Anti-Inflammatory Effect of Fermented Cabbage Extract Containing Nitric Oxide Metabolites with Silica
Abstract
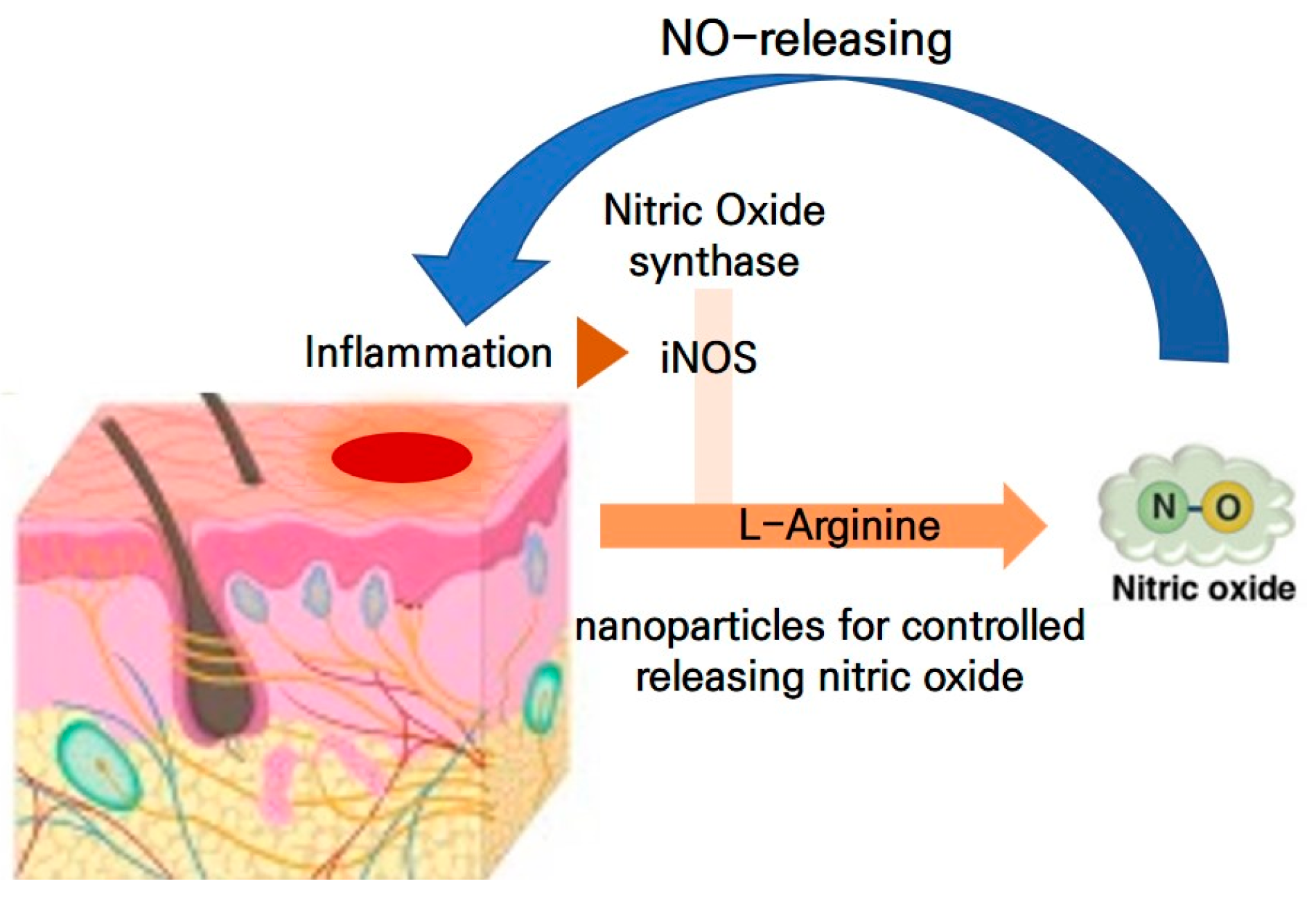
1. Introduction
2. Results
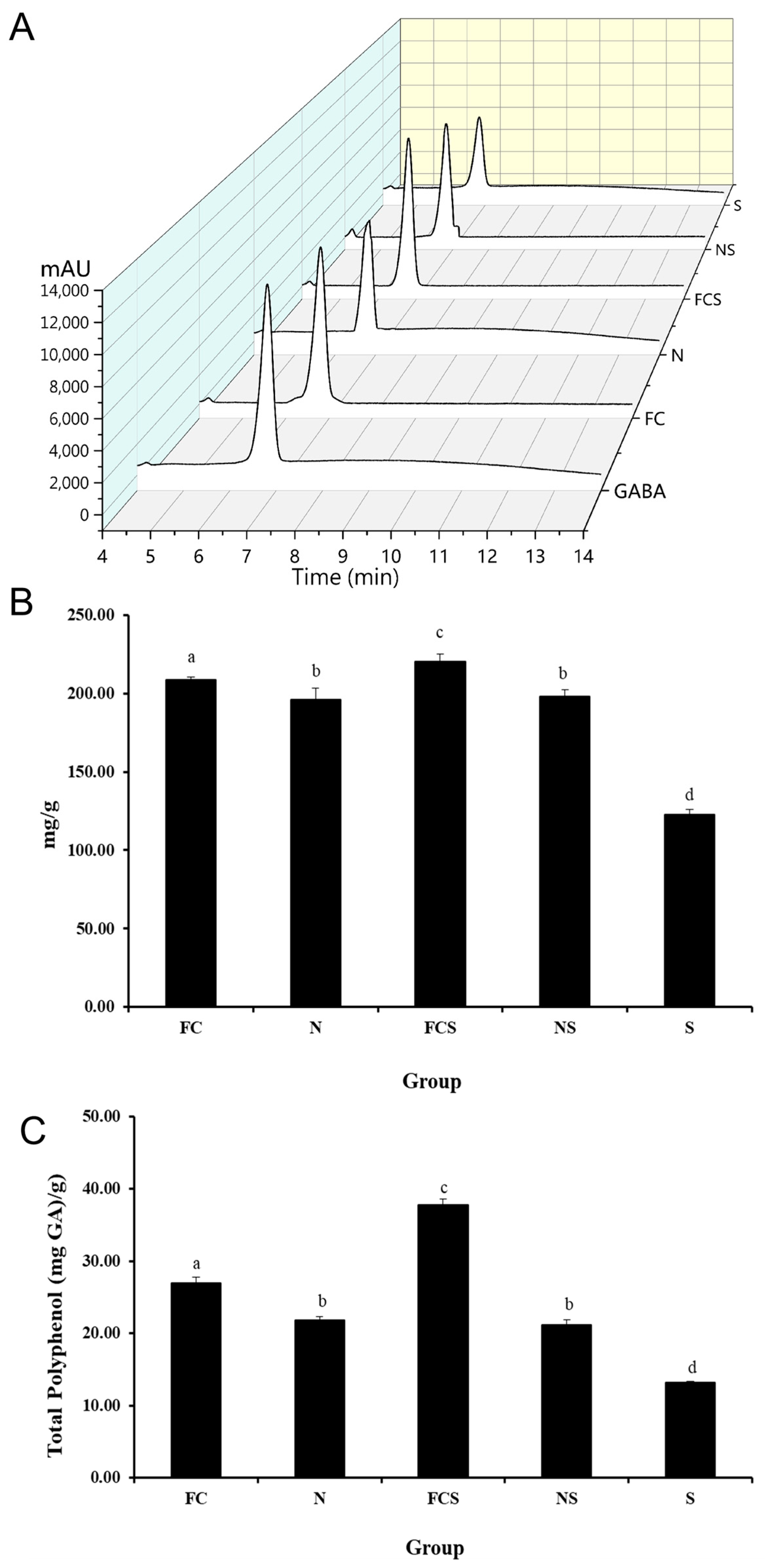
2.1. Determination of Fermented Cabbage Extract Containing Nitric Oxide
2.2. GABA and Total Polyphenol Content of Fermented Cabbage Extract with Silica (FCS)
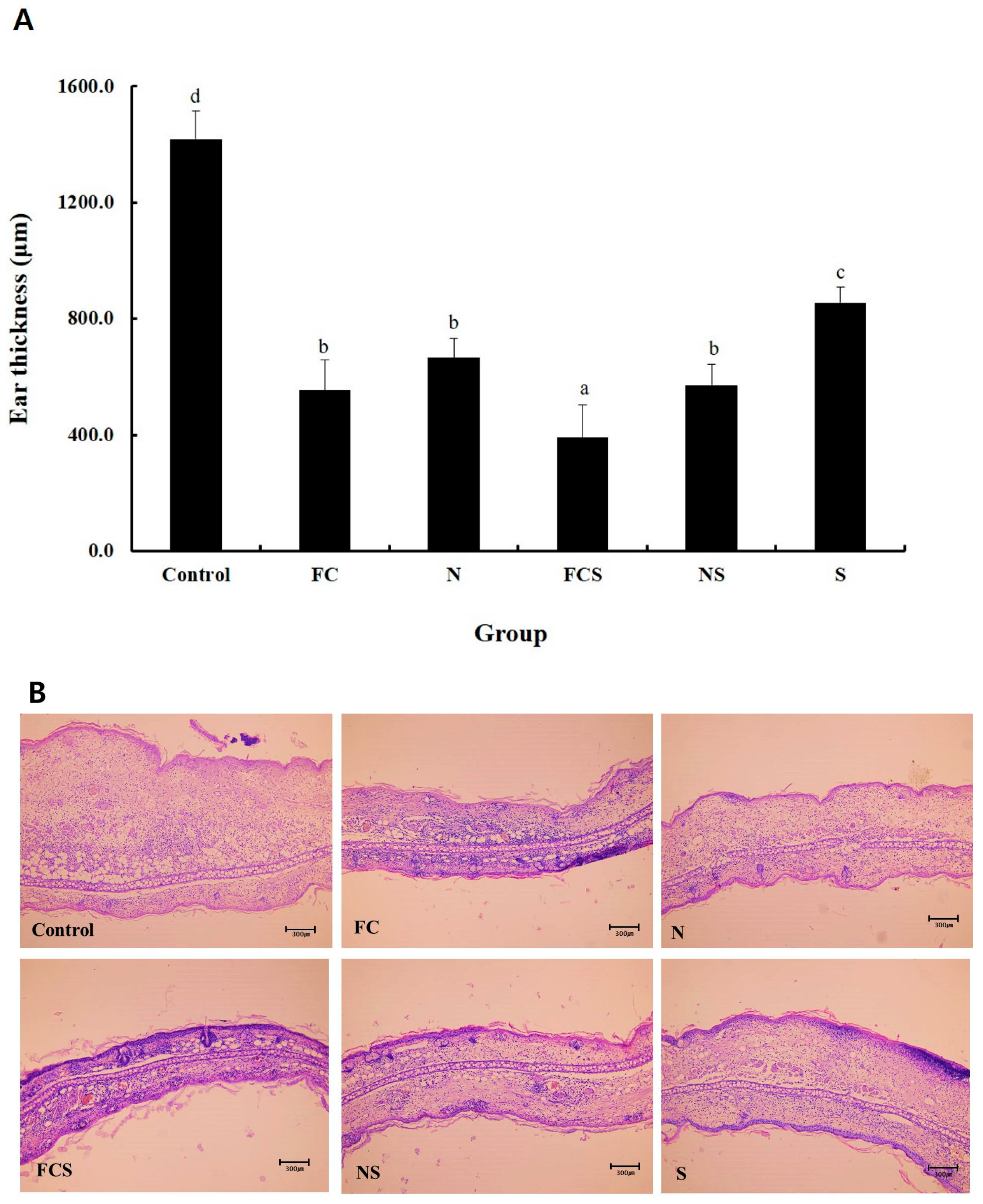
2.3. Fermented Cabbage Extract with Silica (FCS) Treatment Reduced AD Severity in DNFB-Induced Atopic Dermatitis Mice
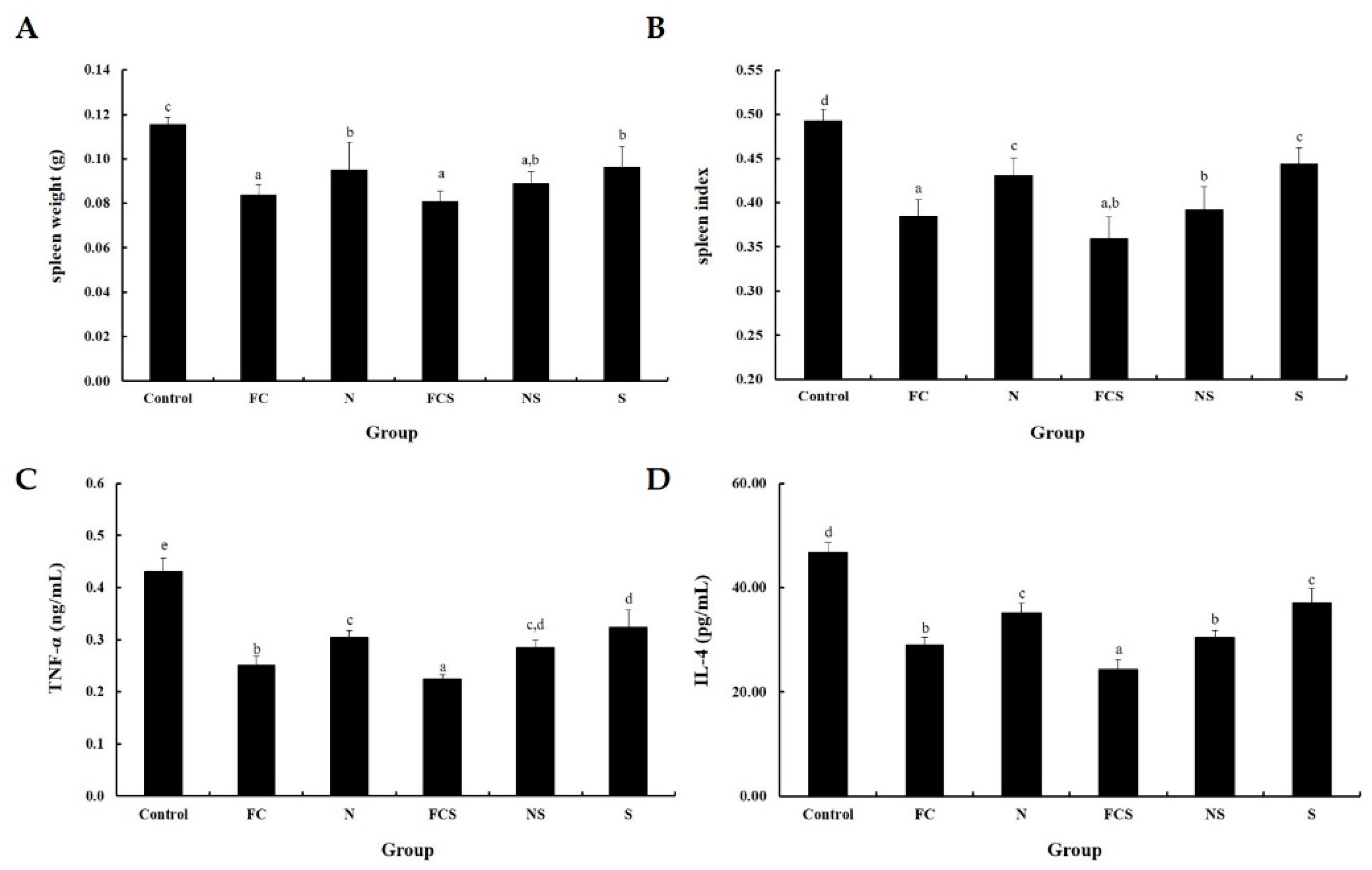
2.4. Effect of Fermented Cabbage Extract with Silica (FCS) to Cytokine and Spleen Index in DNFB-Induced AD Mice
2.5. Histological Analysis from Ear Tissue of DNFB-Induced AD Mice
3. Discussion
4. Materials and Methods
4.1. Preparation of Fermented Cabbage Extract
4.2. GABA and Total Polyphenol Content
4.3. Experimental Animals and Group Design
4.4. Clinical Assessment of Atopic Dermatitis
4.5. Measurement of Ear Thickness
4.6. Spleen Index
4.7. TNF-α and Interleukin (IL)-4 Levels in Serum
4.8. Histopathological Examination
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, H.J. Atopic dermatitis mouse models. Allergy Asthma Respir. Dis. 2019, 7, 113–115. [Google Scholar] [CrossRef]
- Ogawa, H.; Yoshiike, T. A speculative view of atopic dermatitis: Barrier dysfunction in pathogenesis. J. Dermatol. Sci. 1993, 5, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Shin, J.U.; Lee, K.H. Atopic dermatitis and skin barrier dysfunction. Allergy Asthma Respir. Dis. 2013, 1, 20–28. [Google Scholar] [CrossRef]
- Matsunaga, T.; Katayama, I.; Yokozeki, H. ICAM-1 expression on keratinocytes in mechanically-injured skin of a patient with atopic dermatitis. J. Dermatol. Sci. 1996, 12, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Bonneville, M.; Chavagnac, C.; Vocanson, M.; Rozieres, A.; Benetiere, J.; Pernet, I.; Denis, A.; Nicolas, J.F.; Hennino, A. Skin contact irritation conditions the development and severity of allergic contact dermatitis. J. Investig. Dermatol. 2007, 127, 1430–1435. [Google Scholar] [CrossRef]
- Kehren, J.; Desvignes, C.; Krasteva, M.; Ducluzeau, M.T.; Assossou, O.; Horand, F.; Hahne, M.; Kägi, D.; Kaiserlian, D.; Nicolas, J.F. Cytotoxicity is mandatory for CD8(+) T cell-mediated contact hypersensitivity. J. Exp. Med. 1999, 189, 779–786. [Google Scholar] [CrossRef]
- Brandon, L.A.; Adam, J.F. Nitric oxide therapy for dermatologic disease. Future Sci. OA 2015, 1, FSO37. [Google Scholar] [CrossRef]
