The Triumph of the Spin Chemistry of Fullerene C60 in the Light of Its Free Radical Copolymerization with Vinyl Monomers
Abstract
1. What Is It, the C60 Fullerene Molecule?
2. A Short Sketch of the UHF Spin Theory of Fullerenes
2.1. The Root of the C60 Fullerene Radicality
2.2. Spin-Density Algorithm of the Virtual Derivatization
2.3. Donor-Acceptor Peculiarity and Dry Polymerization of Fullerene C60
3. Wet Polymerization of Fullerene C60
3.1. A Short Sketch of Empirical Observations
3.2. Virtual FRCP of Vinyl Monomers with C60 Fullerene in Light of the Spin Theory of the Molecule
4. Main Results Related to the Initial Stage of the Free-Radical Copolymerization of Vinyl Monomers with C60 Fullerene
5. Star-Branched Polymers of the Fullerene C60 Generated in the Course of Its FRCP with Vinyls
6. Concluding Comments
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Katz, E.A. Fullerenes, Carbon Nanotubes and Nanoclusters. Pedigree of Shapes and Concepts; IKK Publ. Co.: Moscow, Russia, 2008. (In Russian) [Google Scholar]
- Cataldo, F.; Graovac, A.; Ori, O. (Eds.) The Mathematics and Topology of Fullerenes; Springer: Dordrecht, The Netherlands, 2011. [Google Scholar]
- Hirsh, A. The Chemistry of Fullerenes; Stuttgart: Thieme, Germany, 1994. [Google Scholar]
- Hirsh, A. Principles of fullerene reactivity. In Fullerenes and Related Structures; Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 1999; Volume 199, pp. 1–65. [Google Scholar]
- Sheka, E.F. Fullerenes. Nanochemistry, Nanomagnetism, Nanomedicine, Nanophotonics; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2011. [Google Scholar]
- Makarova, T.L.; Sundqvist, B.; Höhne, R.; Esquinazi, P.; Kopelevich, Y.; Scharff, P.; Davydov, V.A.; Kashevarova, L.S.; Rakhmanina, A.V. Magnetic carbon. Nature 2001, 413, 716–718. [Google Scholar] [CrossRef] [PubMed]
- Makarova, T.; Palacio, F. (Eds.) Carbon Based Magnetism. An Overview of the Magnetism of Metal Free Carbon-Based Compounds and Materials; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Piotrovski, L.B.; Kiselev, O.I. Fullerenes in Biology; Rostok: St. Petersburg, Russia, 2006. (In Russian) [Google Scholar]
- Dumpis, M.A.; Nikolaev, D.N.; Litasova, E.V.; Iljin, V.V.; Brusina, M.A.; Piotrovsky, L.B. Biological activity of fullerenes—Reality and prospects. Rev. Clin. Pharmacol. Drug Ther. 2018, 16, 4–20. [Google Scholar] [CrossRef]
- Andreev, S.M.; Bashkatova, E.N.; Purgina, D.D.; Shershakova, N.N.; Haitov, M.R. Fullerenes: Biomedical aspects. Immunologiya 2015, 36, 57–61. (In Russian) [Google Scholar]
- Cataldo, F.; Da Ros, T. (Eds.) Medicinal Chemistry and Pharmacologocal Potential of Fullerenes and Carbon Nanotubes; Springer: Berlin, Germany, 2008. [Google Scholar]
- Buseck, P.R.; Tsipursky, S.J.; Hettich, R. Fullerenes from the geological environment. Science 1992, 257, 215–217. [Google Scholar] [CrossRef]
- Kroto, H.W.; Heath, J.R.; Obrien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Cami, J.; Bernard-Salas, J.; Peeters, E.; Malek, S.E. Detection of C60 and C70 in a young planetary Nebula. Science 2010, 329, 1180–1182. [Google Scholar] [CrossRef]
