Aptamer-Based Recognition of Breast Tumor Cells: A New Era for Breast Cancer Diagnosis
Abstract
1. Introduction
2. Results
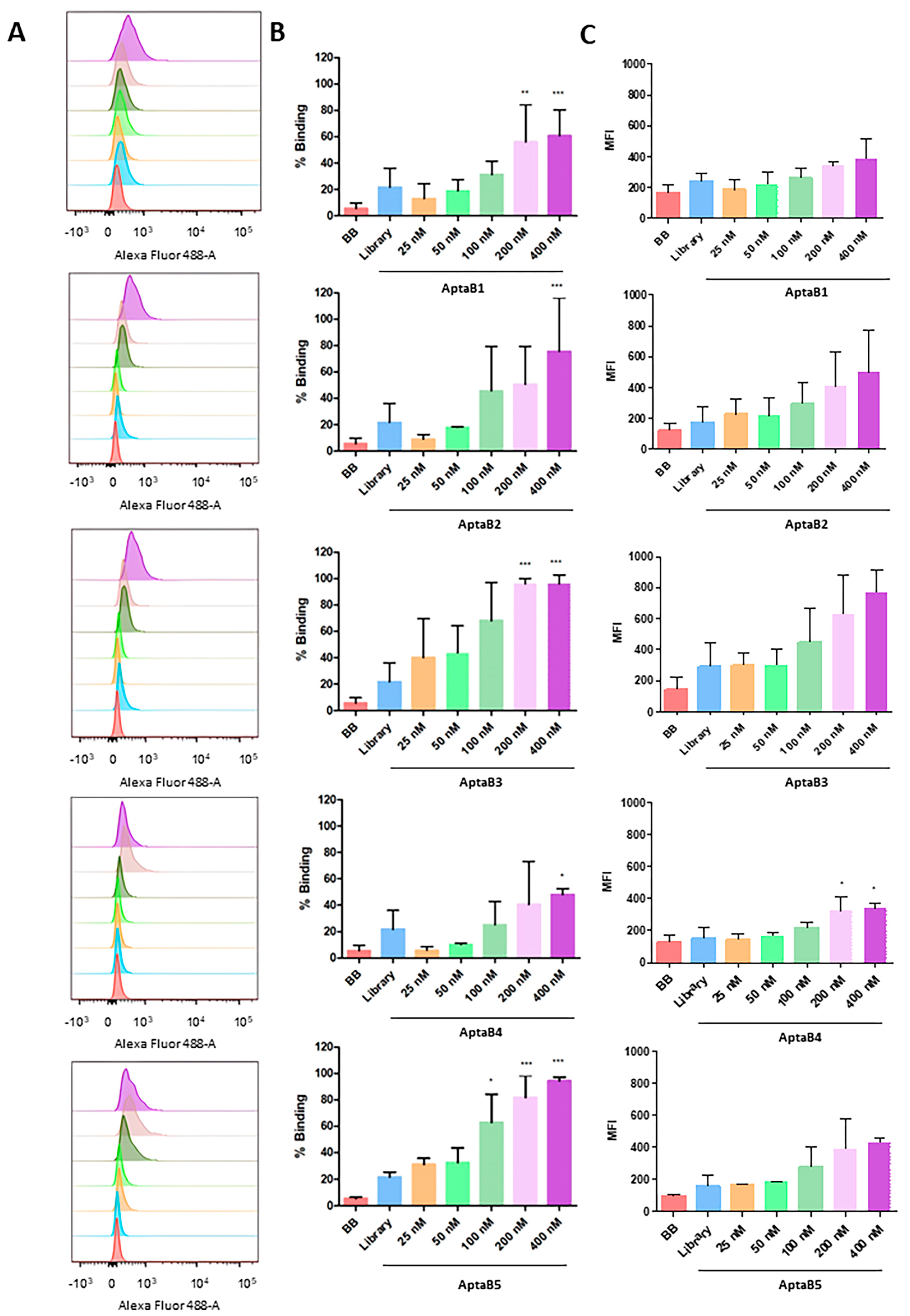
2.1. Selected Aptamers Specifically Recognize Breast Tumor Cells
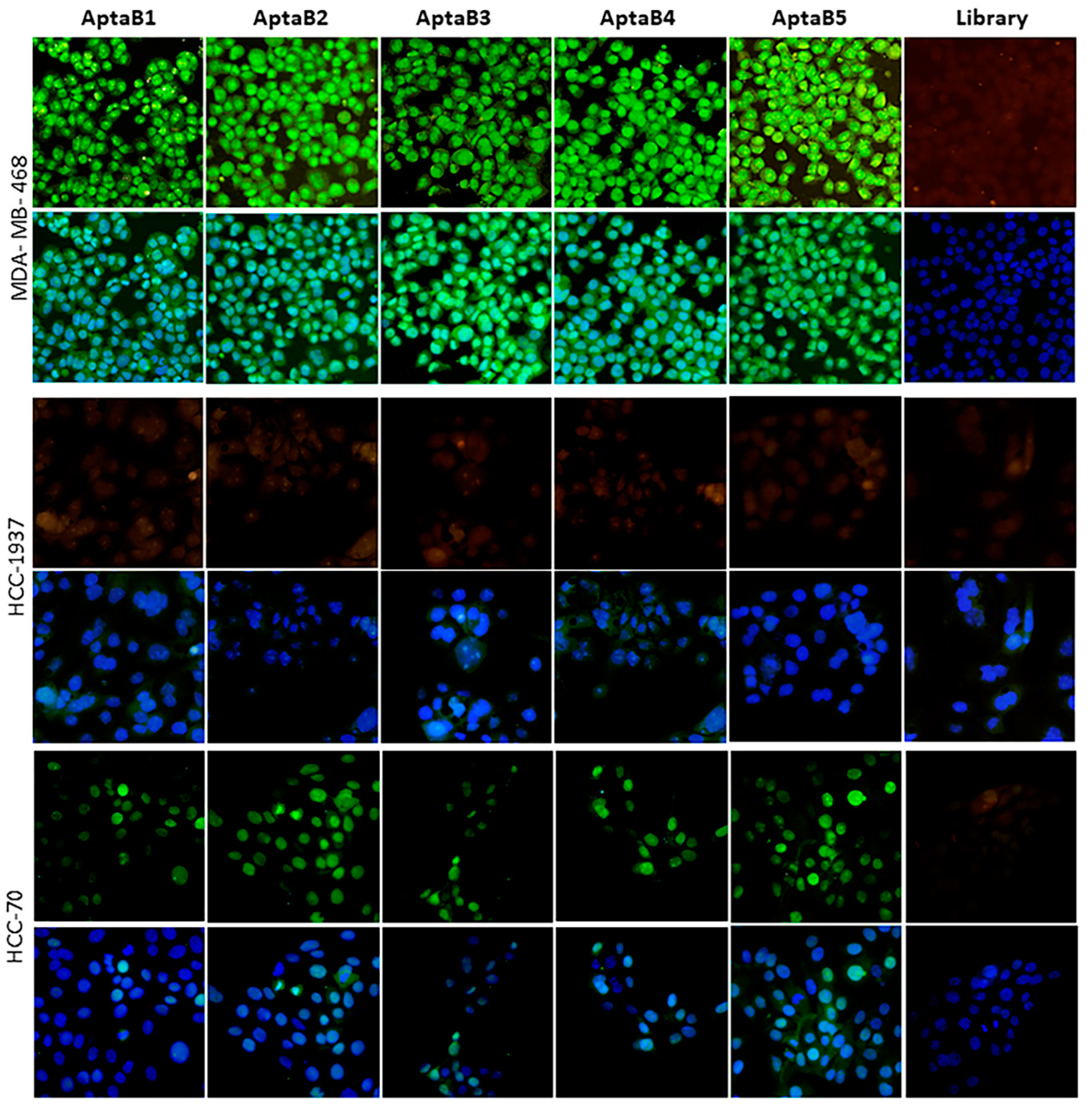
2.2. Subcellular Localization of Aptamers in MDA-MB-231 Cells
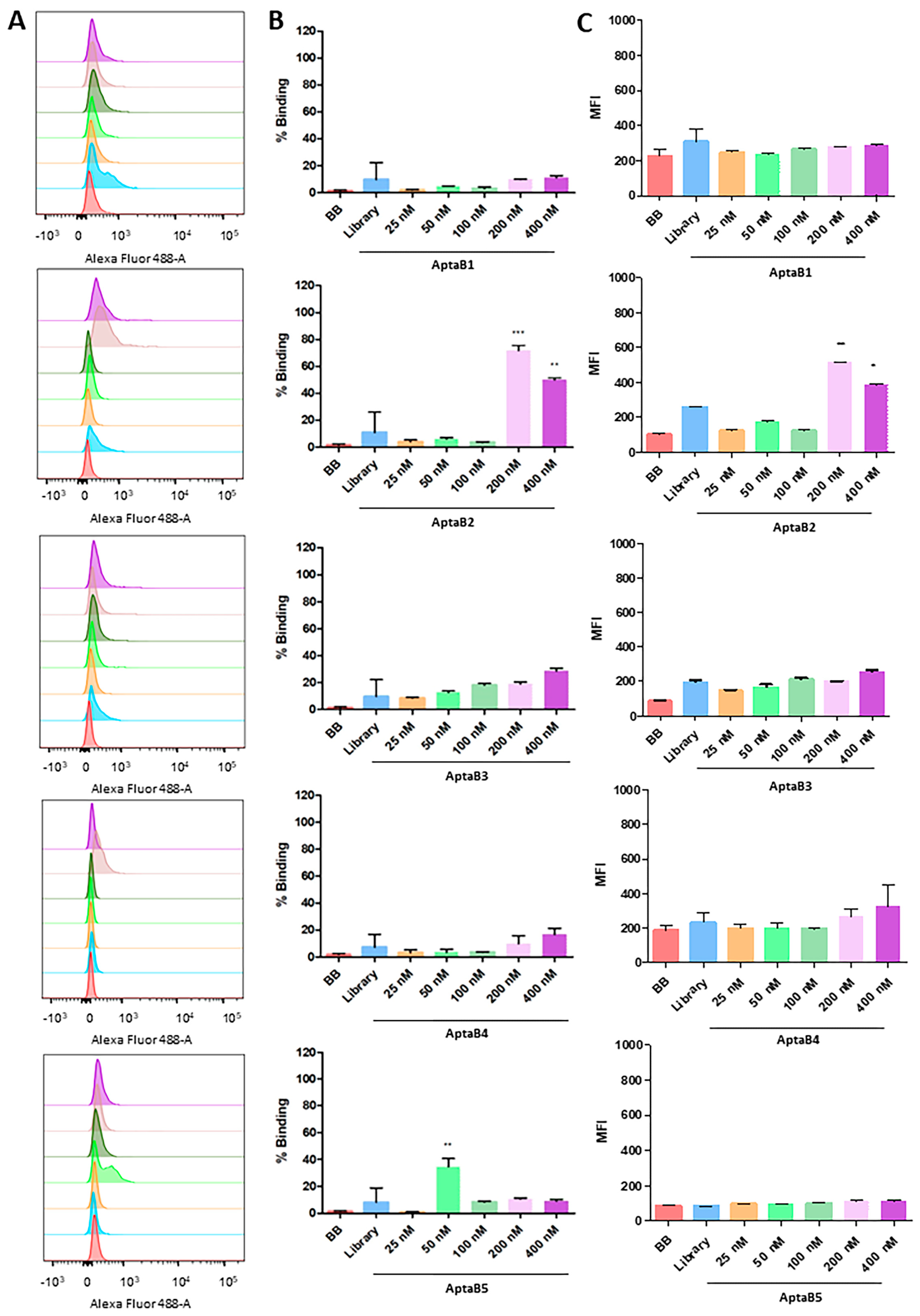
2.3. Aptamers Detect an Expanded Panel of Triple-Negative Breast Tumor Cells
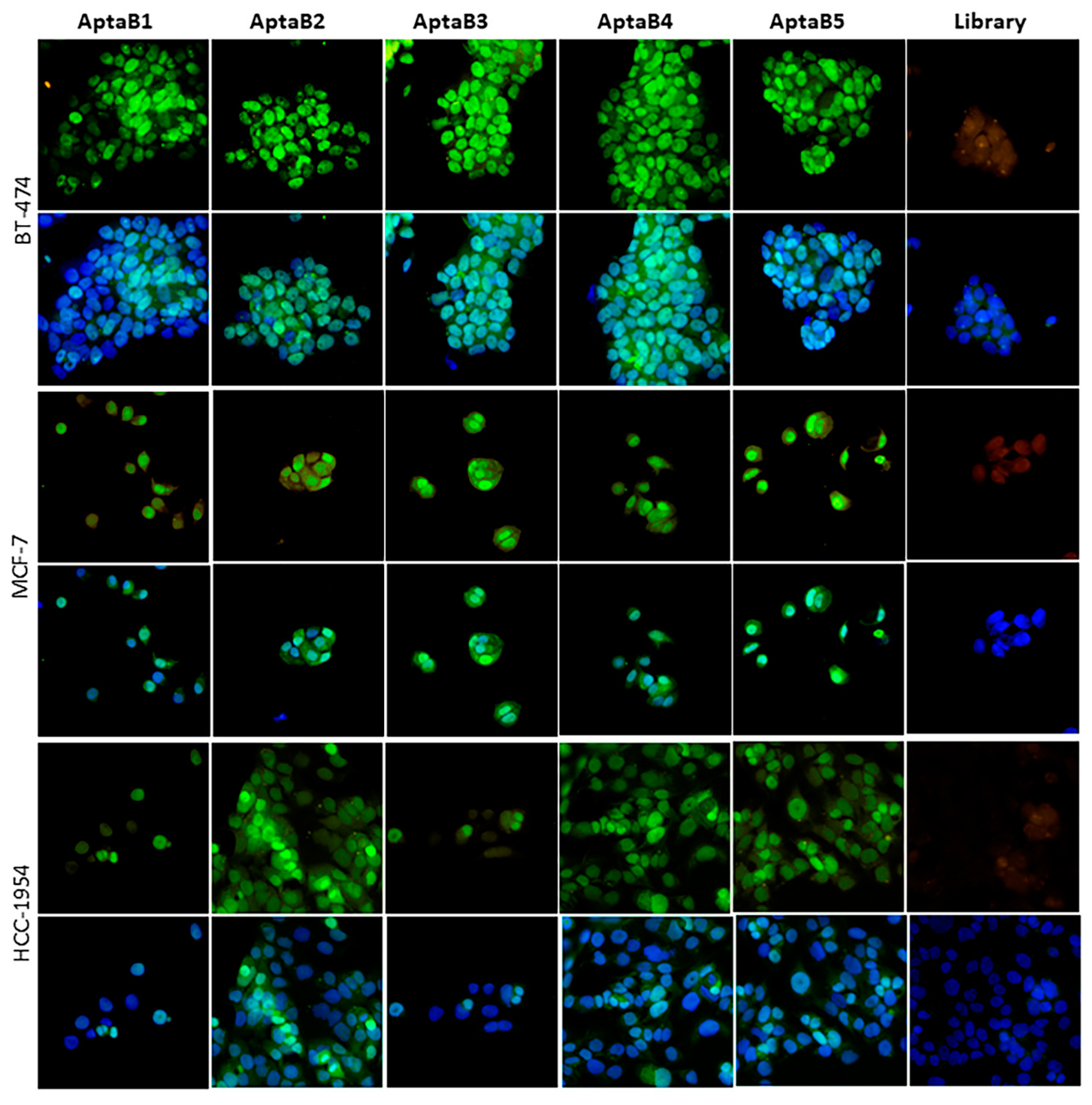
2.4. The Aptamer Panel Detects Breast Tumor Cells from the Luminal A, Luminal B, and HER 2 Molecular Subtypes
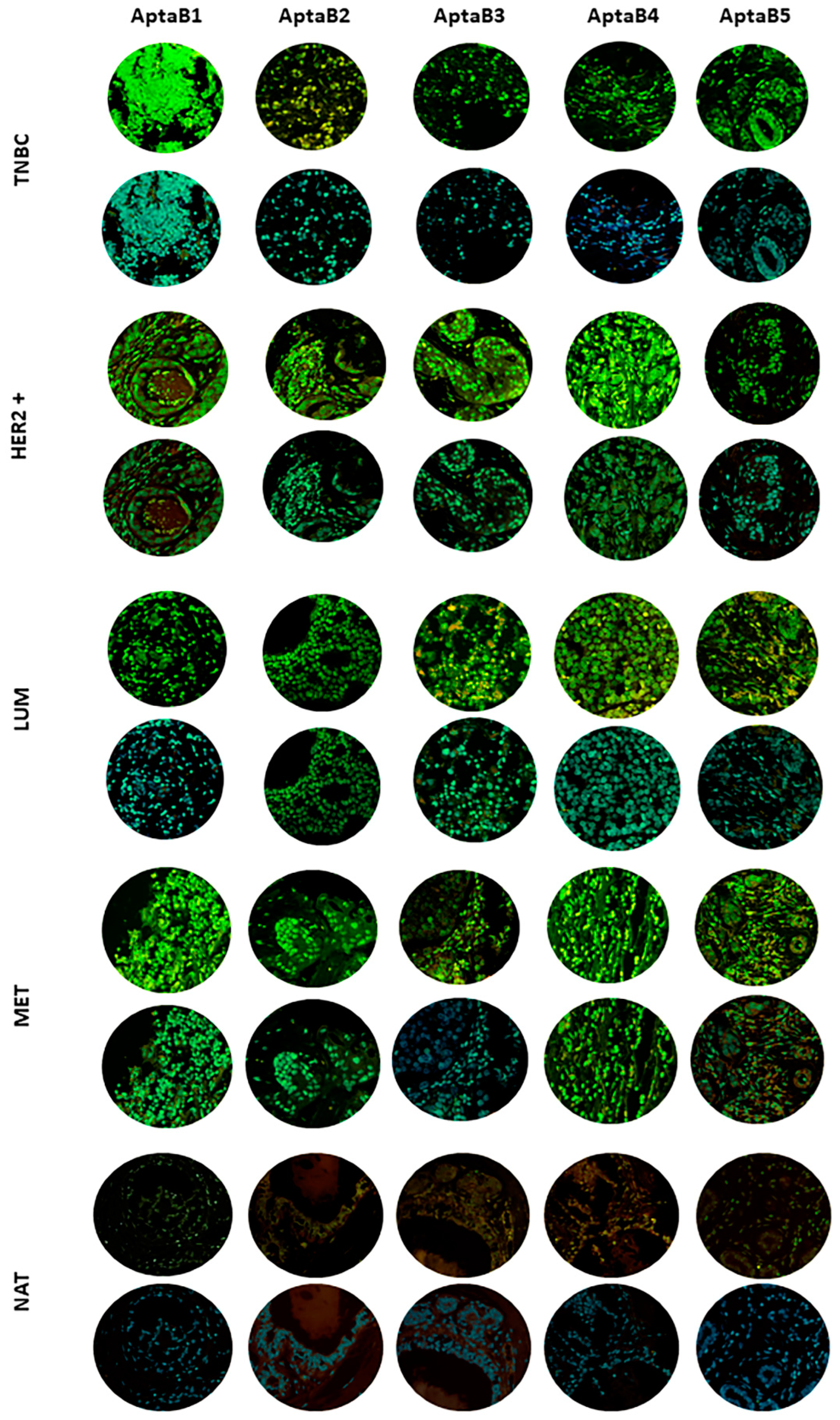
2.5. Aptamers Detect Human Breast Tumor Clinical Samples from Different Subtypes
2.6. Evaluation of the Aptamer Panel as a Diagnostic Tool
2.7. In Silico Characterization of the Three-Dimensional Structure of Aptamers and Selection of Potential Recognition Targets
2.8. Three-Dimensional Structural Characterization of Selected Aptamers for MDA-MB-231 Cells
2.9. Aptamer Structures Are Stable in Aqueous Solution
2.10. Search for Potential Aptamer Targets in MDA-MB-231 Cell Lines
2.11. Characterizing Protein–Aptamer Complexes using Molecular Docking
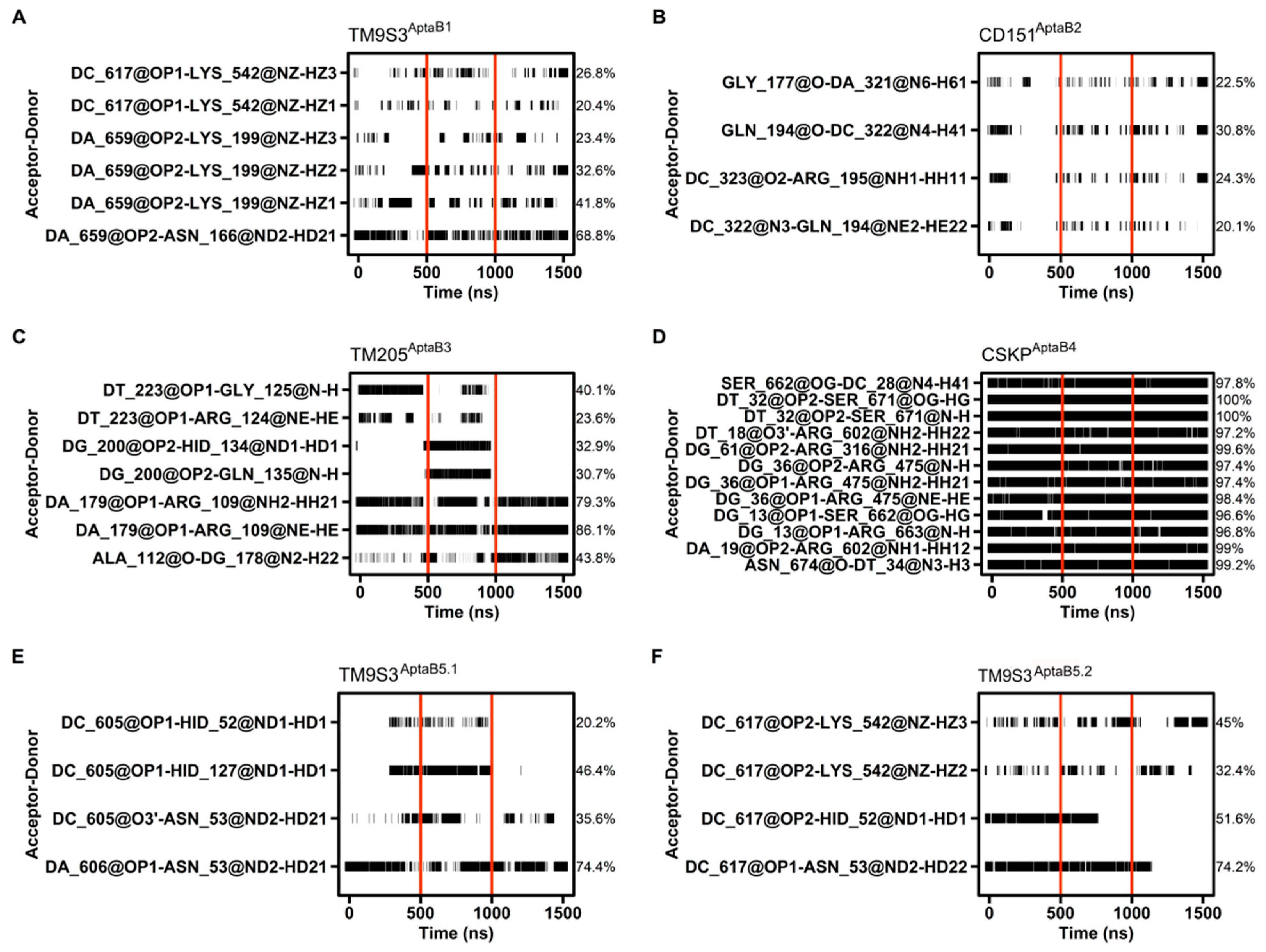
2.12. Molecular Dynamics Details the Interactions between Proteins and Aptamers
2.13. Affinity Calculations Suggest Spontaneous Interactions between the Aptamers and the Proteins
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Culture Conditions
4.2. Cell-SELEX
4.3. Identification and Analysis of Selected Aptamer Sequences for MDA-MB-231 Cells
4.4. Evaluation of Specificity of Aptamers Using Flow Cytometry
4.5. Analysis of the Dissociation Constant (Kd)
4.6. Aptafluorescence
4.7. Three-Dimensional In Vitro Tumor Model
4.8. Validation of Aptamer Recognition in Breast Cancer Samples Using Tissue Microarray (TMA)
4.9. Analysis of the Efficiency of Recognition of Aptamers for Diagnostic Application
