Phosphorylation of Thr-225 and Ser-262 on ERD7 Promotes Age-Dependent and Stress-Induced Leaf Senescence through the Regulation of Hydrogen Peroxide Accumulation in Arabidopsis thaliana
Abstract
1. Introduction
2. Results
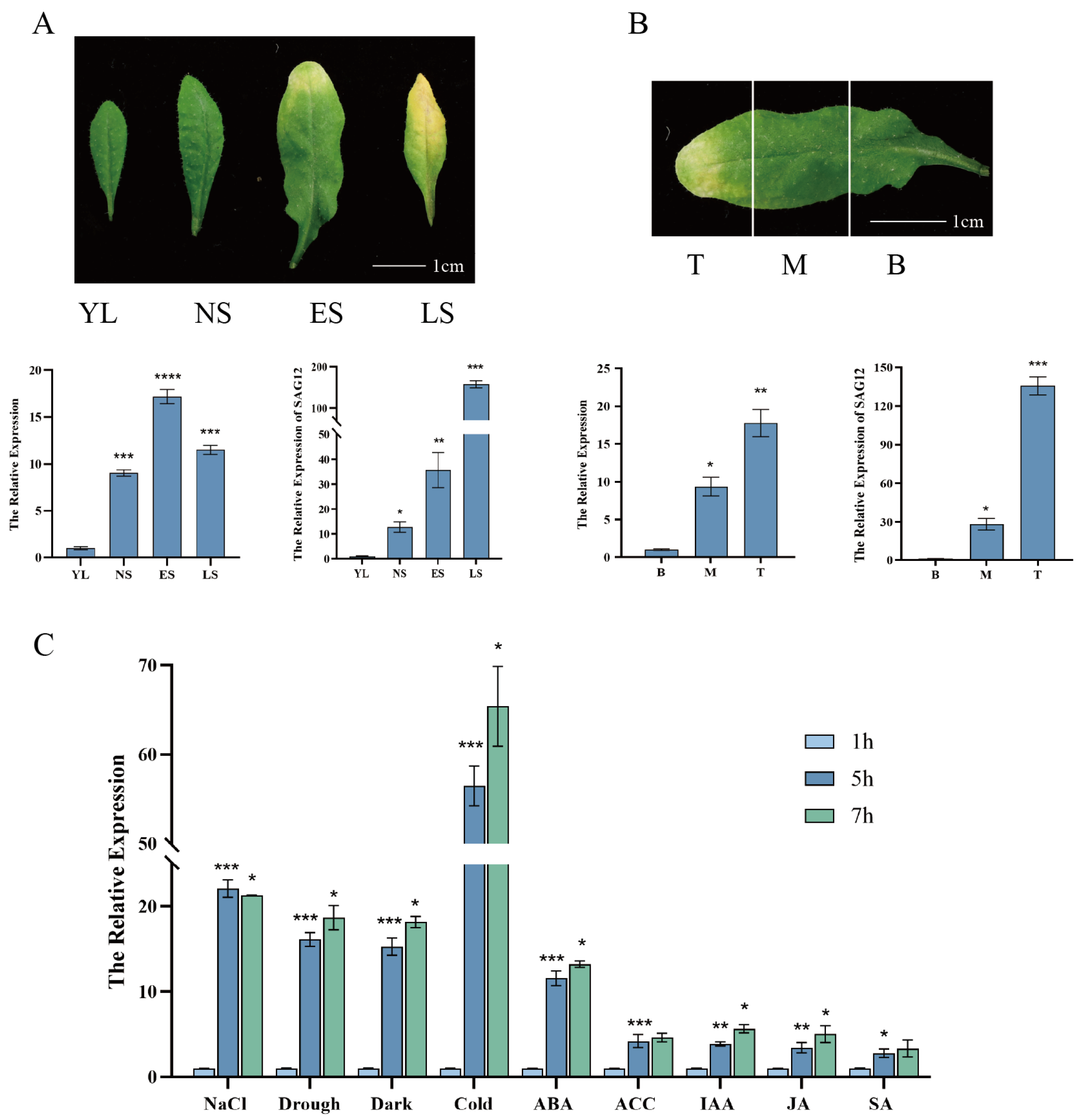
2.1. ERD7 Expression Is Associated with Leaf Senescence and Can Be Induced by Multiple Stresses
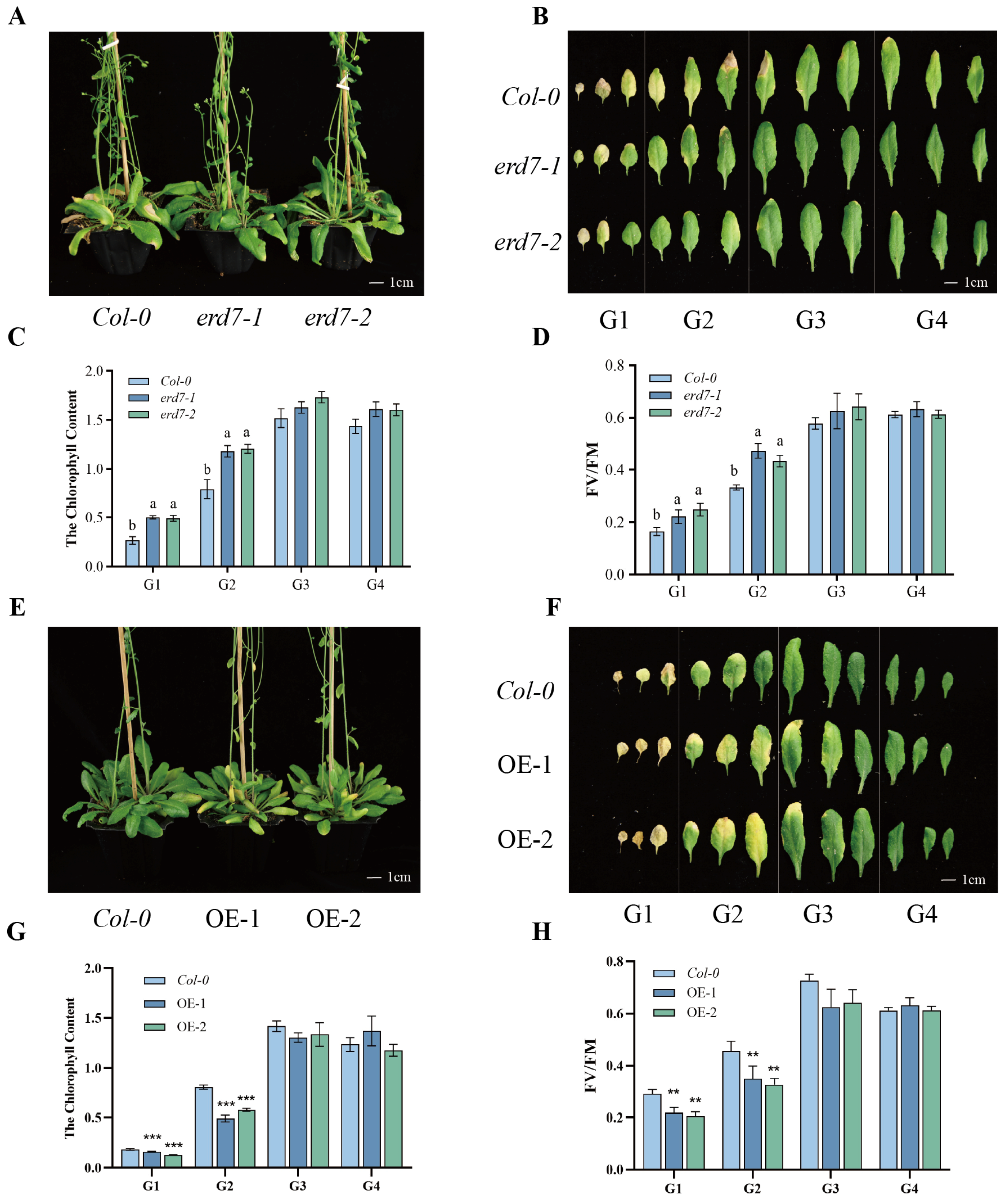
2.2. ERD7 Plays a Positive Role in Leaf Senescence Regulation
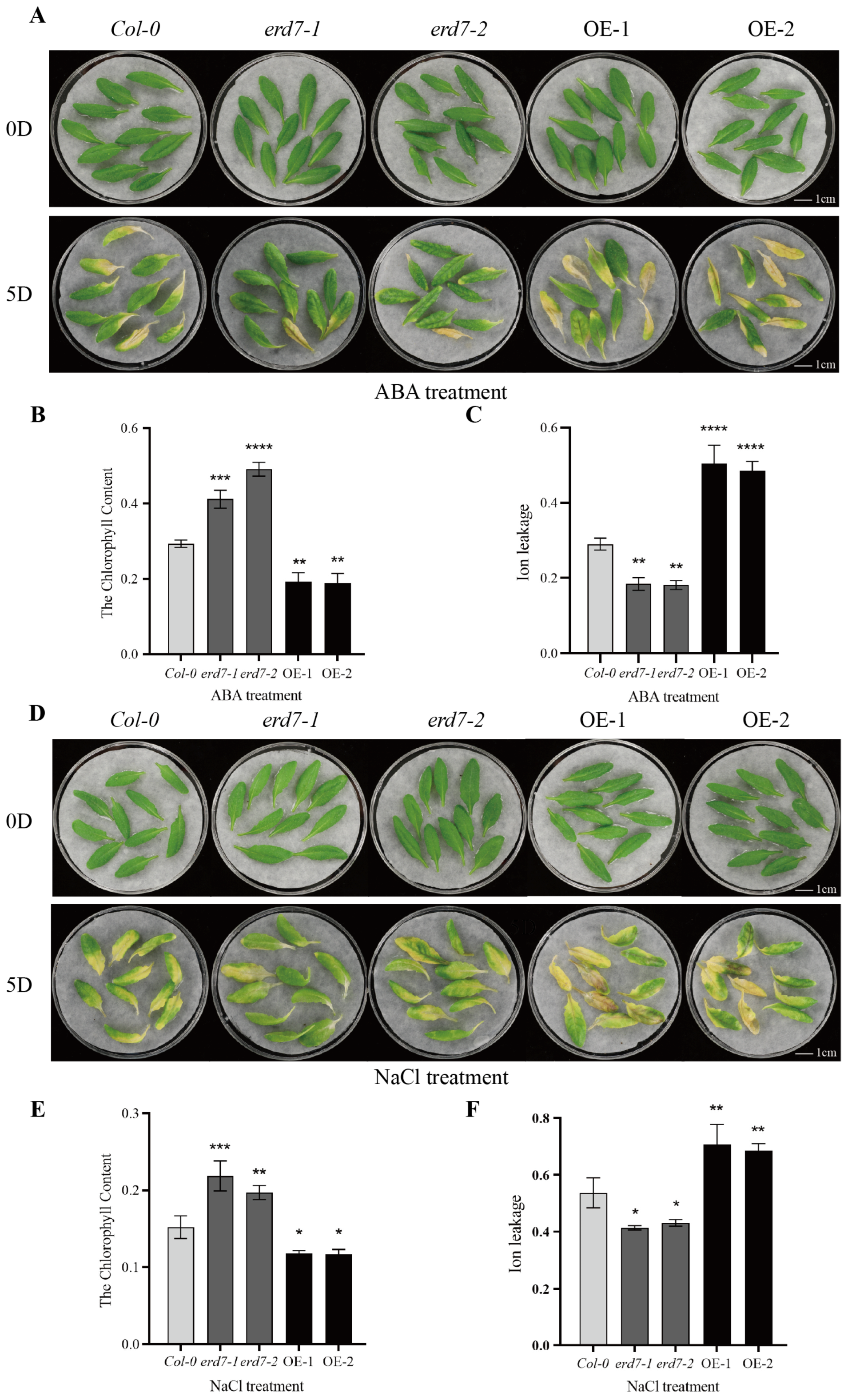
2.3. ERD7 Is Involved in Leaf Senescence Induced by ABA and NaCl
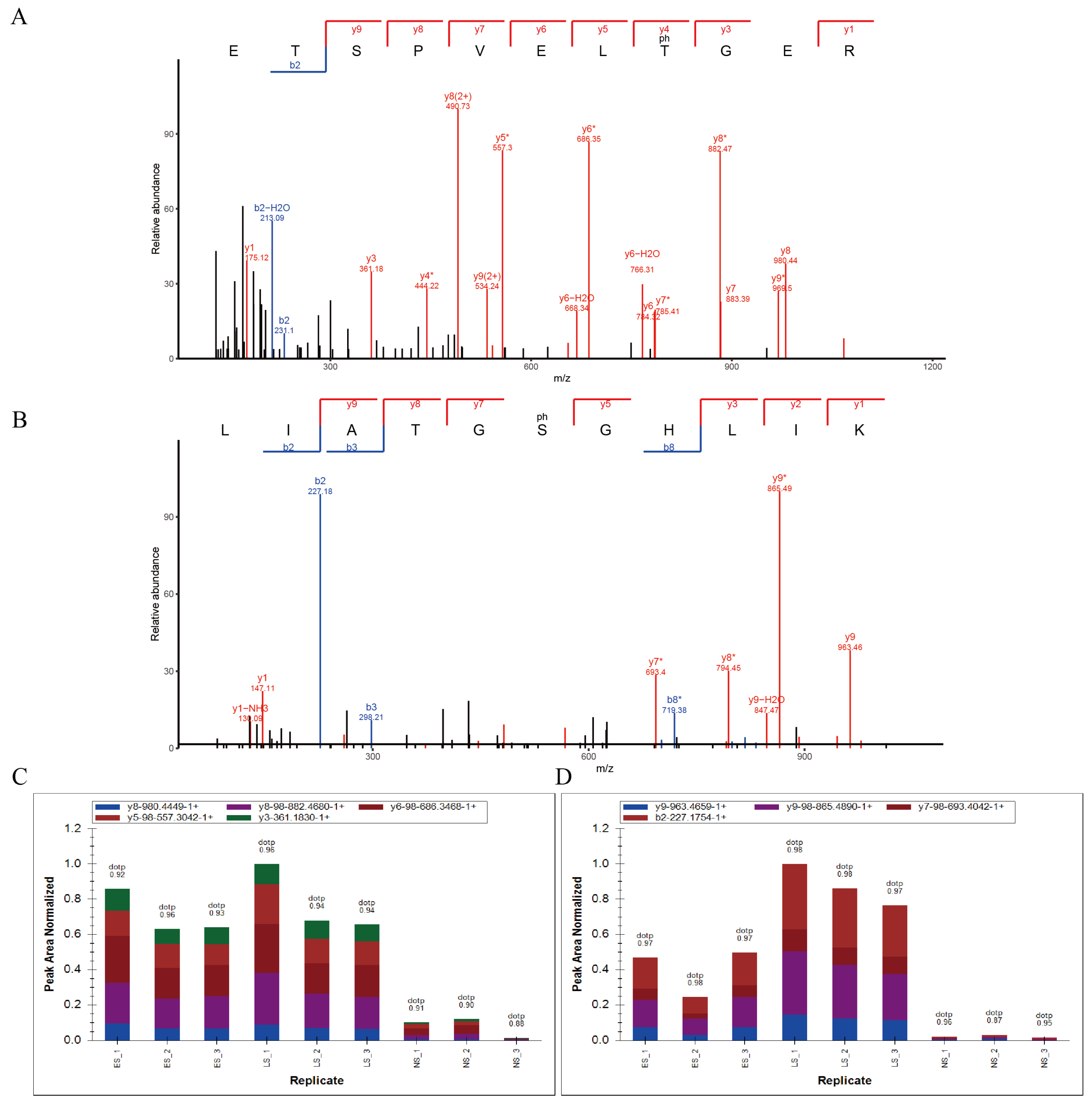
2.4. The Phosphorylation Modification of Thr-225 and Ser-262 Residues Is Up-Regulated during Leaf Senescence
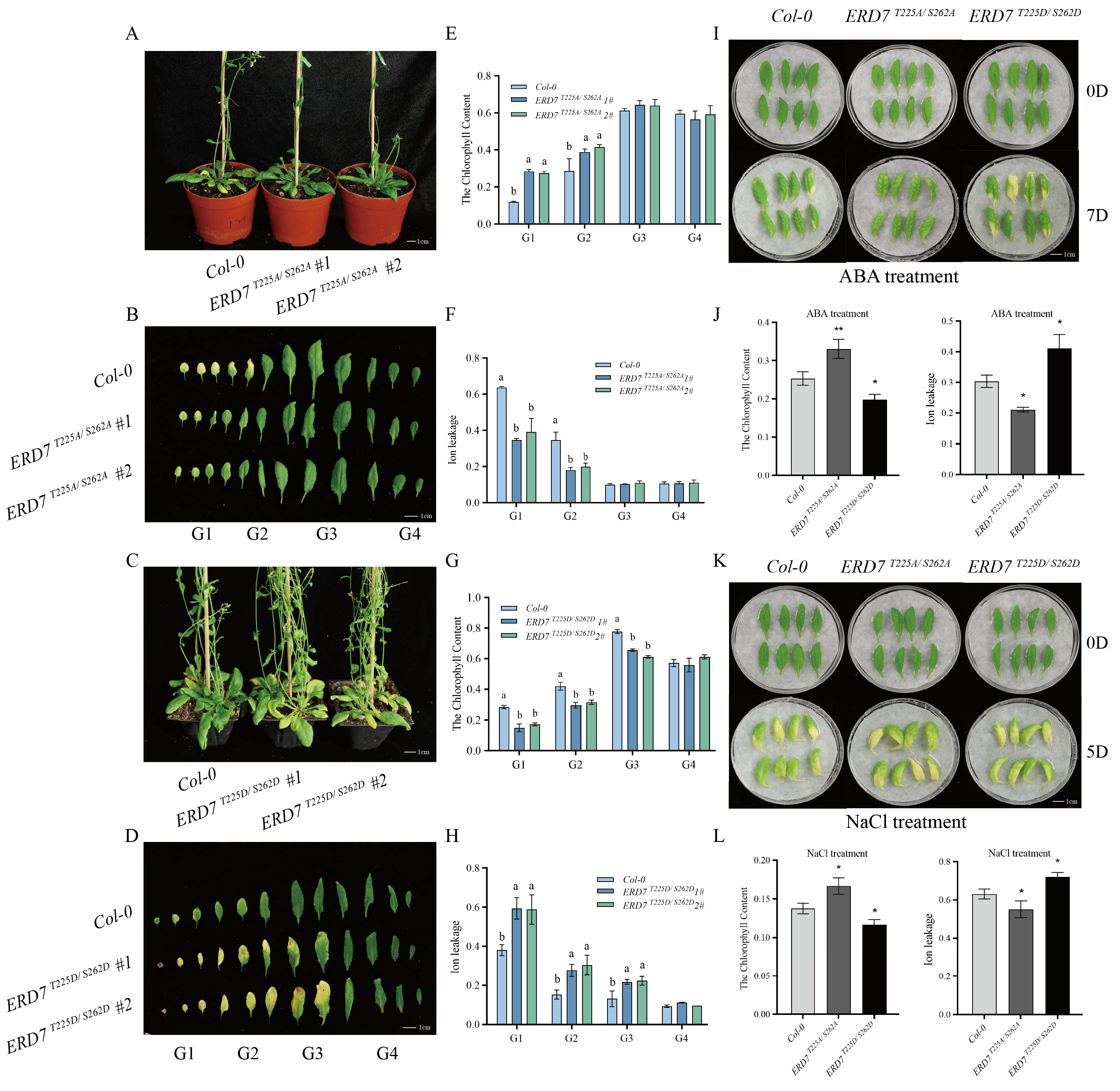
2.5. The Phosphorylation State of Thr-225 and Ser-262 Residues Is Critical for the Function of ERD7 in Regulating Leaf Senescence
2.6. ERD7 Regulates Hydrogen Peroxide Accumulation Depending on T225 and S262 Phosphorylation
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Construct Generation and Arabidopsis Transformation
4.3. Chlorophyll, Fv/Fm, and Electrolyte (Ion) Leakage Rate Measurements
4.4. Induction of Senescence by Detached Leaf Treatments
4.5. Phytohormone and Stress Treatments
4.6. ROS Detection Experiments
4.7. Quantitative RT-PCR Assays
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lim, P.O.; Kim, H.J.; Nam, H.G. Leaf senescence. Annu. Rev. Plant Biol. 2007, 58, 115–136. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Ren, G.; Zhang, K.; Li, Z.; Miao, Y.; Guo, H. Leaf senescence: Progression, regulation, and application. Mol. Hortic. 2021, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Gan, S.; Amasino, R.M. Making Sense of Senescence (Molecular Genetic Regulation and Manipulation of Leaf Senescence). Plant Physiol. 1997, 113, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Buchanan-Wollaston, V.; Page, T.; Harrison, E.; Breeze, E.; Lim, P.O.; Nam, H.G.; Lin, J.F.; Wu, S.H.; Swidzinski, J.; Ishizaki, K.; et al. Comparative transcriptome analysis reveals significant differences in gene expression and signalling pathways between developmental and dark/starvation-induced senescence in Arabidopsis. Plant J. 2005, 42, 567–585. