Faster Gastrointestinal Transit, Reduced Small Intestinal Smooth Muscle Tone and Dysmotility in the Nlgn3R451C Mouse Model of Autism
Abstract
1. Introduction
2. Results
2.1. Faster Gastrointestinal Transit in Nlgn3R451C Mice
2.2. A Reduced Small Intestinal Smooth Muscle Tone in Nlgn3R451C Mice
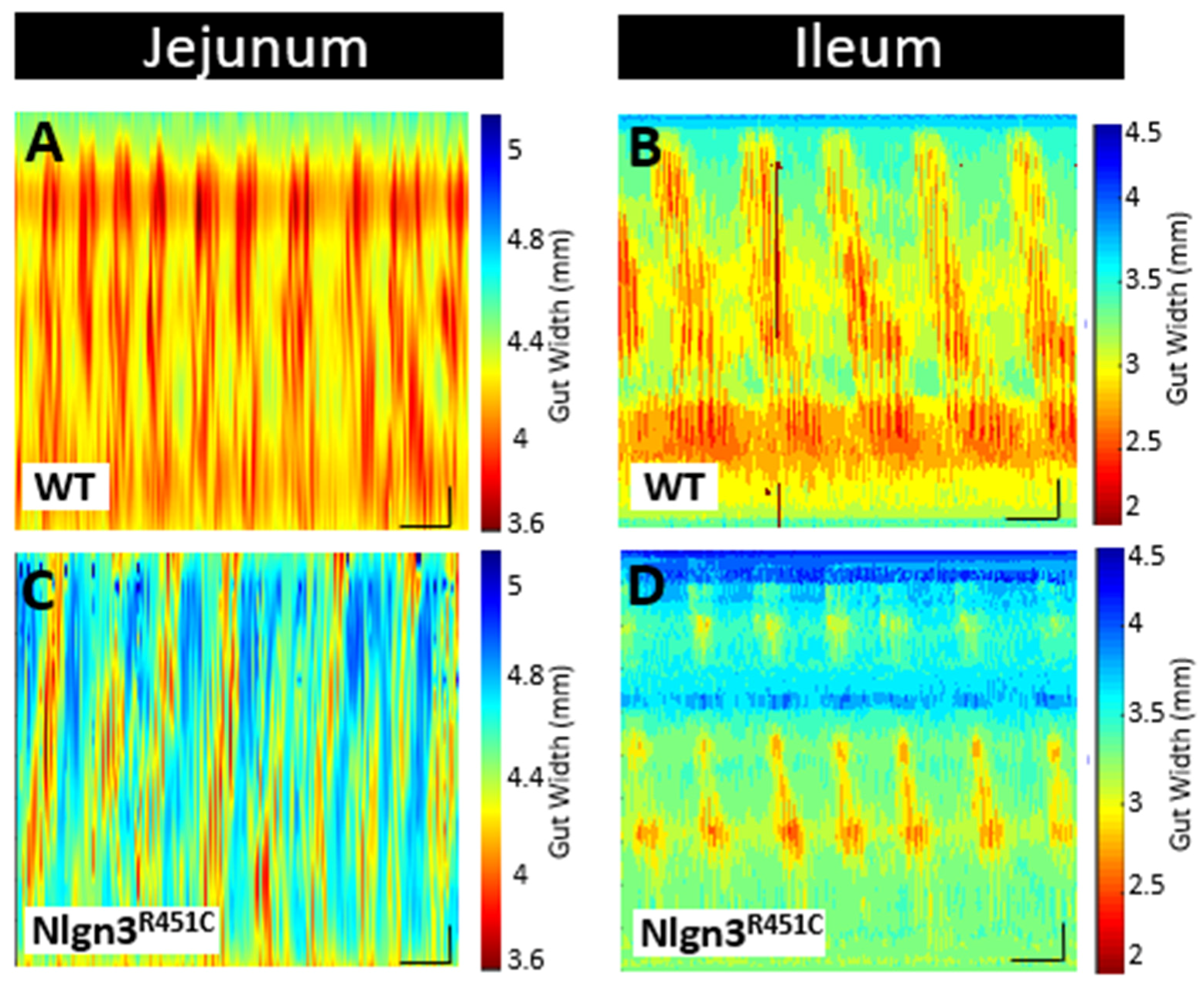
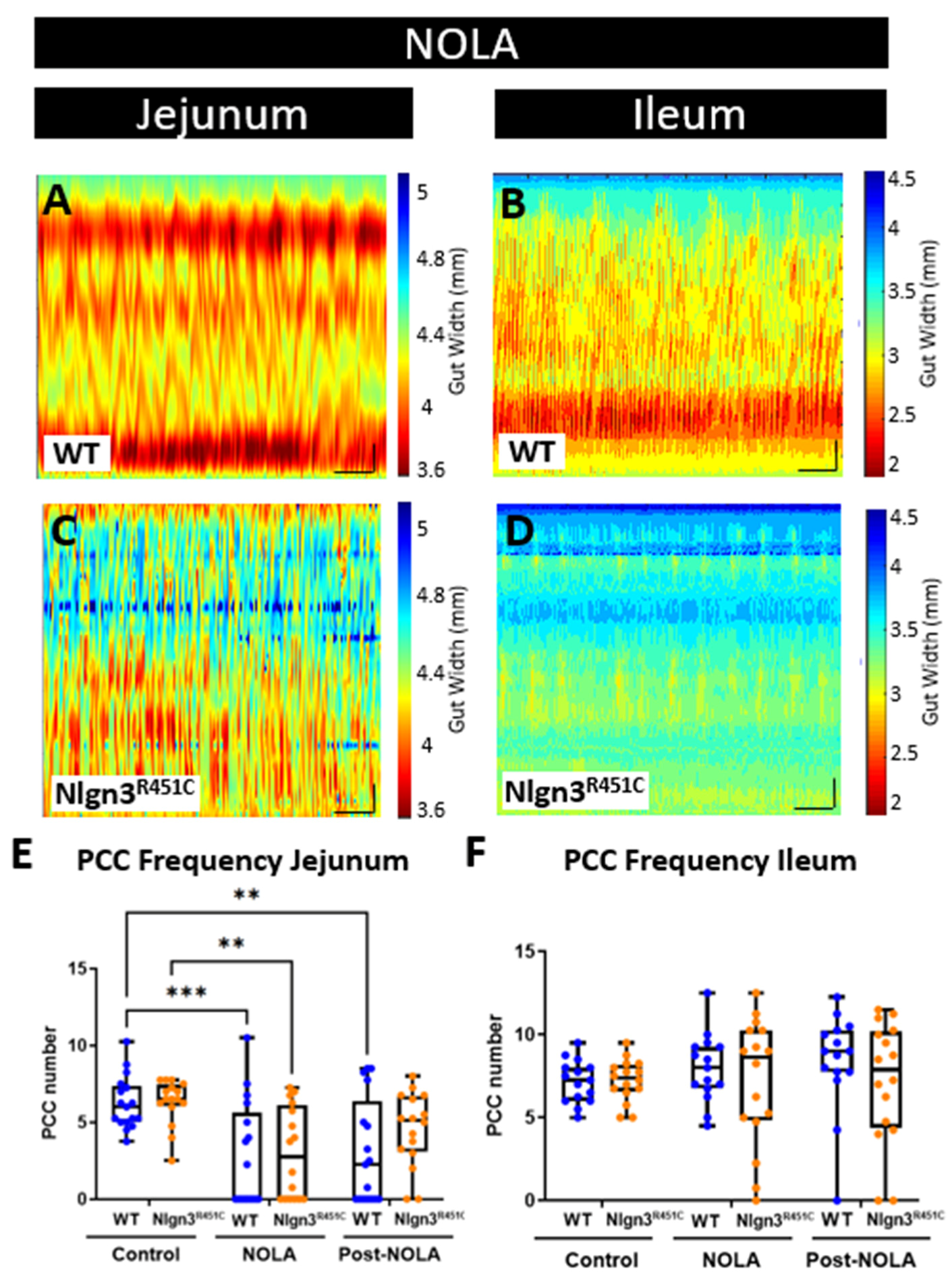
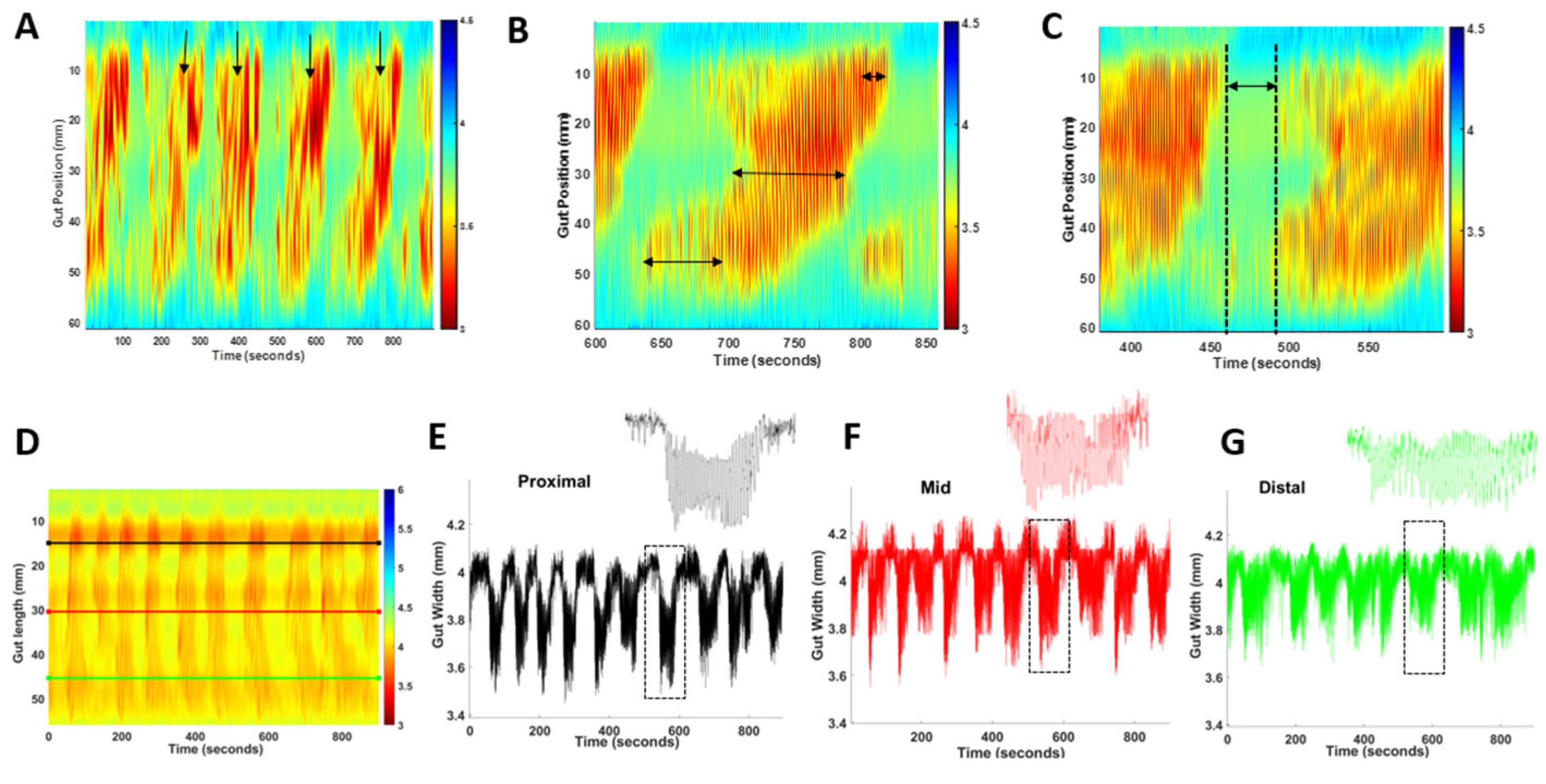
2.3. A Reduced Small Intestinal Resting Diameter and Increased Intra-PCC Frequency in Nlgn3R451C Mice
2.4. The Increased Short Colonic Contractions in Nlgn3R451C Mice
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Whole Body X-ray Imaging
4.3. Gut Length Measurement
4.4. Video Imaging Intestinal Motility
4.5. The Measurement of PCC Parameters: Frequency, Duration, Length and Velocity
4.6. Regional PCC Parameters
4.7. Maximum and Minimum Gut Diameter and Slow Wave Frequency
4.8. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- White, S.W.; Oswald, D.; Ollendick, T.; Scahill, L. Anxiety in children and adolescents with autism spectrum disorders. Clin. Psychol. Review. 2009, 29, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, S.E.; Srivorakiat, L.; Wink, L.K.; Pedapati, E.V.; Erickson, C.A. Aggression in autism spectrum disorder: Presentation and treatment options. Neuropsychiatr. Dis. Treat. 2016, 2016, 1525–1538. [Google Scholar] [CrossRef]
- McElhanon, B.O.; McCracken, C.; Karpen, S.; Sharp, W.G. Gastrointestinal symptoms in autism spectrum disorder: A meta-analysis. Pediatrics 2014, 133, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Margolis, K.G.; Buie, T.M.; Turner, J.B.; Silberman, A.E.; Feldman, J.F.; Murray, K.F.; McSwiggan-Hardin, M.; Levy, J.; Bauman, M.L.; Veenstra-VanderWeele, J.; et al. Development of a brief parent-report screen for common gastrointestinal disorders in autism spectrum disorder. J. Autism Dev. Disord. 2019, 49, 349–362. [Google Scholar] [CrossRef]
- Buie, T.; Campbell, D.B.; Fuchs, I.I.I.G.J.; Furuta, G.T.; Levy, J.; VandeWater, J.; Whitaker, A.H.; Atkins, D.; Bauman, M.L.; Beaudet, A.L.; et al. Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASDs: A consensus report. Pediatrics 2010, 125 (Supp. 1), S1–S8. [Google Scholar] [CrossRef]
- Coury, D.L.; Ashwood, P.; Fasano, A.; Fuchs, G.; Geraghty, M.; Kaul, A.; Mawe, G.; Patterson, P.; Jones, N.E. Gastrointestinal conditions in children with autism spectrum disorder: Developing a research agenda. Pediatrics 2012, 130 (Supp. 2), S160–S168. [Google Scholar] [CrossRef]
- Kohane, I.S.; McMurry, A.; Weber, G.; MacFadden, D.; Rappaport, L.; Kunkel, L.; Bickel, J.; Wattanasin, N.; Spence, S.; Murphy, S.; et al. The co-morbidity burden of children and young adults with autism spectrum disorders. PLoS ONE 2012, 7, e33224. [Google Scholar] [CrossRef]
- De Magistris, L.; Familiari, V.; Pascotto, A.; Sapone, A.; Frolli, A.; Iardino, P.; Carteni, M.; De Rosa, M.; Francavilla, R.; Riegler, G.; et al. Alterations of the intestinal barrier in patients with autism spectrum disorders and in their first-degree relatives. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 418–424. [Google Scholar] [CrossRef]