4.10. Three-Dimensional Structural Characterization of Aptamers
4.11. Molecular Dynamics Simulations of the Aptamers
4.12. Trajectory Analysis
4.13. Selection of Candidate Proteins for Possible Targets Recognized by the Aptamer Using an In Silico Approach
4.14. Obtaining the Three-Dimensional Structure of the Candidate Proteins for Molecular Docking
4.15. Spatial Orientation Analysis and Electrostatic Potential Characterization of Selected Proteins
4.16. Molecular Docking
4.17. Molecular Dynamics Simulations of the Complexes
4.18. Target–Aptamers Binding Evaluation
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- WHO. Available online: www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 20 August 2023).
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef]
- Parker, J.S.; Mullins, M.; Cheang, M.C.; Leung, S.; Voduc, D.; Vickery, T.; Davies, S.; Fauron, C.; He, X.; Hu, Z.; et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J. Clin. Oncol. 2009, 27, 1160. [Google Scholar] [CrossRef]
- Kolovskaya, O.S.; Zyuzyukina, A.V.; Dassie, J.P.; Zamay, G.S.; Zamay, T.N.; Boyakova, N.V.; Khorzhevskii, V.A.; Kirichenko, D.A.; Lapin, I.N.; Shchugoreva, I.A.; et al. Monitoring of breast cancer progression via aptamer-based detection of circulating tumor cells in clinical blood samples. Front. Mol. Biosci. 2023, 10, 1184285. [Google Scholar] [CrossRef]
- Wu, Y.X.; Kwon, Y.J. Aptamers: The “evolution” of SELEX. Methods 2016, 106, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Z.; Yu, Y.; Wang, M.; Li, J.; Zhang, Z.; Liu, J.; Wu, X.; Lu, A.; Zhang, G.; Zhang, B. Recent advances in SELEX technology and aptamer applications in biomedicine. Int. J. Mol. Sci. 2017, 18, 2142. [Google Scholar] [CrossRef]
- Li, X.; Zhang, W.; Liu, L.; Zhu, Z.; Ouyang, G.; An, Y.; Zhao, C.; Yang, C.J. In vitro selection of DNA aptamers for metastatic breast cancer cell recognition and tissue imaging. Anal. Chem. 2014, 86, 6596–6603. [Google Scholar] [CrossRef] [PubMed]