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, J.H.; Lyu, J.I.; Woo, H.R.; Lim, P.O. New insights into the regulation of leaf senescence in Arabidopsis. J. Exp. Bot. 2018, 69, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Schippers, J.H. Transcriptional networks in leaf senescence. Curr. Opin. Plant Biol. 2015, 27, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Schippers, J.H.; Schmidt, R.; Wagstaff, C.; Jing, H.C. Living to Die and Dying to Live: The Survival Strategy behind Leaf Senescence. Plant Physiol. 2015, 169, 914–930. [Google Scholar] [CrossRef]
- Quint, M.; Delker, C.; Franklin, K.A.; Wigge, P.A.; Halliday, K.J.; Van Zanten, M. Molecular and genetic control of plant thermomorphogenesis. Nat. Plants 2016, 2, 15190. [Google Scholar] [CrossRef]
- Balazadeh, S.; Siddiqui, H.; Allu, A.D.; Matallana-Ramirez, L.P.; Caldana, C.; Mehrnia, M.; Zanor, M.I.; Köhler, B.; Mueller-Roeber, B. A gene regulatory network controlled by the NAC transcription factor ANAC092/AtNAC2/ORE1 during salt-promoted senescence. Plant J. 2010, 62, 250–264. [Google Scholar] [CrossRef]
- Sade, N.; Del Mar Rubio-Wilhelmi, M.; Umnajkitikorn, K.; Blumwald, E. Stress-induced senescence and plant tolerance to abiotic stress. J. Exp. Bot. 2018, 69, 845–853. [Google Scholar] [CrossRef]
- Jajic, I.; Sarna, T.; Strzalka, K. Senescence, Stress, and Reactive Oxygen Species. Plants 2015, 4, 393–411. [Google Scholar] [CrossRef] [PubMed]
- Jibran, R.; Hunter, D.A.; Dijkwel, P.P. Hormonal regulation of leaf senescence through integration of developmental and stress signals. Plant Mol. Biol. 2013, 82, 547–561. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Peng, J.; Wen, X.; Guo, H. Ethylene-insensitive3 is a senescence-associated gene that accelerates age-dependent leaf senescence by directly repressing miR164 transcription in Arabidopsis. Plant Cell 2013, 25, 3311–3328. [Google Scholar] [CrossRef]
