Evaluating the State of Glomerular Disease by Analyzing Urinary Sediments: mRNA Levels and Immunofluorescence Staining for Various Markers
Abstract
1. Introduction
2. Results
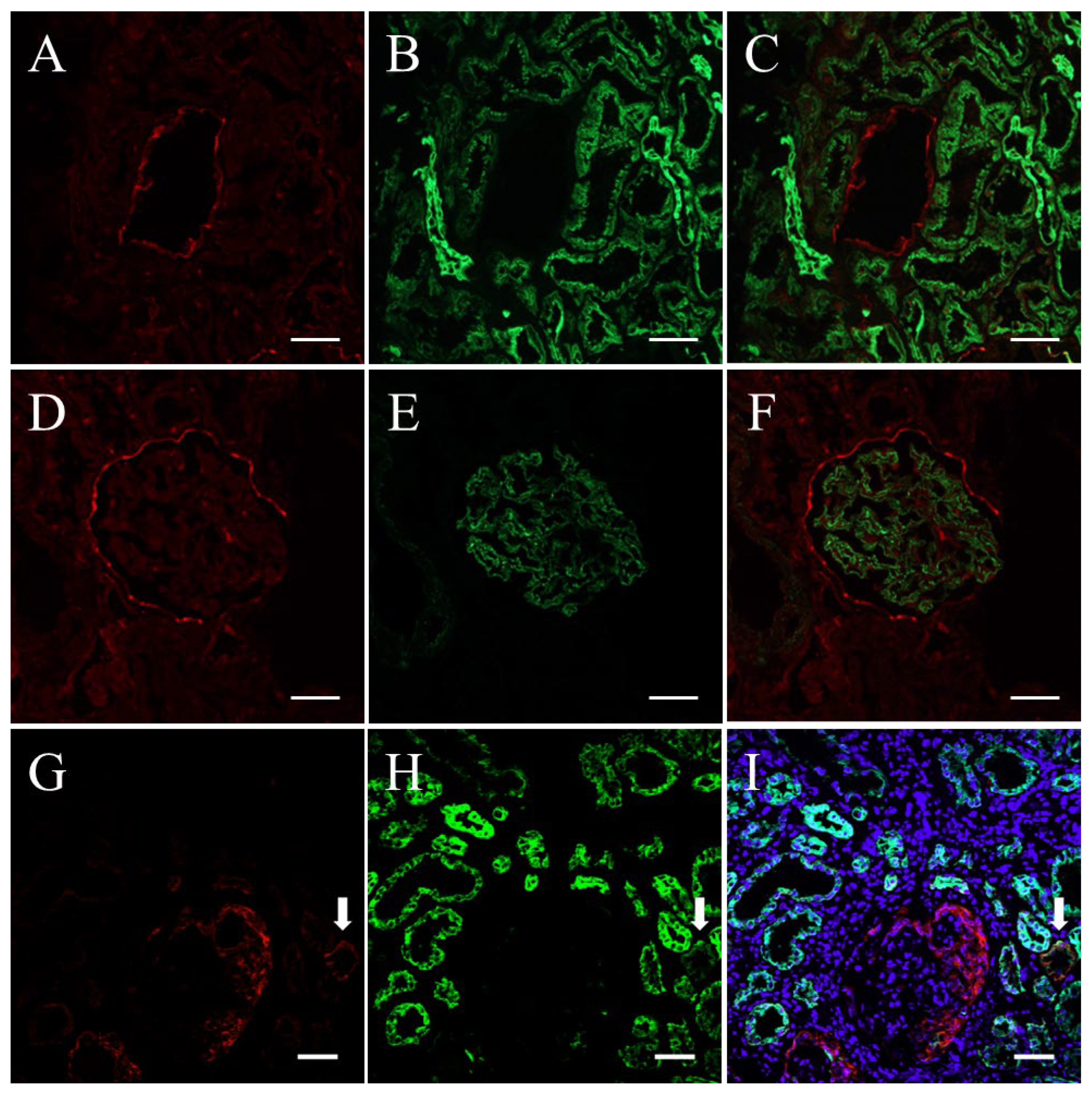
2.1. Verification of Cytokeratin, Synaptopodin, and Claudin1 as Markers of Renal Parenchymal Cells
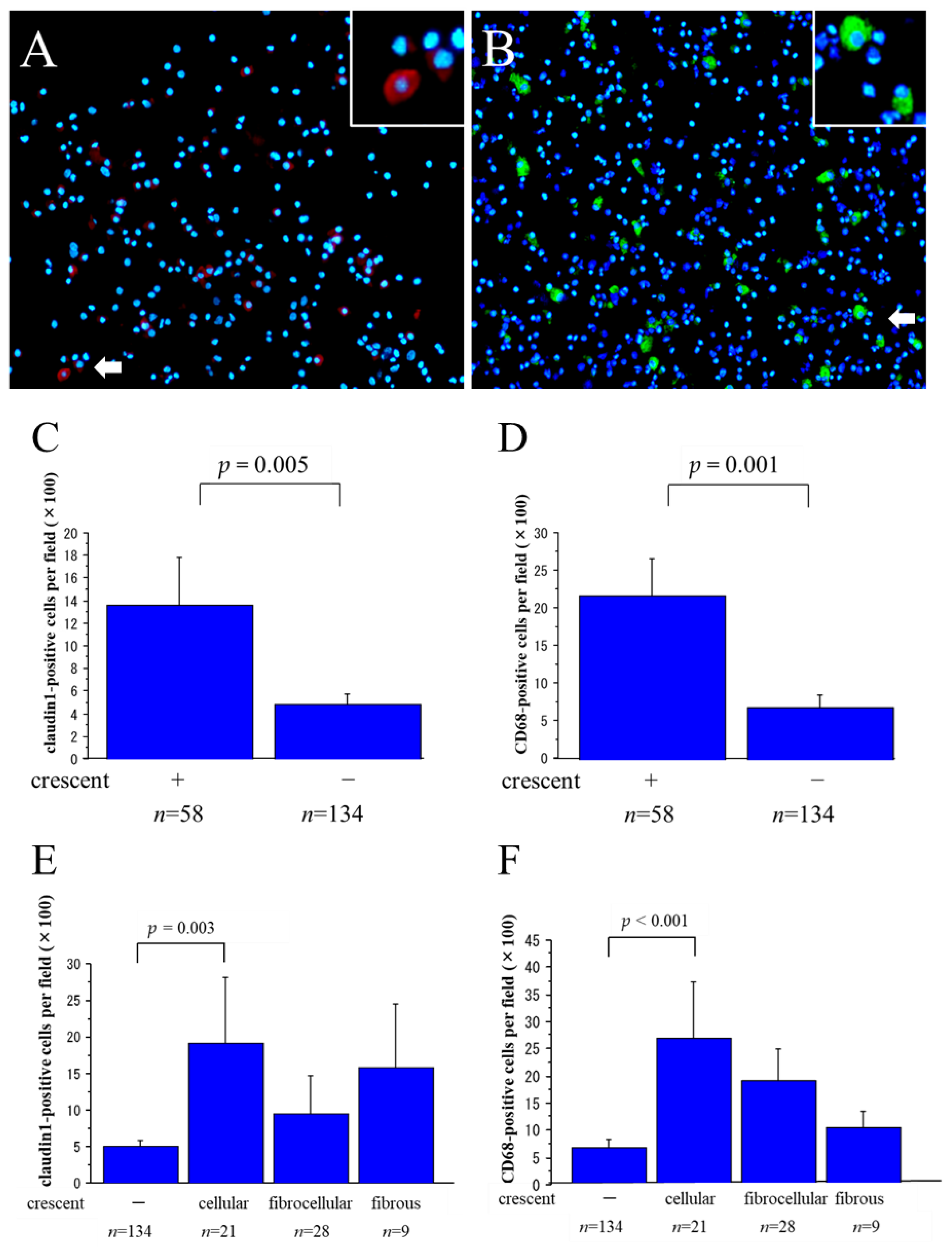
2.2. Claudin1-Positive and CD68-Positive Cells in Urinary Sediments
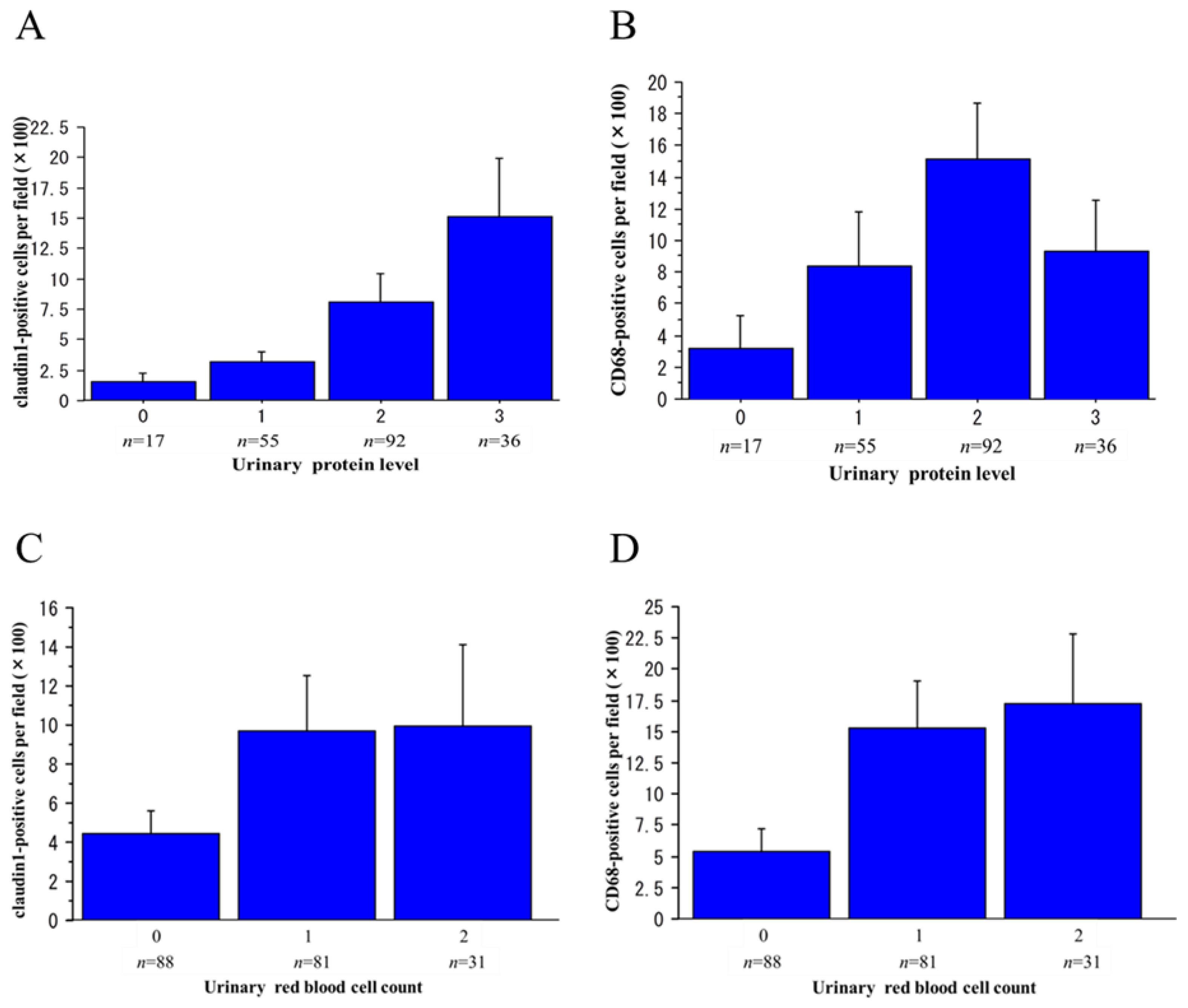
2.3. Claudin1 and CD68 mRNA Levels in Urinary Sediment
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Collection and Treatment of Urine Specimens
4.3. IF Staining
4.4. RNA Extraction and Real-Time RT-PCR
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, Y.; Chen, S.; Wang, F.; Zhang, P.; Liu, M.; Liu, C.; Zhong, X.; Qin, J.; Li, G.; Wang, W. The Significance of Crescents on the Clinical Features and Outcomes of Primary Immunoglobin A Nephropathy. Front. Med. 2022, 9, 864667. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhou, Q.; Hong, L.; Chen, W.; Yang, S.; Yang, Q.; Chen, W.; Yu, X. Clinical outcomes of IgA nephropathy patients with different proportions of crescents. Medicine 2017, 96, e6190. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Liu, L.; Chen, W.; Yang, H.; Zhang, J.; Wang, Y. Clinicopathological features and prognosis of primary membranous nephropathy in combination with crescent. Int. Urol. Nephrol. 2023, 55, 1523–1530. [Google Scholar] [CrossRef] [PubMed]
- Hotta, O.; Taguma, Y.; Sudo, K.; Kurosawa, K. Limitation of kidney biopsy in detecting crescentic lesions in IgA nephropathy. Nephron 1993, 65, 472–473. [Google Scholar] [CrossRef] [PubMed]
- Perazella, M.A. The urine sediment as a biomarker of kidney disease. Am. J. Kidney Dis. 2015, 66, 748–755. [Google Scholar] [CrossRef]
