Nanoparticulated WO3/NiWO4 Using Cellulose as a Template and Its Application as an Auxiliary Co-Catalyst to Pt for Ethanol and Glycerol Electro-Oxidation
Abstract
:1. Introduction
2. Results
2.1. Nanocrystalline Cellulose
2.2. Nanosized NiT/TO
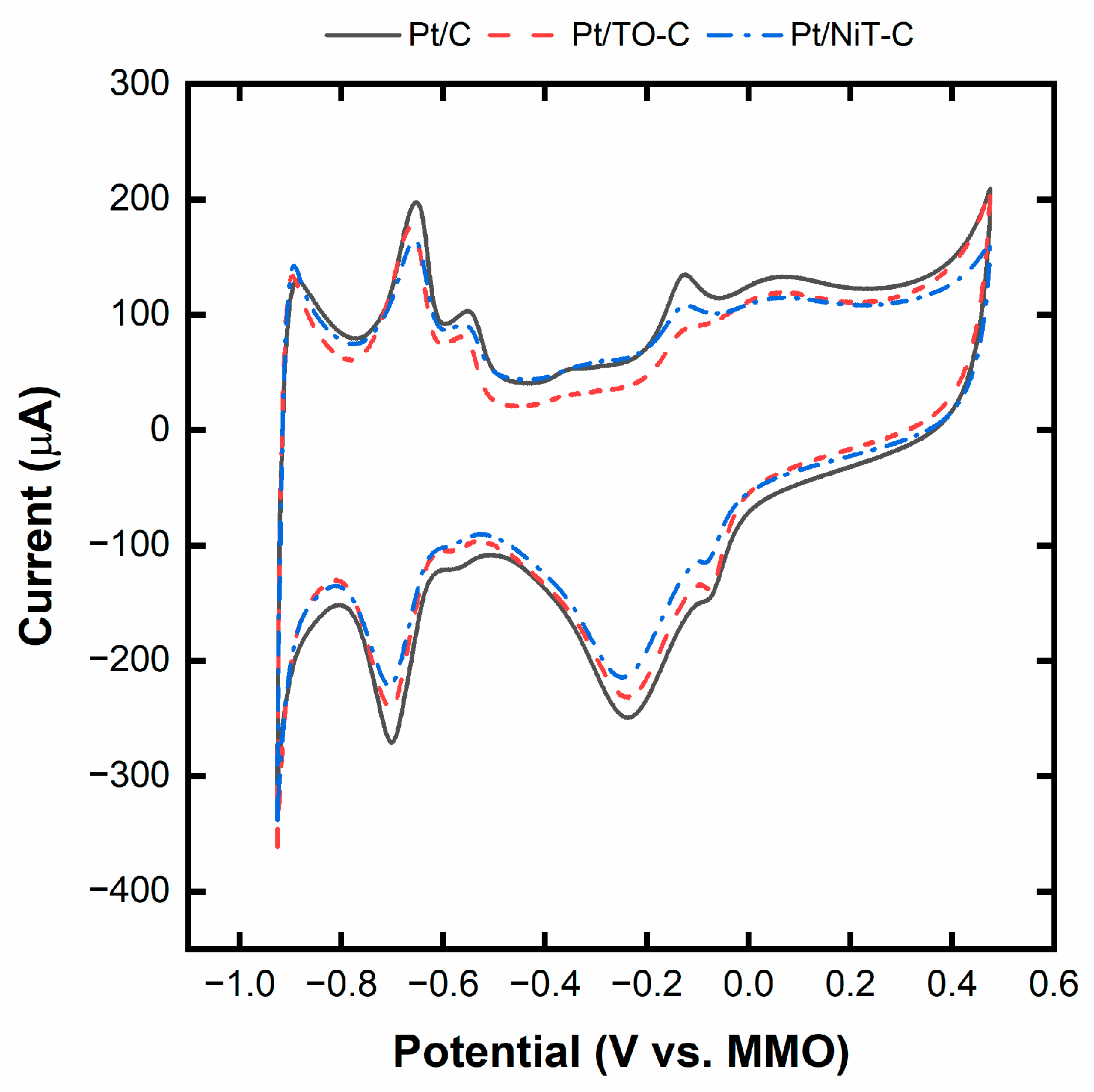
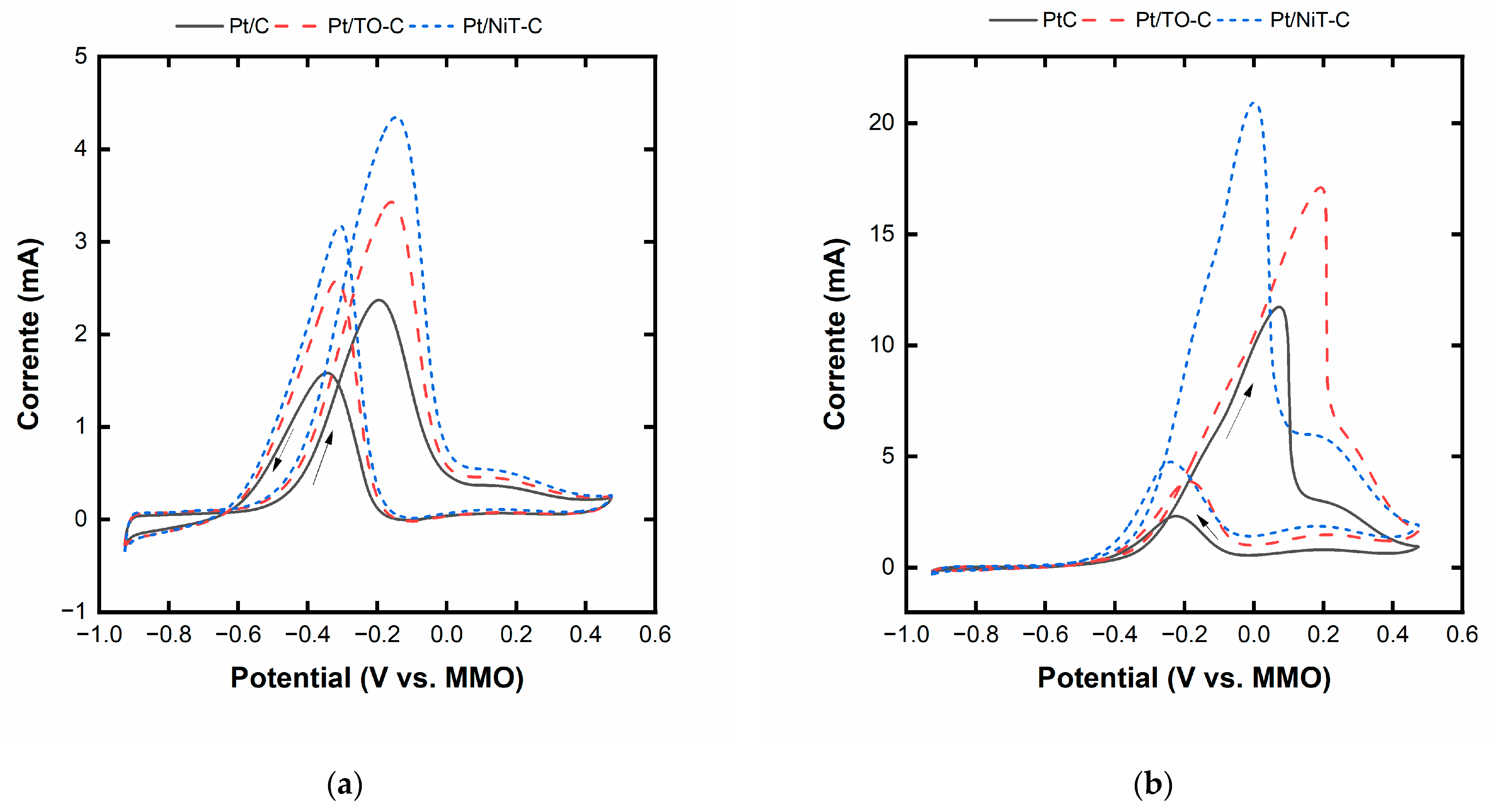
2.3. Pt/TO-C and Pt/NiT-C Electrocatalysts
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sertolli, A.; Gabnai, Z.; Lengyel, P.; Bai, A. Biomass Potential and Utilization in Worldwide Research Trends—A Bibliometric Analysis. Sustainability 2022, 14, 5515. [Google Scholar] [CrossRef]
- Gielen, D.; Boshell, F.; Saygin, D.; Bazilian, M.D.; Wagner, N.; Gorini, R. The Role of Renewable Energy in the Global Energy Transformation. Energy Strategy Rev. 2019, 24, 38–50. [Google Scholar] [CrossRef]
- Singh, S.P.; Jawaid, M.; Chandrasekar, M.; Senthilkumar, K.; Yadav, B.; Saba, N.; Siengchin, S. Sugarcane Wastes into Commercial Products: Processing Methods, Production Optimization and Challenges. J. Clean. Prod. 2021, 328, 129453. [Google Scholar] [CrossRef]
- Ungureanu, N.; Vlăduț, V.; Biriș, S.-Ș. Sustainable Valorization of Waste and By-Products from Sugarcane Processing. Sustainability 2022, 14, 11089. [Google Scholar] [CrossRef]
- Kabeyi, M.J.B.; Olanrewaju, O.A. Bagasse Electricity Potential of Conventional Sugarcane Factories. J. Energy 2023, 2023, 1–25. [Google Scholar] [CrossRef]
- Macrelli, S.; Mogensen, J.; Zacchi, G. Techno-Economic Evaluation of 2nd Generation Bioethanol Production from Sugar Cane Bagasse and Leaves Integrated with the Sugar-Based Ethanol Process. Biotechnol. Biofuels 2012, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.O.S.; Junqueira, T.L.; Cavalett, O.; Cunha, M.P.; Jesus, C.D.F.; Rossell, C.E.V.; Maciel Filho, R.; Bonomi, A. Integrated versus Stand-Alone Second Generation Ethanol Production from Sugarcane Bagasse and Trash. Bioresour. Technol. 