- Sheka, E.F.; Popova, N.A.; Popova, V.A. Physics and chemistry of graphene. Emergentness, magnetism, mechanophysics and mechanochemistry. Phys. Usp. 2018, 61, 645–691. [Google Scholar] [CrossRef]
- Pople, J.A.; Nesbet, R.K. Self-consistent orbitals for radicals. J. Chem. Phys. 1954, 22, 571–572. [Google Scholar] [CrossRef]
- Anderson, P.W. More Is different: Broken symmetry and the nature of the hierarchical structure of science. Science 1972, 177, 393–396. [Google Scholar] [CrossRef]
- Löwdin, P.-O. Quantum theory of many-particle systems. III. Extension of the Hartree-Fock scheme to include degenerate systems and correlation effects. Phys. Rev. 1955, 97, 1509–1520. [Google Scholar] [CrossRef]
- Löwdin, P.-O. Correlation problem in many-electron quantum mechanics. 1. Review of different approaches and discussion of some current ideas. Adv. Chem. Phys. 1958, 2, 209–322. [Google Scholar]
- Fucutome, H. Unrestricted Hartree–Fock theory and its applications to molecules and chemical reactions. Int. J. Quant. Chem. 1981, 20, 955–964. [Google Scholar] [CrossRef]
- Takatsuka, K.; Fueno, T.; Yamaguchi, K. Distribution of odd electrons in ground-state molecules. Theor. Chim. Acta 1978, 48, 175–183. [Google Scholar] [CrossRef]
- Staroverov, V.N.; Davidson, E.R. Distribution of effectively unpaired electrons. Chem. Phys. Lett. 2000, 330, 161–168. [Google Scholar] [CrossRef]
- Dewar, M.J.S.; Zoebisch, E.G.; Healey, E.F.; Stewart, J.J.P. AM1: A new general-purpose quantum mechanical molecular model. J. Am. Chem. Soc. 1985, 107, 3902–3909. [Google Scholar] [CrossRef]
- Thiel, W. Semiempirical quantum–chemical methods. WIRE Comp. Mol. Sci. 2014, 14, 145–157. [Google Scholar] [CrossRef]
- Sheka, E.F. Fullerenes as polyradicals. Cent. Eur. J. Phys. 2004, 2, 160–182. [Google Scholar]
- Sheka, E.F. Odd electrons and covalent bonding in fullerenes. Int. J. Quant. Chem. 2004, 100, 375–387. [Google Scholar] [CrossRef]
- Sheka, E.F.; Zayets, V.A. The radical nature of fullerene and its chemical activity. Russ. J. Phys. Chem. 2005, 79, 2009–2014. [Google Scholar]
- Sheka, E.F. Chemical portrait of fullerene molecules. J. Struct. Chem. 2006, 47, 593–599. [Google Scholar] [CrossRef]
- Sheka, E.F. Chemical susceptibility of fullerenes in view of Hartree-Fock approach. Int. J. Quant. Chem. 2007, 107, 2803–2816. [Google Scholar] [CrossRef]
- Seifert, G.; Joswig, J.-O. Density-functional tight binding—An approximate density-functional theory method. WIRE Comp. Mol. Sci. 2012, 2, 456–465. [Google Scholar] [CrossRef]
- Kaplan, I. Problems in DFT with the total spin and degenerate states. Int. J. Quant. Chem. 2007, 107, 2595–2603. [Google Scholar] [CrossRef]
- Kaplan, I.G. Symmetry properties of the electron density and following from it limits on the KS-DFT applications. Mol. Phys. 2018, 116, 658–665. [Google Scholar] [CrossRef]
