Kick-Starting Wound Healing: A Review of Pro-Healing Drugs
Abstract
1. Introduction
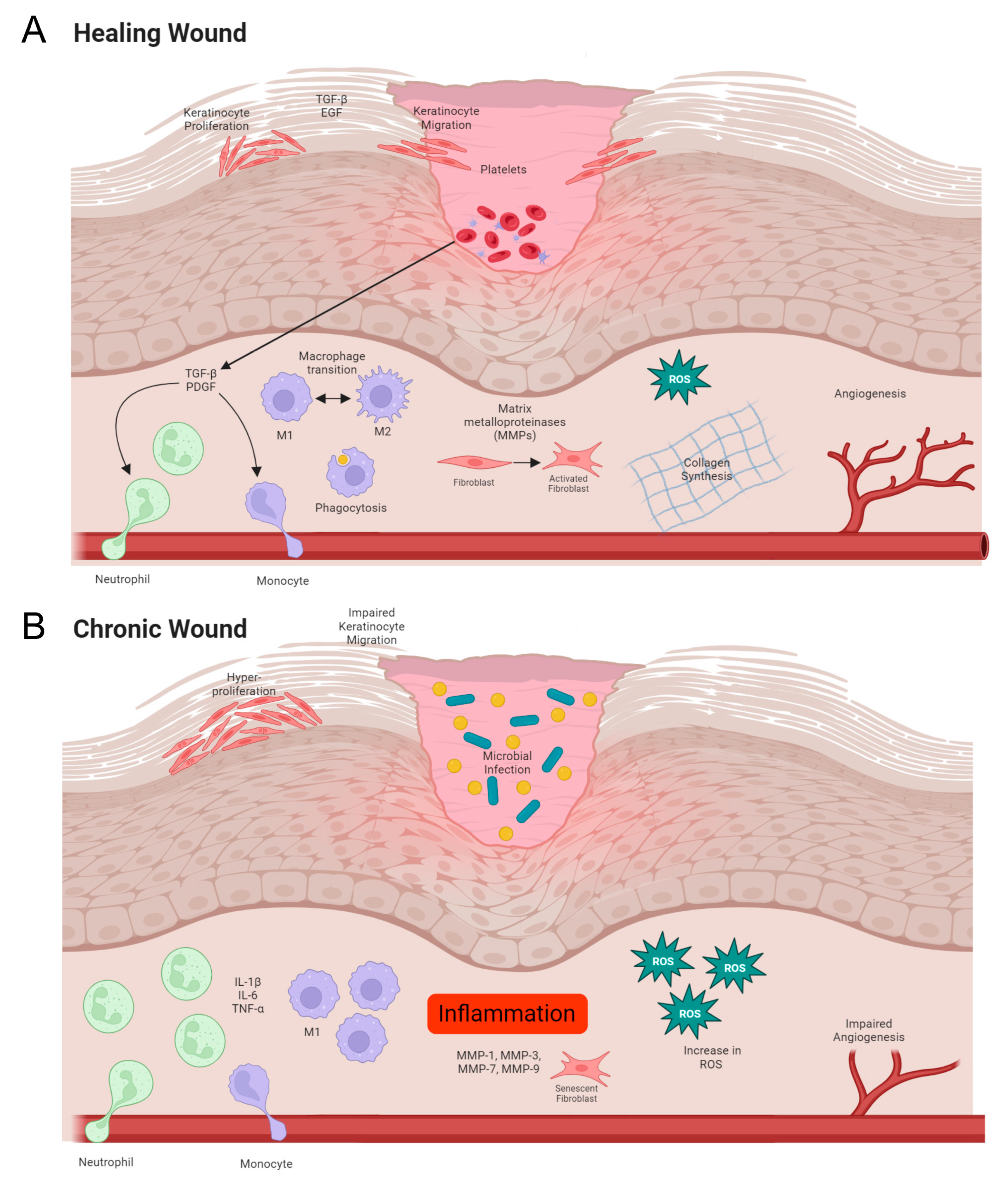
1.1. Normal Wound Healing
1.2. Impaired Wound Healing in Chronic Wounds
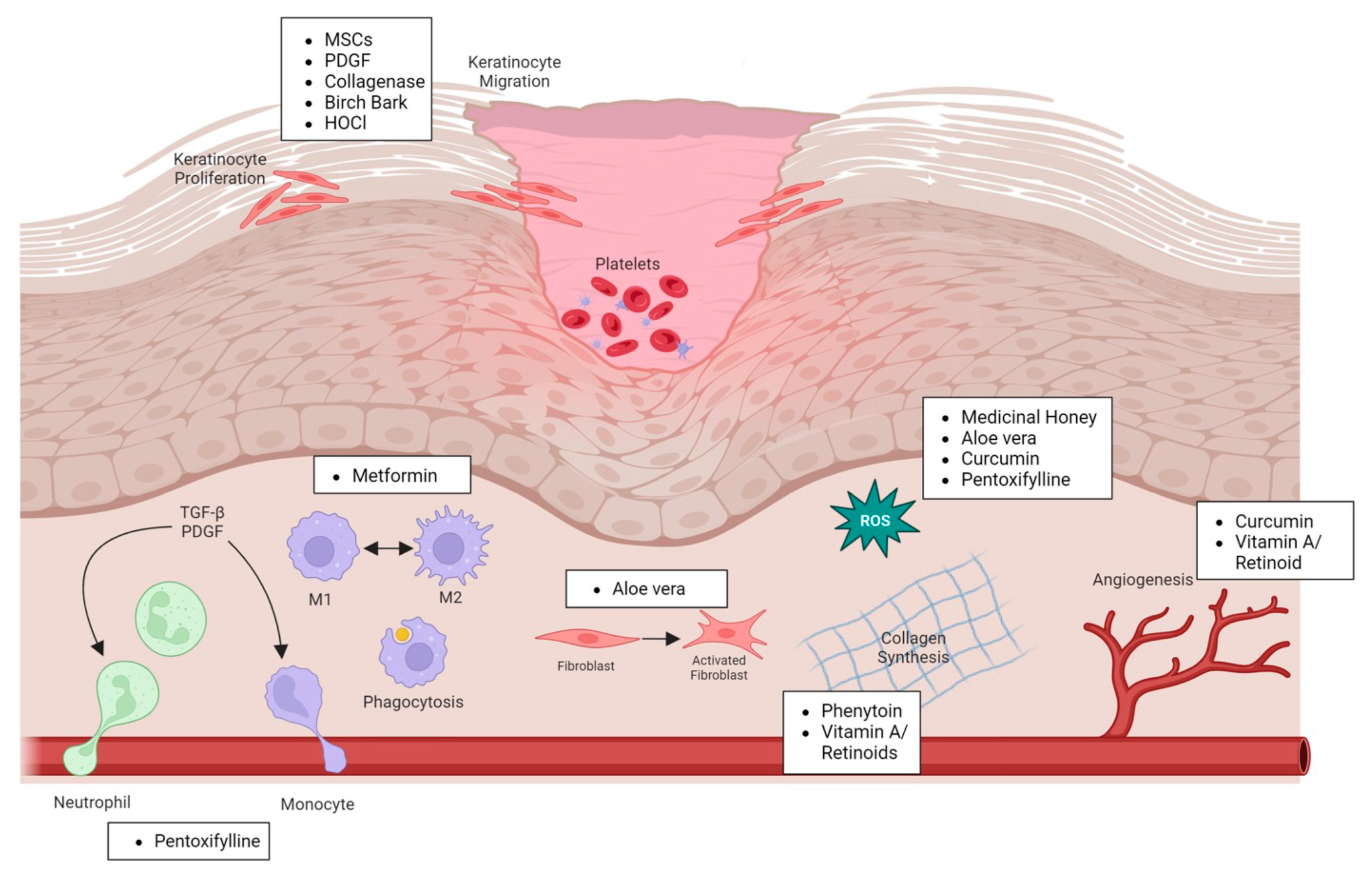
2. Current Therapeutics for Wound Healing
2.1. Natural Products
2.1.1. Antibiotics
2.1.2. Silver
2.1.3. Medicinal Honey
2.1.4. Curcumin
2.1.5. Aloe Vera
2.1.6. Birch Bark
2.2. Human-Derived Factors
2.2.1. Mesenchymal Stem Cells
2.2.2. Macrophages
2.2.3. Collagenase
2.2.4. Placental-Derived Products
2.2.5. Autologous Leucocyte/Platelet/Fibrin Patch
2.3. Pharmaceutical Drugs
2.3.1. PDGF (Becapletmin)
2.3.2. Phenytoin
2.3.3. Vitamin A/Retinoids
2.3.4. Hypochlorous Acid
2.3.5. Pentoxifylline
2.3.6. Metformin
3. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morton, L.M.; Phillips, T.J. Wound healing and treating wounds: Differential diagnosis and evaluation of chronic wounds. J. Am. Acad. Dermatol. 2016, 74, 589–605. [Google Scholar] [CrossRef] [PubMed]
- Enyedi, B.; Kala, S.; Nikolich-Zugich, T.; Niethammer, P. Tissue damage detection by osmotic surveillance. Nat. Cell Biol. 2013, 15, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Niethammer, P.; Grabher, C.; Look, A.T.; Mitchison, T.J. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature 2009, 459, 996–999. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.D. M1 and M2 Macrophages: Oracles of Health and Disease. Crit. Rev. Immunol. 2012, 32, 463–488. [Google Scholar] [CrossRef]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef]
- Haensel, D.; Dai, X. Epithelial-to-mesenchymal transition in cutaneous wound healing: Where we are and where we are heading. Dev. Dyn. 2018, 247, 473–480. [Google Scholar] [CrossRef]
- Zhao, M.; Song, B.; Pu, J.; Wada, T.; Reid, B.; Tai, G.; Wang, F.; Guo, A.; Walczysko, P.; Gu, Y.; et al. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature 2006, 442, 457–460. [Google Scholar] [CrossRef]
- Liu, Y.; Cox, S.R.; Morita, T.; Kourembanas, S. Hypoxia Regulates Vascular Endothelial Growth Factor Gene Expression in Endothelial Cells. Circ. Res. 1995, 77, 638–643. [Google Scholar] [CrossRef]
- Cucina, A.; Borrelli, V.; Randone, B.; Coluccia, P.; Sapienza, P.; Cavallaro, A. Vascular endothelial growth factor increases the migration and proliferation of smooth muscle cells through the mediation of growth factors released by endothelial cells. J. Surg. Res. 2003, 109, 16–23. [Google Scholar] [CrossRef]
- Gilmore, M.A. Phases of wound healing. Dimens. Oncol. Nurs. J. Div. Nurs. 1991, 5, 32–34. [Google Scholar]
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef] [PubMed]
- Falanga, V.; Isseroff, R.R.; Soulika, A.M.; Romanelli, M.; Margolis, D.; Kapp, S.; Granick, M.; Harding, K. Chronic wounds. Nat. Rev. Dis. Primers 2022, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- Järbrink, K.; Ni, G.; Sönnergren, H.; Schmidtchen, A.; Pang, C.; Bajpai, R.; Car, J. The humanistic and economic burden of chronic wounds: A protocol for a systematic review. Syst. Rev. 2017, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive oxygen species (ROS) and wound healing: The functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. Wound J. 2017, 14, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Moseley, R.; Stewart, J.E.; Stephens, P.; Waddington, R.J.; Thomas, D.W. Extracellular matrix metabolites as potential biomarkers of disease activity in wound fluid: Lessons learned from other inflammatory diseases? Br. J. Dermatol. 2004, 150, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, M.; Werner, S. Oxidative stress in normal and impaired wound repair. Pharmacol. Res. 2008, 58, 165–171. [Google Scholar] [CrossRef] [PubMed]
- James, G.A.; Swogger, E.; Wolcott, R.; Pulcini, E.; Secor, P.; Sestrich, J.; Costerton, J.W.; Stewart, P.S. Biofilms in chronic wounds. Wound Repair. Regen. 2008, 16, 37–44. [Google Scholar] [CrossRef]
- Percival, S.L.; McCarty, S.M.; Lipsky, B. Biofilms and wounds: An overview of the evidence. Adv. Wound Care 2015, 4, 373–381. [Google Scholar] [CrossRef]
