Apolar Extracts of St. John’s Wort Alleviate the Effects of β-Amyloid Toxicity in Early Alzheimer’s Disease
Abstract
:1. Introduction
2. Results
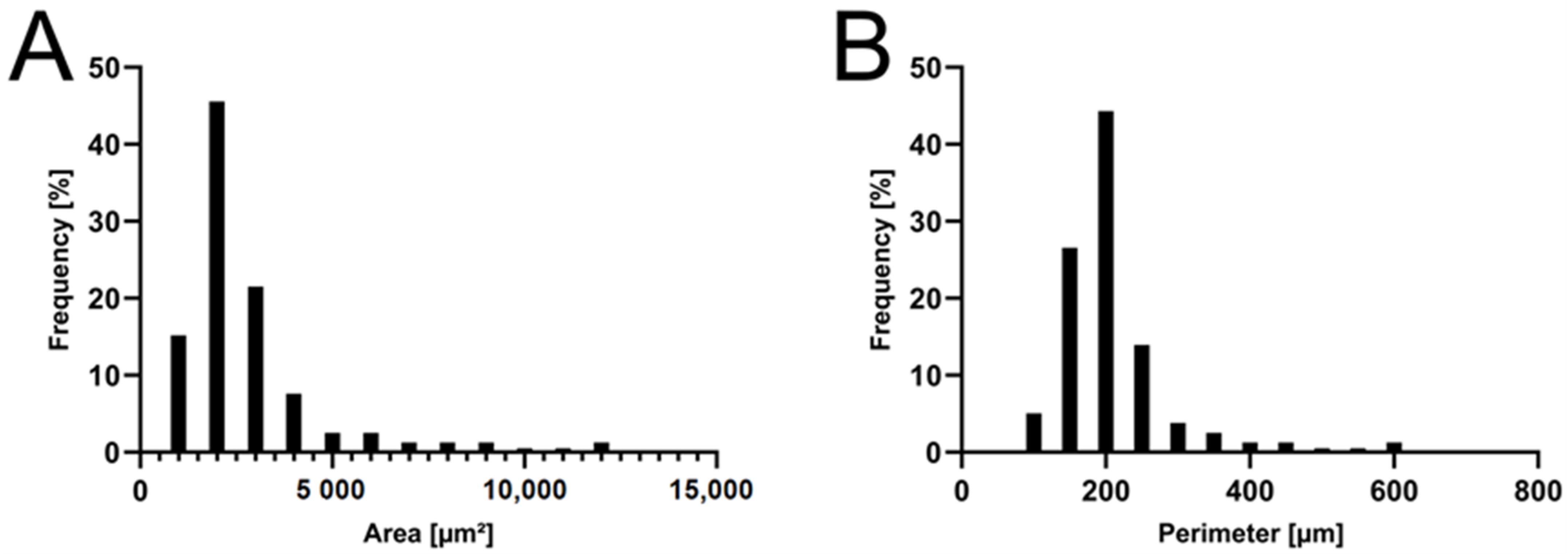
2.1. Formulation and Characterization of the APOL Extract
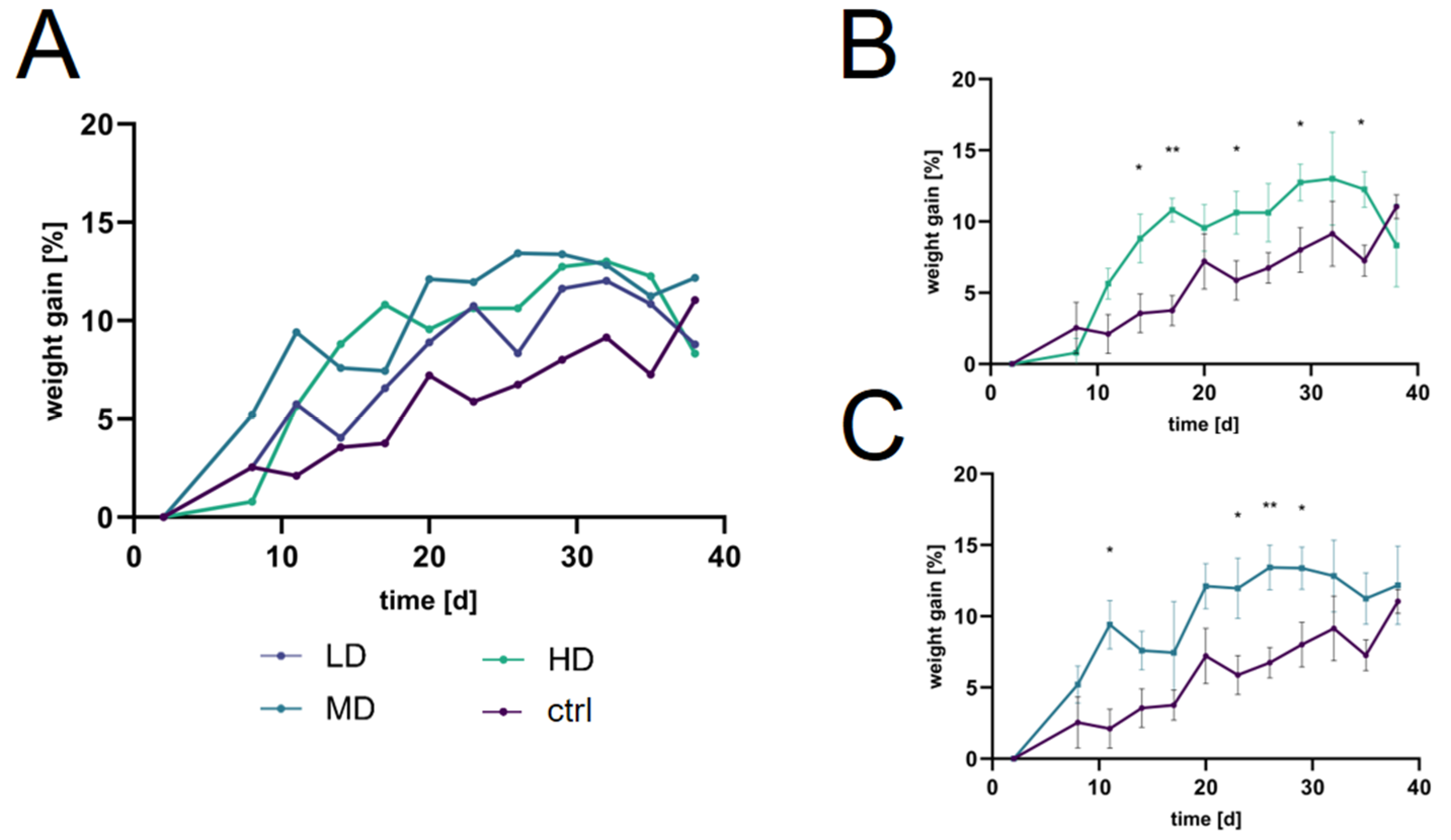
2.2. Toxicity Observations and Treatment Adjustments
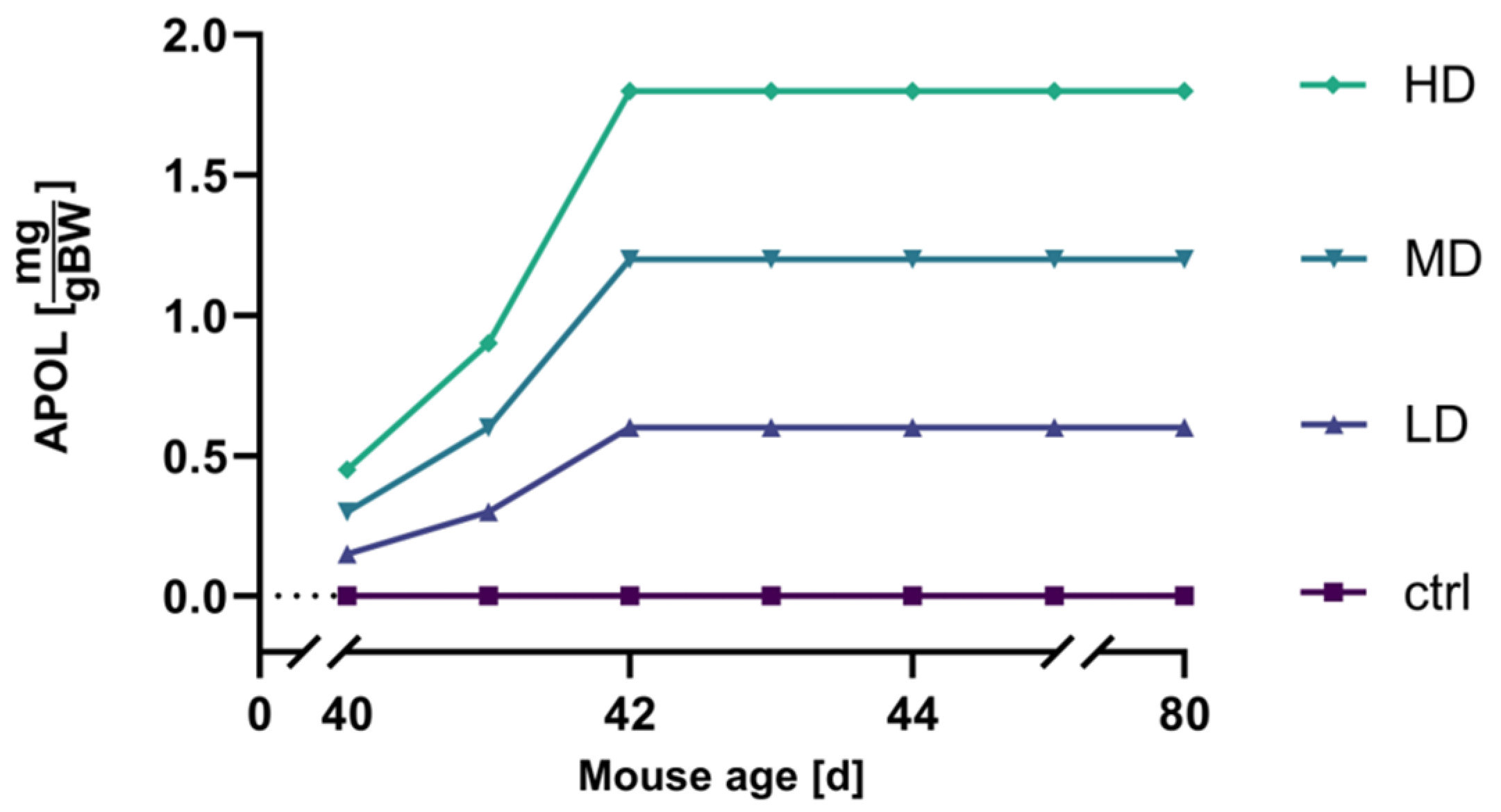
2.3. Dosage strategy and Optimization
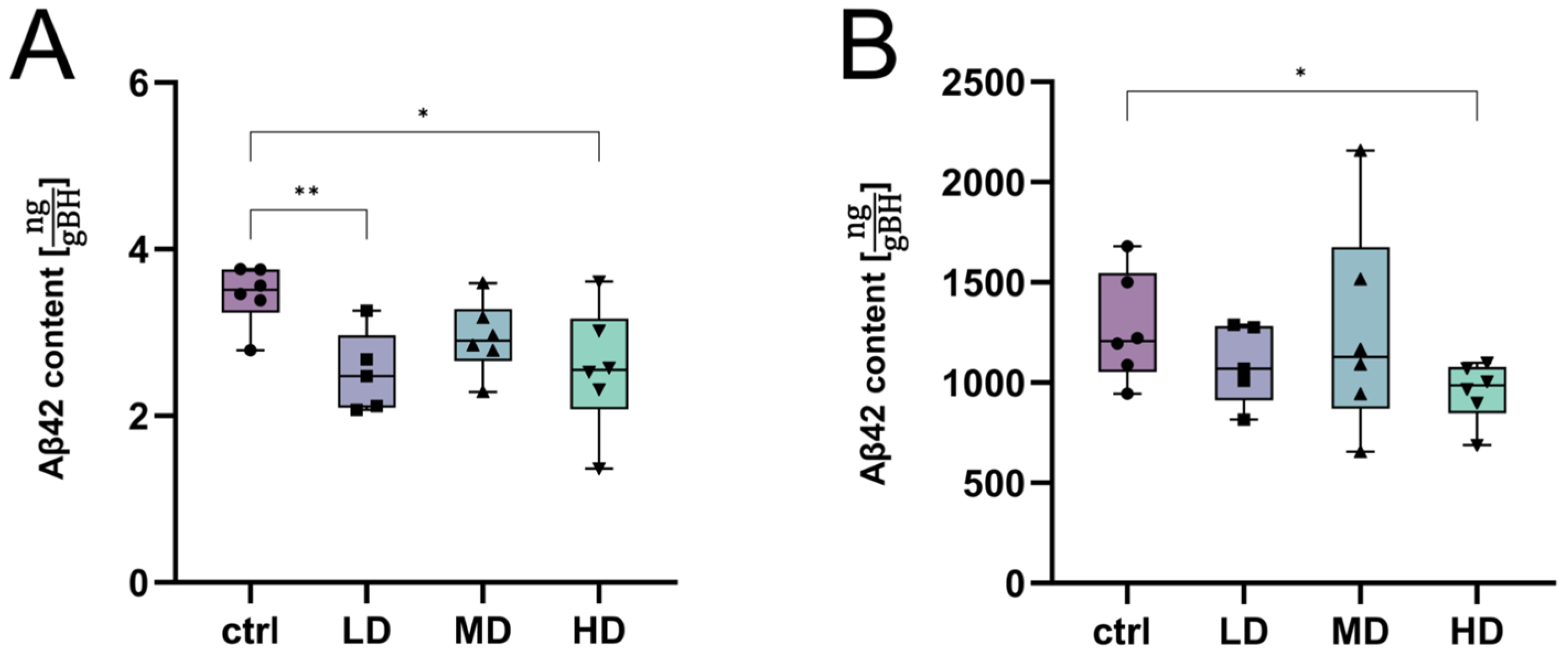
2.4. Assessment of the Treatment Effects of H. perforatum Extracts
2.4.1. Determination of Most Efficient Extract Fraction
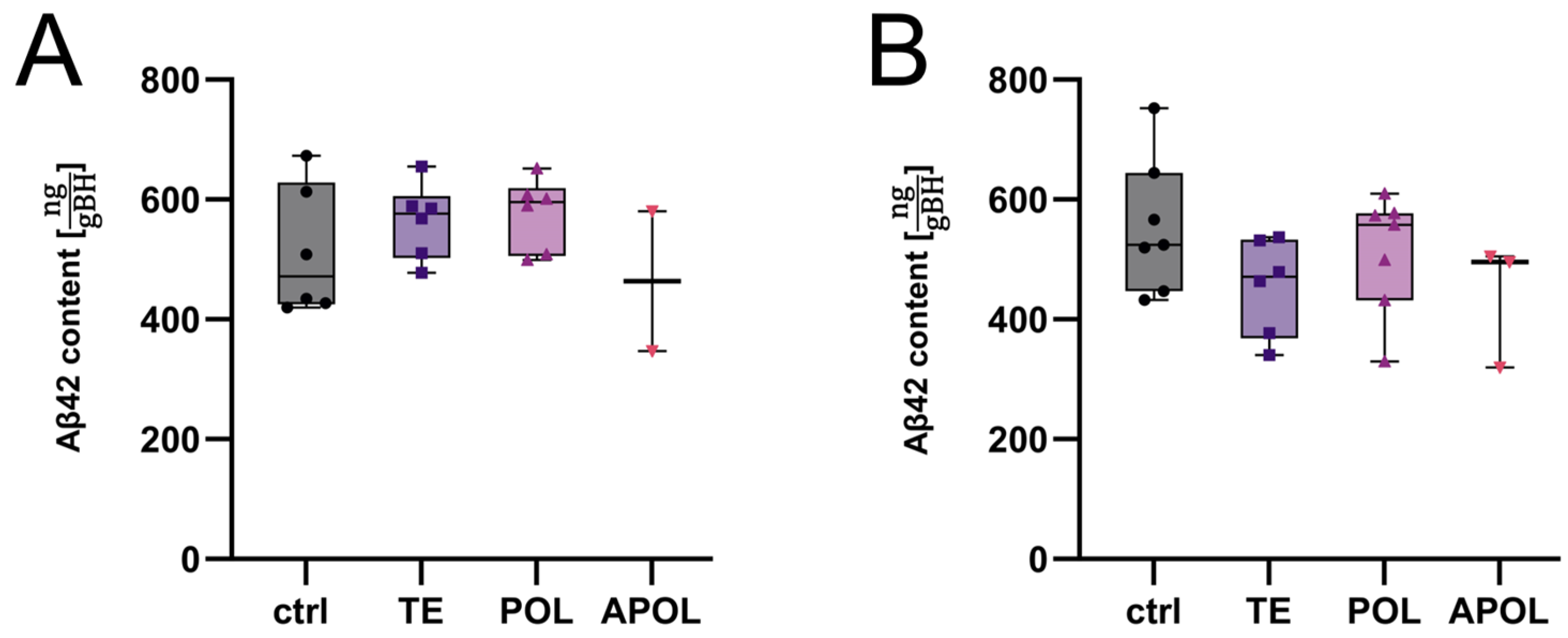
2.4.2. Further Exploration of the APOL Extract
2.4.3. Characterization of Major Extract Metabolites via NMR Spectroscopy
3. Discussion
4. Materials and Methods
4.1. Animal Models and Breeding Scheme
4.2. Production of Plant Material
4.3. The Extraction and Formulation of H. perforatum
4.4. Treatment Schemes
4.4.1. Treatment Scheme to Test Hypericum Perforatum Extracts (TE, POL, and APOL)
4.4.2. Dosage Titration of the APOL Extract
4.4.3. Early Treatment Paradigm to Assess Efficacy of the APOL Extract Fraction
4.5. Tissue Harvesting
4.6. Protein Extraction
4.7. Quantification of Aβ42
4.8. NMR Spectroscopy of Extracts
4.9. Statictics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Bature, F.; Guinn, B.A.; Pang, D.; Pappas, Y. Signs and symptoms preceding the diagnosis of Alzheimer’s disease: A systematic scoping review of literature from 1937 to 2016. BMJ Open 2017, 7, e015746. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.M., 3rd; Cookson, M.R.; Van Den Bosch, L.; Zetterberg, H.; Holtzman, D.M.; Dewachter, I. Hallmarks of neurodegenerative diseases. Cell 2023, 186, 693–714. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.X.; Tian, Y.; Wang, Z.T.; Ma, Y.H.; Tan, L.; Yu, J.T. The Epidemiology of Alzheimer’s Disease Modifiable Risk Factors and Prevention. J. Prev. Alzheimers Dis. 2021, 8, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Prvulovic, D.; Teipel, S.; Jessen, F.; Luckhaus, C.; Frolich, L.; Riepe, M.W.; Dodel, R.; Leyhe, T.; Bertram, L.; et al. The future of Alzheimer’s disease: The next 10 years. Prog. Neurobiol. 2011, 95, 718–728. [Google Scholar] [CrossRef] [PubMed]
- Aisen, P.S.; Vellas, B.; Hampel, H. Moving towards early clinical trials for amyloid-targeted therapy in Alzheimer’s disease. Nat. Rev. Drug Discov. 2013, 12, 324. [Google Scholar] [CrossRef] [PubMed]