- Needham, B.D.; Adame, M.D.; Serena, G.; Rose, D.R.; Preston, G.M.; Conrad, M.C.; Campbell, A.S.; Donabedian, D.H.; Fasano, A.; Ashwood, P.; et al. Plasma and fecal metabolite profiles in autism spectrum disorder. Biol. Psychiatry 2021, 89, 451–462. [Google Scholar] [CrossRef]
- Sharon, G.; Cruz, N.J.; Kang, D.W.; Gandal, M.J.; Wang, B.; Kim, Y.M.; Zink, E.M.; Casey, C.P.; Taylor, B.C.; Lane, C.J.; et al. Human gut microbiota from autism spectrum disorder promote behavioral symptoms in mice. Cell 2019, 177, 1600–1618. [Google Scholar] [CrossRef]
- Arpi, M.N.T.; Simpson, T.I. SFARI genes and where to find them; modelling Autism Spectrum Disorder specific gene expression dysregulation with RNA-seq data. Sci. Rep. 2022, 12, 10158. [Google Scholar] [CrossRef] [PubMed]
- Etherton, M.; Földy, C.; Sharma, M.; Tabuchi, K.; Liu, X.; Shamloo, M.; Malenka, R.C.; Südhof, T.C. Autism-linked neuroligin-3 R451C mutation differentially alters hippocampal and cortical synaptic function. Proc. Natl. Acad. Sci. USA 2011, 108, 13764–13769. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Chen, J.; Chen, Q.; Ren, K.; Feng, D.; Mao, H.; Yao, H.; Yang, J.; Liu, H.; Liu, Y. Anterior cingulate cortex dysfunction underlies social deficits in Shank3 mutant mice. Nat. Neurosci. 2019, 22, 1223–1234. [Google Scholar] [CrossRef] [PubMed]
- Hosie, S.; Malone, D.T.; Liu, S.; Glass, M.; Adlard, P.A.; Hannan, A.J.; Hill-Yardin, E.L. Altered Amygdala Excitation and CB1 Receptor Modulation of Aggressive Behavior in the Neuroligin-3(R451C) Mouse Model of Autism. Front. Cell Neurosci. 2018, 12, 234. [Google Scholar] [CrossRef]
- Peça, J.; Feliciano, C.; Ting, J.T.; Wang, W.; Wells, M.F.; Venkatraman, T.N.; Lascola, C.D.; Fu, Z.; Feng, G. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature 2011, 472, 437–442. [Google Scholar] [CrossRef]
- Rothwell, P.E.; Fuccillo, M.V.; Maxeiner, S.; Hayton, S.J.; Gokce, O.; Lim, B.K.; Fowler, S.C.; Malenka, R.C.; Südhof, T.C. Autism-associated neuroligin-3 mutations commonly impair striatal circuits to boost repetitive behaviors. Cell 2014, 158, 198–212. [Google Scholar] [CrossRef]
- Schmeisser, M.J.; Ey, E.; Wegener, S.; Bockmann, J.; Stempel, A.V.; Kuebler, A.; Janssen, A.-L.; Udvardi, P.T.; Shiban, E.; Spilker, C.; et al. Autistic-like behaviours and hyperactivity in mice lacking ProSAP1/Shank2. Nature 2012, 486, 256–260. [Google Scholar] [CrossRef]
- Tabuchi, K.; Blundell, J.; Etherton, M.R.; Hammer, R.E.; Liu, X.; Powell, C.M.; Südhof, T.C. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science 2007, 318, 71–76. [Google Scholar] [CrossRef]
- Chai, A.P.; Chen, X.F.; Xu, X.S.; Zhang, N.; Li, M.; Li, J.N.; Zhang, L.; Zhang, D.; Zhang, X.; Mao, R.R.; et al. A temporal activity of CA1 neurons underlying short-term memory for social recognition altered in PTEN mouse models of autism spectrum disorder. Front. Cell. Neurosci. 2021, 15, 699315. [Google Scholar] [CrossRef]
- Földy, C.; Malenka, R.C.; Südhof, T.C. Autism-associated neuroligin-3 mutations commonly disrupt tonic endocannabinoid signaling. Neuron 2013, 78, 498–509. [Google Scholar] [CrossRef]
