Cinnamyl Alcohol Attenuates Adipogenesis in 3T3-L1 Cells by Arresting the Cell Cycle
Abstract
:1. Introduction
2. Results
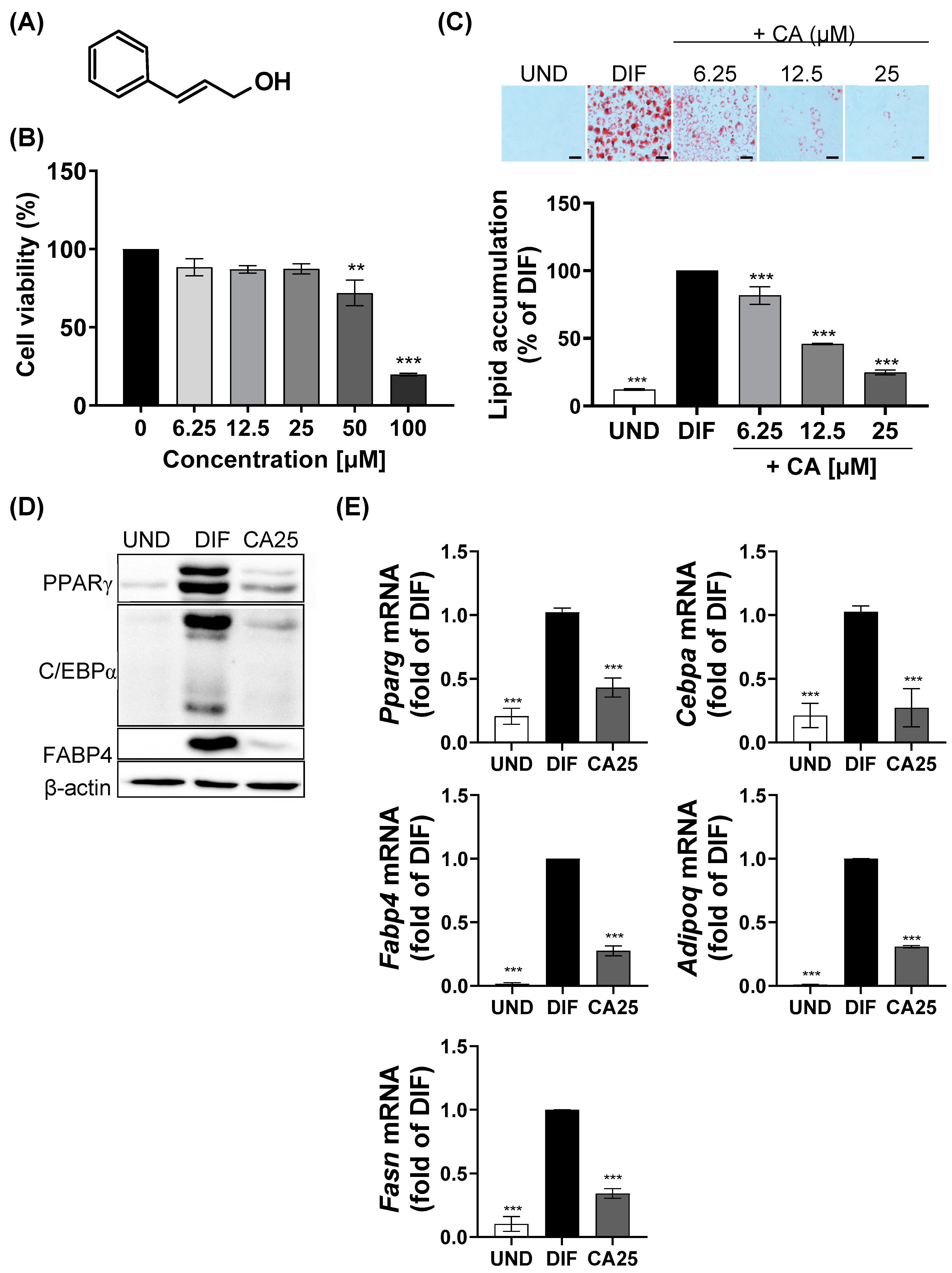
2.1. Effect of CA on Cytotoxicity and Lipid Accumulation in 3T3-L1 Adipocytes
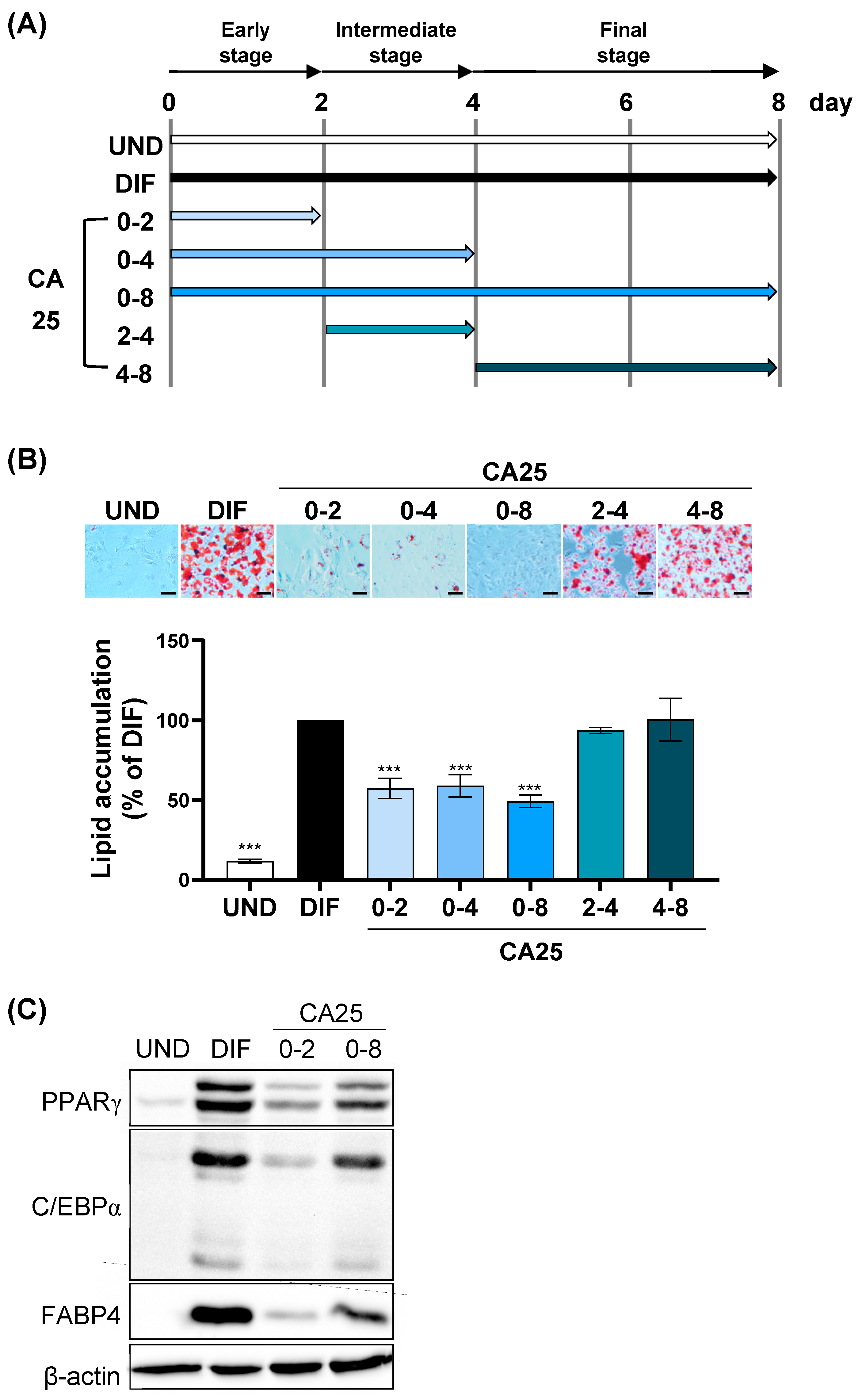
2.2. Regulation of Adipogenesis by CA at the Early Phases
2.3. Inhibition of Cell Cycle Progression during MCE by CA
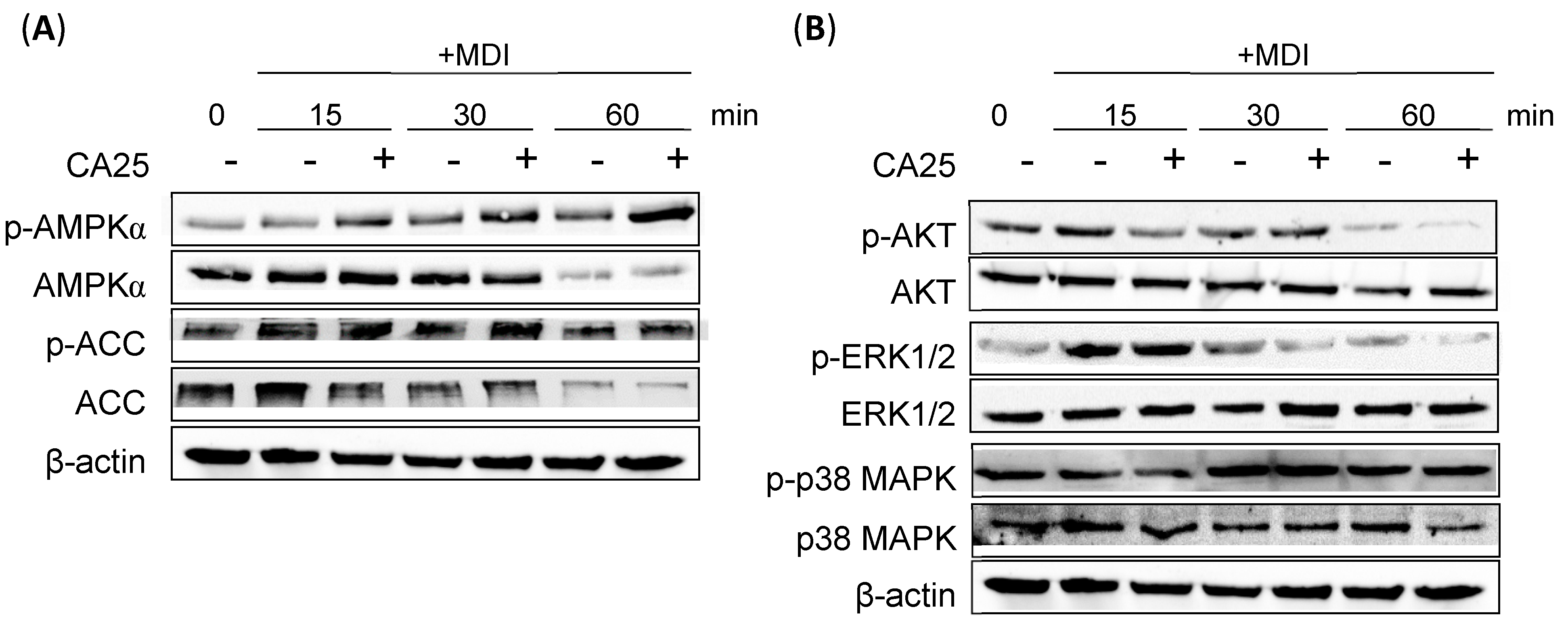
2.4. Effect of CA on the AMPKα and ERK1/2 Signaling Pathways
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture and Differentiation
4.3. Cytotoxicity
4.4. Trypan Blue Staining
4.5. Oil Red O (ORO) Staining
4.6. Western Blot
4.7. Real-Time Polymerase Chain Reaction (RT-PCR)
4.8. Cell Cycle Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lakshmy, K.V.; Mehta, P.G.; Sheth, J.P.; Trivedi, G.K. A new cerium(III)-Catalysed route to cinnamyl alcohols. Org. Prep. Proced. Int. 1985, 17, 251–253. [Google Scholar] [CrossRef]
- Scheman, A.; Scheman, N.; Rakowski, E.M. European Directive Fragrances in Natural Products. Dermatitis 2014, 25, 51–55. [Google Scholar] [CrossRef]
- Api, A.M.; Belsito, D.; Biserta, S.; Botelho, D.; Bruze, M.; Burton, G.A.; Buschmann, J.; Cancellieri, M.A.; Dagli, M.L.; Date, M.; et al. RIFM fragrance ingredient safety assessment, cinnamyl alcohol, CAS Registry Number 104-54-1. Food Chem. Toxicol. 2020, 141, 111337. [Google Scholar] [CrossRef]
- Zhu, G.; Xiao, Z. Flavors and Fragrances: Structure of Various Flavors with Food Ingredients. In Flavors and Fragrances in Food Processing: Preparation and Characterization Methods; ACS Publications: Washington, DC, USA, 2022; pp. 21–188. [Google Scholar]
- Zhang, C.; Xu, Q.; Hou, H.; Wu, J.; Zheng, Z.; Ouyang, J. Efficient biosynthesis of cinnamyl alcohol by engineered Escherichia coli overexpressing carboxylic acid reductase in a biphasic system. Microb. Cell Fact. 2020, 19, 163. [Google Scholar] [CrossRef]
- Nouni, C.; Theodosis-Nobelos, P.; Rekka, E.A. Antioxidant and Hypolipidemic Activities of Cinnamic Acid Derivatives. Molecules 2023, 28, 6732. [Google Scholar] [CrossRef]
