Monomeric CXCL12-Engineered Adipose-Derived Stem Cells Transplantation for the Treatment of Ischemic Stroke
Abstract
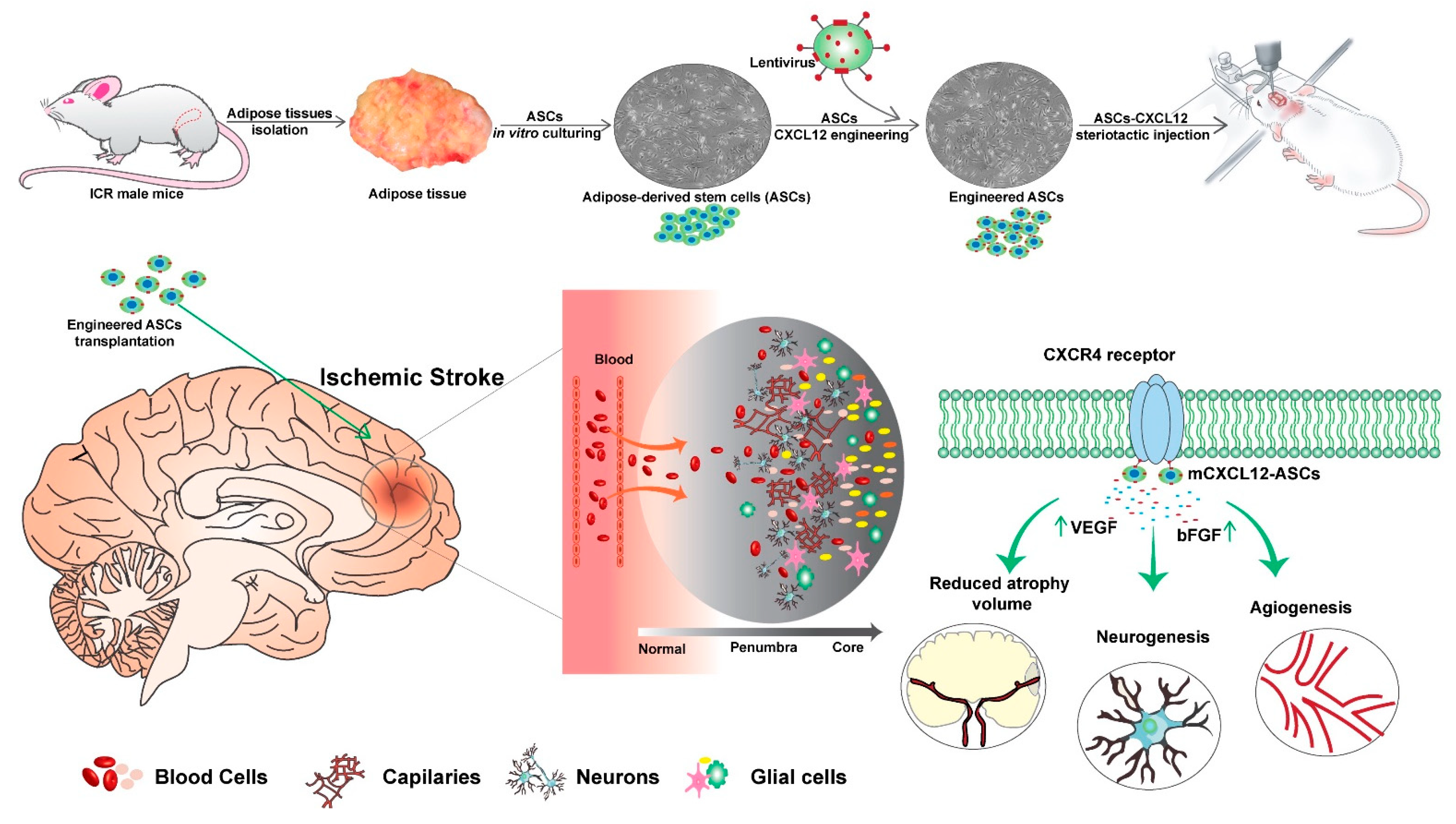
:1. Introduction
2. Results
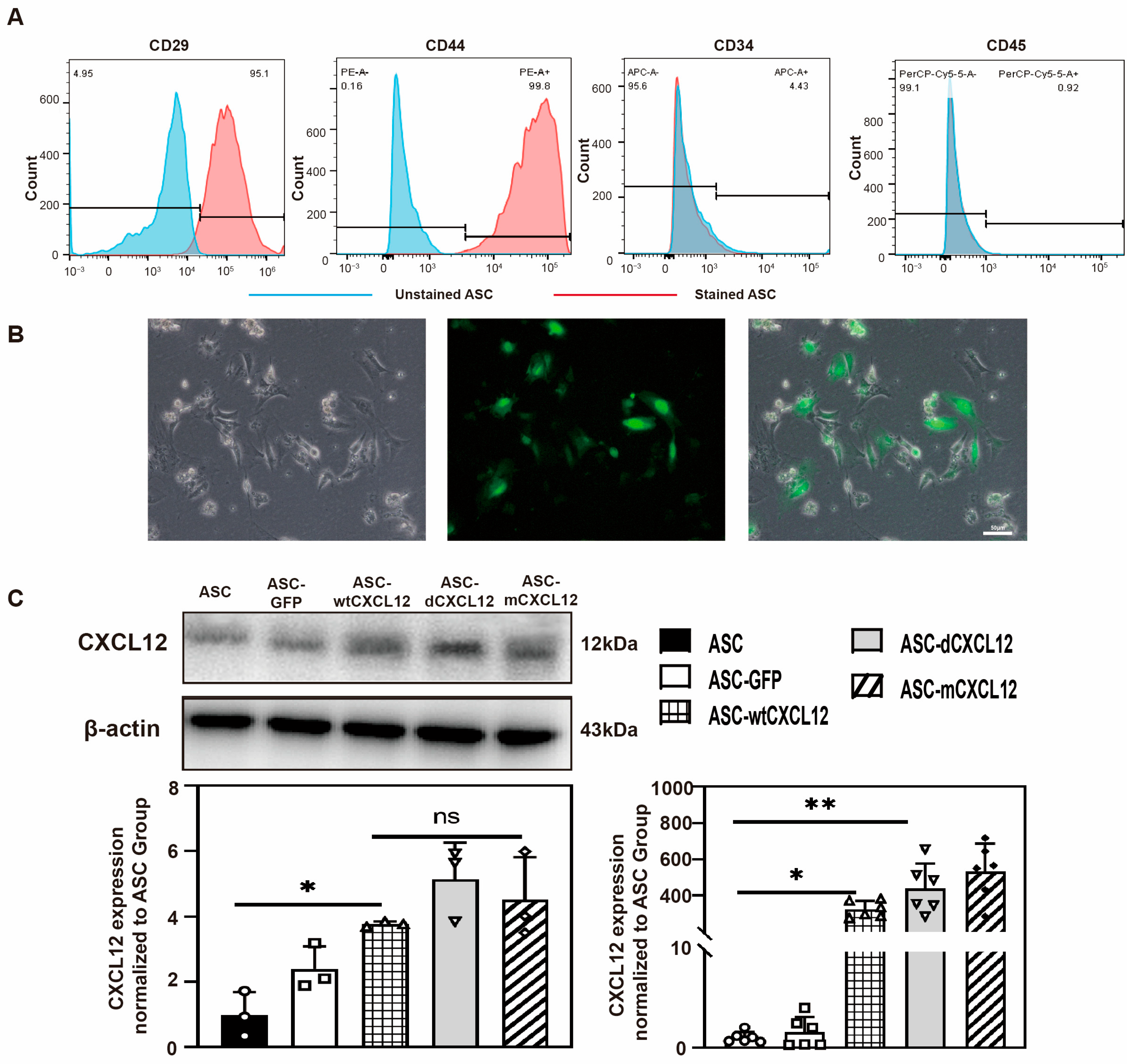
2.1. Identification of ASCs’ and CXCL12/Variants’ Gene Overexpression
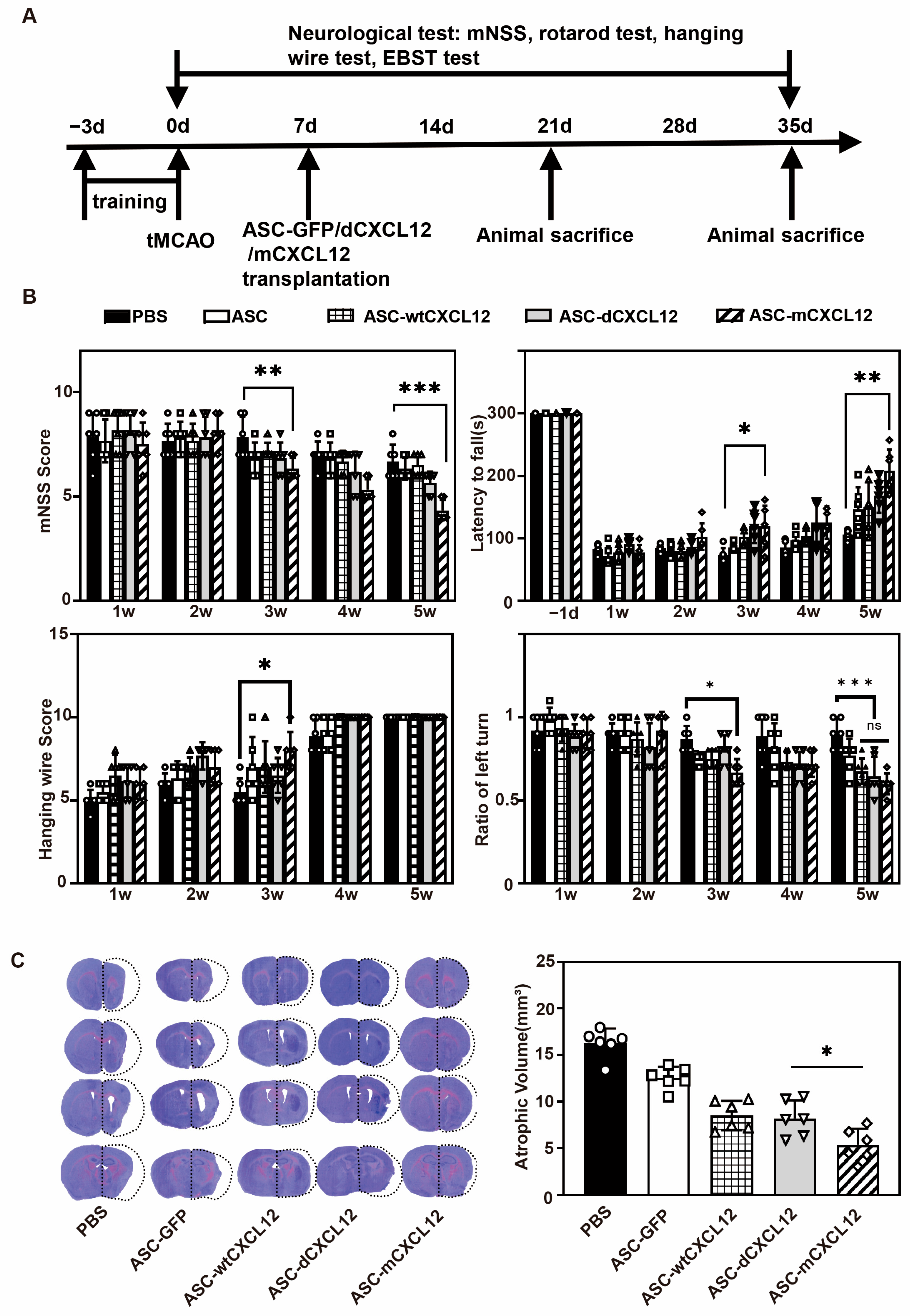
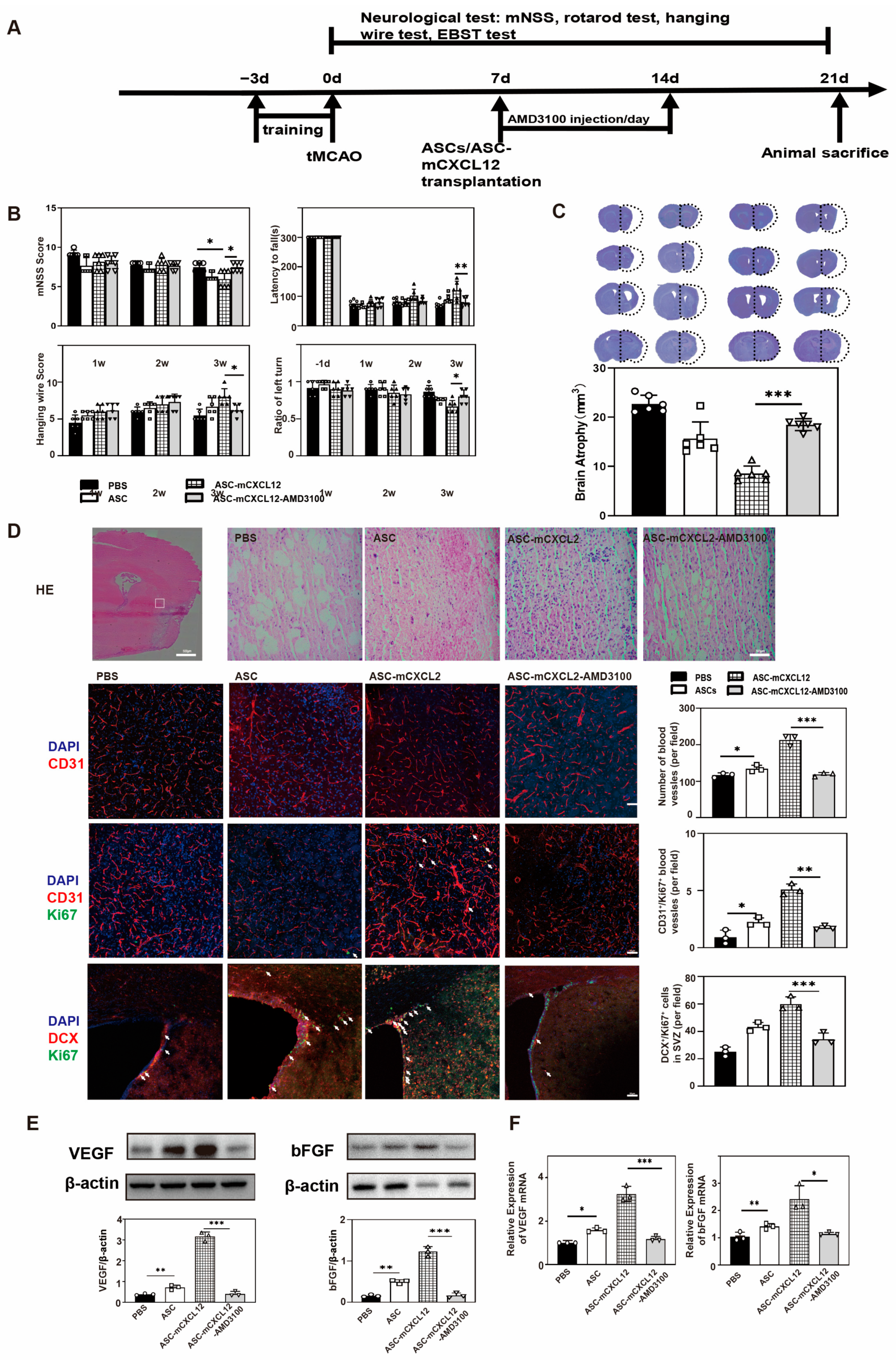
2.2. ASC-mCXCL12 Transplantation Improved Neurobehavioral Recovery and Reduced Brain Atrophy in Ischemic Mice
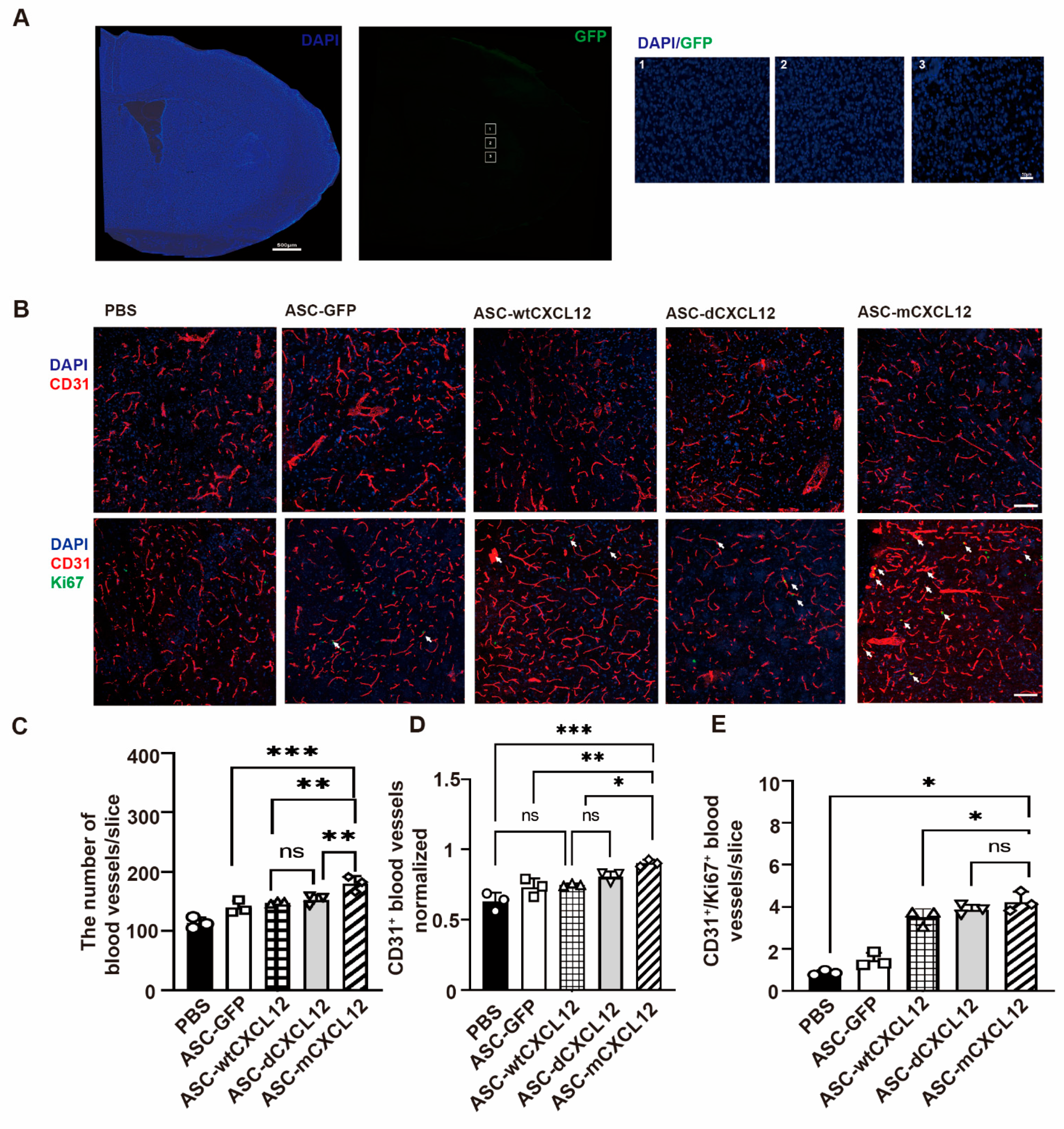
2.3. ASC-mCXCL12 Transplantation Improved Angiogenesis of Ischemic Mice
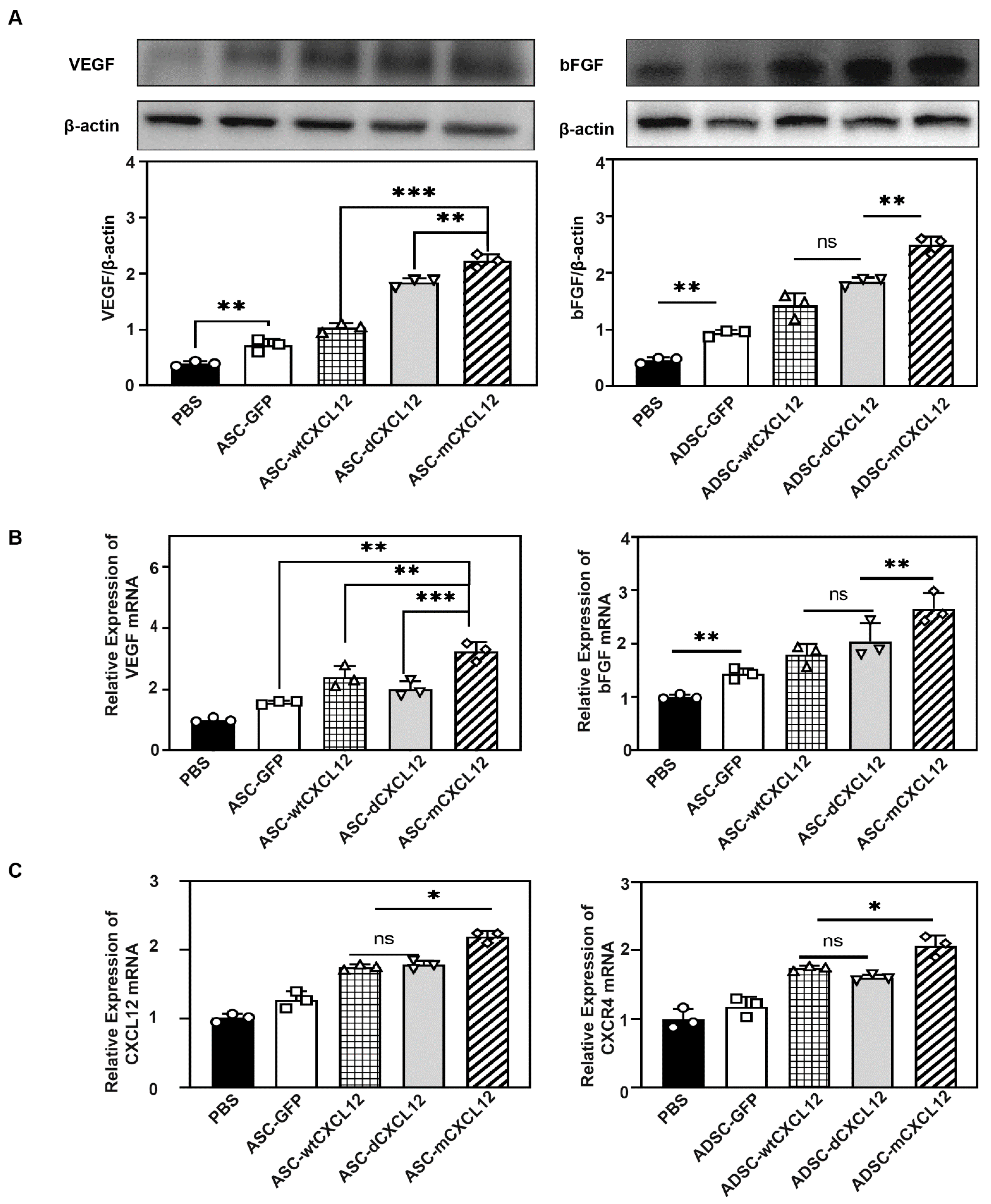
2.4. ASCs-mCXCL12 Promoted the Expression of VEGF and bFGF after Ischemic Stroke
2.5. ASC-mCXCL12 Transplantation Promoted Angiogenesis and Neurogenesis in Ischemic Mouse Brain
2.6. The Regulation of ASC-mCXCL12 Functions in Ischemic Mice Is Mediated Using the CXCL12/CXCR4 Signaling Axis
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. PLenti-CXCL12-IRES-GFP Vector Construction and Lentiviral Vector Production
4.3. ASCs Isolation and Characterization
4.4. Transient Middle Cerebral Artery Occlusion in Mice (tMCAO)
4.5. ASCs, ASC-CXCL12 Variant Transplantation
4.6. Administration of AMD3100
4.7. Neurological Behavioral Evaluation
4.8. Ischemic Brain Infarct Volume and Atrophy Assessment
4.9. Immunohistochemistry and HE Staining
4.10. Cell and Vessel Counting
4.11. Western Blot Analysis
4.12. Real-Time PCR Analysis
- GAPDH:
- Forward: CTGGGCTACACTGAGCACC;
- Reverse: AAGTGGTCGTTGAGGGCAATG,
- VEGF:
- Forward: CTGCCGTCCGATTGAGACC;
- Reverse: CCCCTCCTTGTACCACTGTC,
- bFGF:
- Forward: GCGACCCACACGTCAAACTA;
- Reverse: TCCCTTGATAGACACAACTCCTC,
- CXCR4:
- Forward: GACTGGCATAGTCGGCAATG;
- Reverse: AGAAGGGGAGTGTGATGACAAA,
- CXCL12:
- Forward: TGCATCAGTGACGGTAAACCA
- Reverse: CACAGTTTGGAGTGTTGAGGAT.
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.; Sacco, R.L.; Hacke, W.; Fisher, M.; Pandian, J.; Lindsay, P. World Stroke Organization (WSO): Global Stroke Fact Sheet 2022. Int. J. Stroke 2022, 17, 18–29. [Google Scholar] [CrossRef]