- Tuerk, C.; Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef]
- Zhang, Y.; Lai, B.S.; Juhas, M. Recent advances in aptamer discovery and applications. Molecules 2019, 24, 941. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Hu, J.; Peng, M.; Liu, J.; Liu, J.; Liu, H.; Zhao, X.; Tan, W. Generating aptamers by cell-SELEX for applications in molecular medicine. Int. J. Mol. Sci. 2012, 13, 3341–3353. [Google Scholar] [CrossRef]
- Que-Gewirth, N.S.; Sullenger, B.A. Gene therapy progress and prospects: RNA aptamers. Gene Ther. 2007, 14, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Ohuchi, S. Cell-SELEX technology. BioRes. Open Access 2012, 1, 265–272. [Google Scholar] [CrossRef]
- Prakash, J.S.; Rajamanickam, K. Aptamers and their significant role in cancer therapy and diagnosis. Biomedicines 2015, 3, 248–269. [Google Scholar] [CrossRef]
- Xiang, D.; Zheng, C.; Zhou, S.F.; Qiao, S.; Tran PH, L.; Pu, C.; Duan, W. Superior performance of aptamer in tumor penetration over antibody: Implication of aptamer-based theranostics in solid tumors. Theranostics 2015, 5, 1083. [Google Scholar] [CrossRef] [PubMed]
- Cruz Da Silva, E.; Foppolo, S.; Lhermitte, B.; Ingremeau, M.; Justiniano, H.; Klein, L.; Chenard, M.-P.; Vauchelles, R.; Abdallah, B.; Lehmann, M.; et al. Bioimaging Nucleic-Acid Aptamers with Different Specificities in Human Glioblastoma Tissues Highlights Tumoral Heterogeneity. Pharmaceutics 2022, 14, 1980. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, R.T.; Perez, E.M.; Hernández, D.; Miller, C.P.; Haas, K.M.; Irie, H.Y.; Lee, S.I.; Blau, C.A.; Villén, J. The proteomic landscape of triple-negative breast cancer. Cell Rep. 2015, 11, 630–644. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, Y.S.; Moresco, J.J.; Tu, P.G.; Yates, J.R., 3rd; Nardulli, A.M. Plasma membrane proteomics of human breast cancer cell lines identifies potential targets for breast cancer diagnosis and treatment. PLoS ONE 2014, 9, e102341. [Google Scholar] [CrossRef]