- Sheka, E.F.; Chernozatonskii, L.A. Broken symmetry approach and chemical susceptibility of carbon nanotubes. Int. J. Quant. Chem. 2010, 110, 1466–1480. [Google Scholar] [CrossRef][Green Version]
- Sheka, E.F.; Chernozatonskii, L.A. Chemical reactivity and magnetism of graphene. Int. J. Quant. Chem. 2010, 110, 1938–1946. [Google Scholar] [CrossRef]
- Sheka, E.F. Spin Chemical Physics of Graphene; Pan Stanford: Singapore, 2018. [Google Scholar]
- Sheka, E.F. Spin effects of sp2 nanocarbons in light of unrestricted Hartree-Fock approach and spin-orbit coupling theory. In Quantum Systems in Physics, Chemistry, and Biology: Advances in Concepts and Applications; Tadjer, A., Pavlov, R., Maruani, J., Brändas, E.J., Delgado-Barrio, G., Eds.; Progress in Theoretical Chemistry and Physics 30; Springer: Cham, Switzerland, 2017; pp. 39–63. [Google Scholar]
- Sheka, E.F. Topochemistry of spatially extended sp2 nanocarbons: Fullerenes, nanotubes, and graphene. In Topological Modelling of Nanostructures and Extended Systems; Ashrafi, A.R., Cataldo, F., Iranmanesh, A., Ori, O., Eds.; Carbon Materials: Chemistry and Physics; Springer: Berlin, Germany, 2013; Volume 7, pp. 137–197. [Google Scholar]
- Mishra, M.; Yagci, Y. (Eds.) Handbook of Vinyl Polymers 2: Radical Polymerization, Process, and Technology, New Edition; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Martin, N.; Giacalone, F. (Eds.) Fullerene Polymers. Synthesis, Properties and Applications; Wiley-VCH: Weinheim, Germany, 2009. [Google Scholar]
- Loy, D.A.; Assink, R.A. Synthesis of a C60—P-Xylylene Copolymer. J. Am. Chem. Soc. 1992, 114, 3977–3978. [Google Scholar] [CrossRef]
- Bunker, C.E.; Lawson, G.E.; Sun, Y.P. Fullerene-styrene random copolymers. Novel optical properties. Macromolecules 1995, 28, 3744–3746. [Google Scholar] [CrossRef]
- Cao, T.; Webber, S.E. Free-radical copolymerization of fullerenes with styrene. Macromolecules 1995, 28, 3741–3743. [Google Scholar] [CrossRef]
- Camp, A.G.; Lary, A.; Ford, W.T. Free-radical copolymerization of methyl methacrylate and styrene with C60. Macromolecules 1995, 28, 7959–7961. [Google Scholar] [CrossRef]
- Sun, Y.P.; Lawson, G.E.; Bunker, C.E.; Johnson, R.A.; Ma, B.; Farmer, C.; Riggs, J.E.; Kitaygorodskiy, A. Preparation and Characterization of Fullerene−Styrene Copolymers. Macromolecules 1996, 29, 8441–8448. [Google Scholar] [CrossRef]
- Cao, T.; Webber, S.E. Radical copolymerization of styrene and C60. Macromolecules 1996, 29, 3826–3830. [Google Scholar] [CrossRef]
- Arsalani, N.; Geckeler, K.E. Radical bulk polymerization of styrene in the presence of fullerene[60]. Fuller. Sci. Technol. 1996, 4, 897–910. [Google Scholar] [CrossRef]
- Steward, D.; Imrie, C.T. Role of C60 in the free radical polymerisation of styrene. Chem. Commun. 1996, 13, 1383–1384. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, K.C. Radical polymerization of styrene in the presence of C60. J. Polym. Sci. A Polym. Chem. 1999, 37, 2969–2975. [Google Scholar] [CrossRef]