- Takumi, T.; Tamada, K.; Hatanaka, F.; Nakai, N.; Bolton, P.F. Behavioral neuroscience of autism. Neurosci. Biobehav. Rev. 2020, 110, 60–76. [Google Scholar] [CrossRef] [PubMed]
- Jurgensen, S.; Castillo, P.E. Selective dysregulation of hippocampal inhibition in the mouse lacking autism candidate gene CNTNAP2. J. Neurosci. 2015, 35, 14681–14687. [Google Scholar] [CrossRef] [PubMed]
- Guang, S.; Pang, N.; Deng, X.; Yang, L.; He, F.; Wu, L.; Chen, C.; Yin, F.; Peng, J. Synaptopathology involved in autism spectrum disorder. Front. Cell. Neurosci. 2018, 12, 470. [Google Scholar] [CrossRef] [PubMed]
- Hosie, S.; Ellis, M.; Swaminathan, M.; Ramalhosa, F.; Seger, G.O.; Balasuriya, G.K.; Gillberg, C.; Rastam, M.; Churilov, L.; McKeown, S.J.; et al. Gastrointestinal dysfunction in patients and mice expressing the autism-associated R451C mutation in neuroligin-3. Autism Res. 2019, 12, 1043–1056. [Google Scholar] [CrossRef]
- James, D.M.; Davidson, E.A.; Yanes, J.; Moshiree, B.; Dallman, J.E. The Gut-Brain-Microbiome Axis and Its Link to Autism: Emerging Insights and the Potential of Zebrafish Models. Front. Cell Dev. Biol. 2021, 9, 662916. [Google Scholar] [CrossRef]
- Lee, C.Y.Q.; Franks, A.E.; Hill-Yardin, E.L. Autism-associated synaptic mutations impact the gut-brain axis in mice. Brain Behav. Immun. 2020, 88, 275–282. [Google Scholar] [CrossRef]
- Lee, C.Y.Q.; Balasuriya, G.K.; Herath, M.; Franks, A.E.; Hill-Yardin, E.L. Impaired cecal motility and secretion alongside expansion of gut-associated lymphoid tissue in the Nlgn3(R451C) mouse model of autism. Sci. Rep. 2023, 13, 12687. [Google Scholar] [CrossRef]
- Sauer, A.K.; Bockmann, J.; Steinestel, K.; Boeckers, T.M.; Grabrucker, A.M. Altered Intestinal Morphology and Microbiota Composition in the Autism Spectrum Disorders Associated SHANK3 Mouse Model. Int. J. Mol. Sci. 2019, 20, 2134. [Google Scholar] [CrossRef]
- Sharna, S.S.; Balasuriya, G.K.; Hosie, S.; Nithianantharajah, J.; Franks, A.E.; Hill-Yardin, E.L. Altered Caecal Neuroimmune Interactions in the Neuroligin-3(R451C) Mouse Model of Autism. Front. Cell Neurosci. 2020, 14, 85. [Google Scholar] [CrossRef]
- De Theije, C.G.; Wopereis, H.; Ramadan, M.; van Eijndthoven, T.; Lambert, J.; Knol, J.; Garssen, J.; Kraneveld, A.D.; Oozeer, R. Altered gut microbiota and activity in a murine model of autism spectrum disorders. Brain Behav. Immun. 2014, 37, 197–206. [Google Scholar] [CrossRef]
- Margolis, K.G.; Li, Z.; Stevanovic, K.; Saurman, V.; Israelyan, N.; Anderson, G.M.; Snyder, I.; Veenstra-VanderWeele, J.; Blakely, R.D.; Gershon, M.D. Serotonin transporter variant drives preventable gastrointestinal abnormalities in development and function. J. Clin. Investig. 2016, 126, 2221–2235. [Google Scholar] [CrossRef] [PubMed]