- Li, Q.; Xu, F.; Chen, Z.; Teng, Z.; Sun, K.; Li, X.; Yu, J.; Zhang, G.; Liang, Y.; Huang, X.; et al. Synergistic interplay of ABA and BR signal in regulating plant growth and adaptation. Nat. Plants 2021, 7, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Gan, S. AtNAP, a NAC family transcription factor, has an important role in leaf senescence. Plant J. 2006, 46, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Chu, C. Towards understanding abscisic acid-mediated leaf senescence. Sci. China Life Sci. 2015, 58, 506–508. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Prochazkova, D.; Wilhelmova, N. Leaf senescence and activitiesof the antioxidant enzymes. Biol. Plant 2007, 51, 401–406. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef]
- Lee, D.; Lal, N.K.; Lin, Z.D.; Ma, S.; Liu, J.; Castro, B.; Toruño, T.; Dinesh-Kumar, S.P.; Coaker, G. Regulation of reactive oxygen species during plant immunity through phosphorylation and ubiquitination of RBOHD. Nat. Commun. 2020, 11, 1838. [Google Scholar] [CrossRef]
- Morales, J.; Kadota, Y.; Zipfel, C.; Molina, A.; Torres, M.A. The Arabidopsis NADPH oxidasesRbohDandRbohFdisplay differential expression patterns and contributions during plant immunity. J. Exp. Bot. 2016, 67, 1663–1676. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. The aging process. Basic Life Sci. 1988, 49, 1057–1065. [Google Scholar] [PubMed]
- Taji, T.; Seki, M.; Yamaguchi-Shinozaki, K.; Kamada, H.; Giraudat, J.; Shinozaki, K. Mapping of 25 drought-inducible genes, RD and ERD, in Arabidopsis thaliana. Plant Cell Physiol. 1999, 40, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Tian, N.; She, F.; Cao, A.; Wu, W.; Zheng, S.; Yang, N. Characteristics analysis of Early Responsive to Dehydration genes in Arabidopsis thaliana (AtERD). Plant Signal Behav. 2022, 2, 2105021. [Google Scholar] [CrossRef] [PubMed]
- Doner, N.M.; Seay, D.; Mehling, M.; Sun, S.; Gidda, S.K.; Schmitt, K.; Braus, G.H.; Ischebeck, T.; Chapman, K.D.; Dyer, J.M.; et al. Arabidopsis thaliana EARLY RESPONSIVE TO DEHYDRATION 7 Localizes to Lipid Droplets via Its Senescence Domain. Front. Plant Sci. 2021, 12, 658961. [Google Scholar] [CrossRef] [PubMed]
- Joshi, D.C.; Bakowska, J.C. SPG20 Protein Spartin Associates with Cardiolipin via Its Plant-Related Senescence Domain and Regulates Mitochondrial Ca2+ Homeostasis. PLoS ONE 2011, 6, e19290. [Google Scholar] [CrossRef] [PubMed]
