Advanced Drug Carriers: A Review of Selected Protein, Polysaccharide, and Lipid Drug Delivery Platforms
Abstract
1. Introduction
- Biocompatibility: natural polymers are often natural components of the body (e.g., hyaluronic acid), which minimizes the risk of immune reactions and provides better biocompatibility compared to synthetic polymers.
- Biodegradability: most natural polymers are naturally degradable in the body, eliminating the need for surgical removal after treatment, which is particularly important, especially in terms of minimizing side effects and body burden.
- Diverse sources: polymers of natural origin, such as proteins, polysaccharides, or nucleic acids, can be obtained from a variety of sources, making it possible to tailor their properties to specific applications.
2. Lipid-Containing Carriers
2.1. Lipids—Short Characteristics
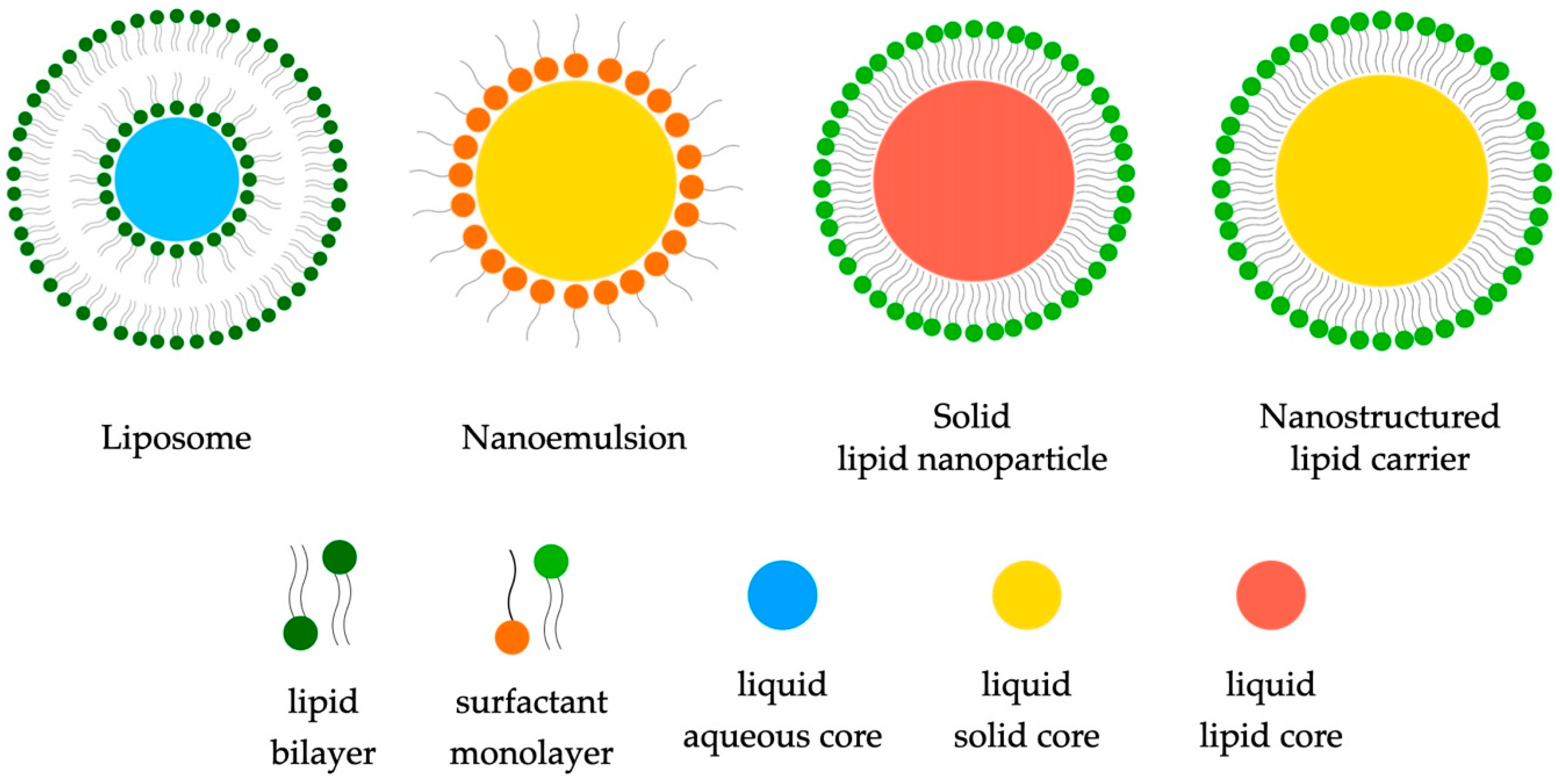
2.1.1. Liposomes
2.1.2. Lipid Nanoemulsions
2.1.3. Solid Lipid Nanoparticles (SLNs) and Nanostructured Lipid Carriers (NLCs)
3. Polysaccharide-Based Nanocomposites
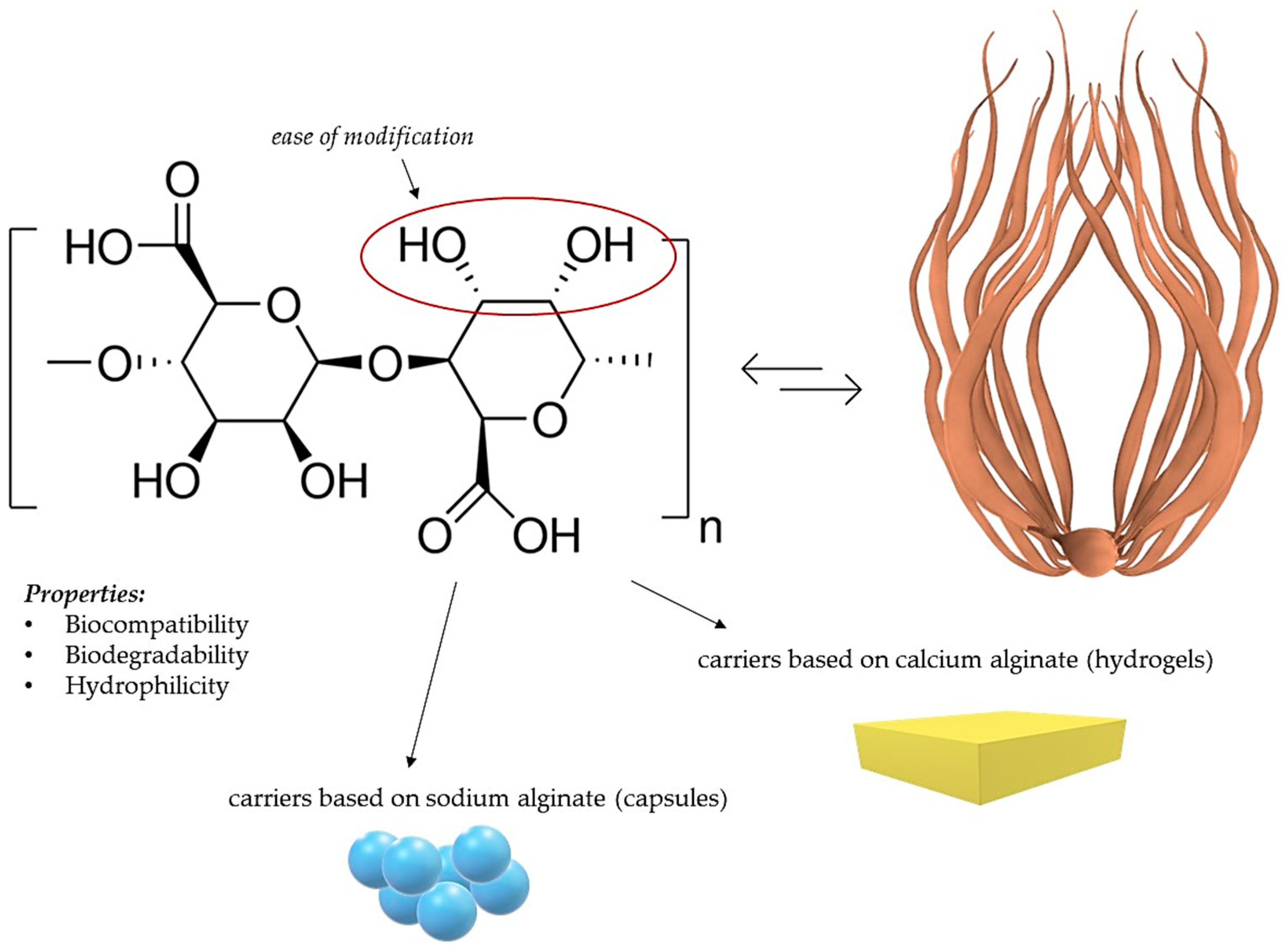
3.1. Alginate-Based Nanocomposites
3.2. Cellulose-Based Nanocomposite Carriers
4. Protein-Based Nanocomposite Carriers
4.1. Gelatin-Based Drug Carriers
4.2. Albumin-Based Drug Carriers
5. Summary
5.1. Conclusions
- In today’s world, the development of modern therapies and active substance delivery strategies requires innovative approaches. Traditional drug delivery methods, such as oral, intravenous, transdermal, or muscle delivery of the active ingredient, have significant limitations, including degradation in the gastrointestinal tract. Lack of selectivity and susceptibility to side effects underscore the need for novel solutions. Bionanocomposites are advanced drug carriers, enabling the precise and targeted delivery of active substances, which could revolutionarily improve the efficacy of therapies, especially in cancer treatment.
- Liposomes represent a promising drug carrier, enabling the encapsulation of both hydrophilic and hydrophobic substances. Their ability to precisely deliver active substances opens new perspectives in anti-cancer therapy, accelerating wound healing or delivering drugs to the eye. In addition, liposomes allow for the controlled release of active substances, which can increase drug stability and reduce side effects.
- Lipid nanoemulsions offer stable solutions for improving the solubility of lipophilic substances. Their ability to efficiently transport active substances, especially in terms of improving bioavailability, makes them attractive drug carriers. In addition, nanoemulsions can be customized, allowing for them to be used in a variety of therapeutic areas, such as the delivery of anti-cancer drugs or the treatment of gastrointestinal diseases.
- Carriers such as solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) represent advanced strategies in drug delivery. Their stability, controlled release of active substances, and ability to improve bioavailability make them promising tools in the field of drug therapy. The use of SLNs, which are constructed from lipids in solid form, and NLCs, which combine liquids and solid oils, presents new possibilities in the efficient transport of drugs, especially in the context of anti-cancer therapy or the delivery of lipophilic substances.
