Initial Molecular Mechanisms of the Pathogenesis of Parkinson’s Disease in a Mouse Neurotoxic Model of the Earliest Preclinical Stage of This Disease
Abstract
:1. Introduction
2. Results
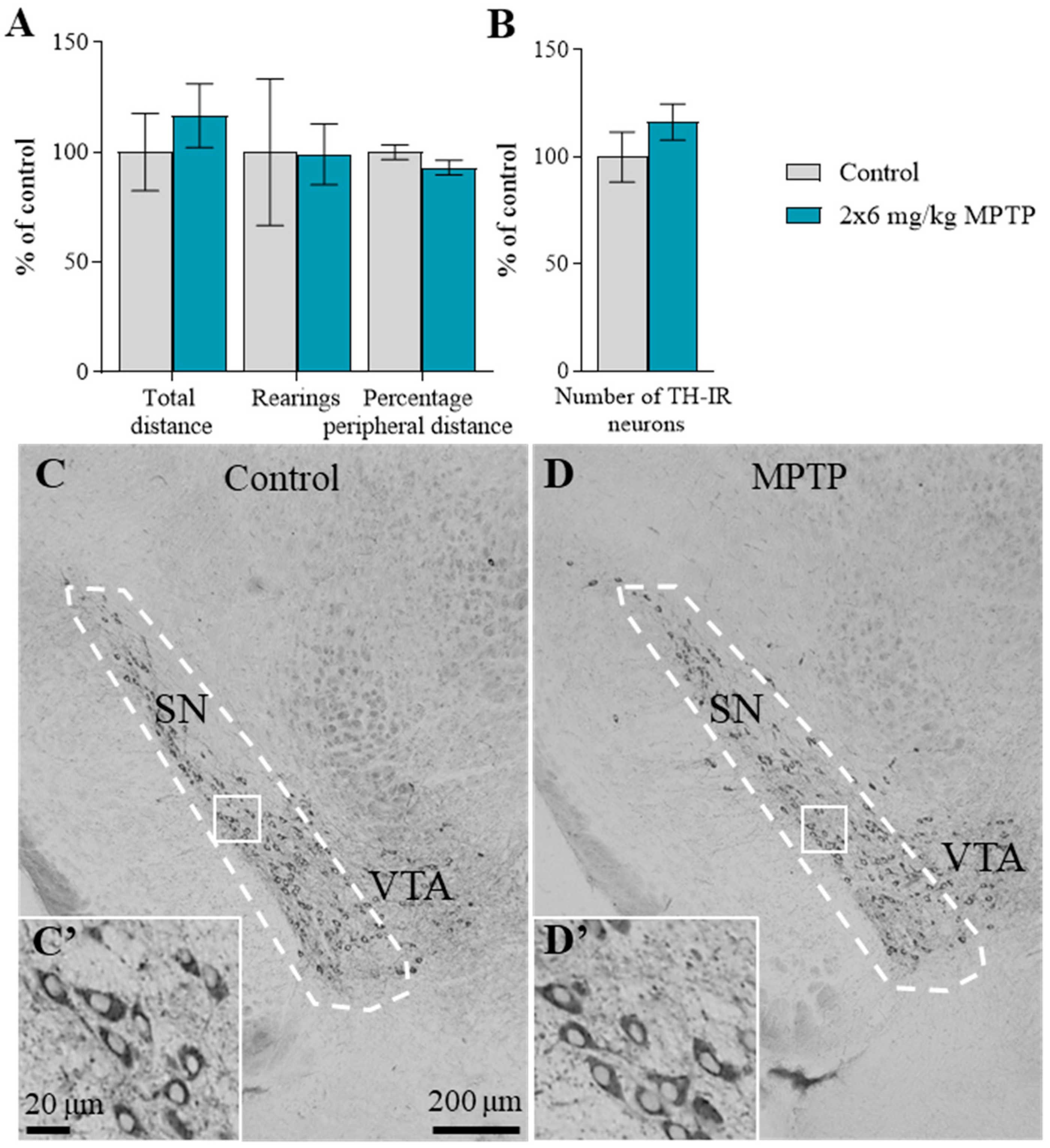
2.1. The Motor Behavior of Mice
2.2. The Number of Tyrosine Hydroxylase-Immunopositive Neurons in the Substantia Nigra
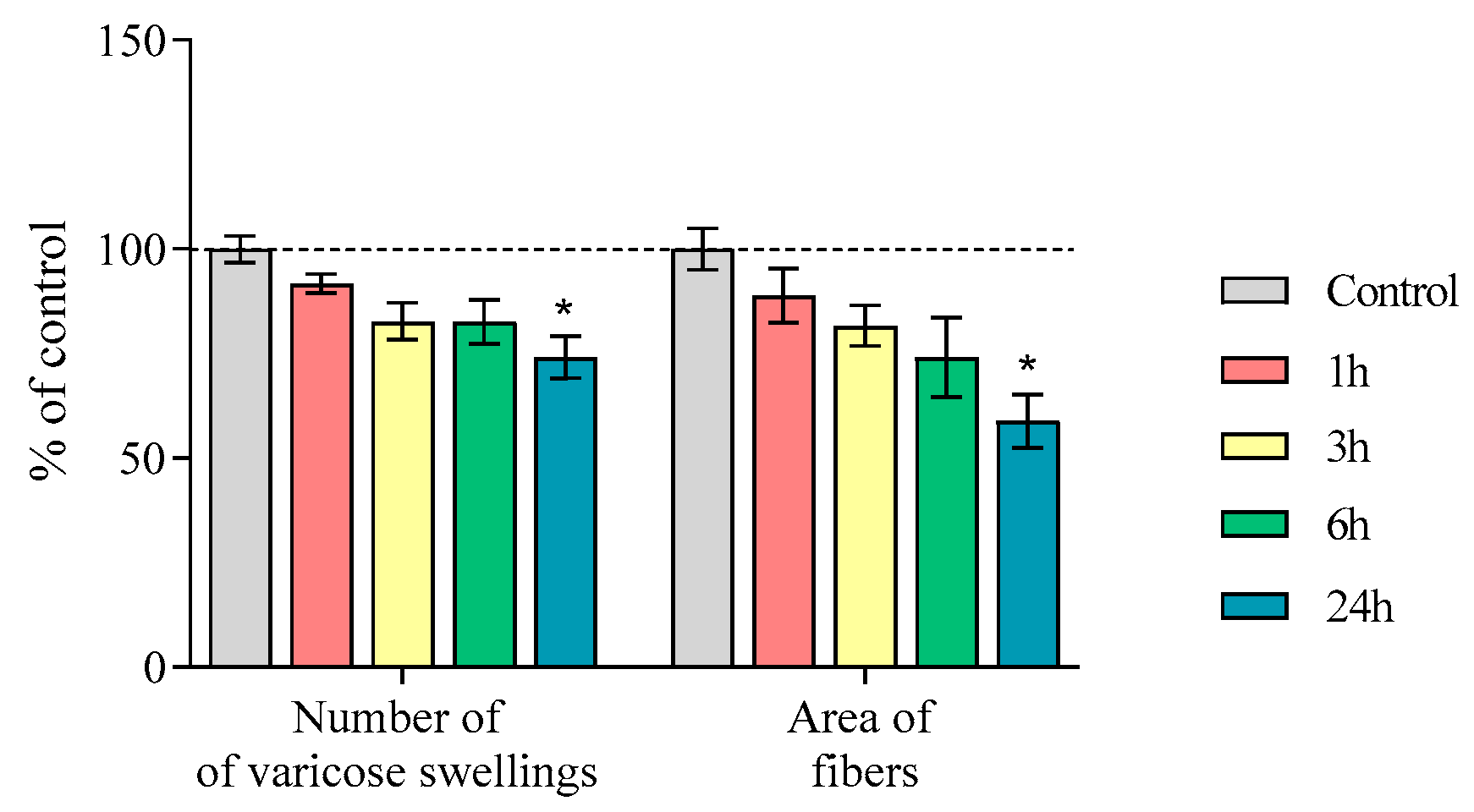
2.3. Nerve Fibers Immunopositive for Tyrosine Hydroxylase, Aromatic L-Amino Acid Decarboxylase, and the Dopamine Transporter in the Striatum of Mice
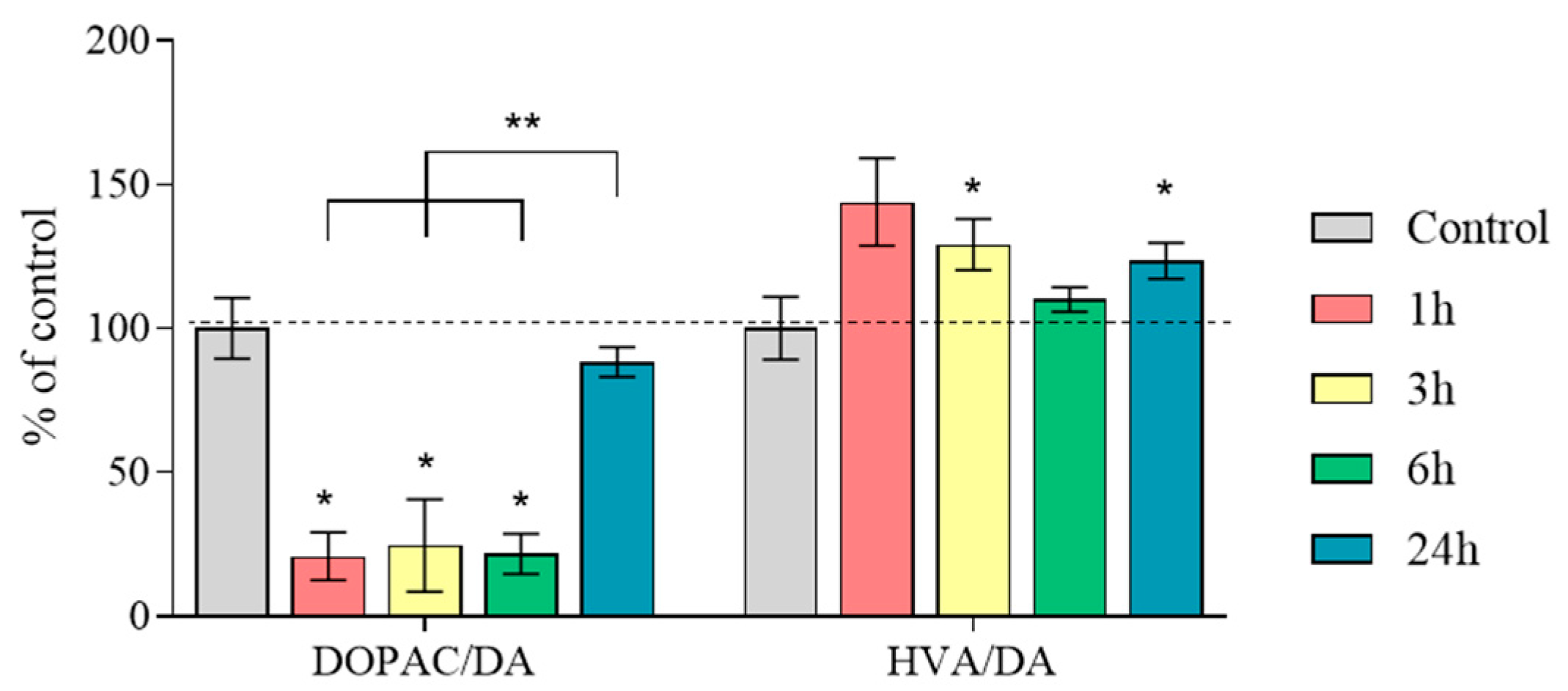
2.4. The Content of Dopamine, Its Metabolites, and MPP+ in the Substantia Nigra
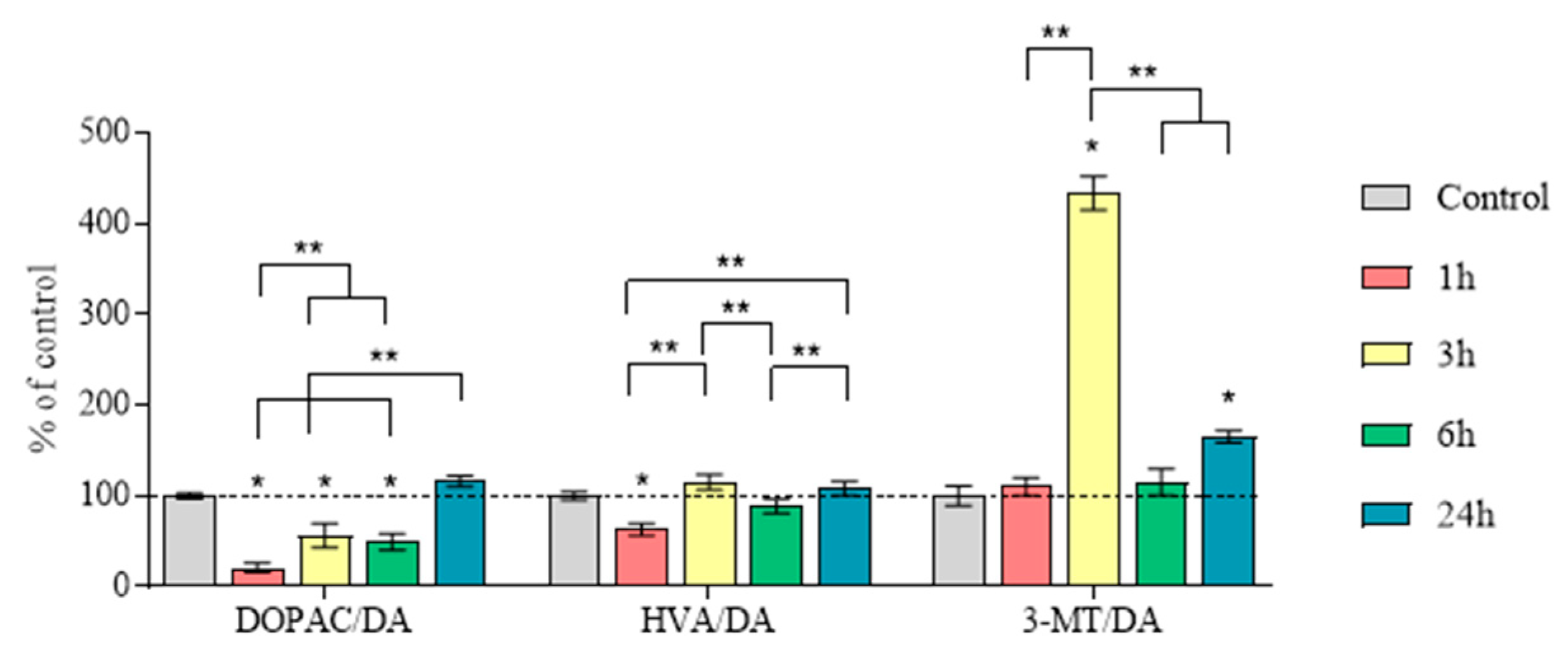
2.5. Concentration of Dopamine, Its Metabolites and MPP+, as Well as the Amount of Dopamine Turnover in the Striatum
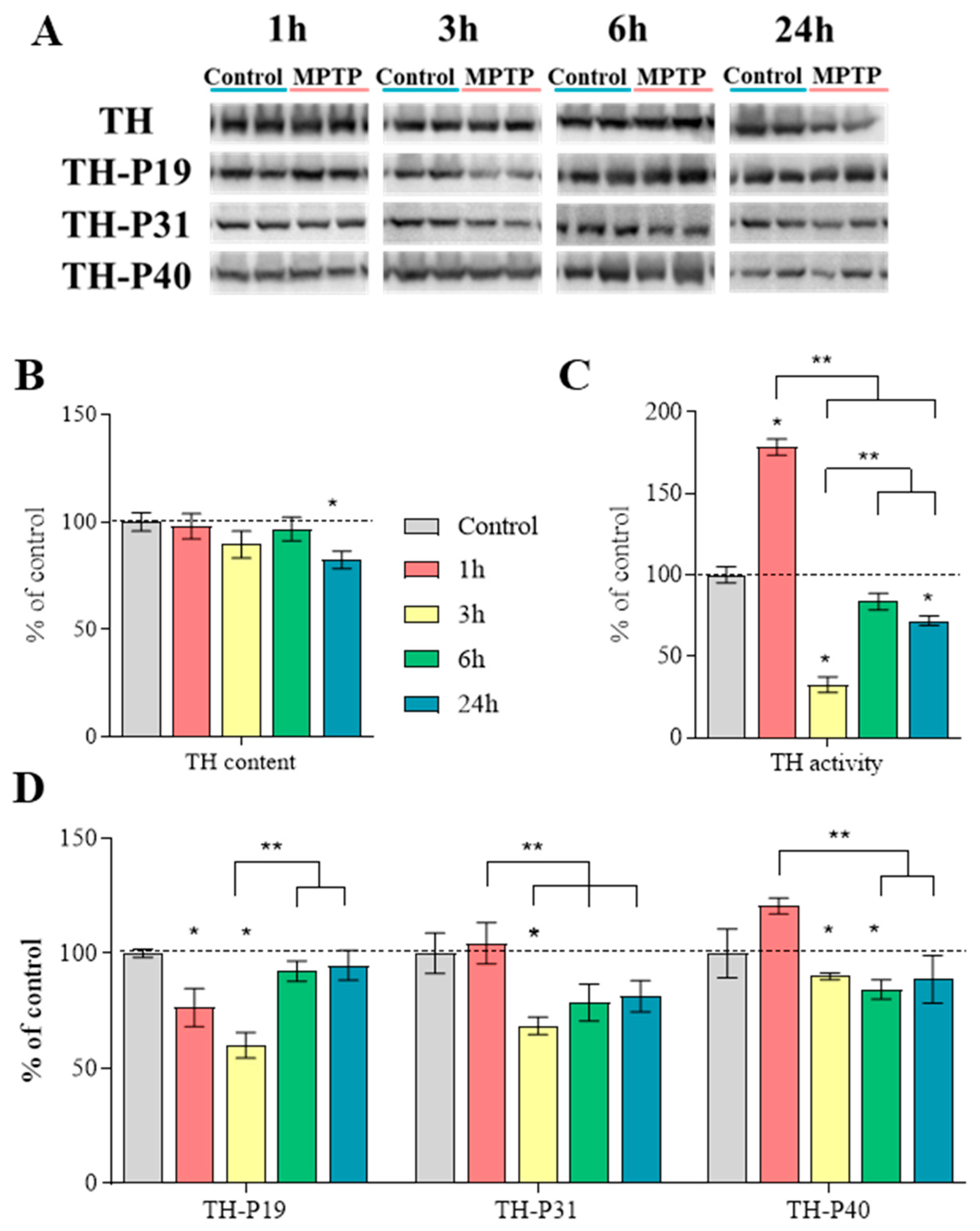
2.6. Characteristics of Tyrosine Hydroxylase in the Striatum
2.7. The Gene Expression of Proteins Involved in Dopamine Metabolism, Neurotransmission, Axonal Transport, Protein Degradation, Neuroprotection, and Neuron Death in the Substantia Nigra
3. Discussion
4. Materials and Methods
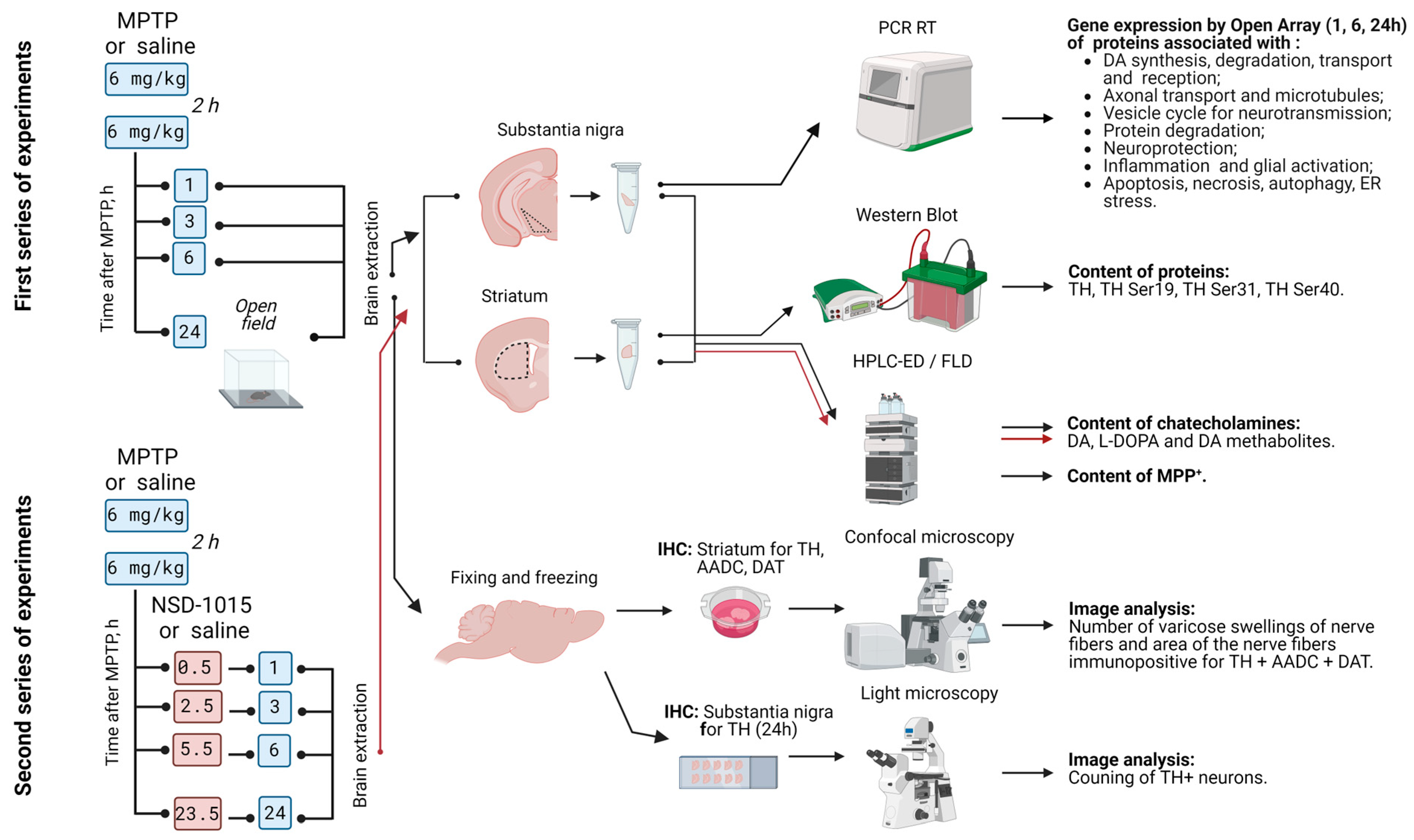
4.1. Animals and Experimental Procedures
4.2. Sample Preparation
4.3. Motor Behavior of Mice
4.4. Immunohistochemistry
4.5. Microscopy
4.6. High-Performance Liquid Chromatography with Electrochemical and Fluorescent Detection
4.7. Assessment of Enzymatic Activity of Tyrosine Hydroxylase
4.8. RT PCR
4.9. Western Blot
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3-MT | 3-methoxytyramine |
| AADC | Aromatic L-amino acid decarboxylase |
| BSA | Bovine serum albumin |
| DA | Dopamine |
| DAergic | Dopaminergic |
| DAT | Dopamine transporter |
| DOPAC | 3,4-dihydroxyphenylacetic acid |
| HPLC with ED/FD | High-performance liquid chromatography with electrochemical and fluorescence detection |
| HVA | Homovanillic acid |
| L-DOPA | L-3,4-dihydroxyphenylalaninePCR |
| MPP+ | 1-methyl-4-phenylpyridinium |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| NSD-1015 | 3-hydroxybenzylhydrazine |
| PBS | Phosphate-buffered saline |
| PD | Parkinson’s disease |
| PCR | Polymerase chain reaction |
| SN | Substantia nigra |
| TH | Tyrosine hydroxylase |
| VMAT2 | Vesicular monoamine transporter 2 |
References
- Kouli, A.; Torsney, K.M.; Kuan, W.-L. Parkinson’s Disease: Etiology, Neuropathology, and Pathogenesis. In Parkinson’s Disease: Pathogenesis and Clinical Aspects; Stoker, T.B., Greenland, J.C., Eds.; Codon Publications: Brisbane, Australia, 2018; ISBN 978-0-9944381-6-4. [Google Scholar]
- Langston, J.W.; Langston, E.B.; Irwin, I. MPTP-Induced Parkinsonism in Human and Non-Human Primates—Clinical and Experimental Aspects. Acta Neurol. Scand. Suppl. 1984, 100, 49–54. [Google Scholar] [PubMed]
- Cao, Y.; Li, B.; Ismail, N.; Smith, K.; Li, T.; Dai, R.; Deng, Y. Neurotoxicity and Underlying Mechanisms of Endogenous Neurotoxins. Int. J. Mol. Sci. 2021, 22, 12805. [Google Scholar] [CrossRef] [PubMed]
- Calabresi, P.; Mechelli, A.; Natale, G.; Volpicelli-Daley, L.; Di Lazzaro, G.; Ghiglieri, V. Alpha-Synuclein in Parkinson’s Disease and Other Synucleinopathies: From Overt Neurodegeneration Back to Early Synaptic Dysfunction. Cell Death Dis. 2023, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Agid, Y. Parkinson’s Disease: Pathophysiology. Lancet 1991, 337, 1321–1324. [Google Scholar] [CrossRef] [PubMed]
- Postuma, R.B.; Berg, D. Prodromal Parkinson’s Disease: The Decade Past, the Decade to Come. Mov. Disord. 2019, 34, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, E.C.; Jenner, P.; Przedborski, S. Pathogenesis of Parkinson’s Disease. Mov. Disord. 2013, 28, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Mahlknecht, P.; Seppi, K.; Poewe, W. The Concept of Prodromal Parkinson’s Disease. J. Parkinsons Dis. 2015, 5, 681–697. [Google Scholar] [CrossRef] [PubMed]
- Kordower, J.H.; Olanow, C.W.; Dodiya, H.B.; Chu, Y.; Beach, T.G.; Adler, C.H.; Halliday, G.M.; Bartus, R.T. Disease Duration and the Integrity of the Nigrostriatal System in Parkinson’s Disease. Brain 2013, 136, 2419–2431. [Google Scholar] [CrossRef]