- Ford, W.T.; Graham, T.D.; Mourey, T.H. Incorporation of C60 into poly(methyl methacrylate) and polystyrene by radical chain polymerization produces branched structures. Macromolecules 1997, 30, 6422–6429. [Google Scholar] [CrossRef]
- Ford, W.T.; Nishioka, T.; McCleskey, S.C.; Mourey, T.H.; Kahol, P. Structure and radical mechanism of formation of copolymers of C60 with styrene and with methyl methacrylate. Macromolecules 2000, 33, 2413–2423. [Google Scholar] [CrossRef]
- Nayak, P.L.; Yang, K.; Dhal, P.K.; Alva, S.; Kumar, J.; Tripathy, S.K. Polyelectrolyte-Containing fullerene I: Synthesis and characterization of the copolymers of 4-vinylbenzoic acid with C60. Chem. Mater. 1998, 10, 2058–2066. [Google Scholar] [CrossRef]
- Jiang, G.; Zheng, Q. Synthesis and application of new fullerene derivative. J. Appl. Polym. Sci. 2005, 97, 2182–2185. [Google Scholar] [CrossRef]
- Ford, W.T.; Nishioka, T.; Qiu, F.; D’Souza, F.; Choi, J.; Kutner, W.; Noworyta, K. Structure determination and electrochemistry of products from the radical reaction of C60 with azo(bisisobutyronitrile). J. Org. Chem. 1999, 64, 6257–6262. [Google Scholar] [CrossRef]
- Kurmaz, S.V.; Pyryaev, A.N.; Obraztsova, N.A. Effect of fullerene on the radical homo_ and copolymerization of N_vinylpyrrolidone and (di)methacrylates. Polym. Sci. Ser. B 2011, 53, 497–504. [Google Scholar] [CrossRef]
- Atovmyan, E.G. On the relationship between the fullerene reactivity and degree of substitution. Russ. Chem. Bull. Int. Ed. 2017, 66, 567–570. [Google Scholar] [CrossRef]
- Atovmyan, E.G.; Grishchuk, A.A.; Estrina, G.A.; Estrin, Y.I. Formation of star-like water-soluble polymeric structures in the process of radical polymerization of N-isopropylacrylamide in the presence of C60. Russ. Chem. Bull. Int. Ed. 2016, 65, 2082–2088. [Google Scholar] [CrossRef]
- Yumagulova, R.K.; Kuznetsov, S.I.; Diniakhmetova, D.R.; Frizen, A.K.; Kraikin, V.A.; Kolesov, S.V. On the Initial Stage of the free-radical polymerizations of styrene and methyl methacryate in the presense of fullerene C60. Kinet. Catal. 2016, 57, 380–387. [Google Scholar] [CrossRef]
- Yumagulova, R.K.; Kolesov, S.V. Specific features of reactions between fullerene C60 and radicals stabilized by conjugation in the process of radical polymerization. Bullet Bashkir Univ. 2020, 25, 47–51. [Google Scholar] [CrossRef][Green Version]
- Tumansky, B.; Kalina, O. Radical Reactions of Fullerenes and Their Derivatives; Kluwer: New York, NY, USA; Boston, FL, USA; Dordrecht, The Netherlands; London, UK; Moscow, Russia, 2002. [Google Scholar]
- Lisenkov, S.V.; Chernozatonskii, L.A. Polymer junctions of fullerene C60 with carbon atoms: Calculations from first principles. Bullet Bashkir Univ. 2003–2004, 8, 152–158, bulletin-vsu-2003-2004.8. [Google Scholar]
- Sabirov, D.S.; Garipova, R.R.; Bulgakov, R.G. Density functional theory study on the decay of fullerenyl radicals RC60•, ROC60•, and ROOC60• (R = tert-butyl and cumyl) and polarizability of the formed fullerene dimers. J. Phys. Chem. A 2013, 117, 13176–13183. [Google Scholar] [CrossRef]