- Patenall, B.L.; Ridgley, J.D.; Jenkins, A.T.A.; Young, A.E. Evidence of bacterial biofilms within acute wounds: A systematic review. J. Wound Care 2023, 32, 273–278. [Google Scholar] [CrossRef]
- Schultz, G.S.; Sibbald, R.G.; Falanga, V.; Ayello, E.A.; Dowsett, C.; Harding, K.; Romanelli, M.; Stacey, M.C.; Teot, L.; Vanscheidt, W. Wound bed preparation: A systematic approach to wound management. Wound Repair Regen. 2003, 11 (Suppl. 1), S1–S28. [Google Scholar] [CrossRef]
- Schaper, N.C.; van Netten, J.J.; Apelqvist, J.; Bus, S.A.; Fitridge, R.; Game, F.; Monteiro-Soares, M.; Senneville, E.; Board, I.E. Practical guidelines on the prevention and management of diabetes-related foot disease (IWGDF 2023 update). Diabetes Metab. Res. Rev. 2023, e3657. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Nomkhondorj, O.; An, C.Y.; Choi, Y.C.; Cho, J. Management of diabetic foot ulcers: A narrative review. J. Yeungnam Med. Sci. 2023, 40, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Jia, Y.; Fu, L.; Guo, K.; Xie, S. The emerging progress on wound dressings and their application in clinic wound management. Heliyon 2023, 9, e22520. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.; Leal, E.C.; Carvalho, E.; Silva, E.A. Innovative Functional Biomaterials as Therapeutic Wound Dressings for Chronic Diabetic Foot Ulcers. Int. J. Mol. Sci. 2023, 24, 9900. [Google Scholar] [CrossRef] [PubMed]
- Brumberg, V.; Astrelina, T.; Malivanova, T.; Samoilov, A. Modern Wound Dressings: Hydrogel Dressings. Biomedicines 2021, 9, 1235. [Google Scholar] [CrossRef]
- Haverkampf, S.; Heider, J.; Weiss, K.T.; Haubner, F.; Ettl, T.; Schreml, J.; Hedtrich, S.; von Susskind-Schwendi, M.; Berneburg, M.; Karrer, S.; et al. NHE1 expression at wound margins increases time-dependently during physiological healing. Exp. Dermatol. 2017, 26, 124–126. [Google Scholar] [CrossRef] [PubMed]
- Schreml, S.; Meier, R.J.; Kirschbaum, M.; Kong, S.C.; Gehmert, S.; Felthaus, O.; Kuchler, S.; Sharpe, J.R.; Woltje, K.; Weiss, K.T.; et al. Luminescent dual sensors reveal extracellular pH-gradients and hypoxia on chronic wounds that disrupt epidermal repair. Theranostics 2014, 4, 721–735. [Google Scholar] [CrossRef]
- Schreml, S.; Meier, R.J.; Wolfbeis, O.S.; Landthaler, M.; Szeimies, R.M.; Babilas, P. 2D luminescence imaging of pH in vivo. Proc. Natl. Acad. Sci. USA 2011, 108, 2432–2437. [Google Scholar] [CrossRef]
- von Scholten, B.J.; Kreiner, F.F.; Gough, S.C.L.; von Herrath, M. Current and future therapies for type 1 diabetes. Diabetologia 2021, 64, 1037–1048. [Google Scholar] [CrossRef]
- Tahrani, A.A.; Barnett, A.H.; Bailey, C.J. Pharmacology and therapeutic implications of current drugs for type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2016, 12, 566–592. [Google Scholar] [CrossRef]
- Stanek, A.; Mosti, G.; Nematillaevich, T.S.; Valesky, E.M.; Planinsek Rucigaj, T.; Boucelma, M.; Marakomichelakis, G.; Liew, A.; Fazeli, B.; Catalano, M.; et al. No More Venous Ulcers-What More Can We Do? J Clin Med 2023, 12, 6153. [Google Scholar] [CrossRef] [PubMed]
- Altoe, L.S.; Alves, R.S.; Sarandy, M.M.; Morais-Santos, M.; Novaes, R.D.; Goncalves, R.V. Does antibiotic use accelerate or retard cutaneous repair? A systematic review in animal models. PLoS ONE 2019, 14, e0223511. [Google Scholar] [CrossRef] [PubMed]
- Norman, G.; Dumville, J.C.; Mohapatra, D.P.; Owens, G.L.; Crosbie, E.J. Antibiotics and antiseptics for surgical wounds healing by secondary intention. Cochrane Database Syst. Rev. 2016, 3, CD011712. [Google Scholar] [CrossRef] [PubMed]
- Hwang, M.R.; Kim, J.O.; Lee, J.H.; Kim, Y.I.; Kim, J.H.; Chang, S.W.; Jin, S.G.; Kim, J.A.; Lyoo, W.S.; Han, S.S.; et al. Gentamicin-loaded wound dressing with polyvinyl alcohol/dextran hydrogel: Gel characterization and in vivo healing evaluation. AAPS PharmSciTech 2010, 11, 1092–1103. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-H.; Lin, J.-H.; Peng, S.-F.; Yeh, C.-L.; Chen, W.-C.; Chang, T.-L.; Liu, M.-J.; Lai, C.-H. Multifunctional gentamicin supplementation of poly(γ-glutamic acid)-based hydrogels for wound dressing application. J. Appl. Polym. Sci. 2011, 120, 1057–1068. [Google Scholar] [CrossRef]
