Ionic Liquid-Based Polymer Matrices for Single and Dual Drug Delivery: Impact of Structural Topology on Characteristics and In Vitro Delivery Efficiency
Abstract
:1. Introduction
2. Results and Discussion
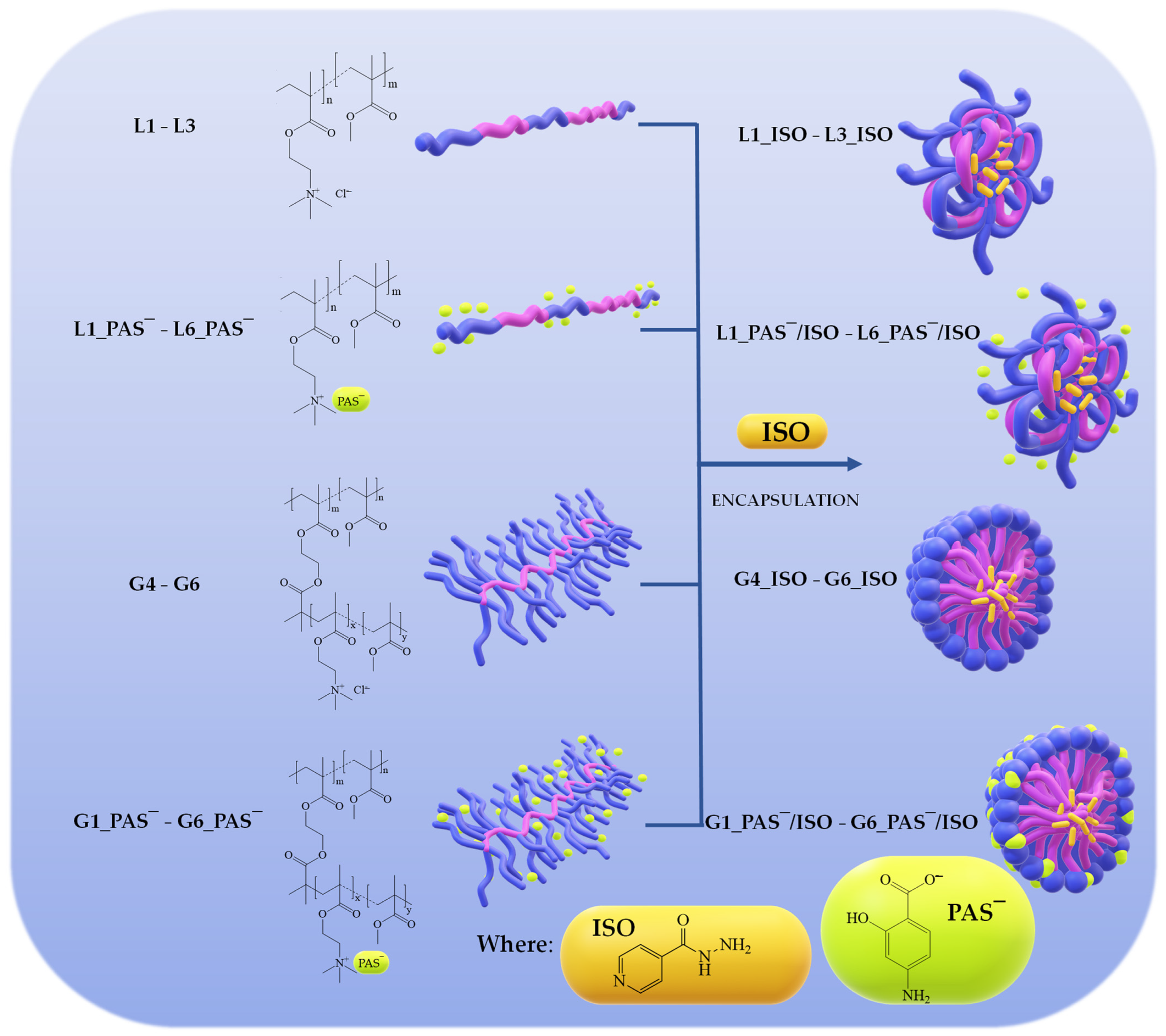
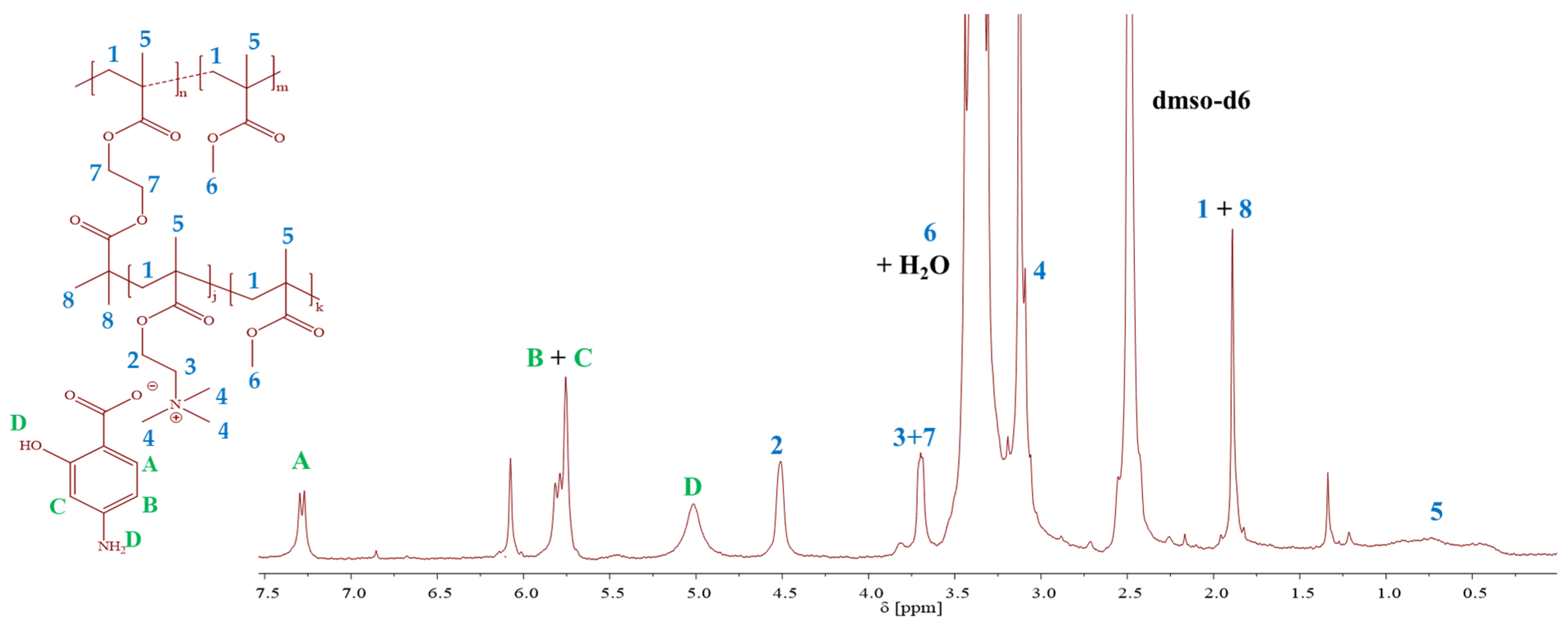
2.1. TMAMA-Based Linear and Graft Copolymers as Drug Carriers
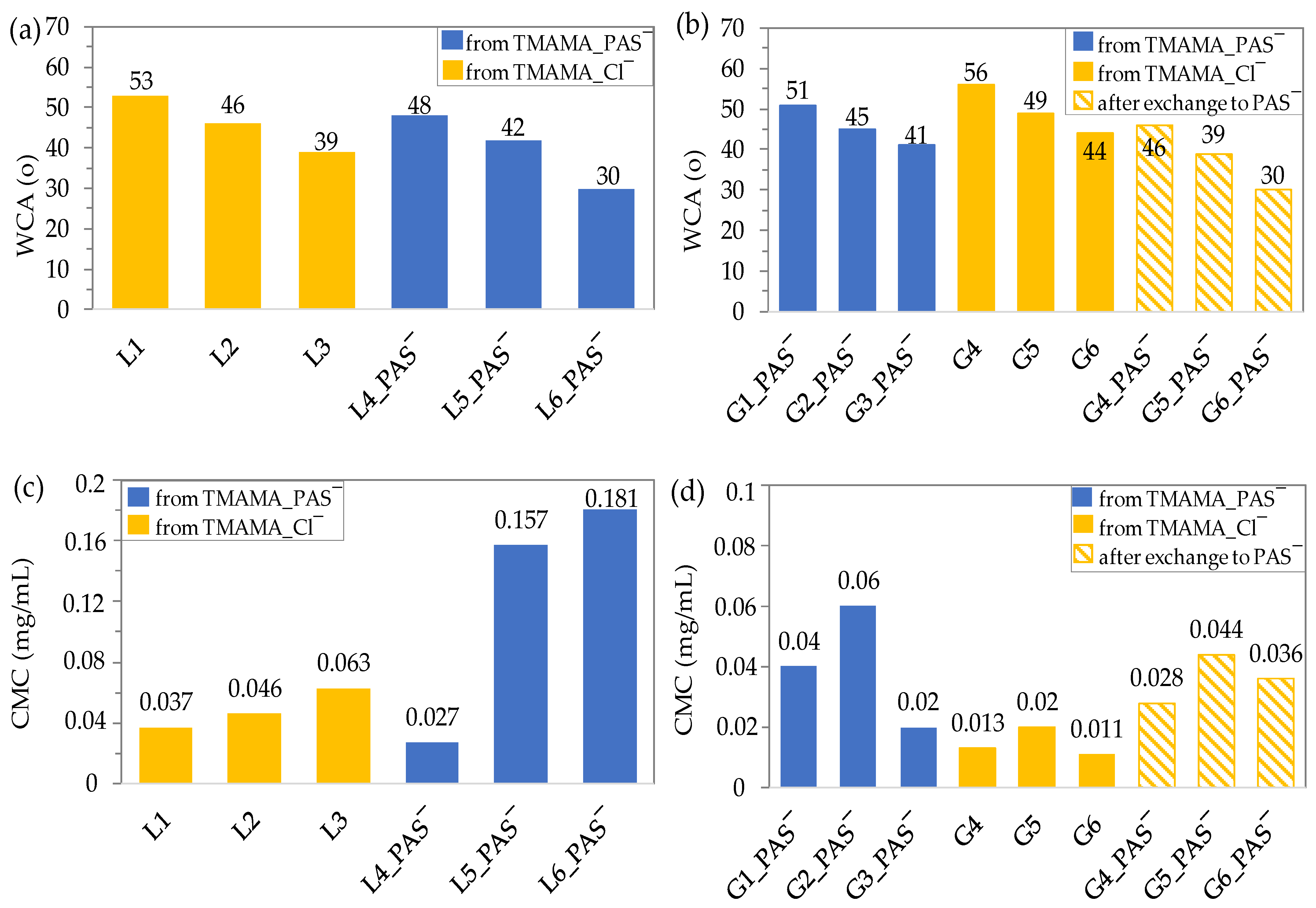
2.2. Wettability and Amphiphilic Properties
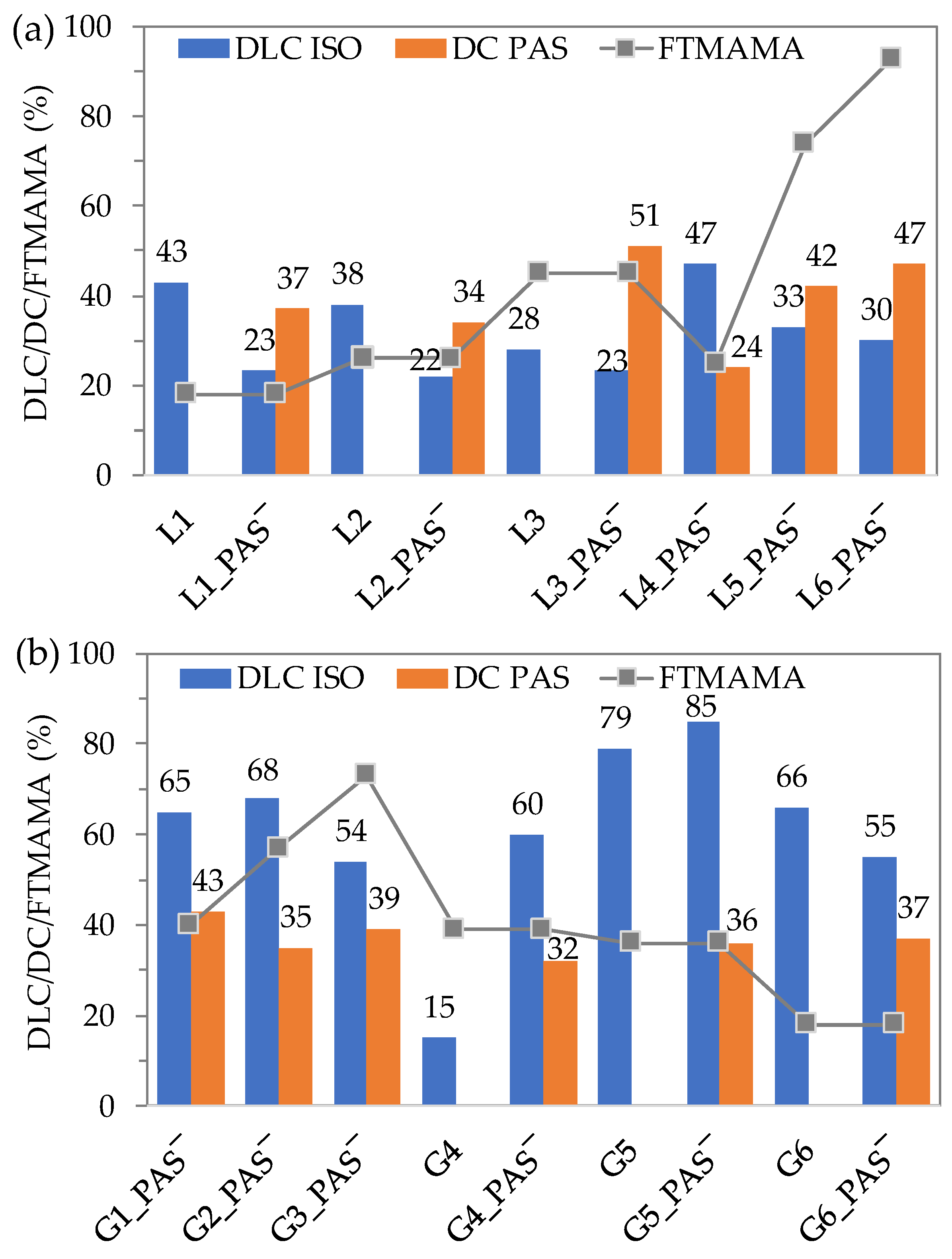
2.3. Encapsulation of Isoniazid
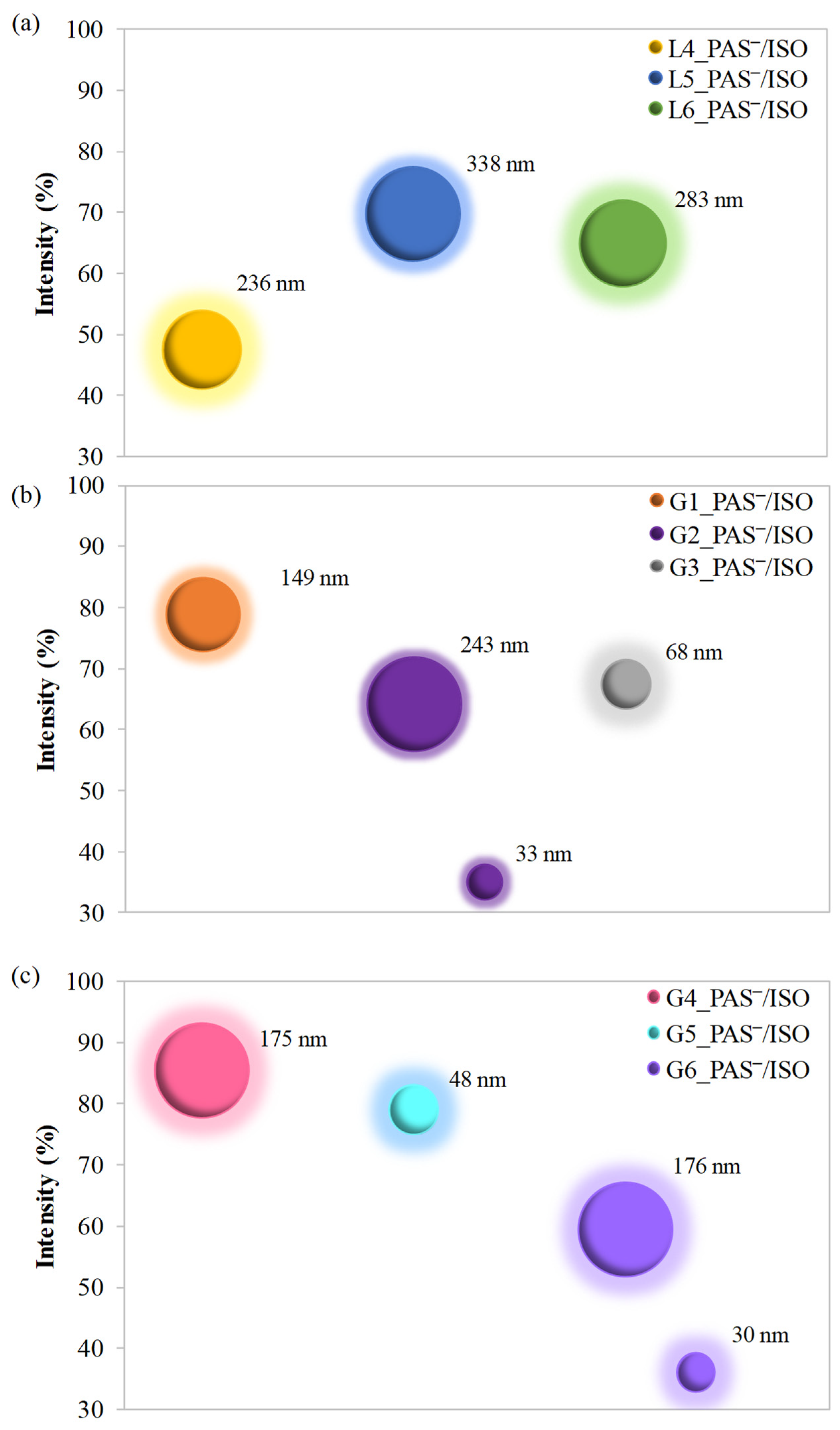
2.4. Hydrodynamic Diameters of Self-Assembled Conjugates
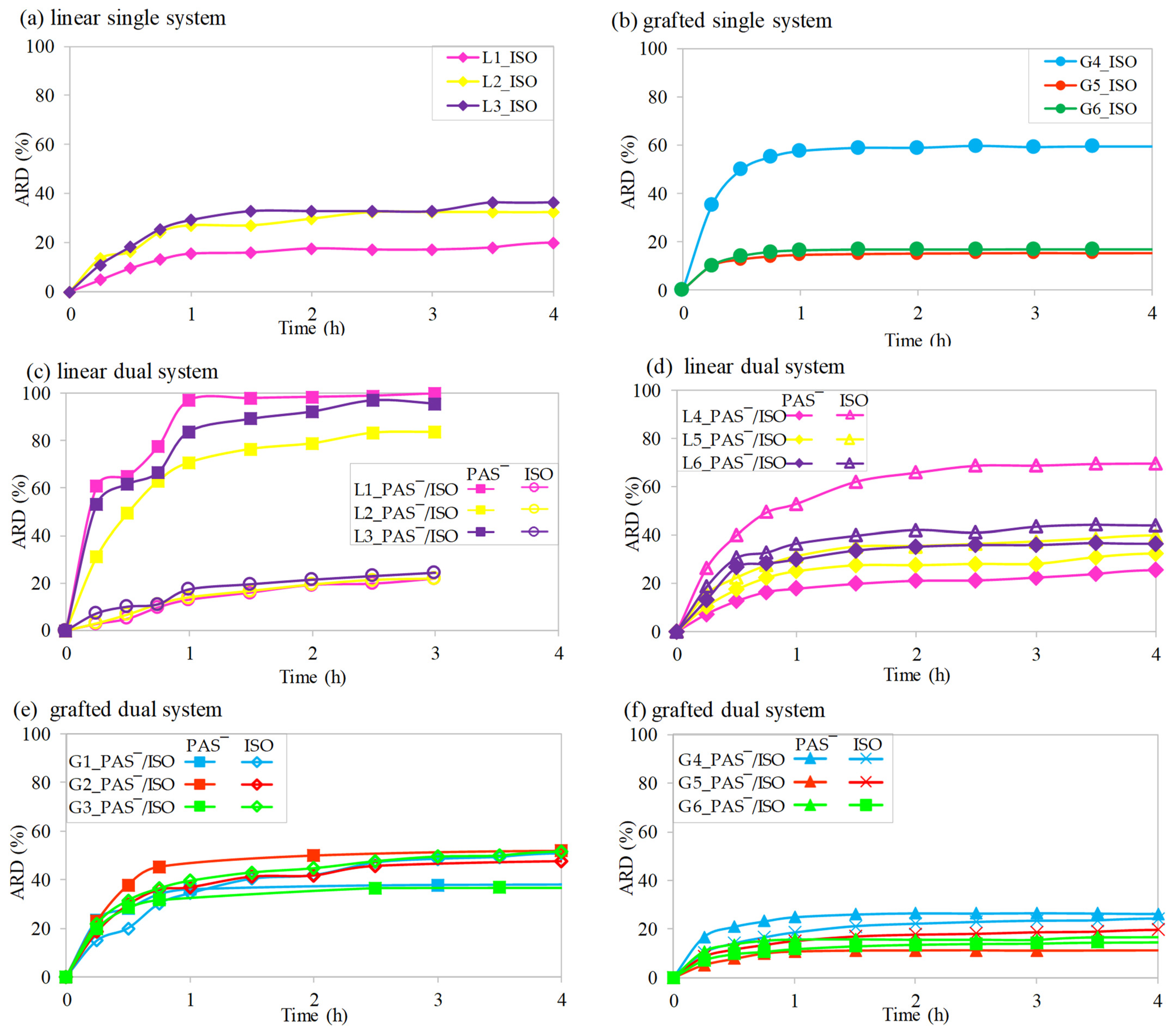
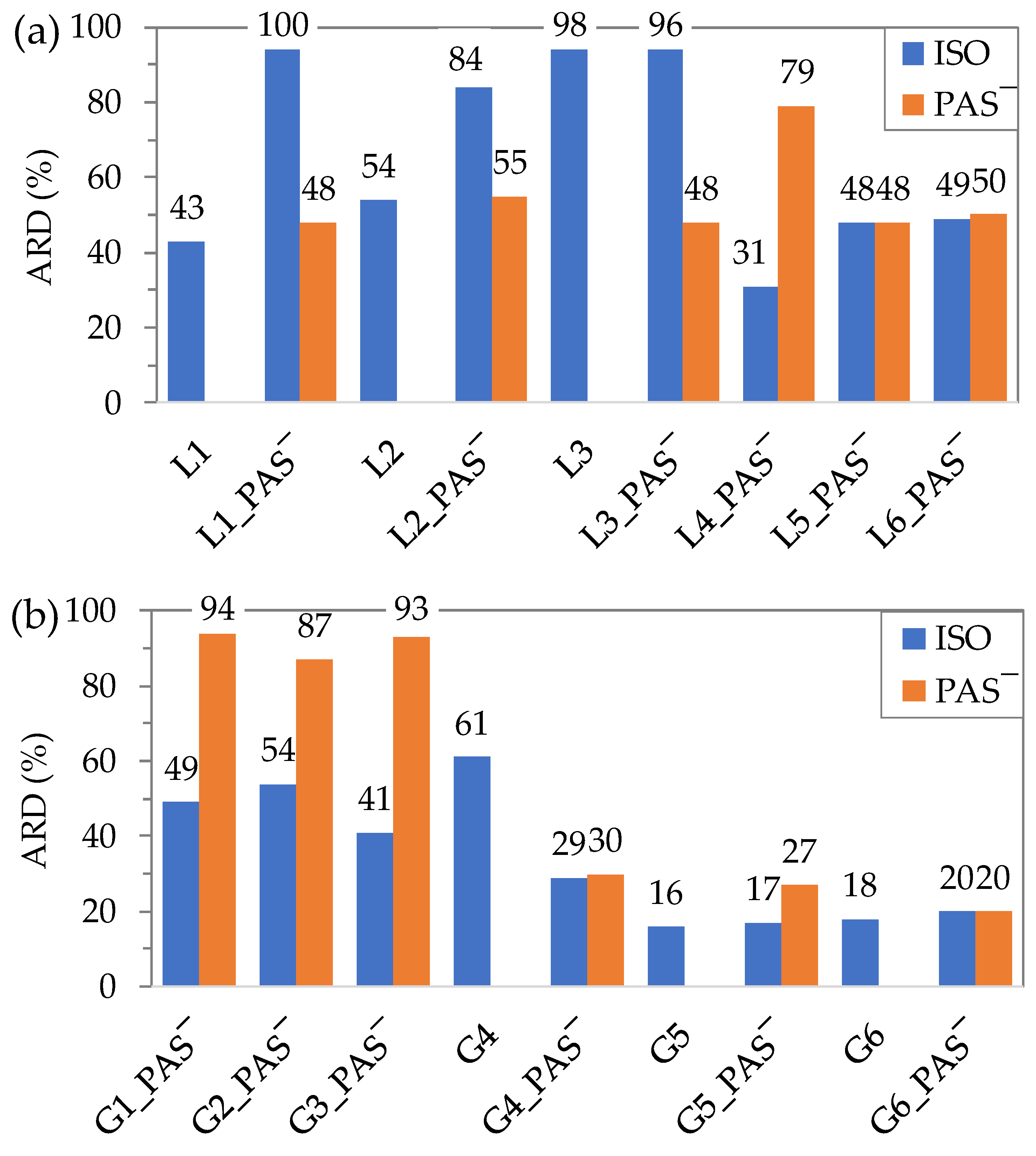
2.5. In Vitro Drug Release Studies
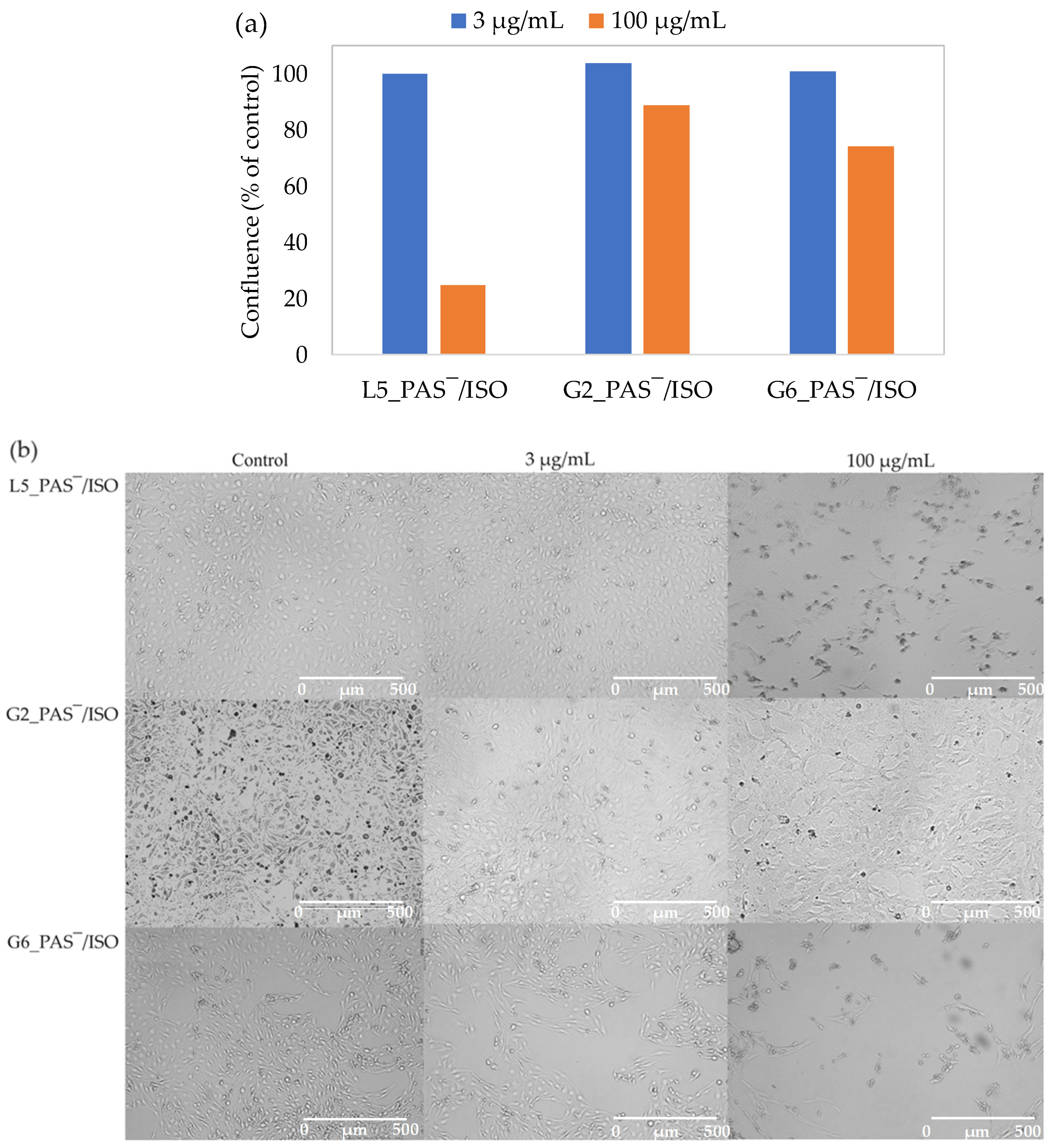
2.6. In Vitro Cytotoxicity Assay
3. Materials and Methods
3.1. Encapsulation of ISO
3.2. In Vitro Drug Release Studies
3.3. Cell Growth and Cytotoxicity Assay
3.4. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Girija, A.R. 12—Medical Applications of Polymer/Functionalized Nanoparticle Systems. In Polymer Composites with Functionalized Nanoparticles; Pielichowski, K., Majka, T.M., Eds.; Elsevier: Amesterdam, The Netherlands, 2019; pp. 381–404. [Google Scholar]
- Demetzos, C.; Pippa, N. Advanced drug delivery nanosystems (aDDnSs): A mini-review. Drug Deliv. 2014, 21, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Din, F.; Aman, W.; Ullah, I.; Qureshi, O.; Mustamoha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef] [PubMed]
- Cukierman, E.; Khan, D. The benefits and challenges associated with the use of drug delivery systems in cancer therapy. Biochem. Pharmacol. 2010, 80, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Allen, T. Drug Delivery Systems: Entering the Mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef]
- Ojewole, E.; Macraj, I.; Naidoo, P.; Govender, T. Exploring the use of novel drug delivery systems for antiretroviral drugs. Eur. J. Pharm. Biopharm. 2008, 70, 697–710. [Google Scholar] [CrossRef] [PubMed]
- Buddolla, A.L.; Kim, S. Recent insights into the development of nucleic acid-based nanoparticles for tumor-targeted drug delivery. Colloids Surf. B 2018, 172, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.; Ma, L.; Zhang, B.; Zhang, Y.; Li, X.; Wang, T.; Zhang, W.; Li, Y.; Sang, S. Construction and application of targeted drug delivery system based on hyaluronic acid and heparin functionalised carbon dots. Colloids Surf. B 2020, 188, 110768. [Google Scholar] [CrossRef]