- Yu, D.; Petermann, A.; Kunter, U.; Rong, S.; Shankland, S.J.; Floege, J. Urinary podocyte loss is a more specific marker of ongoing glomerular damage than proteinuria. J. Am. Soc. Nephrol. 2005, 16, 1733–1741. [Google Scholar] [CrossRef]
- Hara, M.; Yanagihara, T.; Kihara, I. Cumulative excretion of urinary podocytes reflects disease progression in IgA nephropathy and Schonlein-Henoch purpura nephritis. Clin. J. Am. Soc. Nephrol. 2007, 2, 231–238. [Google Scholar] [CrossRef]
- Wang, G.; Lai, F.M.; Lai, K.B.; Chow, K.M.; Li, K.T.; Szeto, C.C. Messenger RNA expression of podocyte-associated molecules in the urinary sediment of patients with diabetic nephropathy. Nephron Clin. Pract. 2007, 106, c169–c179. [Google Scholar] [CrossRef]
- Sato, Y.; Wharram, B.L.; Lee, S.K.; Wickman, L.; Goyal, M.; Venkatareddy, M.; Chang, J.W.; Wiggins, J.E.; Lienczewski, C.; Kretzler, M.; et al. Urine podocyte mRNAs mark progression of renal disease. J. Am. Soc. Nephrol. 2009, 20, 1041–1052. [Google Scholar] [CrossRef]
- Wickman, L.; Afshinnia, F.; Wang, S.Q.; Yang, Y.; Wang, F.; Chowdhury, M.; Graham, D.; Hawkins, J.; Nishizono, R.; Tanzer, M.; et al. Urine podocyte mRNAs, proteinuria, and progression in human glomerular diseases. J. Am. Soc. Nephrol. 2013, 24, 2081–2095. [Google Scholar] [CrossRef]
- Achenbach, J.; Mengel, M.; Tossidou, I.; Peters, I.; Park, J.K.; Haubitz, M.; Ehrich, J.H.; Haller, H.; Schiffer, M. Parietal epithelia cells in the urine as a marker of disease activity in glomerular diseases. Nephrol. Dial. Transplant. 2008, 23, 3138–3145. [Google Scholar] [CrossRef] [PubMed]
- Hotta, O.; Yusa, N.; Kitamura, H.; Taguma, Y. Urinary macrophages as activity markers of renal injury. Clin. Chim. Acta 2000, 297, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Suthanthiran, M.; Schwartz, J.E.; Ding, R.; Abecassis, M.; Dadhania, D.; Samstein, B.; Knechtle, S.J.; Friedewald, J.; Becker, Y.T.; Sharma, V.K.; et al. Urinary-Cell mRNA Profile and Acute Cellular Rejection in Kidney Allografts. N. Engl. J. Med. 2013, 369, 20–31. [Google Scholar] [CrossRef]
