Furanocoumarins as Enhancers of Antitumor Potential of Sorafenib and LY294002 toward Human Glioma Cells In Vitro
Abstract
1. Introduction
2. Results
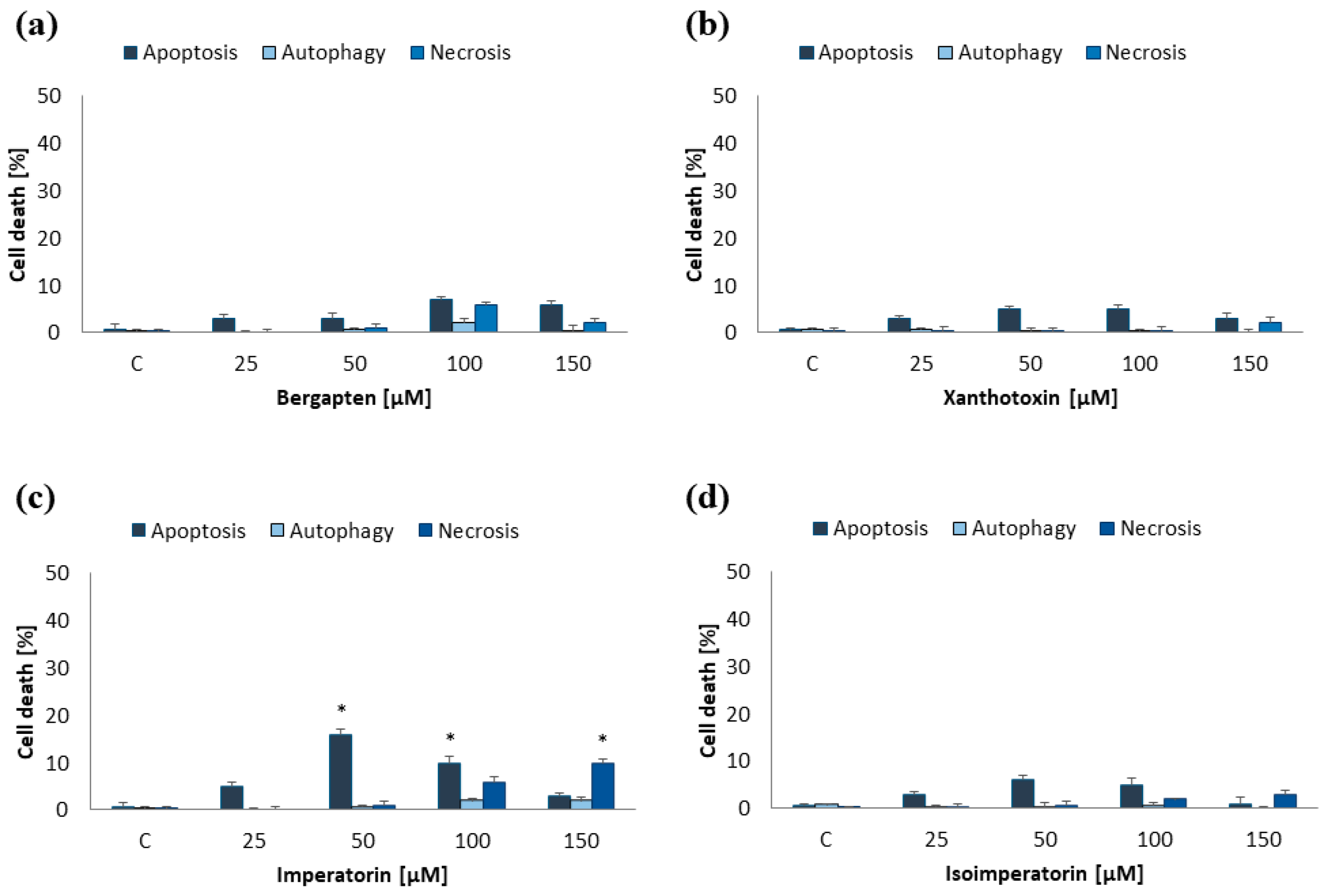
2.1. Induction of Programmed Cell Death by Furanocoumarins: Bergapten, Xanthotoxin, Imperatorin, and Isoimperatorin
2.2. Effect of Furanocoumarins (Bergapten, Xanthotoxin, Imperatorin and Isoimperatorin) in Combination with Sorafenib and LY294002 on Programmed Cell Death Induction
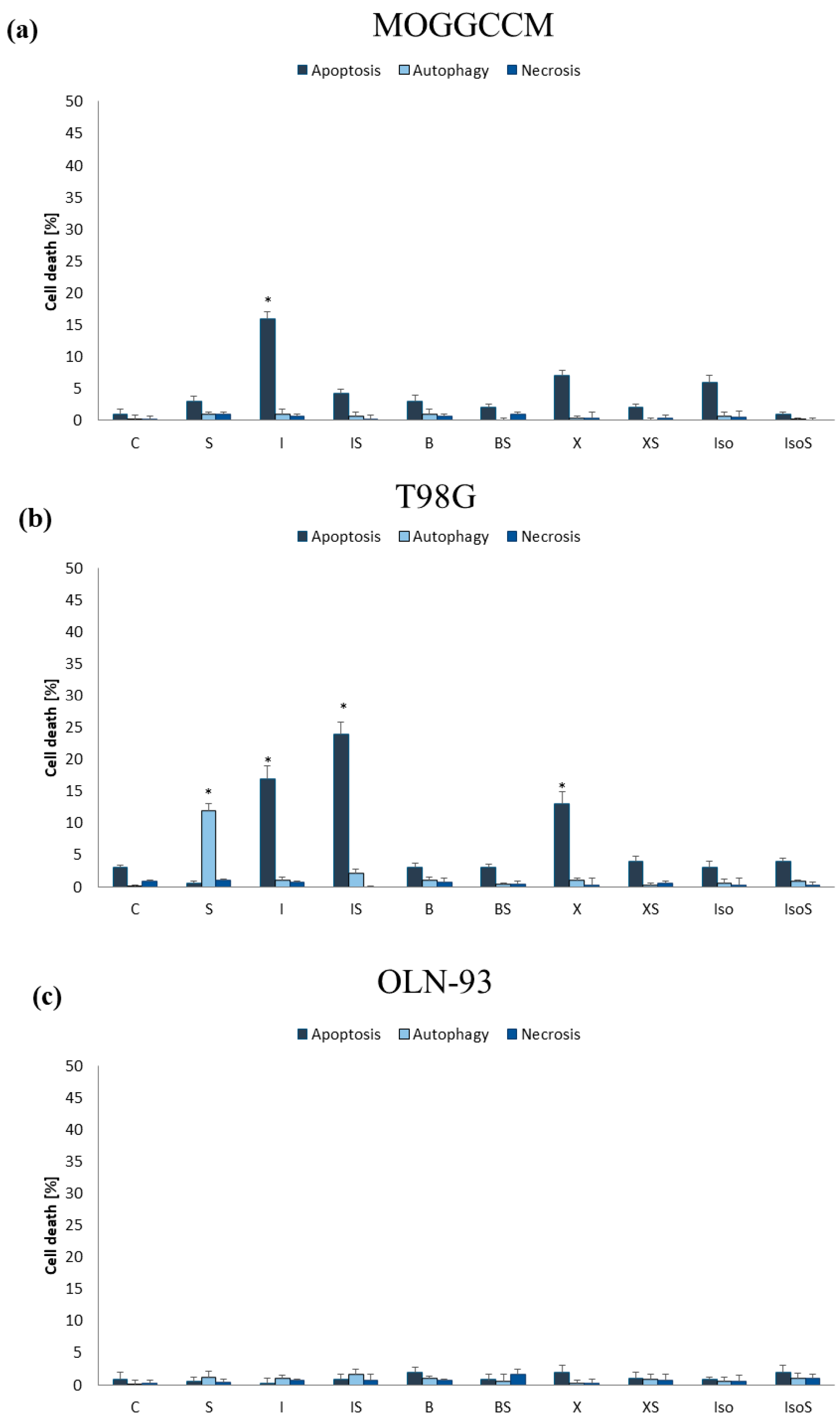
2.2.1. Furanocoumarins in Combination with Sorafenib
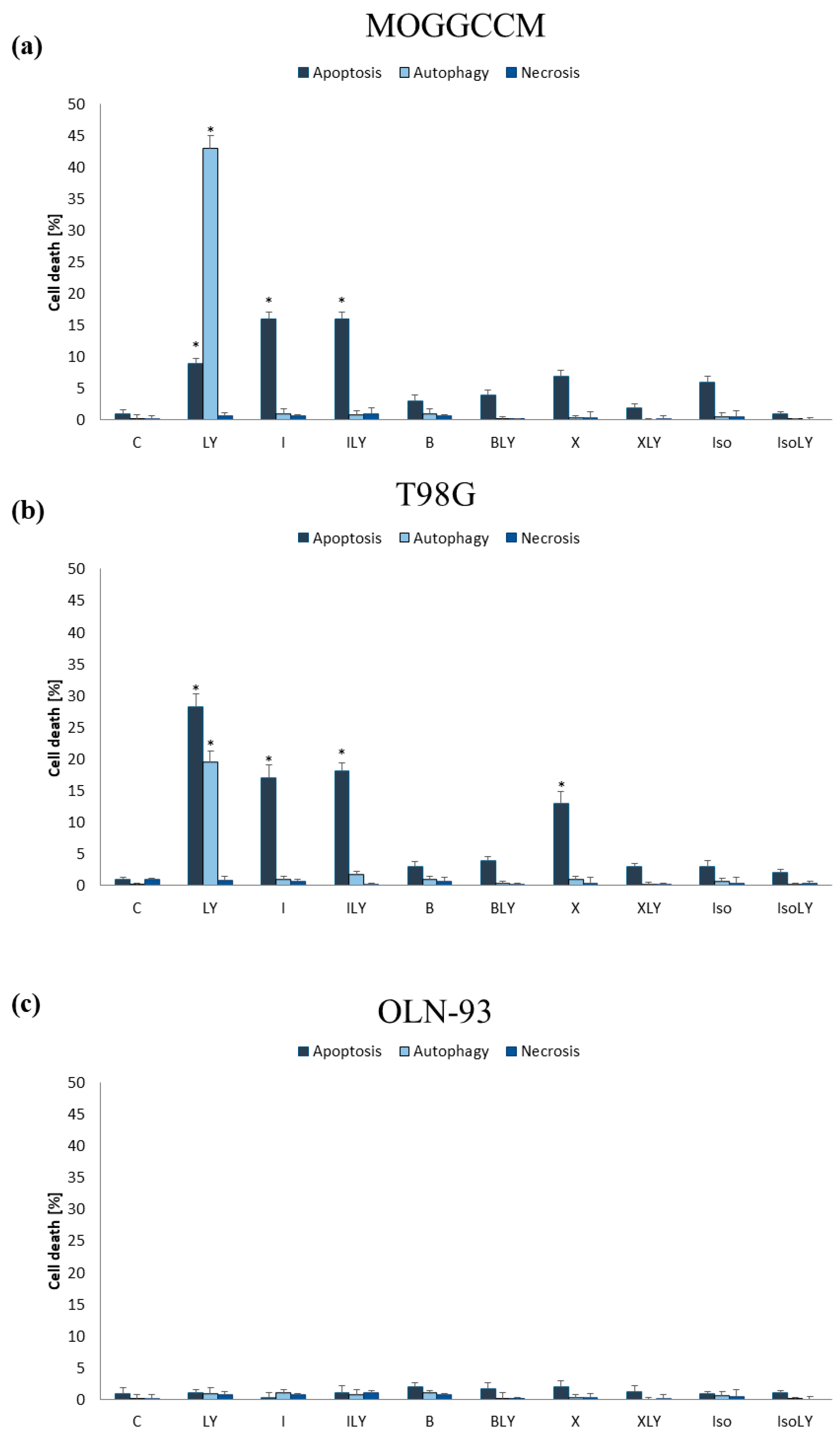
2.2.2. Furanocoumarins in Combination with LY294002
2.3. Estimation of the Level of Beclin 1 and Procaspase 3 and Activity of Caspase 3
2.4. Apoptosis, Autophagy, and Necrosis Induction upon Inhibition of RAF and PI3K Expression
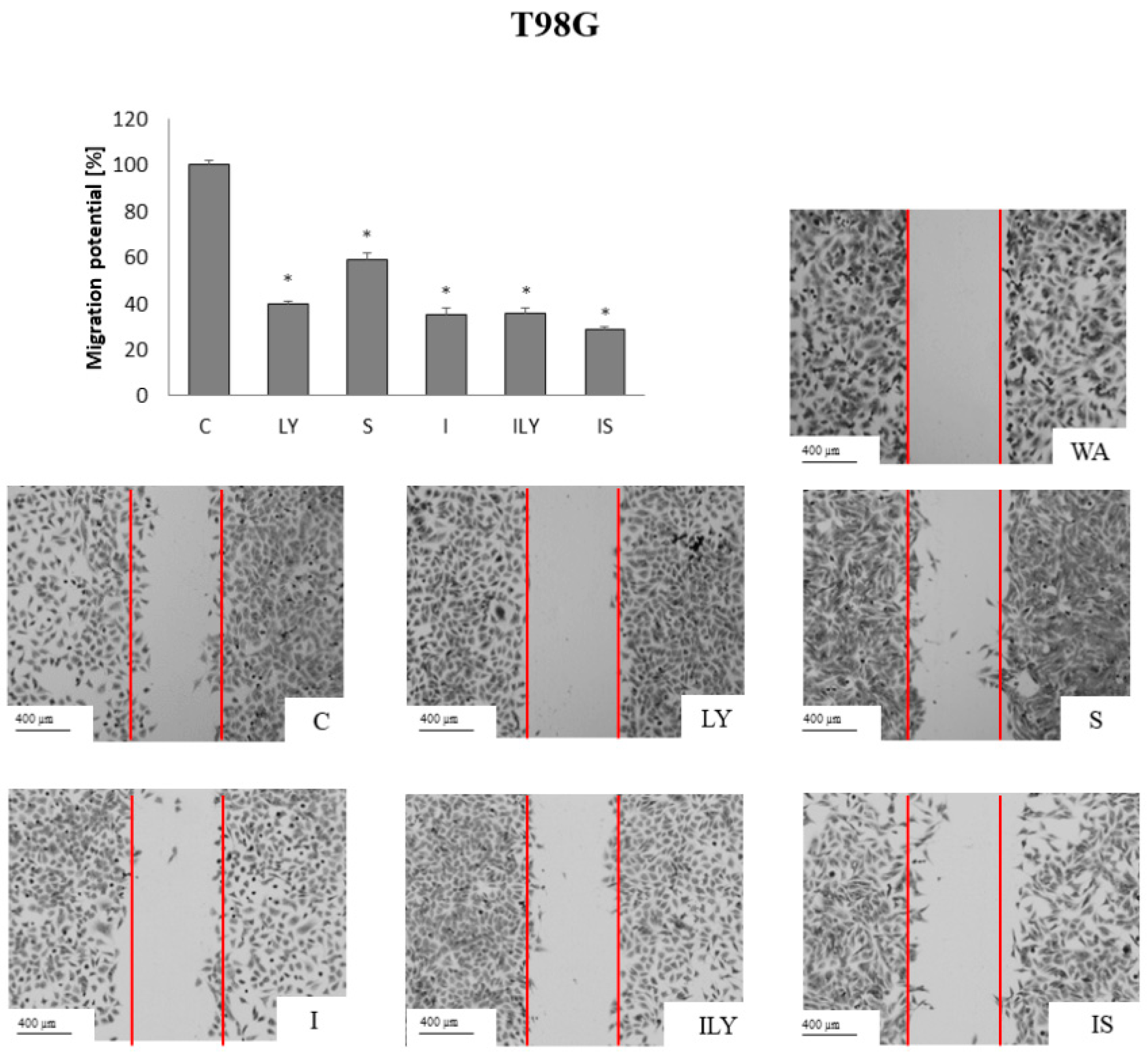
2.5. Effect of Imperatorin, Alone and in Combination with Sorafenib or LY294002 on the Migration Potential
3. Discussion
4. Materials and Methods
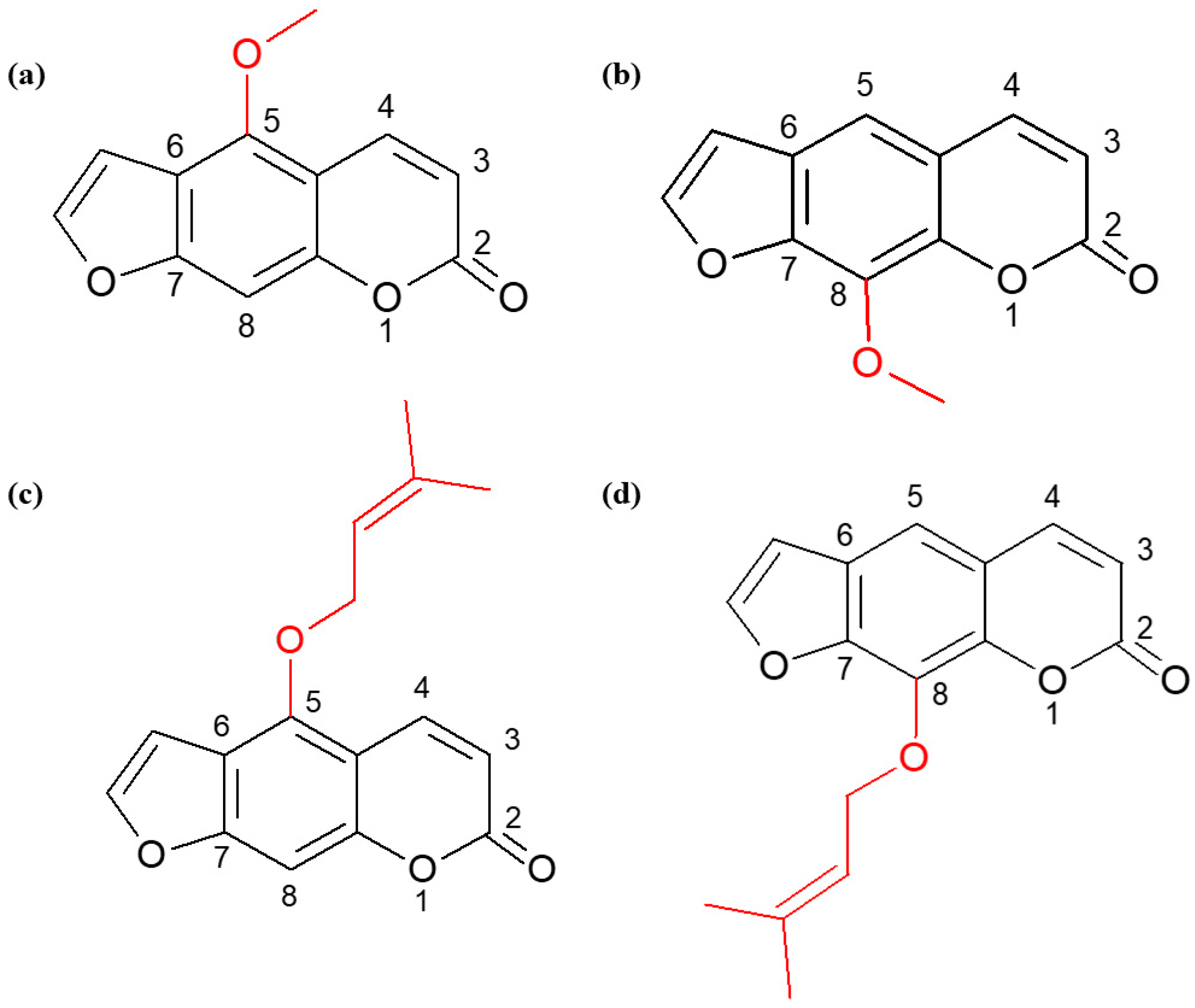
4.1. Isolation of Furanocoumarins
4.2. Cells and Culture Conditions
4.3. Drug Treatment
4.4. Apoptosis, Autophagy, and Necrosis Identification and Level Evaluation
4.5. Immunoblotting
4.6. Caspase 3 Activity Assay
4.7. MOGGCCM and T98G Transfection with siRNA