- Li, H.; Williams, G.R.; Wu, J.; Lv, Y.; Sun, X.; Wu, H.; Zhu, L.M. Thermosensitive nanofibers loaded with ciprofloxacin as antibacterial wound dressing materials. Int. J. Pharm. 2017, 517, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Sim, W.; Barnard, R.T.; Blaskovich, M.A.T.; Ziora, Z.M. Antimicrobial Silver in Medicinal and Consumer Applications: A Patent Review of the Past Decade (2007–2017). Antibiotics 2018, 7, 93. [Google Scholar] [CrossRef]
- Leaper, D. Appropriate use of silver dressings in wounds: International consensus document. Int. Wound J. 2012, 9, 461–464. [Google Scholar] [CrossRef]
- Warriner, R.; Burrell, R. Infection and the chronic wound: A focus on silver. Adv. Ski. Wound Care 2005, 18 (Suppl. S1), 2–12. [Google Scholar] [CrossRef]
- Storm-Versloot, M.N.; Vos, C.G.; Ubbink, D.T.; Vermeulen, H. Topical silver for preventing wound infection. Cochrane Database Syst. Rev. 2010, 3, Cd006478. [Google Scholar] [CrossRef]
- Khansa, I.; Schoenbrunner, A.R.; Kraft, C.T.; Janis, J.E. Silver in Wound Care-Friend or Foe?: A Comprehensive Review. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2390. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.J.; Hollyoak, M.A.; Moaveni, Z.; Brown, T.L.H.; Herndon, D.N.; Heggers, J.P. Retardation of wound healing by silver sulfadiazine is reversed by Aloe vera and nystatin. Burns 2003, 29, 834–836. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Konkel, K.; McCulley, L.; Diak, I.-L. Cases of Argyria Associated With Colloidal Silver Use. Ann. Pharmacother. 2019, 53, 867–870. [Google Scholar] [CrossRef]
- Toussaint, J.; Chung, W.T.; Osman, N.; McClain, S.A.; Raut, V.; Singer, A.J. Topical Antibiotic Ointment Versus Silver-containing Foam Dressing for Second-degree Burns in Swine. Acad. Emerg. Med. 2015, 22, 927–933. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Innes, M.E.; Umraw, N.; Fish, J.S.; Gomez, M.; Cartotto, R.C. The use of silver coated dressings on donor site wounds: A prospective, controlled matched pair study. Burns 2001, 27, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lee, P.Y.; Ho, C.M.; Lui, V.C.; Chen, Y.; Che, C.M.; Tam, P.K.; Wong, K.K. Silver nanoparticles mediate differential responses in keratinocytes and fibroblasts during skin wound healing. ChemMedChem 2010, 5, 468–475. [Google Scholar] [CrossRef]
- Oryan, A.; Alemzadeh, E.; Tashkhourian, J.; Nami Ana, S.F. Topical delivery of chitosan-capped silver nanoparticles speeds up healing in burn wounds: A preclinical study. Carbohydr. Polym. 2018, 200, 82–92. [Google Scholar] [CrossRef]
- Stojkovska, J.; Djurdjevic, Z.; Jancic, I.; Bufan, B.; Milenkovic, M.; Jankovic, R.; Miskovic-Stankovic, V.; Obradovic, B. Comparative in vivo evaluation of novel formulations based on alginate and silver nanoparticles for wound treatments. J. Biomater. Appl. 2018, 32, 1197–1211. [Google Scholar] [CrossRef]
- Lee, D.S.; Sinno, S.; Khachemoune, A. Honey and Wound Healing. Am. J. Clin. Dermatol. 2011, 12, 181–190. [Google Scholar] [CrossRef]
- Hadagali, M.D.; Chua, L.S. The anti-inflammatory and wound healing properties of honey. Eur. Food Res. Technol. 2014, 239, 1003–1014. [Google Scholar] [CrossRef]
- Tashkandi, H. Honey in wound healing: An updated review. Open Life Sci. 2021, 16, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Robson, V.; Dodd, S.; Thomas, S. Standardized antibacterial honey (Medihoney™) with standard therapy in wound care: Randomized clinical trial. J. Adv. Nurs. 2009, 65, 565–575. [Google Scholar] [CrossRef]
- Biglari, B.; vd Linden, P.H.; Simon, A.; Aytac, S.; Gerner, H.J.; Moghaddam, A. Use of Medihoney as a non-surgical therapy for chronic pressure ulcers in patients with spinal cord injury. Spinal Cord. 2012, 50, 165–169. [Google Scholar] [CrossRef]