- Sgritta, M.; Dooling, S.W.; Buffington, S.A.; Momin, E.N.; Francis, M.B.; Britton, R.A.; Costa-Mattioli, M. Mechanisms underlying microbial-mediated changes in social behavior in mouse models of autism spectrum disorder. Neuron 2019, 101, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Bernier, R.; Golzio, C.; Xiong, B.; Stessman, H.A.; Coe, B.P.; Penn, O.; Witherspoon, K.; Gerdts, J.; Baker, C.; Vulto-van Silfhout, A.T.; et al. Disruptive CHD8 mutations define a subtype of autism early in development. Cell 2014, 158, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Baudouin, S.J.; Gaudias, J.; Gerharz, S.; Hatstatt, L.; Zhou, K.; Punnakkal, P.; Tanaka, K.F.; Spooren, W.; Hen, R.; De Zeeuw, C.I.; et al. Shared synaptic pathophysiology in syndromic and nonsyndromic rodent models of autism. Science 2012, 338, 128–132. [Google Scholar] [CrossRef]
- Budreck, E.C.; Scheiffele, P. Neuroligin-3 is a neuronal adhesion protein at GABAergic and glutamatergic synapses. Eur. J. Neurosci. 2007, 26, 1738–1748. [Google Scholar] [CrossRef]
- Uchigashima, M.; Leung, M.; Watanabe, T.; Cheung, A.; Le, T.; Pallat, S.; Dinis, A.L.M.; Watanabe, M.; Kawasawa, Y.I.; Futai, K. Neuroligin3 splice isoforms shape inhibitory synaptic function in the mouse hippocampus. J. Biol. Chem. 2020, 295, 8589–8595. [Google Scholar] [CrossRef]
- Herath, M.; Cho, E.; Marklund, U.; Franks, A.E.; Bornstein, J.C.; Hill-Yardin, E.L. Quantitative Spatial Analysis of Neuroligin-3 mRNA Expression in the Enteric Nervous System Reveals a Potential Role in Neuronal-Glial Synapses and Reduced Expression in Nlgn3(R451C) Mice. Biomolecules 2023, 13, 1063. [Google Scholar] [CrossRef]
- Gilbert, M.; Smith, J.; Roskams, A.J.; Auld, V.J. Neuroligin 3 is a vertebrate gliotactin expressed in the olfactory ensheathing glia, a growth-promoting class of macroglia. Glia 2001, 34, 151–164. [Google Scholar] [CrossRef]
- Jamain, S.; Quach, H.; Betancur, C.; Råstam, M.; Colineaux, C.; Gillberg, I.C.; Soderstrom, H.; Giros, B.; Leboyer, M.; Gillberg, C. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat. Genet. 2003, 34, 27–29. [Google Scholar] [CrossRef]
- Chubykin, A.A.; Liu, X.; Comoletti, D.; Tsigelny, I.; Taylor, P.; Südhof, T.C. Dissection of synapse induction by neuroligins: Effect of a neuroligin mutation associated with autism. J. Biol. Chem. 2005, 280, 22365–22374. [Google Scholar] [CrossRef]
- Comoletti, D.; De Jaco, A.; Jennings, L.L.; Flynn, R.E.; Gaietta, G.; Tsigelny, I.; Ellisman, M.H.; Taylor, P. The Arg451Cys-neuroligin-3 mutation associated with autism reveals a defect in protein processing. J. Neurosci. 2004, 24, 4889–4893. [Google Scholar] [CrossRef] [PubMed]
- Burrows, E.L.; Laskaris, L.; Koyama, L.; Churilov, L.; Bornstein, J.C.; Hill-Yardin, E.L.; Hannan, A.J. A neuroligin-3 mutation implicated in autism causes abnormal aggression and increases repetitive behavior in mice. Mol. Autism 2015, 6, 62. [Google Scholar] [CrossRef] [PubMed]
- Chadman, K.K.; Gong, S.; Scattoni, M.L.; Boltuck, S.E.; Gandhy, S.U.; Heintz, N.; Crawley, J.N. Minimal aberrant behavioral phenotypes of neuroligin-3 R451C knockin mice. Autism Res. 2008, 1, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Herath, M.; Bornstein, J.C.; Hill-Yardin, E.L.; Franks, A.E. The autism-associated Neuroligin-3 R451C mutation alters mucus density and the spatial distribution of bacteria in the mouse gastrointestinal tract. bioRxiv 2022. bioRxiv:06.27.497808. [Google Scholar] [CrossRef]