- Masters, C.L.; Simms, G.; Weinman, N.A.; Multhaup, G.; McDonald, B.L.; Beyreuther, K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc. Natl. Acad. Sci. USA 1985, 82, 4245–4249. [Google Scholar] [CrossRef]
- Iwatsubo, T.; Irizarry, M.C.; Lewcock, J.W.; Carrillo, M.C. Alzheimer’s Targeted Treatments: Focus on Amyloid and Inflammation. J. Neurosci. 2023, 43, 7894–7898. [Google Scholar] [CrossRef]
- Reiss, A.B.; Arain, H.A.; Stecker, M.M.; Siegart, N.M.; Kasselman, L.J. Amyloid toxicity in Alzheimer’s disease. Rev. Neurosci. 2018, 29, 613–627. [Google Scholar] [CrossRef]
- Pahnke, J.; Wolkenhauer, O.; Krohn, M.; Walker, L.C. Clinico-pathologic function of cerebral ABC transporters-implications for the pathogenesis of Alzheimer’s disease. Curr. Alzheimer Res. 2008, 5, 396–405. [Google Scholar] [CrossRef]
- Murphy, M.P.; LeVine, H., 3rd. Alzheimer’s disease and the amyloid-beta peptide. J. Alzheimers Dis. 2010, 19, 311–323. [Google Scholar] [CrossRef]
- Cummings, J.; Apostolova, L.; Rabinovici, G.D.; Atri, A.; Aisen, P.; Greenberg, S.; Hendrix, S.; Selkoe, D.; Weiner, M.; Petersen, R.C.; et al. Lecanemab: Appropriate Use Recommendations. J. Prev. Alzheimers Dis. 2023, 10, 362–377. [Google Scholar] [CrossRef]
- Budd Haeberlein, S.; O’Gorman, J.; Chiao, P.; Bussiere, T.; von Rosenstiel, P.; Tian, Y.; Zhu, Y.; von Hehn, C.; Gheuens, S.; Skordos, L.; et al. Clinical Development of Aducanumab, an Anti-Abeta Human Monoclonal Antibody Being Investigated for the Treatment of Early Alzheimer’s Disease. J. Prev. Alzheimers Dis. 2017, 4, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Sun, F. Aducanumab: The first targeted Alzheimer’s therapy. Drug Discov. Ther. 2021, 15, 166–168. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-Beta Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef] [PubMed]
- De-Paula, V.J.; Radanovic, M.; Diniz, B.S.; Forlenza, O.V. Alzheimer’s disease. Subcell. Biochem. 2012, 65, 329–352. [Google Scholar] [CrossRef] [PubMed]