- Kiuchi-Saishin, Y.; Gotoh, S.; Furuse, M.; Takasuga, A.; Tano, Y.; Tsukita, S. Differential expression patterns of claudins, tight junction membrane proteins, in mouse nephron segments. J. Am. Soc. Nephrol. 2002, 13, 875–886. [Google Scholar] [CrossRef] [PubMed]
- Furuse, M.; Fujita, K.; Hiiragi, T.; Fujimoto, K.; Tsukita, S. Claudin-1 and -2: Novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J. Cell Biol. 1998, 141, 1539–1550. [Google Scholar] [CrossRef] [PubMed]
- Ohse, T.; Chang, A.M.; Pippin, J.W.; Jarad, G.; Hudkins, K.L.; Alpers, C.E.; Miner, J.H.; Shankland, S.J. A new function for parietal epithelial cells: A second glomerular barrier. Am. J. Physiol. Renal Physiol. 2009, 297, F1566–F1574. [Google Scholar] [CrossRef] [PubMed]
- Smeets, B.; Uhlig, S.; Fuss, A.; Mooren, F.; Wetzels, J.F.; Floege, J.; Moeller, M.J. Tracing the origin of glomerular extracapillary lesions from parietal epithelial cells. J. Am. Soc. Nephrol. 2009, 20, 2604–2615. [Google Scholar] [CrossRef]
- Sicking, E.M.; Fuss, A.; Uhlig, S.; Jirak, P.; Dijkman, H.; Wetzels, J.; Engel, D.R.; Urzynicok, T.; Heidenreich, S.; Kriz, W.; et al. Subtotal ablation of parietal epithelial cells induces crescent formation. J. Am. Soc. Nephrol. 2012, 23, 629–640. [Google Scholar] [CrossRef]
- Morimoto, M.; Namba-Hamano, T.; Notsu, S.; Iwata, Y.; Yasuhara, Y.; Yamato, M.; Isaka, Y. Diabetic nephropathy with marked extra-capillary cell proliferation: A case report. BMC Nephrol. 2023, 24, 139. [Google Scholar] [CrossRef]
- Kirk, A.; Campbell, S.; Bass, P.; Mason, J.; Collins, J. Differential expression of claudin tight junction proteins in the human cortical nephron. Nephrol. Dial. Transplant. 2010, 25, 2107–2119. [Google Scholar] [CrossRef]