- Dauer, W.; Przedborski, S. Parkinson’s Disease: Mechanisms and Models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA 2020, 323, 548–560. [Google Scholar] [CrossRef]
- Olanow, C.W.; Obeso, J.A.; Stocchi, F. Continuous Dopamine-Receptor Treatment of Parkinson’s Disease: Scientific Rationale and Clinical Implications. Lancet Neurol. 2006, 5, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Ugrumov, M. Development of Early Diagnosis of Parkinson’s Disease: Illusion or Reality? CNS Neurosci. Ther. 2020, 26, 997–1009. [Google Scholar] [CrossRef]
- Lama, J.; Buhidma, Y.; Fletcher, E.J.R.; Duty, S. Animal Models of Parkinson’s Disease: A Guide to Selecting the Optimal Model for Your Research. Neuronal Signal. 2021, 5, NS20210026. [Google Scholar] [CrossRef] [PubMed]
- Calne, D.B.; Langston, J.W.; Martin, W.R.W.; Stoessl, A.J.; Ruth, T.J.; Adam, M.J.; Pate, B.D.; Schulzer, M. Positron Emission Tomography after MPTP: Observations Relating to the Cause of Parkinson’s Disease. Nature 1985, 317, 246–248. [Google Scholar] [CrossRef]
- Ugrumov, M.V.; Khaindrava, V.G.; Kozina, E.A.; Kucheryanu, V.G.; Bocharov, E.V.; Kryzhanovsky, G.N.; Kudrin, V.S.; Narkevich, V.B.; Klodt, P.M.; Rayevsky, K.S.; et al. Modeling of Presymptomatic and Symptomatic Stages of Parkinsonism in Mice. Neuroscience 2011, 181, 175–188. [Google Scholar] [CrossRef]
- Kolacheva, A.; Bannikova, A.; Pavlova, E.; Bogdanov, V.; Ugrumov, M. Modeling of the Progressive Degradation of the Nigrostriatal Dopaminergic System in Mice to Study the Mechanisms of Neurodegeneration and Neuroplasticity in Parkinson’s Disease. Int. J. Mol. Sci. 2022, 24, 683. [Google Scholar] [CrossRef]
- Yasuda, Y.; Shimoda, T.; Uno, K.; Tateishi, N.; Furuya, S.; Yagi, K.; Suzuki, K.; Fujita, S. The Effects of MPTP on the Activation of Microglia/Astrocytes and Cytokine/Chemokine Levels in Different Mice Strains. J. Neuroimmunol. 2008, 204, 43–51. [Google Scholar] [CrossRef]
- Fan, Y.; Kong, H.; Shi, X.; Sun, X.; Ding, J.; Wu, J.; Hu, G. Hypersensitivity of Aquaporin 4-Deficient Mice to 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyrindine and Astrocytic Modulation. Neurobiol. Aging 2008, 29, 1226–1236. [Google Scholar] [CrossRef]
- Cosi, C.; Marien, M. Decreases in Mouse Brain NAD+ and ATP Induced by 1-Methyl-4-Phenyl-1, 2,3,6-Tetrahydropyridine (MPTP): Prevention by the Poly(ADP-Ribose) Polymerase Inhibitor, Benzamide. Brain Res. 1998, 809, 58–67. [Google Scholar] [CrossRef]
- Horsager, J.; Andersen, K.B.; Knudsen, K.; Skjærbæk, C.; Fedorova, T.D.; Okkels, N.; Schaeffer, E.; Bonkat, S.K.; Geday, J.; Otto, M.; et al. Brain-First versus Body-First Parkinson’s Disease: A Multimodal Imaging Case-Control Study. Brain 2020, 143, 3077–3088. [Google Scholar] [CrossRef]
- Borghammer, P.; Van Den Berge, N. Brain-First versus Gut-First Parkinson’s Disease: A Hypothesis. J. Park. Dis. 2019, 9, S281–S295. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Tredici, K.D.; Rüb, U.; de Vos, R.A.I.; Jansen Steur, E.N.H.; Braak, E. Staging of Brain Pathology Related to Sporadic Parkinson’s Disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Bernheimer, H.; Birkmayer, W.; Hornykiewicz, O.; Jellinger, K.; Seitelberger, F. Brain Dopamine and the Syndromes of Parkinson and Huntington Clinical, Morphological and Neurochemical Correlations. J. Neurol. Sci. 1973, 20, 415–455. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.-C.; Ulane, C.M.; Burke, R.E. Clinical Progression in Parkinson Disease and the Neurobiology of Axons. Ann. Neurol. 2010, 67, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Rudow, G.; O’Brien, R.; Savonenko, A.V.; Resnick, S.M.; Zonderman, A.B.; Pletnikova, O.; Marsh, L.; Dawson, T.M.; Crain, B.J.; West, M.J.; et al. Morphometry of the Human Substantia Nigra in Ageing and Parkinson’s Disease. Acta Neuropathol. 2008, 115, 461–470. [Google Scholar] [CrossRef]
- Ma, S.Y.; Röyttä, M.; Rinne, J.O.; Collan, Y.; Rinne, U.K. Correlation between Neuromorphometry in the Substantia Nigra and Clinical Features in Parkinson’s Disease Using Disector Counts. J. Neurol. Sci. 1997, 151, 83–87. [Google Scholar] [CrossRef]