- Chacko, I.A.; Ghate, V.M.; Dsouza, L.; Lewis, S.A. Lipid vesicles: A versatile drug delivery platform for dermal and transdermal applications. Colloids Surf. B 2020, 195, 111262. [Google Scholar] [CrossRef]
- Singh, S.; Pandey, V.; Tewari, R.; Agarwal, V. Nanoparticle based drug delivery system: Advantages and applications. Indian J. Sci. Technol. 2011, 4, 177–180. [Google Scholar] [CrossRef]
- Gelperina, S.; Kisich, K.; Iseman, M.; Heifets, L. The potential Advantages of Nanoparticle Drug Delivery Systems in Chemotherapy of Tuberculosis. Am. J. Respir. Crit. Care Med. 2005, 172, 1487–1490. [Google Scholar] [CrossRef]
- Yokoyama, M. Drug targeting with nano-sized carrier systems. J. Artif. Organs 2005, 8, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Couvreur, P.; Puisieux, F. Nano- and microparticles for the delivery of polypeptides and proteins. Adv. Drug Deliv. Rev. 1993, 10, 141–162. [Google Scholar] [CrossRef]
- Ahmad, Z.; Shah, A.; Siddiq, M.; Kraatz, H.B. Polymeric micelles as drug delivery vehicles. RSC Adv. 2014, 4, 17028–17038. [Google Scholar] [CrossRef]
- García, M.C. 13-Stimuli-responsive polymersomes for drug delivery applications. In Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications; Makhlouf, A.S., Abu-Thabit, N.Y., Eds.; Woodhead Publishing: Sawston, UK, 2019; Volume 2, pp. 345–392. [Google Scholar]
- Torchilin, V. Structure and design of polymeric surfactant-based drug delivery systems. J. Control. Release 2001, 73, 137–172. [Google Scholar] [CrossRef] [PubMed]
- Atanase, L.I. Micellar Drug Delivery Systems Based on Natural Biopolymers. Polymers 2021, 13, 477. [Google Scholar] [CrossRef] [PubMed]
- Kulthe, S.; Choudhari, M.; Inamdar, N.; Mourya, V. Polymeric micelles: Authoritative aspects for drug delivery. Des. Monomers Polym. 2012, 15, 465–521. [Google Scholar] [CrossRef]
- Yokoyama, M. Polymeric micelles as drug carriers: Their lights and shadows. J. Drug Target. 2014, 22, 576–583. [Google Scholar] [CrossRef]
- Haag, R. Supramolecular Drug-Delivery Systems Based on Polymeric Core–Shell Architectures. Angew. Chem. Int. Ed. 2004, 43, 278–282. [Google Scholar] [CrossRef]
