Unravelling the Transcriptional Response of Agaricus bisporus under Lecanicillium fungicola Infection
Abstract
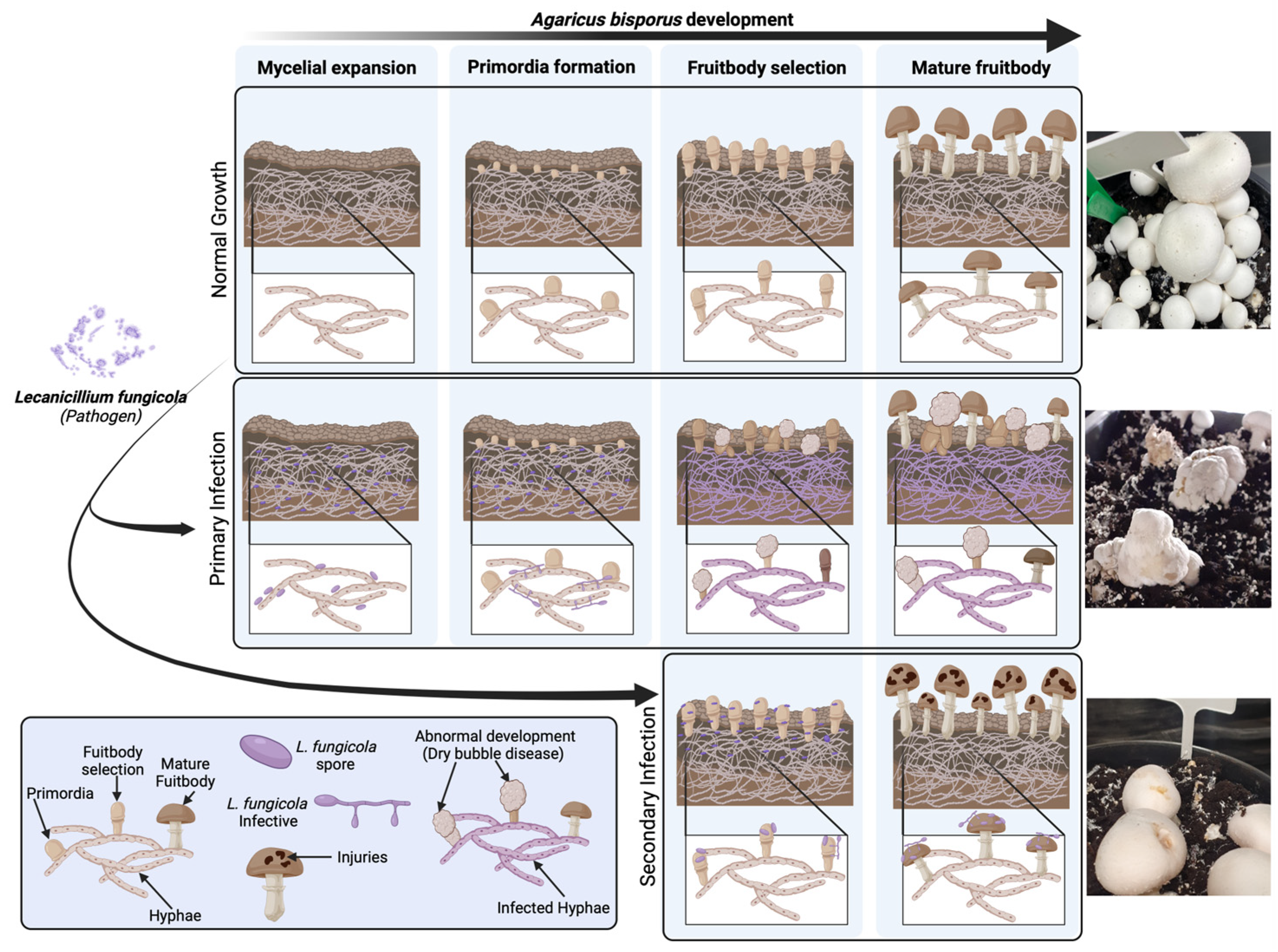
1. Introduction
2. Results
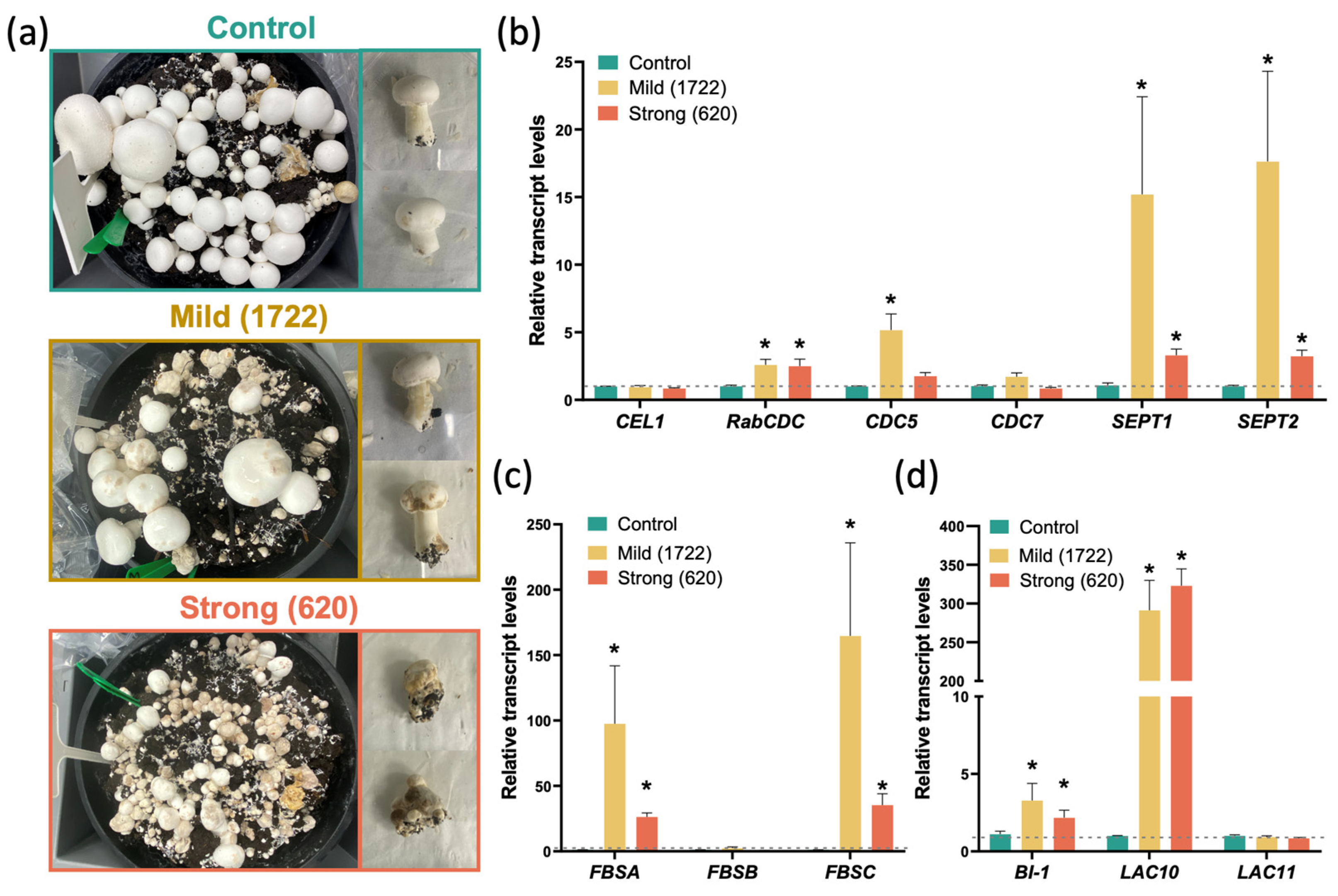
2.1. L. fungicola Infection Affects the Transcription of Genes Involved in the Control of Cell Division, Fruiting Body Development, and Apoptosis
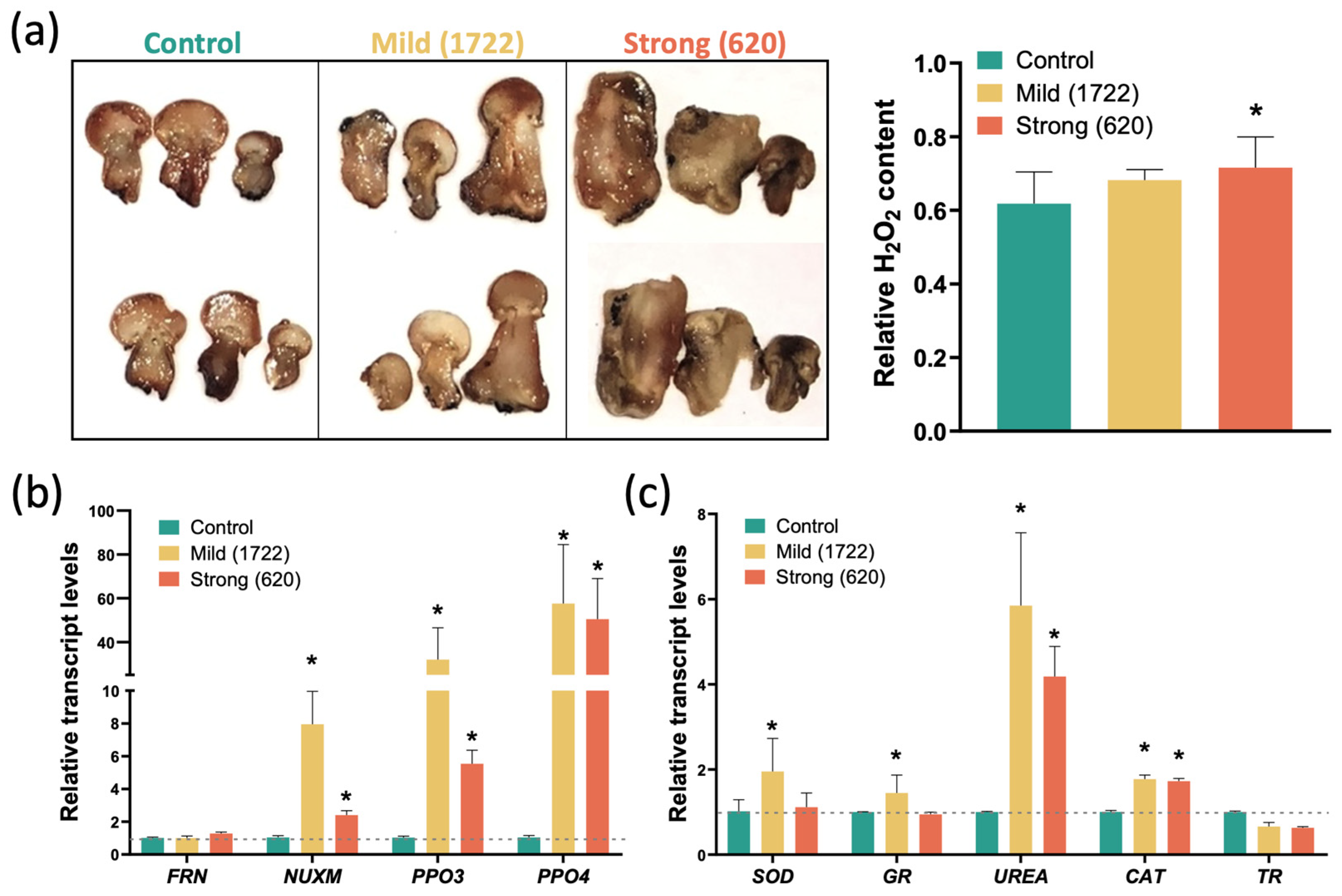
2.2. L. fungicola Infection Produces Impaired Reactive Oxygen Species (ROS) Levels in A. bisporus
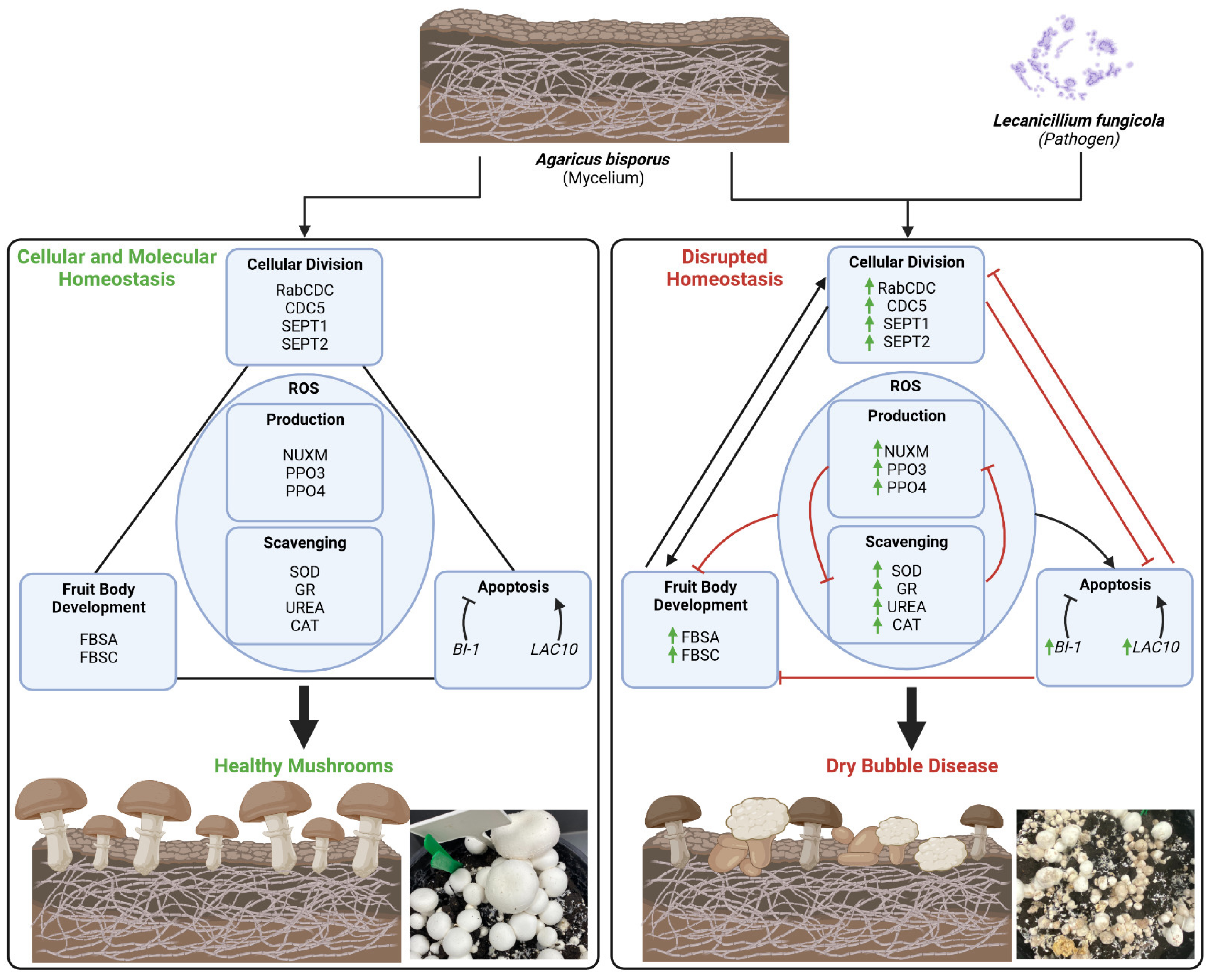
3. Discussion
4. Materials and Methods
4.1. A. bisporus Infection with L. fungicola Strains
4.2. Primer Design
4.3. RNA Extraction and Quantitative (qRT-PCR)
4.4. A. bisporus DAB Staining
4.5. Data Analysis and Visualization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blumfield, M.; Abbott, K.; Duve, E.; Cassettari, T.; Marshall, S.; Fayet-Moore, F. Examining the health effects and bioactive components in Agaricus bisporus mushrooms: A scoping review. J. Nutr. Biochem. 2020, 84, 108453. [Google Scholar] [CrossRef]
- Stamets, P. Mycelium Running: How Mushrooms Can Help Save the World; Ten Speed Press: Emeryville, CA, USA, 2005. [Google Scholar]
- Singh, M.; Kamal, S.; Sharma, V.P. Status and trends in world mushroom production-III-World Production of different mushroom species in 21st century. Mushroom Res. 2020, 29. [Google Scholar] [CrossRef]
- Gea, F.J.; Navarro, M.J.; Santos, M.; Diánez, F.; Carrasco, J. Control of Fungal Diseases in Mushroom Crops while Dealing with Fungicide Resistance: A Review. Microorganisms 2021, 9, 585. [Google Scholar] [CrossRef]
- Soković, M.; Van Griensven, L.J. Antimicrobial activity of essential oils and their components against the three major pathogens of the cultivated button mushroom, Agaricus bisporus. Eur. J. Plant Pathol. 2006, 116, 211–224. [Google Scholar] [CrossRef]
- Fletcher, J.T.; Gaze, R.H. Mushroom Pest and Disease Control: A Color Handbook; Elsevier: Amsterdam, The Netherlands; CRC Press: London, UK, 2007. [Google Scholar] [CrossRef]
- Fletcher, J.; Yarham, D. The incidence of benomyl tolerance in Verticillium fungicola, Mycogone perniciosa and Hypomyces rosellus in mushroom crops. Ann. Appl. Biol. 1976, 84, 343–353. [Google Scholar] [CrossRef]