- Chiu, Y.W.; Hori, Y.; Ebinuma, I.; Sato, H.; Hara, N.; Ikeuchi, T.; Tomita, T. Identification of Calcium and Integrin-Binding Protein 1 as a Novel Regulator of Production of Amyloid β Peptide Using CRISPR/Cas9-Based Screening System. FASEB J. 2020, 34, 7661–7674. [Google Scholar] [CrossRef]
- Chia, S.J.; Tan, E.K.; Chao, Y.X. Historical Perspective: Models of Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 2464. [Google Scholar] [CrossRef]
- Chmielarz, P.; Domanskyi, A. Alpha-Synuclein Preformed Fibrils: A Tool to Understand Parkinson’s Disease and Develop Disease Modifying Therapy. Neural Regen. Res. 2021, 16, 2219–2221. [Google Scholar] [CrossRef]
- Thakur, P.; Breger, L.S.; Lundblad, M.; Wan, O.W.; Mattsson, B.; Luk, K.C.; Lee, V.M.Y.; Trojanowski, J.Q.; Björklund, A. Modeling Parkinson’s Disease Pathology by Combination of Fibril Seeds and α-Synuclein Overexpression in the Rat Brain. Proc. Natl. Acad. Sci. USA 2017, 114, E8284–E8293. [Google Scholar] [CrossRef]
- Bezard, E.; Dovero, S.; Prunier, C.; Ravenscroft, P.; Chalon, S.; Guilloteau, D.; Crossman, A.R.; Bioulac, B.; Brotchie, J.M.; Gross, C.E. Relationship between the Appearance of Symptoms and the Level of Nigrostriatal Degeneration in a Progressive 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Lesioned Macaque Model of Parkinson’s Disease. J. Neurosci. 2001, 21, 6853–6861. [Google Scholar] [CrossRef] [PubMed]
- Shimoji, M.; Zhang, L.; Mandir, A.S.; Dawson, V.L.; Dawson, T.M. Absence of Inclusion Body Formation in the MPTP Mouse Model of Parkinson’s Disease. Brain Res. Mol. Brain Res. 2005, 134, 103–108. [Google Scholar] [CrossRef] [PubMed]
- McCormack, A.L.; Mak, S.K.; Shenasa, M.; Langston, W.J.; Forno, L.S.; Di Monte, D.A. Pathologic Modifications of Alpha-Synuclein in 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine (MPTP)-Treated Squirrel Monkeys. J. Neuropathol. Exp. Neurol. 2008, 67, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Masilamoni, G.J.; Smith, Y. Chronic MPTP Administration Regimen in Monkeys: A Model of Dopaminergic and Non-Dopaminergic Cell Loss in Parkinson’s Disease. J. Neural Transm. 2018, 125, 337–363. [Google Scholar] [CrossRef] [PubMed]
- Kozina, E.A.; Kim, A.R.; Kurina, A.Y.; Ugrumov, M.V. Cooperative Synthesis of Dopamine by Non-Dopaminergic Neurons as a Compensatory Mechanism in the Striatum of Mice with MPTP-Induced Parkinsonism. Neurobiol. Dis. 2017, 98, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Mingazov, E.R.; Khakimova, G.R.; Kozina, E.A.; Medvedev, A.E.; Buneeva, O.A.; Bazyan, A.S.; Ugrumov, M.V. MPTP Mouse Model of Preclinical and Clinical Parkinson’s Disease as an Instrument for Translational Medicine. Mol. Neurobiol. 2018, 55, 2991–3006. [Google Scholar] [CrossRef] [PubMed]
- Alieva, A.K.; Filatova, E.V.; Kolacheva, A.A.; Rudenok, M.M.; Slominsky, P.A.; Ugrumov, M.V.; Shadrina, M.I. Transcriptome Profile Changes in Mice with MPTP-Induced Early Stages of Parkinson’s Disease. Mol. Neurobiol. 2017, 54, 6775–6784. [Google Scholar] [CrossRef]
- Ugrumov, M.V. Brain Neurons Partly Expressing Dopaminergic Phenotype: Location, Development, Functional Significance, and Regulation. Adv. Pharmacol. 2013, 68, 37–91. [Google Scholar] [CrossRef]
- Tagliaferro, P.; Burke, R.E. Retrograde Axonal Degeneration in Parkinson Disease. J Parkinsons Dis 2016, 6, 1–15. [Google Scholar] [CrossRef]
- Orimo, S.; Uchihara, T.; Nakamura, A.; Mori, F.; Kakita, A.; Wakabayashi, K.; Takahashi, H. Axonal Alpha-Synuclein Aggregates Herald Centripetal Degeneration of Cardiac Sympathetic Nerve in Parkinson’s Disease. Brain 2008, 131, 642–650. [Google Scholar] [CrossRef]
- Block, E.R.; Nuttle, J.; Balcita-Pedicino, J.J.; Caltagarone, J.; Watkins, S.C.; Sesack, S.R.; Sorkin, A. Brain Region-Specific Trafficking of the Dopamine Transporter. J. Neurosci. 2015, 35, 12845–12858. [Google Scholar] [CrossRef] [PubMed]
- Fornai, F.; Alessandrì, M.G.; Torracca, M.T.; Bassi, L.; Corsini, G.U. Effects of Noradrenergic Lesions on MPTP/MPP+ Kinetics and MPTP-Induced Nigrostriatal Dopamine Depletions. J. Pharmacol. Exp. Ther. 1997, 283, 100–107. [Google Scholar] [PubMed]