- Bhushan, A.; Gonsalves, A.; Menon, J.U. Current State of Breast Cancer Diagnosis, Treatment, and Theranostics. Pharmaceutics 2021, 13, 723. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, H.; Merkher, Y.; Chen, L.; Liu, N.; Leonov, S.; Chen, Y. Recent advances in therapeutic strategies for triple-negative breast cancer. J. Hematol. Oncol. 2022, 15, 121. [Google Scholar] [CrossRef]
- Li, L.; Zhang, F.; Liu, Z.; Fan, Z. Immunotherapy for Triple-Negative Breast Cancer: Combination Strategies to Improve Outcome. Cancers 2023, 15, 321. [Google Scholar] [CrossRef]
- Rakha, E.A.; Pareja, F.G. New Advances in Molecular Breast Cancer Pathology. Semin. Cancer Biol. 2021, 72, 102–113. [Google Scholar] [CrossRef]
- James, W. Nucleic acid and polypeptide aptamers: A powerful approach to ligand discovery. Curr. Opin. Pharmacol. 2001, 1, 540–546. [Google Scholar] [CrossRef]
- Kumar Kulabhusan, P.; Hussain, B.; Yüce, M. Current perspectives on aptamers as diagnostic tools and therapeutic agents. Pharmaceutics 2020, 12, 646. [Google Scholar] [CrossRef] [PubMed]
- Marusyk, A.; Polyak, K. Tumor heterogeneity: Causes and consequences. Biochim. Et. Biophys. Acta (BBA)-Rev. Cancer 2010, 1805, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Mascini, M.; Palchetti, I.; Tombelli, S. Nucleic acid and peptide aptamers: Fundamentals and bioanalytical aspects. Angew. Chem. Int. Ed. 2012, 51, 1316–1332. [Google Scholar] [CrossRef] [PubMed]
- Norton, K.A.; Popel, A.S.; Pandey, N.B. Heterogeneity of chemokine cell-surface receptor expression in triple-negative breast cancer. Am. J. Cancer Res. 2015, 5, 1295. [Google Scholar]
- Wang, X.; Liu, Y.; Zhou, K.; Zhang, G.; Wang, F.; Ren, J. Isolation and characterization of CD105+/CD90+ subpopulation in breast cancer MDA-MB-231 cell line. Int. J. Clin. Exp. Pathol. 2015, 8, 5105–5112. [Google Scholar]