- Citarella, A.; Moi, D.; Pedrini, M.; Pérez-Peña, H.; Pieraccini, S.; Dimasi, A.; Stagno, C.; Micale, N.; Schirmeister, T.; Sibille, G. Synthesis of SARS-CoV-2 M pro inhibitors bearing a cinnamic ester warhead with in vitro activity against human coronaviruses. Org. Biomol. Chem. 2023, 21, 3811–3824. [Google Scholar] [CrossRef]
- Zou, L.Y.; Li, C.; Chen, X.L.; Yu, F.; Huang, Q.; Chen, L.J.; Wu, W.W.; Liu, Q. The anti-inflammatory effects of cinnamyl alcohol on sepsis-induced mice via the NLRP3 inflammasome pathway. Ann. Transl. Med. 2022, 10, 48. [Google Scholar] [CrossRef]
- Yue, L.; Sun, D.; Khan, I.M.; Liu, X.L.; Jiang, Q.X.; Xia, W.S. Cinnamyl alcohol modified chitosan oligosaccharide for enhancing antimicrobial activity. Food Chem. 2020, 309, 125513. [Google Scholar] [CrossRef]
- Sarma, S.; Sockalingam, S.; Dash, S. Obesity as a multisystem disease: Trends in obesity rates and obesity-related complications. Diabetes Obes. Metab. 2021, 23, 3–16. [Google Scholar] [CrossRef]
- Vishvanath, L.; Gupta, R.K. Contribution of adipogenesis to healthy adipose tissue expansion in obesity. J. Clin. Investig. 2019, 129, 4022–4031. [Google Scholar] [CrossRef]
- Tang, Q.Q.; Otto, T.C.; Lane, M.D. Mitotic clonal expansion: A synchronous process required for adipogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 44–49. [Google Scholar] [CrossRef]
- Patel, Y.M.; Lane, M.D. Mitotic Clonal Expansion during Preadipocyte Differentiation: Calpain-mediated Turnover of p27. J. Biol. Chem. 2000, 275, 17653–17660. [Google Scholar] [CrossRef]
- Jiang, Y.; Guo, L.; Xie, L.-Q.; Zhang, Y.-Y.; Liu, X.-H.; Zhang, Y.; Zhu, H.; Yang, P.-Y.; Lu, H.-J.; Tang, Q.-Q. Proteome Profiling of Mitotic Clonal Expansion during 3T3-L1 Adipocyte Differentiation Using iTRAQ-2DLC-MS/MS. J. Proteome Res. 2014, 13, 1307–1314. [Google Scholar] [CrossRef]
- Merrett, J.E.; Bo, T.; Psaltis, P.J.; Proud, C.G. Identification of DNA response elements regulating expression of CCAAT/enhancer-binding protein (C/EBP) β and δ and MAP kinase-interacting kinases during early adipogenesis. Adipocyte 2020, 9, 427–442. [Google Scholar] [CrossRef]