- Fornai, F.; Schlüter, O.M.; Lenzi, P.; Gesi, M.; Ruffoli, R.; Ferrucci, M.; Lazzeri, G.; Busceti, C.L.; Pontarelli, F.; Battaglia, G.; et al. Parkinson-like Syndrome Induced by Continuous MPTP Infusion: Convergent Roles of the Ubiquitin-Proteasome System and Alpha-Synuclein. Proc. Natl. Acad. Sci. USA 2005, 102, 3413–3418. [Google Scholar] [CrossRef] [PubMed]
- Ransom, B.R.; Kunis, D.M.; Irwin, I.; Langston, J.W. Astrocytes Convert the Parkinsonism Inducing Neurotoxin, MPTP, to Its Active Metabolite, MPP+. Neurosci. Lett. 1987, 75, 323–328. [Google Scholar] [CrossRef]
- Beninger, R.J. The Role of Dopamine in Locomotor Activity and Learning. Brain Res. Rev. 1983, 6, 173–196. [Google Scholar] [CrossRef]
- Troshev, D.; Blokhin, V.; Ukrainskaya, V.; Kolacheva, A.; Ugrumov, M. Isolation of Living Dopaminergic Neurons Labeled with a Fluorescent Ligand of the Dopamine Transporter from Mouse Substantia Nigra as a New Tool for Basic and Applied Research. Front. Mol. Neurosci. 2022, 15, 1020070. [Google Scholar] [CrossRef]
- Palfi, S.; Leventhal, L.; Chu, Y.; Ma, S.Y.; Emborg, M.; Bakay, R.; Déglon, N.; Hantraye, P.; Aebischer, P.; Kordower, J.H. Lentivirally Delivered Glial Cell Line-Derived Neurotrophic Factor Increases the Number of Striatal Dopaminergic Neurons in Primate Models of Nigrostriatal Degeneration. J. Neurosci. 2002, 22, 4942–4954. [Google Scholar] [CrossRef] [PubMed]
- San Sebastián, W.; Guillén, J.; Manrique, M.; Belzunegui, S.; Ciordia, E.; Izal-Azcárate, A.; Garrido-Gil, P.; Vázquez-Claverie, M.; Luquin, M.R. Modification of the Number and Phenotype of Striatal Dopaminergic Cells by Carotid Body Graft. Brain 2007, 130, 1306–1316. [Google Scholar] [CrossRef]
- Depboylu, C.; Klietz, M.; Maurer, L.; Oertel, W.H.; Kobayashi, K.; Weihe, E.; Höglinger, G.U.; Schäfer, M.K.-H. Transcriptional and Structural Plasticity of Tyrosine Hydroxylase Expressing Neurons in Both Striatum and Nucleus Accumbens Following Dopaminergic Denervation. J. Chem. Neuroanat. 2014, 61–62, 169–175. [Google Scholar] [CrossRef]
- Ünal, B.; Shah, F.; Kothari, J.; Tepper, J.M. Anatomical and Electrophysiological Changes in Striatal TH Interneurons after Loss of the Nigrostriatal Dopaminergic Pathway. Brain Struct. Funct. 2015, 220, 331–349. [Google Scholar] [CrossRef]
- Troshev, D.; Bannikova, A.; Blokhin, V.; Pavlova, E.; Kolacheva, A.; Ugrumov, M. Compensatory Processes in Striatal Neurons Expressing the Tyrosine Hydroxylase Gene in Transgenic Mice in a Model of Parkinson’s Disease. Int. J. Mol. Sci. 2023, 24, 16245. [Google Scholar] [CrossRef] [PubMed]
- Hadjiconstantinou, M.; Neff, N.H. Differential Recovery of Dopamine Synthetic Enzymes Following MPTP and the Consequences of GM1 Ganglioside Treatment. Eur. J. Pharmacol. 1990, 181, 137–139. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, A. Proteasomal Degradation of Tyrosine Hydroxylase and Neurodegeneration: Review. J. Neurochem. 2012, 120, 199–201. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, N.; Xu, Z.Q.; Lindskog, M.; Herrera-Marschitz, M.; Goiny, M.; Haycock, J.; Goldstein, M.; Hökfelt, T.; Fisone, G. Regulation of Tyrosine Hydroxylase Activity and Phosphorylation at Ser(19) and Ser(40) via Activation of Glutamate NMDA Receptors in Rat Striatum. J. Neurochem. 2000, 74, 2470–2477. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, M.F.; Garcia-Espana, A.; Goldstein, M.; Deutch, A.Y.; Haycock, J.W. Stoichiometry of Tyrosine Hydroxylase Phosphorylation in the Nigrostriatal and Mesolimbic Systems In Vivo: Effects of Acute Haloperidol and Related Compounds. J. Neurochem. 2001, 75, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Dunkley, P.R.; Dickson, P.W. Tyrosine Hydroxylase Phosphorylation In Vivo. J. Neurochem. 2019, 149, 706–728. [Google Scholar] [CrossRef] [PubMed]
- Kolacheva, A.; Alekperova, L.; Pavlova, E.; Bannikova, A.; Ugrumov, M.V. Changes in Tyrosine Hydroxylase Activity and Dopamine Synthesis in the Nigrostriatal System of Mice in an Acute Model of Parkinson’s Disease as a Manifestation of Neurodegeneration and Neuroplasticity. Brain Sci. 2022, 12, 779. [Google Scholar] [CrossRef]