- Costa, M.; Spencer, N.J.; Brookes, S.J. The role of enteric inhibitory neurons in intestinal motility. Auton. Neurosci. 2021, 235, 102854. [Google Scholar] [CrossRef]
- Hennig, G.W.; Costa, M.; Chen, B.N.; Brookes, S.J. Quantitative analysis of peristalsis in the guinea-pig small intestine using spatio-temporal maps. J. Physiol. 1999, 517 (Pt. 2), 575–590. [Google Scholar] [CrossRef]
- Neal, K.B.; Parry, L.J.; Bornstein, J.C. Strain-specific genetics, anatomy and function of enteric neural serotonergic pathways in inbred mice. J. Physiol. 2009, 587, 567–586. [Google Scholar] [CrossRef]
- Abdu, F.; Hicks, G.A.; Hennig, G.; Allen, J.P.; Grundy, D. Somatostatin sst(2) receptors inhibit peristalsis in the rat and mouse jejunum. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 282, G624–G633. [Google Scholar] [CrossRef]
- Costa, M.; Hibberd, T.J.; Keightley, L.J.; Wiklendt, L.; Arkwright, J.W.; Dinning, P.G.; Brookes, S.J.H.; Spencer, N.J. Neural motor complexes propagate continuously along the full length of mouse small intestine and colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G99–G108. [Google Scholar] [CrossRef]
- Seerden, T.C.; Lammers, W.J.; De Winter, B.Y.; De Man, J.G.; Pelckmans, P.A. Spatiotemporal electrical and motility mapping of distension-induced propagating oscillations in the murine small intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 289, G1043–G1051. [Google Scholar] [CrossRef]
- Balasuriya, G.K.; Nugapitiya, S.S.; Hill-Yardin, E.L.; Bornstein, J.C. Nitric Oxide Regulates Estrus Cycle Dependent Colonic Motility in Mice. Front. Neurosci. 2021, 15, 647555. [Google Scholar] [CrossRef] [PubMed]
- Leembruggen, A.J.; Balasuriya, G.K.; Zhang, J.; Schokman, S.; Swiderski, K.; Bornstein, J.C.; Nithianantharajah, J.; Hill-Yardin, E.L. Colonic dilation and altered ex vivo gastrointestinal motility in the Neuroligin-3 knockout mouse. Autism Res. 2020, 13, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, M.; Hill-Yardin, E.; Ellis, M.; Zygorodimos, M.; Johnston, L.A.; Gwynne, R.M.; Bornstein, J.C. Video Imaging and Spatiotemporal Maps to Analyze Gastrointestinal Motility in Mice. J. Vis. Exp. 2016, 108, 53828. [Google Scholar] [CrossRef]
- Abo-Shaban, T.; Lee, C.Y.Q.; Hosie, S.; Balasuriya, G.K.; Mohsenipour, M.; Johnston, L.A.; Hill-Yardin, E.L. GutMap: A New Interface for Analysing Regional Motility Patterns in ex vivo Mouse Gastrointestinal Preparations. Bio Protoc. 2023, 13, e4831. [Google Scholar] [CrossRef]
- Seifi, M.; Brown, J.F.; Mills, J.; Bhandari, P.; Belelli, D.; Lambert, J.J.; Rudolph, U.; Swinny, J.D. Molecular and functional diversity of GABA-A receptors in the enteric nervous system of the mouse colon. J. Neurosci. 2014, 34, 10361–10378. [Google Scholar] [CrossRef]
- Koussoulas, K.; Swaminathan, M.; Fung, C.; Bornstein, J.C.; Foong, J.P.P. Neurally Released GABA Acts via GABA(C) Receptors to Modulate Ca(2+) Transients Evoked by Trains of Synaptic Inputs, but Not Responses Evoked by Single Stimuli, in Myenteric Neurons of Mouse Ileum. Front. Physiol. 2018, 9, 97. [Google Scholar] [CrossRef]