- Veitch, D.P.; Weiner, M.W.; Aisen, P.S.; Beckett, L.A.; Cairns, N.J.; Green, R.C.; Harvey, D.; Jack, C.R., Jr.; Jagust, W.; Morris, J.C.; et al. Understanding disease progression and improving Alzheimer’s disease clinical trials: Recent highlights from the Alzheimer’s Disease Neuroimaging Initiative. Alzheimers Dement. 2019, 15, 106–152. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, L.; Wang, H.F.; Liu, Y.; Hao, X.K.; Tan, C.C.; Jiang, T.; Liu, B.; Zhang, D.Q.; Yu, J.T.; et al. Multiple Effect of APOE Genotype on Clinical and Neuroimaging Biomarkers Across Alzheimer’s Disease Spectrum. Mol. Neurobiol. 2016, 53, 4539–4547. [Google Scholar] [CrossRef]
- Miculas, D.C.; Negru, P.A.; Bungau, S.G.; Behl, T.; Hassan, S.S.U.; Tit, D.M. Pharmacotherapy Evolution in Alzheimer’s Disease: Current Framework and Relevant Directions. Cells 2022, 12, 131. [Google Scholar] [CrossRef]
- Shi, M.; Chu, F.; Zhu, F.; Zhu, J. Impact of Anti-Amyloid-Beta Monoclonal Antibodies on the Pathology and Clinical Profile of Alzheimer’s Disease: A Focus on Aducanumab and Lecanemab. Front. Aging Neurosci. 2022, 14, 870517. [Google Scholar] [CrossRef]
- van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. New Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Suh, C.H.; Shim, W.H.; Lim, J.S.; Lee, J.H.; Kim, S.J. Incidence of Amyloid-Related Imaging Abnormalities in Patients with Alzheimer Disease Treated with Anti-Beta-Amyloid Immunotherapy: A Meta-Analysis. Neurology 2022, 99, e2092–e2101. [Google Scholar] [CrossRef] [PubMed]
- Sperling, R.A.; Jack, C.R., Jr.; Black, S.E.; Frosch, M.P.; Greenberg, S.M.; Hyman, B.T.; Scheltens, P.; Carrillo, M.C.; Thies, W.; Bednar, M.M.; et al. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: Recommendations from the Alzheimer’s Association Research Roundtable Workgroup. Alzheimers Dement. 2011, 7, 367–385. [Google Scholar] [CrossRef]
- Atwood, C.S.; Perry, G. Playing Russian Roulette with Alzheimer’s Disease Patients: Do the Cognitive Benefits of Lecanemab Outweigh the Risk of Edema, Stroke and Encephalitis? J. Alzheimers Dis. 2023, 92, 799–801. [Google Scholar] [CrossRef] [PubMed]
- Hegrand, M.; Pahnke, J. [Approval first, research afterwards?]. Tidsskr. Nor Laegeforen. 2021, 141. [Google Scholar] [CrossRef]
- Pahnke, J.; Langer, O.; Krohn, M. Alzheimer’s and ABC transporters--new opportunities for diagnostics and treatment. Neurobiol. Dis. 2014, 72 Pt A, 54–60. [Google Scholar] [CrossRef]
- Pahnke, J.; Walker, L.C.; Scheffler, K.; Krohn, M. Alzheimer’s disease and blood-brain barrier function-Why have anti-beta-amyloid therapies failed to prevent dementia progression? Neurosci. Biobehav. Rev. 2009, 33, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.P. Targeting multidrug resistance protein 1 (MRP1, ABCC1): Past, present, and future. Annu. Rev. Pharmacol. Toxicol. 2014, 54, 95–117. [Google Scholar] [CrossRef]
