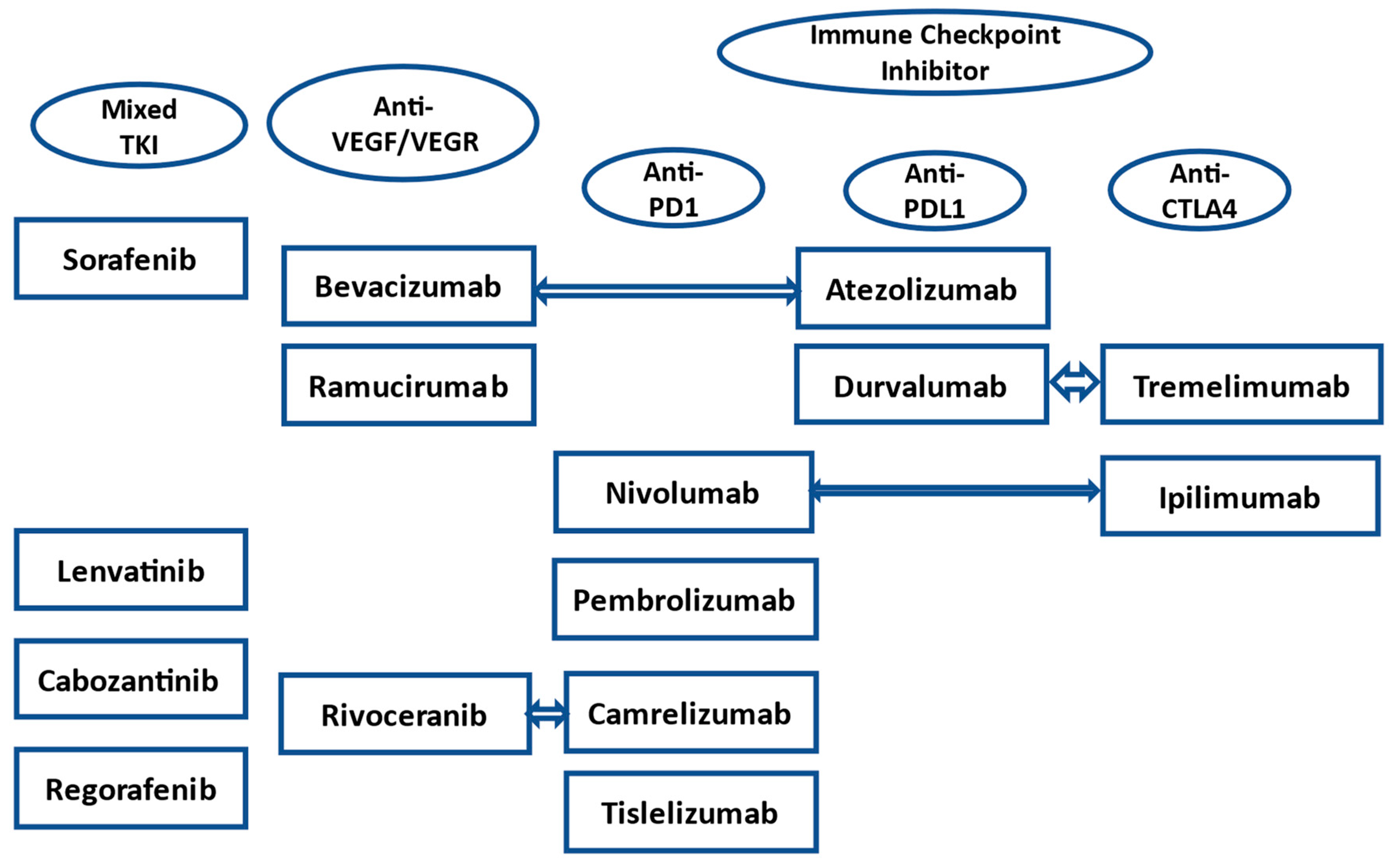
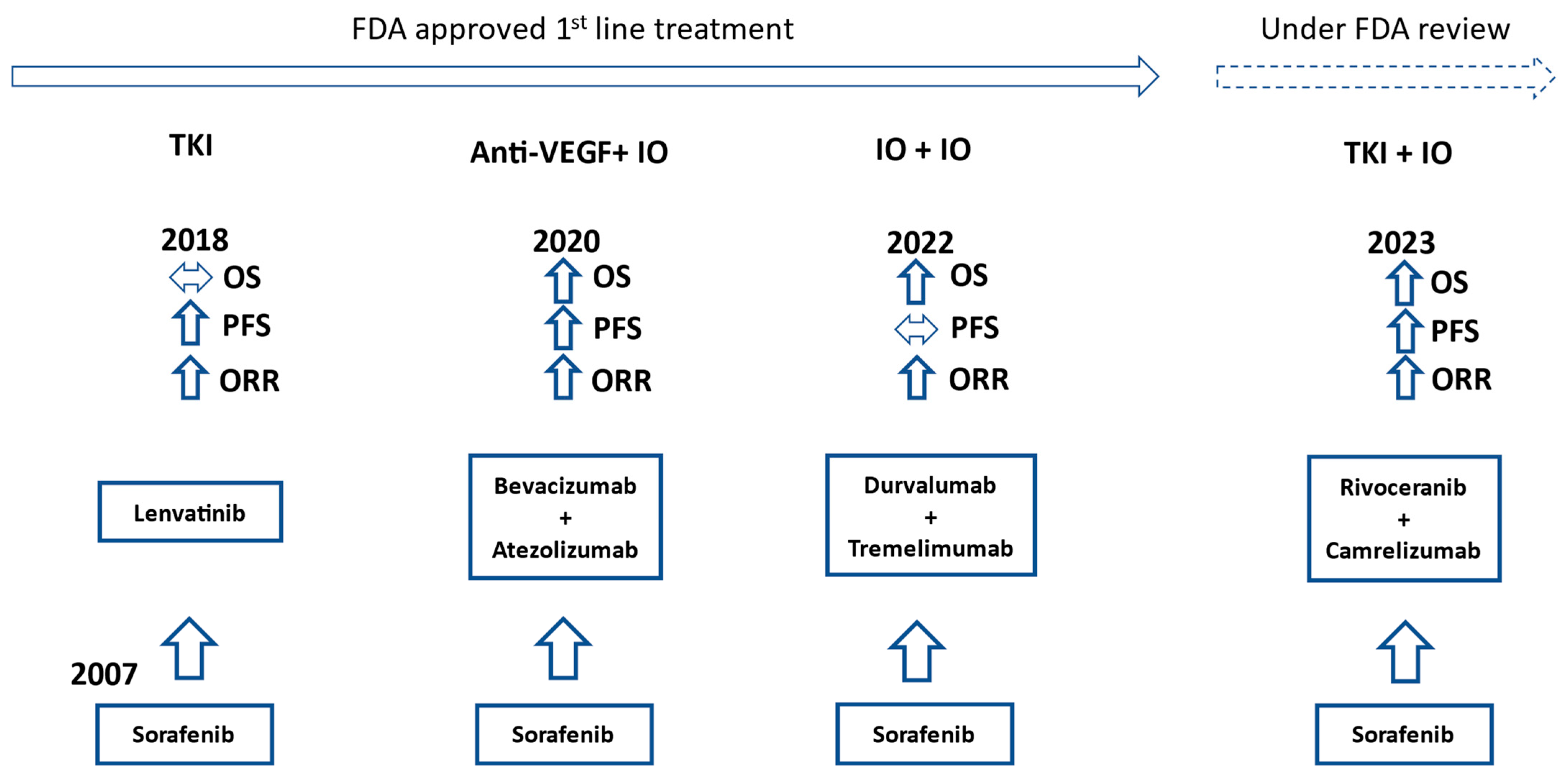
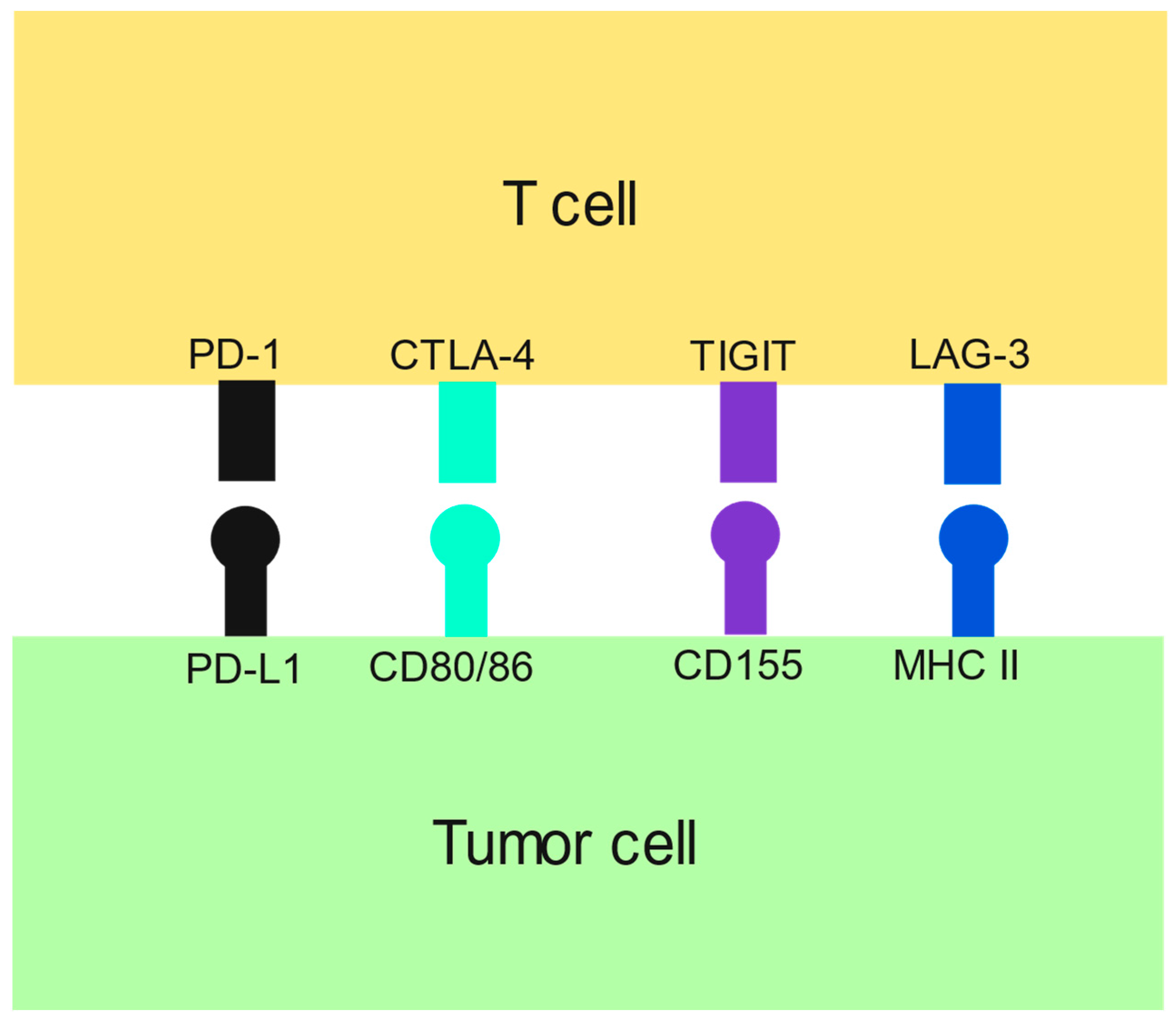
| Drug (Trial) | Control | Total Number of Patients | Drug Median OS (Months) | Control Median OS (Months) | HR (95% CI) |
|---|---|---|---|---|---|
| Regorafenib (RESORCE) | placebo | 573 | 10.6 | 7.8 | 0.63 (0.5–0.79) |
| Ramucirumab (REACH-2) | placebo | 292 | 8.5 | 7.3 | 0.71 (0.53–0.95) |
| Cabozantinib (CELESTIAL) | placebo | 707 | 10.2 | 8 | 0.76 (0.63–0.92) |
| Pembrolizumab (KEYNOTE-240) | placebo | 413 | 13.9 | 10.6 | 0.78 (0.61–0.998) |
| Pembrolizumab (KETNOTE-394) | placebo | 453 | 14.6 | 13 | 0.79 (0.63–0.99) |
| Nivolumab + Ipilimumab (CheckMate 040) | n/a | 148 | 22.8 | n/a | n/a |
| Trial ID | Medications | Comparison | Line of Therapy | Phase |
|---|---|---|---|---|
| NCT04194775 | Nofazinlimab with Lenvatinib | Lenvatinib | 1st | III |