- Diener, H.-C.; Easton, J.D.; Hart, R.G.; Kasner, S.; Kamel, H.; Ntaios, G. Review and update of the concept of embolic stroke of undetermined source. Nat. Rev. Neurol. 2022, 18, 455–465. [Google Scholar] [CrossRef]
- Markus, H.S.; Michel, P. Treatment of posterior circulation stroke: Acute management and secondary prevention. Int. J. Stroke 2022, 17, 723–732. [Google Scholar] [CrossRef]
- Herpich, F.; Rincon, F. Management of Acute Ischemic Stroke. Crit. Care Med. 2020, 48, 1654–1663. [Google Scholar] [CrossRef]
- O’Connor, R.E.; McGraw, P.; Edelsohn, L. Thrombolytic Therapy for Acute Ischemic Stroke: Why the Majority of Patients Remain Ineligible for Treatment. Ann. Emerg. Med. 1999, 33, 9–14. [Google Scholar] [CrossRef]
- Emberson, J.; Lees, K.R.; Lyden, P.; Blackwell, L.; Albers, G.; Bluhmki, E.; Brott, T.; Cohen, G.; Davis, S.; Donnan, G.; et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: A meta-analysis of individual patient data from randomised trials. Lancet 2014, 384, 1929–1935. [Google Scholar] [CrossRef]
- Bai, Q.-K.; Zhao, Z.-G.; Lu, L.-J.; Shen, J.; Zhang, J.-Y.; Sui, H.-J.; Xie, X.-H.; Chen, J.; Yang, J.; Chen, C.-R. Treating ischaemic stroke with intravenous tPA beyond 4.5 hours under the guidance of a MRI DWI/T2WI mismatch was safe and effective. Stroke Vasc. Neurol. 2019, 4, 8–13. [Google Scholar] [CrossRef]
- Boese, A.C.; Le, Q.-S.E.; Pham, D.; Hamblin, M.H.; Lee, J.-P. Neural stem cell therapy for subacute and chronic ischemic stroke. Stem Cell Res. Ther. 2018, 9, 154. [Google Scholar] [CrossRef]
- Kashiyama, N.; Kormos, R.L.; Matsumura, Y.; D’Amore, A.; Miyagawa, S.; Sawa, Y.; Wagner, W.R. Adipose-derived stem cell sheet under an elastic patch improves cardiac function in rats after myocardial infarction. J. Thorac. Cardiovasc. Surg. 2022, 163, e261–e272. [Google Scholar] [CrossRef]