- Sabirov, D.S.; Garipova, R.R.; Bulgakov, R.G. What fullerene is more reactive toward peroxyl radicals? A comparative DFT study on ROO• addition to C60 and C70 fullerenes. Fuller. Nanotub. Carbon Nanostruct. 2015, 23, 1051–1057. [Google Scholar] [CrossRef]
- Diniakhmetova, D.R.; Friesen, A.K.; Kolesov, S.V. Quantum chemical modeling of the addition reactions of 1-n- phenylpropyl radicals to C60 fullerene. Int. J. Quant. Chem. 2016, 116, 489–496. [Google Scholar] [CrossRef]
- Diniakhmetova, D.R.; Friesen, A.K.; Kolesov, S.V. Quantum chemical analysis of the mechanism of the participation of C60 fullerene in the radical polymerization of styrene and MMA initiated by benzoyl peroxide or azobisisobutyronitrile. Russ. J. Phys. Chem. B 2017, 11, 492–498. [Google Scholar] [CrossRef]
- Tukhbatullina, A.A.; Shepelevich, I.S.; Sabirov, D.S. Exaltation of polarizability as a common property of fullerene dimers with diverse intercage bridges. Fuller. Nanotub. Carbon Nanostruct. 2018, 26, 661–666. [Google Scholar] [CrossRef]
- Diniakhmetova, D.R.; Friesen, A.K.; Yumagulova, R.K.; Kolesov, S.V. Simulation of potentially possible reactions at the initial stages of free-radical polymerization of styrene and methyl methacrylate in the presence of fullerene C60. Polym. Sci. B 2018, 60, 414–420. [Google Scholar] [CrossRef]
- Diniakhmetova, D.R.; Friesen, A.K.; Kolesov, S.V. Reactions of fullerene C60 with methyl methacrylate radicals: A density functional theory study. Int. J. Quant. Chem. 2020, 120, e26335. [Google Scholar] [CrossRef]
- Diniakhmetova, D.R.; Friesen, A.K.; Kolesov, S.V. Multiple addition of 2-cyano-iso-propyl radicals to fullerene C60. Russ. J. Phys. Chem. B 2020, 14, 922–928. [Google Scholar] [CrossRef]
- Diniakhmetova, D.R.; Kolesov, S.V. Interaction of styrene and methyl methacrylate with C60 fullerenyl radicals–trisadduts. Bullet Bashkir Univ. 2021, 26, 614–619. [Google Scholar] [CrossRef]
- Sheka, E.F. Digital Twins in the graphene technology. arXiv 2022, arXiv:2208.14926. [Google Scholar]
- Sheka, E.F. Virtual free radical polymerization of vinyl monomers in view of digital twins. Polymers 2023, 15, 2999. [Google Scholar] [CrossRef] [PubMed]
- Sheka, E.F.; Razbirin, B.S.; Nelson, D.K. Continuous symmetry of C60 fullerene and its derivatives. J. Phys. Chem. A 2011, 115, 3480–3490. [Google Scholar] [CrossRef]
- Sheka, E.F. Stretching and breaking of chemical bonds, correlation of electrons, and radical properties of covalent species. Adv. Quant. Chem. 2015, 70, 111–161. [Google Scholar]
- Krusic, P.; Wasserman, E.; Keizer, P.; Morton, J.; Preston, K. Radical reactions of C60. Science 1991, 254, 1183–1185. [Google Scholar] [CrossRef]
- Morton, J.R.F.; Negri, K.; Preston, F. Review of recent EPR and theoretical studies on the addition of free radicals to C60 and C70. Magn. Res. Chem. 1995, 33, 20–27. [Google Scholar] [CrossRef]
- Sheka, E.F. Donor-acceptor origin of fullerene C60 dimerization. Int. J. Quant. Chem. 2007, 107, 2361–2371. [Google Scholar] [CrossRef]