2012, 103, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Chakrabarty, D. Isolation of Nanocellulose from Waste Sugarcane Bagasse (SCB) and Its Characterization. Carbohydr. Polym. 2011, 86, 1291–1299. [Google Scholar] [CrossRef]
- Lu, Q.; Cai, Z.; Lin, F.; Tang, L.; Wang, S.; Huang, B. Extraction of Cellulose Nanocrystals with a High Yield of 88% by Simultaneous Mechanochemical Activation and Phosphotungstic Acid Hydrolysis. ACS Sustain. Chem. Eng. 2016, 4, 2165–2172. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef]
- Thomas, B.; Raj, M.C.; Joy, J.; Moores, A.; Drisko, G.L.; Sanchez, C. Nanocellulose, a Versatile Green Platform: From Biosources to Materials and Their Applications. Chem. Rev. 2018, 118, 11575–11625. [Google Scholar] [CrossRef] [PubMed]
- Anžlovar, A.; Žagar, E. Cellulose Structures as a Support or Template for Inorganic Nanostructures and Their Assemblies. Nanomaterials 2022, 12, 1837–1907. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Boutry, S.; Muller, R.N. Metal Oxide Particles and Their Prospects for Applications. In Iron Oxide Nanoparticles for Biomedical Applications; Mahmoudi, M., Laurent, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 3–42. ISBN 978-0-08-101925-2. [Google Scholar]
- Danish, M.S.S.; Bhattacharya, A.; Stepanova, D.; Mikhaylov, A.; Grilli, M.L.; Khosravy, M.; Senjyu, T. A Systematic Review of Metal Oxide Applications for Energy and Environmental Sustainability. Metals 2020, 10, 1604. [Google Scholar] [CrossRef]