- Figueiras, A.; Domingues, C.; Jarak, I.; Santos, A.; Parra, A.; Pais, A.; Alvarez-Lorenzo, C.; Concheiro, A.; Kabanov, A.; Cabral, H.; et al. New Advances in Biomedical Application of Polymeric Micelles. Pharmecautics 2022, 14, 1700. [Google Scholar] [CrossRef]
- Aliabadi, H.M.; Lavasanifar, A. Polymeric micelles for drug delivery. Expert Opin. Drug Deliv. 2005, 3, 139–162. [Google Scholar] [CrossRef]
- Ke, X.; Ng, V.; Ono, R.; Chan, J.; Krishnamurthy, S.; Wang, Y.; Hedrick, J.; Yang, Y. Role of non-covalent and covalent interactions in cargo loading capacity and stability of polymeric micelles. J. Control. Relelase 2014, 193, 9–26. [Google Scholar] [CrossRef] [PubMed]
- De, R.; Mahata, M.K.; Kim, K.T. Structure-Based Varieties of Polymeric Nanocarriers and Influences of Their Physicochemical Properties on Drug Delivery Profiles. Adv. Sci. 2022, 9, 2105373. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; He, C.; Tian, H.; Ding, J.; Hsiao, B.S.; Chu, B.; Chem, X. Polymeric nanostructured materials for biomedical applications. Prog. Polym. Sci. 2016, 60, 86–128. [Google Scholar] [CrossRef]
- Ghosh, B.; Biswas, S. Polymeric micelles in cancer therapy: State of the art. J. Control. Release 2021, 332, 127–147. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, A.M.; Torchilin, V.P. Multifunctional polymeric micelles for delivery of drugs and siRNA. Front. Pharmacol. 2014, 5, 77. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Qin, L.; Mu, T.; Xue, Z.; Gao, G. Are Ionic Liquids Chemically Stable? Chem. Rev. 2017, 117, 7113–7131. [Google Scholar] [CrossRef] [PubMed]
- Mallakpour, S.; Dinari, M. Ionic Liquids as Green Solvents: Progress and Prospects. In Green Solvents II: Properties and Applications of Ionic Liquids; Mohammad, A., Inamuddin, Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 1–32. [Google Scholar]
- Zhao, H. Methods for stabilizing and activating enzymes in ionic liquids—A review. J. Chem. Technol. Biotechnol. 2010, 7, 891–907. [Google Scholar] [CrossRef]
- Sidat, Z.; Marimuthu, T.; Kumar, P.; du Toit, L.C.; Kondiah, P.P.D.; Choonara, Y.E.; Pillay, V. Ionic Liquids as Potential and Synergistic Permeation Enhancers for Transdermal Drug Delivery. Pharmaceutics 2019, 11, 96. [Google Scholar] [CrossRef]
- Vijayakrishna, K.; Mecerreyes, D.; Gnanou, Y.; Taton, D. Polymeric Vesicles and Micelles Obtained by Self-Assembly of Ionic Liquid-Based Block Copolymers Triggered by Anion or Solvent Exchange. Macromolecules 2009, 42, 5167–5174. [Google Scholar] [CrossRef]