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brito, C.; Azevedo, A.; Esteves, S.; Marques, A.R.; Martins, C.; Costa, I.; Mafra, M.; Bravo Marques, J.M.; Roque, L.; Pojo, M. Clinical insights gained by refining the 2016 WHO classification of diffuse gliomas with: EGFR amplification, TERT mutations, PTEN deletion and MGMT methylation. BMC Cancer 2019, 19, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Szala, S.; Jarosz, M.; Smolarczyk, R.; Cichoń, T. Błędne koła glejaków: Unaczynienie i inwazyjność. Post. Hig. 2012, 66, 888–900. [Google Scholar] [CrossRef]
- Nakada, M.; Kita, D.; Watanabe, T.; Hayashi, Y.; Teng, L.; Pyko, I.V.; Hamada, J. Aberrant signaling pathways in glioma. Cancers 2011, 3, 3242–3278. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, F.; Pinna, C.; Dallavalle, S.; Tamborini, L.; Pinto, A. An overview of coumarin as a versatile and readily accessible scaffold with broad-ranging biological activities. Int. J. Mol. Sci. 2020, 21, 4618. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Sun, S.; Wu, B.; Liu, J. Coumarins as Potential Anti-drug Resistant Cancer Agents: A Mini Review. Top. Med. Chem. 2021, 21, 1725–1736. [Google Scholar] [CrossRef] [PubMed]
- Bielawska, K.; Malinowska, M.; Cyuńczyk, M. Wpływ kumaryn na organizm człowieka. Bromat. Chem. Toksykol. 2014, 47, 213–221. [Google Scholar]
- Ahmed, S.; Khan, H.; Aschner, M.; Mirzae, H.; Küpeli Akkol, E.; Capasso, R. Anticancer Potential of Furanocoumarins: Mechanistic and Therapeutic Aspects. Int. J. Mol. Sci. 2020, 21, 5622. [Google Scholar] [CrossRef]
- Widelski, J.; Kukula-Koch, W.; Baj, T.; Kedzierski, B.; Fokialakis, N.; Magiatis, P.; Pozarowski, P.; Rolinski, J.; Graikou, K.; Chinou, I.; et al. Rare Coumarins Induce Apoptosis, G1 Cell Block and Reduce RNA Content in HL60 Cells. Open Chem. 2017, 15, 1–6. [Google Scholar] [CrossRef]
