Recent Advances in Synthesis of Non-Alternating Polyketone Generated by Copolymerization of Carbon Monoxide and Ethylene
Abstract
:1. Introduction
2. Chain Structure of PKs without a Perfect Alternating Structure
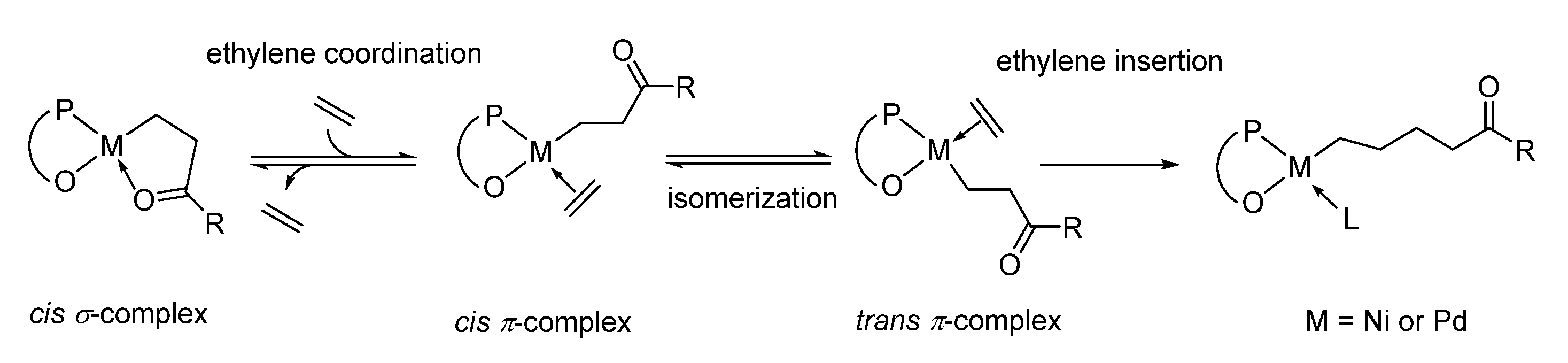
3. Catalytic Copolymerization Mechanism
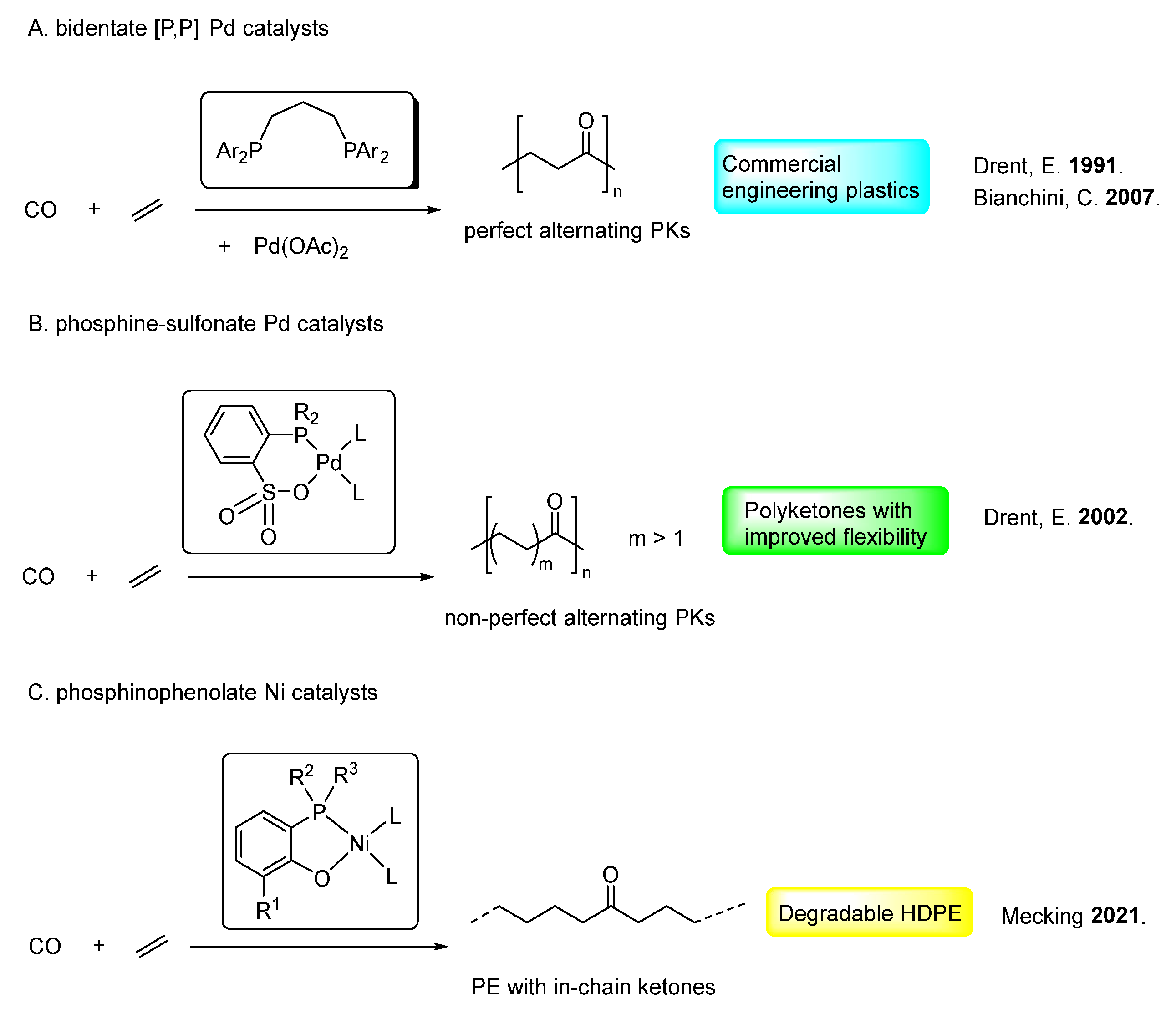
4. Non-Alternating Copolymerization Catalysts
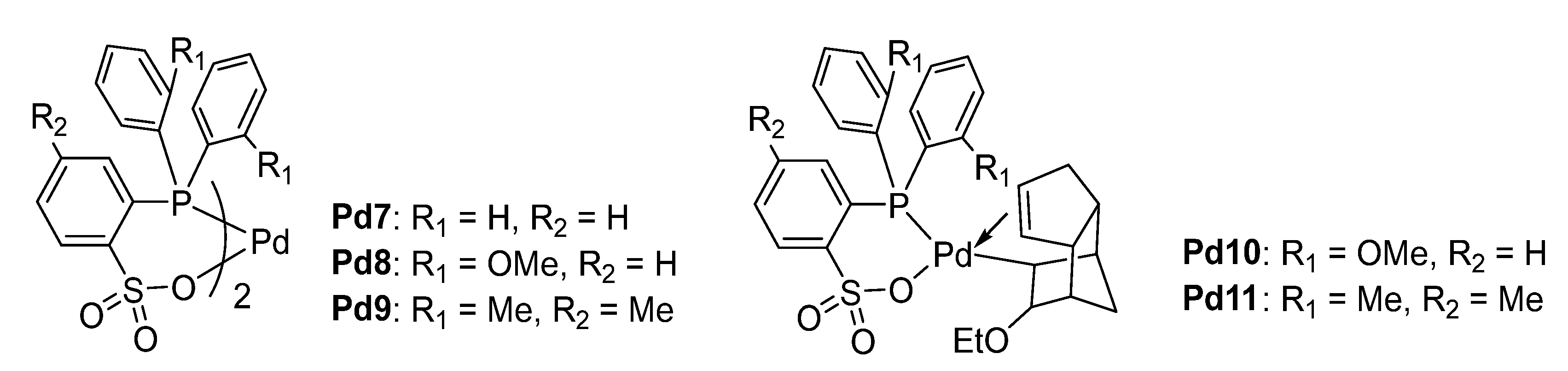
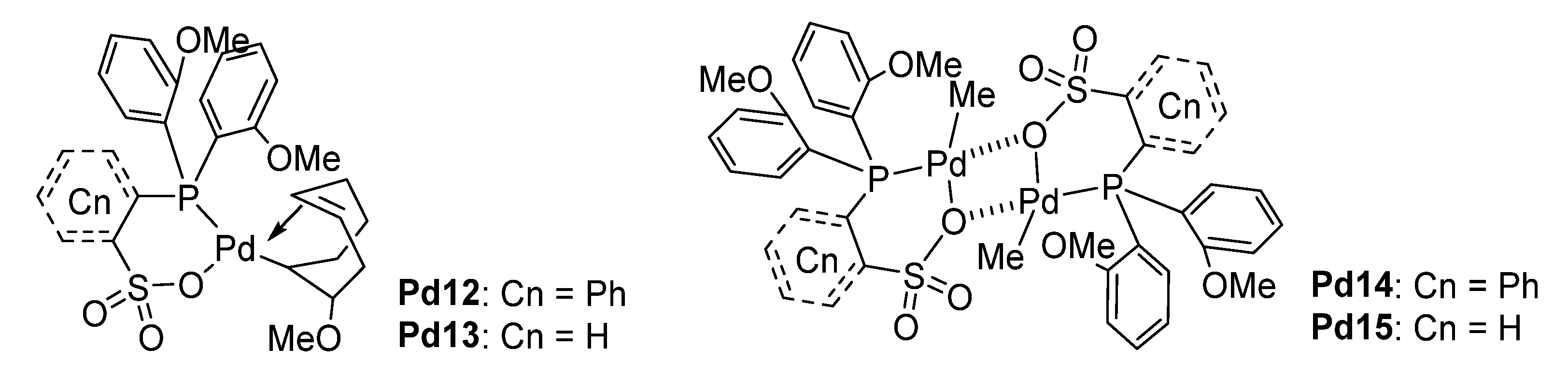
4.1. Phosphine–Sulfonate Pd Catalysts
4.2. Diphosphazane Monoxide Pd/Ni Catalysts
4.3. Phosphinophenolate Ni Catalysts
5. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Drent, E.; Budzelaar, P.H. Palladium-catalyzed alternating copolymerization of alkenes and carbon monoxide. Chem. Rev. 1996, 96, 663–682. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Ito, S.; Nozaki, K. Coordination-insertion copolymerization of fundamental polar monomers. Chem. Rev. 2009, 109, 5215–5244. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, C.; Meli, A. Alternating copolymerization of carbon monoxide and olefins by single-site metal catalysis. Coordin. Chem. Rev. 2002, 225, 35–66. [Google Scholar] [CrossRef]
- Müller, G.; Rieger, B. Propene based thermoplastic elastomers by early and late transition metal catalysis. Prog. Polym. Sci. 2002, 27, 815–851. [Google Scholar] [CrossRef]
- Durand, J.; Milani, B. The role of nitrogen-donor ligands in the palladium-catalyzed polyketones synthesis. Coordin. Chem. Rev. 2006, 250, 542–560. [Google Scholar] [CrossRef]
- Xiao, Z.; Zheng, H.; Du, C.; Zhong, L.; Liao, H.; Gao, J.; Gao, H.; Wu, Q. Enhancement on alternating copolymerization of carbon monoxide and styrene by dibenzobarrelene-based α-diimine palladium catalysts. Macromolecules 2018, 51, 9110–9121. [Google Scholar] [CrossRef]
- Liao, G.; Xiao, Z.; Chen, X.; Du, C.; Zhong, L.; Cheung, C.S.; Gao, H. Fast and regioselective polymerization of para-alkoxystyrene by palladium catalysts for precision production of high-molecular-weight polystyrene derivatives. Macromolecules 2019, 53, 256–266. [Google Scholar] [CrossRef]
- Du, C.; Chu, H.; Xiao, Z.; Zhong, L.; Zhou, Y.; Qin, W.; Liang, G.; Gao, H. Alternating vinylarene–carbon monoxide copolymers: Simple and efficient nonconjugated luminescent macromolecules. Macromolecules 2020, 53, 9337–9344. [Google Scholar] [CrossRef]
- Wang, S.; Wang, L.; Zhong, L.; Xu, R.; Wang, X.; Kang, W.; Gao, H. C1-symmetric tert-butyl substituted pyridylamido hafnium complex for ethylene, α-olefin, and styrene polymerizations. Eur. Polym. J. 2020, 131, 109709–109719. [Google Scholar] [CrossRef]
- Du, C.; Cheung, C.S.; Zheng, H.; Li, D.; Du, W.; Gao, H.; Liang, G.; Gao, H. Bathochromic-shifted emissions by postfunctionalization of nonconjugated polyketones. ACS Appl. Mater. Interfaces 2021, 13, 59288–59297. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhong, L.; Du, C.; Du, W.; Zheng, H.; Cheung, C.S.; Wang, L.; Gao, H. Unprecedented steric and positioning effects of comonomer substituents on α-diimine palladium-catalyzed vinyl arene/CO copolymerization. Macromolecules 2021, 54, 687–695. [Google Scholar] [CrossRef]
- Cheung, C.S.; Shi, X.B.; Pei, L.X.; Du, C.; Gao, H.; Qiu, Z.L.; Gao, H.Y. Alternating copolymerization of carbon monoxide and vinyl arenes using [N,N] bidentate palladium catalysts. J. Polym. Sci. 2022, 60, 1448–1467. [Google Scholar] [CrossRef]
- Abu-Surrah, A.S.; Lappalainen, K.; Kettunen, M.; Repo, T.; Leskelä, M.; Hodali, H.A.; Rieger, B. Homo- and copolymerization of strained cyclic olefins with new palladium(II) complexes bearing ethylene-bridged heterodonor ligands. Macromo. Chem. Phys. 2001, 202, 599–603. [Google Scholar] [CrossRef]
- Kettunen, M.; Abu-Surrah, A.S.; Repo, T.; Leskelä, M. Copolymerization of carbon monoxide with-methylenecycloalkane and dienes:: Synthesis of functionalized aliphatic polyketones. Polym. Int. 2001, 50, 1223–1227. [Google Scholar] [CrossRef]