- Sheka, E.F.; Shaymardanova, L.K. C60-based composites in view of topochemical reactions. J. Mater. Chem. 2011, 21, 17128–17146. [Google Scholar] [CrossRef]
- Ecklund, P.C.; Rao, A.M.; Zhou, P.; Wang, Y.; Holden, J.M. Photochemical transformation of C60 and C70 films. Thin Solid Film. 1995, 257, 185–203. [Google Scholar] [CrossRef]
- Iwasa, Y.; Arima, T.; Fleming, R.M.; Siegrist, T.; Zhou, O.; Haddon, R.C.; Rothberg, L.I.; Lyons, K.B.; Carter, H.L., Jr.; Hebard, A.F.; et al. New phases of C60 synthesized at high pressure. Science 1994, 264, 1570–1572. [Google Scholar] [CrossRef] [PubMed]
- Yamawaki, H.; Yoshida, M.; Kakudate, Y.; Usuba, S.; Yokoi, H.; Fujiwara, S.; Aoki, K.; Ruoff, R.; Malhotra, R.; Lorentz, D. Infrared study of vibrational property and polymerization of fullerene C60 and C70 under pressure. J. Phys. Chem. 1993, 97, 11161–11163. [Google Scholar] [CrossRef]
- Takahashi, N.; Dock, H.; Matsuzawa, N.; Ata, M. Plasma-polymerized C60/C70 mixture films: Electric conductivity and structure. J. Appl. Phys. 1993, 74, 5790–5798. [Google Scholar] [CrossRef]
- Zhao, I.B.; Poirier, D.M.; Pechman, R.J.; Weaver, J.H. Electron stimulated polymerization of solid C60. Appl. Phys. Lett. 1994, 64, 577–580. [Google Scholar] [CrossRef]
- Nakaya, M.; Kuwahara, Y.; Aono, M.; Nakayama, T. Reversibility-controlled single molecular level chemical reaction in a C60 monolayer via ionization induced by scanning transmission microscopy. Small 2008, 4, 538–541. [Google Scholar] [CrossRef]
- Dzyabchenko, A.V.; Agafonov, V.N.; Davydov, V.A. Theoretical molecular packings and the structural model of solid-phase polymerization of fullerene C60 under high pressures. Crystallogr. Rep. 1999, 44, 18–24. [Google Scholar]
- Sheka, E.F.; Zaets, V.A.; Ginzburg, I.Y. Nanostructural magnetism of polymeric fullerene crystals. J. Eksp. Theor. Phys. 2006, 103, 728–739. [Google Scholar] [CrossRef]
- Makarova, T. Magnetism in polymerized fullerenes. In Frontiers of Multifunctional Integrated Nanosystems; Buzaneva, E., Scharff, P., Eds.; Kluwer: Dordrecht, The Netherland, 2004; pp. 331–342. [Google Scholar]
- Harris, P.J.F. Fullerene polymers: A brief review. C 2020, 6, 71. [Google Scholar] [CrossRef]
- Sheka, E.F. Digital twins kinetics of virtual free-radical copolymerization of vinyl monomers with stable radicals. 1. Methyl methacrylate. arXiv 2023, arXiv:2309.11616. [Google Scholar]
- Sheka, E.F. Digital twins kinetics of virtual free-radical copolymerization of vinyl monomers with stable radicals. 2. Styrene. arXiv 2023, arXiv:2311.02752. [Google Scholar]
- Sheka, E.F. Digital twins kinetics of virtual free-radical copolymerization of vinyl monomers with stable radicals. 3. NIPA. arXiv 2023, arXiv:2311.06866. [Google Scholar]
- Sheka, E.F. Digitalization of free-radical polymerization. arXiv 2023, arXiv:2312.14163. [Google Scholar]
- Goclon, J.; Winkler, R.; Margraf, J.T. Theoretical investigation of interactions between palladium and fullerene in polymer. RSC Adv. 2017, 7, 2202–2210. [Google Scholar] [CrossRef]