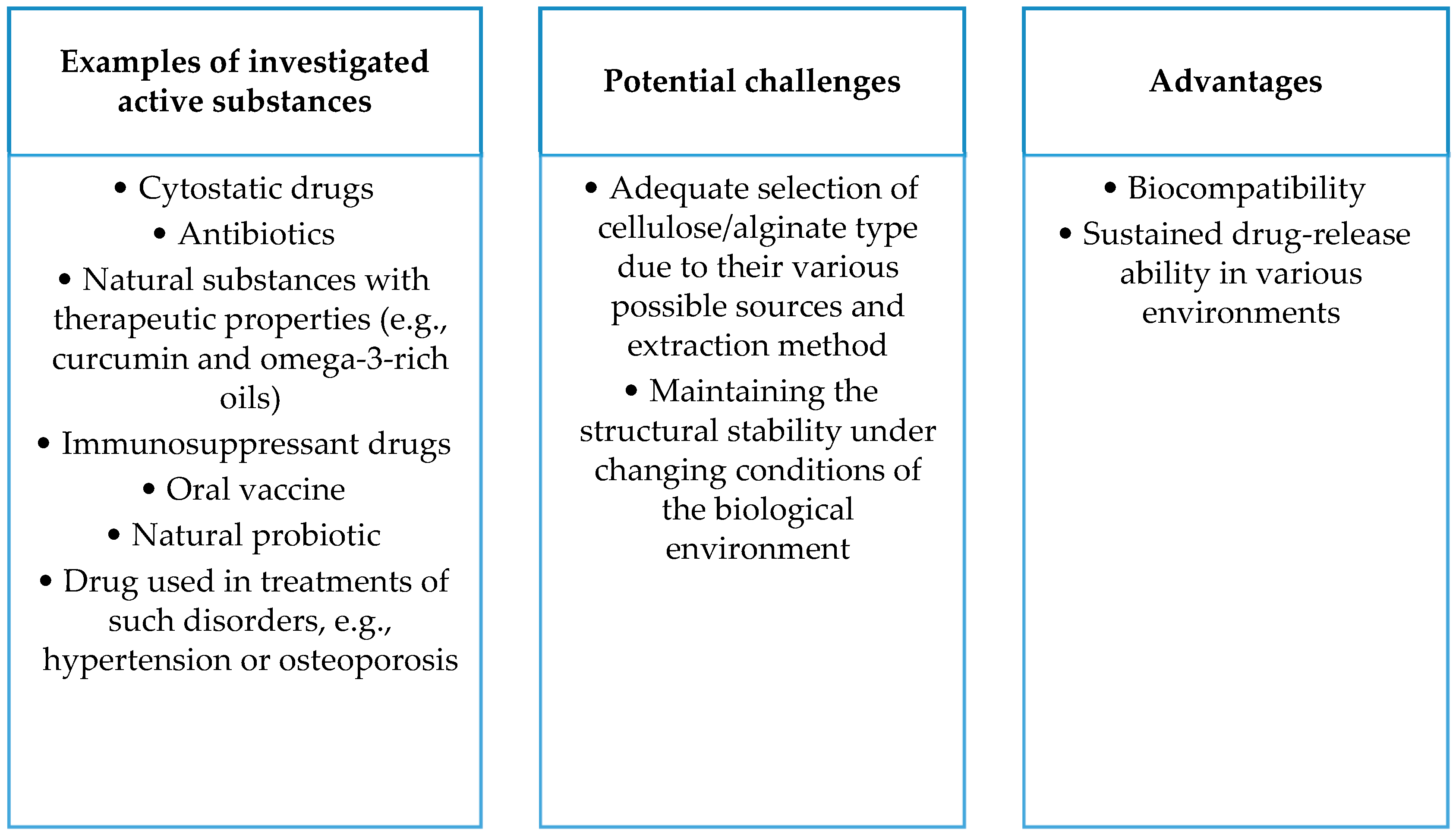
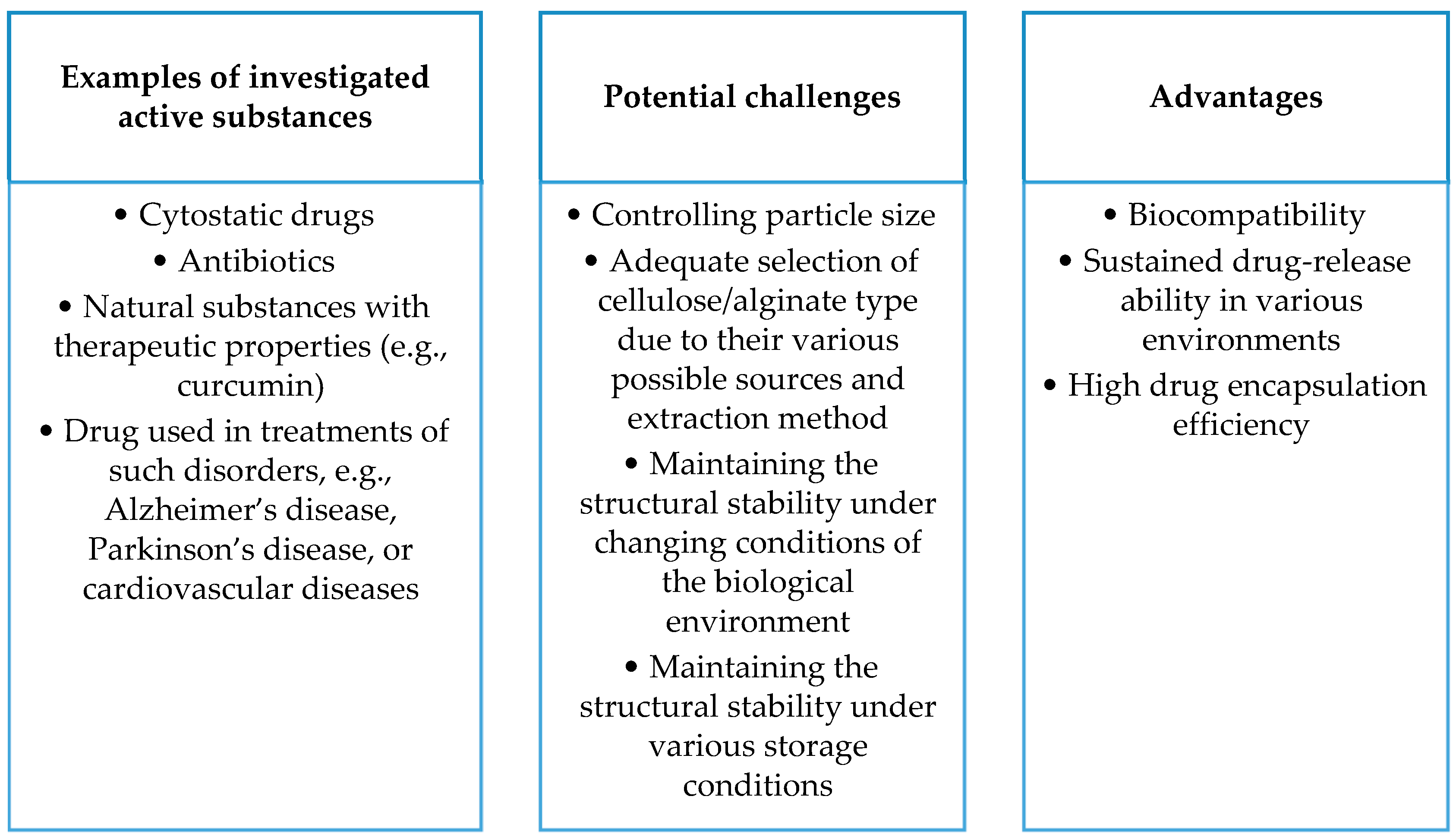
- Sodium alginate, being biodegradable, biocompatible, and readily available, holds promise as a material for drug delivery systems. Alginate-based nanocomposites, such as composites with hydroxyapatite and ciprofloxacin, exhibit controlled drug release, enhancing therapeutic efficacy. Alginate-based nanocomposites also show promise as carriers for curcumin, increasing its solubility and demonstrating potential antibacterial properties. Alginate is applied in delivering drugs against tuberculosis and in anti-cancer therapy, indicating its significant potential in medicine.
- Cellulose, being biocompatible and suitable as a drug carrier, demonstrates potential in delivering antibiotics, especially in nanocomposites with gold nanoparticles. Copolymer hydrogels with cellulose nanocrystals effectively transport doxorubicin, showing controlled drug release. Nanocellulose gains recognition as an effective drug carrier, indicating a promising path for future research.
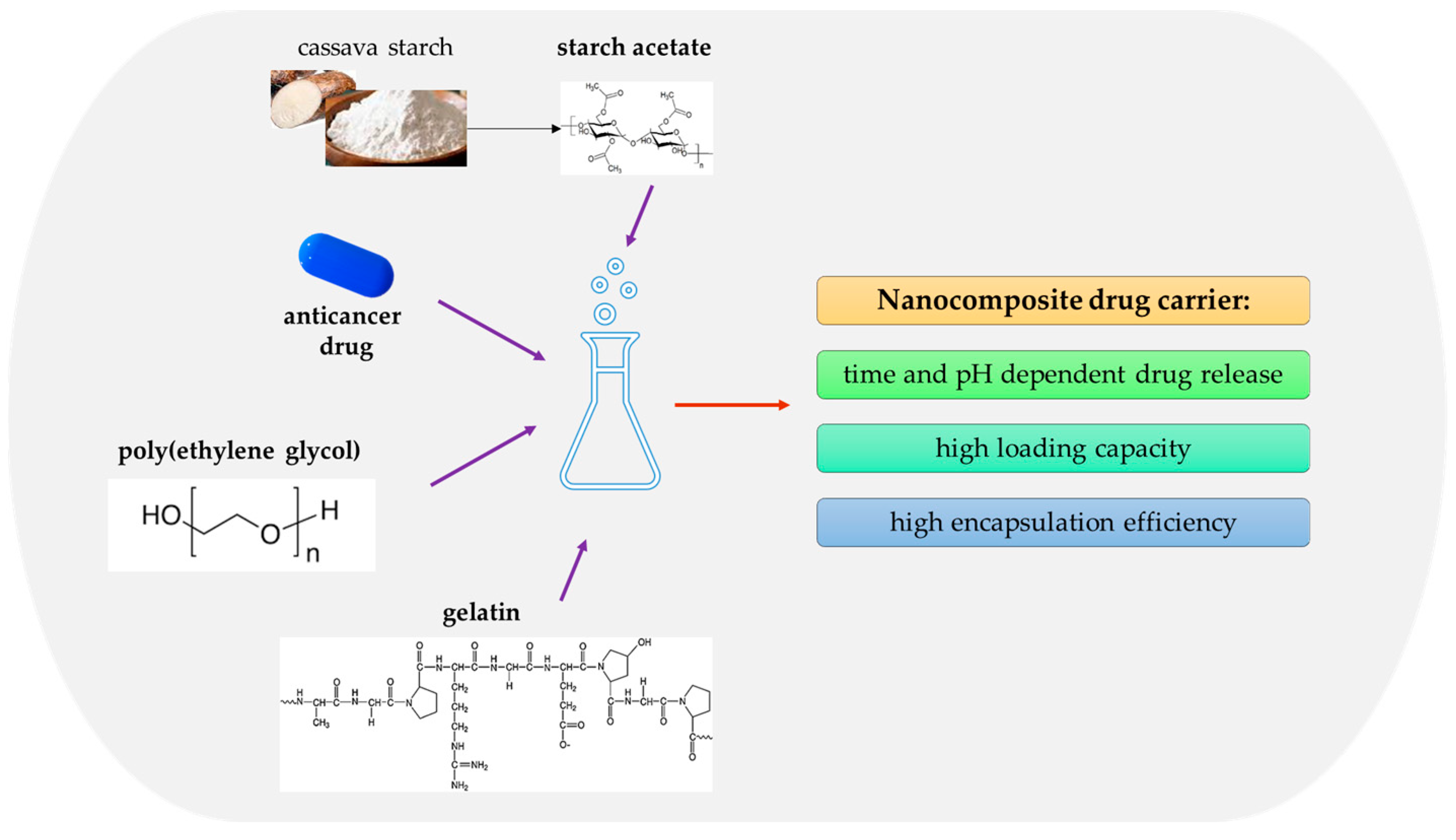
- Gelatin, with its ability to form matrices for active substances, is emerging as a promising material in the field of drug delivery. Its biocompatibility, biodegradability, and ability to form drug carrier systems, such as microspheres, may increase drug stability and improve their therapeutic efficacy. Moreover, this protein is being intensively studied as a component of nanocomposite carriers of anti-cancer drugs. Incorporating active compounds, such as cisplatin, quercetin, or doxorubicin, into gelatin-based nanocarriers shows promise regarding drug-release efficiency and their cytotoxic effects against cancer cells.
- Albumin has potential for controlled drug release due to its ability to specifically bind and transport various molecules. In addition, albumin exhibits biodegradability and biocompatibility, significantly reducing the risk of immune reactions, which is important for its potential use as a drug carrier. Many studies have confirmed the potential of albumin to deliver drugs to tumor tissues. The ability of albumin to be biodistributed across cancer cells and the ability of these cells to internalize the protein have been demonstrated. This finding indicates the potential for using albumin in targeted drug delivery to cancer cells. Research on albumin-containing drug carriers is not just focused on cancer therapy. Numerous studies show the potential of albumin in the delivery of antibacterial, analgesic, and antifungal drugs, as well as active substances to prevent obesity.