- Megía, P.J.; Vizcaíno, A.J.; Calles, J.A.; Carrero, A. Hydrogen Production Technologies: From Fossil Fuels toward Renewable Sources. A Mini Review. Energy Fuels 2021, 35, 16403–16415. [Google Scholar] [CrossRef]
- Abohamzeh, E.; Salehi, F.; Sheikholeslami, M.; Abbassi, R.; Khan, F. Review of Hydrogen Safety during Storage, Transmission, and Applications Processes. J. Loss Prev. Process Ind. 2021, 72, 104569. [Google Scholar] [CrossRef]
- Bristowe, G.; Smallbone, A. The Key Techno-Economic and Manufacturing Drivers for Reducing the Cost of Power-to-Gas and a Hydrogen-Enabled Energy System. Hydrogen 2021, 2, 273–300. [Google Scholar] [CrossRef]
- Siwal, S.S.; Thakur, S.; Zhang, Q.B.; Thakur, V.K. Electrocatalysts for Electrooxidation of Direct Alcohol Fuel Cell: Chemistry and Applications. Mater. Today Chem. 2019, 14, 100182. [Google Scholar] [CrossRef]
- Miller, H.A.; Lavacchi, A.; Vizza, F. Storage of Renewable Energy in Fuels and Chemicals through Electrochemical Reforming of Bioalcohols. Curr. Opin. Electrochem. 2020, 21, 140–145. [Google Scholar] [CrossRef]
- Martins, C.A.; Fernández, P.S.; Troiani, H.E.; Martins, M.E.; Camara, G.A. Ethanol vs. Glycerol: Understanding the Lack of Correlation between the Oxidation Currents and the Production of CO2 on Pt Nanoparticles. J. Electroanal. Chem. 2014, 717–718, 231–236. [Google Scholar] [CrossRef]
- Souza, F.M.; Pinheiro, V.S.; Gentil, T.C.; Lucchetti, L.E.B.; Silva, J.C.M.; Santos, M.L.M.G.; De Oliveira, I.; Dourado, W.M.C.; Amaral-Labat, G.; Okamoto, S.; et al. Alkaline Direct Liquid Fuel Cells: Advances, Challenges and Perspectives. J. Electroanal. Chem. 2022, 922, 116712. [Google Scholar] [CrossRef]