- Gimble, J.M.; Katz, A.J.; Bunnell, B.A. Adipose-Derived Stem Cells for Regenerative Medicine. Circ. Res. 2007, 100, 1249–1260. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human Adipose Tissue Is a Source of Multipotent Stem Cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef]
- Chan, T.-M.; Chen, J.Y.-R.; Ho, L.-I.; Lin, H.-P.; Hsueh, K.-W.; Liu, D.D.; Chen, Y.-H.; Hsieh, A.-C.; Tsai, N.-M.; Hueng, D.-Y.; et al. ADSC Therapy in Neurodegenerative Disorders. Cell Transplant. 2014, 23, 549–557. [Google Scholar] [CrossRef]
- Gir, P.; Oni, G.; Brown, S.A.; Mojallal, A.; Rohrich, R.J. Human Adipose Stem Cells. Plast. Reconstr. Surg. 2012, 129, 1277–1290. [Google Scholar] [CrossRef]
- Li, Y.; Chang, S.; Li, W.; Tang, G.; Ma, Y.; Liu, Y.; Yuan, F.; Zhang, Z.; Yang, G.-Y.; Wang, Y. cxcl12-engineered endothelial progenitor cells enhance neurogenesis and angiogenesis after ischemic brain injury in mice. Stem Cell Res. Ther. 2018, 9, 139. [Google Scholar] [CrossRef]
- Wang, Z.; Moresco, P.; Yan, R.; Li, J.; Gao, Y.; Biasci, D.; Yao, M.; Pearson, J.; Hechtman, J.F.; Janowitz, T.; et al. Carcinomas assemble a filamentous CXCL12–keratin-19 coating that suppresses T cell–mediated immune attack. Proc. Natl. Acad. Sci. USA 2022, 119, e2119463119. [Google Scholar] [CrossRef]
- Drury, L.J.; Ziarek, J.J.; Gravel, S.; Veldkamp, C.T.; Takekoshi, T.; Hwang, S.T.; Heveker, N.; Volkman, B.F.; Dwinell, M.B. Monomeric and dimeric CXCL12 inhibit metastasis through distinct CXCR4 interactions and signaling pathways. Proc. Natl. Acad. Sci. USA 2011, 108, 17655–17660. [Google Scholar] [CrossRef]
- Chang, S.; Li, Y.; Yuan, F.; Qu, M.; Song, Y.; Zhang, Z.; Yang, G.-Y.; Wang, Y. Monomeric CXCL12 outperforms its dimeric and wild type variants in the promotion of human endothelial progenitor cells’ function. Biochem. Biophys. Res. Commun. 2017, 488, 303–310. [Google Scholar] [CrossRef]
- Komi, D.E.A.; Khomtchouk, K.; Santa Maria, P.L. A Review of the Contribution of Mast Cells in Wound Healing: Involved Molecular and Cellular Mechanisms. Clin. Rev. Allergy Immunol. 2020, 58, 298–312. [Google Scholar] [CrossRef]