- Hofrichter, J.; Krohn, M.; Schumacher, T.; Lange, C.; Feistel, B.; Walbroel, B.; Heinze, H.J.; Crockett, S.; Sharbel, T.F.; Pahnke, J. Reduced Alzheimer’s disease pathology by St. John’s Wort treatment is independent of hyperforin and facilitated by ABCC1 and microglia activation in mice. Curr. Alzheimer Res. 2013, 10, 1057–1069. [Google Scholar] [CrossRef]
- Krohn, M.; Lange, C.; Hofrichter, J.; Scheffler, K.; Stenzel, J.; Steffen, J.; Schumacher, T.; Bruning, T.; Plath, A.S.; Alfen, F.; et al. Cerebral amyloid-beta proteostasis is regulated by the membrane transport protein ABCC1 in mice. J. Clin. Investig. 2011, 121, 3924–3931. [Google Scholar] [CrossRef]
- Pahnke, J.; Frohlich, C.; Paarmann, K.; Krohn, M.; Bogdanovic, N.; Arsland, D.; Winblad, B. Cerebral ABC transporter-common mechanisms may modulate neurodegenerative diseases and depression in elderly subjects. Arch. Med. Res. 2014, 45, 738–743. [Google Scholar] [CrossRef]
- Ng, Q.X.; Venkatanarayanan, N.; Ho, C.Y. Clinical use of Hypericum perforatum (St John’s wort) in depression: A meta-analysis. J. Affect. Disord. 2017, 210, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, C.S. John’s wort—Ancient herbal protector. Pharm. Hist. 1990, 32, 166–169. [Google Scholar] [PubMed]
- Guo, Y.; Huang, F.; Sun, W.; Zhou, Y.; Chen, C.; Qi, C.; Yang, J.; Li, X.N.; Luo, Z.; Zhu, H.; et al. Unprecedented polycyclic polyprenylated acylphloroglucinols with anti-Alzheimer’s activity from St. John’s wort. Chem. Sci. 2021, 12, 11438–11446. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Modes of Action of Herbal Medicines and Plant Secondary Metabolites. Medicines 2015, 2, 251–286. [Google Scholar] [CrossRef]
- Brenn, A.; Grube, M.; Jedlitschky, G.; Fischer, A.; Strohmeier, B.; Eiden, M.; Keller, M.; Groschup, M.H.; Vogelgesang, S.S. John’s Wort reduces beta-amyloid accumulation in a double transgenic Alzheimer’s disease mouse model-role of P-glycoprotein. Brain Pathol. 2014, 24, 18–24. [Google Scholar] [CrossRef]
- Shen, S.; Zhang, W. ABC transporters and drug efflux at the blood-brain barrier. Rev. Neurosci. 2010, 21, 29–53. [Google Scholar] [CrossRef]