- Adawiyah, N.; Moniruzzaman, M.; Hawatulaila, S.; Goto, M. Ionic liquids as a potential tool for drug delivery systems. Med. Chem. Commun. 2016, 7, 1881–1897. [Google Scholar] [CrossRef]
- Marsh, K.N.; Boxall, J.A.; Lichtenthaler, R. Room temperature ionic liquids and their mixtures—A review. Fluid Phase Equilibria 2004, 219, 93–98. [Google Scholar] [CrossRef]
- Earle, M.J.; Seddon, K.R. Ionic liquids. Green solvents for the future. Pure Appl. Chem. 2000, 72, 1391–1398. [Google Scholar] [CrossRef]
- Antonietti, M.; Kuang, D.; Smarsly, B.; Zhou, Y. Ionic Liquids for the Convenient Synthesis of Functional Nanoparticles and Other Inorganic Nanostructures. Angew. Chem. Int. Ed. 2004, 43, 4988–4992. [Google Scholar] [CrossRef] [PubMed]
- Kubisa, P. Application of Ionic Liquids as Solvents for Polymerization Processes. Prog. Polym. Sci. 2004, 29, 3–12. [Google Scholar] [CrossRef]
- Ueki, T.; Watanabe, M. Macromolecules in Ionic Liquids: Progress, Challenges, and Opportunities. Macromolecules 2008, 41, 3739–3749. [Google Scholar] [CrossRef]
- Lu, J.; Yan, F.; Texter, J. Advanced applications of ionic liquids in polymer science. Prog. Polym. Sci. 2009, 34, 431–448. [Google Scholar] [CrossRef]
- Halayqa, M.; Zawadzki, M.; Domańska, U.; Plichta, A. Polymer–Ionic liquid–Pharmaceutical conjugates as drug delivery systems. J. Mol. Struct. 2019, 1180, 573–584. [Google Scholar] [CrossRef]
- Banerjee, P.; Anas, M.; Jana, S.; Mandal, T. Recent developments in stimuli-responsive poly(ionic liquid)s. J. Polym. Res. 2020, 27, 177. [Google Scholar] [CrossRef]
- He, Y.; Li, Z.; Simone, P.; Lodge, T.P. Self-Assembly of Block Copolymer Micelles in an Ionic Liquid. J. Am. Chem. Soc. 2006, 128, 2745–2750. [Google Scholar] [CrossRef]
- Tao, D.J.; Cheng, Z.; Chen, F.F.; Li, Z.M.; Hu, N.; Chen, X.S. Synthesis and Thermophysical Properties of Biocompatible Cholinium-Based Amino Acid Ionic Liquids. J. Chem. Eng. Data 2013, 58, 1542–1548. [Google Scholar] [CrossRef]
- Muhammad, N.; Hossain, M.I.; Man, Z.; El-Harbawi, M.; Bustam, M.A.; Noaman, Y.A.; Alitheen, N.; Ng, M.; Hefter, G.; Yin, C.-Y. Synthesis and Physical Properties of Choline Carboxylate Ionic Liquids. J. Chem. Eng. Data 2012, 57, 2191–2196. [Google Scholar] [CrossRef]