5.2. Perspectives and Future Challenges
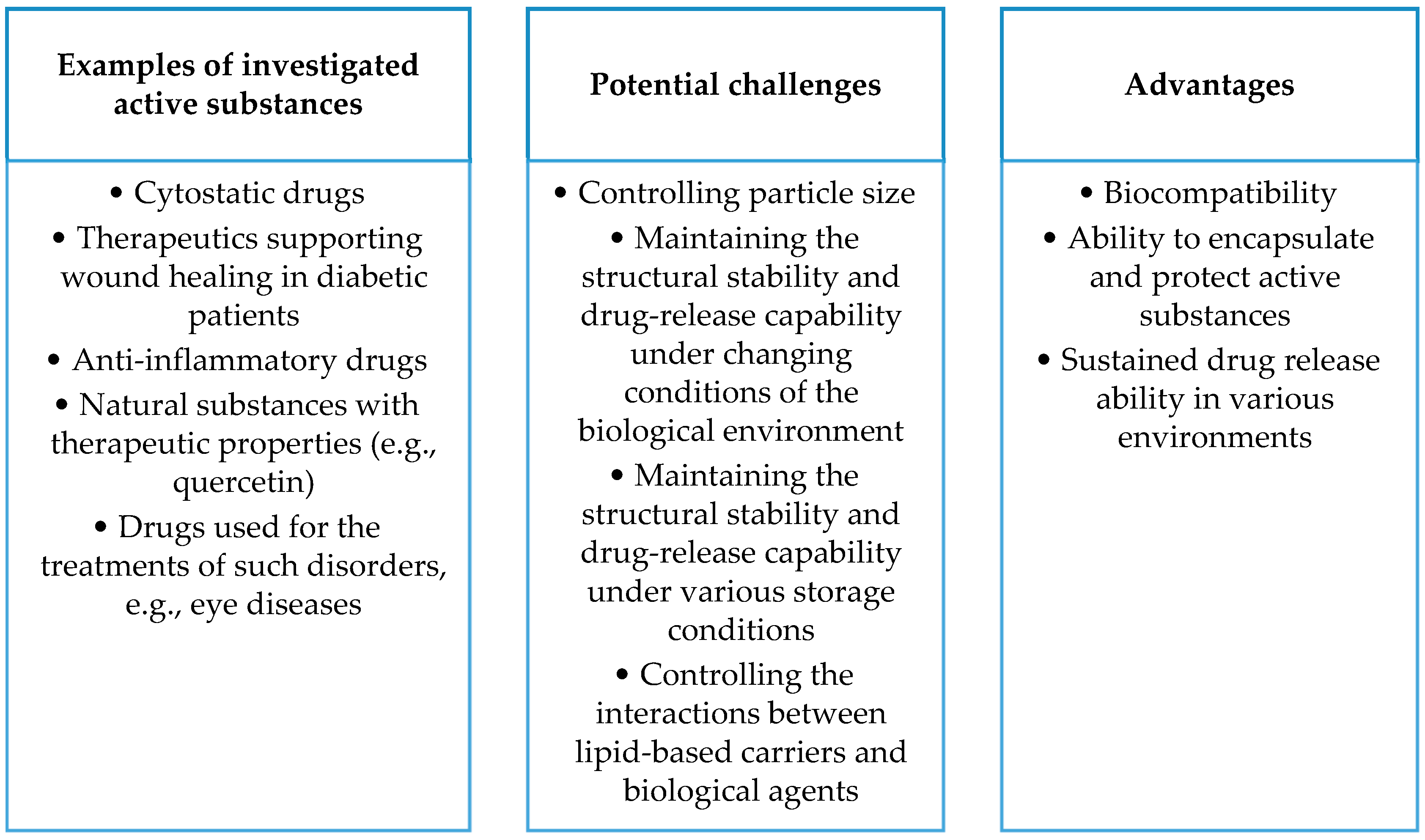
- Studies on proteins, polysaccharides, and lipid-containing nanosystems (including liposomes, lipid nanoemulsions, solid lipid nanoparticles, and nanostructured lipid carriers) show promising results in terms of therapeutic efficacy and improvement in the stability and bioavailability of active substances. Future research may focus on further refining nanocomposite technologies, increasing their specificity and effectiveness in drug delivery across various medical, pharmacological, and cosmetic applications.
- The development of drug carriers based on proteins, polysaccharides, and lipids promises to improve therapeutic efficacy but brings with it complex issues. Liposomes can disintegrate and react with digestive enzymes, requiring in-depth studies in the context of stability, release, and interaction with the immune system. Analogous issues apply to nanoemulsions. In the case of solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs), difficulties related to stability, particle size control, and biological interactions are significant. Despite the high biocompatibility of gelatin, further research is needed to maintain it under physicochemical conditions, and solubility regulation is a key challenge. Polysaccharides, such as alginates, face difficulties in terms of physicochemical stability in biological environments, affecting the persistence and control of drug release. Cellulose, despite its biocompatibility, requires research on maintaining stability under physicochemical conditions, especially control of solubility in different biological environments. Issues related to maintaining the stability of cellulose carriers during storage, controlling particle size, and affecting bioavailability are areas for further research.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kandula, S.; Singh, P.K.; Kaur, G.A.; Tiwari, A. Trends in smart drug delivery systems for targeting cancer cells. Mater. Sci. Eng. B 2023, 297, 116816. [Google Scholar] [CrossRef]
- Srivastav, A.K.; Karpathak, S.; Rai, M.K.; Kumar, D.; Misra, D.P.; Agarwal, V. Lipid based drug delivery systems for oral, transdermal and parenteral delivery: Recent strategies for targeted delivery consistent with different clinical application. J. Drug Deliv. Sci. Technol. 2023, 85, 104526. [Google Scholar] [CrossRef]
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef] [PubMed]
- Sultana, A.; Zare, M.; Thomas, V.; Sampath Kumar, T.S.; Ramakrishna, S. Nano-based drug delivery systems: Conventional drug delivery routes, recent developments and future prospects. Med. Drug Discov. 2022, 15, 100134. [Google Scholar] [CrossRef]
- Hua, S. Advances in oral drug delivery for regional targeting in the gastrointestinal tract-influence of physiological, pathophysiological and pharmaceutical factors. Front. Pharmacol. 2020, 11, 524. [Google Scholar] [CrossRef] [PubMed]
- Ezike, T.C.; Okpala, U.S.; Onoja, U.L.; Nwike, C.P.; Ezeako, E.C.; Okpara, O.J.; Okoroafor, C.C.; Eze, S.C.; Kalu, O.L.; Odoh, E.C.; et al. Advances in drug delivery systems, challenges and future directions. Heliyon 2023, 9, e17488. [Google Scholar] [CrossRef] [PubMed]
- Tewabe, A.; Abate, A.; Tamrie, M.; Seyfu, A.; Abdela Siraj, E. Targeted Drug Delivery—From Magic Bullet to Nanomedicine: Principles, Challenges, and Future Perspectives. J. Multidiscip. Healthc. 2021, 14, 1711–1724. [Google Scholar] [CrossRef]
- Veselov, V.V.; Nosyrev, A.E.; Jicsinszky, L.; Alyautdin, R.N.; Cravotto, G. Targeted Delivery Methods for Anticancer Drugs. Cancers 2022, 14, 622. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yang, Y.; Chen, Y.; Xu, Y.; Peng, J. Cell-based drug delivery systems and their in vivo fate. Adv. Drug Deliv. Rev. 2022, 187, 114394. [Google Scholar] [CrossRef]
- Zhao, Z.; Ukidve, A.; Kim, J.; Mitragotri, S. Targeting strategies for tissue-specific drug delivery. Cell 2020, 181, 151–167. [Google Scholar] [CrossRef]
- Wu, J. The enhanced permeability and retention (EPR) effect: The significance of the concept and methods to enhance its application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Sharma, A.R.; Lee, S.S.; Bhattacharya, M.; Nam, J.S.; Chakraborty, C. Advances in nanocarriers enabled brain targeted drug delivery across blood brain barrier. Int. J. Pharm. 2019, 559, 360–372. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hong, W.; Ren, W.; Xu, T.; Qian, Z.; He, Z. Recent progress in targeted delivery vectors based on biomimetic nanoparticles. Signal Transduct. Target. Ther. 2021, 6, 225. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.; Ke, W.; Dirisala, A.; Toh, K.; Tanaka, M.; Li, J. Stealth and pseudo-stealth nanocarriers. Adv. Drug Deliv. Rev. 2023, 198, 114895. [Google Scholar] [CrossRef]
- Manzari, M.T.; Shamay, Y.; Kiguchi, H.; Rosen, N.; Scaltriti, M.; Heller, D.A. Targeted drug delivery strategies for precision medicines. Nat. Rev. Mater. 2021, 6, 351–370. [Google Scholar] [CrossRef]
- Finbloom, J.A.; Sousa, F.; Stevens, M.M.; Desai, T.A. Engineering the drug carrier biointerface to overcome biological barriers to drug delivery. Adv. Drug Deliv. Rev. 2020, 167, 89–108. [Google Scholar] [CrossRef]
- Di, J.; Gao, X.; Du, Y.; Zhang, H.; Gao, J.; Zheng, A. Size, shape, charge and “stealthy” surface: Carrier properties affect the drug circulation time in vivo. Asian J. Pharm. Sci. 2021, 16, 444–458. [Google Scholar] [CrossRef]
- Trucillo, P. Drug carriers: Classification, administration, release profiles, and industrial approach. Processes 2021, 9, 470. [Google Scholar] [CrossRef]
- Yoo, J.; Park, C.; Yi, G.; Lee, D.; Koo, H. Active targeting strategies using biological ligands for nanoparticle drug delivery systems. Cancers 2019, 11, 640. [Google Scholar] [CrossRef]
- Nelemans, L.C.; Gurevich, L. Drug delivery with polymeric nanocarriers—Cellular uptake mechanisms. Materials 2020, 13, 366. [Google Scholar] [CrossRef]
- Li, J.; Wang, Q.; Xia, G.; Adilijiang, N.; Li, Y.; Hou, Z.; Fan, Z.; Li, J. Recent Advances in Targeted Drug Delivery Strategy for Enhancing Oncotherapy. Pharmaceutics 2023, 15, 2233. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S. Natural polymers vs. synthetic polymer. In Natural Polymer Drug Delivery Systems: Nanoparticles, Plants, and Algae; Bhatia, S., Ed.; Springer: New York, NY, USA, 2016; pp. 95–118. [Google Scholar]
- Mogoşanu, G.D.; Grumezescu, A.M.; Bejenaru, L.E.; Bejenaru, C. Chapter 8—Natural and synthetic polymers for drug delivery and targeting. In Nanobiomaterials in Drug Delivery. Applications of Nanobiomaterials; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 229–284. [Google Scholar]
- Tong, X.; Pan, W.; Su, T.; Zhang, M.; Dong, W.; Qi, X. Recent advances in natural polymer-based drug delivery systems. React. Funct. Polym. 2020, 148, 104501. [Google Scholar] [CrossRef]
- George, A.; Shah, P.A.; Shrivastav, P.S. Natural biodegradable polymers based nano-formulations for drug delivery: A review. Int. J. Pharm. 2019, 561, 244–264. [Google Scholar] [CrossRef] [PubMed]
- Raveendran, S.; Rochani, A.K.; Maekawa, T.; Kumar, D.S. Smart carriers and nanohealers: A nanomedical insight on natural polymers. Materials 2017, 10, 929. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yang, F.; Xiong, F.; Gu, N. The smart drug delivery system and its clinical potential. Theranostics 2016, 6, 1306–1323. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Khan, M.; Umar, M.N.; Oh, D.H. Nanobiotechnology and its applications in drug delivery system: A review. IET Nanobiotechnol. 2015, 9, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Zhou, G.; Wen, Y.; Ye, J.; Li, X.; Wang, X. Recent advances on stimuli-responsive biopolymer-based nanocomposites for drug delivery. Compos. B Eng. 2023, 266, 111018. [Google Scholar] [CrossRef]
- Omanovic-Miklicanin, E.; Badnjević, A.; Kazlagić, A.; Hajlovac, M. Nanocomposites: A brief review. Health Technol. 2020, 10, 51–59. [Google Scholar] [CrossRef]
- Chen, J.; Ashames, A.; Buabeid, M.A.; Fahelelbom, K.M.; Ijaz, M.; Murtaza, G. Nanocomposites drug delivery systems for the healing of bone fractures. Int. J. Pharm. 2020, 585, 119477. [Google Scholar] [CrossRef]
- Jayakumar, A.; Mathew, S.; Radoor, S.; Kim, J.T.; Rhim, J.; Siengchin, S. Recent advances in two-dimensional nanomaterials: Properties, antimicrobial, and drug delivery application of nanocomposites. Mater. Today Chem. 2023, 30, 101492. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Inamuddin, A.; Mohammad, A. Applications of Nanocomposite Materials in Drug Delivery Sawston; Woodhead Publishing: Sawston, UK, 2018; pp. 509–573. [Google Scholar]
- Harugade, A.; Sherje, A.P.; Pethe, A. Chitosan: A review on properties, biological activities and recent progress in biomedical applications. React. Funct. Polym. 2023, 191, 105634. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Rodrigues, J.; Pan, S.; Danquah, M.K. Chapter 8—Cellulose-based bionanocomposites: Synthesis, properties, and applications. In Advances in Bionanocomposites. Materials, Applications, and Life Cycle. Micro and Nano Technologies; Sharma, B., Thomas, S., Bajpai, P.K., Ghosal, K., Shekhar, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 191–210. [Google Scholar]
- Kang, J.H.; Ko, Y.T. Lipid-coated gold nanocomposites for enhanced cancer therapy. Int. J. Nanomed. 2015, 10, 33–45. [Google Scholar]
- Puglia, C.; Lauro, M.R.; Tirendi, G.G.; Fassari, G.E.; Carbone, C.; Bonina, F.; Puglisi, G. Modern drug delivery strategies applied to natural active compounds. Expert Opin. Drug Deliv. 2017, 14, 755–768. [Google Scholar] [CrossRef]
- Kumari, A.; Singla, R.; Guliani, A.; Yadav, S.K. Nanoencapsulation for drug delivery. EXCLI J. 2014, 13, 265. [Google Scholar]