- Stanojević, O.; Berić, T.; Potočnik, I.; Rekanović, E.; Stanković, S.; Milijašević-Marčić, S. Biological control of green mould and dry bubble diseases of cultivated mushroom (Agaricus bisporus L.) by Bacillus spp. Crop Prot. 2019, 126, 104944. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Baars, J.J.P.; Kalkhove, S.C.; Lugones, L.G.; Wosten, H.A.B.; Bakker, P.A.H.M. Lecanicillium fungicola: Causal agent of dry bubble disease in white-button mushroom. Mol. Plant Pathol. 2010, 11, 585–595. [Google Scholar] [CrossRef]
- Savoie, J.-M.; Mata, G.; Largeteau, M. New prospects in pathogen control of button mushroom cultures. In Mushroom Biotechnology; Elsevier: Oxford, UK, 2016; pp. 93–110. [Google Scholar]
- Bailey, A.M.; Collopy, P.D.; Thomas, D.J.; Sergeant, M.R.; Costa, A.M.S.B.; Barker, G.L.A.; Mills, P.R.; Challen, M.P.; Foster, G.D. Transcriptomic analysis of the interactions between Agaricus bisporus and Lecanicillium fungicola. Fungal Genet. Biol. 2013, 55, 67–76. [Google Scholar] [CrossRef]
- Banks, A.M.; Aminuddin, F.; Williams, K.; Batstone, T.; Barker, G.L.A.; Foster, G.D.; Bailey, A.M. Genome Sequence of Lecanicillium fungicola 150-1, the Causal Agent of Dry Bubble Disease. Microbiol. Resour. Announc. 2019, 8, e00340-19. [Google Scholar] [CrossRef]
- Balint-Kurti, P. The plant hypersensitive response: Concepts, control and consequences. Mol. Plant Pathol. 2019, 20, 1163–1178. [Google Scholar] [CrossRef]
- Santana Nunes, J.; Rocha de Brito, M.; Cunha Zied, D.; Aparecida das Graças Leite, E.; Souza Dias, E.; Alves, E. Evaluation of the infection process by Lecanicillium fungicola in Agaricus bisporus by scanning electron microscopy. Rev. Iberoam. Micol. 2017, 34, 36–42. [Google Scholar] [CrossRef][Green Version]
- Carrasco, J.; Tello, M.L.; de Toro, M.; Tkacz, A.; Poole, P.; Pérez-Clavijo, M.; Preston, G. Casing microbiome dynamics during button mushroom cultivation: Implications for dry and wet bubble diseases. Microbiology 2019, 165, 611–624. [Google Scholar] [CrossRef]
- Clarke, J.; Grogan, H.; Fitzpatrick, D.; Kavanagh, K. Characterising the proteomic response of mushroom pathogen Lecanicillium fungicola to Bacillus velezensis QST 713 and Kos biocontrol agents. Eur. J. Plant Pathol. 2022, 163, 369–379. [Google Scholar] [CrossRef]