- Bousoik, E.; Nabiee, R.; Amirrad, F.; Nichols, A.; Witt, R.; Mahdipoor, P.; Montazeri Aliabadi, H. Heterogeneity and Plasticity of Human Breast Cancer Cells in Response to Molecularly-Targeted Drugs. Front. Oncol. 2019, 9, 1070. [Google Scholar] [CrossRef]
- Camorani, S.; Passariello, M.; Agnello, L.; Esposito, S.; Collina, F.; Cantile, M.; Di Bonito, M.; Ulasov, I.V.; Fedele, M.; Zannetti, A.; et al. Aptamer targeted therapy potentiates immune checkpoint blockade in triple-negative breast cancer. J. Exp. Clin. Cancer Res. 2020, 39, 1–16. [Google Scholar] [CrossRef]
- Ray, P.; White, R.R. Aptamers for targeted drug delivery. Pharmaceuticals 2010, 3, 1761–1778. [Google Scholar] [CrossRef]
- Mladkova, J.; Sanda, M.; Matouskova, E.; Selicharova, I. Phenotyping breast cancer cell lines EM-G3, HCC1937, MCF7 and MDA-MB-231 using 2-D electrophoresis and affinity chromatography for glutathione-binding proteins. BMC Cancer 2010, 10, 1–10. [Google Scholar] [CrossRef]
- Aran, D.; Camarda, R.; Odegaard, J.; Paik, H.; Oskotsky, B.; Krings, G.; Goga, A.; Sirota, M.; Butte, A.J. Comprehensive analysis of normal adjacent to tumor transcriptomes. Nat. Commun. 2017, 8, 1077. [Google Scholar] [CrossRef]
- Slaughter, D.P.; Southwick, H.W.; Smejkal, W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer 1953, 6, 963–968. [Google Scholar] [CrossRef]
- Abreu, R.d.S.; Antunes, D.; Moreira, A.d.S.; Passetti, F.; Mendonça, J.B.; de Araújo, N.S.; Sassaro, T.F.; Alberto, A.V.P.; Carrossini, N.; Fernandes, P.V.; et al. Next Generation of Ovarian Cancer Detection Using Aptamers. Int. J. Mol. Sci. 2023, 24, 6315. [Google Scholar] [CrossRef] [PubMed]
- Rabal, O.; Pastor, F.; Villanueva, H.; Soldevilla, M.M.; Hervas-Stubbs, S.; Oyarzabal, J. In Silico Aptamer Docking Studies: From a Retrospective Validation to a Prospective Case Study-TIM3 Aptamers Binding. Mol. Ther. Nucleic Acids 2016, 5, e376. [Google Scholar] [CrossRef]