- Wala, M.; Simka, W. Effect of Anode Material on Electrochemical Oxidation of Low Molecular Weight Alcohols—A Review. Molecules 2021, 26, 2144. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Fang, C.; Hu, J.; Zhang, D. Platinum-Based Electrocatalysts for Direct Alcohol Fuel Cells: Enhanced Performances toward Alcohol Oxidation Reactions. Chempluschem 2021, 86, 574–586. [Google Scholar] [CrossRef] [PubMed]
- Vyas, A.N.; Saratale, G.D.; Sartale, S.D. Recent Developments in Nickel Based Electrocatalysts for Ethanol Electrooxidation. Int. J. Hydrogen Energy 2020, 45, 5928–5947. [Google Scholar] [CrossRef]
- Tsurumura, T.; Yoshimoto, N.; Egashira, M.; Morita, M. Electrocatalytic Activity of Platinum Nano-Particle Deposited on Tungsten Oxide for Anodic Oxidation of Ethanol. Electrochemistry 2012, 80, 916–918. [Google Scholar] [CrossRef]
- Miecznikowski, K. WO3 Decorated Carbon Nanotube Supported PtSn Nanoparticles with Enhanced Activity towards Electrochemical Oxidation of Ethylene Glycol in Direct Alcohol Fuel Cells. Arab. J. Chem. 2020, 13, 1020–1031. [Google Scholar] [CrossRef]
- Zhang, D.-Y.; Ma, Z.-F.; Wang, G.; Konstantinov, K.; Yuan, X.; Liu, H.-K. Electro-Oxidation of Ethanol on Pt-WO3/C Electrocatalyst. Electrochem. Solid-State Lett. 2006, 9, A423–A426. [Google Scholar] [CrossRef]
- Tseung, A.C.C.; Chen, K.Y. Hydrogen Spill-over Effect on Pt/WO3 Anode Catalysts. Catal. Today 1997, 38, 439–443. [Google Scholar] [CrossRef]
- Tseung, A.C.C.; Shen, P.K.; Chen, K.Y. Precious Metal/Hydrogen Bronze Anode Catalysts for the Oxidation of Small Organic Molecules and Impure Hydrogen. J. Power Sources 1996, 61, 223–225. [Google Scholar] [CrossRef]