- Wang, X.; Seidel, F.W.; Nozaki, K. Synthesis of polyethylene with in-chain alpha,beta-unsaturated ketone and isolated ketone units: Pd-catalyzed ring-opening copolymerization of cyclopropenone with ethylene. Angew. Chem. Int. Ed. Engl. 2019, 58, 12955–12959. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Luo, J.; Liu, T.; Jia, L. Dual-site catalysis for sustainable polymers to replace current commodity polymers—Carbonylative copolymerization of ethylene, ethylene oxide, and tetrahydrofuran. Chem. Commun. 2020, 56, 15341–15344. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, S.Y.; Bao, R.Y.; Liu, Z.Y.; Yang, M.B.; Tan, C.B.; Yang, W. Progress in polyketone materials: Blends and composites. Polym. Int. 2018, 67, 1478–1487. [Google Scholar] [CrossRef]
- Zong, Y.L.; Li, Q.K.; Mu, H.L.; Jian, Z.B. Palladium promoted copolymerization of carbon monoxide with polar or non-polar olefinic monomers. Curr. Org. Chem. 2021, 25, 287–300. [Google Scholar] [CrossRef]
- Nozaki, K.; Kosaka, N.; Graubner, V.M.; Hiyama, T. Methylenation of optically active γ-polyketones. Polym. J. 2002, 34, 376–382. [Google Scholar] [CrossRef]
- Kosaka, N.; Hiyama, T.; Nozaki, K. Baeyer-Villiger oxidation of an optically active 1,4-polyketone. Macromolecules 2004, 37, 4484–4487. [Google Scholar] [CrossRef]
- Kosaka, N.; Oda, T.; Hiyama, T.; Nozaki, K. Synthesis and photoisomerization of optically active 1,4-polyketones substituted by azobenzene side chains. Macromolecules 2004, 37, 3159–3164. [Google Scholar] [CrossRef]
- Pérez-Foullerat, D.; Hild, S.; Mücke, A.; Rieger, B. Synthesis and properties of poly (ketone-co-alcohol) materials: Shape memory thermoplastic elastomers by control of the glass transition process. Macromo. Chem. Phys. 2004, 205, 374–382. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Broekhuis, A.A.; Stuart, M.C.A.; Picchioni, F. Polymeric Amines by chemical modifications of alternating aliphatic polyketones. J. Appl. Polym. Sci. 2008, 107, 262–271. [Google Scholar] [CrossRef]
- Toncelli, C.; Bouwhuis, S.; Broekhuis, A.A.; Picchioni, F. Cyclopentadiene-functionalized polyketone as self-cross-linking thermo-reversible thermoset with increased softening temperature. J. Appl. Polym. Sci. 2015, 133, 42924. [Google Scholar] [CrossRef]
- Ataollahi, N.; Girardi, F.; Cappelletto, E.; Vezzù, K.; Di Noto, V.; Scardi, P.; Callone, E.; Di Maggio, R. Chemical modification and structural rearrangements of polyketone-based polymer membrane. J. Appl. Polym. Sci. 2017, 134, 46549. [Google Scholar] [CrossRef]
- Hwang, S.Y.; Cho, Y.S.; Kim, T.; Jung, Y.S.; Hwang, T.S. Synthesis of highly durable sulfonated polyketone fibers by direct sulfonation reaction and their adsorption properties for heavy metals. Macromol. Res. 2020, 28, 336–342. [Google Scholar] [CrossRef]
- Luleburgaz, S.; Hizal, G.; Tunca, U.; Durmaz, H. Modification of polyketone via chlorodimethylsilane-mediated reductive etherification reaction: A practical way for alkoxy-functional polymers. Macromolecules 2021, 54, 5106–5116. [Google Scholar] [CrossRef]
- Agostinelli, E.; Belli, F.; Tempera, G.; Mura, A.; Floris, G.; Toniolo, L.; Vavasori, A.; Fabris, S.; Momo, F.; Stevanato, R. Polyketone polymer: A new support for direct enzyme immobilization. J. Biotechnol. 2007, 127, 670–678. [Google Scholar] [CrossRef] [PubMed]
- El Ghanem, H.M.; Abdul Jawad, S.D.; Al-Saleh, M.H.; Hussain, Y.A.; Abu-Surrah, A.S. Electrical impedance spectroscopic study of CNT/ethylene-alt-CO/propylene-alt-CO polyketones nanocomposite. J. Macromol. Sci. 2014, 53, 878–892. [Google Scholar] [CrossRef]
- Won, J.S.; Jin, D.Y.; Lee, J.E.; Lee, S.G. Effects of finishing conditions on the properties of polyketone fiber. Fiber. Polym. 2015, 16, 1908–1916. [Google Scholar] [CrossRef]
- Ataollahi, N.; Vezzù, K.; Nawn, G.; Pace, G.; Cavinato, G.; Girardi, F.; Scardi, P.; Di Noto, V.; Di Maggio, R. A polyketone-based anion exchange membrane for electrochemical applications: Synthesis and characterization. Electrochim. Acta 2017, 226, 148–157. [Google Scholar] [CrossRef]
- Mu, J.L.; Fan, W.J.; Shan, S.Y.; Su, H.Y.; Wu, S.S.; Jia, Q.M. Thermal degradation kinetics of polyketone based on styrene and carbon monoxide. Thermochim. Acta 2014, 579, 74–79. [Google Scholar] [CrossRef]