- Sang, Q.; Young, H.M. Chemical coding of neurons in the myenteric plexus and external muscle of the small and large intestine of the mouse. Cell Tissue Res. 1996, 284, 39–53. [Google Scholar] [CrossRef]
- Schieve, L.A.; Rice, C.; Yeargin-Allsopp, M.; Boyle, C.A.; Kogan, M.D.; Drews, C.; Devine, O. Parent-reported prevalence of autism spectrum disorders in US-born children: An assessment of changes within birth cohorts from the 2003 to the 2007 National Survey of Children’s Health. Matern. Child Health J. 2012, 16 (Suppl. S1), S151–S157. [Google Scholar] [CrossRef]
- McQuade, R.M.; Stojanovska, V.; Donald, E.; Abalo, R.; Bornstein, J.C.; Nurgali, K. Gastrointestinal dysfunction and enteric neurotoxicity following treatment with anticancer chemotherapeutic agent 5-fluorouracil. Neurogastroenterol. Motil. 2016, 28, 1861–1875. [Google Scholar] [CrossRef]
- McQuade, R.M.; Stojanovska, V.; Stavely, R.; Timpani, C.; Petersen, A.C.; Abalo, R.; Bornstein, J.C.; Rybalka, E.; Nurgali, K. Oxaliplatin-induced enteric neuronal loss and intestinal dysfunction is prevented by co-treatment with BGP-15. Br. J. Pharmacol. 2018, 175, 656–677. [Google Scholar] [CrossRef]
- McQuade, R.M.; Stojanovska, V.; Donald, E.L.; Rahman, A.A.; Campelj, D.G.; Abalo, R.; Rybalka, E.; Bornstein, J.C.; Nurgali, K. Irinotecan-Induced Gastrointestinal Dysfunction Is Associated with Enteric Neuropathy, but Increased Numbers of Cholinergic Myenteric Neurons. Front. Physiol. 2017, 8, 391. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.M.; Rahman, A.A.; Carbone, S.E.; Randall-Demllo, S.; Filippone, R.; Bornstein, J.C.; Eri, R.; Nurgali, K. Alterations of colonic function in the Winnie mouse model of spontaneous chronic colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G85–G102. [Google Scholar] [CrossRef] [PubMed]
- Sahakian, L.; Filippone, R.T.; Stavely, R.; Robinson, A.M.; Yan, X.S.; Abalo, R.; Eri, R.; Bornstein, J.C.; Kelley, M.R.; Nurgali, K. Inhibition of APE1/Ref-1 Redox Signaling Alleviates Intestinal Dysfunction and Damage to Myenteric Neurons in a Mouse Model of Spontaneous Chronic Colitis. Inflamm. Bowel Dis. 2021, 27, 388–406. [Google Scholar] [CrossRef] [PubMed]
- Venkataramana, S.; Lourenssen, S.; Miller, K.G.; Blennerhassett, M.G. Early inflammatory damage to intestinal neurons occurs via inducible nitric oxide synthase. Neurobiol. Dis. 2015, 75, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Huizinga, J.D.; Chen, J.H.; Fang Zhu, Y.; Pawelka, A.; McGinn, R.J.; Bardakjian, B.L.; Parsons, S.P.; Kunze, W.A.; Wu, R.Y.; Bercik, P.; et al. The origin of segmentation motor activity in the intestine. Nat. Commun. 2014, 5, 3326. [Google Scholar] [CrossRef]
- Spencer, N.J.; Hu, H. Enteric nervous system: Sensory transduction, neural circuits and gastrointestinal motility. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 338–351. [Google Scholar] [CrossRef]
- Brenman, J.E.; Chao, D.S.; Gee, S.H.; McGee, A.W.; Craven, S.E.; Santillano, D.R.; Wu, Z.; Huang, F.; Xia, H.; Peters, M.F.; et al. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell 1996, 84, 757–767. [Google Scholar] [CrossRef]