- Umar, M.H.; Van Griensven, L.J. Studies on the morphogenesis of Agaricus bisporus: The dilemma of normal versus abnormal fruit body development. Mycol. Res. 1999, 103, 1235–1244. [Google Scholar] [CrossRef]
- Lu, B.C. Cell degeneration and gill remodelling during basidiocarp development in the fungus Coprinus cinereus. Can. J. Bot. 1991, 69, 1161–1169. [Google Scholar] [CrossRef]
- Krizsán, K.; Almási, É.; Merényi, Z.; Sahu, N.; Virágh, M.; Kószó, T.; Mondo, S.; Kiss, B.; Bálint, B.; Kües, U.; et al. Transcriptomic atlas of mushroom development reveals conserved genes behind complex multicellularity in fungi. Proc. Natl. Acad. Sci. USA 2019, 116, 7409–7418. [Google Scholar] [CrossRef]
- Zhang, L.; Gong, W.; Li, C.; Shen, N.; Gui, Y.; Bian, Y.; Kwan, H.S.; Cheung, M.K.; Xiao, Y. RNA-Seq-based high-resolution linkage map reveals the genetic architecture of fruiting body development in shiitake mushroom, Lentinula edodes. Comput. Struct. Biotechnol. J. 2021, 19, 1641–1653. [Google Scholar] [CrossRef]
- Marquardt, J.; Chen, X.; Bi, E. Septin Assembly and Remodeling at the Cell Division Site during the Cell Cycle. Front. Cell Dev. Biol. 2021, 9, 793920. [Google Scholar] [CrossRef]
- Brochu, V.; Girard-Martel, M.; Duval, I.; Lerat, S.; Grondin, G.; Domingue, O.; Beaulieu, C.; Beaudoin, N. Habituation to thaxtomin A in hybrid poplar cell suspensions provides enhanced and durable resistance to inhibitors of cellulose synthesis. BMC Plant Biol. 2010, 10, 272. [Google Scholar] [CrossRef]
- Yang, X.X.; Sun, J.D.; Cui, F.C.; Ji, J.; Wang, L.P.; Zhang, Y.Z.; Sun, X.L. An eco-friendly sensor based on CQD@MIPs for detection of N-acylated homoserine lactones and its 3D printing applications. Talanta 2020, 219, 121343. [Google Scholar] [CrossRef]
- Kwok, A.C.; Wong, J.T. Cellulose synthesis is coupled to cell cycle progression at G1 in the dinoflagellate Crypthecodinium cohnii. Plant Physiol. 2003, 131, 1681–1691. [Google Scholar] [CrossRef]
- Gibieža, P.; Prekeris, R. Rab GTPases and cell division. Small GTPases 2018, 9, 107–115. [Google Scholar] [CrossRef]
- De Groot, P.W.J.; Schaap, P.J.; Van Griensven, L.; Visser, J. Isolation of developmentally regulated genes from the edible mushroom Agaricus bisporus. Microbiol. (Read.) 1997, 143 Pt 6, 1993–2001. [Google Scholar] [CrossRef]