- Reeves, J.A.; Allegrezza, M.L.; Konkolewicz, D. Rise and Fall: Poly(phenyl vinyl ketone) photopolymerization and photodegradation under visible and uv radiation. Macromol. Rapid. Commun. 2017, 38, 1600623. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Pearson, A.; Kazemi, Y.; Kakroodi, A.; Hammami, A.; Heydrich, M.; Xu, B.; Naguib, H.E. Influence of hygrothermal conditioning on the chemical structure and thermal mechanical properties of aliphatic polyketone. Polym. Degrad. Stabil. 2020, 179, 109260. [Google Scholar] [CrossRef]
- Morgen, T.O.; Baur, M.; Gottker-Schnetmann, I.; Mecking, S. Photodegradable branched polyethylenes from carbon monoxide copolymerization under benign conditions. Nat. Commun. 2020, 11, 3693. [Google Scholar] [CrossRef] [PubMed]
- Bovey, F.A.; Gooden, R.; Schilling, F.C.; Winslow, F.H. Carbon-13 NMR study of the solid-state photochemistry of poly(ethylene-co-carbon monoxide). Macromolecules 2002, 21, 938–944. [Google Scholar] [CrossRef]
- Xu, F.Y.; Chien, J.C.W. Photodegradation of alpha-olefin/carbon monoxide alternating copolymer. Macromolecules 2002, 26, 3485–3489. [Google Scholar] [CrossRef]
- Walter, R.; August, M. Production of Ketonic Bodies. U.S. Patent No. 2,577,208, 4 December 1951. [Google Scholar]
- Keim, W.; Maas, H. Copolymerization of ethylene and carbon monoxide by phosphinite-modified palladium catalysts. J. Organomet. Chem. 1996, 514, 271–276. [Google Scholar] [CrossRef]
- Bianchini, C.; Lee, H.M.; Meli, A.; Moneti, S.; Vizza, F.; Fontani, M.; Zanello, P. Copolymerization of carbon monoxide with ethene catalyzed by palladium(ii) complexes of 1,3-bis(diphenylphosphino)propane ligands bearing different substituents on the carbon backbone. Macromolecules 1999, 32, 4183–4193. [Google Scholar] [CrossRef]
- Doherty, S.; Eastham, G.R.; Tooze, R.P.; Scanlan, T.H.; Williams, D.; Elsegood, M.R.J.; Clegg, W. Palladium complexes of C2-, C3-, and C4-bridged bis(phospholyl) ligands: remarkably active catalysts for the copolymerization of ethylene and carbon monoxide. Organometallics 1999, 18, 3558–3560. [Google Scholar] [CrossRef]
- Lindner, E.; Schmid, M.; Wegner, P.; Nachtigal, C.; Steimann, M.; Fawzi, R. Palladium(II) complexes with hemilabile etherdiphos ligands in the alternating copolymerization of carbon monoxide with olefins. Inorganica Chim. Acta. 1999, 296, 103–113. [Google Scholar] [CrossRef]
- Bianchini, C.; Lee, H.M.; Meli, A.; Oberhauser, W.; Vizza, F.; Brüggeller, P.; Rainer, H.B.; Langes, C. New structurally rigid palladium catalysts for the alternating copolymerization of carbon monoxide and ethene. Chem. Commun. 2000, 9, 777–778. [Google Scholar] [CrossRef]
- Verspui, G.; Schanssema, F.; Sheldon, R.A. A stable, conspicuously active, water-soluble pd catalyst for the alternating copolymerization of ethene and CO in water. Angew. Chem. Int. Ed. Engl. 2000, 39, 804–806. [Google Scholar] [CrossRef]
- Doherty, S.; Robins, E.G.; Knight, J.G.; Newman, C.R.; Rhodes, B.; Champkin, P.A.; Clegg, W. Selectivity for the methoxycarbonylation of ethylene versus CO ethylene copolymerization with catalysts based on C4-bridged bidentate phosphines and phospholes. J. Organomet. Chem. 2001, 640, 182–196. [Google Scholar] [CrossRef]
- Lu, C.C.; Peters, J.C. Catalytic copolymerization of CO and ethylene with a charge neutral palladium(II) zwitterion. J. Am. Chem. Soc. 2002, 124, 5272–5273. [Google Scholar] [CrossRef]
- Bianchini, C.; Meli, A.; Oberhauser, W.; Claver, C.; Garcia Suarez, E.J. Unraveling the o-methoxy effect in the CO/ethene copolymerization reaction by diphosphanepalladium(II) catalysis. Eur. J. Inorg. Chem. 2007, 2007, 2702–2710. [Google Scholar] [CrossRef]
- Drent, E.; Vanbroekhoven, J.A.M.; Doyle, M.J. Efficient palladium catalysts for the copolymerization of carbon-monoxide with olefins to produce perfectly alternating polyketones. J. Organomet. Chem. 1991, 417, 235–251. [Google Scholar] [CrossRef]
- Hyosung. Available online: https://www.hyosung.com/ (accessed on 28 December 2023.).