- Amiri, M.; Khazaeli, P.; Salehabadi, A.; Salavati-Niasari, M. Hydrogel beads-based nanocomposites in novel drug delivery platforms: Recent trends and developments. Adv. Colloid Interface Sci. 2021, 288, 102316. [Google Scholar] [CrossRef]
- Mumtaz, S.; Khattak, S.; Rehman, F.U.; Muhammad, P.; Hanif, S. Chapter 13—Bionanocomposites as a new platform for drug delivery systems. In Woodhead Publishing Series in Biomaterials. Novel Platforms for Drug Delivery Applications; Das, S., Thomas, S., Das, P.P., Eds.; Woodhead Publishing: Cambridge, UK, 2023; pp. 289–315. [Google Scholar]
- Çalış, S.; Atar, K.Ö.; Arslan, F.B.; Eroğlu, H.; Çapan, Y. Chapter 4—Nanopharmaceuticals as Drug-Delivery Systems: For, Against, and Current Applications. In Micro and Nano Technologies. Nanocarriers for Drug Delivery; Mohapatra, S.S., Ranjan, S., Dasgupta, N., Mishra, R.K., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 133–154. [Google Scholar]
- Seo, Y.; Lim, H.; Park, H.; Yu, J.; An, J.; Yoo, H.Y.; Lee, T. Recent Progress of Lipid Nanoparticles-Based Lipophilic Drug Delivery: Focus on Surface Modifications. Pharmaceutics 2023, 15, 772. [Google Scholar] [CrossRef]
- Manning, S.R. Microalgal lipids: Biochemistry and biotechnology. Curr. Opin. Biotechnol. 2022, 74, 1–7. [Google Scholar] [CrossRef]
- Guo, R.; Chen, Y.; Borgard, H.; Jijiwa, M.; Nasu, M.; He, M.; Deng, Y. The Function and Mechanism of Lipid Molecules and Their Roles in The Diagnosis and Prognosis of Breast Cancer. Molecules 2020, 25, 4864. [Google Scholar] [CrossRef]
- Kerr, B.J.; Kellner, T.A.; Shurson, G.C. Characteristics of lipids and their feeding value in swine diets. J. Anim. Sci. Biotechnol. 2015, 6, 1–23. [Google Scholar] [CrossRef]
- Angellotti, G.; Presentato, A.; Murgia, D.; Di Prima, G.; D’Agostino, F.; Scarpaci, A.G.; D’Oca, M.C.; Alduina, R.; Campisi, G.; De Caro, V. Lipid Nanocarriers-Loaded Nanocomposite as a Suitable Platform to Release Antibacterial and Antioxidant Agents for Immediate Dental Implant Placement Restorative Treatment. Pharmaceutics 2021, 13, 2072. [Google Scholar] [CrossRef] [PubMed]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef] [PubMed]
- Nakhaei, P.; Margiana, R.; Bokov, D.O.; Abdelbasset, W.K.; Jadidi Kouhbanani, M.A.; Varma, R.S.; Marofi, F.; Jarahian, M.; Beheshtkhoo, N. Liposomes: Structure, Biomedical Applications, and Stability Parameters with Emphasis on Cholesterol. Front. Bioeng. Biotechnol. 2021, 9, 705886. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M.; Gholami, T.; Amiri, O.; Pardakhti, A.; Ahmadi, M.; Akbari, A.; Salavati-Niasari, M. The magnetic inorganic-organic nanocomposite based on ZnFe2O4-Imatinib-liposome for biomedical applications, in vivo and in vitro study. J. Alloys Compd. 2020, 849, 156604. [Google Scholar] [CrossRef]
- Ding, Q.; Ding, C.; Liu, X.; Zheng, Y.; Zhao, Y.; Zhang, S.; Liu, W. Preparation of nanocomposite membranes loaded with taxifolin liposome and its mechanism of wound healing in diabetic mice. Int. J. Biol. Macromol. 2023, 241, 124537. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhu, X.; Zhang, M.; Jiang, X.; Guo, W.; Jiang, F.; Cao, F. In vitro and in vivo assessment of structural integrity for HPCD complex@ Liposome nanocomposites from ocular surface to the posterior segment of the eye. Carbohydr. Polym. 2023, 315, 120960. [Google Scholar] [CrossRef]
- Justine, R.Y.; Janssen, M.; Liang, B.J.; Huang, H.C.; Fisher, J.P. A liposome/gelatin methacrylate nanocomposite hydrogel system for delivery of stromal cell-derived factor-1α and stimulation of cell migration. Acta Biomater. 2020, 108, 67–76. [Google Scholar]
- Zhao, Y.; Zhao, J.; Shan, G.; Yan, D.; Chen, Y.; Liu, Y. SERS-active liposome@ Ag/Au nanocomposite for NIR light-driven drug release. Colloids Surf. B Biointerfaces 2017, 154, 150–159. [Google Scholar] [CrossRef]
- Zhang, N.; Wu, Y.; Xu, W.; Li, Z.; Wang, L. Synergic fabrication of multifunctional liposomes nanocomposites for improved radiofrequency ablation combination for liver metastasis cancer therapy. Drug Deliv. 2022, 29, 506–518. [Google Scholar] [CrossRef]
- Nguyen, V.D.; Min, H.; Kim, C.; Han, J.; Park, J.; Choi, E. Folate receptor-targeted liposomal nanocomplex for effective synergistic photothermal-chemotherapy of breast cancer in vivo. Colloids Surf. B Biointerfaces 2019, 173, 539–548. [Google Scholar] [CrossRef]
- Yu, J.Y.; Chuesiang, P.; Shin, G.H.; Park, H.J. Post-processing techniques for the improvement of liposome stability. Pharmaceutics 2021, 13, 1023. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of liposomes as drug delivery system for therapeutic applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef] [PubMed]
- Šturm, L.; Poklar Ulrih, N. Basic Methods for Preparation of Liposomes and Studying Their Interactions with Different Compounds, with the Emphasis on Polyphenols. Int. J. Mol. Sci. 2021, 22, 6547. [Google Scholar] [CrossRef] [PubMed]
- Sawaftah, N.A.; Paul, V.; Awad, N.; Husseini, G.A. Modeling of Anti-Cancer Drug Release Kinetics from Liposomes and Micelles: A Review. IEEE Trans. 2021, 20, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Zahednezhad, F.; Saadat, M.; Valizadeh, H.; Zakeri-Milani, P.; Baradaran, B. Liposome and immune system interplay: Challenges and potentials. J. Control. Release 2019, 305, 194–209. [Google Scholar] [CrossRef] [PubMed]
- Inglut, C.T.; Sorrin, A.J.; Kuruppu, T.; Vig, S.; Cicalo, J.; Ahmad, H.; Huang, H.C. Immunological and toxicological considerations for the design of liposomes. Nanomaterials 2020, 10, 190. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Zhang, T.; Wang, C.; Huang, Z.; Luo, X.; Deng, Y. A review on phospholipids and their main applications in drug delivery systems. Asian J. Pharm. Sci. 2015, 10, 81–98. [Google Scholar] [CrossRef]
- Sakdiset, P.; Okada, A.; Todo, H.; Sugibayashi, K. Selection of phospholipids to design liposome preparations with high skin penetration-enhancing effects. J. Drug Deliv. Sci. Technol. 2018, 44, 58–64. [Google Scholar] [CrossRef]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, composition, types, and clinical applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef]
- Hörmann, K.; Zimmer, A. Drug delivery and drug targeting with parenteral lipid nanoemulsions—A review. J. Control. Release 2016, 223, 85–98. [Google Scholar] [CrossRef]
- Parchekani, J.; Allahverdi, A.; Taghdir, M.; Naderi-Manesh, H. Design and simulation of the liposomal model by using a coarse-grained molecular dynamics approach towards drug delivery goals. Sci. Rep. 2022, 12, 2371. [Google Scholar] [CrossRef] [PubMed]
- Sabjan, K.B.; Munawar, S.M.; Rajendiran, D.; Vinoji, S.K.; Kasinathan, K. Nanoemulsion as Oral Drug Delivery—A Review. Curr. Drug Res. Rev. 2020, 12, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Andretto, V.; Taurino, G.; Guerriero, G.; Guérin, H.; Lainé, E.; Bianchi, M.G.; Lollo, G. Nanoemulsions Embedded in Alginate Beads as Bioadhesive Nanocomposites for Intestinal Delivery of the Anti-Inflammatory Drug Tofacitinib. Biomacromolecules 2023, 24, 2892–2907. [Google Scholar] [CrossRef] [PubMed]
- Hinger, D.; Navarro, F.; Käch, A.; Thomann, J.S.; Mittler, F.; Couffin, A.C.; Maake, C. Photoinduced effects of m-tetrahydroxyphenylchlorin loaded lipid nanoemulsions on multicellular tumor spheroids. J. Nanobiotechnol. 2016, 14, 68. [Google Scholar] [CrossRef] [PubMed]
- Samadi, A.; Pourmadadi, M.; Yazdian, F.; Rashedi, H.; Navaei-Nigjeh, M. Ameliorating quercetin constraints in cancer therapy with pH-responsive agarose-polyvinylpyrrolidone-hydroxyapatite nanocomposite encapsulated in double nanoemulsion. Int. J. Biol. Macromol. 2021, 182, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, H.; Pourmadadi, M.; Abdouss, M.; Rahdar, A.; Díez-Pascual, A.M. Formulation of double nanoemulsions based on pH-sensitive poly acrylic acid/agarose/ZnO for quercetin controlled release. J. Mol. Liq. 2023, 391, 123363. [Google Scholar] [CrossRef]
- Shamsabadipour, A.; Pourmadadi, M.; Rashedi, H.; Yazdian, F.; Navaei-Nigjeh, M. Nanoemulsion carriers of porous γ-alumina modified by polyvinylpyrrolidone and carboxymethyl cellulose for pH-sensitive delivery of 5-fluorouracil. Int. J. Biol. Macromol. 2023, 233, 123621. [Google Scholar] [CrossRef]
- Manzoor, M.; Sharma, P.; Murtaza, M.; Jaiswal, A.K.; Jaglan, S. Fabrication, characterization, and interventions of protein, polysaccharide and lipid-based nanoemulsions in food and nutraceutical delivery applications: A review. Int. J. Biol. Macromol. 2023, 241, 124485. [Google Scholar] [CrossRef]
- Mushtaq, A.; Wani, S.M.; Malik, A.R.; Gull, A.; Ramniwas, S.; Nayik, G.A.; Ercisli, S.; Marc, R.A.; Bari, A. Recent insights into Nanoemulsions: Their preparation, properties and applications. Food Chem. X 2023, 18, 100684. [Google Scholar] [CrossRef]
- Choi, S.J.; McClements, D.J. Nanoemulsions as delivery systems for lipophilic nutraceuticals: Strategies for improving their formulation, stability, functionality and bioavailability. Food Sci. Biotechnol. 2020, 29, 149–168. [Google Scholar] [CrossRef]
- Singh, Y.; Meher, J.G.; Raval, K.; Khan, F.A.; Chaurasia, M.; Jain, N.K.; Chourasia, M.K. Nanoemulsion: Concepts, development and applications in drug delivery. J. Control. Release 2017, 252, 28–49. [Google Scholar] [CrossRef]
- Marhamati, M.; Ranjbar, G.; Rezaie, M. Effects of emulsifiers on the physicochemical stability of Oil-in-water Nanoemulsions: A critical review. J. Mol. Liq. 2021, 340, 117218. [Google Scholar] [CrossRef]
- Sen Gupta, S.; Ghosh, M. Formulation development and process parameter optimization of lipid nanoemulsions using an alginate-protein stabilizer. J. Food Sci. Technol. 2015, 52, 2544–2557. [Google Scholar] [CrossRef][Green Version]