- Cole, K.A.; Harmon, A.W.; Harp, J.B.; Patel, Y.M. Rb regulates C/EBPβ-DNA-binding activity during 3T3-L1 adipogenesis. Am. J. Physiol. Cell Physiol. 2004, 286, C349–C354. [Google Scholar] [CrossRef]
- Li, Q.; Hagberg, C.E.; Silva Cascales, H.; Lang, S.; Hyvönen, M.T.; Salehzadeh, F.; Chen, P.; Alexandersson, I.; Terezaki, E.; Harms, M.J.; et al. Obesity and hyperinsulinemia drive adipocytes to activate a cell cycle program and senesce. Nat. Med. 2021, 27, 1941–1953. [Google Scholar] [CrossRef]
- Sadowski, H.B.; Wheeler, T.T.; Young, D.A. Gene expression during 3T3-L1 adipocyte differentiation. Characterization of initial responses to the inducing agents and changes during commitment to differentiation. J. Biol. Chem. 1992, 267, 4722–4731. [Google Scholar] [CrossRef]
- Guo, L.; Li, X.; Tang, Q.Q. Transcriptional Regulation of Adipocyte Differentiation: A Central Role for CCAAT/Enhancer-binding Protein (C/EBP) β. J. Biol. Chem. 2015, 290, 755–761. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, J.; Liu, M.; Zhou, Y.; Zhang, L.; Li, Y. The new role of AMP-activated protein kinase in regulating fat metabolism and energy expenditure in adipose tissue. Biomolecules 2021, 11, 1757. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, L.; Li, B.; Jiang, H.; Duan, Y.; Xie, Z.; Shuai, L.; Li, J.; Li, J. AMP-Activated Protein Kinase (AMPK) Regulates Energy Metabolism through Modulating Thermogenesis in Adipose Tissue. Front. Physiol. 2018, 9, 122. [Google Scholar] [CrossRef]
- Hwang, D.I.; Won, K.-J.; Kim, D.-Y.; Kim, B.; Lee, H.M. Cinnamyl Alcohol, the Bioactive Component of Chestnut Flower Absolute, Inhibits Adipocyte Differentiation in 3T3-L1 Cells by Downregulating Adipogenic Transcription Factors. Am. J. Chin. Med. 2017, 45, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Ghaben, A.L.; Scherer, P.E. Adipogenesis and metabolic health. Nat. Rev. Mol. Cell Biol. 2019, 20, 242–258. [Google Scholar] [CrossRef] [PubMed]