- Kiyosue, T.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Cloning of cDNAs for genes that are early-responsive to dehydration stress (ERDs) in Arabidopsis thaliana L.: Identification of three ERDs as HSP cognate genes. Plant Mol. Biol. 1994, 25, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Smart, C.M.; Hosken, S.E.; Thomas, H.; Greaves, J.A.; Blair, B.G.; Schuch, W. The timing of maize leaf senescence and characterisation of senescence-related cDNAs. Physiol. Plant 2006, 93, 673–682. [Google Scholar] [CrossRef]
- Buchanan-Wollaston, V.; Ainsworth, C. Leaf senescence in Brassica napus: Cloning of senescence related genes by subtractive hybridisation. Plant Mol. Biol. 1997, 33, 821–834. [Google Scholar] [CrossRef]
- Rampitsch, C.; Bykova, N.V. The beginnings of crop phosphoproteomics: Exploring early warning systems of stress. Front. Plant Sci. 2012, 3, 144. [Google Scholar] [CrossRef]
- Hashiguchi, A.; Komatsu, S. Impact of Post-Translational Modifications of Crop Proteins under Abiotic Stress. Proteomes 2016, 4, 42. [Google Scholar] [CrossRef] [PubMed]
- Silva-Sanchez, C.; Li, H.; Chen, S. Recent advances and challenges in plant phosphoproteomics. Proteomics 2015, 15, 1127–1141. [Google Scholar] [CrossRef] [PubMed]
- Kosová, K.; Vítámvás, P.; Prášil, I.T.; Klíma, M.; Renaut, J. Plant Proteoforms under Environmental Stress: Functional Proteins Arising From a Single Gene. Front. Plant Sci. 2021, 12, 793113. [Google Scholar] [CrossRef]
- Sewelam, N.; Kazan, K.; Schenk, P.M. Global Plant Stress Signaling: Reactive Oxygen Species at the Cross-Road. Front. Plant Sci. 2016, 7, 187. [Google Scholar] [CrossRef] [PubMed]
- Vermot, A.; Petit-Härtlein, I.; Smith, S.M.E.; Fieschi, F. NADPH Oxidases (NOX): An Overview from Discovery, Molecular Mechanisms to Physiology and Pathology. Antioxidants 2021, 10, 890. [Google Scholar] [CrossRef] [PubMed]
- Ben Rejeb, K.; Benzarti, M.; Debez, A.; Bailly, C.; Savouré, A.; Abdelly, C. NADPH oxidase-dependent H2O2 production is required for salt-induced antioxidant defense in Arabidopsis thaliana. J. Plant Physiol. 2015, 174, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Bengoa Luoni, S.; Astigueta, F.H.; Nicosia, S.; Moschen, S.; Fernandez, P.; Heinz, R. Transcription Factors Associated with Leaf Senescence in Crops. Plants 2019, 8, 411. [Google Scholar] [CrossRef]