- Fang, D.; Zhu, L.; Yang, Z.; Hua, X. Isolation and identification of bergapten in dry root of Glehnia littoralis and preliminary determination of its antitumor activity in vitro. J. Plant Resour. Environ. 2010, 19, 95–96. [Google Scholar]
- Hung, W.L.; Suh, J.H.; Wang, Y. Chemistry and health effects of furanocoumarins in grapefruit. J. Food Drug Anal. 2017, 25, 71–83. [Google Scholar] [CrossRef]
- Panno, M.; Giordano, F. Effects of psoralens as anti-tumoral agents in breast cancer cells. World J. Clin. Oncol. 2014, 5, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Xie, L.; Liu, K.; Cao, Y.; Dai, X.; Wang, X.; Lu, J.; Zhang, X.; Li, X. Bergapten: A review of its pharmacology, pharmacokinetics, and toxicity. Phytother. Res. 2021, 35, 6131–6147. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xiong, J.; Luo, W.; Yang, J.; Xi, T. 8-Methoxypsoralen Induces Intrinsic Apoptosis in HepG2 Cells: Involvement of Reactive Oxygen Species Generation and ERK1/2 Pathway Inhibition. Cell Physiol. Biochem. 2015, 37, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Lu, J.; Zhong, G.; Lu, L.; Qu, Y.; Zhang, C. Xanthotoxin (8-methoxypsoralen): A review of its chemistry, pharmacology, pharmacokinetics, and toxicity. Phytother. Res. 2022, 36, 3805–3832. [Google Scholar] [CrossRef] [PubMed]
- Am, J.U.; Gong, W.J.; Su, Y.; Mou, Z.B. Imperatorin shows selective antitumor effects in SGC-7901 human gastric adenocarcinoma cells by inducing apoptosis, cell cycle arrest and targeting PI3K/Akt/m-TOR signalling pathway. J. BUON 2017, 22, 1471–1476. [Google Scholar] [PubMed]