- Kozina, E.A.; Khakimova, G.R.; Khaindrava, V.G.; Kucheryanu, V.G.; Vorobyeva, N.E.; Krasnov, A.N.; Georgieva, S.G.; Kerkerian-Le Goff, L.; Ugrumov, M.V. Tyrosine Hydroxylase Expression and Activity in Nigrostriatal Dopaminergic Neurons of MPTP-Treated Mice at the Presymptomatic and Symptomatic Stages of Parkinsonism. J. Neurol. Sci. 2014, 340, 198–207. [Google Scholar] [CrossRef]
- Myöhänen, T.T.; Schendzielorz, N.; Männistö, P.T. Distribution of Catechol-O-Methyltransferase (COMT) Proteins and Enzymatic Activities in Wild-Type and Soluble COMT Deficient Mice. J. Neurochem. 2010, 113, 1632–1643. [Google Scholar] [CrossRef]
- Schendzielorz, N.; Oinas, J.-P.; Myöhänen, T.T.; Reenilä, I.; Raasmaja, A.; Männistö, P.T. Catechol-O-Methyltransferase (COMT) Protein Expression and Activity after Dopaminergic and Noradrenergic Lesions of the Rat Brain. PLoS ONE 2013, 8, e61392. [Google Scholar] [CrossRef]
- Choi, S.J.; Panhelainen, A.; Schmitz, Y.; Larsen, K.E.; Kanter, E.; Wu, M.; Sulzer, D.; Mosharov, E.V. Changes in Neuronal Dopamine Homeostasis Following 1-Methyl-4-Phenylpyridinium (MPP+) Exposure. J. Biol. Chem. 2015, 290, 6799–6809. [Google Scholar] [CrossRef] [PubMed]
- Rana, D.; Colombani, T.; Mohammed, H.S.; Eggermont, L.J.; Johnson, S.; Annabi, N.; Bencherif, S.A. Strategies to Prevent Dopamine Oxidation and Related Cytotoxicity Using Various Antioxidants and Nitrogenation. Emergent Mater. 2019, 2, 209–217. [Google Scholar] [CrossRef]
- Rollema, H.; Damsma, G.; Horn, A.S.; De Vries, J.B.; Westerink, B.H. Brain Dialysis in Conscious Rats Reveals an Instantaneous Massive Release of Striatal Dopamine in Response to MPP+. Eur. J. Pharmacol. 1986, 126, 345–346. [Google Scholar] [CrossRef]
- Pileblad, E.; Fornstedt, B.; Clark, D.; Carlsson, A. Acute Effects of 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine on Dopamine Metabolism in Mouse and Rat Striatum. J. Pharm. Pharmacol. 1985, 37, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, M.J.; Acheson, A.L.; Stachowiak, M.K.; Stricker, E.M. Neurochemical Compensation after Nigrostriatal Bundle Injury in an Animal Model of Preclinical Parkinsonism. Arch. Neurol. 1984, 41, 856–861. [Google Scholar] [CrossRef]
- Altar, C.A.; Boyar, W.C.; Oei, E.; Wood, P.L. Dopamine Autoreceptors Modulate the in Vivo Release of Dopamine in the Frontal, Cingulate and Entorhinal Cortices. J. Pharmacol. Exp. Ther. 1987, 242, 115–120. [Google Scholar] [PubMed]
- Roffler-Tarlov, S.; Sharman, D.F.; Tegerdine, P. 3,4-Dihydroxyphenylacetic Acid and 4-Hydroxy-3-Methoxyphenylacetic Acid in the Mouse Striatum: A Reflection of Intra- and Extra-Neuronal Metabolism of Dopamine? Br. J. Pharmacol. 1971, 42, 343–351. [Google Scholar] [CrossRef]
- Westerink, B.H. Sequence and Significance of Dopamine Metabolism in the Rat Brain. Neurochem. Int. 1985, 7, 221–227. [Google Scholar] [CrossRef]
- Riederer, P.; Konradi, C.; Schay, V.; Kienzl, E.; Birkmayer, G.; Danielczyk, W.; Sofic, E.; Youdim, M.B. Localization of MAO-A and MAO-B in Human Brain: A Step in Understanding the Therapeutic Action of L-Deprenyl. Adv. Neurol. 1987, 45, 111–118. [Google Scholar]
- Cartelli, D.; Casagrande, F.; Busceti, C.L.; Bucci, D.; Molinaro, G.; Traficante, A.; Passarella, D.; Giavini, E.; Pezzoli, G.; Battaglia, G.; et al. Microtubule Alterations Occur Early in Experimental Parkinsonism and The Microtubule Stabilizer Epothilone D Is Neuroprotective. Sci. Rep. 2013, 3, 1837. [Google Scholar] [CrossRef]
- Heeringa, M.J.; Abercrombie, E.D. Biochemistry of Somatodendritic Dopamine Release in Substantia Nigra: An in Vivo Comparison with Striatal Dopamine Release. J. Neurochem. 1995, 65, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.M.; Callahan, L.M.; Casaceli, C.; Chen, L.; Kiser, G.L.; Chui, B.; Kaysser-Kranich, T.M.; Sendera, T.J.; Palaniappan, C.; Federoff, H.J. Dysregulation of Gene Expression in the 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Lesioned Mouse Substantia Nigra. J. Neurosci. 2004, 24, 7445–7454. [Google Scholar] [CrossRef] [PubMed]