- Kothandan, R.; Uthayasooriyan, P.; Vairamani, S. Search for RNA aptamers against non-structural protein of SARS-CoV-2: Design using molecular dynamics approach. Beni-Suef Univ. J. Basic Appl. Sci. 2021, 10, 64. [Google Scholar] [CrossRef] [PubMed]
- Niazi, S.; Purohit, M.; Sonawani, A.; Niazi, J.H. Revealing the molecular interactions of aptamers that specifically bind to the extracellular domain of HER2 cancer biomarker protein: An in silico assessment. J. Mol. Graph. Model. 2018, 83, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.-L.; Fu, Z.-X.; Fang, M.; Zhou, Q.-Y.; Zhao, Q.-N.; Guo, J.-B.; Lu, W.-D.; Wang, H. High expression of CASK correlates with progression and poor prognosis of colorectal cancer. Tumor Biol. 2014, 35, 9185–9194. [Google Scholar] [CrossRef]
- Qu, J.; Zhou, Y.; Li, Y.; Yu, J.; Wang, W. CASK regulates Notch pathway and functions as a tumor promoter in pancreatic cancer. Arch. Biochem. Biophys. 2021, 701, 108789. [Google Scholar] [CrossRef]
- Ding, B.; Bao, C.; Jin, L.; Xu, L.; Fan, W.; Lou, W. CASK Silence overcomes sorafenib resistance of hepatocellular carcinoma through activating apoptosis and autophagic cell death. Front. Oncol. 2021, 11, 681683. [Google Scholar] [CrossRef]
- Liu, Q.; Dong, H.-T.; Zhao, T.; Yao, F.; Xu, Y.; Chen, B.; Wu, Y.; Jin, F.; Xing, P. Cancer-associated adipocytes release FUCA2 to promote aggressiveness in TNBC. Endocr. Relat. Cancer 2022, 29, 139–149. [Google Scholar] [CrossRef]
- Moreira, A.S. Programa de Biologia Celular e Molecular-Instituto Oswaldo Cruz/Fiocruz. Ph.D. Thesis, Instituto de Comunicação e Informação Científica e Tecnológica em Saúde (Icict/Fiocruz), Rio de Janeiro, Brazil, 2018. Desenvolvimento de Aptâmeros Como Agentes Antitumorais. [Google Scholar]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Seqtk. Available online: https://github.com/lh3/seqtk (accessed on 20 August 2022).