- Sunagawa, M.; Magae, Y. Isolation of genes differentially expressed during the fruit body development of Pleurotus ostreatus by differential display of RAPD. FEMS Microbiol. Lett. 2005, 246, 279–284. [Google Scholar] [CrossRef]
- Reimers, K.; Choi, C.Y.; Bucan, V.; Vogt, P.M. The Bax Inhibitor-1 (BI-1) family in apoptosis and tumorigenesis. Curr. Mol. Med. 2008, 8, 148–156. [Google Scholar] [CrossRef]
- Chang, W.; Feng, W.; Yang, Y.; Shen, Y.; Song, T.; Li, Y.; Cai, W. Metagenomics analysis of the effects of Agaricus bisporus mycelia on microbial diversity and CAZymes in compost. PeerJ 2022, 10, e14426. [Google Scholar] [CrossRef]
- Srivastava, S.; Brychkova, G.; Yarmolinsky, D.; Soltabayeva, A.; Samani, T.; Sagi, M. Aldehyde Oxidase 4 Plays a Critical Role in Delaying Silique Senescence by Catalyzing Aldehyde Detoxification. Plant Physiol. 2017, 173, 1977–1997. [Google Scholar] [CrossRef]
- Bandyopadhyay, U.; Das, D.; Banerjee, R.K. Reactive oxygen species: Oxidative damage and pathogenesis. Curr. Sci. 1999, 77, 658–666. [Google Scholar]
- Camejo, D.; Guzmán-Cedeño, Á.; Moreno, A. Reactive oxygen species, essential molecules, during plant–pathogen interactions. Plant Physiol. Biochem. 2016, 103, 10–23. [Google Scholar] [CrossRef]
- Yang, X.; Yang, K.; Wang, X.; Wang, Y.; Zhao, Z.; Meng, D. Transcriptomic analysis reveals the mechanism of bacterial disease resistance of postharvest button mushroom (Agaricus bisporus). Physiol. Mol. Plant Pathol. 2022, 122, 101903. [Google Scholar] [CrossRef]
- Zeng, X.; Yang, M.; Ye, T.; Feng, J.; Xu, X.; Yang, H.; Wang, X.; Bao, L.; Li, R.; Xue, B.; et al. Mitochondrial GRIM-19 loss in parietal cells promotes spasmolytic polypeptide-expressing metaplasia through NLR family pyrin domain-containing 3 (NLRP3)-mediated IL-33 activation via a reactive oxygen species (ROS) -NRF2- Heme oxygenase-1(HO-1)-NF-кB axis. Free Radic Biol. Med. 2023, 202, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Krause, K.H.; Xenarios, I.; Soldati, T.; Boeckmann, B. Evolution of the ferric reductase domain (FRD) superfamily: Modularity, functional diversification, and signature motifs. PLoS ONE 2013, 8, e58126. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Wen, Y.; Yu, Y.; Li, H.; Wang, J.; Sun, B. Ignored role of polyphenol in boosting reactive oxygen species generation for polyphenol/chemodynamic combination therapy. Mater Today Bio 2022, 16, 100436. [Google Scholar] [CrossRef] [PubMed]