- Kühn, K.; Wellen, J.; Link, N.; Maskri, L.; Lübbert, H.; Stichel, C.C. The Mouse MPTP Model: Gene Expression Changes in Dopaminergic Neurons. Eur. J. Neurosci. 2003, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ford, C.P. The Role of D2-Autoreceptors in Regulating Dopamine Neuron Activity and Transmission. Neuroscience 2014, 282, 13–22. [Google Scholar] [CrossRef]
- Mirza, F.J.; Zahid, S. The Role of Synapsins in Neurological Disorders. Neurosci. Bull. 2017, 34, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, Y.; Hu, M.; Chai, Z.; Wu, Q.; Huang, R.; Han, W.; Zhang, C.X.; Zhou, Z. Synaptotagmin-11 Inhibits Clathrin-Mediated and Bulk Endocytosis. EMBO Rep. 2016, 17, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Slevin, J.T.; Whiteheart, S.W. Cellular Functions of NSF: Not Just SNAPs and SNAREs. FEBS Lett. 2007, 581, 2140–2149. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, Z.; Du, S.; Wei, H.; Li, X.; Li, X.; Shen, J.; Chen, X.; Cai, Z. The Increase of α-Synuclein and Alterations of Dynein in A53T Transgenic and Aging Mouse. J. Clin. Neurosci. 2022, 96, 154–162. [Google Scholar] [CrossRef]
- Polymeropoulos, M.H.; Lavedan, C.; Leroy, E.; Ide, S.E.; Dehejia, A.; Dutra, A.; Pike, B.; Root, H.; Rubenstein, J.; Boyer, R.; et al. Mutation in the Alpha-Synuclein Gene Identified in Families with Parkinson’s Disease. Science 1997, 276, 2045–2047. [Google Scholar] [CrossRef]
- Polymeropoulos, M.H.; Higgins, J.J.; Golbe, L.I.; Johnson, W.G.; Ide, S.E.; Di Iorio, G.; Sanges, G.; Stenroos, E.S.; Pho, L.T.; Schaffer, A.A.; et al. Mapping of a Gene for Parkinson’s Disease to Chromosome 4q21-Q23. Science 1996, 274, 1197–1199. [Google Scholar] [CrossRef]
- D′Andrea, M.R.; Ilyin, S.; Plata-Salaman, C.R. Abnormal Patterns of Microtubule-Associated Protein-2 (MAP-2) Immunolabeling in Neuronal Nuclei and Lewy Bodies in Parkinson’s Disease Substantia Nigra Brain Tissues. Neurosci. Lett. 2001, 306, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yuan, Y.-H.; Sun, J.-D.; Li, J.; Li, Z.-P.; Chen, N.-H. Nigrostriatal Dynein Changes in A53T Alpha-Synuclein Transgenic Mice. F1000Res 2014, 3, 68. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Conde, L.D.; Ramos-Acevedo, R.; Reyes-Hernández, M.A.; Balbuena-Olvera, A.J.; Morales-Moreno, I.D.; Argüero-Sánchez, R.; Schüle, B.; Guerra-Crespo, M. Alpha-Synuclein Physiology and Pathology: A Perspective on Cellular Structures and Organelles. Front. Neurosci. 2020, 13, 1399. [Google Scholar] [CrossRef] [PubMed]
- Calì, T.; Ottolini, D.; Negro, A.; Brini, M. α-Synuclein Controls Mitochondrial Calcium Homeostasis by Enhancing Endoplasmic Reticulum-Mitochondria Interactions. J. Biol. Chem. 2012, 287, 17914–17929. [Google Scholar] [CrossRef] [PubMed]
- Mady, L.J.; Haleem, F.; Christakos, S. Calcium-Buffering Proteins: Calbindin. In Encyclopedia of Biological Chemistry, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; pp. 284–289. [Google Scholar]
- Schnapp, B.J.; Reese, T.S. Dynein Is the Motor for Retrograde Axonal Transport of Organelles. Proc. Natl. Acad. Sci. USA 1989, 86, 1548–1552. [Google Scholar] [CrossRef]
- Dehmelt, L.; Halpain, S. The MAP2/Tau Family of Microtubule-Associated Proteins. Genome Biol. 2005, 6, 204. [Google Scholar] [CrossRef]
- Nishida, K.; Matsumura, K.; Tamura, M.; Nakamichi, T.; Shimamori, K.; Kuragano, M.; Kabir, A.M.R.; Kakugo, A.; Kotani, S.; Nishishita, N.; et al. Effects of Three Microtubule-Associated Proteins (MAP2, MAP4, and Tau) on Microtubules’ Physical Properties and Neurite Morphology. Sci. Rep. 2023, 13, 8870. [Google Scholar] [CrossRef]
- Cartelli, D.; Ronchi, C.; Maggioni, M.G.; Rodighiero, S.; Giavini, E.; Cappelletti, G. Microtubule Dysfunction Precedes Transport Impairment and Mitochondria Damage in MPP+ -Induced Neurodegeneration. J. Neurochem. 2010, 115, 247–258. [Google Scholar] [CrossRef]
- Kim-Han, J.S.; Antenor-Dorsey, J.A.; O’Malley, K.L. The Parkinsonian Mimetic, MPP+, Specifically Impairs Mitochondrial Transport in Dopamine Axons. J. Neurosci. 2011, 31, 7212–7221. [Google Scholar] [CrossRef]