- Arya, A.; Nahar, L.; Khan, H.U.; Sarker, S.D. Chapter Thirteen-Anti-obesity natural products. In Annual Reports in Medicinal Chemistry; Sarker, S.D., Nahar, L., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 55, pp. 411–433. [Google Scholar]
- Tandon, P.; Wafer, R.; Minchin, J.E.N. Adipose morphology and metabolic disease. J. Exp. Biol. 2018, 221, jeb164970. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-E.; Schmidt, H.; Lai, B.; Ge, K. Transcriptional and Epigenomic Regulation of Adipogenesis. Mol. Cell. Biol. 2019, 39, e00601-18. [Google Scholar] [CrossRef] [PubMed]
- Farmer, S.R. Transcriptional control of adipocyte formation. Cell Metab. 2006, 4, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wu, D.; Fan, Z.; Yang, J.; Li, Y.; Meng, Y.; Gao, C.; Zhan, H. FABP4 in obesity-associated carcinogenesis: Novel insights into mechanisms and therapeutic implications. Front. Mol. Biosci. 2022, 9, 973955. [Google Scholar] [CrossRef]
- Kwan, H.Y.; Wu, J.H.; Su, T.; Chao, X.J.; Liu, B.; Fu, X.Q.; Chan, C.L.; Lau, R.H.Y.; Tse, A.K.W.; Han, Q.B.; et al. Cinnamon induces browning in subcutaneous adipocytes. Sci. Rep. 2017, 7, 2447. [Google Scholar] [CrossRef]
- Huang, B.; Yuan, H.D.; Kim, D.Y.; Quan, H.Y.; Chung, S.H. Cinnamaldehyde prevents adipocyte differentiation and adipogenesis via regulation of peroxisome proliferator-activated receptor-gamma (PPARgamma) and AMP-activated protein kinase (AMPK) pathways. J. Agric. Food Chem. 2011, 59, 3666–3673. [Google Scholar] [CrossRef]
- Hoi, J.K.; Lieder, B.; Liebisch, B.; Czech, C.; Hans, J.; Ley, J.P.; Somoza, V. TRPA1 Agonist Cinnamaldehyde Decreases Adipogenesis in 3T3-L1 Cells More Potently than the Non-agonist Structural Analog Cinnamyl Isobutyrate. Acs Omega 2020, 5, 33305–33313. [Google Scholar] [CrossRef]
- Imai, M.; Kumaoka, T.; Hosaka, M.; Sato, Y.; Li, C.; Sudoh, M.; Tamada, Y.; Yokoe, H.; Saito, S.; Tsubuki, M.; et al. Inhibitory effects of hydroxylated cinnamoyl esters on lipid absorption and accumulation. Bioorg. Med. Chem. 2015, 23, 3788–3795. [Google Scholar]