- Zhang, H.; Annich, G.M.; Meyerhoff, M.E. Nitric Oxide-Releasing Fumed Silica Particles: Synthesis, Characterization, and Biomedical Application. J. Am. Chem. Soc. 2003, 125, 5015–5024. [Google Scholar] [CrossRef]
- Makrides, S.C.; Ryan, U.S. Overview of the Endothelium. In Thrombosis and Hemorrhage, 2nd ed.; Loscalzo, J., Schafer, A.I., Eds.; Williams & Wilkins: Baltimore, MD, USA, 1998; Volume 13, pp. 295–306. [Google Scholar]
- Radomski, M.K.; Palmer, R.M.; Moncada, S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet 1987, 2, 1057–1058. [Google Scholar] [CrossRef]
- Vaughn, M.W.; Kuo, L.; Liao, J.C. Effective diffusion distance of nitric oxide in the microcirculation. Am. J. Physiol. 1998, 274, H1705–H1714. [Google Scholar] [CrossRef]
- Caimi, G.; Hopps, E.; Montana, M.; Carollo, C.; Calandrino, V.; Incalcaterra, E.; Canino, B.; Lo Presti, R. Nitric oxide metabolites (nitrite and nitrate) in several clinical condition. Clin. Hemorheol. Microcirc. 2014, 56, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Frost, M.C.; Meyerhoff, M.E. Synthesis, characterization, and controlled nitric oxide release from S-nitrosothiol-derivatized fumed silica polymer filler particles. J. Biomed. Mater. Res. A 2005, 72, 409–419. [Google Scholar] [CrossRef]
- Park, J.U.; Jung, H.D.; Song, E.H.; Choi, T.H.; Kim, H.E.; Song, J.; Kim, S. The accelerating effect of chitosan-silica hybrid dressing materials on the early phase of wound healing. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1828–1839. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, Q.; Gao, W.; Zhang, Z.; Lou, Y.; Jin, H.; Chen, X.; Lei, B.; Xu, H.; Mao, C. Highly efficient local delivery of endothelial progenitor cells significantly potentiates angiogenesis and full-thickness wound healing. Acta Biomater. 2018, 69, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, F.; Wang, S.; Lu, J.; Li, J.; Du, Y.; Sun, X.; Chen, X.; Gao, J.; Ling, D. Ceria nanocrystals decorated mesoporous silica nanoparticle based ROS-scavenging tissue adhesive for highly efficient regenerative wound healing. Biomaterials 2018, 151, 66–77. [Google Scholar] [CrossRef]
- Meddahi-Pellé, A.; Legrand, A.; Marcellan, A.; Louedec, L.; Letourneur, D.; Leibler, L. Organ repair, hemostasis, and in vivo bonding of medical devices by aqueous solutions of nanoparticles. Angew. Chem. Int. Ed. 2014, 53, 6369–6373. [Google Scholar] [CrossRef]