- Ni, R.; Luo, C.; Ci, H.; Sun, D.; An, R.; Wang, Z.; Yang, J.; Li, Y.; Sun, J. Construction of vascularized tissue-engineered breast with dual angiogenic and adipogenic micro-tissues. Mater. Today Bio 2023, 18, 100539. [Google Scholar] [CrossRef]
- Tao, Z.; Liu, L.; Wu, M.; Wang, Q.; Wang, Y.; Xiong, J.; Xue, C. Metformin promotes angiogenesis by enhancing VEGFa secretion by adipose-derived stem cells via the autophagy pathway. Regen. Biomater. 2023, 10, rbad043. [Google Scholar] [CrossRef]
- Wu, J.; Yang, Q.; Wu, S.; Yuan, R.; Zhao, X.; Li, Y.; Wu, W.; Zhu, N. Adipose-Derived Stem Cell Exosomes Promoted Hair Regeneration. Tissue Eng. Regen. Med. 2021, 18, 685–691. [Google Scholar] [CrossRef]
- Chen, J.; Tang, Y.-X.; Liu, Y.-M.; Chen, J.; Hu, X.-Q.; Liu, N.; Wang, S.-X.; Zhang, Y.; Zeng, W.-G.; Ni, H.-J.; et al. Transplantation of Adipose-Derived Stem Cells is Associated with Neural Differentiation and Functional Improvement in a Rat Model of Intracerebral Hemorrhage. CNS Neurosci. Ther. 2012, 18, 847–854. [Google Scholar] [CrossRef]
- Kim, J.-M.; Lee, S.-T.; Chu, K.; Jung, K.-H.; Song, E.-C.; Kim, S.-J.; Sinn, D.-I.; Kim, J.-H.; Park, D.-K.; Kang, K.-M.; et al. Systemic transplantation of human adipose stem cells attenuated cerebral inflammation and degeneration in a hemorrhagic stroke model. Brain Res. 2007, 1183, 43–50. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, H.; Hu, Y.; Pan, C.; Wu, G.; Li, Q.; Tang, Z. Adipose-derived mesenchymal stem cells stereotactic transplantation alleviate brain edema from intracerebral hemorrhage. J. Cell. Biochem. 2019, 120, 14372–14382. [Google Scholar] [CrossRef]
- Oh, S.-H.; Choi, C.; Chang, D.-J.; Shin, D.-A.; Lee, N.; Jeon, I.; Sung, J.-H.; Lee, H.; Hong, K.-S.; Ko, J.J.; et al. Early neuroprotective effect with lack of long-term cell replacement effect on experimental stroke after intra-arterial transplantation of adipose-derived mesenchymal stromal cells. Cytotherapy 2015, 17, 1090–1103. [Google Scholar] [CrossRef]