- Akbik, D.; Ghadiri, M.; Chrzanowski, W.; Rohanizadeh, R. Curcumin as a wound healing agent. Life Sci. 2014, 116, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Li, Y.; Wen, Y.; Chen, Y.F.; Na, L.X.; Li, S.T.; Sun, C.H. Curcumin, a potential inhibitor of up-regulation of TNF-alpha and IL-6 induced by palmitate in 3T3-L1 adipocytes through NF-kappaB and JNK pathway. Biomed. Environ. Sci. 2009, 22, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Gupta, S.C.; Sung, B. Curcumin: An orally bioavailable blocker of TNF and other pro-inflammatory biomarkers. Br. J. Pharmacol. 2013, 169, 1672–1692. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, D.; Ahmed, M.R.; Gomathi, K.; Chitra, K.; Sehgal, P.K.; Jayakumar, R. Dermal wound healing processes with curcumin incorporated collagen films. Biomaterials 2004, 25, 1911–1917. [Google Scholar] [CrossRef]
- Gadekar, R.; Saurabh, M.K.; Thakur, G.S.; Saurabh, A. Study of formulation, characterisation and wound healing potential of transdermal patches of curcumin. Asian J Pharm Clin Res 2012, 5, 225–230. [Google Scholar]
- Phan, T.-T.; See, P.; Lee, S.-T.; Chan, S.-Y. Protective effects of curcumin against oxidative damage on skin cells in vitro: Its implication for wound healing. J. Trauma Acute Care Surg. 2001, 51, 927–931. [Google Scholar] [CrossRef]
- Sung, C.K. The history of Aloe. In New Perspectives on Aloe; Park, Y.I., Lee, S.K., Eds.; Springer: Boston, MA, USA, 2006; pp. 7–17. [Google Scholar]
- Lee, S.K. Overview of Aloe study. In New Perspectives on Aloe; Park, Y.I., Lee, S.K., Eds.; Springer: Boston, MA, USA, 2006; pp. 1–5. [Google Scholar]
- Eshghi, F.; Hosseinimehr, S.J.; Rahmani, N.; Khademloo, M.; Norozi, M.S.; Hojati, O. Effects of Aloe vera cream on posthemorrhoidectomy pain and wound healing: Results of a randomized, blind, placebo-control study. J. Altern. Complement. Med. 2010, 16, 647–650. [Google Scholar] [CrossRef]
- Liang, J.; Cui, L.; Li, J.; Guan, S.; Zhang, K.; Li, J. Aloe vera: A medicinal plant used in skin wound healing. Tissue Eng. Part B Rev. 2021, 27, 455–474. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.H.; Donato, J.; Hartman, G.M.; Haas, R.C. Anti-inflammatory and wound healing activity of a growth substance in Aloe vera. J. Am. Podiatr. Med. Assoc. 1994, 84, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.-C.; Kim, S.Y.; Kim, Y.T.; Kim, E.-A.; Lee, S.-H.; Ko, S.-C.; Wijesinghe, W.A.J.P.; Samarakoon, K.W.; Kim, Y.-S.; Cho, J.H.; et al. In vitro and in vivo antioxidant activities of polysaccharide purified from aloe vera (Aloe barbadensis) gel. Carbohydr. Polym. 2014, 99, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Ozsoy, N.; Candoken, E.; Akev, N. Implications for Degenerative Disorders: Antioxidative Activity, Total Phenols, Flavonoids, Ascorbic Acid, β-Carotene and β-Tocopherol inAloe vera. Oxid. Med. Cell. Longev. 2009, 2, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Erichsen-Brown, C. Medicinal and Other Uses of North American Plants: A Historical Survey with Special Reference to the Eastern Indian Tribes; Courier Corporation: Chelmsford, MA, USA, 2013. [Google Scholar]
- Scheffler, A. The Wound Healing Properties of Betulin from Birch Bark from Bench to Bedside. Planta Med. 2019, 85, 524–527. [Google Scholar] [CrossRef] [PubMed]
- Emrich, S.; Schuster, A.; Schnabel, T.; Oostingh, G.J. Antimicrobial Activity and Wound-Healing Capacity of Birch, Beech and Larch Bark Extracts. Molecules 2022, 27, 2817. [Google Scholar] [CrossRef]
- Ebeling, S.; Naumann, K.; Pollok, S.; Wardecki, T.; Vidal-y-Sy, S.; Nascimento, J.M.; Boerries, M.; Schmidt, G.; Brandner, J.M.; Merfort, I. From a Traditional Medicinal Plant to a Rational Drug: Understanding the Clinically Proven Wound Healing Efficacy of Birch Bark Extract. PLoS ONE 2014, 9, e86147. [Google Scholar] [CrossRef]
- Barret, J.P.; Podmelle, F.; Lipový, B.; Rennekampff, H.O.; Schumann, H.; Schwieger-Briel, A.; Zahn, T.R.; Metelmann, H.R. Accelerated re-epithelialization of partial-thickness skin wounds by a topical betulin gel: Results of a randomized phase III clinical trials program. Burns 2017, 43, 1284–1294. [Google Scholar] [CrossRef]
- Frew, Q.; Rennekampff, H.-O.; Dziewulski, P.; Moiemen, N.; Zahn, T.; Hartmann, B. Betulin wound gel accelerated healing of superficial partial thickness burns: Results of a randomized, intra-individually controlled, phase III trial with 12-months follow-up. Burns 2019, 45, 876–890. [Google Scholar] [CrossRef]