- Coley, A.A.; Gao, W.J. PSD95: A synaptic protein implicated in schizophrenia or autism? Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 82, 187–194. [Google Scholar] [CrossRef]
- Irie, M.; Hata, Y.; Takeuchi, M.; Ichtchenko, K.; Toyoda, A.; Hirao, K.; Takai, Y.; Rosahl, T.W.; Südhof, T.C. Binding of neuroligins to PSD-95. Science 1997, 277, 1511–1515. [Google Scholar] [CrossRef]
- Costa, M.; Wiklendt, L.; Arkwright, J.; Spencer, N.; Omari, T.; Brookes, S.J.; Dinning, P. An experimental method to identify neurogenic and myogenic active mechanical states of intestinal motility. Front. Syst. Neurosci. 2013, 11, 7. [Google Scholar] [CrossRef]
- Huizinga, J.D.; Chen, J.-H. Interstitial cells of Cajal: Update on basic and clinical science. Curr. Gastroenterol. Rep. 2014, 16, 363. [Google Scholar] [CrossRef] [PubMed]
- Huizinga, J.D.; Lammers, W.J.E.P. Gut peristalsis is governed by a multitude of cooperating mechanisms. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G1–G8. [Google Scholar] [CrossRef] [PubMed]
- Sanders, K.M.; Koh, S.D.; Ward, S.M. Interstitial cells of Cajal as pacemakers in the gastrointestinal tract. Annu. Rev. Physiol. 2006, 68, 307–343. [Google Scholar] [CrossRef] [PubMed]
- Swiderski, K.; Bindon, R.; Trieu, J.; Naim, T.; Schokman, S.; Swaminathan, M.; Leembruggen, A.J.L.; Hill-Yardin, E.L.; Koopman, R.; Bornstein, J.C.; et al. Spatiotemporal Mapping Reveals Regional Gastrointestinal Dysfunction in mdx Dystrophic Mice Ameliorated by Oral L-arginine Supplementation. J. Neurogastroenterol. Motil. 2020, 26, 133–146. [Google Scholar] [CrossRef]




| Small Intestinal Motility Parameter | Jejunum | Ileum | ||||
|---|---|---|---|---|---|---|
| WT n = 17 | Nlgn3R451C n = 16 | Normality Passed? | WT n = 16 | Nlgn3R451C n = 16 | Normality Passed? | |
| Full length PCC Frequency (No. of PCCs/15 min) | 6.3 ± 0.4 | 6.3 ± 0.4 | Yes. WT: w = 0.9, p = 0.3. Nlgn3R451C: w = 0.9, p = 0.051 | 7.2 ± 0.3 | 7.2 ± 0.3 | Yes. WT: w = 0.99, p > 0.99. Nlgn3R451C: w = 0.97, p = 0.8 |
| Full length PCC Quiescence (s) | 39.4 ± 5.3 | 37.6 ± 5.8 | Yes. WT: w = 0.9, p = 0.2. Nlgn3R451C: w = 0.9, p = 0.3 | 35.8 ± 2.7 | 54.3 ± 6.8 * | Yes. WT: w = 0.96, p = 0.6. Nlgn3R451C: w = 0.9, p = 0.06 |
| Full length PCC Velocity (mm/s) | 0.5 ± 0.02 | 0.6 ± 0.06 | Yes. WT: w = 0.9, p = 0.3. Nlgn3R451C: w = 0.9, p = 0.4 | −0.3 ± 0.1 | −0.4 ± 0.2 | Yes. WT: w = 0.9, p = 0.4. Nlgn3R451C: w = 0.98, p = 0.9 |
| Proximal region PCC duration (s) | 30.9 ± 4 | 30.8 ± 4.5 | Yes. WT: w = 0.9, p = 0.1. Nlgn3R451C: w = 0.96, p = 0.7 | 74.1 ± 5.7 | 58.5 ± 4 | Yes. WT: w = 0.9, p = 0.2. Nlgn3R451C: w = 0.96, p = 0.7 |
| Mid region PCC duration (s) | 41.6 ± 4.3 | 39.4 ± 5.6 | Yes. WT: w = 0.9, p = 0.1. Nlgn3R451C: w = 0.9, p = 0.4 | 85.2 ± 5.6 | 63.4 ± 3.7 † | Yes. WT: w = 0.98, p = 0.98. Nlgn3R451C: w = 0.96, p = 0.7 |
| Distal region PCC duration (s) | 34.5 ± 2.8 | 34.4 ± 7.8 | Yes. WT: w = 0.97, p = 0.7. Nlgn3R451C: w = 0.9, p = 0.4 | 88.3 ± 6.1 | 61.4 ± 6.5 † | Yes. WT: w = 0.99, p = 0.99. Nlgn3R451C: w = 0.9, p = 0.2 |
| Small Intestinal Motility Parameters | Jejunum | Ileum | |||||
|---|---|---|---|---|---|---|---|