- Kumar, R.; Bhuvana, T.; Sharma, A. Nickel Tungstate–Graphene Nanocomposite for Simultaneous Electrochemical Detection of Heavy Metal Ions with Application to Complex Aqueous Media. RSC Adv. 2017, 7, 42146–42158. [Google Scholar] [CrossRef]
- Kumar, R.; Gupta, P.K.; Agrawal, A.; Nagarale, R.K.; Sharma, A. Hydrothermally Synthesized Reduced Graphene Oxide-NiWO4 Nanocomposite for Lithium-Ion Battery Anode. J. Electrochem. Soc. 2017, 164, A785–A795. [Google Scholar] [CrossRef]
- Du, X.; Shao, Q.; Zhang, X. Metal Tungstate Dominated NiCo2O4@NiWO4 Nanorods Arrays as an Efficient Electrocatalyst for Water Splitting. Int. J. Hydrogen Energy 2019, 44, 2883–2888. [Google Scholar] [CrossRef]
- Srirapu, V.K.V.P.; Kumar, A.; Srivastava, P.; Singh, R.N.; Sinha, A.S.K. Nanosized CoWO4 and NiWO4 as Efficient Oxygen-Evolving Electrocatalysts. Electrochim. Acta 2016, 209, 75–84. [Google Scholar] [CrossRef]
- Hai, G.; Huang, J.; Cao, L.; Kajiyoshi, K.; Wang, L.; Feng, L. Hierarchical W18O49/NiWO4/NF Heterojunction with Tuned Composition and Charge Transfer for Efficient Water Splitting. Appl. Surf. Sci. 2021, 562, 150145. [Google Scholar] [CrossRef]
- Somacescu, S.; Osiceanu, P.; Calderon Moreno, J.M.; Culita, D.C.; Neațu, F.; Trandafir, M.M.; Neațu, Ș.; Kuncser, A.; Szijjártó, G.P.; Tálas, E.; et al. Design of Electrocatalysts with Reduced Pt Content Supported on Mesoporous NiWO4 and NiWO4-Graphene Nanoplatelets Composite for Oxygen Reduction and Hydrogen Oxidation in Acidic Medium. Int. J. Hydrogen Energy 2023, 48, 6317–6335. [Google Scholar] [CrossRef]