- Clevers, H. What is an adult stem cell? Science 2015, 350, 1319–1320. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Discher, D.E.; Peault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, M.; O’Garra, A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010, 10, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Gadani, S.P.; Cronk, J.C.; Norris, G.T.; Kipnis, J. IL-4 in the brain: A cytokine to remember. J. Immunol. 2012, 189, 4213–4219. [Google Scholar] [CrossRef]
- Guillamat-Prats, R. The Role of MSC in Wound Healing, Scarring and Regeneration. Cells 2021, 10, 1729. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.-S.; Mohammadi, A.A.; Kabiri, H.; Hashempoor, M.R.; Mahmoodi, M.; Amini, M.; Mehrabani, D. The healing effect of Wharton’s jelly stem cells seeded on biological scaffold in chronic skin ulcers: A randomized clinical trial. J. Cosmet. Dermatol. 2019, 18, 1961–1967. [Google Scholar] [CrossRef]
- Vojtaššák, J.; Danišovič, L.; Kubeš, M.; Bakoš, D.; Jarabek, L.; Uličná, M.; Blaško, M. Autologous biograft and mesenchymal stem cells in treatment of the diabetic foot. Neuroendocrinol. Lett. 2006, 27, 134–137. [Google Scholar] [PubMed]
- Hansen, S.B.; Højgaard, L.D.; Kastrup, J.; Ekblond, A.; Follin, B.; Juhl, M. Optimizing an immunomodulatory potency assay for Mesenchymal Stromal Cell. Front. Immunol. 2022, 13, 1085312. [Google Scholar] [CrossRef]
- Zhou, T.; Yuan, Z.; Weng, J.; Pei, D.; Du, X.; He, C.; Lai, P. Challenges and advances in clinical applications of mesenchymal stromal cells. J. Hematol. Oncol. 2021, 14, 24. [Google Scholar] [CrossRef]
- Nuschke, A. Activity of mesenchymal stem cells in therapies for chronic skin wound healing. Organogenesis 2014, 10, 29–37. [Google Scholar] [CrossRef]
- Lee, D.E.; Ayoub, N.; Agrawal, D.K. Mesenchymal stem cells and cutaneous wound healing: Novel methods to increase cell delivery and therapeutic efficacy. Stem Cell Res. Ther. 2016, 7, 37. [Google Scholar] [CrossRef]
- Goren, I.; Müller, E.; Schiefelbein, D.; Christen, U.; Pfeilschifter, J.; Mühl, H.; Frank, S. Systemic Anti-TNFα Treatment Restores Diabetes-Impaired Skin Repair in ob/ob Mice by Inactivation of Macrophages. J. Investig. Dermatol. 2007, 127, 2259–2267. [Google Scholar] [CrossRef] [PubMed]
- Danon, D.; Madjar, J.; Edinov, E.; Knyszynski, A.; Brill, S.; Diamantshtein, L.; Shinar, E. Treatment of human ulcers by application of macrophages prepared from a blood unit. Exp. Gerontol. 1997, 32, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Zuloff-Shani, A.; Adunsky, A.; Even-Zahav, A.; Semo, H.; Orenstein, A.; Tamir, J.; Regev, E.; Shinar, E.; Danon, D. Hard to heal pressure ulcers (stage III–IV): Efficacy of injected activated macrophage suspension (AMS) as compared with standard of care (SOC) treatment controlled trial. Arch. Gerontol. Geriatr. 2010, 51, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.; Winter, H. Considerations for the Use of Clostridial Collagenase in Clinical Practice. Clin. Drug Investig. 1998, 15, 245–252. [Google Scholar] [CrossRef]
- Alipour, H.; Raz, A.; Zakeri, S.; Dinparast Djadid, N. Therapeutic applications of collagenase (metalloproteases): A review. Asian Pac. J. Trop. Biomed. 2016, 6, 975–981. [Google Scholar] [CrossRef]
- Sheets, A.R.; Demidova-Rice, T.N.; Shi, L.; Ronfard, V.; Grover, K.V.; Herman, I.M. Identification and Characterization of Novel Matrix-Derived Bioactive Peptides: A Role for Collagenase from Santyl® Ointment in Post-Debridement Wound Healing? PLoS ONE 2016, 11, e0159598. [Google Scholar] [CrossRef]
- Tallis, A.; Motley, T.A.; Wunderlich, R.P.; Dickerson, J.E.; Waycaster, C.; Slade, H.B. Clinical and Economic Assessment of Diabetic Foot Ulcer Debridement with Collagenase: Results of a Randomized Controlled Study. Clin. Ther. 2013, 35, 1805–1820. [Google Scholar] [CrossRef]
- Riley, K.N.; Herman, I.M. Collagenase promotes the cellular responses to injury and wound healing in vivo. J. Burn. Wounds 2005, 4, e8. [Google Scholar]