- Hu, J.; Xu, C.; Cheng, B.; Jin, L.; Li, J.; Gong, Y.; Lin, W.; Pan, Z.; Pan, C. Imperatorin acts as a cisplatin sensitizer via downregulating Mcl-1 expression in HCC chemotherapy. Tumour Biol. 2016, 37, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.W.; Sun, J.G.; Chan, J.Y.; Yang, L.; Wu, S.H.; Fung, K.P.; Liu, F.Y. Anticancer effects of imperatorin isolated from Angelica dahurica: Induction of apoptosis in HepG2 cells through both death-receptor- and mitochondria-mediated pathways. Chemotherapy 2011, 57, 449–459. [Google Scholar] [CrossRef]
- Kang, J.; Lee, S.; Yim, D. Effect of Isoimperatorin on the Proliferation of Prostate Cancer Cell Line DU145 Cells. Biomol. Ther. 2005, 13, 185–189. [Google Scholar]
- Haas, B.; Klinger, V.; Keksel, C.; Bonigut, V.; Kiefer, D.; Caspers, J.; Walther, J.; Wos-Maganga, M.; Weickhardt, S.; Röhn, G.; et al. Inhibition of the PI3K but not the MEK/ERK pathway sensitizes human glioma cells to alkylating drugs. Cancer Cell Int. 2018, 18, 69. [Google Scholar] [CrossRef]
- Hassler, M.R.; Ackerl, M.; Flechl, B.; Sax, C.; Wöhrer, A.; Widhalm, G.; Dieckmann, K.; Hainfellner, J.; Preusser, M.; Marosi, C. Sorafenib for patients with pretreated recurrent or progressive high-grade glioma: A retrospective, single-institution study. Anticancer. Drugs. 2014, 25, 723–728. [Google Scholar] [CrossRef]