- Kim, H.K.; Choi, Y.H.; Verpoorte, R. NMR-based metabolomic analysis of plants. Nat. Protoc. 2010, 5, 536–549. [Google Scholar] [CrossRef]
- Bilia, A.R.; Bergonzi, M.C.; Mazzi, G.; Vincieri, F.F. NMR spectroscopy: A useful tool for characterisation of plant extracts, the case of supercritical CO2 arnica extract. J. Pharm. Biomed. Anal. 2002, 30, 321–330. [Google Scholar] [CrossRef]
- Bascunana, P.; Brackhan, M.; Mohle, L.; Wu, J.; Bruning, T.; Eiriz, I.; Jansone, B.; Pahnke, J. Time- and Sex-Dependent Effects of Fingolimod Treatment in a Mouse Model of Alzheimer’s Disease. Biomolecules 2023, 13, 331. [Google Scholar] [CrossRef]
- Negres, S.; Scutari, C.; Ionica, F.E.; Gonciar, V.; Velescu, B.S.; Seremet, O.C.; Zanfirescu, A.; Zbarcea, C.E.; Stefanescu, E.; Ciobotaru, E.; et al. Influence of hyperforin on the morphology of internal organs and biochemical parameters, in experimental model in mice. Rom. J. Morphol. Embryol. 2016, 57, 663–673. [Google Scholar]
- Lee, J.Y.; Duke, R.K.; Tran, V.H.; Hook, J.M.; Duke, C.C. Hyperforin and its analogues inhibit CYP3A4 enzyme activity. Phytochemistry 2006, 67, 2550–2560. [Google Scholar] [CrossRef] [PubMed]
- Tatsis, E.C.; Boeren, S.; Exarchou, V.; Troganis, A.N.; Vervoort, J.; Gerothanassis, I.P. Identification of the major constituents of Hypericum perforatum by LC/SPE/NMR and/or LC/MS. Phytochemistry 2007, 68, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Rusalepp, L.; Raal, A.; Püssa, T.; Mäeorg, U. Comparison of chemical composition of Hypericum perforatum and H. maculatum in Estonia. Biochem. Syst. Ecol. 2017, 73, 41–46. [Google Scholar] [CrossRef]
- Barnes, J.; Anderson, L.A.; Phillipson, J.D. St John’s wort (Hypericum perforatum L.): A review of its chemistry, pharmacology and clinical properties. J. Pharm. Pharmacol. 2001, 53, 583–600. [Google Scholar] [CrossRef] [PubMed]
- Muller, D.; Niederberger, M.; Trenckmann, U.; Volz, H.P. Laif 900 in treatment of depression. Proven reliability in first line therapy. MMW Fortschr. Med. 2010, 152, 54–55. [Google Scholar]
- Uebelhack, R.; Gruenwald, J.; Graubaum, H.J.; Busch, R. Efficacy and tolerability of Hypericum extract STW 3-VI in patients with moderate depression: A double-blind, randomized, placebo-controlled clinical trial. Adv. Ther. 2004, 21, 265–275. [Google Scholar] [CrossRef]
- Bennett, S.; Thomas, A.J. Depression and dementia: Cause, consequence or coincidence? Maturitas 2014, 79, 184–190. [Google Scholar] [CrossRef]