- Drent, E.; van Dijk, R.; van Ginkel, R.; van Oort, B.; Pugh, R.I. The first example of palladium catalysed non-perfectly alternating copolymerisation of ethene and carbon monoxide. Chem. Commun. 2002, 9, 964–965. [Google Scholar] [CrossRef] [PubMed]
- Baur, M.; Lin, F.; Morgen, T.O.; Odenwald, L.; Mecking, S. Polyethylene materials with in-chain ketones from nonalternating catalytic copolymerization. Science 2021, 374, 604–607. [Google Scholar] [CrossRef]
- Wang, C.; Xia, J.; Zhang, Y.; Hu, X.; Jian, Z. Photodegradable polar-functionalized polyethylenes. Natl. Sci. Rev. 2023, 10, nwad039. [Google Scholar] [CrossRef]
- Sommazzi, A.; Garbassi, F. Olefin-carbon monoxide copolymers. Prog. Polym. Sci. 1997, 22, 1547–1605. [Google Scholar] [CrossRef]
- Belov, G.P. Cationic Pd-II, Ni-II, and Ru-II complexes in the synthesis of alternating copolymers of CO with vinyl monomers. Russ. Chem. Bull. 2002, 51, 1605–1615. [Google Scholar] [CrossRef]
- Bianchini, C.; Meli, A.; Oberhauser, W. Catalyst design and mechanistic aspects of the alternating copolymerisation of ethene and carbon monoxide by diphosphine-modified palladium catalysis. Dalton. Trans. 2003, 13, 2627–2635. [Google Scholar] [CrossRef]
- García Suárez, E.J.; Godard, C.; Ruiz, A.; Claver, C. Alternating and non-alternating Pd-catalysed co-and terpolymerisation of carbon monoxide and alkenes. Eur. J. Inorg. Chem. 2007, 2007, 2582–2593. [Google Scholar] [CrossRef]
- Cavinato, G.; Toniolo, L. Carbonylation of ethene catalysed by Pd(II)-phosphine complexes. Molecules 2014, 19, 15116–15161. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Pan, R.C.; Chen, M.; Liu, Y.; Chen, C.; Lu, X.B. Synthesis of nonalternating polyketones using cationic diphosphazane monoxide-palladium complexes. J. Am. Chem. Soc. 2021, 143, 10743–10750. [Google Scholar] [CrossRef]
- Luo, R.; Newsham, D.K.; Sen, A. Palladium-catalyzed nonalternating copolymerization of ethene and carbon monoxide: Scope and mechanism. Organometallics 2009, 28, 6994–7000. [Google Scholar] [CrossRef]
- Tang, S.; Seidel, F.W.; Nozaki, K. High density polyethylenes bearing isolated in-chain carbonyls. Angew. Chem. Int. Ed. Engl. 2021, 60, 26506–26510. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Song, Y.-H.; Jiao, S.; Zou, C.; Li, S.-H.; Chen, C.; Lu, X.-B.; Liu, Y. Carbonyl functionalized polyethylene materials via Ni- and Pd-diphosphazane monoxide catalyzed nonalternating copolymerization. J. Catal. 2023, 417, 334–340. [Google Scholar] [CrossRef]
- Yonezaki, G.; Seidel, F.W.; Takahashi, K.; Nozaki, K. Nickel-catalyzed selective incorporation of isolated in-chain carbonyls into ethylene/carbon monoxide copolymer using metal carbonyls as a carbonyl source. Bull. Chem. Soc. Jpn. 2023, 96, 545–549. [Google Scholar] [CrossRef]
- Li, S.H.; Pan, R.C.; Ren, B.H.; Yang, J.W.; Kang, X.; Liu, Y. Cationic palladium catalyzed nonalternating copolymerization of ethylene with carbon monoxide: Microstructure analysis and computational study. Chin. J. Chem. 2022, 41, 417–423. [Google Scholar] [CrossRef]
- Voccia, M.; Odenwald, L.; Baur, M.; Lin, F.; Falivene, L.; Mecking, S.; Caporaso, L. Mechanistic insights into Ni(ii)-catalyzed nonalternating ethylene-carbon monoxide copolymerization. J. Am. Chem. Soc. 2022, 144, 15111–15117. [Google Scholar] [CrossRef]
- Soomro, S.S.; Cozzula, D.; Leitner, W.; Vogt, H.; Müller, T.E. The microstructure and melt properties of CO–ethylene copolymers with remarkably low CO content. Polym. Chem. 2014, 5, 3831–3837. [Google Scholar] [CrossRef]