| WT n = 17 | Nlgn3R451C n = 16 | Normality Passed? | WT n = 16 | Nlgn3R451C n = 16 | Normality Passed? | ||
| Resting diameter (mm) | Prox | 4.7 ± 0.1 | 4.2 ± 0.1 * | Yes. WT: w = 0.9, p = 0.3. Nlgn3R451C: w = 0.9, p = 0.1 | 3.4 ± 0.1 | 3.6 ± 0.1 | Yes. WT: w = 0.98, p = 0.96. Nlgn3R451C: w = 0.9, p = 0.07 |
| Mid | 4.7 ± 0.1 | 4.2 ± 0.1 * | Yes. WT: w = 0.9, p = 0.3. Nlgn3R451C: w = 0.96, p = 0.6 | 3.3 ± 0.1 | 3.4 ± 0.1 | Yes. WT: w = 0.98, p = 0.9. Nlgn3R451C: w = 0.9, p = 0.07 | |
| Distal | 4.9 ± 0.1 | 4.3 ± 0.1 † | Yes. WT: w = 0.9, p = 0.4. Nlgn3R451C: w = 0.95, p = 0.5 | 3.2 ± 0.1 | 3.3 ± 0.1 | Yes. WT: w = 0.9, p = 0.2. Nlgn3R451C: w = 0.9, p = 0.1 | |
| Constricted diameter (mm) | Prox | 3.9 ± 0.1 | 3.5 ± 0.1 | Yes. WT: w = 0.9, p = 0.3. Nlgn3R451C: w = 0.9, p = 0.1 | 2.7 ± 0.1 | 2.9 ± 0.1 | Yes. WT: w = 0.95, p = 0.6. Nlgn3R451C: w = 0.9, p = 0.06 |
| Mid | 3.7 ± 0.1 | 3.5 ± 0.1 | Yes. WT: w = 0.9, p = 0.3. Nlgn3R451C: w = 0.95, p = 0.5 | 2.5 ± 0.1 | 2.7 ± 0.1 | Yes. WT: w = 0.98, p = 0.9. Nlgn3R451C: w = 0.9, p = 0.2 | |
| Distal | 4.0 ± 0.1 | 3.6 ± 0.1 * | Yes. WT: w = 0.98, p = 0.95. Nlgn3R451C: w = 0.9, p = 0.4 | 2.5 ± 0.1 | 2.7 ± 0.1 | Yes. WT: w = 0.9, p = 0.3. Nlgn3R451C: w = 0.9, p = 0.1 | |
| Intra PCC frequency (sec−1) | Prox | CI: 0.5–0.7 | CI: 0.6–0.7 * | No. w = 0.95, p = 0.0002 | CI: 0.3–0.6 | CI: 0.4–0.5 | No. w = 0.91, p < 0.0001 |
| Mid | CI:0.5–0.7 | CI: 0.6–0.7 * | CI: 0.3–0.6 | CI: 0.4–0.6 | |||
| Distal | CI: 0.5–0.7 | CI: 0.5–0.6 * | CI: 0.4–0.6 | CI: 0.4–0.5 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hosie, S.; Abo-Shaban, T.; Mou, K.; Balasuriya, G.K.; Mohsenipour, M.; Alamoudi, M.U.; Filippone, R.T.; Belz, G.T.; Franks, A.E.; Bornstein, J.C.; et al. Faster Gastrointestinal Transit, Reduced Small Intestinal Smooth Muscle Tone and Dysmotility in the Nlgn3R451C Mouse Model of Autism. Int. J. Mol. Sci. 2024, 25, 832. https://doi.org/10.3390/ijms25020832
Hosie S, Abo-Shaban T, Mou K, Balasuriya GK, Mohsenipour M, Alamoudi MU, Filippone RT, Belz GT, Franks AE, Bornstein JC, et al. Faster Gastrointestinal Transit, Reduced Small Intestinal Smooth Muscle Tone and Dysmotility in the Nlgn3R451C Mouse Model of Autism. International Journal of Molecular Sciences. 2024; 25(2):832. https://doi.org/10.3390/ijms25020832
Chicago/Turabian StyleHosie, Suzanne, Tanya Abo-Shaban, Kevin Mou, Gayathri K. Balasuriya, Mitra Mohsenipour, Mohammed U. Alamoudi, Rhiannon T. Filippone, Gabrielle T. Belz, Ashley E. Franks, Joel C. Bornstein, and et al. 2024. "Faster Gastrointestinal Transit, Reduced Small Intestinal Smooth Muscle Tone and Dysmotility in the Nlgn3R451C Mouse Model of Autism" International Journal of Molecular Sciences 25, no. 2: 832. https://doi.org/10.3390/ijms25020832
APA StyleHosie, S., Abo-Shaban, T., Mou, K., Balasuriya, G. K., Mohsenipour, M., Alamoudi, M. U., Filippone, R. T., Belz, G. T., Franks, A. E., Bornstein, J. C., Nurgali, K., & Hill-Yardin, E. L. (2024). Faster Gastrointestinal Transit, Reduced Small Intestinal Smooth Muscle Tone and Dysmotility in the Nlgn3R451C Mouse Model of Autism. International Journal of Molecular Sciences, 25(2), 832. https://doi.org/10.3390/ijms25020832