- Ahmad, B.; Serpell, C.J.; Fong, I.L.; Wong, E.H. Molecular Mechanisms of Adipogenesis: The Anti-adipogenic Role of AMP-Activated Protein Kinase. Front. Mol. Biosci. 2020, 7, 76. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.Y.; Chen, C.W.; Chen, L.K.; Chou, H.Y.; Chang, C.L.; Juan, C.C. Curcumin Attenuates Adipogenesis by Inducing Preadipocyte Apoptosis and Inhibiting Adipocyte Differentiation. Nutrients 2019, 11, 2307. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.-M.; Chun, S. Ginsenoside CK inhibits the early stage of adipogenesis via the AMPK, MAPK, and AKT signaling pathways. Antioxidants 2022, 11, 1890. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, M.; Cai, G.H.; Chen, Y.; Shi, X.C.; Zhang, C.C.; Xia, B.; Xie, B.C.; Liu, H.; Zhang, R.X.; et al. A Potential Nutraceutical Candidate Lactucin Inhibits Adipogenesis through Downregulation of JAK2/STAT3 Signaling Pathway-Mediated Mitotic Clonal Expansion. Cells 2020, 9, 331. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-A.; Kang, K.; Lee, H.-J.; Kim, M.; Kim, C.Y.; Nho, C.W. Apigenin isolated from Daphne genkwa Siebold et Zucc. inhibits 3T3-L1 preadipocyte differentiation through a modulation of mitotic clonal expansion. Life Sci. 2014, 101, 64–72. [Google Scholar] [CrossRef]
- Hishida, T.; Naito, K.; Osada, S.; Nishizuka, M.; Imagawa, M. Crucial roles of D-type cyclins in the early stage of adipocyte differentiation. Biochem. Biophys. Res. Commun. 2008, 370, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Audano, M.; Pedretti, S.; Caruso, D.; Crestani, M.; De Fabiani, E.; Mitro, N. Regulatory mechanisms of the early phase of white adipocyte differentiation: An overview. Cell. Mol. Life Sci. 2022, 79, 139. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Alonso, D.; Malumbres, M. Mammalian cell cycle cyclins. In Seminars in Cell & Developmental Biology; Academic Press: Cambridge, MA, USA, 2020; pp. 28–35. [Google Scholar]
- Daval, M.; Foufelle, F.; Ferré, P. Functions of AMP-activated protein kinase in adipose tissue. J. Physiol. 2006, 574, 55–62. [Google Scholar] [CrossRef]