- Mizuno, H.; Tobita, M.; Uysal, A.C. Concise Review: Adipose-Derived Stem Cells as a Novel Tool for Future Regenerative Medicine. Stem Cells 2012, 30, 804–810. [Google Scholar] [CrossRef]
- Otsuki, Y.; Nakamura, Y.; Harada, S.; Yamamoto, Y.; Ogino, K.; Morikawa, K.; Ninomiya, H.; Miyagawa, S.; Sawa, Y.; Hisatome, I.; et al. Adipose stem cell sheets improved cardiac function in the rat myocardial infarction, but did not alter cardiac contractile responses to β-adrenergic stimulation. Biomed. Res. 2015, 36, 11–19. [Google Scholar] [CrossRef]
- Ren, S.; Chen, J.; Duscher, D.; Liu, Y.; Guo, G.; Kang, Y.; Xiong, H.; Zhan, P.; Wang, Y.; Wang, C.; et al. Microvesicles from human adipose stem cells promote wound healing by optimizing cellular functions via AKT and ERK signaling pathways. Stem Cell Res. Ther. 2019, 10, 47. [Google Scholar] [CrossRef]
- Lombardi, F.; Palumbo, P.; Augello, F.R.; Cifone, M.G.; Cinque, B.; Giuliani, M. Secretome of Adipose Tissue-Derived Stem Cells (ASCs) as a Novel Trend in Chronic Non-Healing Wounds: An Overview of Experimental In Vitro and In Vivo Studies and Methodological Variables. Int. J. Mol. Sci. 2019, 20, 3721. [Google Scholar] [CrossRef]
- Ma, Q.; Jones, D.; Springer, T.A. The Chemokine Receptor CXCR4 Is Required for the Retention of B Lineage and Granulocytic Precursors within the Bone Marrow Microenvironment. Immunity 1999, 10, 463–471. [Google Scholar] [CrossRef]
- Scala, S. Molecular Pathways: Targeting the CXCR4–CXCL12 Axis—Untapped Potential in the Tumor Microenvironment. Clin. Cancer Res. 2015, 21, 4278–4285. [Google Scholar] [CrossRef]
- Strazza, M.; Azoulay-Alfaguter, I.; Peled, M.; Smrcka, A.V.; Skolnik, E.Y.; Srivastava, S.; Mor, A. PLCε1 regulates SDF-1α–induced lymphocyte adhesion and migration to sites of inflammation. Proc. Natl. Acad. Sci. USA 2017, 114, 2693–2698. [Google Scholar] [CrossRef]