- Tavanti, T.R.; Melo, A.A.R.d.; Moreira, L.D.K.; Sanchez, D.E.J.; Silva, R.d.S.; Silva, R.M.d.; Reis, A.R.d. Micronutrient fertilization enhances ROS scavenging system for alleviation of abiotic stresses in plants. Plant Physiol. Biochem. 2021, 160, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Brychkova, G.; Alikulov, Z.; Fluhr, R.; Sagi, M. A critical role for ureides in dark and senescence-induced purine remobilization is unmasked in the Atxdh1 Arabidopsis mutant. Plant J. 2008, 54, 496–509. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Kono, K.; Lowery, D.M.; Bartolini, S.; Yaffe, M.B.; Ohya, Y.; Pellman, D. Polo-like Kinase Cdc5 Controls the Local Activation of Rho1 to Promote Cytokinesis. Science 2006, 313, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Sourirajan, A.; Lichten, M. Polo-like kinase Cdc5 drives exit from pachytene during budding yeast meiosis. Genes Dev. 2008, 22, 2627–2632. [Google Scholar] [CrossRef]
- Mishra, P.K.; Olafsson, G.; Boeckmann, L.; Westlake, T.J.; Jowhar, Z.M.; Dittman, L.E.; Baker, R.E.; D’Amours, D.; Thorpe, P.H.; Basrai, M.A. Cell cycle–dependent association of polo kinase Cdc5 with CENP-A contributes to faithful chromosome segregation in budding yeast. Mol. Biol. Cell 2019, 30, 1020–1036. [Google Scholar] [CrossRef]
- Takai, N.; Hamanaka, R.; Yoshimatsu, J.; Miyakawa, I. Polo-like kinases (Plks) and cancer. Oncogene 2005, 24, 287–291. [Google Scholar] [CrossRef]
- Raab, C.A.; Raab, M.; Becker, S.; Strebhardt, K. Non-mitotic functions of polo-like kinases in cancer cells. Biochim. Biophys. Acta (BBA) Rev. Cancer 2021, 1875, 188467. [Google Scholar] [CrossRef]
- Liu, X.; Erikson, R.L. Polo-like kinase (Plk)1 depletion induces apoptosis in cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 5789–5794. [Google Scholar] [CrossRef]
- Liu, X.-B.; Xia, E.-H.; Li, M.; Cui, Y.-Y.; Wang, P.-M.; Zhang, J.-X.; Xie, B.-G.; Xu, J.-P.; Yan, J.-J.; Li, J.; et al. Transcriptome data reveal conserved patterns of fruiting body development and response to heat stress in the mushroom-forming fungus Flammulina filiformis. PLoS ONE 2020, 15, e0239890. [Google Scholar] [CrossRef] [PubMed]