- Koob, T.J.; Rennert, R.; Zabek, N.; Massee, M.; Lim, J.J.; Temenoff, J.S.; Li, W.W.; Gurtner, G. Biological properties of dehydrated human amnion/chorion composite graft: Implications for chronic wound healing. Int. Wound J. 2013, 10, 493–500. [Google Scholar] [CrossRef]
- Tettelbach, W.; Cazzell, S.; Sigal, F.; Caporusso, J.M.; Agnew, P.S.; Hanft, J.; Dove, C. A multicentre prospective randomised controlled comparative parallel study of dehydrated human umbilical cord (EpiCord) allograft for the treatment of diabetic foot ulcers. Int. Wound J. 2019, 16, 122–130. [Google Scholar] [CrossRef]
- Zelen, C.M. An evaluation of dehydrated human amniotic membrane allografts in patients with DFUs. J. Wound Care 2013, 22, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Londahl, M.; Tarnow, L.; Karlsmark, T.; Lundquist, R.; Nielsen, A.M.; Michelsen, M.; Nilsson, A.; Zakrzewski, M.; Jorgensen, B. Use of an autologous leucocyte and platelet-rich fibrin patch on hard-to-heal DFUs: A pilot study. J. Wound Care 2015, 24, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Game, F.; Jeffcoate, W.; Tarnow, L.; Day, F.; Fitzsimmons, D.; Jacobsen, J. The LeucoPatch(R) system in the management of hard-to-heal diabetic foot ulcers: Study protocol for a randomised controlled trial. Trials 2017, 18, 469. [Google Scholar] [CrossRef] [PubMed]
- Game, F.; Jeffcoate, W.; Tarnow, L.; Jacobsen, J.L.; Whitham, D.J.; Harrison, E.F.; Ellender, S.J.; Fitzsimmons, D.; Londahl, M.; LeucoPatch, I.I.t.t. LeucoPatch system for the management of hard-to-heal diabetic foot ulcers in the UK, Denmark, and Sweden: An observer-masked, randomised controlled trial. Lancet Diabetes Endocrinol. 2018, 6, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Embil, J.M.; Nagai, M.K. Becaplermin: Recombinant platelet derived growth factor, a new treatment for healing diabetic foot ulcers. Expert Opin. Biol. Ther. 2002, 2, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Steed, D.L. Clinical evaluation of recombinant human platelet-derived growth factor for the treatment of lower extremity diabetic ulcers. Diabetic Ulcer Study Group. J. Vasc. Surg. 1995, 21, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Smiell, J.M.; Wieman, T.J.; Steed, D.L.; Perry, B.H.; Sampson, A.R.; Schwab, B.H. Efficacy and safety of becaplermin (recombinant human platelet-derived growth factor-BB) in patients with nonhealing, lower extremity diabetic ulcers: A combined analysis of four randomized studies. Wound Repair Regen. 1999, 7, 335–346. [Google Scholar] [CrossRef]
- Pierce, G.F.; Mustoe, T.A.; Senior, R.M.; Reed, J.; Griffin, G.L.; Thomason, A.; Deuel, T.F. In vivo incisional wound healing augmented by platelet-derived growth factor and recombinant c-sis gene homodimeric proteins. J. Exp. Med. 1988, 167, 974–987. [Google Scholar] [CrossRef]
- LeGrand, E.K. Preclinical promise of becaplermin (rhPDGF-BB) in wound healing. Am. J. Surg. 1998, 176, 48s–54s. [Google Scholar] [CrossRef]
- Winterfield, L.; Vleugels, R.A.; Park, K.K. The Value of the Black Box Warning in Dermatology. J. Drugs Dermatol. 2015, 14, 660–666. [Google Scholar]
- Ziyadeh, N.; Fife, D.; Walker, A.M.; Wilkinson, G.S.; Seeger, J.D. A matched cohort study of the risk of cancer in users of becaplermin. Adv. Ski. Wound Care 2011, 24, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, J.D.; Queiroz-Junior, C.M.; Costa, J.E.; Teixeira, A.L.; Silva, T.A. Phenytoin-induced gingival overgrowth: A review of the molecular, immune, and inflammatory features. ISRN Dent. 2011, 2011, 497850. [Google Scholar] [CrossRef] [PubMed]
- Goebel, R.W. Sodium diphenylhydantoin association with oral healing. J. Oral Surg. 1972, 30, 191–195. [Google Scholar] [PubMed]
- Shafer, W.G.; Beatty, R.K.; Davis, W.B. Effect of dilantin sodium on tensile strength of healing wounds. Proc. Soc. Exp. Biol. Med. 1958, 98, 348–350. [Google Scholar] [CrossRef] [PubMed]
- Sayar, H.; Gergerlioglu, N.; Seringec, N.; Ozturk, P.; Bulbuloglu, E.; Karabay, G. Comparison of efficacy of topical phenytoin with hypericin in second-degree burn wound healing: An experimental study in rats. Med. Sci. Monit. Basic Res. 2014, 20, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, P.; Rwanyuma, L.; Mkony, C. A comparison of topical Phenytoin with Silverex in the treatment of superficial dermal burn wounds. Cent. Afr. J. Med. 2002, 48, 105–108. [Google Scholar] [PubMed]