- Sierro, F.; Biben, C.; Martínez-Muñoz, L.; Mellado, M.; Ransohoff, R.M.; Li, M.; Woehl, B.; Leung, H.; Groom, J.; Batten, M.; et al. Disrupted cardiac development but normal hematopoiesis in mice deficient in the second CXCL12/SDF-1 receptor, CXCR7. Proc. Natl. Acad. Sci. USA 2007, 104, 14759–14764. [Google Scholar] [CrossRef]
- Uto-Konomi, A.; McKibben, B.; Wirtz, J.; Sato, Y.; Takano, A.; Nanki, T.; Suzuki, S. CXCR7 agonists inhibit the function of CXCL12 by down-regulation of CXCR4. Biochem. Biophys. Res. Commun. 2013, 431, 772–776. [Google Scholar] [CrossRef]
- Pozzobon, T.; Goldoni, G.; Viola, A.; Molon, B. CXCR4 signaling in health and disease. Immunol. Lett. 2016, 177, 6–15. [Google Scholar] [CrossRef]
- Mousavi, A. CXCL12/CXCR4 signal transduction in diseases and its molecular approaches in targeted-therapy. Immunol. Lett. 2020, 217, 91–115. [Google Scholar] [CrossRef]
- Luker, G.D.; Yang, J.; Richmond, A.; Scala, S.; Festuccia, C.; Schottelius, M.; Wester, H.-J.; Zimmermann, J. At the Bench: Pre-clinical evidence for multiple functions of CXCR4 in cancer. J. Leukoc. Biol. 2020, 109, 969–989. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, H.; Lirussi, F.; Garrido, C.; Ye, X.-Y.; Xie, T. Dual inhibitors of histone deacetylases and other cancer-related targets: A pharmacological perspective. Biochem. Pharmacol. 2020, 182, 114224. [Google Scholar] [CrossRef]
- Huang, J.; Li, Y.; Tang, Y.; Tang, G.; Yang, G.-Y.; Wang, Y. CXCR4 Antagonist AMD3100 Protects Blood–Brain Barrier Integrity and Reduces Inflammatory Response After Focal Ischemia in Mice. Stroke 2013, 44, 190–197. [Google Scholar] [CrossRef]
- Heuser, R.R. The Role for Cardiologists in Stroke Intervention. Prog. Cardiovasc. Dis. 2017, 59, 549–554. [Google Scholar] [CrossRef]
- Jovin, T.G.; Chamorro, A.; Cobo, E.; de Miquel, M.A.; Molina, C.A.; Rovira, A.; San Román, L.; Serena, J.; Abilleira, S.; Ribó, M.; et al. Thrombectomy within 8 Hours after Symptom Onset in Ischemic Stroke. N. Engl. J. Med. 2015, 372, 2296–2306. [Google Scholar] [CrossRef]