- Shi, M.; Tong, X.; Li, W.; Fang, J.; Chen, L.; Ma, C. Enhanced Electrocatalytic Oxygen Reduction on NiWO x Solid Solution with Induced Oxygen Defects. ACS Appl. Mater. Interfaces 2017, 9, 34990–35000. [Google Scholar] [CrossRef] [PubMed]
- Adzic, R.R.; Marinkovic, N.S. Electrocatalyst for Alcohol Oxidation in Fuel Cells. US Patent 6,183,894 B1, 6 February 2001. [Google Scholar]
- Kumaresan, A.; Arun, A.; Kalpana, V.; Vinupritha, P.; Sundaravadivel, E. Polymer-Supported NiWO4 Nanocomposites for Visible Light Degradation of Toxic Dyes. J. Mater. Sci. Mater. Electron. 2022, 33, 9660–9668. [Google Scholar] [CrossRef]
- Rosal, F.J.O.; Gouveia, A.F.; Sczancoski, J.C.; Lemos, P.S.; Longo, E.; Zhang, B.; Cavalcante, L.S. Electronic Structure, Growth Mechanism, and Sonophotocatalytic Properties of Sphere-like Self-Assembled NiWO4 Nanocrystals. Inorg. Chem. Commun. 2018, 98, 34–40. [Google Scholar] [CrossRef]
- Afify, H.H.; Hassan, S.A.; Obaida, M.; Moussa, I.; Abouelsayed, A. Preparation, Characterization, and Optical Spectroscopic Studies of Nanocrystalline Tungsten Oxide WO3. Opt. Laser Technol. 2019, 111, 604–611. [Google Scholar] [CrossRef]
- Sharmila, B.; Divyashree, P.; Dhanekar, S.; Dwivedi, P. Sensing Demonstration and Scalable Production of Nanostructured WO3 FET. Opt. Mater. 2022, 134, 113027. [Google Scholar] [CrossRef]
- Monshi, A.; Foroughi, M.R.; Monshi, M.R. Modified Scherrer Equation to Estimate More Accurately Nano-Crystallite Size Using XRD. World J. Nano Sci. Eng. 2012, 02, 154–160. [Google Scholar] [CrossRef]
- Zhao, H.; Kwak, J.; Conrad Zhang, Z.; Brown, H.; Arey, B.; Holladay, J. Studying Cellulose Fiber Structure by SEM, XRD, NMR and Acid Hydrolysis. Carbohydr. Polym. 2007, 68, 235–241. [Google Scholar] [CrossRef]