- Lingayat, V.J.; Zarekar, N.S.; Shendge, R.S. Solid lipid nanoparticles: A review. Nanosci. Nanotechnol. Res. 2017, 4, 67–72. [Google Scholar]
- Samiun, W.S.; Ashari, S.E.; Salim, N.; Ahmad, S. Optimization of Processing Parameters of Nanoemulsion Containing Aripiprazole Using Response Surface Methodology. Int. J. Nanomed. 2020, 15, 1585–1594. [Google Scholar] [CrossRef]
- Naseri, N.; Valizadeh, H.; Zakeri-Milani, P. Solid lipid nanoparticles and nanostructured lipid carriers: Structure, preparation and application. Adv. Pharm. Bull. 2015, 5, 305. [Google Scholar] [CrossRef]
- Beloqui, A.; Solinís, M.Á.; Rodríguez-Gascón, A.; Almeida, A.J.; Préat, V. Nanostructured lipid carriers: Promising drug delivery systems for future clinics. Nanomedicine 2016, 12, 143–161. [Google Scholar] [CrossRef]
- Vigani, B.; Valentino, C.; Sandri, G.; Listro, R.; Fagiani, F.; Collina, S.; Ferrari, F. A composite nanosystem as a potential tool for the local treatment of glioblastoma: Chitosan-coated solid lipid nanoparticles embedded in electrospun nanofibers. Polymers 2021, 13, 1371. [Google Scholar] [CrossRef]
- Shu, X.; Liu, J.; Mao, L.; Yuan, F.; Gao, Y. Composite hydrogels filled with rhamnolipid-based nanoemulsion, nanostructured lipid carrier, or solid lipid nanoparticle: A comparative study on gel properties and the delivery of lutein. Food Hydrocoll. 2024, 146, 109264. [Google Scholar] [CrossRef]
- Gordillo-Galeano, A.; Mora-Huertas, C.E. Solid lipid nanoparticles and nanostructured lipid carriers: A review emphasizing on particle structure and drug release. Eur. J. Pharm. Biopharm. 2018, 133, 285–308. [Google Scholar] [CrossRef]
- Bunjes, H. Structural properties of solid lipid based colloidal drug delivery systems. Curr. Opin. Colloid Interface Sci. 2011, 16, 405–411. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Khan, R.A.; Singh, V.; Yusuf, M.; Akhtar, N.; Sulaiman, G.M.; Albukhaty, S.; Abdellatif, A.A.H.; Khan, M.; Mohammed, S.A.A.; et al. Solid lipid nanoparticles for targeted natural and synthetic drugs delivery in high-incidence cancers, and other diseases: Roles of preparation methods, lipid composition, transitional stability, and release profiles in nanocarriers’ development. Nanotechnol. Rev. 2023, 12, 20220517. [Google Scholar] [CrossRef]
- Sakellari, G.; Zafeiri, J.; Batchelor, H.; Spyropoulos, F. Solid lipid nanoparticles and nanostructured lipid carriers of dual functionality at emulsion interfaces. Part I: Pickering stabilization functionality. Colloids Surf. A Physicochem. Eng. Asp. 2022, 654, 130135. [Google Scholar] [CrossRef]
- Azhar Shekoufeh Bahari, L.; Hamishehkar, H. The Impact of Variables on Particle Size of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers; A Comparative Literature Review. Adv. Pharm. Bull. 2016, 6, 143–151. [Google Scholar] [CrossRef]
- Guo, X.; Wang, Y.; Qin, Y.; Shen, P.; Peng, Q. Structures, properties and application of alginic acid: A review. Int. J. Biol. Macromol. 2020, 162, 618–628. [Google Scholar] [CrossRef]
- Taubner, T.; Marounek, M.; Synytsya, A. Preparation and characterization of amidated derivatives of alginic acid. Int. J. Biol. Macromol. 2017, 103, 202–207. [Google Scholar] [CrossRef]
- Kashif, M.; Ngaini, Z.; Harry, A.V.; Vekariya, R.L.; Ahmad, A.; Zuo, Z.; Sahari, S.K.; Hussain, S.; Khan, Z.A.; Alarifi, A. An experimental and DFT study on novel dyes incorporated with natural dyes on titanium dioxide (TiO2) towards solar cell application. Appl. Phys. A 2020, 126, 716. [Google Scholar] [CrossRef]
- Ahmad, A.; Mubarak, N.M.; Jannat, F.T.; Ashfaq, T.; Santulli, C.; Rizwan, M.; Najda, A.; Bin-Jumah, M.; Abdel-Daim, M.M.; Hussain, S.; et al. A Critical Review on the Synthesis of Natural Sodium Alginate Based Composite Materials: An Innovative Biological Polymer for Biomedical Delivery Applications. Processes 2021, 9, 137. [Google Scholar] [CrossRef]
- Yang, J.; Pan, J. Hydrothermal synthesis of silver nanoparticles by sodium alginate and their applications in surface-enhanced Raman scattering and catalysis. Acta Mater. 2012, 60, 4753–4758. [Google Scholar] [CrossRef]
- Jadach, B.; Świetlik, W.; Froelich, A. Sodium Alginate as a Pharmaceutical Excipient: Novel Applications of a Well-known Polymer. J. Pharm. Sci. 2022, 111, 1250–1261. [Google Scholar] [CrossRef]
- Karim, A.; Rehman, A.; Feng, J.; Noreen, A.; Assadpour, E.; Kharazmi, M.S.; Lianfu, Z.; Jafari, S.M. Alginate-based nanocarriers for the delivery and controlled-release of bioactive compounds. Adv. Colloid Interface Sci. 2022, 307, 102744. [Google Scholar] [CrossRef]
- Abourehab, M.A.S.; Rajendran, R.R.; Singh, A.; Pramanik, S.; Shrivastav, P.; Ansari, M.J.; Manne, R.; Amaral, L.S.; Deepak, A. Alginate as a Promising Biopolymer in Drug Delivery and Wound Healing: A Review of the State-of-the-Art. Int. J. Mol. Sci. 2022, 23, 9035. [Google Scholar] [CrossRef]
- Hegde, V.; Uthappa, U.T.; Altalhi, T.; Jung, H.; Han, S.S.; Kurkuri, M.D. Alginate based polymeric systems for drug delivery, antibacterial/microbial, and wound dressing applications. Mater. Today Commun. 2022, 33, 104813. [Google Scholar] [CrossRef]
- Raus, R.A.; Nawawi, W.M.F.W.; Nasaruddin, R.R. Alginate and alginate composites for biomedical applications. Asian J. Pharm. Sci. 2021, 16, 280–306. [Google Scholar] [CrossRef]
- Venkatasubbu, G.D.; Ramasamy, S.; Ramakrishnan, V.; Kumar, J. Hydroxyapatite-alginate nanocomposite as drug delivery matrix for sustained release of ciprofloxacin. J. Biomed. Nanotechnol. 2011, 7, 759–767. [Google Scholar] [CrossRef]
- Soumia, A.; Adel, M.; Amina, S.; Bouhadjar, B.; Amal, D.; Farouk, Z.; Abdelkader, B.; Mohamed, S. Fe3O4-alginate nanocomposite hydrogel beads material: One-pot preparation, release kinetics and antibacterial activity. Int. J. Biol. Macromol. 2020, 145, 466–475. [Google Scholar] [CrossRef]
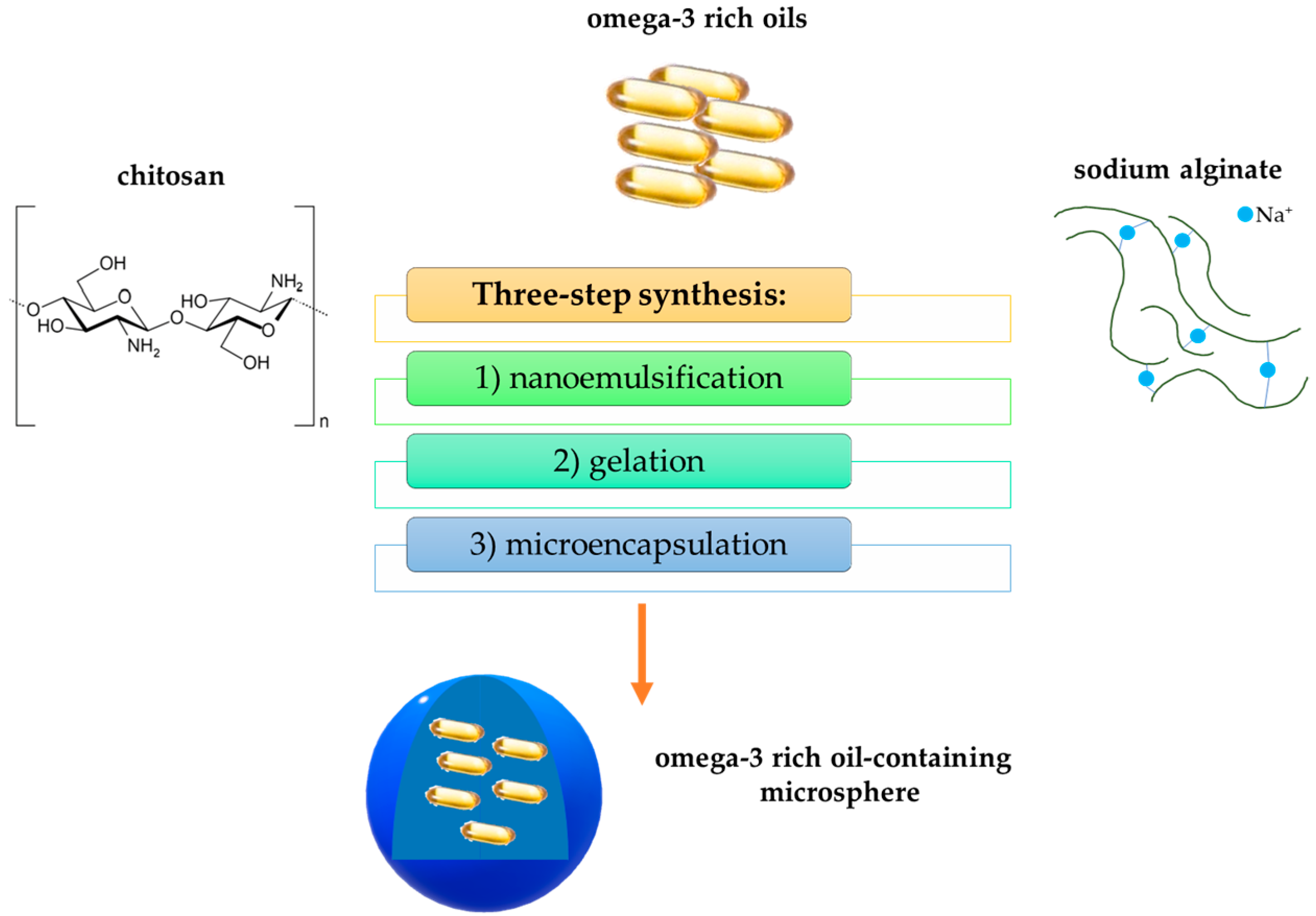
- Hamed, S.F.; Hashim, A.F.; Hamid, H.A.A.; Abd-Elsalam, K.A.; Golonka, I.; Musiał, W.; El-Sherbiny, I.M. Edible alginate/chitosan-based nanocomposite microspheres as delivery vehicles of omega-3 rich oils. Carbohydr. Polym. 2020, 239, 116201. [Google Scholar] [CrossRef]
- Yu, X.; Wen, T.; Cao, P.; Shan, L.; Li, L. Alginate-chitosan coated layered double hydroxide nanocomposites for enhanced oral vaccine delivery. J. Colloid Interface Sci. 2019, 556, 258–265. [Google Scholar] [CrossRef]
- Malesu, V.K.; Sahoo, D.; Nayak, P.L. Chitosan-Sodium Alginate Nanocomposites Blended with Cloisite 30B As a Novel Drug Delivery System for Anticancer Drug Curcumin. Int. J. Appl. Biol. Pharm. 2011, 2, 402–411. [Google Scholar]
- Nguyen-Ngo, C.; Willcox, J.C.; Lappas, M. Anti-inflammatory effects of phenolic acids punicalagin and curcumin in human placenta and adipose tissue. Placenta 2020, 100, 1–12. [Google Scholar] [CrossRef]
- Cui, J.; Zhou, J.; Huang, L.; Jing, J.; Wang, N.; Wang, L. Curcumin encapsulation and protection based on lysozyme nanoparticles. Food Sci. Nutr. 2019, 7, 2702–2707. [Google Scholar] [CrossRef]
- Chegeni, M.; Rozbahani, Z.S.; Ghasemian, M.; Mehri, M. Synthesis and application of the calcium alginate/SWCNT-Gl as a bio-nanocomposite for the curcumin delivery. Int. J. Biol. Macromol. 2020, 156, 504–513. [Google Scholar] [CrossRef]
- El-Din, H.M.N.; Ibraheim, D.M.; Rabie, A.G.M. Characterization and drug delivery characters of nanocomposite hydrogels based on gamma-radiation copolymerization of poly (vinyl pyrrolidone) (PVP)/sodium alginate (AG)/silver NPs. Int. J. Biol. Macromol. 2023, 234, 123674. [Google Scholar]
- Kim, J.; Hlaing, S.P.; Lee, J.; Saparbayeva, A.; Kim, S.; Hwang, D.S.; Lee, E.H.; Yoon, I.; Yun, H.; Kim, M.; et al. Exfoliated bentonite/alginate nanocomposite hydrogel enhances intestinal delivery of probiotics by resistance to gastric pH and on-demand disintegration. Carbohydr. Polym. 2021, 272, 118462. [Google Scholar] [CrossRef]
- Khushbu; Jindal, R. RSM-CCD optimized microwave assisted synthesis of chitosan and sodium alginate based nanocomposite containing inclusion complexes of β-cyclodextrin and amlodipine besylate for sustained drug delivery systems. J. Drug Deliv. Sci. Technol. 2021, 61, 102325. [Google Scholar] [CrossRef]
- Fan, Y.; Wu, W.; Lei, Y.; Gaucher, C.; Pei, S.; Zhang, J.; Xia, X. Edaravone-Loaded Alginate-Based Nanocomposite Hydrogel Accelerated Chronic Wound Healing in Diabetic Mice. Mar. Drugs 2019, 17, 285. [Google Scholar] [CrossRef]
- Agili, F.A.; Aly, S.F.M. Physicochemical characterization and release properties of oral drug delivery: A pH-sensitive nanocomposite based on sodium alginate–pectin–tannic acid–silver. Polym. Polym. Compos. 2020, 28, 598–608. [Google Scholar] [CrossRef]
- Shabanpour, S.; Shariati, F.P.; Khatibani, A.B. Potential Alendronate Sodium drug carrier by preparation and characterization of sodium alginate cross-linked Montmorillonite. Braz. J. Pharm. Sci. 2022, 58, 20243. [Google Scholar] [CrossRef]
- Evelyna, A.; Astifanni, T.K.; Ruth, I.; Asri, L.; Purwasasmita, B.S. Preparation of Nanocellulose-Alginate Nanocomposites for Chlorhexidine Digluconate Drug Carrier. IOP Conf. Ser. 2019, 547, 012046. [Google Scholar] [CrossRef]
- Lakkakula, J.; Roy, A.; Krishnamoorthy, K.; Alghamdi, S.; Almehmadi, M.; Gujarathi, P.; Pansare, P.; Allahyani, M.; Abdulaziz, O.; Velhal, K.; et al. Alginate-Based Nanosystems for Therapeutic Applications. J. Nanomater. 2022, 2022, 6182815. [Google Scholar] [CrossRef]
- Iliescu, R.I.; Andronescu, E.; Ghitulica, C.D.; Voicu, G.; Ficai, A.; Hoteteu, M. Montmorillonite–alginate nanocomposite as a drug delivery system—incorporation and in vitro release of irinotecan. Int. J. Pharm. 2014, 463, 184–192. [Google Scholar] [CrossRef]
- Lei, H.; Xie, M.; Zhao, Y.; Zhang, F.; Xu, Y.; Xie, J. Chitosan/sodium alginate modificated graphene oxide-based nanocomposite as a carrier for drug delivery. Ceram. Int. 2016, 42, 17798–17805. [Google Scholar] [CrossRef]