- Jeddi, I.; Saiz, L. Three-dimensional modeling of single stranded DNA hairpins for aptamer-based biosensors. Sci. Rep. 2017, 7, 1178. [Google Scholar] [CrossRef] [PubMed]
- Adams, P.D.; Afonine, P.V.; Bunkóczi, G.; Chen, V.B.; Davis, I.W.; Echols, N.; Headd, J.J.; Hung, L.W.; Kapral, G.J.; Grosse-Kunstleve, R.W.; et al. PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 2010, 66 Pt 2, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Case, D.A.; Belfon, K.; Ben-Shalom, I.Y.; Brozell, S.R.; Cerutti, D.S.; Cheatham, T.E.; Cruzeiro, V.W.D.; Darden, T.A.; Duke, R.E.; Giambasu, G.; et al. Amber; University of California: San Francisco, CA, USA, 2020. [Google Scholar]
- Case, D.A.; Cheatham, T.E., III; Darden, T.; Gohlke, H.; Luo, R.; Merz, K.M., Jr.; Onufriev, A.; Simmerling, C.; Wang, B.; Woods, R.J. The Amber Biomolecular Simulation Programs. J. Comput. Chem. 2005, 26, 1668–1688. [Google Scholar] [CrossRef] [PubMed]
- Mark, P.; Nilsson, L. Structure and Dynamics of the TIP3P, SPC, and SPC/E Water Models at 298 K. J. Phys. Chem. A 2001, 105, 9954–9960. Available online: http://pubs.acs.org/doi/abs/10.1021/jp003020w (accessed on 20 August 2023). [CrossRef]
- Norberto De Souza, O.; Ornstein, R.L. Effect of periodic box size on aqueous molecular dynamics simulation of a DNA dodecamer with particle-mesh Ewald method. Biophys. J. 1997, 72, 2395–2397. [Google Scholar] [CrossRef] [PubMed]
- Darden, T.; Perera, L.; Li, L.; Pedersen, L. New tricks for modelers from the crystallography toolkit: The particle mesh Ewald algorithm and its use in nucleic acid simulations. Structure 1999, 7, 55–60. Available online: http://biomednet.com/elecref/09692126007R0055 (accessed on 20 August 2023). [CrossRef]
- Berendsen, H.J.C.; Postma, J.P.M.; Van Gunsteren, W.F.; Dinola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Nanzer, A.P.; Huber, T.; Torda, A.E.; van Gunsteren, W.F. Molecular dynamics simulation using weak-coupling NOE distance restraining. J. Biomol. NMR 1996, 8, 285–291. [Google Scholar] [CrossRef]
- Amadei, A.; Linssen, A.B.M.; de Groot, B.L.; van Aalten, D.M.F.; Berendsen, H.J.C. An efficient method for sampling the essential subspace of proteins. J. Biomol. Struct. Dyn. 1996, 13, 615–625. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. Gromacs: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1, 19–25. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Jurrus, E.; Engel, D.; Star, K.; Monson, K.; Brandi, J.; Felberg, L.E.; Brookes, D.H.; Wilson, L.; Chen, J.; Liles, K.; et al. Improvements to the APBS biomolecular solvation software suite. Protein Sci. 2018, 27, 112–128. [Google Scholar] [CrossRef]
- Gordon, J.C.; Myers, J.B.; Folta, T.; Shoja, V.; Heath, L.S.; Onufriev, A. H++: A server for estimating pKas and adding missing hydrogens to macromolecules. Nucleic Acids Res. 2005, 33, W368–W371. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Lim, J.B.; Klauda, J.B.; Im, W. CHARMM-GUI membrane builder for mixed bilayers and its application to yeast membranes. Biophys. J. 2009, 97, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Kim, T.; Im, W. Automated builder and database of protein/membrane complexes for molecular dynamics simulations. PLoS ONE 2007, 2, e880. [Google Scholar] [CrossRef]
- Genheden, S.; Ryde, U. The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Informa 2015, 10, 449–461. [Google Scholar] [CrossRef]
- Wang, E.; Sun, H.; Wang, J.; Wang, Z.; Liu, H.; Zhang, J.Z.H.; Hou, T. End-Point Binding Free Energy Calculation with MM/PBSA and MM/GBSA: Strategies and Applications in Drug Design. Chem. Rev. 2019, 119, 9478–9508. [Google Scholar] [CrossRef]