- Emma, G.Y.; Richard, L.G.; Ana, B.P.; Teruaki, N.; Randall, L.; Ning, Z.; Elizabeth, M.; Tomoko, M.C. A Nitric Oxide-Releasing Topical Medication as a Potential Treatment Option for Atopic Dermatitis through Antimicrobial and Anti-Inflammatory Activity. J. Investig. Dermatol. 2020, 140, 2531–2535.e2. [Google Scholar] [CrossRef]
- Mitchell, J.; Jorge, G.G.; Alain, L.; Mirko, G.; Christopher, W.; Christopher, M.; Satya, P. Novel nitric oxide producing probiotic wound healing patch: Preparation and in vivo analysis in a New Zealand white rabbit model of ischaemic and infected wounds. Int. Wound J. 2012, 9, 330–343. [Google Scholar] [CrossRef]
- Ali, B.H.; Al-Salam, S.; Suleimani, Y.A.; Al Kalbani, J.A.; Al Bahlani, S.; Ashique, M.; Manoj, P.; Al-Dhahli, B.; AlAbri, N.; Naser, H.T.; et al. Curcumin ameliorates kidney function and oxidative stress in experimental chronic kidney disease. Basic Clin. Pharmacol. Toxicol. 2018, 122, 65–73. [Google Scholar] [CrossRef]
- Ali, S.O.; Darwish, H.A.; Ismail, N.A. Modulatory effects of curcumin, silybin-phytosome and alpha-R-lipoic acid against thioacetamide induced liver cirrhosis in rats. Chem.-Biol. Interact. 2014, 216, 26–33. [Google Scholar] [CrossRef]
- Asiwe, J.N.; Asiwe, N.; Onuh, J.E. The effect of high dietary salt consumption on renal function in Streptozotocin-induced diabetic male Wistar rats. Int. J. Nutr. Sci. 2021, 6, 201–207. [Google Scholar] [CrossRef]
- El-Maddawy, Z.K.; El-Sayed, Y.S. Comparative analysis of the protective effects of curcumin and N-acetyl cysteine against paracetamol-induced hepatic, renal, and testicular toxicity in Wistar rats. Environ. Sci. Pollut. Res. Int. 2018, 25, 3468–3479. [Google Scholar] [CrossRef] [PubMed]
- Alghasham, A.; Salem, T.A.; Meki, A.R.M. Effect of cadmium-polluted water on plasma levels of tumor necrosis factor-α, interleukin-6 and oxidative status biomarkers in rats: Protective effect of curcumin. Food Chem. Toxicol. 2013, 59, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Prasad, S.; Kim, J.H.; Patchva, W.L.J.; Priyadarsini, I.K.; Aggarwal, B.B. Multitargeting by curcumin as revealed by molecular interaction studies. Nat. Prod. Rep. 2011, 28, 1937–1955. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Hosseinzadeh, H. Antidotal or protective effects of Curcuma longa (turmeric) and its active ingredient, curcumin, against natural and chemical toxicities: A review. Biomed. Pharmacother. 2018, 99, 411–421. [Google Scholar] [CrossRef]