- Nagy, L.; Vonk, P.; Künzler, M.; Földi, C.; Virágh, M.; Ohm, R.; Hennicke, F.; Bálint, B.; Csernetics, Á.; Hegedüs, B. Lessons on fruiting body morphogenesis from genomes and transcriptomes of Agaricomycetes. Stud. Mycol. 2023, 104, 1–85. [Google Scholar] [CrossRef]
- Xu, Q.; Reed, J.C. Bax inhibitor-1, a mammalian apoptosis suppressor identified by functional screening in yeast. Mol. Cell 1998, 1, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Lebeaupin, C.; Blanc, M.; Vallée, D.; Keller, H.; Bailly-Maitre, B. BAX inhibitor-1: Between stress and survival. FEBS J. 2020, 287, 1722–1736. [Google Scholar] [CrossRef]
- Robinson, K.; Clements, A.; Williams, A.; Berger, C.N.; Frankel, G. Bax inhibitor 1 in apoptosis and disease. Oncogene 2011, 30, 2391–2400. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.-W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef]
- Sauer, H.; Wartenberg, M.; Hescheler, J. Reactive Oxygen Species as Intracellular Messengers during Cell Growth and Differentiation. Cell. Physiol. Biochem. 2001, 11, 173–186. [Google Scholar] [CrossRef]
- Khan, A.Q.; Rashid, K.; AlAmodi, A.A.; Agha, M.V.; Akhtar, S.; Hakeem, I.; Raza, S.S.; Uddin, S. Reactive oxygen species (ROS) in cancer pathogenesis and therapy: An update on the role of ROS in anticancer action of benzophenanthridine alkaloids. Biomed. Pharmacother. 2021, 143, 112142. [Google Scholar] [CrossRef]
- Brychkova, G.; Yarmolinsky, D.; Fluhr, R.; Sagi, M. The determination of sulfite levels and its oxidation in plant leaves. Plant Sci. 2012, 190, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Radi, R. Interplay of carbon dioxide and peroxide metabolism in mammalian cells. J. Biol. Chem. 2022, 298, 102358. [Google Scholar] [CrossRef] [PubMed]
- Toshniwal, A.G.; Gupta, S.; Mandal, L.; Mandal, S. ROS Inhibits Cell Growth by Regulating 4EBP and S6K, Independent of TOR, during Development. Dev. Cell 2019, 49, 473–489.e479. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Wang, L.Y.; Liao, D.J.; Lu, X.P.; Hu, D.J.; Liang, X.; Zhao, J.; Mo, Z.Y.; Li, S.P. Potential molecular mechanisms for fruiting body formation of Cordyceps illustrated in the case of Cordyceps sinensis. Mycology 2017, 8, 231–258. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.-R.; Chang, Y.-S. Laccase-mediated oxidation of small organics: Bifunctional roles for versatile applications. Trends Biotechnol. 2013, 31, 335–341. [Google Scholar] [CrossRef]