- Armento, A.; Ehlers, J.; Schotterl, S.; Naumann, U. Molecular mechanisms of glioma cell motility. W: De Vleeschouwer S. (red.), Glioblastoma. Codon Publ. 2017, 53, 89–96. [Google Scholar] [CrossRef]
- Langhans, J.; Schneele, L.; Trenkler, N.; von Bandemer, H.; Nonnenmacher, L.; Karpel-Massler, G.; Siegelin, M.D.; Zhou, S.; Halatsch, M.E.; Debatin, K.M.; et al. The effects of PI3K-mediated signalling on glioblastoma cell behaviour. Oncogenesis 2017, 6, 398. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.L.; Li, M.; Zhang, K.P. Imperatorin inhibits the invasion and migration of breast cancer cells by regulating HMGB2. J. Biol. Regul. Homeost. Agents 2021, 35, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Sumorek-Wiadro, J.; Zając, A.; Bądziul, D.; Langner, E.; Skalicka-Woźniak, K.; Maciejczyk, A.; Wertel, I.; Rzeski, W.; Jakubowicz-Gil, J. Coumarins modulate the anti-glioma properties of temozolomide. Eur. J. Pharmacol. 2020, 881, 173207. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Tang, X.; Zhao, G.; Jiang, Q.; Liao, Z.; Liang, X. Effects of coumarins from Angelica dahurica on chemotherapeutic sensitivity of human breast cancer cells. Chin. J. Clin. Pharmacol. Ther. 2019, 24, 140–146. [Google Scholar] [CrossRef]
- Okuyama, T.; Takata, M.; Nishino, H.; Nishino, A.; Takayasu, J.; Lwashima, A. Studies on the antitumor-promoting activity of naturally occurring substances. II. Inhibition of tumor-promoter-enhanced phospholipid metabolism by umbelliferous materials. Chem. Pharm. Bull. 1990, 38, 1084–1086. [Google Scholar] [CrossRef] [PubMed]