- Soliman, M.M.; Baiomy, A.A.; Yassin, M.H. Molecular and histopathological study on the ameliorative effects of curcumin against lead acetate-induced hepatotoxicity and nephrototoxicity in Wistar rats. Biol. Trace Elem. Res. 2015, 167, 91–102. [Google Scholar] [CrossRef]
- Ranjana, S.; Prakrati, G.; Pradeep, K.; Shashi, K.B.; Saurabh, K. Microbial Fermentation and Its Role in Quality Improvement of Fermented Foods. Fermentation 2020, 6, 106. [Google Scholar] [CrossRef]
- Velat, C.; Burcin, B.; Mehtap, Y.; Pinar, G.O.; Necdet, S. Do traditional fermented foods protect against infantile atopic dermatitis. Pediatr Allergy Immunol. 2019, 30, 540–545. [Google Scholar] [CrossRef]
- Park, S.M.; Bae, J.H. Fermented food intake is associated with a reduced likelihood of atopic dermatitis in an adult population (Korean National Health and Nutrition Examination). Nutr Res. 2016, 36, 125–133. [Google Scholar] [CrossRef]
- Bhat, R.; Axtell, R.; Mitra, A.; Miranda, M.; Lock, C.; Tsien, R.; Steinman, L. Inhibitory role for GABA in autoimmune inflammation. Proc. Natl. Acad. Sci. USA 2010, 107, 2580–2585. [Google Scholar] [CrossRef]
- Salvo, E.D.; Gangemi, S.; Genovese, C.; Cicero, N.; Casciaro, M. Polyphenols from Mediterranean Plants: Biological Activities for Skin Photoprotection in Atopic Dermatitis, Psoriasis, and Chronic Urticaria. Plants 2023, 12, 3579. [Google Scholar] [CrossRef] [PubMed]
- Guttman-Yassky, E.; Nograles, K.E.; Krueger, J.G. Contrasting pathogenesis of atopic dermatitis and psoriasis—Part I: Clinical and pathologic concepts. J. Allergy Clin. Immunol. 2011, 127, 1110–1118. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.W.; Kim, M.R.; Lee, E.Y.; Kim, J.H.; Kim, Y.S.; Jeon, S.G.; Yang, J.M.; Lee, B.J.; Pyun, B.Y.; Gho, Y.S.; et al. Extracellular vesicles derived from Staphylococcus aureus induce atopic dermatitis-like skin inflammation. Allergy 2011, 66, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Furue, M. Regulation of Skin Barrier Function via Competition between AHR Axis versus IL-13/IL-4‒JAK‒STAT6/STAT3 Axis: Pathogenic and Therapeutic Implications in Atopic Dermatitis. J. Clin. Med. 2020, 9, 3741. [Google Scholar] [CrossRef] [PubMed]