- Woo, H.R.; Kim, H.J.; Lim, P.O.; Nam, H.G. Leaf Senescence: Systems and Dynamics Aspects. Annu. Rev. Plant Biol. 2019, 70, 347–376. [Google Scholar] [CrossRef]
- Hilker, M.; Schmülling, T. Stress priming, memory, and signalling in plants. Plant Cell Environ. 2019, 42, 753–761. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, D.; Wang, Y.; Zhou, R.; Wang, L.; Zhang, Y.; Yu, J.; Gong, H.; You, J.; Zhang, X. Genome-wide identification and comprehensive analysis of the NAC transcription factor family in Sesamum indicum. PLoS ONE 2018, 13, e0199262. [Google Scholar] [CrossRef]
- Pascual, M.; Torre, F.D.L.; Cañas, R.A.; Cánovas, F.M.; Ávila, C. NAC Transcription Factors in Woody Plants. Prog. Bot. 2018, 80, 195–222. [Google Scholar]
- Guo, Y.; Gan, S.S. Convergence and divergence in gene expression profiles induced by leaf senescence and 27 senescence-promoting hormonal, pathological and environmental stress treatments. Plant Cell Environ. 2011, 35, 644–655. [Google Scholar] [CrossRef] [PubMed]
- Besseau, S.; Li, J.; Palva, E.T. WRKY54 and WRKY70 co-operate as negative regulators of leaf senescence in Arabidopsis thaliana. J. Exp. Bot. 2012, 63, 2667–2679. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Lv, B.; Luo, L.; He, J.; Mao, C.; Xi, D.; Ming, F. The NAC-type transcription factor OsNAC2 regulates ABA-dependent genes and abiotic stress tolerance in rice. Sci. Rep. 2017, 7, 40641. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Wang, Y.; Li, B.; Chang, J.; Chen, M.; Li, K.; Yang, G.; He, G. TaNAC29, a NAC transcription factor from wheat, enhances salt and drought tolerance in transgenic Arabidopsis. BMC Plant Biol. 2015, 15, 268. [Google Scholar] [CrossRef]
- Kiyosue, T.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Characterization of two cDNAs (ERD10 and ERD14) corresponding to genes that respond rapidly to dehydration stress in Arabidopsis thaliana. Plant Cell Physiol. 1994, 35, 225–231. [Google Scholar] [PubMed]
- Kiyosue, T.; Abe, H.; Yamaguchi-Shinozaki, K.; Shinozaki, K. ERD6, a cDNA clone for an early dehydration-induced gene of Arabidopsis, encodes a putative sugar transporter. Biochim. Biophys. Acta 1998, 1370, 187–191. [Google Scholar] [CrossRef]