- Gouveia, W.; Jorge, T.F.; Martins, S.; Meireles, M.; Carolino, M.; Cruz, C.; Almeida, T.; Araujo, M. Toxicity of ionic liquids prepared from biomaterials. Chemosphere 2014, 104, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.M.; Silva, S.S.; Reis, R.L. Biocompatible ionic liquids: Fundamental behaviours and applications. Chem. Soc. Rev. 2019, 48, 4317–4335. [Google Scholar] [CrossRef]
- Li, X.; Ma, N.; Zhang, L.; Ling, G.; Zhang, P. Applications of choline-based ionic liquids in drug delivery. Int. J. Pharm. 2022, 612, 121366. [Google Scholar] [CrossRef] [PubMed]
- Bielas, R.; Siewniak, A.; Skonieczna, M.; Adamiec, M.; Mielańczyk, Ł.; Neugebauer, D. Choline based polymethacrylate matrix with pharmaceutical cations as co-delivery system for antibacterial and anti-inflammatory combined therapy. J. Mol. Liq. 2019, 285, 114–122. [Google Scholar] [CrossRef]
- Niesyto, K.; Mazur, A.; Neugebauer, D. Dual-Drug Delivery via the Self-Assembled Conjugates of Choline-Functionalized Graft Copolymers. Materials 2022, 15, 4457. [Google Scholar] [CrossRef] [PubMed]
- Niesyto, K.; Neugebauer, D. Linear Copolymers Based on Choline Ionic Liquid Carrying Anti-Tuberculosis Drugs: Influence of Anion Type on Physicochemical Properties and Drug Release. Int. J. Mol. Sci. 2021, 22, 284. [Google Scholar] [CrossRef] [PubMed]
- Niesyto, K.; Neugebauer, D. Synthesis and Characterization of Ionic Graft Copolymers: Introduction and In Vitro Release of Antibacterial Drug by Anion Exchange. Polymers 2020, 12, 2159. [Google Scholar] [CrossRef]
- Mazur, A.; Niesyto, K.; Neugebauer, D. Pharmaceutical Functionalization of Monomeric Ionic Liquid for the Preparation of Ionic Graft Polymer Conjugates. Int. J. Mol. Sci. 2022, 23, 14731. [Google Scholar] [CrossRef]
- Bielas, R.; Mielańczyk, A.; Skonieczna, M.; Mielańczyk, Ł.; Neugebauer, D. Choline supported poly(ionic liquid) graft copolymers as novel delivery systems of anionic pharmaceuticals for anti-inflammatory and anti-coagulant therapy. Sci. Rep. 2019, 9, 14410. [Google Scholar] [CrossRef]