- Sigurdsson, S.; Ögmundsdottir, H.; Gudbjarnason, S. Antiproliferative Effect of Angelica archangelica Fruits. Z. Naturforsch. 2004, 59, 523–527. [Google Scholar] [CrossRef]
- Khabibov, M.; Garifullin, A.; Boumber, Y.; Khaddour, K.; Fernandez, M.; Khamitov, F.; Khalikova, L.; Kuznetsova, N.; Kit, O.; Kharin, L. Signaling pathways and therapeutic approaches in glioblastoma multiforme (Review). Int. J. Oncol. 2022, 60, 69. [Google Scholar] [CrossRef]
- Jo, Y.; Kim, E.H.; Sai, S.; Kim, J.S.; Cho, J.M.; Kim, H.; Baek, J.H.; Kim, J.Y.; Hwang, S.G.; Yoon, M. Functional Biological Activity of Sorafenib as a Tumor-Treating Field Sensitizer for Glioblastoma Therapy. Int. J. Mol. Sci. 2018, 19, 3684. [Google Scholar] [CrossRef]
- Siegelin, M.D.; Raskett, C.M.; Gilbert, C.A.; Ross, A.H.; Altieri, D.C. Sorafenib exerts anti-glioma activity in vitro and in vivo. Neurosci. Lett. 2010, 478, 165–170. [Google Scholar] [CrossRef]
- Maciejczyk, A.; Kapral-Piotrowska, J.; Sumorek-Wiadro, J.; Zając, A.; Grela, E.; Luchowski, R.; Gruszecki, W.; Lemieszek, M.; Wertel, I.; Pecio, Ł.; et al. Lensoside Aβ as an Adjuvant to the Anti-Glioma Potential of Sorafenib. Cancers 2021, 13, 2637. [Google Scholar] [CrossRef] [PubMed]
- Sumorek-Wiadro, J.; Zając, A.; Langner, E.; Skalicka-Woźniak, K.; Maciejczyk, A.; Rzeski, W.; Jakubowicz-Gil, J. Antiglioma potential of coumarins combined with sorafenib. Molecules 2020, 25, 5192. [Google Scholar] [CrossRef] [PubMed]