- Gallino, J.P.; Ruibal, C.; Casaretto, E.; Fleitas, A.L.; Bonnecarrère, V.; Borsani, O.; Vidal, S. A Dehydration-Induced Eukaryotic Translation Initiation Factor iso4G Identified in a Slow Wilting Soybean Cultivar Enhances Abiotic Stress Tolerance in Arabidopsis. Front. Plant Sci. 2009, 9, 542–548. [Google Scholar] [CrossRef]
- Tan, S.; Sha, Y.; Sun, L.; Li, Z. Abiotic Stress-Induced Leaf Senescence: Regulatory Mechanisms and Application. Int. J. Mol. Sci. 2023, 24, 11996. [Google Scholar] [CrossRef]
- Cheng, M.C.; Liao, P.M.; Kuo, W.W.; Lin, T.P. The Arabidopsis ETHYLENE RESPONSE FACTOR1 Regulates Abiotic Stress-Responsive Gene Expression by Binding to Different cis-Acting Elements in Response to Different Stress Signals. Plant Physiol. 2013, 162, 1566–1582. [Google Scholar] [CrossRef]
- Rasmussen, S.; Barah, P.; Suarez-Rodriguez, M.C.; Bressendorff, S.; Friis, P.; Costantino, P.; Bones, A.M.; Nielsen, H.B.; Mundy, J. Transcriptome Responses to Combinations of Stresses in Arabidopsis. Plant Physiol. 2013, 161, 1783–1794. [Google Scholar] [CrossRef]
- Bray, E.A. Genes commonly regulated by water-deficit stress in Arabidopsis thaliana. J. Exp. Bot. 2004, 55, 2331–2341. [Google Scholar] [CrossRef] [PubMed]
- Barajas-Lopez, J.D.; Tiwari, A.; Zarza, X.; Shaw, M.W.; Pascual, J.S.; Punkkinen, M.; Bakowska, J.C.; Munnik, T.; Fujii, H. EARLY RESPONSE TO DEHYDRATION 7 Remodels Cell Membrane Lipid Composition during Cold Stress in Arabidopsis. Plant Cell Physiol. 2021, 62, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Panavas, T.; Pikula, A.; Reid, P.D.; Rubinstein, B.; Walker, E.L. Identification of senescence-associated genes from daylily petals. Plant Mol. Biol. 1999, 40, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Olsen, J.V.; Blagoev, B.; Gnad, F.; Macek, B.; Kumar, C.; Mortensen, P.; Mann, M. Global, In Vivo, and Site-Specific Phosphorylation Dynamics in Signaling Networks. Cell 2006, 127, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Fujii, H.; Zhu, J.K. An autophosphorylation site of the protein kinase SOS2 is important for salt tolerance in Arabidopsis. Mol. Plant 2009, 2, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Cui, Y.; Xu, F.; Xu, X.; Gao, G.; Wang, Y.; Guo, Z.; Wang, D.; Wang, N.N. SENESCENCE-SUPPRESSED PROTEIN PHOSPHATASE Directly Interacts with the Cytoplasmic Domain of SENESCENCE-ASSOCIATED RECEPTOR-LIKE KINASE and Negatively Regulates Leaf Senescence in Arabidopsis. Plant Physiol. 2015, 169, 1275–1291. [Google Scholar] [CrossRef] [PubMed]