- Hasegawa, K.; Wakino, S.; Simic, P.; Sakamaki, Y.; Minakuchi, H.; Fujimura, K.; Hosoya, K.; Komatsu, M.; Kaneko, Y.; Kanda, T.; et al. Renal tubular Sirt1 attenuates diabetic albuminuria by epigenetically suppressing Claudin-1 overexpression in podocytes. Nat. Med. 2013, 19, 1496–1504. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Sunq, A.; Roth, R.A.; Hou, J. Inducible Expression of Claudin-1 in Glomerular Podocytes Generates Aberrant Tight Junctions and Proteinuria through Slit Diaphragm Destabilization. J. Am. Soc. Nephrol. 2017, 28, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Sevillano, A.M.; Gutiérrez, E.; Yuste, C.; Cavero, T.; Mérida, E.; Rodríguez, P.; García, A.; Morales, E.; Fernández, C.; Martínez, M.A.; et al. Remission of Hematuria Improves Renal Survival in IgA Nephropathy. J. Am. Soc. Nephrol. 2017, 28, 3089–3099. [Google Scholar] [CrossRef] [PubMed]
- Rhee, R.L.; Davis, J.C.; Ding, L.; Fervenza, F.C.; Hoffman, G.S.; Kallenberg, C.G.M.; Langford, C.A.; McCune, W.J.; Monach, P.A.; Seo, P.; et al. The Utility of Urinalysis in Determining the Risk of Renal Relapse in ANCA-Associated Vasculitis. Clin. J. Am. Soc. Nephrol. 2018, 13, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, P.F.; Fujii, N.; Roy, J.; Chen, H.Y.; Lee Hamm, L.; Sondheimer, J.H.; He, J.; Fischer, M.J.; Rincon-Choles, H.; Krishnan, G.; et al. Hematuria as a risk factor for progression of chronic kidney disease and death: Findings from the Chronic Renal Insufficiency Cohort (CRIC) Study. BMC Nephrol. 2018, 19, 150. [Google Scholar] [CrossRef] [PubMed]