- Iliescu, R.I.; Andronescu, E.; Ghiţulică, C.D.; Berger, D.; Ficai, A. Montmorillonite-alginate nanocomposite beads as drug carrier for oral administration of carboplatin—preparation and characterization. U.P.B. Sci. Bull. Ser. B 2011, 73, 3–16. [Google Scholar]
- Rosiak, P.; Latanska, I.; Paul, P.; Sujka, W.; Kolesinska, B. Modification of Alginates to Modulate Their Physic-Chemical Properties and Obtain Biomaterials with Different Functional Properties. Molecules 2021, 26, 7264. [Google Scholar] [CrossRef]
- Goh, C.H.; Heng, P.W.S.; Chan, L.W. Alginates as a useful natural polymer for microencapsulation and therapeutic applications. Carbohydr. Polym. 2012, 88, 1–12. [Google Scholar] [CrossRef]
- Kroneková, Z.; Pelach, M.; Mazancová, P.; Uhelská, L.; Treľová, D.; Rázga, F.; Némethová, V.; Szalai, S.; Chorvát, D.; McGarrigle, J.J.; et al. Structural changes in alginate-based microspheres exposed to in vivo environment as revealed by confocal Raman microscopy. Sci. Rep. 2018, 8, 1637. [Google Scholar] [CrossRef]
- Abka-Khajouei, R.; Tounsi, L.; Shahabi, N.; Patel, A.K.; Abdelkafi, S.; Michaud, P. Structures, Properties and Applications of Alginates. Mar. Drugs 2022, 20, 364. [Google Scholar] [CrossRef]
- Hasnain, S.; Jameel, E.; Mohanta, B.; Dhara, A.K.; Alkahtani, S.; Nayak, A.K. Chapter 1—Alginates: Sources, structure, and properties. In Alginates in Drug Delivery; Nayak, A.K., Hasnain, S., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 1–17. [Google Scholar]
- Wang, B.-T.; Hu, S.; Yu, X.-Y.; Jin, L.; Zhu, Y.-J.; Jin, F.-J. Studies of Cellulose and Starch Utilization and the Regulatory Mechanisms of Related Enzymes in Fungi. Polymers 2020, 12, 530. [Google Scholar] [CrossRef]
- Moreira, L.R.; Filho, E.X. Insights into the mechanism of enzymatic hydrolysis of xylan. Appl. Microbiol. Biotechnol. 2016, 100, 5205–5214. [Google Scholar] [CrossRef]
- Zhang, C.; Keten, S.; Derome, D.; Carmeliet, J. Hydrogen bonds dominated frictional stick-slip of cellulose nanocrystals. Carbohydr. Polym. 2021, 258, 117682. [Google Scholar] [CrossRef]
- Chang, S.; Weng, Z.; Zhang, C.; Jiang, S.; Duan, G. Cellulose-Based Intelligent Responsive Materials: A Review. Polymers 2023, 15, 3905. [Google Scholar] [CrossRef]
- Ningtyas, K.R.; Agassi, T.N.; Putri, P.G. Utilization of Waste Cellulose Raw Material for Making Paper Pulp. IOP Conf. Ser. 2022, 1012, 012091. [Google Scholar]
- Mäkelä, M.; Rissanen, M.; Sixta, H. Identification of cellulose textile fibers. Analyst 2021, 146, 7503–7509. [Google Scholar] [CrossRef]
- Chen, C.; Xi, Y.; Weng, Y. Recent Advances in Cellulose-Based Hydrogels for Tissue Engineering Applications. Polymers 2022, 14, 3335. [Google Scholar] [CrossRef]
- Joseph, B.; Sagarika, V.K.; Sabu, C.; Kalarikkal, N.; Sabu, T. Cellulose nanocomposites: Fabrication and biomedical applications. J. Bioresour. Bioprod. 2020, 5, 223–237. [Google Scholar] [CrossRef]
- Huo, Y.; Liu, Y.; Xia, M.; Du, H.; Lin, Z.; Li, B.; Liu, H. Nanocellulose-Based Composite Materials Used in Drug Delivery Systems. Polymers 2022, 14, 2648. [Google Scholar] [CrossRef]
- Prusty, K.; Swain, S.K. Release of ciprofloxacin drugs by nano gold embedded cellulose grafted polyacrylamide hybrid nanocomposite hydrogels. Int. J. Biol. Macromol. 2019, 126, 765–775. [Google Scholar] [CrossRef]
- Ji, L.; Zhang, F.; Zhu, L.; Jiang, J. An in-situ fabrication of bamboo bacterial cellulose/sodium alginate nanocomposite hydrogels as carrier materials for controlled protein drug delivery. Int. J. Biol. Macromol. 2021, 170, 459–468. [Google Scholar] [CrossRef]
- Rana, A.K.; Scarpa, F.; Thakur, V.K. Cellulose/polyaniline hybrid nanocomposites: Design, fabrication, and emerging multidimensional applications. Ind. Crops Prod. 2022, 187, 115356. [Google Scholar] [CrossRef]
- Shahzadi, I.; Islam, M.; Saeed, H.; Shahzadi, A.; Haider, J.; Haider, A.; Imran, M.; Rathore, H.A.; Ul-Hamid, A.; Nabgan, W.; et al. Facile synthesis of copolymerized cellulose grafted hydrogel doped calcium oxide nanocomposites with improved antioxidant activity for anti-arthritic and controlled release of doxorubicin for anti-cancer evaluation. Int. J. Biol. Macromol. 2023, 235, 123874. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Mohamed, A.S.; El-Sherbeeny, A.M.; Nadeem, A.; Ahmad, S.F. Synthesis of exfoliate bentonite/cellulose nanocomposite as a delivery system for Oxaliplatin drug with enhanced loading and release properties; cytotoxicity and pharmacokinetic studies. Chem. Phys. Lett. 2020, 755, 137818. [Google Scholar] [CrossRef]
- Das, S.; Ghosh, B.; Sarkar, K. Nanocellulose as sustainable biomaterials for drug delivery. Sens. Int. 2022, 3, 100135. [Google Scholar] [CrossRef]
- Patil, T.V.; Patel, D.K.; Dutta, S.D.; Ganguly, K.; Santra, T.S.; Lim, K. Nanocellulose, a versatile platform: From the delivery of active molecules to tissue engineering applications. Bioact. Mater. 2022, 9, 566–589. [Google Scholar] [CrossRef] [PubMed]
- Gholamali, I.; Yadollahi, M. Doxorubicin-loaded carboxymethyl cellulose/Starch/ZnO nanocomposite hydrogel beads as an anticancer drug carrier agent. Int. J. Biol. Macromol. 2020, 160, 724–735. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, C.; Chowdhuri, A.R.; Kumar, A.; Laha, D.; Garai, S.; Chakraborty, J.; Sahu, S.K. One pot synthesis of carbon dots decorated carboxymethyl cellulose-hydroxyapatite nanocomposite for drug delivery, tissue engineering and Fe3+ ion sensing. Carbohydr. Polym. 2018, 181, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Rakhshaei, R.; Namazi, H.; Hamishehkar, H.; Rahimi, M. Graphene quantum dot cross-linked carboxymethyl cellulose nanocomposite hydrogel for pH-sensitive oral anticancer drug delivery with potential bioimaging properties. Int. J. Biol. Macromol. 2020, 150, 1121–1129. [Google Scholar] [CrossRef]
- Rasoulzadeh, M.; Namazi, H. Carboxymethyl cellulose/graphene oxide bio-nanocomposite hydrogel beads as anticancer drug carrier agent. Carbohydr. Polym. 2017, 168, 320–326. [Google Scholar] [CrossRef]
- Salahuddin, N.; Gaber, M.; Mousa, M.; Elfiky, M. Dopamine/Artesunate loaded polyhydroxybutyrate-g-cellulose- magnetite zinc oxide core shell nanocomposites: Synergistic antimicrobial and anticancer efficacy. Int. J. Biol. Macromol. 2023, 248, 125348. [Google Scholar] [CrossRef]
- Ghawanmeh, A.A.; Tan, L.L.; Ali, G.A.M.; Assiri, M.A.; Chong, K.F. Optimization of carboxymethyl cellulose-gum Arab-based hydrogel beads for anticancer drugs delivery. J. Mol. Liq. 2024, 393, 123631. [Google Scholar] [CrossRef]
- Ostovar, S.; Pourmadadi, M.; Zaker, M.A. Co-biopolymer of chitosan/carboxymethyl cellulose hydrogel improved by zinc oxide and graphene quantum dots nanoparticles as pH-sensitive nanocomposite for quercetin delivery to brain cancer treatment. Int. J. Biol. Macromol. 2023, 253, 127091. [Google Scholar] [CrossRef]
- Mandal, B.; Rameshbabu, A.P.; Dhara, S.; Pal, S. Nanocomposite hydrogel derived from poly (methacrylic acid)/carboxymethyl cellulose/AuNPs: A potential transdermal drugs carrier. Polymer 2017, 120, 9–19. [Google Scholar] [CrossRef]
- Alsaaed, F.A.T.; El-Lateef, H.M.A.; Khalaf, M.M.; Mohamed, I.M.A.; Al-Omair, M.A.; Gouda, M. Drug Delivery System Based on Carboxymethyl Cellulose Containing Metal-Organic Framework and Its Evaluation for Antibacterial Activity. Polymers 2022, 14, 3815. [Google Scholar] [CrossRef] [PubMed]
- Moon, R.J.; Schueneman, G.T.; Simonsen, J. Overview of cellulose nanomaterials, their capabilities and applications. JOM 2016, 68, 2383–2394. [Google Scholar] [CrossRef]
- Varghese, R.T.; Cherian, R.M.; Chirayil, C.J.; Antony, T.; Kargarzadeh, H.; Thomas, S. Nanocellulose as an Avenue for Drug Delivery Applications: A Mini-Review. J. Compos. Sci. 2023, 7, 210. [Google Scholar] [CrossRef]
- Chin, S.F.; Jimmy, F.B.; Pang, S.C. Size controlled fabrication of cellulose nanoparticles for drug delivery applications. J. Drug Deliv. Sci. Technol. 2018, 43, 262–266. [Google Scholar] [CrossRef]
- Mujtaba, M.; Negi, A.; King, A.W.T.; Zare, M.; Kuncova-Kallio, J. Surface modifications of nanocellulose for drug delivery applications; a critical review. Curr. Opin. Biomed. Eng. 2023, 28, 100475. [Google Scholar] [CrossRef]
- Chua, L.; Lim, P.; Thoo, Y.; Neo, Y.; Tan, T. Extraction and characterization of gelatin derived from acetic acid-treated black soldier fly larvae. Food Chem. Adv. 2023, 2, 100282. [Google Scholar] [CrossRef]
- Ahmad, M.I.; Li, Y.; Pan, J.; Liu, F.; Dai, H.; Fu, Y.; Huang, T.; Farooq, S.; Zhang, H. Collagen and gelatin: Structure, properties, and applications in food industry. Int. J. Biol. Macromol. 2024, 254, 128037. [Google Scholar] [CrossRef]
- Rather, J.A.; Akhter, N.; Ashraf, Q.S.; Mir, S.A.; Makroo, H.A.; Darakshan Majid, D.; Barba, F.J.; Khaneghah, A.M.; Dar, B.N. A comprehensive review on gelatin: Understanding impact of the sources, extraction methods, and modifications on potential packaging applications. Food Pack. Shelf Life 2022, 34, 100945. [Google Scholar] [CrossRef]
- Luo, Q.; Hossen, M.A.; Zeng, Y.; Dai, J.; Li, S.; Qin, W.; Liu, Y. Gelatin-based composite films and their application in food packaging: A review. J. Food Eng. 2022, 313, 110762. [Google Scholar] [CrossRef]
- Jiang, X.; Du, Z.; Zhang, X.; Zaman, F.; Song, Z.; Guan, Y.; Yu, T.; Huang, Y. Gelatin-based anticancer drug delivery nanosystems: A mini review. Front. Bioeng. Biotechnol. 2023, 11, 1158749. [Google Scholar] [CrossRef] [PubMed]
- Milano, F.; Masi, A.; Madaghiele, M.; Sannino, A.; Salvatore, L.; Gallo, N. Current Trends in Gelatin-Based Drug Delivery Systems. Pharmaceutics 2023, 15, 1499. [Google Scholar] [CrossRef]