- Enache, D.; Winblad, B.; Aarsland, D. Depression in dementia: Epidemiology, mechanisms, and treatment. Curr. Opin. Psychiatry 2011, 24, 461–472. [Google Scholar] [CrossRef]
- Oliveira, A.I.; Pinho, C.; Sarmento, B.; Dias, A.C. Neuroprotective Activity of Hypericum perforatum and Its Major Components. Front. Plant Sci. 2016, 7, 1004. [Google Scholar] [CrossRef]
- Rasmussen, B.; Cloarec, O.; Tang, H.; Staerk, D.; Jaroszewski, J.W. Multivariate analysis of integrated and full-resolution 1H-NMR spectral data from complex pharmaceutical preparations: St. John’s wort. Planta Med. 2006, 72, 556–563. [Google Scholar] [CrossRef]
- Brattstrom, A. Long-term effects of St. John’s wort (Hypericum perforatum) treatment: A 1-year safety study in mild to moderate depression. Phytomedicine Int. J. Phytother. Phytopharm. 2009, 16, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Wolf-Klein, G.P.; Silverstone, F.A.; Levy, A.P. Nutritional patterns and weight change in Alzheimer patients. Int. Psychogeriatr./IPA 1992, 4, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; DiLuca, C.; Growdon, J.H. Weight loss in Alzheimer’s disease. J Geriatr Psychiatry Neurol 1993, 6, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Sarasua, S.M.; Davis, N.J.; DeLuca, J.M.; Thielke, S.M.; Yu, C.E. Weight Loss Is a Strong Predictor of Memory Disorder Independent of Genetic Influences. Genes 2023, 14, 1563. [Google Scholar] [CrossRef] [PubMed]
- Madabushi, R.; Frank, B.; Drewelow, B.; Derendorf, H.; Butterweck, V. Hyperforin in St. John’s wort drug interactions. Eur. J. Clin. Pharmacol. 2006, 62, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Godtel-Armbrust, U.; Metzger, A.; Kroll, U.; Kelber, O.; Wojnowski, L. Variability in PXR-mediated induction of CYP3A4 by commercial preparations and dry extracts of St. John’s wort. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2007, 375, 377–382. [Google Scholar] [CrossRef]
- Kuhn, J.; Sharman, T. Cerebral Amyloid Angiopathy. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Davis, J.; Van Nostrand, W.E. Enhanced pathologic properties of Dutch-type mutant amyloid beta-protein. Proc. Natl. Acad. Sci. USA 1996, 93, 2996–3000. [Google Scholar] [CrossRef]