- Dukes, A.A.; Bai, Q.; Van Laar, V.S.; Zhou, Y.; Ilin, V.; David, C.N.; Agim, Z.S.; Bonkowsky, J.L.; Cannon, J.R.; Watkins, S.C.; et al. Live Imaging of Mitochondrial Dynamics in CNS Dopaminergic Neurons in Vivo Demonstrates Early Reversal of Mitochondrial Transport Following MPP(+) Exposure. Neurobiol. Dis. 2016, 95, 238–249. [Google Scholar] [CrossRef]
- Stamer, K.; Vogel, R.; Thies, E.; Mandelkow, E.; Mandelkow, E.-M. Tau Blocks Traffic of Organelles, Neurofilaments, and APP Vesicles in Neurons and Enhances Oxidative Stress. J. Cell Biol. 2002, 156, 1051–1063. [Google Scholar] [CrossRef] [PubMed]
- Ebneth, A.; Godemann, R.; Stamer, K.; Illenberger, S.; Trinczek, B.; Mandelkow, E.-M.; Mandelkow, E. Overexpression of Tau Protein Inhibits Kinesin-Dependent Trafficking of Vesicles, Mitochondria, and Endoplasmic Reticulum: Implications for Alzheimer’s Disease. J. Cell Biol. 1998, 143, 777–794. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.F.; Gan, Z.Y.; Komander, D.; Dewson, G. Ubiquitin Signalling in Neurodegeneration: Mechanisms and Therapeutic Opportunities. Cell Death Differ. 2021, 28, 570–590. [Google Scholar] [CrossRef]
- Geisler, S.; Vollmer, S.; Golombek, S.; Kahle, P.J. The Ubiquitin-Conjugating Enzymes UBE2N, UBE2L3 and UBE2D2/3 Are Essential for Parkin-Dependent Mitophagy. J. Cell Sci. 2014, 127, 3280–3293. [Google Scholar] [CrossRef] [PubMed]
- Palazón-Riquelme, P.; Worboys, J.D.; Green, J.; Valera, A.; Martín-Sánchez, F.; Pellegrini, C.; Brough, D.; López-Castejón, G. USP7 and USP47 Deubiquitinases Regulate NLRP3 Inflammasome Activation. EMBO Rep. 2018, 19, e44766. [Google Scholar] [CrossRef] [PubMed]
- Simunovic, F.; Yi, M.; Wang, Y.; Macey, L.; Brown, L.T.; Krichevsky, A.M.; Andersen, S.L.; Stephens, R.M.; Benes, F.M.; Sonntag, K.C. Gene Expression Profiling of Substantia Nigra Dopamine Neurons: Further Insights into Parkinson’s Disease Pathology. Brain 2009, 132, 1795–1809. [Google Scholar] [CrossRef] [PubMed]
- Duke, D.C.; Moran, L.B.; Kalaitzakis, M.E.; Deprez, M.; Dexter, D.T.; Pearce, R.K.B.; Graeber, M.B. Transcriptome Analysis Reveals Link between Proteasomal and Mitochondrial Pathways in Parkinson’s Disease. Neurogenetics 2006, 7, 139–148. [Google Scholar] [CrossRef]
- Vyas, I.; Heikkila, R.E.; Nicklas, W.J. Studies on the Neurotoxicity of 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine: Inhibition of NAD-Linked Substrate Oxidation by Its Metabolite, 1-Methyl-4-Phenylpyridinium. J. Neurochem. 1986, 46, 1501–1507. [Google Scholar] [CrossRef]
- Berthet, A.; Margolis, E.B.; Zhang, J.; Hsieh, I.; Zhang, J.; Hnasko, T.S.; Ahmad, J.; Edwards, R.H.; Sesaki, H.; Huang, E.J.; et al. Loss of Mitochondrial Fission Depletes Axonal Mitochondria in Midbrain Dopamine Neurons. J. Neurosci. 2014, 34, 14304–14317. [Google Scholar] [CrossRef]
- Rappold, P.M.; Cui, M.; Grima, J.C.; Fan, R.Z.; de Mesy-Bentley, K.L.; Chen, L.; Zhuang, X.; Bowers, W.J.; Tieu, K. Drp1 Inhibition Attenuates Neurotoxicity and Dopamine Release Deficits In Vivo. Nat. Commun. 2014, 5, 5244. [Google Scholar] [CrossRef]
- Yamamoto, M.; Kensler, T.W.; Motohashi, H. The KEAP1-NRF2 System: A Thiol-Based Sensor-Effector Apparatus for Maintaining Redox Homeostasis. Physiol. Rev. 2018, 98, 1169–1203. [Google Scholar] [CrossRef] [PubMed]
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef] [PubMed]
- Cassarino, D.S.; Fall, C.P.; Swerdlow, R.H.; Smith, T.S.; Halvorsen, E.M.; Miller, S.W.; Parks, J.P.; Parker, W.D.; Bennett, J.P. Elevated Reactive Oxygen Species and Antioxidant Enzyme Activities in Animal and Cellular Models of Parkinson’s Disease. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 1997, 1362, 77–86. [Google Scholar] [CrossRef]
- Carlsson, A.; Lindqvist, M. In-Vivo Measurements of Tryptophan and Tyrosine Hydroxylase Activities in Mouse Brain. J. Neural Transm. 1973, 34, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Franklin, K.B.J. The Mouse Brain in Stereotaxic Coordinates, 2nd ed.; References Scientific Research Publishing; Academic Press: San Diego, CA, USA, 2001; Available online: https://www.scirp.org/(S(lz5mqp453edsnp55rrgjct55))/reference/referencespapers.aspx?referenceid=2090083 (accessed on 12 October 2023).