- Dong, Z.; Meng, X.; Yang, W.; Zhang, J.; Sun, P.; Zhang, H.; Fang, X.; Wang, D.; Fan, C. Progress of gelatin-based microspheres (GMSs) as delivery vehicles of drug and cell. Mater. Sci. Eng. C 2021, 122, 111949. [Google Scholar] [CrossRef] [PubMed]
- Raj, V.; Prabha, G. Synthesis, characterization and in vitro drug release of cisplatin loaded Cassava starch acetate–PEG/gelatin nanocomposites. J. Assoc. Arab Univ. Basic Appl. Sci. 2016, 21, 10–16. [Google Scholar] [CrossRef]
- Najafabadi, A.P.; Pourmadadi, M.; Yazdian, F.; Rashedi, H.; Rahdar, A.; Díez-Pascual, A.M. pH-sensitive ameliorated quercetin delivery using graphene oxide nanocarriers coated with potential anticancer gelatin-polyvinylpyrrolidone nanoemulsion with bitter almond oil. J. Drug Deliv. Sci. Technol. 2023, 82, 104339. [Google Scholar] [CrossRef]
- Mdlovu, N.V.; Juang, R.; Weng, M.; Lin, K. Green synthesis and characterization of silicate nanostructures coated with Pluronic F127/gelatin for triggered drug delivery in tumor microenvironments. Int. J. Biol. Macromol. 2023, 251, 126337. [Google Scholar] [CrossRef] [PubMed]
- Ostovar, S.; Pourmadadi, M.; Shamsabadipour, A.; Mashayekh, P. Nanocomposite of chitosan/gelatin/carbon quantum dots as a biocompatible and efficient nanocarrier for improving the Curcumin delivery restrictions to treat brain cancer. Int. J. Biol. Macromol. 2023, 242, 124986. [Google Scholar] [CrossRef] [PubMed]
- Kulshrestha, A.; Sharma, S.; Singh, K.; Kumar, A. Magnetoresponsive biocomposite hydrogels comprising gelatin and valine based magnetic ionic liquid surfactant as controlled release nanocarrier for drug delivery. Mater. Adv. 2022, 3, 484. [Google Scholar] [CrossRef]
- Moya-Lopez, C.; Juan, A.; Donizeti, M.; Valcarcel, J.; Vazquez, J.A.; Solano, E.; Chapron, D.; Bourson, P.; Bravo, I.; Alonso-Moreno, C.; et al. Multifunctional PLA/Gelatin Bionanocomposites for Tailored Drug Delivery Systems. Pharmaceutics 2022, 14, 1138. [Google Scholar] [CrossRef]
- Gheysoori, P.; Paydayesh, A.; Jafari, M.; Peidayesh, H. Thermoresponsive nanocomposite hydrogels based on Gelatin/poly (N–isopropylacrylamide) (PNIPAM) for controlled drug delivery. Eur. Polym. J. 2023, 186, 111846. [Google Scholar] [CrossRef]
- Bhattacharyya, S.K.; Dule, M.; Paul, R.; Dash, J.; Anas, M.; Mandal, T.K.; Das, P.; Das, N.C.; Banerjee, S. Carbon Dot Cross-Linked Gelatin Nanocomposite Hydrogel for pH-Sensing and pH-Responsive Drug Delivery. ACS Biomater. Sci. Eng. 2020, 6, 5662–5674. [Google Scholar] [CrossRef] [PubMed]
- Bakravi, A.; Ahamadian, Y.; Hashemi, H.; Namazi, H. Synthesis of gelatin-based biodegradable hydrogel nanocomposite and their application as drug delivery agent. Adv. Polym. Technol. 2018, 37, 2625–2635. [Google Scholar] [CrossRef]
- Bora, A.; Sarmah, D.; Rather, M.A.; Mandal, M.; Karak, N. Nanocomposite of starch, gelatin and itaconic acid-based biodegradable hydrogel and ZnO/cellulose nanofiber: A pH-sensitive sustained drug delivery vehicle. Int. J. Biol. Macromol. 2024, 256, 128253. [Google Scholar] [CrossRef] [PubMed]
- Tanwar, A.; Kalode, P.; Roshni, V.; Prema, B.K.; Doshi, P.; Ottoor, D. Influence of nanofillers (Ag NPs and C. dots) on the controlled drug release profile of gelatin-grafted-polyacrylamide hydrogel: An in vitro study. Mater. Today Commun. 2023, 35, 105922. [Google Scholar] [CrossRef]
- Li, C.; Li, F.; Wang, K.; Wang, Q.; Liu, H.; Sun, X.; Xie, D. Synthesis, characterizations, and release mechanisms of carboxymethyl chitosan-graphene oxide-gelatin composite hydrogel for controlled delivery of drug. Inorg. Chem. Commun. 2023, 155, 110965. [Google Scholar] [CrossRef]
- Singh, H.; Yadav, I.; Sheikh, W.M.; Dan, A.; Darban, Z.; Shah, S.A.; Mishra, N.C.; Shahabuddin, S.; Hassan, S.; Bashir, S.M.; et al. Dual cross-linked gellan gum/gelatin-based multifunctional nanocomposite hydrogel scaffold for full-thickness wound healing. Int. J. Biol. Macromol. 2023, 251, 126349. [Google Scholar] [CrossRef] [PubMed]
- Hezari, S.; Olad, A.; Dilmaghani, A. Modified gelatin/iron- based metal-organic framework nanocomposite hydrogel as wound dressing: Synthesis, antibacterial activity, and Camellia sinensis release. Int. J. Biol. Macromol. 2022, 218, 488–505. [Google Scholar] [CrossRef]
- Alarçin, E.; Dokgöz, A.B.; Akgüner, Z.P.; Seki, H.K.; Bal-Öztürk, A. Gelatin methacryloyl/nanosilicate nanocomposite hydrogels encapsulating dexamethasone with a tunable crosslinking density for bone repair. J. Drug Deliv. Sci. Technol. 2022, 77, 103844. [Google Scholar] [CrossRef]
- Rahmani, S.; Olad, A.; Rahmani, Z. Preparation of self-healable nanocomposite hydrogel based on Gum Arabic/gelatin and graphene oxide: Study of drug delivery behavior. Polym. Bull. 2023, 80, 4117–4138. [Google Scholar] [CrossRef]
- Jaberifard, F.; Arsalani, N.; Ghorbani, M.; Mostafavi, H. Incorporating halloysite nanotube/carvedilol nanohybrids into gelatin microsphere as a novel oral pH-sensitive drug delivery system. Colloids Surf. A 2022, 637, 128122. [Google Scholar] [CrossRef]
- Mathew, S.A.; Arumainathan, S. Crosslinked Chitosan–Gelatin Biocompatible Nanocomposite as a Neuro Drug Carrier. ACS Omega 2022, 7, 18732–18744. [Google Scholar] [CrossRef]
- Baydin, T.; Aarstad, O.A.; Dille, M.J.; Hattrem, M.N.; Draget, K.I. Long-term storage stability of type A and type B gelatin gels: The effect of Bloom strength and co-solutes. Food Hydrocoll. 2022, 127, 107535. [Google Scholar] [CrossRef]
- Dranca, I.; Vyazovkin, S. Thermal stability of gelatin gels: Effect of preparation conditions on the activation energy barrier to melting. Polymer 2009, 50, 4859–4867. [Google Scholar] [CrossRef]
- Tan, Y.; Zi, Y.; Peng, J.; Shi, C.; Zheng, Y.; Zhong, J. Gelatin as a bioactive nanodelivery system for functional food applications. Food Chem. 2023, 423, 136265. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Manikandan, R.; Singh, S. Stability testing for gelatin-based formulations: Rapidly evaluating the possibility of a reduction in dissolution rates. Pharm. Technol. 2000, 24, 58–72. [Google Scholar]
- Foox, M.; Zilberman, M. Drug delivery from gelatin-based systems. Expert Opin. Drug Deliv. 2015, 12, 1547–1563. [Google Scholar] [CrossRef]
- Alipal, J.; Mohd Pu’ad, N.A.S.; Lee, T.C.; Nayan, N.H.M.; Sahari, N.; Basri, H.; Idris, M.I.; Abdullah, H.Z. A review of gelatin: Properties, sources, process, applications, and commercialisation. Mater. Today Proc. 2021, 42, 240–250. [Google Scholar] [CrossRef]
- Hutapea, T.P.H.; Madurani, K.A.; Syahputra, M.Y.; Hudha, M.N.; Asriana, A.N.; Suprapto; Kurniawan, F. Albumin: Source, preparation, determination, applications, and prospects. J. Sci. Adv. Mater. Devices 2023, 8, 100549. [Google Scholar] [CrossRef]
- Tiwari, R.; Sethiya, N.K.; Gulbake, A.S.; Mehra, N.K.; Murty, U.S.N.; Gulbake, A. A review on albumin as a biomaterial for ocular drug delivery. Int. J. Biol. Macromol. 2021, 191, 591–599. [Google Scholar] [CrossRef]
- Pompili, E.; Zaccherini, G.; Baldassarre, M.; Iannone, G.; Caraceni, P. Albumin administration in internal medicine: A journey between effectiveness and futility. Eur. J. Intern. Med. 2023, 117, 28–37. [Google Scholar] [CrossRef]
- Mishra, V.; Heath, R.J. Structural and Biochemical Features of Human Serum Albumin Essential for Eukaryotic Cell Culture. Int. J. Mol. Sci. 2021, 22, 8411. [Google Scholar] [CrossRef] [PubMed]
- Wouw, J.; Joles, J.A. Albumin is an interface between blood plasma and cell membrane, and not just a sponge. Clin Kidney J. 2022, 15, 624–634. [Google Scholar] [CrossRef]
- Xu, X.; Jinyu Hu, J.; Xue, H.; Hu, Y.; Liu, Y.; Lin, G.; Liu, L.; Xu, R. Applications of human and bovine serum albumins in biomedical engineering: A review. Int. J. Biol. Macromol. 2023, 253, 126914. [Google Scholar] [CrossRef] [PubMed]
- Spada, A.; Emami, J.; Tuszynski, J.A.; Lavasanifar, A. The Uniqueness of Albumin as a Carrier in Nanodrug Delivery. Mol. Pharm. 2021, 18, 1862–1894. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Iqbal, H.; Ur-Rehman, U.; Zhai, L.; Yuan, Z.; Razzaq, A.; Lv, M.; Wei, H.; Ning, X.; Xin, J.; et al. Albumin-based nanodevices for breast cancer diagnosis and therapy. J. Drug Deliv. Sci. Technol. 2023, 79, 104072. [Google Scholar] [CrossRef]
- Hoogenboezem, E.N.; Duvall, C.L. Harnessing Albumin as a Carrier for Cancer Therapies. Adv. Drug Deliv. Rev. 2018, 130, 73–89. [Google Scholar] [CrossRef]
- Ma, N.; Liu, J.; He, W.; Li, Z.; Luan, Y.; Song, Y.; Garg, S. Folic acid-grafted bovine serum albumin decorated graphene oxide: An efficient drug carrier for targeted cancer therapy. J. Colloid Interface Sci. 2017, 490, 598–607. [Google Scholar] [CrossRef]
- Akbal, O.; Vural, T.; Malekghasemi, S.; Bozdoğan, B.; Denkbaş, E.B. Saponin loaded montmorillonite-human serum albumin nanocomposites as drug delivery system in colorectal cancer therapy. Appl. Clay Sci. 2018, 166, 214–222. [Google Scholar] [CrossRef]
- Yu, X.; Xu, Z.; Wang, X.; Xu, Q.; Chen, J. Bactrian camel serum albumins-based nanocomposite as versatile biocargo for drug delivery, biocatalysis and detection of hydrogen peroxide. Mater. Sci. Eng. C 2020, 109, 110627. [Google Scholar] [CrossRef]
- Elgohary, M.M.; Helmy, M.W.; Abdelfattah, E.A.; Ragab, D.M.; Mortada, S.M.; Fang, J.; Elzoghby, A.O. Targeting sialic acid residues on lung cancer cells by inhalable boronic acid-decorated albumin nanocomposites for combined chemo/herbal therapy. J. Control. Release 2018, 285, 230–243. [Google Scholar] [CrossRef]
- Chen, Z.; Hong, G.; Liu, Z.; Yang, D.; Kankala, R.K.; Wu, W. Synergistic antitumor efficacy of doxorubicin and gambogic acid-encapsulated albumin nanocomposites. Colloids Surf. B 2020, 196, 111286. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, X.; Zhang, C.; Lin, K.; Yang, J.; Yi Zhang, Y.; Hao, J.; Tian, F. Folic acid-coupled bovine serum albumin-modified magnetic nanocomposites from quantum-sized Fe3O4 and layered double hydroxide for actively targeted delivery of 5-fluorouracil. Int. J. Biol. Macromol. 2024, 256, 128385. [Google Scholar] [CrossRef] [PubMed]