- Peng, B. Stability and strength of monolayer polymeric C60. Nano Lett. 2023, 23, 652–658. [Google Scholar] [CrossRef]
- Starkweather, H.W.; Taylor, G.B. The kinetics of the polymerization of vinyl acetate. J. Am. Chem. Soc. 1930, 52, 4708–4714. [Google Scholar] [CrossRef]
- Semenov, N.N. Tsepnyie Reakcii (Chain Reactions); Goschimizdat: Moscow, Russia, 1934. (In Russian) [Google Scholar]
- Bagdasar’yan, K.S. Teoriya Radikal’noi Polimerizatsii (Free_Radical Polymerization Theory); Nauka: Moscow, Russia, 1966. (In Russian) [Google Scholar]
- Pross, A. Theoretical and Physical Principles of Organic Reactivity; Wiley: New York, NY, USA, 1995. [Google Scholar]
- Denisov, E.T. Constanty skorosni gomoliticheskikh zhidkofaznykh reakciy (Rate Constants of Homolytic Liquid-Phase Reactions); Nauka: Moscow, Russia, 1971. [Google Scholar]
- Heuts, J.P.A. Theory of radical reactions. In Handbook of Radical Polymerization; Matyjaszewski, K., Davis, T.P., Eds.; John Wiley and Sons: Hoboken, NJ, USA, 2002; pp. 1–76. [Google Scholar]
- Denisov, E.T.; Sarkisov, O.M.; Likhtenshtein, G.I. Chemical Kinetics: Fundamentals and Recent Developments; Elsevier: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Denisov, E.T.; Afanas’ev, I.B. Oxidation and Antioxidants in Organic Chemistry and Biology; CRC Press. Taylor and Francis Group: Boca Raton, FL, USA, 2005. [Google Scholar]
- Matyjaszewski, K.; Davis, T.P. (Eds.) Handbook of Radical Polymerization; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2002. [Google Scholar]
- Mathis, C. Star—Shaped polymers with a fullerene core. In Fullerene Polymers. Synthesis, Properties and Applications; Martin, N., Giacalone, F., Eds.; Wilry-VCH: Weinheim, Germany, 2009; pp. 97–127. [Google Scholar]
- Atovmyan, E.G.; Badamshina, E.R.; Gafurova, M.P.; Grishchuk, A.A.; Estrin, Y.I. Synthesis of new polyhydroxylated fullerenes. Dokl. Chem. 2005, 402, 75–76. [Google Scholar] [CrossRef]
- Hirsch, A.; Brettreich, M. Fullerenes Chemistry and Reactions; Wiley-VCH: London, UK, 2004. [Google Scholar]
- Atovmyan, E.G.; Grishchuk, A.A.; Fedotova, T.N. Polymerization of [60] fullerene activated with butyl lithium. Russ. Chem. Bull. 2011, 60, 1505–1507. [Google Scholar] [CrossRef]
- Vinogradova, L.V. Star-shaped polymers with the fullerene C60 branching center. Russ. Chem. Bull. 2012, 61, 907–922. [Google Scholar] [CrossRef]
- Stephens, P.W.; Bortel, G.; Faigel, G.; Tegze, M.; Janossy, A.; Pekker, S.; Oszlanyi, G.; Forro, L. Polymeric fullerene chains in RbC60 and KC60. Nature 1994, 370, 636–639. [Google Scholar] [CrossRef]
- Sheka, E.F. Computational synthesis of hydrogenated fullerenes from C60 to C60H60. J. Mol. Mod. 2011, 17, 1973–1984. [Google Scholar] [CrossRef]
- Sheka, E.F. Step-wise computational synthesis of fullerene C60 derivatives. Fluorinated fullerenes C60F2k. J. Exp. Theor. Phys. 2010, 111, 397–414. [Google Scholar] [CrossRef]
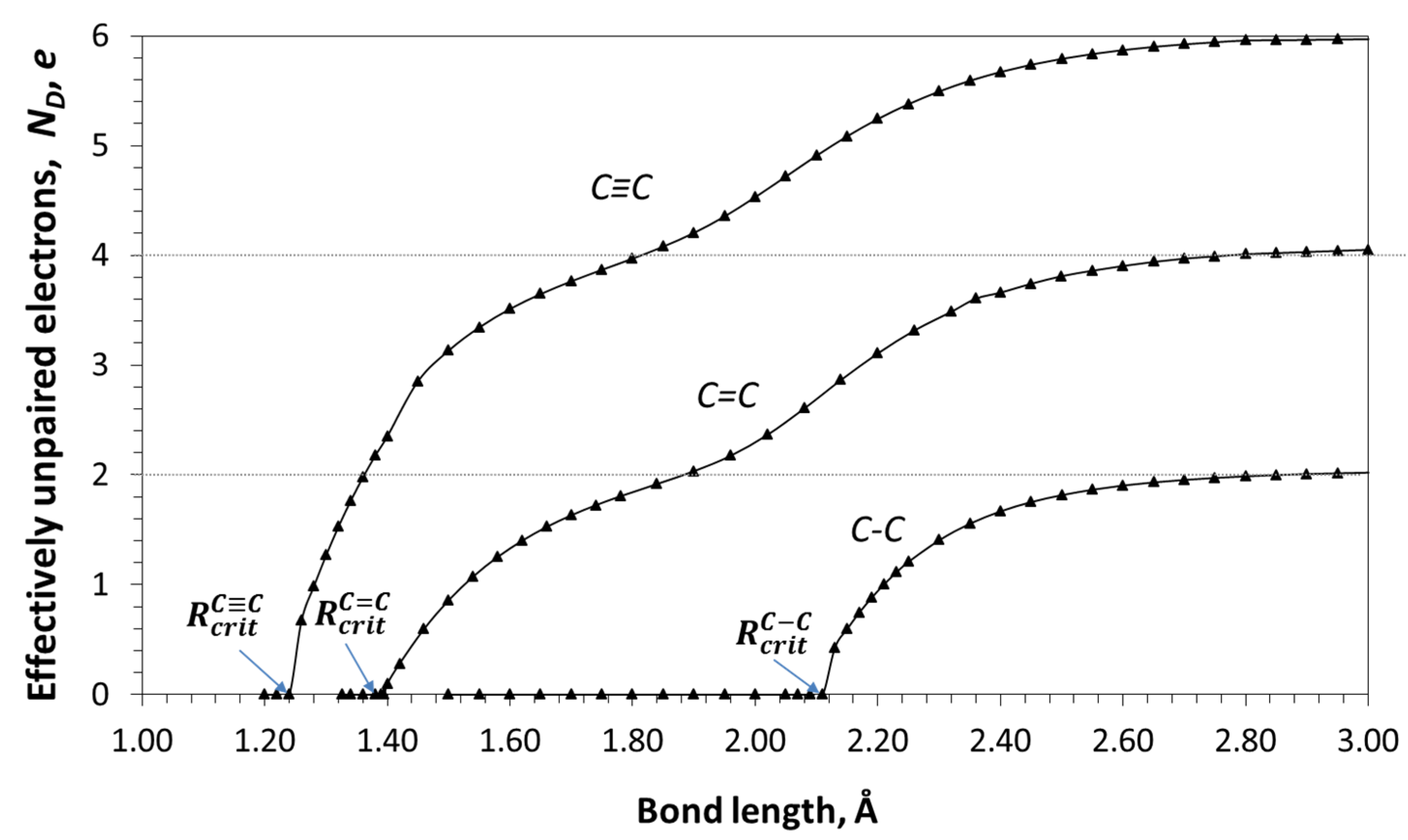
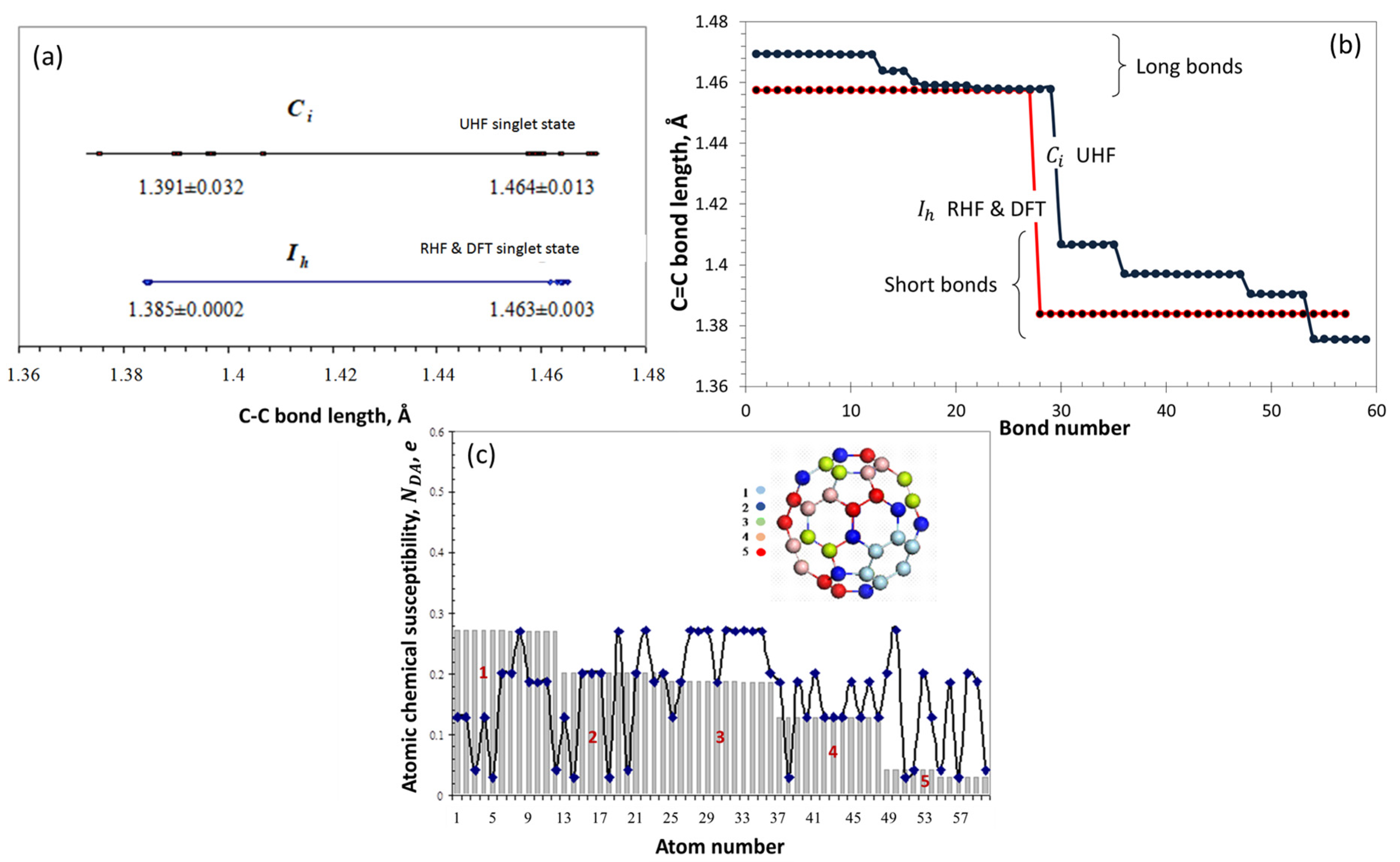
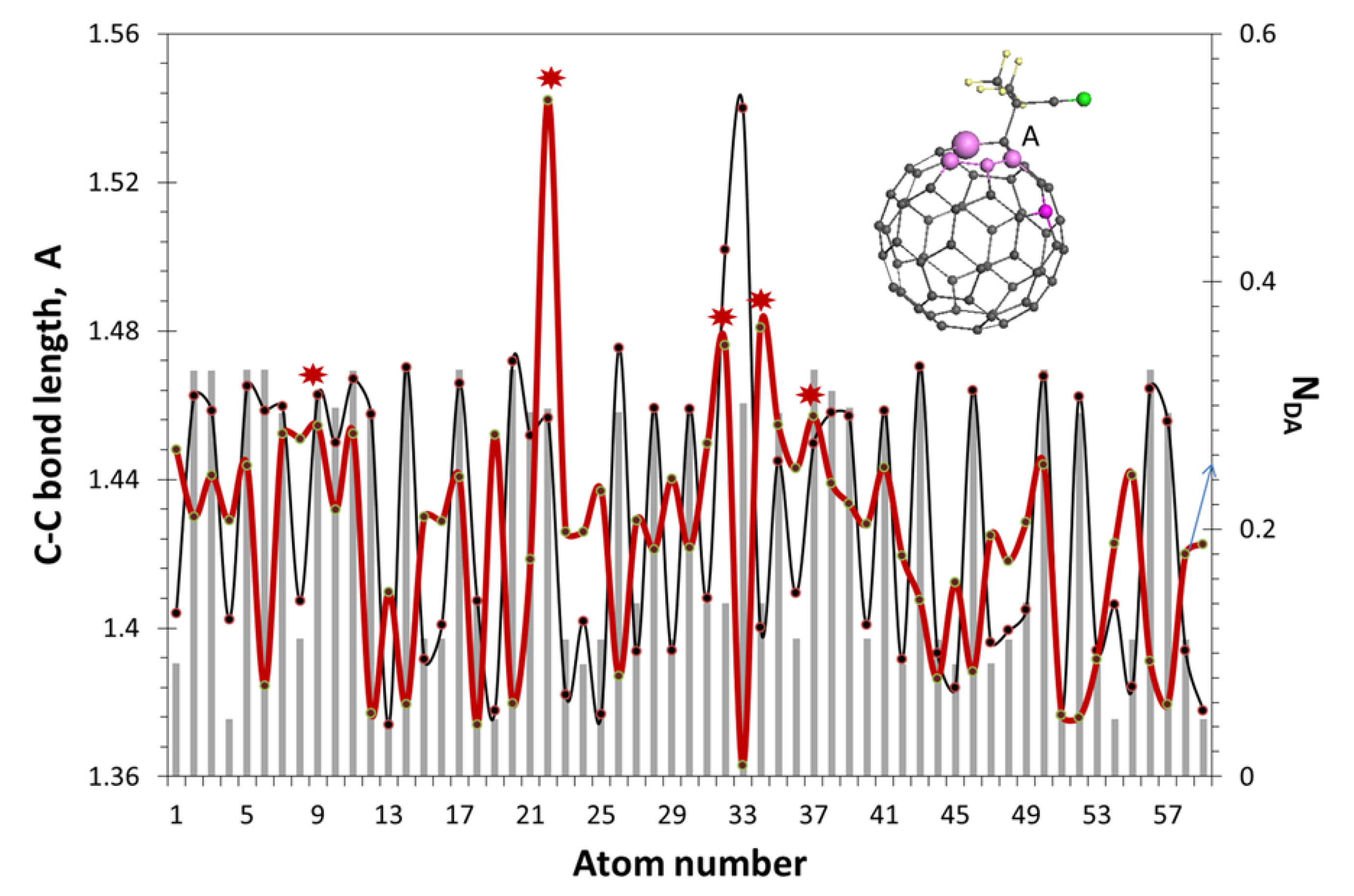
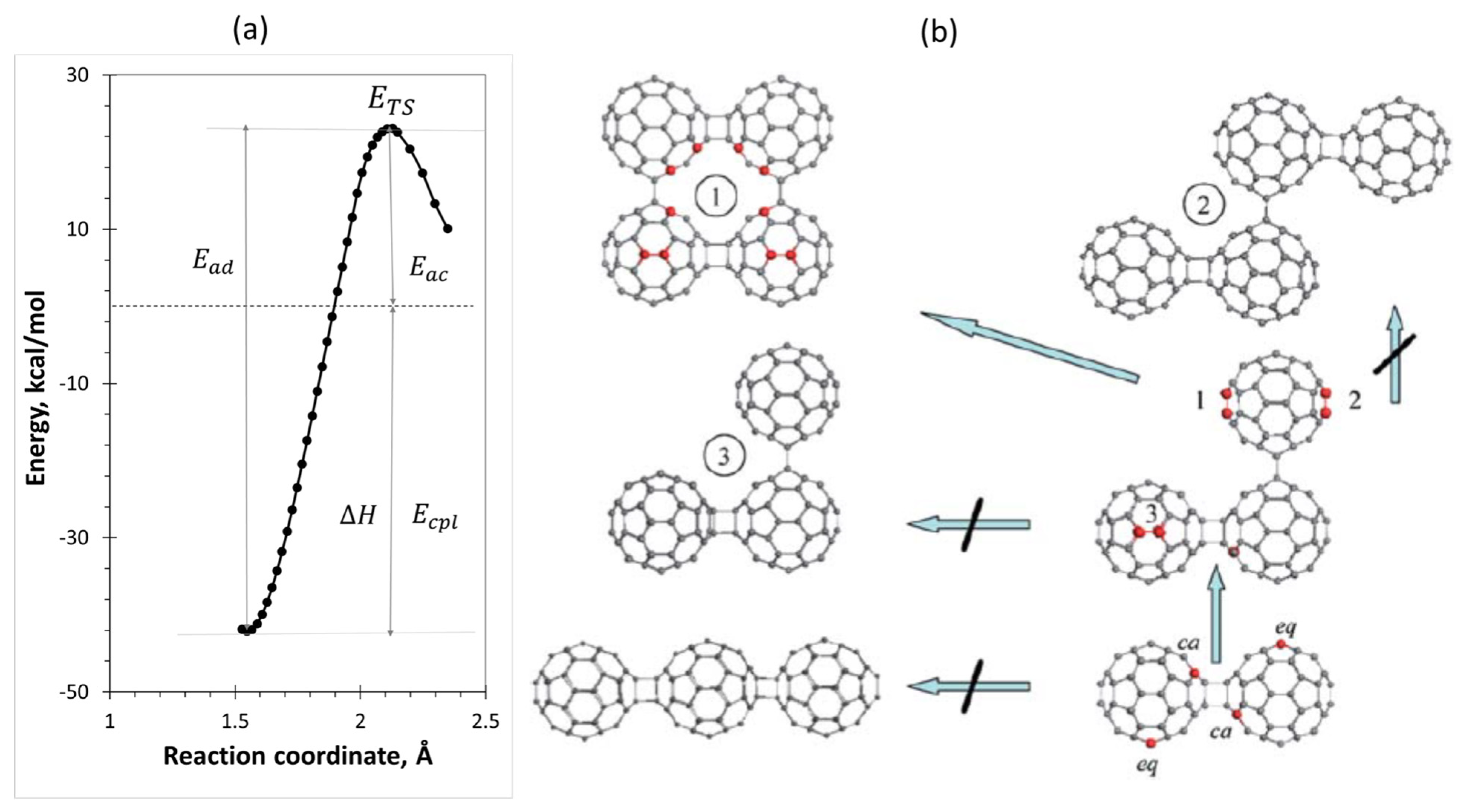