- Oda, T.; Hotta, O.; Taguma, Y.; Kitamura, H.; Sugai, H.; Onodera, S.; Horigome, I.; Suzuki, K.; Shouji, Y.; Furuta, T.; et al. Clinicopathological significance of intratubular giant macrophages in progressive glomerulonephritis. Kidney Int. 1998, 53, 1190–1200. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Huen, S.; Nishio, H.; Nishio, S.; Lee, H.K.; Choi, B.S.; Ruhrberg, C.; Cantley, L.G. Distinct macrophage phenotypes contribute to kidney injury and repair. J. Am. Soc. Nephrol. 2011, 22, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Anders, H.J.; Ryu, M. Renal microenvironments and macrophage phenotypes determine progression or resolution of renal inflammation and fibrosis. Kidney Int. 2011, 80, 915–925. [Google Scholar] [CrossRef]
- Kushiyama, T.; Oda, T.; Yamada, M.; Higashi, K.; Yamamoto, K.; Oshima, N.; Sakurai, Y.; Miura, S.; Kumagai, H. Effects of liposome-encapsulated clodronate on chlorhexidine gluconate-induced peritoneal fibrosis in rats. Nephrol. Dial. Transplant. 2011, 26, 3143–3154. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]






| Sex (male/female) | 100/92 |
| Age (years) | 44 ± 18 |
| Serum creatinine (mg/dL) | 1.13 ± 1.25 |
| Urinary red blood cell (URBC) count (0/1/2) | 88/81/31 |
| Urinary protein (g/gCr) | 2.14 ± 3.32 |
| Serum albumin (g/dL) | 3.43 ± 0.99 |
| Number | |
|---|---|
| Minimal change nephrotic syndrome (MCNS) | 15 (1) |
| Focal segmental glomerulosclerosis (FSGS) | 22 (1) |
| Membranous nephropathy (MN) | 19 (3) |
| Membranoproliferative glomerulonephritis (MPGN) | 1 |
| IgA nephropathy (IgAN) | 73 (32) |
| Mesangial proliferative non-IgA nephropathy (non-IgAN) | 5 |
| Acute poststreptococcal glomerulonephritis (APSGN) | 4 (1) |
| Henoch–Schönlein purpura nephritis (HSPN) | 10 (6) |
| ANCA-associated vasculitis (AAV) | 11 (11) |
| Lupus nephritis (LN) | 9 (2) |
| Diabetic nephropathy (DMN) | 8 |
| Amyloidosis | 5 (1) |
| Thin basement membrane disease (TBMD) | 3 |
| Fabry disease | 1 |
| Minor glomerular abnormality (MGA) | 6 |
| Total | 192 (58) |