- Xinyu, Y.; Adilijiang, X.; Qilan, X.; Azhati, Z.; Yiyan, S.; Ling, C.; Jin, C. GSH-responsive curcumin/doxorubicin encapsulated Bactrian camel serum albumin nanocomposites with synergistic effect against lung cancer cells. J. Biomed. Sci. 2020, 34, 54–66. [Google Scholar]
- Bardania, H.; Jafari, F.; Baneshi, M.; Mahmoudi, R.; Ardakani, M.T.; Safari, F.; Barmak, M.J. Folic Acid-Functionalized Albumin/Graphene Oxide Nanocomposite to Simultaneously Deliver Curcumin and 5-Fluorouracil into Human Colorectal Cancer Cells: An In Vitro Study. Biomed Res. Int. 2023, 2023, 8334102. [Google Scholar] [CrossRef] [PubMed]
- Jalali, E.S.; Shojaosadati, S.A.; Hamedi, S. Green synthesis of bovine serum albumin/oxidized gum Arabic nanocomposite as pH-responsive carrier for controlled release of piperine and the molecular docking study. Int. J. Biol. Macromol. 2023, 225, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Jing, W.; Jiang, M.; Fu, X.; Yang, J.; Chen, L.; Leng, F.; Xu, P.; Huang, W.; Yang, C.Y.Z. Self-assembly drug-albumin nanocomposites for nonalcoholic fatty liver disease treatment. Int. J. Biol. Macromol. 2022, 214, 697–707. [Google Scholar] [CrossRef]
- Madeira, P.P.; Rocha, I.L.D.; Rosa, M.E.; Freire, M.G.; Coutinho, J.A.P. On the aggregation of bovine serum albumin. J. Mol. Liq. 2022, 349, 118183. [Google Scholar] [CrossRef]
- Karimi, M.; Bahrami, S.; Ravari, S.B.; Zangabad, P.S.; Mirshekari, H.; Bozorgomid, M.; Shahreza, S.; Sori, M.; Hamblin, M.R. Albumin nanostructures as advanced drug delivery systems. Expert Opin. Drug Deliv. 2016, 13, 1609–1623. [Google Scholar] [CrossRef]
- Larsen, M.T.; Kuhlmann, M.; Hvam, M.L.; Howard, K.A. Albumin-based drug delivery: Harnessing nature to cure disease. Mol. Cell. Ther. 2016, 4, 3. [Google Scholar] [CrossRef]



| Structure | Nanocomposite Matrix | Drug/Active Substance | Application | Ref. |
|---|---|---|---|---|
| Liposome | Soy lecithin, cetyltrimethylammonium chloride phosphate buffer, ZnFe2O4, and hyaluronic acid | Imatinib | drug delivery (anti-cancer therapy) | [50] |
| Liposome | Poly(vinyl alcohol) and chitosan | Taxifolin | Accelerating wound healing in diabetic patients | [51] |
| Liposome | Hydroxypropyl-β-cyclodextrin | Dexamethasone | Topical drug delivery system for the posterior segment of the eye | [52] |
| Liposome | Gelatin and methacrylate | Chemokinin SDF-1α | Stimulation of cell migration | [53] |
| Liposome | Ag/Au | Doxorubicin | Drug delivery (anti-cancer therapy) | [54] |
| Liposome | Fullerene and PEGylated iron oxide nanoparticles | Doxorubicin | Multi-mechanism cancer treatment based on radiofrequency-induced imaging and targeted drug delivery via an external magnetic field | [55] |
| Liposome | Folic acid and gold nanorods | Doxorubicin | Cancer treatment via both chemotherapy and photothermal therapy | [56] |
| Active Agent | Active Substance Properties | Nanocomposite Structure | Ref. |
|---|---|---|---|
| Prednisolone | Immunosuppressant drug used to treat some inflammatory diseases and some types of cancer | Poly(vinyl pyrrolidone)/sodium alginate copolymer incorporated with silver nanoparticles | [109] |
| Lactobacillus rhamnosus GG | Natural probiotic positively altering the gut microbiome composition | Exfoliated bentonite/alginate nanocomposite hydrogels | [110] |
| Amlodipine besylate | Calcium channel blocker applied in angina and hypertension | Nanocomposite matrix based on alginate, chitosan, and graphene oxide incorporated with inclusion complexes of amlodipine besylate and β-Cyclodextrin | [111] |
| Edaravone | Free radical scavenger approved for acute cerebral infarction treatment | Nanocomposite hydrogels based on alginate and positively charged Eudragit nanoparticles incorporated with the drug | [112] |
| Propranolol | Drug applied for cardiac treatment; shows anti-anxiety and anti-migraine effects | Sodium alginate/pectin/tannic acid—silver nanoparticle-based nanocomposite prepared via microwave irradiation | [113] |
| Alendronate sodium | Drug applied for osteoporosis treatment | Sodium alginate cross-linked montmorillonite nanocomposite beads | [114] |
| Chlorhexidine digluconate | Drug with antibacterial activity applied in dentinal tubules infections | Alginate/nanocellulose-based nanocomposites | [115] |
| Tofacitinib | Drug applied for autoimmune disease treatment | Alginate-based beads containing drug-incorporated nanoemulsions | [69] |
| Active Agent | Active Substance Properties | Nanocomposite Structure | Ref. |
|---|---|---|---|
| Doxorubicin | Anti-cancer drug | Carboxymethyl cellulose/ZnO/starch-based nanocomposite hydrogel beads | [141] |
| Doxorubicin | Anti-cancer drug | Nanocomposites based on carbon dots conjugated carboxymethyl cellulose and hydroxyapatite | [142] |
| Doxorubicin | Anti-cancer drug | Nanocomposite hydrogel based on graphene quantum dot crosslinked carboxymethyl cellulose | [143] |
| Doxorubicin | Anti-cancer drug | Nanocomposite hydrogel beads based on carboxymethyl cellulose/graphene oxide | [144] |
| Artesunate | Anti-malarial drug also showing anti-cancer efficacy | Nanocomposites based on polyhydroxybutyrate and functionalized carboxymethylcellulose and additionally containing zinc oxide and Fe3O4 magnetic nanoparticles | [145] |
| 5-fluorouracil (5-FU) | Anti-cancer drug | Nanocomposite hydrogel beads based on carboxymethylcellulose and Arabic gum | [146] |
| Tetracycline | Antibiotic | Nanocomposite based on carboxymethyl cellulose containing Zn-melamine and Cu-melamine framework | [147] |
| Diclofenac sodium | Nonsteroidal anti-inflammatory drug | Nanocomposite based on poly(methacrylic acid) crosslinked with carboxymethyl cellulose and incorporated with in situ-formed silver nanoparticles | [148] |
| Active Substance | Active Substance Properties | Nanocomposite Structure | Ref. |
|---|---|---|---|
| Acetaminophen (paracetamol) | Analgesic and antipyretic activity | Gelatin-based nanocomposite hydrogel incorporated with drug-loaded poly(N–isopropylacrylamide) nanoparticles | [167] |
| Cefadroxil | Antibacterial activity | Gelatin-based nanocomposites incorporated with carbon dots | [168] |
| Cephalexin | Antibacterial activity | chemically crosslinked gelatin-based hydrogel nanocomposites incorporated with CuO nanoparticles | [169] |
| Ciprofloxacin | Antibacterial activity | Gelatin, starch, and itaconic acid-based hydrogel nanocomposites containing ZnO and cellulose nanofibers | [170] |
| Ciprofloxacin | Antibacterial activity | Gelatin-grafted polyacrylamide nanocomposite hydrogels containing silver nanoparticles and carbon dots | [171] |
| Ibuprofen | Analgesic, anti-inflammatory, and antipyretic activity | Gelatin/carboxymethyl chitosan/graphene oxide-based nanocomposite hydrogel | [172] |
| Flurbiprofen | Analgesic, anti-inflammatory, and antipyretic activity | Dual crosslinked gelatin/gellan gum-based nanocomposite hydrogel incorporated with cerium oxide nanoparticles | [173] |
| Camellia sinensis | Herbal drug showing antibacterial activity | Nanocomposite hydrogel based on methacrylic anhydride, modified gelatin, and an iron-based metal–organic framework | [174] |
| Dexamethasone | Anti-inflammatory, analgesic, anti-allergic, and immunosuppressive activity | Gelatin methacryloyl/nanosilicate-based nanocomposite hydrogels | [175] |
| Drug/Active Substance | Application | Nanocomposite Structure | Ref. |
|---|---|---|---|
| Doxorubicin | Anti-cancer therapy | Folic acid-grafted bovine serum albumin/graphene oxide-based nanocomposite | [194] |
| Saponin | Colorectal cancer treatment | Montmorillonite loaded with saponine/human serum albumin-based nanocomposite | [195] |
| Doxorubicin | Anti-cancer therapy | Bactrian camel serum albumin-based nanocomposite | [196] |
| Etoposide | Lung cancer treatment | Boronic acid-modified albumin-based nanocomposites | [197] |
| Doxorubicin, gambogic acid | Liver cancer treatment | Albumin-based nanocomposites | [198] |
| 5-fluorouracil | Liver cancer treatment | Nanocomposites consisting of folic acid, bovine serum albumin, layered double hydroxide, and quantum-sized Fe3O4 | [199] |
| Doxorubicin | Lung cancer treatment | Bactrian camel serum albumin-based nanocomposites incorporated with glutathione-responsive curcumin | [200] |
| 5-fluorouracil, curcumin | Colorectal cancer treatment | Nanocomposites based on graphene oxide and folic acid-functionalized albumin | [201] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamroży, M.; Kudłacik-Kramarczyk, S.; Drabczyk, A.; Krzan, M. Advanced Drug Carriers: A Review of Selected Protein, Polysaccharide, and Lipid Drug Delivery Platforms. Int. J. Mol. Sci. 2024, 25, 786. https://doi.org/10.3390/ijms25020786
Jamroży M, Kudłacik-Kramarczyk S, Drabczyk A, Krzan M. Advanced Drug Carriers: A Review of Selected Protein, Polysaccharide, and Lipid Drug Delivery Platforms. International Journal of Molecular Sciences. 2024; 25(2):786. https://doi.org/10.3390/ijms25020786
Chicago/Turabian StyleJamroży, Mateusz, Sonia Kudłacik-Kramarczyk, Anna Drabczyk, and Marcel Krzan. 2024. "Advanced Drug Carriers: A Review of Selected Protein, Polysaccharide, and Lipid Drug Delivery Platforms" International Journal of Molecular Sciences 25, no. 2: 786. https://doi.org/10.3390/ijms25020786
APA StyleJamroży, M., Kudłacik-Kramarczyk, S., Drabczyk, A., & Krzan, M. (2024). Advanced Drug Carriers: A Review of Selected Protein, Polysaccharide, and Lipid Drug Delivery Platforms. International Journal of Molecular Sciences, 25(2), 786. https://doi.org/10.3390/ijms25020786