- Ortmann, P.; Wimmer, F.P.; Mecking, S. Long-spaced polyketones from ADMET copolymerizations as ideal models for ethylene/CO copolymers. ACS Macro. Lett. 2015, 4, 704–707. [Google Scholar] [CrossRef]
- Hearley, A.K.; Nowack, R.A.J.; Rieger, B. New single-site palladium catalysts for the nonalternating copolymerization of ethylene and carbon monoxide. Organometallics 2005, 24, 2755–2763. [Google Scholar] [CrossRef]
- Bettucci, L.; Bianchini, C.; Claver, C.; Suarez, E.J.; Ruiz, A.; Meli, A.; Oberhauser, W. Ligand effects in the non-alternating CO-ethylene copolymerization by palladium(II) catalysis. Dalton. Trans. 2007, 47, 5590–5602. [Google Scholar] [CrossRef] [PubMed]
- Newsham, D.K.; Borkar, S.; Sen, A.; Conner, D.M.; Goodall, B.L. Inhibitory role of carbon monoxide in palladium(II)-catalyzed nonalternating ethene/carbon monoxide copolymerizations and the synthesis of polyethene-poly(ethene-alt-carbon monoxide). Organometallics 2007, 26, 3636–3638. [Google Scholar] [CrossRef]
- Haras, A.; Michalak, A.; Rieger, B.; Ziegler, T. Theoretical analysis of factors controlling the nonalternating CO/C2H4 copolymerization. J. Am. Chem. Soc. 2005, 127, 8765–8774. [Google Scholar] [CrossRef]
- Haras, A.; Michalak, A.; Rieger, B.; Ziegler, T. Comparative study on catalytic systems for the alternating and nonalternating CO/ethene copolymerization. Organometallics 2006, 25, 946–953. [Google Scholar] [CrossRef]
- Conley, M.P.; Jordan, R.F. cis/trans isomerization of phosphinesulfonate palladium(II) complexes. Angew. Chem. Int. Ed. Engl. 2011, 50, 3744–3746. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Anselment, T.M.; Claverie, J.; Goodall, B.; Jordan, R.F.; Mecking, S.; Rieger, B.; Sen, A.; van Leeuwen, P.W.; Nozaki, K. Ortho-phosphinobenzenesulfonate: A superb ligand for palladium-catalyzed coordination-insertion copolymerization of polar vinyl monomers. Acc. Chem. Res. 2013, 46, 1438–1449. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Munakata, K.; Ito, S.; Kochi, T.; Chung, L.W.; Morokuma, K.; Nozaki, K. Pd-catalyzed copolymerization of methyl acrylate with carbon monoxide: Structures, properties and mechanistic aspects toward ligand design. J. Am. Chem. Soc. 2011, 133, 6761–6779. [Google Scholar] [CrossRef] [PubMed]
- Noda, S.; Nakamura, A.; Kochi, T.; Chung, L.W.; Morokuma, K.; Nozaki, K. Mechanistic studies on the formation of linear polyethylene chain catalyzed by palladium phosphine-sulfonate complexes: Experiment and theoretical studies. J. Am. Chem. Soc. 2009, 131, 14088–14100. [Google Scholar] [CrossRef] [PubMed]
- Zuidema, E.; Bo, C.; van Leeuwen, P.W. Ester versus polyketone formation in the palladium-diphosphine catalyzed carbonylation of ethene. J. Am. Chem. Soc. 2007, 129, 3989–4000. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, C.; Meli, A.; Oberhauser, W.; Segarra, A.M.; Passaglia, E.; Lamac, M.; Stepnicka, P. Palladium(II) complexes with phosphanylferrocenecarboxylate ligands and their use as catalyst precursors for semialternating CO-ethylene copolymerization. Eur. J. Inorg. Chem. 2008, 2008, 441–452. [Google Scholar] [CrossRef]
- Zhu, L.H.; Gaire, S.; Ziegler, C.J.; Jia, L. Nickel catalysts for non-alternating CO-ethylene copolymerization. ChemCatChem 2022, 14, e202200974. [Google Scholar] [CrossRef]