- Fujii, M. Current Understanding of Pathophysiological Mechanisms of Atopic Dermatitis: Interactions among Skin Barrier Dysfunction, Immune Abnormalities and Pruritus. Biol. Pharm. Bull. 2020, 43, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Lee, E.; Lee, B.H.; Cho, W.K.; Ma, J.Y.; Park, K.I. Ethanolic Extracts of Artemisia apiacea Hance Improved Atopic Dermatitis-Like Skin Lesions In Vivo and Suppressed TNF-Alpha/IFN-Gamma–Induced Proinflammatory Chemokine Production In Vitro. Nutrients 2018, 10, 806. [Google Scholar] [CrossRef] [PubMed]
- Stephan, W.; Natalija, N. Atopic dermatitis. Lancet 2016, 387, 1109–1122. [Google Scholar] [CrossRef]
- Abe, M.; Thomson, A.W. Influence of immunosuppressive drugs on dendritic cells. Transpl. Immunol. 2003, 11, 357–365. [Google Scholar] [CrossRef]
- Fang, F.C. Mechanisms of nitric oxide-related antimicrobial activity. J. Clin. Investig. 1997, 99, 2818–2825. [Google Scholar] [CrossRef]
- Fang, F.C. Antimicrobial reactive oxygen and nitrogen species: Concepts and controversies. Nat. Rev. Microbiol. 2004, 2, 820–832. [Google Scholar] [CrossRef]
- Raulli, R.; McElhaney-Feser, G.; Hrabie, J.A.; Cihlar, R.L. Antimicrobial properties of nitric oxide using diazeniumdiolates as the nitric oxide donor. Recent Res. Dev. Microbiol. 2002, 6, 177–183. [Google Scholar]
- Sharma, J.N.; Omran, A.A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Moncada, S.; Palmer, R.M.J.; Higgs, E.A. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991, 43, 109–142. [Google Scholar] [PubMed]
- Gonon, A.T.; Erbas, D.; Broijerswen, A.; Valen, G.; Pernow, J. Nitric oxide mediates protective effect of endothelin receptors antagonism during myocardial ischemia and perfusion. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H1767–H1774. [Google Scholar] [CrossRef]
- Stenger, S.; Thuring, H.; Rollinghoff, M.; Bogdan, C. Tissue expression of inducible nitric oxide synthase is closely associated with resistance to Leishmania major. J. Exp. Med. 1994, 180, 783–793. [Google Scholar] [CrossRef]
- Heck, D.E.; Laskin, D.L.; Gardner, C.R.; Laskin, J.D. Epidermal Growth Factor Suppresses Nitric Oxide and Hydrogen Peroxide Production by Keratinocytes. J. Biol. Chem. 1992, 267, 21277–21280. [Google Scholar] [CrossRef] [PubMed]
- Kolb-Bachofen, V.; Fehsel, K.; Michel, G.; Ruzicka, T. Epidermal keratinocyte expression of inducible nitric oxide synthase in skin lesions of psoriasis vulgaris. Lancet 1994, 344, 139. [Google Scholar] [CrossRef]
- Goldsmith, P.C.; Leslie, T.A.; Hayes, N.A.; Levell, N.J.; Dowd, P.M.; Foreman, J.C. Inhibitors of nitric oxide synthase in human skin. J. Investig. Dermatol. 1996, 106, 113–118. [Google Scholar] [CrossRef]