- Keihankhadiv, S.; Neugebauer, D. Synthesis and Characterization of Linear Copolymers Based on Pharmaceutically Functionalized Monomeric Choline Ionic Liquid for Delivery of p-Aminosalicylate. Pharmaceutics 2023, 15, 860. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.K.; Moshikur, R.M.; Wakabayashi, R.; Moniruzzaman, M.; Goto, M. Biocompatible Ionic Liquid-Mediated Micelles for Enhanced Transdermal Delivery of Paclitaxel. ACS Appl. Mater. Interfaces 2021, 13, 19745–19755. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.; Sharma, R.; Mahajan, R.K. An Investigation of Drug Binding Ability of a Surface Active Ionic Liquid: Micellization, Electrochemical, and Spectroscopic Studies. Langmuir 2012, 28, 17238–17246. [Google Scholar] [CrossRef] [PubMed]
- Ghatak, C.; Rao, V.G.; Mandal, S.; Ghosh, S.; Sarkar, N. An Understanding of the Modulation of Photophysical Properties of Curcumin inside a Micelle Formed by an Ionic Liquid: A New Possibility of Tunable Drug Delivery System. J. Phys. Chem. B 2012, 116, 3369–3379. [Google Scholar] [CrossRef] [PubMed]
- Kurnik, I.S.; D’Angelo, N.A.; Mazzola, P.G.; Chorilli, M.; Kamei, D.T.; Pereira, J.F.; Vicente, A.A.; Lopes, A.M. Polymeric micelles using cholinium-based ionic liquids for the encapsulation and release of hydrophobic drug molecules. Biomater. Sci. 2021, 9, 2183–2196. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Li, Y.; Wang, Z.; Wang, B.; Pan, X.; Zhao, W.; Ma, X.; Zhang, J. A dual responsive hyaluronic acid graft poly(ionic liquid) block copolymer micelle for an efficient CD44-targeted antitumor drug delivery. New J. Chem. 2019, 43, 12275–12282. [Google Scholar] [CrossRef]
- Lu, B.; Zhou, G.; Xiao, F.; He, Q.; Zhang, J. Stimuli-responsive poly(ionic liquid) nanoparticles for controlled drug delivery. J. Mater. Chem. B 2020, 8, 7994–8001. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Tahara, Y.; Tamura, M.; Kamiya, N.; Goto, M. Ionic liquid-assisted transdermal delivery of sparingly soluble drugs. Chem. Comm. 2010, 46, 1452–1454. [Google Scholar] [CrossRef]
- Sawdon, A.J.; Peng, C.A. Polymeric micelles for acyclovir drug delivery. Colloids Surf. B 2014, 122, 738–745. [Google Scholar] [CrossRef]
- Keihankhadiv, S.; Neugebauer, D. Self-Assembling Polymers with p-Aminosalicylate Anions Supported by Encapsulation of p-Aminosalicylate for the Improvement of Drug Content and Release Efficiency. Pharmaceuticals 2023, 16, 1502. [Google Scholar] [CrossRef]
- Mazur, A.; Neugebauer, D. Characterization of Graft Copolymers Synthesized from p-Aminosalicylate Functionalized Monomeric Choline Ionic Liquid. Pharmaceutics 2023, 15, 2556. [Google Scholar] [CrossRef] [PubMed]