- Mohle, L.; Stefan, K.; Bascunana, P.; Brackhan, M.; Bruning, T.; Eiriz, I.; El Menuawy, A.; van Genderen, S.; Santos-Garcia, I.; Gorska, A.M.; et al. ABC Transporter C1 Prevents Dimethyl Fumarate from Targeting Alzheimer’s Disease. Biology 2023, 12, 932. [Google Scholar] [CrossRef]
- Mohle, L.; Brackhan, M.; Bascunana, P.; Pahnke, J. Dimethyl fumarate does not mitigate cognitive decline and beta-amyloidosis in female APPPS1 mice. Brain Res. 2021, 1768, 147579. [Google Scholar] [CrossRef]
- Radde, R.; Bolmont, T.; Kaeser, S.A.; Coomaraswamy, J.; Lindau, D.; Stoltze, L.; Calhoun, M.E.; Jaggi, F.; Wolburg, H.; Gengler, S.; et al. Abeta42-driven cerebral amyloidosis in transgenic mice reveals early and robust pathology. EMBO Rep. 2006, 7, 940–946. [Google Scholar] [CrossRef]
- Scheffler, K.; Stenzel, J.; Krohn, M.; Lange, C.; Hofrichter, J.; Schumacher, T.; Bruning, T.; Plath, A.S.; Walker, L.; Pahnke, J. Determination of spatial and temporal distribution of microglia by 230 nm-high-resolution, high-throughput automated analysis reveals different amyloid plaque populations in an APP/PS1 mouse model of Alzheimer’s disease. Curr. Alzheimer Res. 2011, 8, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Hespeler, D.; El Nomeiri, S.; Kaltenbach, J.; Muller, R.H. Nanoporous smartPearls for dermal application-Identification of optimal silica types and a scalable production process as prerequisites for marketed products. Beilstein J. Nanotechnol. 2019, 10, 1666–1678. [Google Scholar] [CrossRef]
- Perez-Roman, I.; Kiekens, F.; Cordoba-Diaz, D.; Garcia-Rodriguez, J.J.; Cordoba-Diaz, M. Development of a Solid Formulation Containing a Microemulsion of a Novel Artemisia Extract with Nematocidal Activity for Oral Administration. Pharmaceutics 2020, 12, 873. [Google Scholar] [CrossRef] [PubMed]
- Choudhari, Y.; Reddy, U.; Monsuur, F.; Pauly, T.; Hoefer, H.; McCarthy, W. Comparative evaluation of porous silica based carriers for lipids and liquid drug formulations. Open Mater. Sci. 2014, 1, 61–74. [Google Scholar] [CrossRef]
- Griciuc, A.; Federico, A.N.; Natasan, J.; Forte, A.M.; McGinty, D.; Nguyen, H.; Volak, A.; LeRoy, S.; Gandhi, S.; Lerner, E.P.; et al. Gene therapy for Alzheimer’s disease targeting CD33 reduces amyloid beta accumulation and neuroinflammation. Hum. Mol. Genet. 2020, 29, 2920–2935. [Google Scholar] [CrossRef]