- Yang, Y.; Torbey, M.T. Angiogenesis and Blood-Brain Barrier Permeability in Vascular Remodeling after Stroke. Curr. Neuropharmacol. 2020, 18, 1250–1265. [Google Scholar] [CrossRef]
- Kanazawa, M.; Hatakeyama, M.; Ninomiya, I. Angiogenesis and neuronal remodeling after ischemic stroke. Neural Regen. Res. 2020, 15, 16–19. [Google Scholar] [CrossRef]
- Jeong, J.O.; Han, J.W.; Kim, J.M.; Cho, H.J.; Park, C.; Lee, N.; Kim, D.W.; Yoon, Y.S. Malignant tumor formation after transplantation of short-term cultured bone marrow mesenchymal stem cells in experimental myocardial infarction and diabetic neuropathy. Circ. Res. 2011, 108, 1340–1347. [Google Scholar] [CrossRef]
- Ji, S.Q.; Cao, J.; Zhang, Q.Y.; Li, Y.Y.; Yan, Y.Q.; Yu, F.X. Adipose tissue-derived stem cells promote pancreatic cancer cell proliferation and invasion. Braz. J. Med. Biol. Res. 2013, 46, 758–764. [Google Scholar] [CrossRef]
- Gutjahr, J.C.; Crawford, K.S.; Jensen, D.R.; Naik, P.; Peterson, F.C.; Samson, G.P.B.; Legler, D.F.; Duchene, J.; Veldkamp, C.T.; Rot, A.; et al. The dimeric form of CXCL12 binds to atypical chemokine receptor 1. Sci. Signal. 2021, 14, eabc9012. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y.; Zang, G.; Wang, T.; Yu, Z.; Wang, S.; Tang, Z.; Liu, J. Adipose-derived stem cells improve erectile function partially through the secretion of IGF-1, bFGF, and VEGF in aged rats. Andrology 2018, 6, 498–509. [Google Scholar] [CrossRef]
- Rehman, J.; Traktuev, D.; Li, J.; Merfeld-Clauss, S.; Temm-Grove, C.J.; Bovenkerk, J.E.; Pell, C.L.; Johnstone, B.H.; Considine, R.V.; March, K.L. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 2004, 109, 1292–1298. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Mollayeva, T.; Mollayeva, S.; Colantonio, A. Traumatic brain injury: Sex, gender and intersecting vulnerabilities. Nat. Rev. Neurol. 2018, 14, 711–722. [Google Scholar] [CrossRef]