- Jakubowicz-Gil, J.; Langner, E.; Bądziul, D.; Wertel, I.; Rzeski, W. Quercetin and sorafenib as a novel and effective couple in programmed cell death induction in human gliomas. Neurotox. Res. 2014, 26, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Zając, A.; Sumorek-Wiadro, J.; Maciejczyk, A.; Langner, E.; Wertel, I.; Rzeski, W.; Jakubowicz-Gil, J. LY294002 and sorafenib as inhibitors of intracellular survival pathways in the elimination of human glioma cells by programmed cell death. Cell Tissue Res. 2021, 386, 17. [Google Scholar] [CrossRef]
- Baccarini, M. An old kinase on a new path: Raf and apoptosis. Cell Death Differ. 2002, 9, 783–785. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Han, L.; Shi, Z.; Zhang, K.; Liu, Y.; Zheng, Y.; Jiang, T.; Pu, P.; Jiang, C.; Kang, C. LY294002 enhances cytotoxicity of temozolomide in glioma by down-regulation of the PI3K/Akt pathway. Mol. Med. Rep. 2012, 5, 575–579. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zając, A.; Sumorek-Wiadro, J.; Langner, E.; Wertel, I.; Maciejczyk, A.; Pawlikowska-Pawlęga, B.; Pawelec, J.; Wasiak, M.; Hułas-Stasiak, M.; Bądziul, D.; et al. Involvement of PI3K Pathway in Glioma Cell Resistance to Temozolomide Treatment. Int. J. Mol. Sci. 2021, 22, 5155. [Google Scholar] [CrossRef] [PubMed]
- Dattachoudhury, S.; Sharma, R.; Kumar, A.; Jaganathan, B.G. Sorafenib Inhibits Proliferation, Migration and Invasion of Breast Cancer Cells. Oncology 2020, 98, 478–486. [Google Scholar] [CrossRef]
- Liu, P.; Xu, B.; Li, J.; Lu, H. LY294002 inhibits leukemia cell invasion and migration through early growth response gene 1 induction independent of phosphatidylinositol 3-kinase-Akt pathway. Biochem. Biophys. Res. Commun. 2008, 377, 187–190. [Google Scholar] [CrossRef]