- Andrew, R.J.; De Rossi, P.; Nguyen, P.; Kowalski, H.R.; Recupero, A.J.; Guerbette, T.; Krause, S.V.; Rice, R.C.; Laury-Kleintop, L.; Wagner, S.L.; et al. Reduction of the expression of the late-onset Alzheimer’s disease (AD) risk-factor BIN1 does not affect amyloid pathology in an AD mouse model. J. Biol. Chem. 2019, 294, 4477–4487. [Google Scholar] [CrossRef]
- Xia, D.; Lianoglou, S.; Sandmann, T.; Calvert, M.; Suh, J.H.; Thomsen, E.; Dugas, J.; Pizzo, M.E.; DeVos, S.L.; Earr, T.K.; et al. Novel App knock-in mouse model shows key features of amyloid pathology and reveals profound metabolic dysregulation of microglia. Mol. Neurodegener. 2022, 17, 41. [Google Scholar] [CrossRef]
- Razali, N.M.; Wah, Y.B. Power comparisons of shapiro-wilk, kolmogorov-smirnov, lilliefors and anderson-darling tests. J. Stat. Model. Anal. 2011, 2, 21–33. [Google Scholar]


| Mouse Age [d] | TE | POL | APOL |
|---|---|---|---|
| 49 | 4.00 | 4.00 | 3.30 |
| 50 | 2.00 | 4.00 | 1.65 |
| 51 | 2.00 | 4.00 | 1.65 |
| 52–75 | 2.00 | 4.00 | 1.65 |
| Mouse Age [d] | APOL1 | APOL2 |
|---|---|---|
| 49 | 0.300 | 0.075 |
| 50 | 0.600 | 0.150 |
| 51 | 1.200 | 0.300 |
| Mouse Age [d] | APOL | Ctrl | ||
|---|---|---|---|---|
| HD | MD | LD | ||
| 39 | 0.0 | 0.0 | 0.0 | 0.0 |
| 40 | 0.600 | 0.150 | 0.075 | 0.600 |
| 41 | 0.900 | 0.300 | 0.150 | 0.900 |
| 42–80 | 1.800 | 0.600 | 0.300 | 1.800 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Menuawy, A.; Brüning, T.; Eiriz, I.; Hähnel, U.; Marthe, F.; Möhle, L.; Górska, A.M.; Santos-García, I.; Wangensteen, H.; Wu, J.; et al. Apolar Extracts of St. John’s Wort Alleviate the Effects of β-Amyloid Toxicity in Early Alzheimer’s Disease. Int. J. Mol. Sci. 2024, 25, 1301. https://doi.org/10.3390/ijms25021301
El Menuawy A, Brüning T, Eiriz I, Hähnel U, Marthe F, Möhle L, Górska AM, Santos-García I, Wangensteen H, Wu J, et al. Apolar Extracts of St. John’s Wort Alleviate the Effects of β-Amyloid Toxicity in Early Alzheimer’s Disease. International Journal of Molecular Sciences. 2024; 25(2):1301. https://doi.org/10.3390/ijms25021301
Chicago/Turabian StyleEl Menuawy, Ahmed, Thomas Brüning, Iván Eiriz, Urs Hähnel, Frank Marthe, Luisa Möhle, Anna Maria Górska, Irene Santos-García, Helle Wangensteen, Jingyun Wu, and et al. 2024. "Apolar Extracts of St. John’s Wort Alleviate the Effects of β-Amyloid Toxicity in Early Alzheimer’s Disease" International Journal of Molecular Sciences 25, no. 2: 1301. https://doi.org/10.3390/ijms25021301