- Khaindrava, V.G.; Ershov, P.V.; Antsiperov, V.E.; Obukhov, I.V.; Nanaev, A.K.; Ugriumov, M.V. Optimization of counting process of dopaminergic neurons in substantia nigra of parkinsonian mice. Tsitologiia 2010, 52, 423–430. [Google Scholar] [PubMed]
- Gerfen, C.R.; Herkenham, M.; Thibault, J. The Neostriatal Mosaic: II. Patch- and Matrix-Directed Mesostriatal Dopaminergic and Non-Dopaminergic Systems. J. Neurosci. 1987, 7, 3915–3934. [Google Scholar] [CrossRef]
- Rydbirk, R.; Folke, J.; Winge, K.; Aznar, S.; Pakkenberg, B.; Brudek, T. Assessment of Brain Reference Genes for RT-qPCR Studies in Neurodegenerative Diseases. Sci. Rep. 2016, 6, 37116. [Google Scholar] [CrossRef]
- Bengtsson, M.; Ståhlberg, A.; Rorsman, P.; Kubista, M. Gene Expression Profiling in Single Cells from the Pancreatic Islets of Langerhans Reveals Lognormal Distribution of mRNA Levels. Genome Res. 2005, 15, 1388–1392. [Google Scholar] [CrossRef]
- Morrison, T.; Hurley, J.; Garcia, J.; Yoder, K.; Katz, A.; Roberts, D.; Cho, J.; Kanigan, T.; Ilyin, S.E.; Horowitz, D.; et al. Nanoliter High Throughput Quantitative PCR. Nucleic Acids Res. 2006, 34, e123. [Google Scholar] [CrossRef]
- Prokopec, S.D.; Watson, J.D.; Waggott, D.M.; Smith, A.B.; Wu, A.H.; Okey, A.B.; Pohjanvirta, R.; Boutros, P.C. Systematic Evaluation of Medium-Throughput mRNA Abundance Platforms. RNA 2013, 19, 51–62. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of Protein Using Bicinchoninic Acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Aldridge, G.M.; Podrebarac, D.M.; Greenough, W.T.; Weiler, I.J. The Use of Total Protein Stains as Loading Controls: An Alternative to High-Abundance Single-Protein Controls in Semi-Quantitative Immunoblotting. J. Neurosci. Methods 2008, 172, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Romero-Calvo, I.; Ocón, B.; Martínez-Moya, P.; Suárez, M.D.; Zarzuelo, A.; Martínez-Augustin, O.; de Medina, F.S. Reversible Ponceau Staining as a Loading Control Alternative to Actin in Western Blots. Anal. Biochem. 2010, 401, 318–320. [Google Scholar] [CrossRef] [PubMed]



| Gene | Protein | Function | Time after 2 × 6 mg/kg MPTP | |||||
|---|---|---|---|---|---|---|---|---|
| 1 h | 6 h | 24 h | ||||||
| Fold Change | p | Fold Change | p | Fold Change | p | |||
| DA synthesis, degradation, transport, and reception | ||||||||
| Th | Tyrosine hydroxylase | DA synthesis | 0.74 | 0.10 | 0.81 | 0.23 | 0.56 | 0.01 |
| Maoa | Monoamine oxidase B | DA degradation | 0.96 | 0.88 | 1.29 | 0.00 | 0.88 | 0.70 |
| Maob | Monoamine oxidase B | DA degradation | 0.89 | 0.50 | 0.62 | 0.01 | 0.91 | 0.67 |
| Comt | Catechol-O-methyltranserase | DA degradation | 1.21 | 0.02 | 1.15 | 0.17 | 1.20 | 0.20 |
| Slc6a3 | Dopamine transporter | DA reuptake | 0.80 | 0.09 | 0.89 | 0.26 | 0.61 | 0.01 |
| Slc18a2 | Vesicular monoamine transporter 2 | DA vesicles uptake | 0.90 | 0.28 | 0.97 | 0.70 | 0.63 | 0.01 |
| Drd2 | Dopamine receptor 2 | DA reception | 0.59 | 0.01 | 0.53 | 0.04 | 0.50 | 0.00 |
| Axonal transport and microtubules | ||||||||
| Dynll1 | Dynein light chain | Axonal transport | 0.55 | 0.02 | 0.62 | 0.02 | 1.01 | 0.96 |
| Mapt | Microtubule-associated tau | Axonal transport | 1.20 | 0.01 | 0.96 | 0.72 | 1.03 | 0.29 |
| Map2 | Microtubule-associated protein 2 | Axonal transport | 1.55 | 0.00 | 1.30 | 0.02 | 1.33 | 0.03 |
| Vesicle cycle for neurotransmission | ||||||||
| Snca | α-Synuclein | Neurotransmission | 1.11 | 0.42 | 1.05 | 0.74 | 0.77 | 0.03 |
| Syn1 | Synapsin 1 | Vesicular cycle | 1.59 | 0.01 | 1.34 | 0.08 | 1.28 | 0.15 |
| Syt11 | Synaptotagmin 11 | Endocytosis | 1.29 | 0.04 | 1.06 | 0.63 | 1.06 | 0.66 |
| Nsf | N-ethylmaleimide sensitive fusion protein | Vesicular cycle | 1.51 | 0.00 | 1.26 | 0.05 | 1.32 | 0.02 |
| Dnm1l | Dynamin 1-like protein | Vesicular cycle Mitochondrial fission | 1.34 | 0.02 | 1.25 | 0.05 | 1.21 | 0.06 |
| Protein degradation | ||||||||
| Ube2n | Ubiquitin conjugating enzyme E2 N | E2 enzyme | 1.07 | 0.41 | 0.85 | 0.04 | 1.05 | 0.69 |
| Psmb4 | Proteasome 20S subunit beta 4 | Proteasome subunits | 1.29 | 0.01 | 1.10 | 0.18 | 0.90 | 0.28 |
| Psmd4 | Proteasome 26S subunit ubiquitin receptor, non-ATPase 4 | Proteasome subunits | 1.20 | 0.05 | 1.48 | 0.00 | 1.31 | 0.23 |
| Usp47 | Ubiquitin specific peptidase 47 | Protein deubiquitination | 1.70 | 0.00 | 1.72 | 0.00 | 1.41 | 0.04 |
| Neuroprotection | ||||||||
| Sod1 | Superoxide dismutase 1 | Antioxidant system | 1.30 | 0.03 | 1.12 | 0.31 | 1.01 | 0.60 |
| Gpx1 | Glutathione peroxidase 1 | Antioxidant system | 1.30 | 0.01 | 1.17 | 0.17 | 1.04 | 0.65 |
| Txnrd1 | Thioredoxin reductase 1 | Antioxidant system | 2.59 | 0.01 | 2.32 | 0.01 | 2.41 | 0.01 |
| Prdx1 | Peroxiredoxin 1 | Antioxidant system | 0.73 | 0.03 | 0.94 | 0.53 | 0.75 | 0.34 |
| Nfe2l2 | Nuclear factor erythroid 2-related factor 2 | Transcriptome factor | 2.31 | 0.02 | 1.82 | 0.12 | 1.84 | 0.12 |
| Keap1 | Kelch-like ECH-associated protein 1 | Regulation of Nfe2l2 | 1.49 | 0.01 | 1.48 | 0.00 | 1.04 | 0.78 |
| Ntrk2 | Neurotrophic receptor tyrosine kinase 2 | Neurotrophic factor receptor | 1.30 | 0.00 | 1.22 | 0.00 | 1.06 | 0.26 |
| Calb1 | Calbindin 1 | Ca2+-binding protein | 1.48 | 0.18 | 1.20 | 0.37 | 2.25 | 0.03 |
| Inflammation and glial activation | ||||||||
| Cnr1 | Cannabinoid receptor 1 | Endocannabinoid system | 1.60 | 0.01 | 1.49 | 0.03 | 1.66 | 0.02 |
| Clk1 | CDC-like kinase 1 | Pre-mRNA processing | 1.63 | 0.00 | 1.06 | 0.70 | 1.55 | 0.00 |
| Cluster of Genes | Genes |
|---|---|
| House-keeping gene | Cyc1 |
| Dopamine synthesis, degradation, transport, and reception | Th, Ddc, Dbh, Pnmt, Maoa, Maob, Comt, Slc6a3, Slc18a2, Slc29a4, Drd1–Drd5 |
| Axonal transport and microtubules | Kif1a, Kif1b, Kif5a, Kif2c, Dync1h1, Dynll1, Dctn1, Mapt, Map2, Mark2, Tubb3, Tuba1a |
| Vesicle cycle for neurotransmission | Snca, Syn1, Stx1a, Syt1, Syt11, Rab5a, Rab7, Nsf, Dnm1l |
| Neuroprotection | Sod1, Gpx1, Gsr, Txnrd1, Nos1, Prdx1, Nfe2l2, Agtr2, Keap1, Sigmar1, Cacna1d, Trpm2, Bdnf, Gdnf, Ngf, Vegfa, Cdnf, Ntrk2, Ntrk1, Ngfr, Nr4a2, Mmp3, Pitx3, Wnt11, Ctnnb1, Calb1 |
| Protein degradation | Park2, Ube2n, Uba3, Psmb4, Psmc3, Psmd4, Usp47, Ubb |
| Inflammation and glial activation | Gfap, Ifng, Tgfb1, Akt1, Cnr1, Ptgs2, Traf1, Cxcl11 |
| Apoptosis, necrosis, autophagy, ER stress | Casp1, Casp3, Parp1, Aifm1, Bcl2l11, Map3k5, Cib1, Trp53, Bax, Fos, Mapk8, Lamp2, Atg16l1, Atg5, Capn1, Tnf, Ctsb, Ern2, Eif2ak3, Atf6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolacheva, A.; Pavlova, E.; Bannikova, A.; Bogdanov, V.; Ugrumov, M. Initial Molecular Mechanisms of the Pathogenesis of Parkinson’s Disease in a Mouse Neurotoxic Model of the Earliest Preclinical Stage of This Disease. Int. J. Mol. Sci. 2024, 25, 1354. https://doi.org/10.3390/ijms25021354
Kolacheva A, Pavlova E, Bannikova A, Bogdanov V, Ugrumov M. Initial Molecular Mechanisms of the Pathogenesis of Parkinson’s Disease in a Mouse Neurotoxic Model of the Earliest Preclinical Stage of This Disease. International Journal of Molecular Sciences. 2024; 25(2):1354. https://doi.org/10.3390/ijms25021354
Chicago/Turabian StyleKolacheva, Anna, Ekaterina Pavlova, Alyona Bannikova, Vsevolod Bogdanov, and Michael Ugrumov. 2024. "Initial Molecular Mechanisms of the Pathogenesis of Parkinson’s Disease in a Mouse Neurotoxic Model of the Earliest Preclinical Stage of This Disease" International Journal of Molecular Sciences 25, no. 2: 1354. https://doi.org/10.3390/ijms25021354
APA StyleKolacheva, A., Pavlova, E., Bannikova, A., Bogdanov, V., & Ugrumov, M. (2024). Initial Molecular Mechanisms of the Pathogenesis of Parkinson’s Disease in a Mouse Neurotoxic Model of the Earliest Preclinical Stage of This Disease. International Journal of Molecular Sciences, 25(2), 1354. https://doi.org/10.3390/ijms25021354