- Osakabe, Y.; Maruyama, K.; Seki, M.; Satou, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Leucine-Rich Repeat Receptor-Like Kinase1 Is a Key Membrane-Bound Regulator of Abscisic Acid Early Signaling in Arabidopsis. Plant Cell 2005, 17, 1105–1119. [Google Scholar] [CrossRef]
- Wang, W.; Chen, S.; Zhong, G.; Gao, C.; Zhang, Q.; Tang, D. MITOGEN-ACTIVATED PROTEIN KINASE3 enhances disease resistance of edr1 mutants by phosphorylating MAPKKK5. Plant Physiol. 2023, 28, 472. [Google Scholar] [CrossRef]
- Zhou, C.; Cai, Z.; Guo, Y.; Gan, S. An arabidopsis mitogen-activated protein kinase cascade, MKK9-MPK6, plays a role in leaf senescence. Plant Physiol. 2009, 150, 167–177. [Google Scholar] [CrossRef]
- Lu, Y.J.; Li, P.; Shimono, M.; Corrion, A.; Higaki, T.; He, S.Y.; Day, B. Arabidopsis calcium-dependent protein kinase 3 regulates actin cytoskeleton organization and immunity. Nat. Commun. 2020, 11, 6234. [Google Scholar] [CrossRef]
- Wang, W.; Chen, D.; Zhang, X.; Liu, D.; Cheng, Y.; Shen, F. Role of plant respiratory burst oxidase homologs in stress responses. Free Radic. Res. 2018, 52, 826–839. [Google Scholar] [CrossRef] [PubMed]
- Kadota, Y.; Sklenar, J.; Derbyshire, P.; Stransfeld, L.; Asai, S.; Ntoukakis, V.; Jones, J.D.; Shirasu, K.; Menke, F.; Jones, A.; et al. Direct regulation of the NADPH oxidase RBOHD by the PRR-associated kinase BIK1 during plant immunity. Mol. Cell 2014, 54, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Lherminier, J.; Elmayan, T.; Fromentin, J.; Elaraqui, K.T.; Vesa, S.; Morel, J.; Verrier, J.L.; Cailleteau, B.; Blein, J.P.; Simon-Plas, F. NADPH oxidase-mediated reactive oxygen species production: Subcellular localization and reassessment of its role in plant defense. Mol. Plant Microbe Interact. 2009, 22, 868–881. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.S.; Yang, C.Y. Physiological Responses and Expression Profile of NADPH Oxidase in Rice (Oryza Sativa) Seedlings under Different Levels of Submergence. Rice 2016, 9, 2. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, J.; Gu, G.; Jin, L.; Chen, C.; Lin, Z.; Song, J.; Xie, X. Integrative Analyses of Biochemical Properties and Transcriptome Reveal the Dynamic Changes in Leaf Senescence of Tobacco (Nicotiana tabacum L.). Front. Genet. 2021, 12, 790167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Guo, Y. Hormone Treatments in Studying Leaf Senescence. Methods Mol. Biol. 2018, 1744, 125–132. [Google Scholar] [PubMed]

| Protein Accession | Peptide Modified Sequence | NS/ES Ratio | NS/ES p Value | NS/LS Ratio | NS/LS p Value |
|---|---|---|---|---|---|
| A0A1P8AYB1 | ETSPVELT(ph)GER | 0.10874 | 0.00147 | 0.09919 | 0.00373 |
| A0A1P8AYB1 | LIATGS(ph)GHLIK | 0.05846 | 0.00889 | 0.02689 | 0.00024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, R.; Pan, X.; Li, W.; Zhang, Z.; Guo, Y. Phosphorylation of Thr-225 and Ser-262 on ERD7 Promotes Age-Dependent and Stress-Induced Leaf Senescence through the Regulation of Hydrogen Peroxide Accumulation in Arabidopsis thaliana. Int. J. Mol. Sci. 2024, 25, 1328. https://doi.org/10.3390/ijms25021328
Wu R, Pan X, Li W, Zhang Z, Guo Y. Phosphorylation of Thr-225 and Ser-262 on ERD7 Promotes Age-Dependent and Stress-Induced Leaf Senescence through the Regulation of Hydrogen Peroxide Accumulation in Arabidopsis thaliana. International Journal of Molecular Sciences. 2024; 25(2):1328. https://doi.org/10.3390/ijms25021328
Chicago/Turabian StyleWu, Rongrong, Xiaolu Pan, Wei Li, Zenglin Zhang, and Yongfeng Guo. 2024. "Phosphorylation of Thr-225 and Ser-262 on ERD7 Promotes Age-Dependent and Stress-Induced Leaf Senescence through the Regulation of Hydrogen Peroxide Accumulation in Arabidopsis thaliana" International Journal of Molecular Sciences 25, no. 2: 1328. https://doi.org/10.3390/ijms25021328
APA StyleWu, R., Pan, X., Li, W., Zhang, Z., & Guo, Y. (2024). Phosphorylation of Thr-225 and Ser-262 on ERD7 Promotes Age-Dependent and Stress-Induced Leaf Senescence through the Regulation of Hydrogen Peroxide Accumulation in Arabidopsis thaliana. International Journal of Molecular Sciences, 25(2), 1328. https://doi.org/10.3390/ijms25021328