- Noshi, M.N. Phosphotungstic Acid Hydrate. In Encyclopedia of Reagents for Organic Synthesis; John Wiley & Sons, Ltd: Chichester, UK, 2013; pp. 1–8. [Google Scholar]
- Pinheiro, A.L.N.; Oliveira-Neto, A.; De Souza, E.C.; Perez, J.; Paganin, V.A.; Ticianelli, E.A.; Gonzalez, E.R. Electrocatalysis on Noble Metal and Noble Metal Alloys Dispersed on High Surface Area Carbon. J. New Mater. Electrochem. Syst. 2003, 6, 1–8. [Google Scholar]
- Fu, X.; Wan, C.; Huang, Y.; Duan, X. Noble Metal Based Electrocatalysts for Alcohol Oxidation Reactions in Alkaline Media. Adv. Funct. Mater. 2022, 32, 2106401. [Google Scholar] [CrossRef]
- Verma, S.; Lu, S.; Kenis, P.J.A. Co-Electrolysis of CO2 and Glycerol as a Pathway to Carbon Chemicals with Improved Technoeconomics Due to Low Electricity Consumption. Nat. Energy 2019, 4, 466–474. [Google Scholar] [CrossRef]
- Koolen, C.D.; Luo, W.; Züttel, A. From Single Crystal to Single Atom Catalysts: Structural Factors Influencing the Performance of Metal Catalysts for CO2 Electroreduction. ACS Catal. 2023, 13, 948–973. [Google Scholar] [CrossRef]
- Chueh, L.-Y.; Kuo, C.-H.; Yang, R.-H.; Tsai, D.-H.; Tsai, M.-H.; Yang, C.-C.; Chen, H.-Y.; Wang, C.-H.; Pan, Y.-T. WOx Nanowire Supported Ultra-Fine Ir-IrOx Nanocatalyst with Compelling OER Activity and Durability. Chem. Eng. J. 2023, 464, 142613. [Google Scholar] [CrossRef]
- Ma, X.; Yang, C.; Zhang, F.; Ke, F.; Cheng, Q.; Zou, L.; Yang, H. Oxygen-Vacancy-Rich Tungsten Oxide Boosted Ultrasmall Iridium Nanoparticles for Acidic Oxygen Evolution. Int. J. Hydrogen Energy 2023, 48, 36776–36783. [Google Scholar] [CrossRef]