- Hetrick, E.M.; Shin, J.H.; Stasko, N.A.; Johnson, C.B.; Wespe, D.A.; Holmuhamedov, E.; Schoenfisch, M.H. Bactericidal efficacy of nitric oxide-releasing silica nanoparticles. ACS Nano 2008, 2, 235–246. [Google Scholar] [CrossRef]
- Hetrick, E.M.; Shin, J.H.; Paul, H.S.; Schoenfisch, M.H. Anti-Biofilm Efficacy of Nitric Oxide-Releasing Silica Nanoparticles. Biomaterials 2009, 30, 2782–2789. [Google Scholar] [CrossRef]
- Lancaster, J.R. A tutorial on the diffusability and reactivity of free nitric oxide. Nitric Oxide Biol. Chem. 1997, 1, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Bagwe, R.P.; Hilliard, L.R.; Tan, W. Surface modification of silica nanoparticles to reduce aggregation and nonspecific binding. Langmuir 2006, 22, 4357–4362. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Lohstreter, S.; Pierce, D.T.; Parisien, J.; Wu, M.; Hall, C.; Julia, X.Z. Silica nanoparticles with continuously tunable sizes: Synthesis and size effects on cellular contrast imaging. Chem. Mater. 2008, 20, 4411–4419. [Google Scholar] [CrossRef]
- Kusznierewicz, B.; Lewandowska, J.; Kruszyna, A.; Piasek, A.; Smiechowska, A.; Namiesnik, J.; Bartoszek, A. The antioxidative properties of white cabbage (Brassica oleracea var. capitata F. Alba) fresh and submitted to culinary processing. J. Food Biochem. 2010, 34, 262–285. [Google Scholar] [CrossRef]
- Wagner, A.E.; Huebbe, P.; Konishi, T.; Rahman, M.M.; Nakahara, M.; Matsugo, S.; Rimbach, G. Free radical scavenging and antioxidant activity of ascorbigen versus ascorbic acid: Studies in vitro and in cultured human keratinocytes. J. Agric. Food Chem. 2008, 56, 11694–11699. [Google Scholar] [CrossRef] [PubMed]
- Hrncirik, K.; Valusek, J.; Velisek, J. A study on the formation and stability of ascorbigen in an aqueous system. Food Chem. 1998, 63, 349–355. [Google Scholar] [CrossRef]
- Ciska, E.; Pathak, D.R. Glucosinolate derivatives in stored fermented cabbage. J. Agric. Food Chem. 2004, 52, 7938–7943. [Google Scholar] [CrossRef]
- Peñas, E.; Pihlava, J.M.; Frias, J.; Vidal-Valverde, C. Influence of fermentation conditions of Brassica oleracea L. var. capitata on the volatile glucosinolate hydrolysis compounds of sauerkrauts. Food Sci. Technol. 2012, 48, 16–23. [Google Scholar] [CrossRef]
- Tolonen, M.; Taipale, M.; Viander, B.; Pihlava, J.M.; Korhonen, H.; Ryhänen, E.L. Plant-derived biomolecules in fermented cabbage. J. Agric. Food Chem. 2002, 50, 6798–6803. [Google Scholar] [CrossRef]