- Veldkamp, C.T.; Seibert, C.; Peterson, F.C.; De la Cruz, N.B.; Haugner, J.C.; Basnet, H.; Sakmar, T.P.; Volkman, B.F. Structural Basis of CXCR4 Sulfotyrosine Recognition by the Chemokine SDF-1/CXCL12. Sci. Signal. 2008, 1, ra4. [Google Scholar] [CrossRef]
- Smith, E.W.; Nevins, A.M.; Qiao, Z.; Liu, Y.; Getschman, A.E.; Vankayala, S.L.; Kemp, M.T.; Peterson, F.C.; Li, R.; Volkman, B.F.; et al. Structure-Based Identification of Novel Ligands Targeting Multiple Sites within a Chemokine–G-Protein-Coupled-Receptor Interface. J. Med. Chem. 2016, 59, 4342–4351. [Google Scholar] [CrossRef]
- Haarmann, A.; Zimmermann, L.; Bieber, M.; Silwedel, C.; Stoll, G.; Schuhmann, M.K. Regulation and release of vasoactive endoglin by brain endothelium in response to hypoxia/reoxygenation in stroke. Int. J. Mol. Sci. 2022, 23, 7085. [Google Scholar] [CrossRef]
- Haroon, K.; Ruan, H.; Zheng, H.; Wu, S.; Liu, Z.; Shi, X.; Tang, Y.; Yang, G.Y.; Zhang, Z. Bio-clickable, small extracellular vesicles-COCKTAIL therapy for ischemic stroke. J. Control. Release 2023, 363, 585–596. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, H.; Haroon, K.; Liu, M.; Hu, X.; Xu, Q.; Tang, Y.; Wang, Y.; Yang, G.-Y.; Zhang, Z. Monomeric CXCL12-Engineered Adipose-Derived Stem Cells Transplantation for the Treatment of Ischemic Stroke. Int. J. Mol. Sci. 2024, 25, 792. https://doi.org/10.3390/ijms25020792
Zheng H, Haroon K, Liu M, Hu X, Xu Q, Tang Y, Wang Y, Yang G-Y, Zhang Z. Monomeric CXCL12-Engineered Adipose-Derived Stem Cells Transplantation for the Treatment of Ischemic Stroke. International Journal of Molecular Sciences. 2024; 25(2):792. https://doi.org/10.3390/ijms25020792
Chicago/Turabian StyleZheng, Haoran, Khan Haroon, Mengdi Liu, Xiaowen Hu, Qun Xu, Yaohui Tang, Yongting Wang, Guo-Yuan Yang, and Zhijun Zhang. 2024. "Monomeric CXCL12-Engineered Adipose-Derived Stem Cells Transplantation for the Treatment of Ischemic Stroke" International Journal of Molecular Sciences 25, no. 2: 792. https://doi.org/10.3390/ijms25020792