- Viana, N.A.; Guimarães, M.G.; de Brasil, A.C.M.; do Vale, A.T.; de Macedo, J.L.; Ghesti, G.F. Gaseificação Da Casca de Jatobá-Do-Cerrado: Caracterização e Comparação Entre Simulação e Ensaios Laboratorais. Rev. Bras. Energ. 2017, 23, 82–104. [Google Scholar]

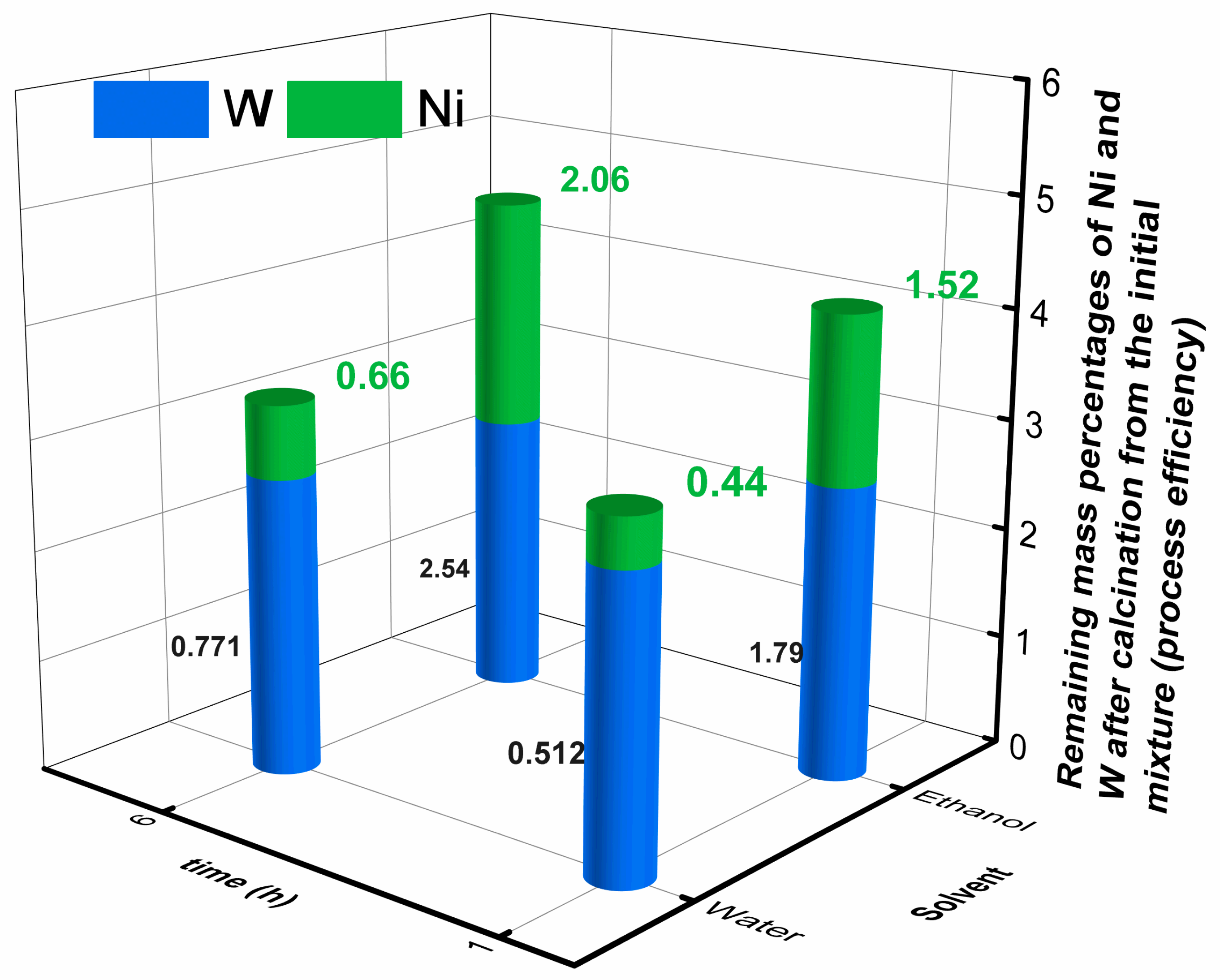
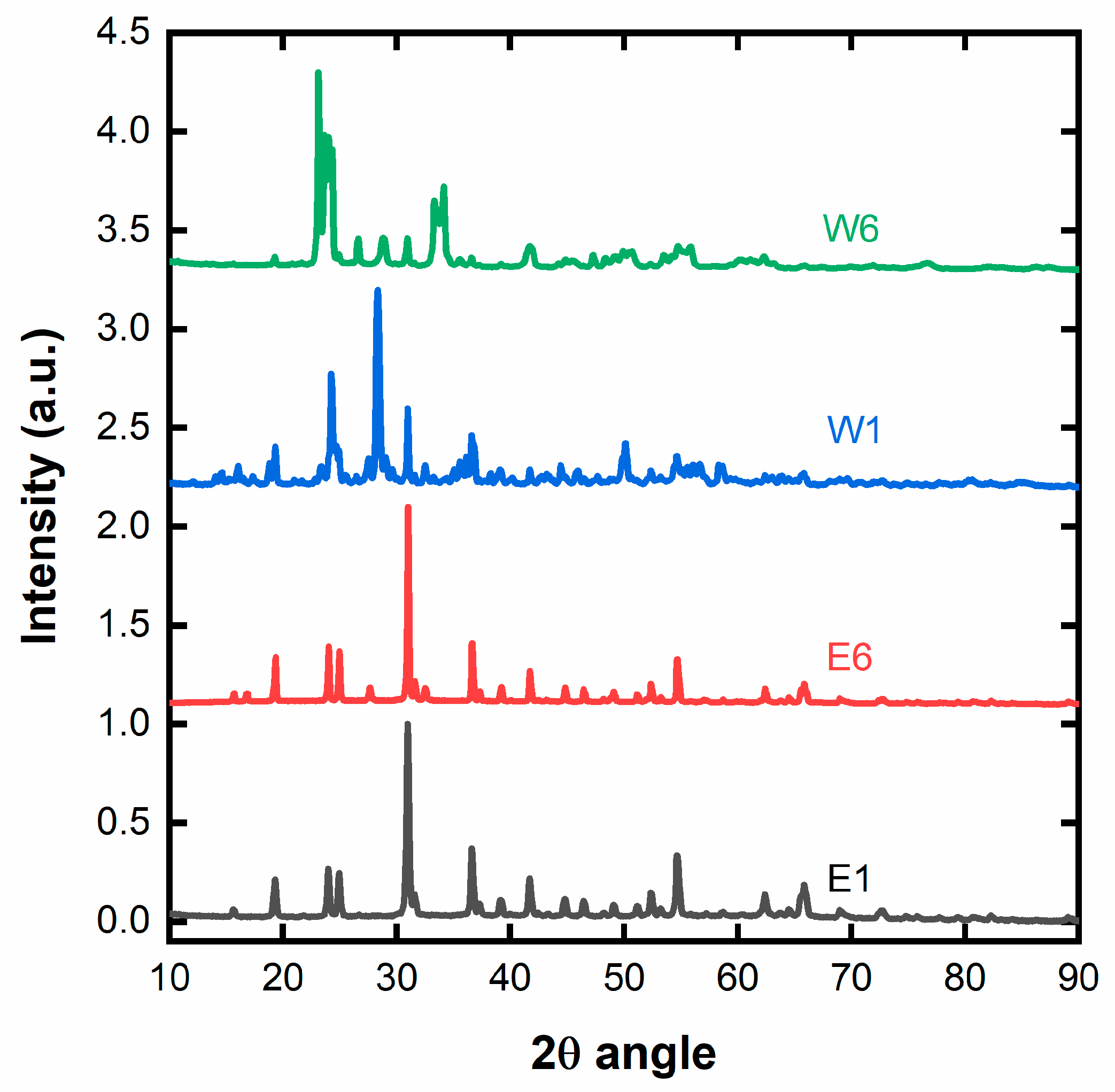

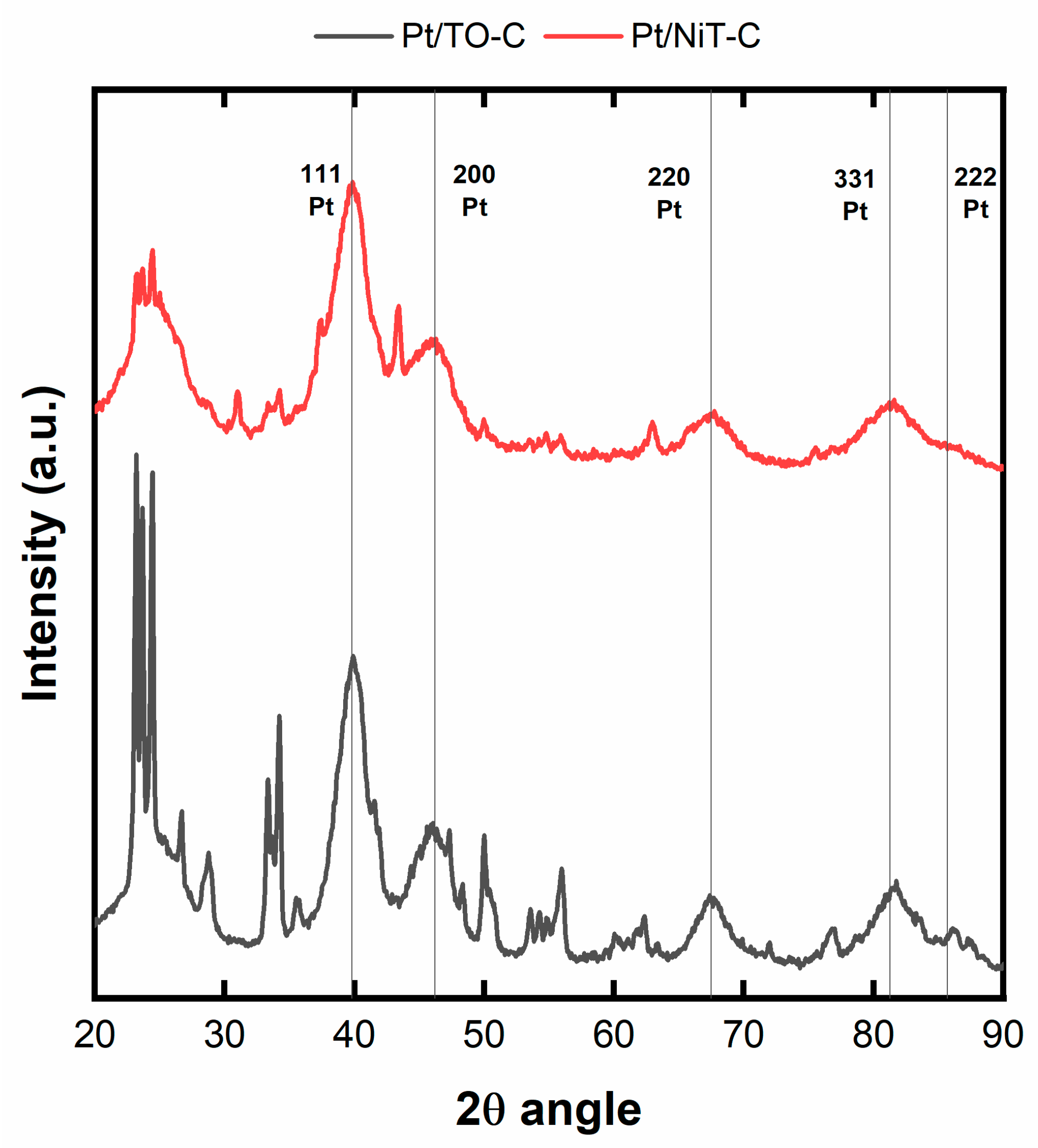
| Sample | TO Phase (nm) | NiT Phase (nm) |
|---|---|---|
| E1 | 23.5 ± 2.3 | 24.8 ± 1.8 |
| E6 | 43.8 ± 4.3 | 45.3 ± 2.6 |
| W1 | 30.9 ± 2.8 | 31.3 ± 2.4 |
| W6 | 41.1 ± 3.8 | 38.6 ± 2.9 |
| Catalyst | Onset Potential for Ethanol/Glycerol (V vs. MMO) | Maximum Current for Ethanol/Glycerol (mA) |
|---|---|---|
| Pt/C | −0.471/−0.327 | 2.3/11.7 |
| Pt/TO-C | −0.500/−0.320 | 3.3/17.2 |
| Pt/NiT-TO-C | −0.508/−0.301 | 4.4/20.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guimarães, M.G.; Macedo, J.L.; Linares, J.J.; Ghesti, G.F. Nanoparticulated WO3/NiWO4 Using Cellulose as a Template and Its Application as an Auxiliary Co-Catalyst to Pt for Ethanol and Glycerol Electro-Oxidation. Int. J. Mol. Sci. 2024, 25, 685. https://doi.org/10.3390/ijms25020685
Guimarães MG, Macedo JL, Linares JJ, Ghesti GF. Nanoparticulated WO3/NiWO4 Using Cellulose as a Template and Its Application as an Auxiliary Co-Catalyst to Pt for Ethanol and Glycerol Electro-Oxidation. International Journal of Molecular Sciences. 2024; 25(2):685. https://doi.org/10.3390/ijms25020685
Chicago/Turabian StyleGuimarães, Munique G., Julio L. Macedo, José J. Linares, and Grace F. Ghesti. 2024. "Nanoparticulated WO3/NiWO4 Using Cellulose as a Template and Its Application as an Auxiliary Co-Catalyst to Pt for Ethanol and Glycerol Electro-Oxidation" International Journal of Molecular Sciences 25, no. 2: 685. https://doi.org/10.3390/ijms25020685