- Ernst, I.M.A.; Wagner, A.E.; Schuemann, C.; Storm, N.; Höppner, W.; Döring, F.; Stocker, A.; Rimbach, G. Allyl, butyl- and phenylethyl-isothiocyanate activate Nrf2 in cultured cells. Pharmacol. Res. 2011, 63, 233–240. [Google Scholar] [CrossRef]
- Guerrero-Beltrán, C.E.; Calderón-Oliver, M.; Pedraza-Chaverri, J.; Chirino, Y.I. Protective effect of sulforaphane against oxidative stress: Recent advances. Exp. Toxicol. Pathol. 2012, 64, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Ju, M.S.; Jeong, H.K.; Kim, H.G.; Park, G.H.; Youn, Y.S.; Kim, Y.O.; Kim, S.Y.; Oh, M.S. Anti-nociceptive and anti-inflammatory effects of Geranii Herba. Korea J. Herbol. 2010, 25, 97–101. [Google Scholar] [CrossRef]
- Sumimoto, S.; Kawai, M.; Kasajima, Y.; Hamamoto, T. Increased plasma tumour necrosis factor-alpha concentration in atopic dermatitis. Arch. Dis. Child. 1992, 67, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Church, M.K.; Clough, G.F. Human skin mast cells: In vitro and in vivo studies. Ann. Allergy Asthma Immunol. 1999, 83, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Church, M.K.; el-Lati, S.; Okayama, Y. Biological properties of human skin mast cells. Clin. Exp. Allergy 1991, 21, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chamlin, S.L.; Kao, J.; Frieden, I.J.; Sheu, M.Y.; Fowler, A.J.; Fluhr, J.W.; Williams, M.L.; Elias, P.M. Ceramide-dominant barrier repair lipids alleviate childhood atopic dermatitis: Changes in barrier function provide a sensitive indicator of disease activity. J. Am. Acad. Dermatol. 2002, 47, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Onoue, A.; Kabashima, K.; Kobayashi, M.; Mori, T.; Tokura, Y. Induction of eosinophil- and Th2-attracting epidermal chemokines and cutaneous late-phase reaction in tape-stripped skin. Exp. Dermatol. 2009, 18, 1036–1043. [Google Scholar] [CrossRef]
- Wood, L.C.; Elias, P.M.; Calhoun, C.; Tsai, J.C.; Grunfeld, C.; Feingold, K.R. Barrier disruption stimulates interleukin-1 alpha expression and release from a pre-formed pool in murine epidermis. J. Investig. Dermatol. 1996, 106, 397–403. [Google Scholar] [CrossRef]
- Park, J.S.; Ryu, J.H.; Kim, B.Y.; Chun, H.S.; Kim, M.S.; Shin, Y.I. Fermented Lettuce Extract Containing Nitric Oxide Metabolites Attenuates Inflammatory Parameters in Model Mice and in Human Fibroblast-Like Synoviocytes. Nutrients 2023, 15, 1106. [Google Scholar] [CrossRef]