| Antibody Type | Clone | Specificity | Supplier | Dilution |
|---|---|---|---|---|
| rabbit anti-claudin1 | - | parietal epithelial cells | Bioworld Technology, Minneapolis, MN, USA | 1:100 |
| mouse anti-CD68 | EBM11 | macrophages | Dako, Glostrup, Denmark | 1:100 |
| rabbit anti- neutrophil elastase | - | neutrophils | Calbiochem, La Jolla, CA, USA | 1:500 |
| mouse anti-synaptopodin | G1D4 | podocytes | Progen, Heidelberg, Germany | 1:1 |
| mouse anti-cytokeratin | KL-1 | tubular epithelial cells | IMMUNOTECH, Monrovia, CA, USA | 1:100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamamoto, K.; Oda, T.; Uchida, T.; Takechi, H.; Oshima, N.; Kumagai, H. Evaluating the State of Glomerular Disease by Analyzing Urinary Sediments: mRNA Levels and Immunofluorescence Staining for Various Markers. Int. J. Mol. Sci. 2024, 25, 744. https://doi.org/10.3390/ijms25020744
Yamamoto K, Oda T, Uchida T, Takechi H, Oshima N, Kumagai H. Evaluating the State of Glomerular Disease by Analyzing Urinary Sediments: mRNA Levels and Immunofluorescence Staining for Various Markers. International Journal of Molecular Sciences. 2024; 25(2):744. https://doi.org/10.3390/ijms25020744
Chicago/Turabian StyleYamamoto, Kojiro, Takashi Oda, Takahiro Uchida, Hanako Takechi, Naoki Oshima, and Hiroo Kumagai. 2024. "Evaluating the State of Glomerular Disease by Analyzing Urinary Sediments: mRNA Levels and Immunofluorescence Staining for Various Markers" International Journal of Molecular Sciences 25, no. 2: 744. https://doi.org/10.3390/ijms25020744
APA StyleYamamoto, K., Oda, T., Uchida, T., Takechi, H., Oshima, N., & Kumagai, H. (2024). Evaluating the State of Glomerular Disease by Analyzing Urinary Sediments: mRNA Levels and Immunofluorescence Staining for Various Markers. International Journal of Molecular Sciences, 25(2), 744. https://doi.org/10.3390/ijms25020744