- Shukla, S.; Maclennan, G.T.; Hartman, D.J.; Fu, P.; Resnick, M.I.; Gupta, S. Activation of PI3K-Akt signaling pathway promotes prostate cancer cell invasion. Int. J. Cancer 2007, 121, 1424–1432. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sumorek-Wiadro, J.; Zając, A.; Skalicka-Woźniak, K.; Rzeski, W.; Jakubowicz-Gil, J. Furanocoumarins as Enhancers of Antitumor Potential of Sorafenib and LY294002 toward Human Glioma Cells In Vitro. Int. J. Mol. Sci. 2024, 25, 759. https://doi.org/10.3390/ijms25020759
Sumorek-Wiadro J, Zając A, Skalicka-Woźniak K, Rzeski W, Jakubowicz-Gil J. Furanocoumarins as Enhancers of Antitumor Potential of Sorafenib and LY294002 toward Human Glioma Cells In Vitro. International Journal of Molecular Sciences. 2024; 25(2):759. https://doi.org/10.3390/ijms25020759
Chicago/Turabian StyleSumorek-Wiadro, Joanna, Adrian Zając, Krystyna Skalicka-Woźniak, Wojciech Rzeski, and Joanna Jakubowicz-Gil. 2024. "Furanocoumarins as Enhancers of Antitumor Potential of Sorafenib and LY294002 toward Human Glioma Cells In Vitro" International Journal of Molecular Sciences 25, no. 2: 759. https://doi.org/10.3390/ijms25020759
APA StyleSumorek-Wiadro, J., Zając, A., Skalicka-Woźniak, K., Rzeski, W., & Jakubowicz-Gil, J. (2024). Furanocoumarins as Enhancers of Antitumor Potential of Sorafenib and LY294002 toward Human Glioma Cells In Vitro. International Journal of Molecular Sciences, 25(2), 759. https://doi.org/10.3390/ijms25020759