- Jeon, J.-R.; Baldrian, P.; Murugesan, K.; Chang, Y.-S. Laccase-catalysed oxidations of naturally occurring phenols: From in vivo biosynthetic pathways to green synthetic applications. Microb. Biotechnol. 2012, 5, 318–332. [Google Scholar] [CrossRef]
- Baldrian, P. Fungal laccases–occurrence and properties. FEMS Microbiol. Rev. 2006, 30, 215–242. [Google Scholar] [CrossRef]
- Matuszewska, A.; Karp, M.; Jaszek, M.; Janusz, G.; Osińska-Jaroszuk, M.; Sulej, J.; Stefaniuk, D.; Tomczak, W.; Giannopoulos, K. Laccase purified from Cerrena unicolor exerts antitumor activity against leukemic cells. Oncol. Lett. 2016, 11, 2009–2018. [Google Scholar] [CrossRef]
- Kim, M.; Jee, S.C.; Sung, J.-S.; Kadam, A.A. Anti-proliferative applications of laccase immobilized on super-magnetic chitosan-functionalized halloysite nanotubes. Int. J. Biol. Macromol. 2018, 118, 228–237. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Kumarasamy, M.; Sosnik, A.; Danino, D. Enhanced Thermostability and Anticancer Activity in Breast Cancer Cells of Laccase Immobilized on Pluronic-Stabilized Nanoparticles. ACS Appl. Mater. Interfaces 2019, 11, 39436–39448. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Yu, B.P. Cellular defenses against damage from reactive oxygen species. Physiol. Rev. 1994, 74, 139–162. [Google Scholar] [CrossRef] [PubMed]
- Bafana, A.; Dutt, S.; Kumar, A.; Kumar, S.; Ahuja, P.S. The basic and applied aspects of superoxide dismutase. J. Mol. Catal. B Enzym. 2011, 68, 129–138. [Google Scholar] [CrossRef]
- Couto, N.; Wood, J.; Barber, J. The role of glutathione reductase and related enzymes on cellular redox homoeostasis network. Free Radic. Biol. Med. 2016, 95, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Sepasi Tehrani, H.; Moosavi-Movahedi, A.A. Catalase and its mysteries. Prog. Biophys. Mol. Biol. 2018, 140, 5–12. [Google Scholar] [CrossRef]
- Zhou, C.; Bhinderwala, F.; Lehman, M.K.; Thomas, V.C.; Chaudhari, S.S.; Yamada, K.J.; Foster, K.W.; Powers, R.; Kielian, T.; Fey, P.D. Urease is an essential component of the acid response network of Staphylococcus aureus and is required for a persistent murine kidney infection. PLOS Pathog. 2019, 15, e1007538. [Google Scholar] [CrossRef]