- Shen, Y.; Honma, N.; Kobayashi, K.; Jia, L.N.; Hosono, T.; Shindo, K.; Ariga, T.; Seki, T. Cinnamon extract enhances glucose uptake in 3T3-L1 adipocytes and C2C12 myocytes by inducing LKB1-AMP-activated protein kinase signaling. PLoS ONE 2014, 9, e87894. [Google Scholar] [CrossRef]
- Prusty, D.; Park, B.H.; Davis, K.E.; Farmer, S.R. Activation of MEK/ERK signaling promotes adipogenesis by enhancing peroxisome proliferator-activated receptor γ (PPARγ) and C/EBPα gene expression during the differentiation of 3T3-L1 preadipocytes. J. Biol. Chem. 2002, 277, 46226–46232. [Google Scholar] [CrossRef]
- Murugan, D.D.; Balan, D.; Wong, P.F. Adipogenesis and therapeutic potentials of antiobesogenic phytochemicals: Insights from preclinical studies. Phytother. Res. 2021, 35, 5936–5960. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.-M.; Lee, Y.-S.; Sin, D.-M.; Lee, S.; Lee, M.K.; Lee, Y.-M.; Hong, J.-T.; Yun, Y.-P.; Yoo, H.-S. Sulforaphane Inhibits Mitotic Clonal Expansion During Adipogenesis Through Cell Cycle Arrest. Obesity 2012, 20, 1365–1371. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Nagasawa, Y.; Fujimori, K. Glycyrrhizic acid suppresses early stage of adipogenesis through repression of MEK/ERK-mediated C/EBPβ and C/EBPδ expression in 3T3-L1 cells. Chem. Biol. Interact. 2021, 346, 109595. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.R.; Kim, Y.-S.; Kim, M.J. Cinnamyl Alcohol Attenuates Adipogenesis in 3T3-L1 Cells by Arresting the Cell Cycle. Int. J. Mol. Sci. 2024, 25, 693. https://doi.org/10.3390/ijms25020693
Choi YR, Kim Y-S, Kim MJ. Cinnamyl Alcohol Attenuates Adipogenesis in 3T3-L1 Cells by Arresting the Cell Cycle. International Journal of Molecular Sciences. 2024; 25(2):693. https://doi.org/10.3390/ijms25020693
Chicago/Turabian StyleChoi, Yae Rim, Young-Suk Kim, and Min Jung Kim. 2024. "Cinnamyl Alcohol Attenuates Adipogenesis in 3T3-L1 Cells by Arresting the Cell Cycle" International Journal of Molecular Sciences 25, no. 2: 693. https://doi.org/10.3390/ijms25020693