- Soedarsono, S.; Jayanti, R.P.; Mertaniasih, N.M.; Kusmiati, T.; Permatasari, A.; Indrawanto, D.W.; Charisma, A.N.; Yuliwulandari, R.; Long, N.P.; Choi, Y.K.; et al. Development of population pharmacokinetics model of isoniazid in Indonesian patients with tuberculosis. Int. J. Infect. Dis. 2022, 117, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.; Schnoes, H.K.; Armstrong, E.L.; Boyle, R.W. Site of inhibitory action of isoniazid in the synthesis of mycolic acids in Mycobacterium tuberculosis. J. Lipid Res. 1975, 16, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Jindani, A.; Aber, V.R.; Edwards, E.A.; Mitchison, D.A. The Early Bactericidal Activity of Drugs in Patients with Pulmonary Tuberculosis. Am. Rev. Respir. Dis. 1980, 121, 939–949. [Google Scholar] [PubMed]
- McIlleron, H.; Wash, P.; Burger, A.; Norman, J.; Folb, P.I.; Smith, P. Determinants of Rifampin, Isoniazid, Pyrazinamide, and Ethambutol Pharmacokinetics in a Cohort of Tuberculosis Patients. Antimicrob. Agents Chemother. 2006, 50, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Kinzig-Schippers, M.; Tomalik-Scharte, D.; Jetter, A.; Scheidel, B.; Jakob, V.; Rodamer, M.; Cascorbi, I.; Doroshyenko, O.; Sorgel, F.; Fuhr, U. Should We Use N-Acetyltransferase Type 2 Genotyping To Personalize Isoniazid Doses? Antimicrob. Agents Chemother. 2005, 49, 1733–1738. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Jiang, G.; Wen, S.; Huo, F.; Wang, F.; Huang, H.; Pang, Y. Para-aminosalicylic acid increases the susceptibility to isoniazid in clinical isolates of Mycobacterium tuberculosis. Infect. Drug Resist. 2019, 12, 825–829. [Google Scholar] [CrossRef]
- Jennne, J.W. Partial purification and properties of the isoniazid transacetylase in human liver. Its relationship to the acetylation of p-aminosalicylic acid. J. Clin. Investig. 1965, 44, 1992–2002. [Google Scholar] [CrossRef]
- Niesyto, K.; Łyżniak, W.; Skonieczna, M.; Neugebauer, D. Biological In Vitro Evaluation of PIL Graft Conjugates: Cytotoxicity Characteristics. Int. J. Mol. Sci. 2021, 22, 7741. [Google Scholar] [CrossRef]
- Niesyto, K.; Skonieczna, M.; Adamiec-Organiściok, M.; Neugebauer, D. Toxicity evaluation of choline ionic liquid-based nanocarriers of pharmaceutical agents for lung treatment. J. Biomed. Mater. Res. Part B Appl. Biomater. 2023, 7, 1374–1385. [Google Scholar] [CrossRef]