- Drent, E.; van Dijk, R.; van Ginkel, R.; van Oort, B.; Pugh, R.I. Palladium catalysed copolymerisation of ethene with alkylacrylates: Polar comonomer built into the linear polymer chain. Chem. Commun. 2002, 7, 744–745. [Google Scholar] [CrossRef]
- Chen, C.; Anselment, T.M.J.; Fröhlich, R.; Rieger, B.; Kehr, G.; Erker, G. o-Diarylphosphinoferrocene sulfonate palladium systems for nonalternating ethene–carbon monoxide copolymerization. Organometallics 2011, 30, 5248–5257. [Google Scholar] [CrossRef]
- Dodge, H.M.; Natinsky, B.S.; Jolly, B.J.; Zhang, H.C.; Mu, Y.; Chapp, S.M.; Tran, T.V.; Diaconescu, P.L.; Do, L.H.; Wang, D.W.; et al. Polyketones from carbon dioxide and ethylene by integrating electrochemical and organometallic catalysis. ACS Catal. 2023, 13, 4053–4059. [Google Scholar] [CrossRef]
- Chen, M.; Chen, C. A versatile ligand platform for palladium- and nickel-catalyzed ethylene copolymerization with polar monomers. Angew. Chem. Int. Ed. Engl. 2018, 57, 3094–3098. [Google Scholar] [CrossRef]
- Xu, M.; Yu, F.; Li, P.; Xu, G.; Zhang, S.; Wang, F. Enhancing chain initiation efficiency in the cationic allyl-nickel catalyzed (co)polymerization of ethylene and methyl acrylate. Inorg. Chem. 2020, 59, 4475–4482. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Liao, D.H.; Pang, W.M.; Chen, M.; Tan, C. Versatile PNPO ligands for palladium and nickel catalyzed ethylene polymerization and copolymerization with polar monomers. J. Catal. 2021, 393, 281–289. [Google Scholar] [CrossRef]
- Klabunde, U.; Tulip, T.H.; Roe, D.C.; Ittel, S.D. Reaction of nickel polymerization catalysts with carbon monoxide. J. Organomet. Chem. 1987, 334, 141–156. [Google Scholar] [CrossRef]
- Desjardins, S.Y.; Cavell, K.J.; Hoare, J.L.; Skelton, B.W.; Sobolev, A.N.; White, A.H.; Keim, W. Single component N-O chelated arylnickel(II) complexes as ethene polymerisation and CO/ethene copolymerisation catalysts. Examples of ligand induced changes to the reaction pathway. J. Organomet. Chem. 1997, 544, 163–174. [Google Scholar] [CrossRef]
- Klaui, W.; Bongards, J.; Reiß, G.J. Novel nickel(ii) complexes for the catalytic copolymerization of ethylene and carbon monoxide: Polyketone synthesis in supercritical carbon dioxide. Angew. Chem. Int. Ed. Engl. 2000, 39, 3894–3896. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Zhang, M.; Pan, F.; Babahan, I.; Ding, K.; Jia, L.; Crandall, L.A.; Ziegler, C.J. Zwitterionic nickel(ii) catalyst for CO–ethylene alternating copolymerization. Organometallics 2015, 34, 4798–4801. [Google Scholar] [CrossRef]
- Chen, S.Y.; Ren, B.H.; Li, S.H.; Song, Y.H.; Jiao, S.; Zou, C.; Chen, C.; Lu, X.B.; Liu, Y. Cationic P,O-coordinated nickel(ii) catalysts for carbonylative polymerization of ethylene: Unexpected productivity via subtle electronic variation. Angew. Chem. Int. Ed. Engl. 2022, 61, e202204126. [Google Scholar] [CrossRef]
- Xin, B.S.; Sato, N.; Tanna, A.; Oishi, Y.; Konishi, Y.; Shimizu, F. Nickel catalyzed copolymerization of ethylene and alkyl acrylates. J. Am. Chem. Soc. 2017, 139, 3611–3614. [Google Scholar] [CrossRef]
- Zhang, Y.; Mu, H.; Pan, L.; Wang, X.; Li, Y. Robust bulky [P,O] neutral nickel catalysts for copolymerization of ethylene with polar vinyl monomers. ACS Catal. 2018, 8, 5963–5976. [Google Scholar] [CrossRef]




| Microstructure | PKs | Range of m | CO Content (%) | Tm (°C) |
|---|---|---|---|---|
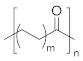 | Alternating PKs | m = 1 | 50 | ~260 |