- Inchingolo, F.; Vermesan, D.; Inchingolo, A.D.; Malcangi, G.; Santacroce, L.; Scacco, S.; Benagiano, V.; Girolamo, F.; Cagiano, R.; Caprio, M.; et al. Bedsores successfully treated with topical phenytoin. Acta Biomed. 2017, 88, 45–48. [Google Scholar] [CrossRef]
- Anstead, G.M.; Hart, L.M.; Sunahara, J.F.; Liter, M.E. Phenytoin in wound healing. Ann. Pharmacother. 1996, 30, 768–775. [Google Scholar] [CrossRef]
- Talas, G.; Brown, R.A.; McGrouther, D.A. Role of phenytoin in wound healing—A wound pharmacology perspective. Biochem. Pharmacol. 1999, 57, 1085–1094. [Google Scholar] [CrossRef]
- Moy, L.S.; Tan, E.M.L.; Holness, R.; Uitto, J. Phenytoin Modulates Connective Tissue Metabolism and Cell Proliferation in Human Skin Fibroblast Cultures. Arch. Dermatol. 1985, 121, 79–83. [Google Scholar] [CrossRef]
- Abdelmalek, M.; Spencer, J. Retinoids and Wound Healing. Dermatol. Surg. 2006, 32, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Zasada, M.; Budzisz, E. Retinoids: Active molecules influencing skin structure formation in cosmetic and dermatological treatments. Postep. Dermatol. Alergol. 2019, 36, 392–397. [Google Scholar] [CrossRef]
- Wicke, C.; Halliday, B.; Allen, D.; Roche, N.S.; Scheuenstuhl, H.; Spencer, M.M.; Roberts, A.B.; Hunt, T.K. Effects of Steroids and Retinoids on Wound Healing. Arch. Surg. 2000, 135, 1265–1270. [Google Scholar] [CrossRef] [PubMed]
- Polcz, M.E.; Barbul, A. The Role of Vitamin A in Wound Healing. Nutr. Clin. Pract. 2019, 34, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Leyden, J.; Stein-Gold, L.; Weiss, J. Why Topical Retinoids Are Mainstay of Therapy for Acne. Dermatol. Ther. 2017, 7, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Vagotis, F.L.; Brundage, S.R. Histologic study of dermabrasion and chemical peel in an animal model after pretreatment with Retin-A. Aesthetic Plast. Surg. 1995, 19, 243–246. [Google Scholar] [CrossRef]
- Orringer, J.S.; Kang, S.; Johnson, T.M.; Karimipour, D.J.; Hamilton, T.; Hammerberg, C.; Voorhees, J.J.; Fisher, G.J. Tretinoin treatment before carbon-dioxide laser resurfacing: A clinical and biochemical analysis. J. Am. Acad. Dermatol. 2004, 51, 940–946. [Google Scholar] [CrossRef]
- Tom, W.L.; Peng, D.H.; Allaei, A.; Hsu, D.; Hata, T.R. The effect of short-contact topical tretinoin therapy for foot ulcers in patients with diabetes. Arch. Dermatol. 2005, 141, 1373–1377. [Google Scholar] [CrossRef]
- Paquette, D.; Badiavas, E.; Falanga, V. Short-contact topical tretinoin therapy to stimulate granulation tissue in chronic wounds. J. Am. Acad. Dermatol. 2001, 45, 382–386. [Google Scholar] [CrossRef]
- Watcher, M.A.; Wheeland, R.G. The role of topical agents in the healing of full-thickness wounds. J. Dermatol. Surg. Oncol. 1989, 15, 1188–1195. [Google Scholar] [CrossRef]
- Sakarya, S.; Gunay, N.; Karakulak, M.; Ozturk, B.; Ertugrul, B. Hypochlorous Acid: An ideal wound care agent with powerful microbicidal, antibiofilm, and wound healing potency. Wounds 2014, 26, 342–350. [Google Scholar] [PubMed]
- Gold, M.H.; Andriessen, A.; Bhatia, A.C.; Bitter, P., Jr.; Chilukuri, S.; Cohen, J.L.; Robb, C.W. Topical stabilized hypochlorous acid: The future gold standard for wound care and scar management in dermatologic and plastic surgery procedures. J. Cosmet. Dermatol. 2020, 19, 270–277. [Google Scholar] [CrossRef] [PubMed]
- da Costa, M.C.; Ferreira, B.A.; de Moura, F.B.R.; de Lima, L.G.; Araujo, F.d.A.; Mota, F.C.D. Evaluation of 4% stabilized Sodium Hypochlorite activity in the repair of cutaneous excisional wounds in mice. Injury 2021, 52, 2075–2083. [Google Scholar] [CrossRef]
- Dharap, S.B.; Ghag, G.S.; Kulkarni, K.P.; Venkatesh, V. Efficacy and safety of oxum in treatment of the venous ulcer. J. Indian. Med. Assoc. 2008, 106, 326–328. [Google Scholar] [PubMed]