- Wolinska, J.; King, K.C. Environment can alter selection in host–parasite interactions. Trends Parasitol. 2009, 25, 236–244. [Google Scholar] [CrossRef]
- Morin, E.; Kohler, A.; Baker, A.R.; Foulongne-Oriol, M.; Lombard, V.; Nagye, L.G.; Ohm, R.A.; Patyshakuliyeva, A.; Brun, A.; Aerts, A.L.; et al. Genome sequence of the button mushroom Agaricus bisporus reveals mechanisms governing adaptation to a humic-rich ecological niche. Proc. Natl. Acad. Sci. USA 2012, 109, 17501–17506. [Google Scholar] [CrossRef]
- Missirlis, F.; Phillips, J.P.; Jäckle, H. Cooperative action of antioxidant defense systems in Drosophila. Curr. Biol. 2001, 11, 1272–1277. [Google Scholar] [CrossRef]
- Tao, Y.; van Peer, A.F.; Huang, Q.; Shao, Y.; Zhang, L.; Xie, B.; Jiang, Y.; Zhu, J.; Xie, B. Identification of novel and robust internal control genes from Volvariella volvacea that are suitable for RT-qPCR in filamentous fungi. Sci. Rep. 2016, 6, 29236. [Google Scholar] [CrossRef] [PubMed]
- Navarro, P.; Billette, C.; Ferrer, N.; Savoie, J.-M. Characterization of the aap1 gene of Agaricus bisporus, a homolog of the yeast YAP1. Comptes Rendus Biol. 2014, 337, 29–43. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quiroz, L.F.; Ciosek, T.; Grogan, H.; McKeown, P.C.; Spillane, C.; Brychkova, G. Unravelling the Transcriptional Response of Agaricus bisporus under Lecanicillium fungicola Infection. Int. J. Mol. Sci. 2024, 25, 1283. https://doi.org/10.3390/ijms25021283
Quiroz LF, Ciosek T, Grogan H, McKeown PC, Spillane C, Brychkova G. Unravelling the Transcriptional Response of Agaricus bisporus under Lecanicillium fungicola Infection. International Journal of Molecular Sciences. 2024; 25(2):1283. https://doi.org/10.3390/ijms25021283
Chicago/Turabian StyleQuiroz, Luis Felipe, Tessa Ciosek, Helen Grogan, Peter C. McKeown, Charles Spillane, and Galina Brychkova. 2024. "Unravelling the Transcriptional Response of Agaricus bisporus under Lecanicillium fungicola Infection" International Journal of Molecular Sciences 25, no. 2: 1283. https://doi.org/10.3390/ijms25021283
APA StyleQuiroz, L. F., Ciosek, T., Grogan, H., McKeown, P. C., Spillane, C., & Brychkova, G. (2024). Unravelling the Transcriptional Response of Agaricus bisporus under Lecanicillium fungicola Infection. International Journal of Molecular Sciences, 25(2), 1283. https://doi.org/10.3390/ijms25021283