| Non-perfect alternating PKs | m > 1 | 10~50 | <260 | |
| PE with in-chain ketones | m > 1 | 0~10 | <140 |
| Structure Unit | Fragments | NMR Shifts | ||
|---|---|---|---|---|
| n | 1H | 13C | ||
| Alternating unit (AU) |  | 1 | / | 212.9 a/207.0 b |
| 2 | ~2.4 c | 35.7 c | ||
| Non-alternating unit (N2) |  | 3 | / | 209.8 b |
| 4 | ~2.2 c | 42.1 c | ||
| 5 | ~1.5 c | 23.0 c | ||
| Non-alternating unit (N3) | 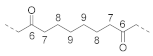 | 6 | / | 210.1 b |
| 7 | ~2.2 c | 42.5 c | ||
| 8 | ~1.5 c | 23.7 c | ||
| 9 | ~1.3 c | 28.8 c | ||
| Non-alternating unit (N4) | 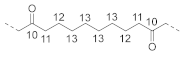 | 10 | / | 210.3 b |
| 11 | ~2.2 c | 42.7 c | ||
| 12 | ~1.5 c | 24.0 c | ||
| 13 | ~1.3 c | 29.2 c | ||
| Isolated carbonyl unit (I) | 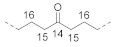 | 14 | / | 210.3 b |
| 15 | ~2.4 b | 42.7 b | ||
| 16 | ~1.6 b | 23.8 b | ||
| Cat. | Add. | Temp. (°C) | Press. (Bar) | CO/Thylene Feed | Act. a | Extra C2 Insertion b (Mol %) | Tm c | Ref |
|---|---|---|---|---|---|---|---|---|
| Pd1 | / | 110 | 50 | 2/3 | 123 | 7.3 | 234 | [50] |
| Pd2 | / | 110 | 50 | 2/3 | 103 | 11.9 | 231 | [50] |
| Pd3 | / | 110 | 50 | 2/3 | 108 | 18.3 | 229 | [50] |
| Pd4 | / | 110 | 40 | 1/3 | <1 | 1.4 | 220~230 | [67] |
| Pd5 | / | 110 | 110 | 1/10 | 12 | 18.3 | 220~230 | [67] |
| Pd5 | / | 110 | 40 | 1/3 | 69 | 2.4 | 220~230 | [67] |
| Pd6 | / | 110 | 40 | 1/3 | 47 | 8.2 | 220~230 | [67] |
| Pd7 | / | 110 | 40 | 1/3 | 2.5 | <1 | 220~230 | [67] |
| Pd8 | / | 110 | 40 | 1/3 | 106 | 4.1 | 220~230 | [67] |
| Pd9 | / | 110 | 40 | 1/3 | 102 | 2.1 | 220~230 | [67] |
| Pd9 | / | 200 | 70 | 1/6 | 75 | 4.9 | 220~230 | [67] |
| Pd10 | B(C6F5)3 e | 110 | 140 | 2/5 | 292 | 3.1 | 220~230 | [67] |
| Pd11 | B(C6F5)3 e | 110 | 140 | 2/5 | 67 | 2.2 | 220~230 | [67] |
| Pd11 | B(C6F5)3 e | 110 | 105 | 1/20 | 89 | 26.4 | 220~230 | [67] |
| Pd12 | / | 120 | 56 | 1/20 | 41 | 27.8 | 193 | [68] |
| Pd12 | BQ e | 120 | 56 | 1/3 | 607 | 2.9 | /d | [68] |
| Pd12 | / | 120 | 56 | 1/3 | 337 | 3.4 | 235 | [68] |
| Pd13 | / | 120 | 56 | 1/3 | 170 | 5.6 | /d | [68] |
| Pd14 | / | 110 | 56 | 1/3 | 0.4 | 0.3 | /d | [68] |
| Pd15 | / | 110 | 56 | 1/3 | 0.4 | 0.3 | /d | [68] |
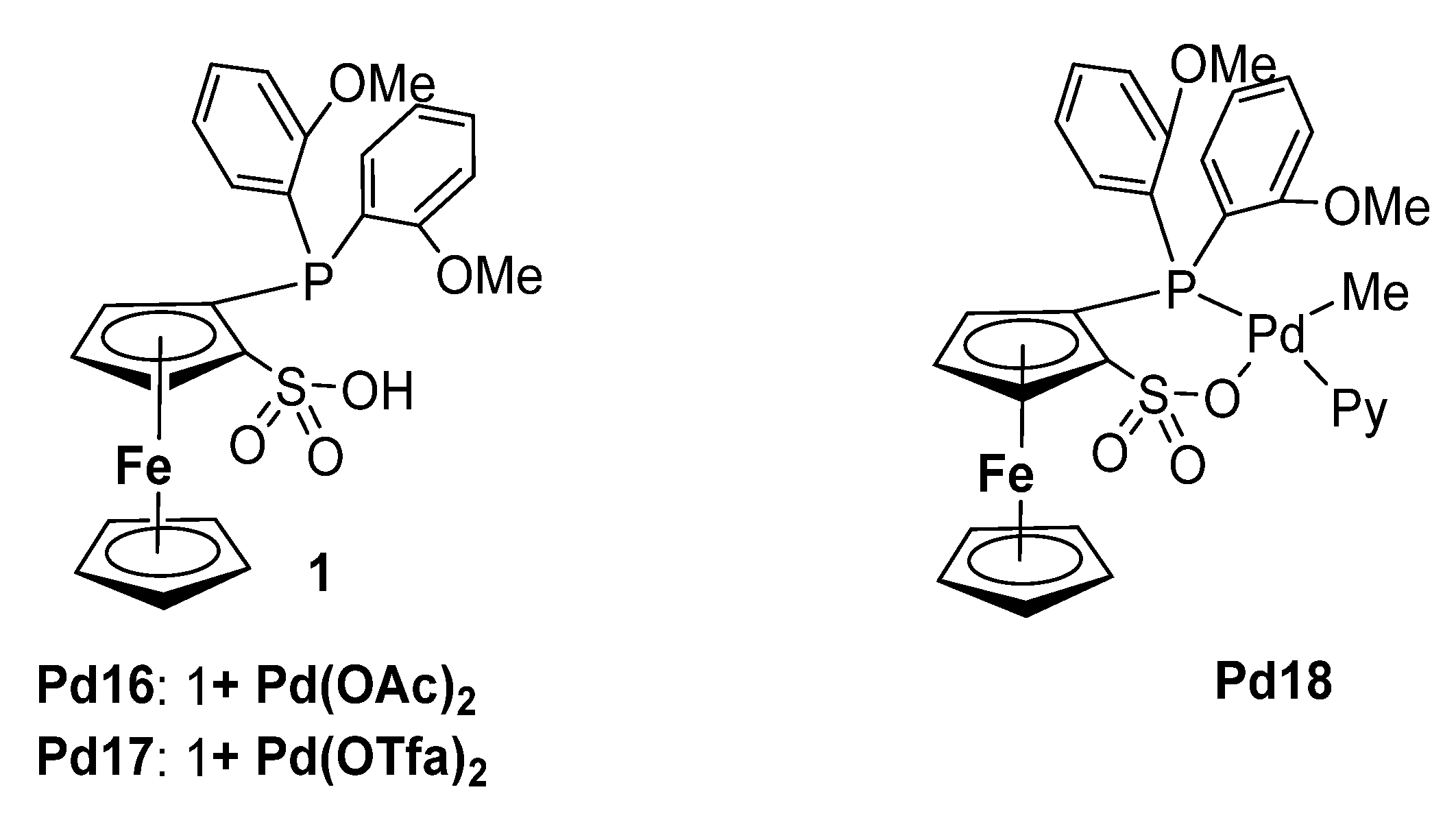
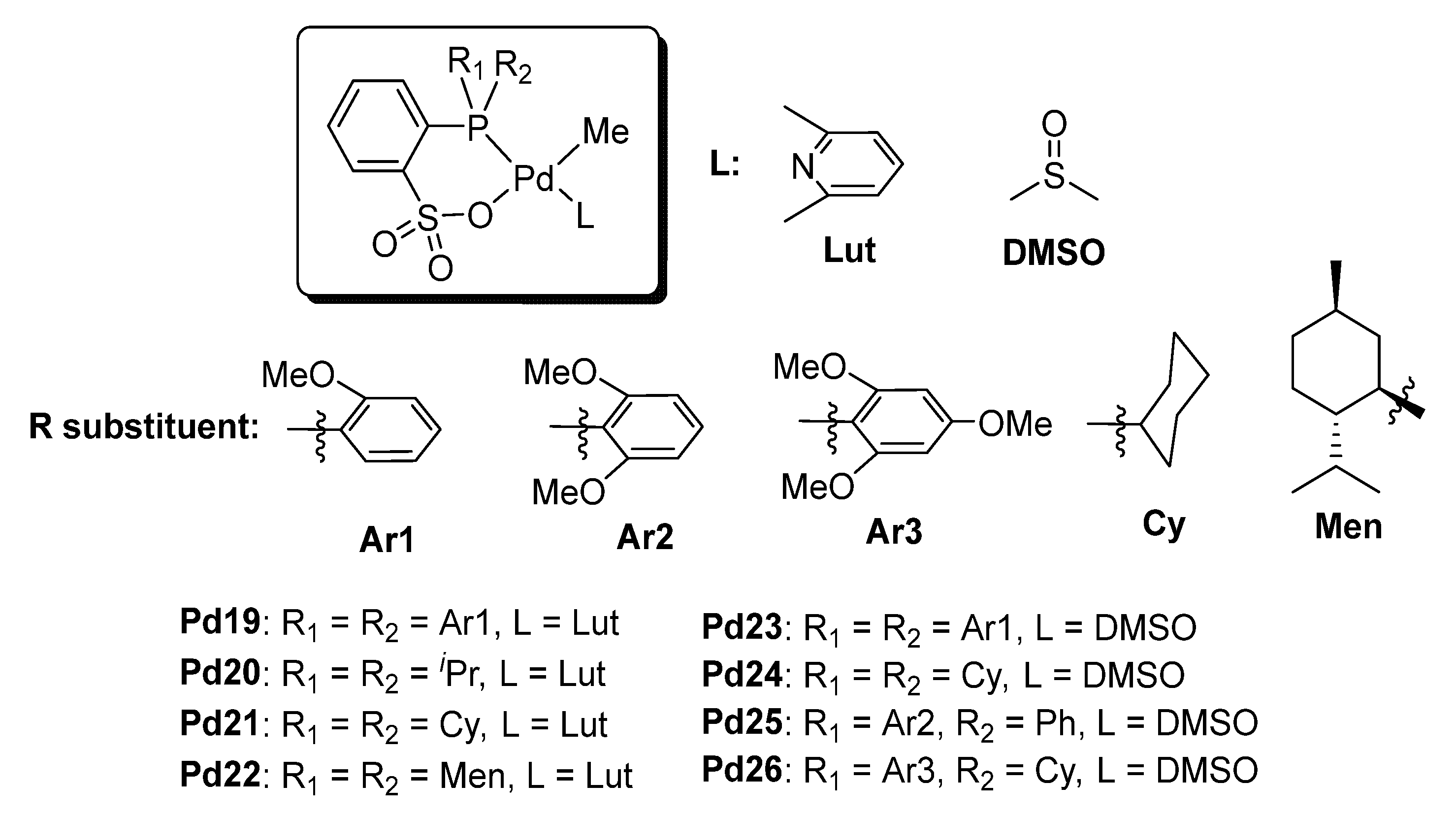
| Pd16 | / | 90 | 55 | 1/10 | 18 | 12 | 199 | [80] |
| Pd16 | / | 110 | 65 | 1/12 | 21 | 25 | 171 | [80] |
| Pd17 | / | 110 | 50 | 2/3 | 75 | 1 | 242 | [80] |
| Pd18 | / | 110 | 50 | 2/3 | 74 | 3 | 233 | [80] |
| Pd18 | B(C6F5)3 e | 110 | 50 | 2/3 | 159 | 1 | 242 | [80] |
| Pd18 | / | 110 | 55 | 1/10 | 18 | 27 | 167 | [80] |
| Cat. | CO/Ethylene Feed | Act. a | Carbonyl Content (Extra C2 Insertion) b (Mol %) | Mn c | PDI c | Tm d | Ref |
|---|---|---|---|---|---|---|---|
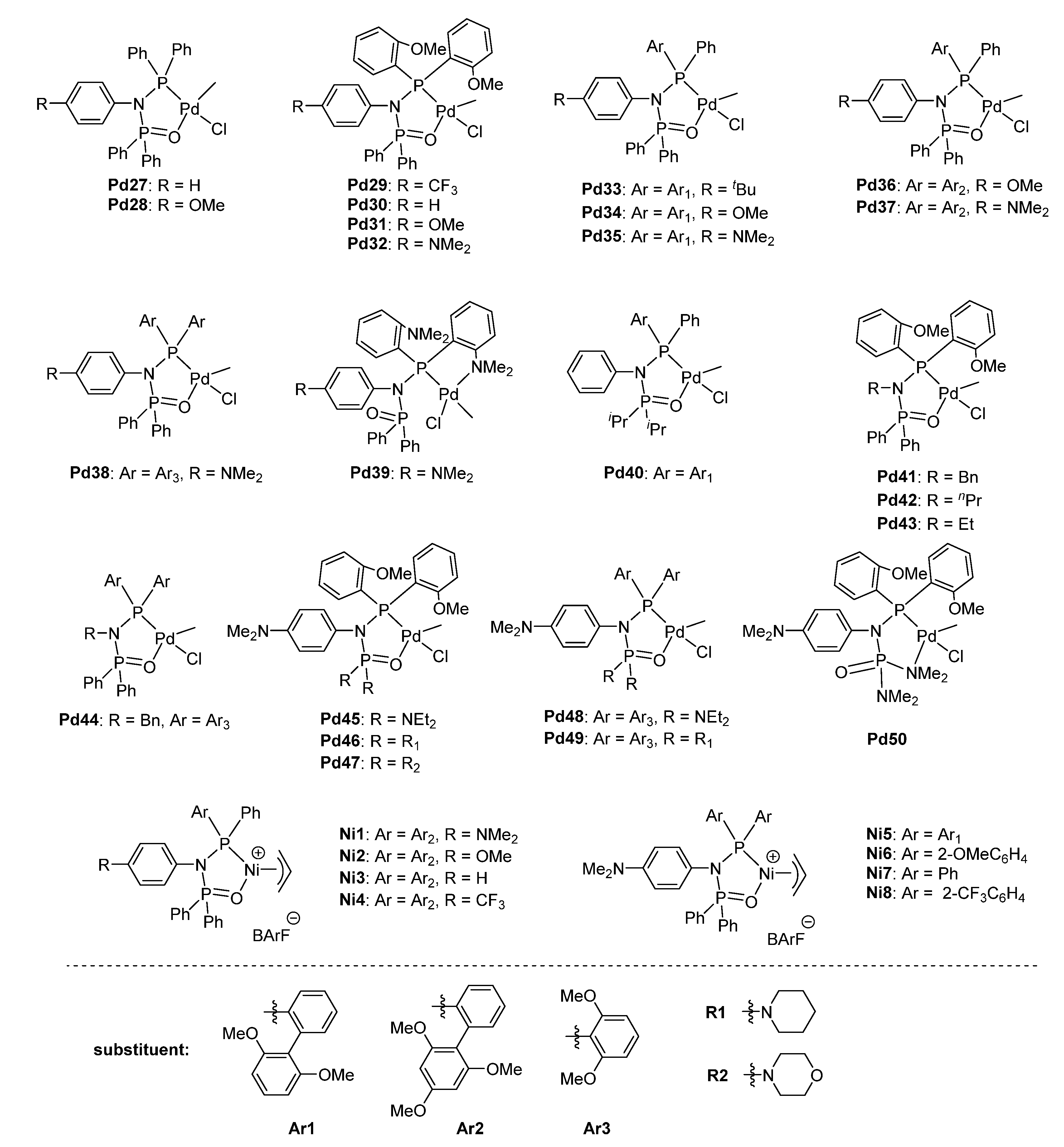
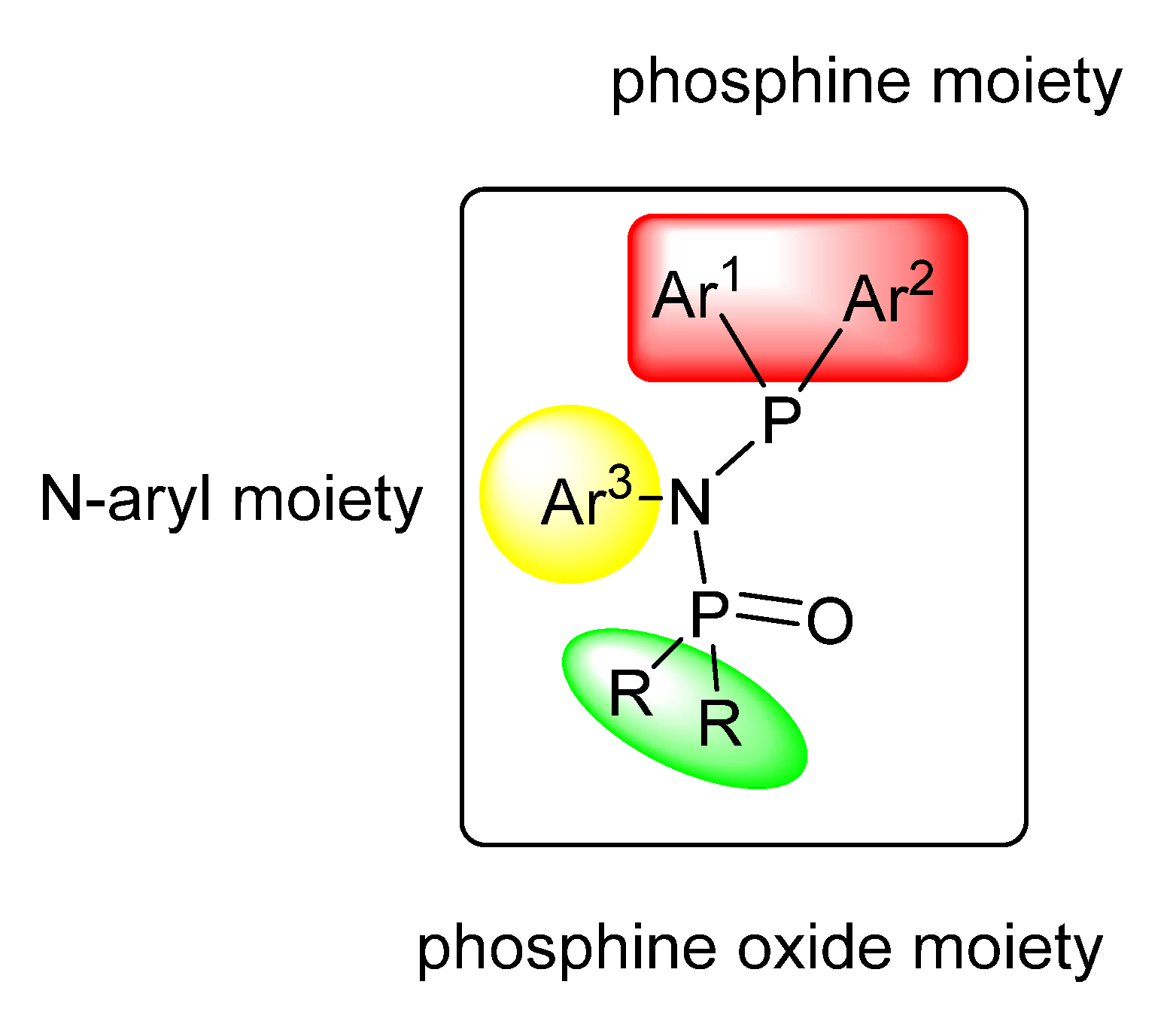
| Pd27 | 1/9 | 15.8 | 49 | 403 | 1.46 | 245 | [58] |
| Pd28 | 1/9 | 19.7 | 49 | 443 | 1.37 | 245 | [58] |
| Pd29 | 1/9 | 28.1 | 49 | 770 | 1.37 | 241 | [58] |
| Pd30 | 1/9 | 38.3 | 47 | 835 | 1.28 | 232 | [58] |
| Pd31 | 1/9 | 40.9 | 47 | 904 | 1.16 | 231 | [58] |
| Pd32 | 1/9 | 51.3 | 46 | 948 | 1.23 | 227 | [58] |
| Pd33 | 1/9 | 31.4 | 48 | 569 | 1.39 | 243 | [58] |
| Pd34 | 1/9 | 35.6 | 48 | 631 | 1.33 | 242 | [58] |
| Pd35 | 1/9 | 36.9 | 47 | 654 | 1.33 | 238 | [58] |
| Pd36 | 1/9 | 26.2 | 48 | 542 | 1.32 | 246 | [58] |
| Pd37 | 1/9 | 28.4 | 48 | 592 | 1.42 | 246 | [58] |
| Pd38 | 1/9 | 70.6 | 43 (17.7) | 915 | 1.09 | 186 | [58] |