- Lu, Y.G.; Zhang, H.; Meng, X.Y.; Wang, L.; Guo, X.N. A validated HPLC method for the determination of GABA by pre-column derivatization with 2,4-dinitrofluorodinitrobenzene and its application to plant GAD activity study. Anal. Lett. 2010, 43, 2663–2671. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Kim, E.; Zhang, M.; Lee, Y.-S.; Ji, B.; Lee, S.-H.; Cheong, Y.E.; Yun, S.-I.; Kim, Y.-S.; Kim, K.H.; et al. Antidiabetic Effect of Noodles Containing Fermented Lettuce Extracts. Metabolites 2021, 11, 520. [Google Scholar] [CrossRef] [PubMed]
- Al-Farsi, M.; Alasalvar, C.; Morris, A.; Baron, M.; Shahidi, F. Comparison of antioxidant activity, anthocyanins, carotenoids, and phenolics of three native fresh and sun-dried date (Phoenix dactylifera L.) varieties grown in Oman. J. Agric. Food Chem. 2005, 53, 7592–7599. [Google Scholar] [CrossRef] [PubMed]



| Group | Left Ear | Right Ear | Material | Dose | n | |
|---|---|---|---|---|---|---|
| 1 | Control | Normal | 0.3% DNFB | Vehicle | - | 5 |
| 2 | FC | Normal | 0.3% DNFB | Fermented cabbage extracts | 200 mg/kg | 5 |
| 3 | N | Normal | 0.3% DNFB | Sodium nitrite (NaNO2) | 200 mg/kg | 5 |
| 4 | FCS | Normal | 0.3% DNFB | Fermented cabbage extracts + silica 0.1% | 200 mg/kg | 5 |
| 5 | NS | Normal | 0.3% DNFB | Sodium nitrite (NaNO2) + silica 0.1% | 200 mg/kg | 5 |
| 6 | S | Normal | 0.3% DNFB | Silica 0.1% | 200 mg/kg | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-S.; Ji, B.-J.; Pae, H.-O.; Cheon, M.-W.; Xu, G.; Chun, H.-S.; Kim, S. Anti-Inflammatory Effect of Fermented Cabbage Extract Containing Nitric Oxide Metabolites with Silica. Int. J. Mol. Sci. 2024, 25, 775. https://doi.org/10.3390/ijms25020775
Lee Y-S, Ji B-J, Pae H-O, Cheon M-W, Xu G, Chun H-S, Kim S. Anti-Inflammatory Effect of Fermented Cabbage Extract Containing Nitric Oxide Metabolites with Silica. International Journal of Molecular Sciences. 2024; 25(2):775. https://doi.org/10.3390/ijms25020775
Chicago/Turabian StyleLee, Yun-Seong, Byeong-Jun Ji, Hyun-Ock Pae, Mu-Weon Cheon, Guangpeng Xu, Hyun-Soo Chun, and Sooah Kim. 2024. "Anti-Inflammatory Effect of Fermented Cabbage Extract Containing Nitric Oxide Metabolites with Silica" International Journal of Molecular Sciences 25, no. 2: 775. https://doi.org/10.3390/ijms25020775
APA StyleLee, Y.-S., Ji, B.-J., Pae, H.-O., Cheon, M.-W., Xu, G., Chun, H.-S., & Kim, S. (2024). Anti-Inflammatory Effect of Fermented Cabbage Extract Containing Nitric Oxide Metabolites with Silica. International Journal of Molecular Sciences, 25(2), 775. https://doi.org/10.3390/ijms25020775