- Jull, A.B.; Arroll, B.; Parag, V.; Waters, J. Pentoxifylline for treating venous leg ulcers. Cochrane Database Syst. Rev. 2012, 12, Cd001733. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Khalili, H. Potential benefits of pentoxifylline on wound healing. Expert. Rev. Clin. Pharmacol. 2016, 9, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Velaei, K.; Bayat, M.; Torkman, G.; Rezaie, F.; Amini, A.; Noruzian, M.; Tavassol, A.; Bayat, M. Evaluating the effects of pentoxifylline administration on experimental pressure sores in rats by biomechanical examinations. Lab. Anim. Res. 2012, 28, 209–215. [Google Scholar] [CrossRef]
- Lim, T.; Taira, B.; Singer, A.; Lin, F.; McClain, S.; Clark, R. 314: Effect of IV Pentoxifylline on Burn Wound Progression. Ann. Emerg. Med. 2009, 54, S98. [Google Scholar] [CrossRef]
- Rawlins, J.M.; Lam, W.L.; Karoo, R.O.; Naylor, I.L.; Sharpe, D.T. Pentoxifylline inhibits mature burn scar fibroblasts in culture. Burns 2006, 32, 42–45. [Google Scholar] [CrossRef]
- Zhao, P.; Sui, B.-D.; Liu, N.; Lv, Y.-J.; Zheng, C.-X.; Lu, Y.-B.; Huang, W.-T.; Zhou, C.-H.; Chen, J.; Pang, D.-L.; et al. Anti-aging pharmacology in cutaneous wound healing: Effects of metformin, resveratrol, and rapamycin by local application. Aging Cell 2017, 16, 1083–1093. [Google Scholar] [CrossRef]
- Qing, L.; Fu, J.; Wu, P.; Zhou, Z.; Yu, F.; Tang, J. Metformin induces the M2 macrophage polarization to accelerate the wound healing via regulating AMPK/mTOR/NLRP3 inflammasome singling pathway. Am. J. Transl. Res. 2019, 11, 655–668. [Google Scholar] [PubMed]
- Han, X.; Tao, Y.; Deng, Y.; Yu, J.; Sun, Y.; Jiang, G. Metformin accelerates wound healing in type 2 diabetic db/db mice. Mol. Med. Rep. 2017, 16, 8691–8698. [Google Scholar] [CrossRef] [PubMed]
- Aragona, M.; Dekoninck, S.; Rulands, S.; Lenglez, S.; Mascre, G.; Simons, B.D.; Blanpain, C. Defining stem cell dynamics and migration during wound healing in mouse skin epidermis. Nat. Commun. 2017, 8, 14684. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.R.; Wang, Y. Drug delivery systems for wound healing. Curr. Pharm. Biotechnol. 2015, 16, 621–629. [Google Scholar] [CrossRef]
- Gaur, N.; Patenall, B.L.; Ghimire, B.; Thet, N.T.; Gardiner, J.E.; Le Doare, K.E.; Ramage, G.; Short, B.; Heylen, R.A.; Williams, C.; et al. Cold Atmospheric Plasma-Activated Composite Hydrogel for an Enhanced and On-Demand Delivery of Antimicrobials. ACS Appl. Mater. Interfaces 2023, 15, 19989–19996. [Google Scholar] [CrossRef]
- Ninan, N.; Forget, A.; Shastri, V.P.; Voelcker, N.H.; Blencowe, A. Antibacterial and Anti-Inflammatory pH-Responsive Tannic Acid-Carboxylated Agarose Composite Hydrogels for Wound Healing. ACS Appl. Mater. Interfaces 2016, 8, 28511–28521. [Google Scholar] [CrossRef]
| Category | Name | Effect on Wound Healing | Commercial Name |
|---|---|---|---|
| Natural Products | Antibiotics |
| Varied |
| Silver sulfadiazine |
| N/A | |
| Medicinal Honey |
| MediHoneyTM | |
| Curcumin |
| N/A | |
| Aloe Vera |
| N/A | |
| Birch Bark |
| Episalvan® | |
| Human-derived Factors | MSCs |
| N/A |
| Collagenase |
| Santyl® | |
| Pharmaceutical Drugs | PDGF (Becapletmin) |
| Regranex® |
| Phenytoin |
| Dilatin | |
| Vitamin A/Retinoids |
| Tretinoin | |
| Hypochlorous Acid |
| N/A | |
| Pentoxifylline |
| Trental® | |
| Metformin |
| Metformin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patenall, B.L.; Carter, K.A.; Ramsey, M.R. Kick-Starting Wound Healing: A Review of Pro-Healing Drugs. Int. J. Mol. Sci. 2024, 25, 1304. https://doi.org/10.3390/ijms25021304
Patenall BL, Carter KA, Ramsey MR. Kick-Starting Wound Healing: A Review of Pro-Healing Drugs. International Journal of Molecular Sciences. 2024; 25(2):1304. https://doi.org/10.3390/ijms25021304
Chicago/Turabian StylePatenall, Bethany L., Kristyn A. Carter, and Matthew R. Ramsey. 2024. "Kick-Starting Wound Healing: A Review of Pro-Healing Drugs" International Journal of Molecular Sciences 25, no. 2: 1304. https://doi.org/10.3390/ijms25021304
APA StylePatenall, B. L., Carter, K. A., & Ramsey, M. R. (2024). Kick-Starting Wound Healing: A Review of Pro-Healing Drugs. International Journal of Molecular Sciences, 25(2), 1304. https://doi.org/10.3390/ijms25021304