| No. | DPTMAMA | DPn | FTMAMA (mol.%) | Mn (g/mol) | Ð | DC (%) |
|---|---|---|---|---|---|---|
| L1 | 71 | 390 | 18 | 46,700 | 1.12 | - |
| L1_PAS‾ | 37 | |||||
| L2 | 52 | 203 | 26 | 26,900 | 1.26 | - |
| L2_PAS‾ | 34 | |||||
| L3 | 224 | 497 | 45 | 73,800 | 1.96 | - |
| L3_PAS‾ | 51 | |||||
| L4_PAS‾ | 68 | 272 | 25 | 42,500 | 1.29 | 24 |
| L5_PAS‾ | 139 | 190 | 74 | 50,300 | 1.33 | 42 |
| L6_PAS‾ | 261 | 279 | 93 | 86,500 | 1.5500 | 47 |
| No. | nsc | DG (%) | DPsc | DPTMAMA | FTMAMA (mol.%) | Mn (g/mol) | Ð | DC (%) |
|---|---|---|---|---|---|---|---|---|
| G1_PAS‾ | 99 | 48 | 89 | 35 | 40 | 841,900 | 1.40 | 43 |
| G2_PAS‾ | 65 | 18 | 35 | 20 | 57 | 431,300 | 1.36 | 35 |
| G3_PAS‾ | 75 | 55 | 73 | 405,000 | 1.46 | 39 | ||
| G4 | 48 | 26 | 35 | 15 | 39 | 273,100 | 1.15 | - |
| G4_PAS‾ | 32 | |||||||
| G5 | 133 | 46 | 28 | 11 | 36 | 583,500 | 1.03 | - |
| G5_PAS‾ | 36 | |||||||
| G6 | 65 | 12 | 18 | 1,090,500 | 1.11 | - | ||
| G6_PAS‾ | 37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niesyto, K.; Keihankhadiv, S.; Mazur, A.; Mielańczyk, A.; Neugebauer, D. Ionic Liquid-Based Polymer Matrices for Single and Dual Drug Delivery: Impact of Structural Topology on Characteristics and In Vitro Delivery Efficiency. Int. J. Mol. Sci. 2024, 25, 1292. https://doi.org/10.3390/ijms25021292
Niesyto K, Keihankhadiv S, Mazur A, Mielańczyk A, Neugebauer D. Ionic Liquid-Based Polymer Matrices for Single and Dual Drug Delivery: Impact of Structural Topology on Characteristics and In Vitro Delivery Efficiency. International Journal of Molecular Sciences. 2024; 25(2):1292. https://doi.org/10.3390/ijms25021292
Chicago/Turabian StyleNiesyto, Katarzyna, Shadi Keihankhadiv, Aleksy Mazur, Anna Mielańczyk, and Dorota Neugebauer. 2024. "Ionic Liquid-Based Polymer Matrices for Single and Dual Drug Delivery: Impact of Structural Topology on Characteristics and In Vitro Delivery Efficiency" International Journal of Molecular Sciences 25, no. 2: 1292. https://doi.org/10.3390/ijms25021292
APA StyleNiesyto, K., Keihankhadiv, S., Mazur, A., Mielańczyk, A., & Neugebauer, D. (2024). Ionic Liquid-Based Polymer Matrices for Single and Dual Drug Delivery: Impact of Structural Topology on Characteristics and In Vitro Delivery Efficiency. International Journal of Molecular Sciences, 25(2), 1292. https://doi.org/10.3390/ijms25021292