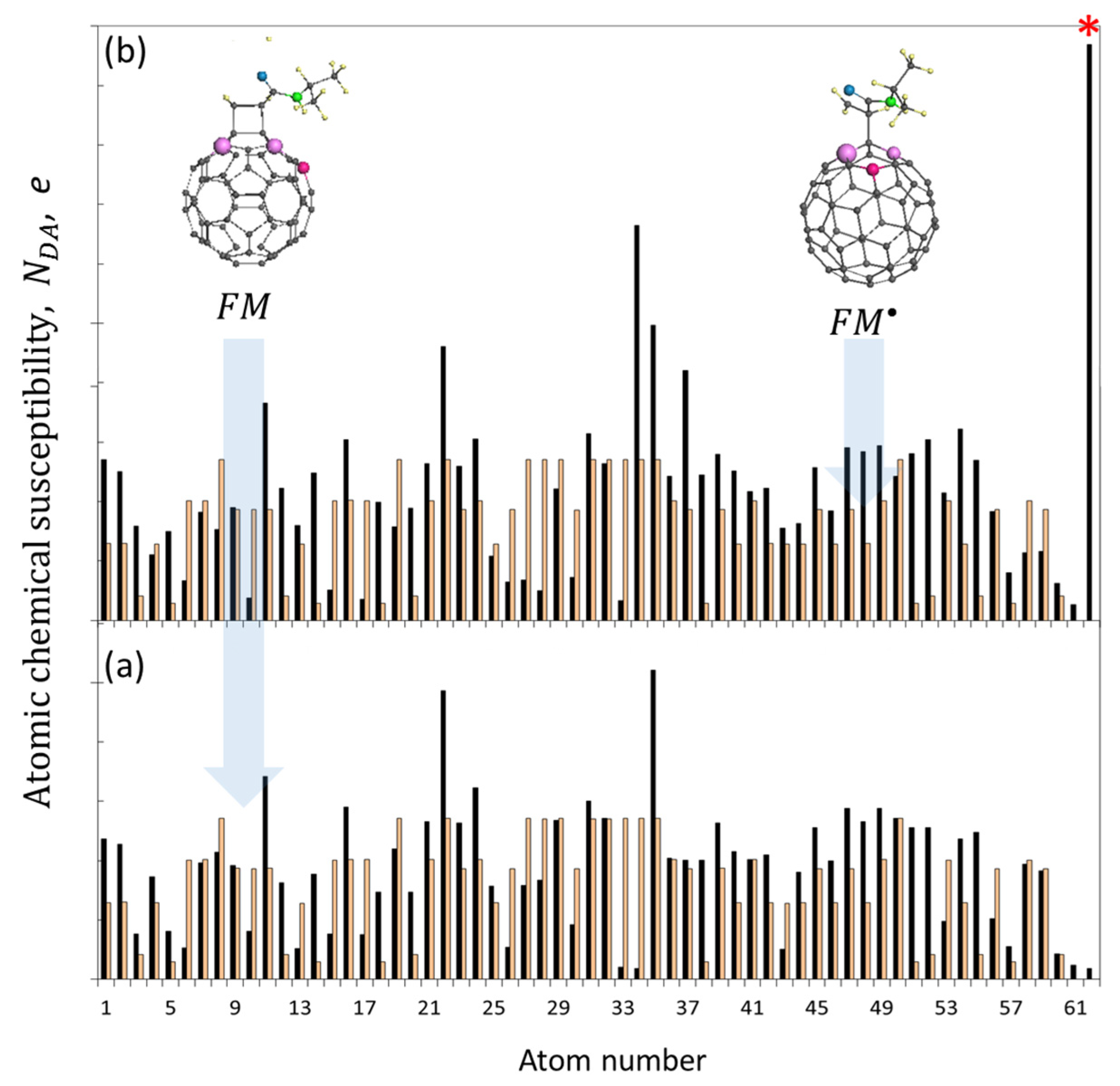
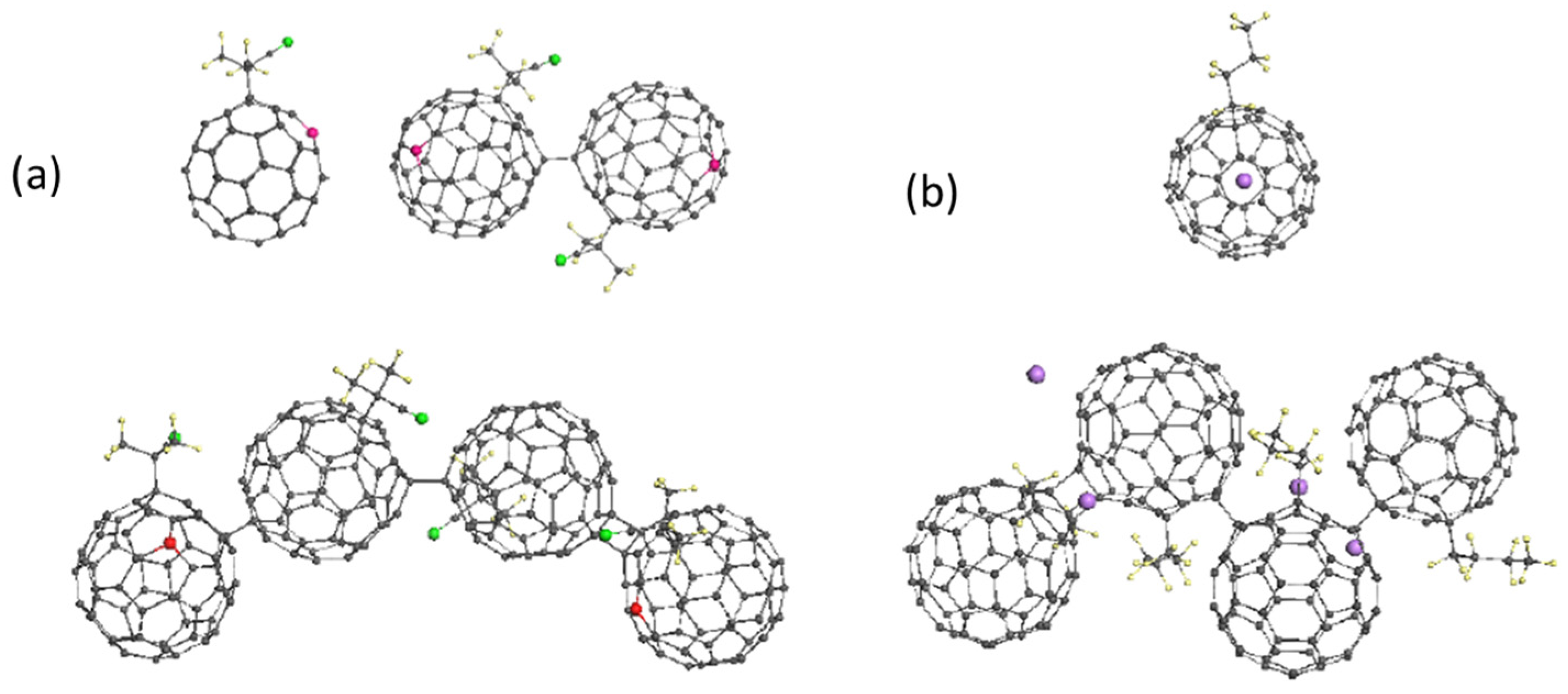
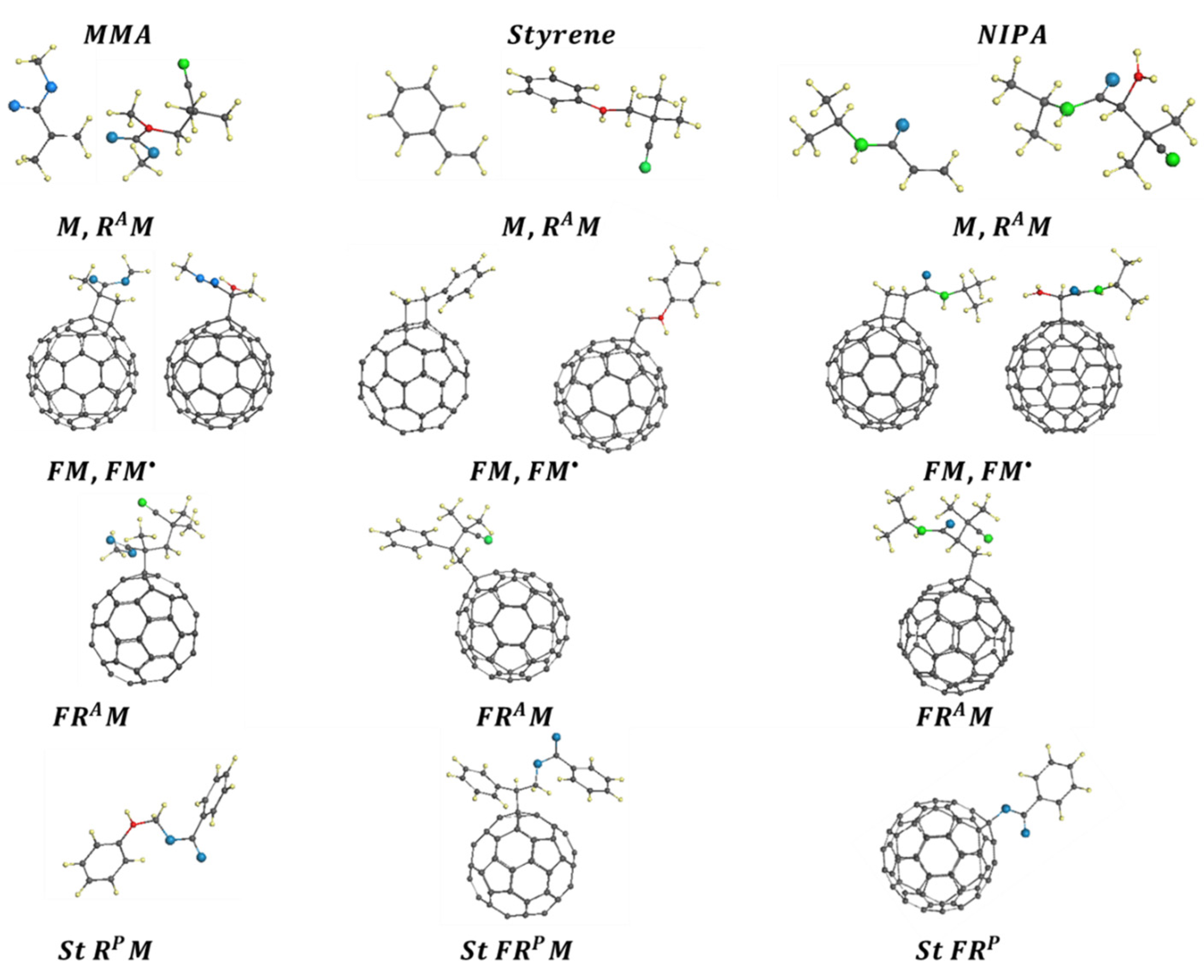
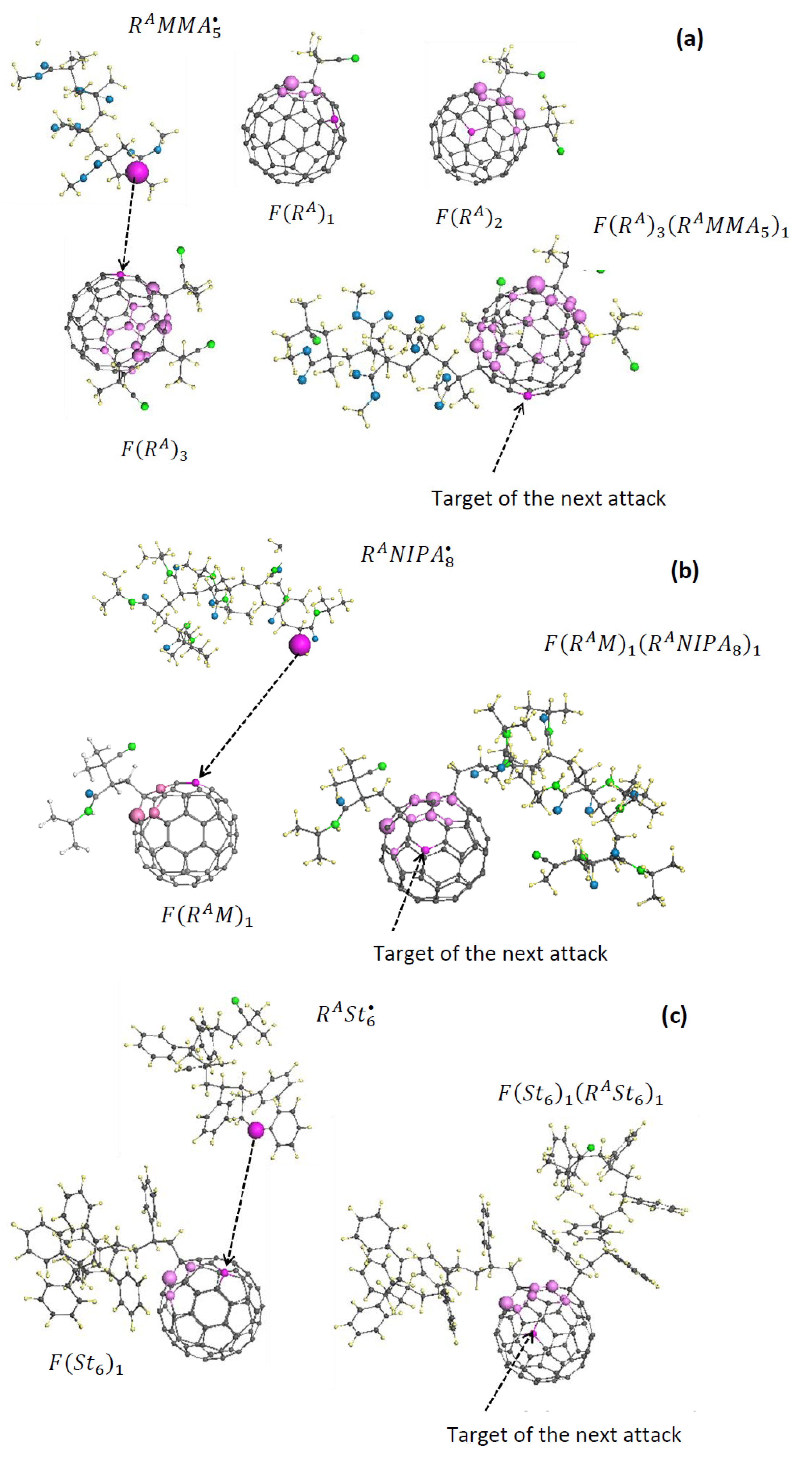
| Reaction Mark | Reaction Equation (1) | Reaction Rate Constant | Reaction Type |
|---|---|---|---|
| (1) | generation of monomer-radicals | ||
| (2) | generation of oligomer-radicals, polymer chain growth | ||
| (3a) | two-dentant grafting of monomer on C60 | ||
| (3b) | one-dentant stable radical grafting of monomer, generation of monomer-radical | ||
| (4) | generation of oligomer-radical anchored to C60, polymer chain growth | ||
| (5) | one-dentant coupling with monomer | ||
| (6) | monomer-radical grafting on C60 | ||
| (7) | monomer-radical capturing with stable radical | ||
| (8) | free radical grafting on C60 | ||
| (9) | free radical capturing with stable radical | ||
| (10) | stable radical grafting on C60 | ||
| (11) | monomer-radical capturing with free radical | ||
| (12) | monomer-radical capturing with radical S |
| Oligomers | C60 + Butyl Li | |
|---|---|---|
| Monomer | −7.07 | −5.96 |
| Dimer | −7.13 | −5.52 |
| Trimer | −6.99 | −5.40 |
| Tetramer | −6.88 | −5.41 |
| () | () | |||||
|---|---|---|---|---|---|---|
| Digital Twins’ set | ||||||
| Two-dentant | One-dentant | |||||
| - | - | - | ||||
| - | - | - | ||||
| - | - | |||||
| Virtual reaction solution of methyl methacrylate (MMA) | ||||||
| 10.46 (2) (2) | 12.28 (1) (2) | >20 (2) (3) | ||||
| - | - | 11.58 | ||||
| - | - | 9.40 | ||||
| Virtual reaction solution of styrene (St) | ||||||
| 12.06 (2) (2) 6.12–16.49 (2–6) (2,4) | 8.50 (1) (2) | 24.52 (2) (3) | 8.38 (1) | |||
| - | - | - | 9.73 | |||
| 7.02 (5) | ||||||
| - | - | - | 9.41 | |||
| - | 2.79 (1) (2,5) 8.78 (2) | - | 28.82 (5) | |||
| 11.25 (2) 2) | - | - | ||||
| Virtual reaction solution of N-isopropyl acrylamide (NIPA) | ||||||
| 8.39 (2) (2) 7.74 (3) (2,4) 8.49 (4) (2,4) | 19.09 (1) 2) | 17.29 (1) (3) 27.79 (2) | 20.01 | |||
| - | - | 0.023 | ||||
| - | - | 9.398 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheka, E.F. The Triumph of the Spin Chemistry of Fullerene C60 in the Light of Its Free Radical Copolymerization with Vinyl Monomers. Int. J. Mol. Sci. 2024, 25, 1317. https://doi.org/10.3390/ijms25021317
Sheka EF. The Triumph of the Spin Chemistry of Fullerene C60 in the Light of Its Free Radical Copolymerization with Vinyl Monomers. International Journal of Molecular Sciences. 2024; 25(2):1317. https://doi.org/10.3390/ijms25021317
Chicago/Turabian StyleSheka, Elena F. 2024. "The Triumph of the Spin Chemistry of Fullerene C60 in the Light of Its Free Radical Copolymerization with Vinyl Monomers" International Journal of Molecular Sciences 25, no. 2: 1317. https://doi.org/10.3390/ijms25021317
APA StyleSheka, E. F. (2024). The Triumph of the Spin Chemistry of Fullerene C60 in the Light of Its Free Radical Copolymerization with Vinyl Monomers. International Journal of Molecular Sciences, 25(2), 1317. https://doi.org/10.3390/ijms25021317