| Pd39 | 1/9 | 0 | /e | /e | /e | /e | [58] |
| Pd40 | 1/9 | 25.5 | 49 | 726 | 1.20 | 244 | [58] |
| Pd38 | 1/1 | 124.0 | 50 (1.1) | 1340 | 1.08 | 248 | [58] |
| Pd38 | 1/3 | 105.0 | 47 (4.7) | 1200 | 1.08 | 226 | [58] |
| Pd38 | 1/5 | 80.1 | 45 (11.9) | 1110 | 1.14 | 214 | [58] |
| Pd38 | 1/20 | 54.7 | 39 (24.2) | 1070 | 1.14 | 165/147 | [58] |
| Pd41 | 1/9 | 70.1 | 48 (2.1) | 142 | 1.61 | 240 | [63] |
| Pd42 | 1/9 | 69.3 | 47 | 134 | 1.76 | 237 | [63] |
| Pd43 | 1/9 | 60.7 | 47 | 126 | 1.61 | 237 | [63] |
| Pd32 | 1/9 | 32.5 | 46 (4.9) | 118 | 1.34 | 229 | [63] |
| Pd44 | 1/9 | 124.1 | 47 (3.3) | 199 | 1.43 | 235 | [63] |
| Pd45 | 1/9 | 25.0 | 44 (12.2) | 67 | 1.35 | 196 | [63] |
| Pd46 | 1/9 | 23.3 | 44 (11.6) | 61 | 1.27 | 194 | [63] |
| Pd47 | 1/9 | 22.6 | 46 (6.7) | 67 | 1.21 | 209 | [63] |
| Pd48 | 1/9 | 59.4 | 44 (12.1) | 149 | 1.45 | 198 | [63] |
| Pd49 | 1/9 | 67.8 | 45 (10.1) | 151 | 1.47 | 206 | [63] |
| Pd50 | 1/9 | 24.1 | 46 (6.5) | 64 | 1.37 | 209 | [63] |
| Ni1 | 0.2% | 66 | 1.1 | 8.94 | 2.64 | 124 | [61] |
| Ni2 | 0.2% | 87 | 0.5 | 8.82 | 2.44 | 122 | [61] |
| Ni3 | 0.2% | 91 | 0.8 | 4.40 | 2.95 | 121 | [61] |
| Ni4 | 0.2% | 42 | 1.1 | 3.08 | 2.32 | 109 | [61] |
| Ni5 | 0.2% | 0 | /e | /e | /e | /e | [61] |
| Ni6 | 0.2% | 263 | 0.2 | 17.8 | 2.31 | 124 | [61] |
| Ni7 | 0.2% | 301 | 0.5 | 5.48 | 2.80 | 118 | [61] |
| Ni8 | 0.2% | 0 | /e | /e | /e | /e | [61] |
| Ni6 | 1.0% | 39 | 0.2 | 24.0 | 2.25 | 126 | [61] |
| Pd29 | 0.2% | 24 | 0.9 | 3.33 | 2.75 | 125 | [61] |
| Pd30 | 0.2% | 33 | 0.8 | 4.08 | 2.33 | 126 | [61] |
| Pd31 | 0.2% | 36 | 0.9 | 7.91 | 2.59 | 126 | [61] |
| Pd32 | 0.2% | 44 | 0.6 | 9.56 | 2.70 | 126 | [61] |
| Pd38 | 0.2% | 68 | 0.8 | 40.4 | 2.62 | 126 | [61] |
| Pd38 | 0.1% | 70 | 0.5 | 93.3 | 1.80 | 133 | [61] |
| Cat. | CO/Ethylene Feed a (%) | Act. b | Carbonyl Content c (Mol %) | IC/NA/AC d | Mn e | PDI e | Tm f | Ref |
|---|---|---|---|---|---|---|---|---|
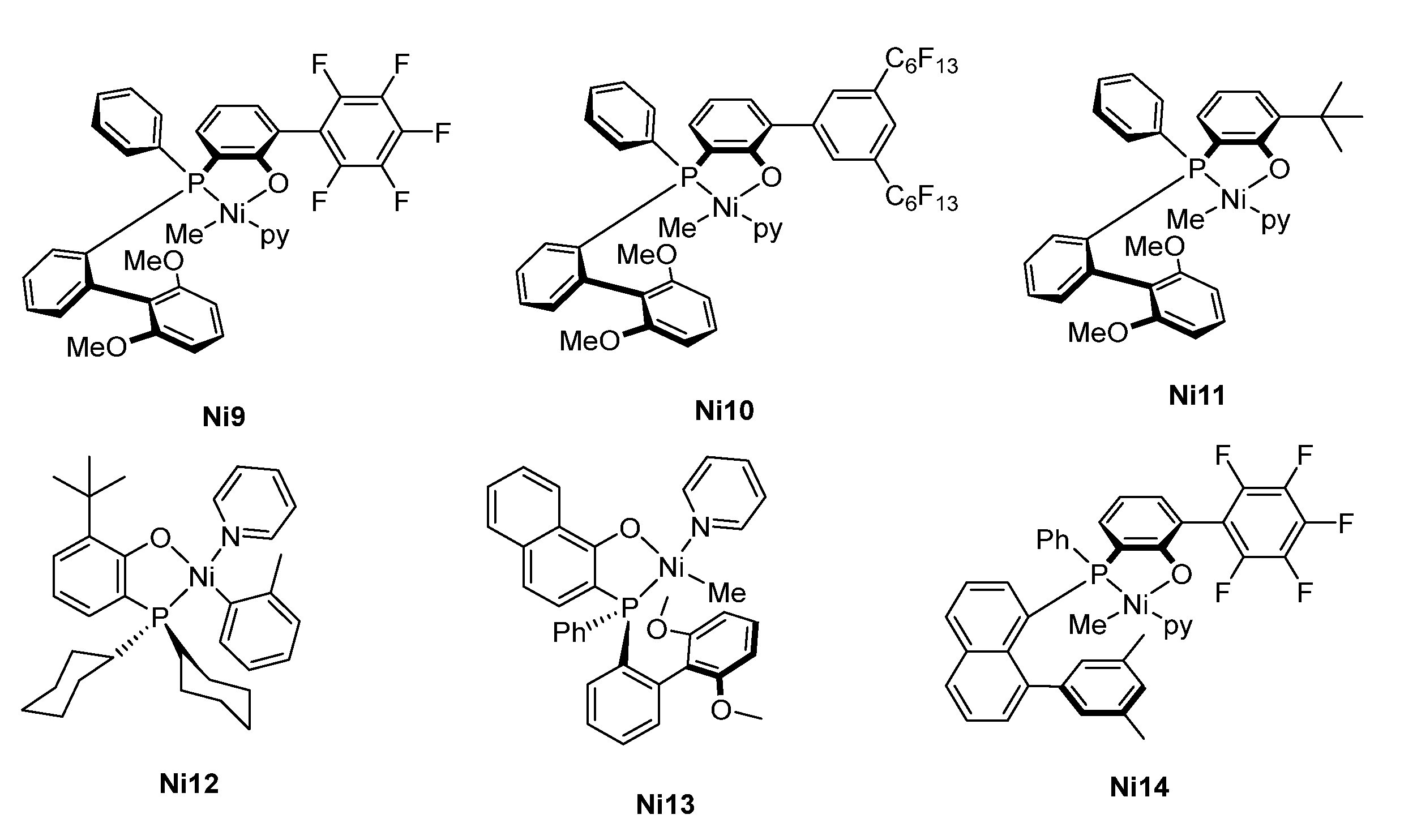
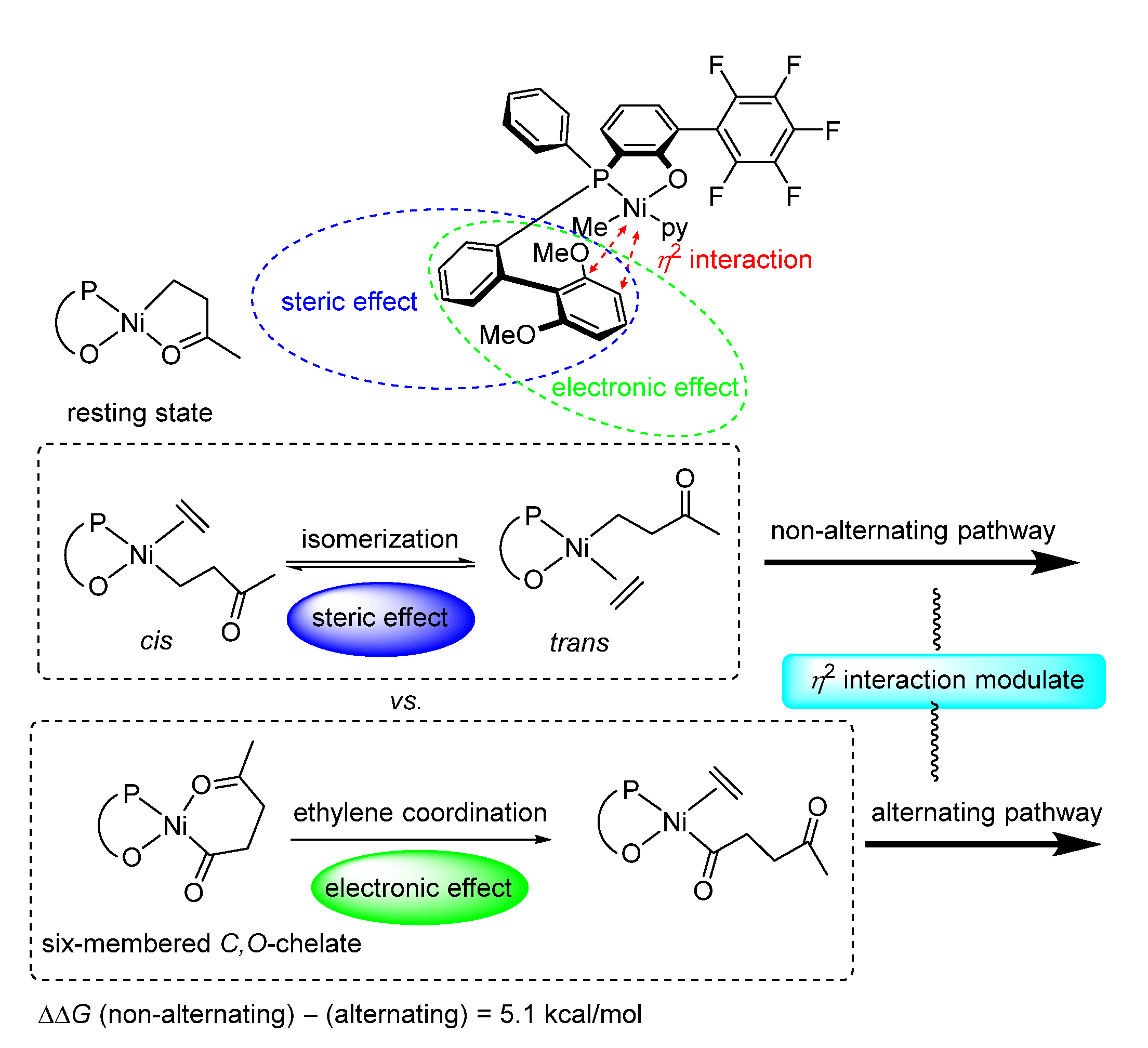
| Ni9 | 0.2 | 213.1 | 0.3 (0.3) | 79/21/0 | 140 | 1.6 | 134 | [51] |
| Ni9 | 0.6 | 98.8 | 0.8 (1.1) | 32/36/32 | 147 | 1.8 | 135 | [51] |
| Ni10 | 0.6 | 56.6 | 1.0 (1.4) | 21/29/49 | 60 | 1.7 | 133 | [51] |
| Ni11 | 0.6 | 12.9 | 3.7 (4.4) | 28/34/38 | 84 | 1.7 | 134 | [51] |
| Ni12 | 0.6 | 22.1 | 1.6 (3.0) | 7/34/59 | 45 | 1.6 | 133 | [51] |
| Ni13 | 0.6 | 41.2 | 1.1 (2.5) | 18/33/49 | 90 | 1.7 | 135 | [51] |
| Ni14 | 0.6 | 0.6 | 50 | 0/0/100 | /g | /g | /g | [51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, X.; Zheng, H.; Gao, H.; Cheng, Z.; Feng, C.; Yang, J.; Gao, H. Recent Advances in Synthesis of Non-Alternating Polyketone Generated by Copolymerization of Carbon Monoxide and Ethylene. Int. J. Mol. Sci. 2024, 25, 1348. https://doi.org/10.3390/ijms25021348
Xiao X, Zheng H, Gao H, Cheng Z, Feng C, Yang J, Gao H. Recent Advances in Synthesis of Non-Alternating Polyketone Generated by Copolymerization of Carbon Monoxide and Ethylene. International Journal of Molecular Sciences. 2024; 25(2):1348. https://doi.org/10.3390/ijms25021348
Chicago/Turabian StyleXiao, Xieyi, Handou Zheng, Heng Gao, Zhaocong Cheng, Chunyu Feng, Jiahao Yang, and Haiyang Gao. 2024. "Recent Advances in Synthesis of Non-Alternating Polyketone Generated by Copolymerization of Carbon Monoxide and Ethylene" International Journal of Molecular Sciences 25, no. 2: 1348. https://doi.org/10.3390/ijms25021348