| NCT04344158 | AK105 with Anlotinib | Sorafenib | 1st | III |
| NCT04401800 | Tislelizumab with Lenvatinib | n/a | 1st | II |
| NCT04720716 | IBI310 with Sintilimab | Sorafenib | 1st | III |
| NCT04770896 | Atezolizumab with Lenvatinib or Sorafenib | Lenvatinib or Sorafenib | 2nd | III |
| NCT03439891 | Sorafenib with Nivolumab | n/a | 1st | II |
| NCT03211416 | Sorafenib with Pembrolizumab | n/a | 1st or 2nd | I/II |
| NCT04039607 | Nivolumab with Ipilimumab | Lenvatinib or Sorafenib | 1st | III |
| Trial ID | CAR T-Cell Target | Phase |
|---|---|---|
| NCT05003895 | GPC3 | I |
| NCT04864054 | GPC3 | I/II |
| NCT05926726 | GPC3 | I |
| NCT05620706 | GPC3 | I |
| NCT05783570 | GPC3 | I |
| NCT05120271 | GPC3 | I/II |
| NCT03198546 | GPC3 | I |
| NCT04951141 | GPC3 | I |
| NCT05131763 | NKG2DL | I |
| NCT05028933 | EPCAM | I |
| NCT04502082 | AFP | 1/II |
| NCT03132792 | AFP | I |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadagopan, N.; He, A.R. Recent Progress in Systemic Therapy for Advanced Hepatocellular Carcinoma. Int. J. Mol. Sci. 2024, 25, 1259. https://doi.org/10.3390/ijms25021259
Sadagopan N, He AR. Recent Progress in Systemic Therapy for Advanced Hepatocellular Carcinoma. International Journal of Molecular Sciences. 2024; 25(2):1259. https://doi.org/10.3390/ijms25021259
Chicago/Turabian StyleSadagopan, Narayanan, and Aiwu Ruth He. 2024. "Recent Progress in Systemic Therapy for Advanced Hepatocellular Carcinoma" International Journal of Molecular Sciences 25, no. 2: 1259. https://doi.org/10.3390/ijms25021259
APA StyleSadagopan, N., & He, A. R. (2024). Recent Progress in Systemic Therapy for Advanced Hepatocellular Carcinoma. International Journal of Molecular Sciences, 25(2), 1259. https://doi.org/10.3390/ijms25021259