- Adasme-Carreño, F.; Muñoz-Gutierrez, C.; Caballero, J.; Alzate-Morales, J.H. Performance of the MM/GBSA scoring using a binding site hydrogen bond network-based frame selection: The protein kinase case. Phys. Chem. Chem. Phys. 2014, 16, 14047–14058. [Google Scholar] [CrossRef]
- Forouzesh, N.; Mishra, N. An effective MM/GBSA protocol for absolute binding free energy calculations: A case study on SARS-CoV-2 spike protein and the human ACE2 receptor. Molecules 2021, 26, 2383. [Google Scholar] [CrossRef]









| APTAMERS | KD VALUES |
|---|---|
| APTAB1 | 139 ± 14 nM |
| APTAB2 | 206 ± 41 nM |
| APTAB3 | 145 ± 31 nM |
| APTAB4 | 194 ± 0.7 nM |
| APTAB5 | 126 ± 19 nM |
| Aptamer | Sample | Recognition/ Total Number | % Recognition | Staining Intensity |
|---|---|---|---|---|
| AptaB1 | Adjacent tissue | 1/10 | 10% | (+) |
| Primary tumor tissue | 7/50 | 14% | (+++) | |
| Metastatic tissue | 5/40 | 12.5% | (+++) | |
| AptaB2 | Adjacent tissue | 1/10 | 10% | (+) |
| Primary tumor tissue | 18 /50 | 36% | (+++) | |
| Metastatic tissue | 5/40 | 12.5% | (++) | |
| AptaB3 | Adjacent tissue | 1/10 | 10% | (+) |
| Primary tumor tissue | 3/50 | 6% | (+++) | |
| Metastatic tissue | 6/40 | 15% | (++) | |
| AptaB4 | Adjacent tissue | 4/10 | 40% | (+) |
| Primary tumor tissue | 25/50 | 50% | (+++) | |
| Metastatic tissue | 15/40 | 37.5% | (+++) | |
| AptaB5 | Adjacent tissue | 3/10 | 30% | (+) |
| Primary tumor tissue | 20/50 | 40% | (+++) | |
| Metastatic tissue | 27/40 | 67.5% | (+++) |
| Aptamer | Molecular Subtype from Primary Tumor | Recognition/ Total Number | % Recognition | Staining Intensity |
|---|---|---|---|---|
| AptaB1 | Luminal | 3/28 | 10.7 | (+++) |
| HER 2 | 3/7 | 42 | (+++) | |
| Triple-negative | 1/10 | 10 | (+++) | |
| AptaB2 | Luminal | 8/28 | 28 | (+++) |
| HER 2 | 5/7 | 70 | (+++) | |
| Triple-negative | 4/10 | 40 | (+++) | |
| AptaB3 | Luminal | 1/28 | 3.5 | (+++) |
| HER 2 | 1/7 | 14 | (+++) | |
| Triple-negative | 1/10 | 10 | (++) | |
| AptaB4 | Luminal | 12/28 | 42 | (+++) |
| HER 2 | 6/7 | 85 | (+++) | |
| Triple-negative | 4/10 | 40 | (++) | |
| AptaB5 | Luminal | 9/28 | 32 | (+++) |
| HER 2 | 5/7 | 70 | (+++) | |
| Triple-negative | 5/10 | 50 | (+++) |
| Aptamer | Molecular Subtype of the Metastatic Sample | Recognition/Number of Samples | % Recognition | Staining Intensity |
|---|---|---|---|---|
| AptaB1 | Luminal | 3/14 | 21% | (+++) |
| HER 2 | 0/9 | - | (−) | |
| Triple-negative | 0/8 | - | (−) | |
| AptaB2 | Luminal | 1/14 | 7.1% | (+++) |
| HER 2 | 2/9 | 22% | (+++) | |
| Triple-negative | 2/8 | 25% | (+++) | |
| AptaB3 | Luminal | 1/14 | 3.5% | (+++) |
| HER 2 | 1/9 | 11% | (+++) | |
| Triple-negative | 3/8 | 37.5% | (++) | |
| AptaB4 | Luminal | 6/14 | 42% | (+++) |
| HER 2 | 1/9 | 11% | (+++) | |
| Triple-negative | 5/8 | 62% | (++) | |
| AptaB5 | Luminal | 10/14 | 32% | (+++) |
| HER 2 | 7/9 | 77% | (+++) | |
| Triple-negative | 5/8 | 62% | (+++) |
| Stage | Number of Samples | AptaB1 | AptaB2 | AptaB3 | AptaB4 | AptaB5 |
|---|---|---|---|---|---|---|
| I | 4 | 0 | 1 | 0 | 3 | 2 |
| II | 39 | 4 | 15 | 3 | 20 | 4 |
| III | 3 | 2 | 1 | 1 | 2 | 3 |
| Grade | Number of Samples | AptaB1 | AptaB2 | AptaB3 | AptaB4 | AptaB5 |
|---|---|---|---|---|---|---|
| I | 8 | 1 | 4 | 0 | 5 | 1 |
| II | 29 | 4 | 10 | 3 | 17 | 11 |
| III | 9 | 1 | 4 | 0 | 4 | 5 |
| TNM | Number of Samples | AptaB1 | AptaB2 | AptaB3 | AptaB4 | AptaB5 |
|---|---|---|---|---|---|---|
| T1N0M0 | 4 | 0 | 1 | 0 | 3 | 2 |
| T2N0M0 | 29 | 4 | 11 | 2 | 15 | 10 |
| T2N1M0 | 6 | 0 | 2 | 1 | 3 | 3 |
| T2N3M0 | 1 | 1 | 0 | 1 | 0 | 1 |
| T3N0M0 | 4 | 0 | 2 | 0 | 2 | 0 |
| T3N1M0 | 1 | 0 | 0 | 0 | 1 | 1 |
| T4N0M0 | 3 | 1 | 2 | 0 | 2 | 2 |
| T4N1M0 | 2 | 1 | 1 | 0 | 1 | 2 |
| Aptamers | Sensitivity | Specificity | Accuracy |
|---|---|---|---|
| AptaB1 | 13% | 90% | 21% |
| AptaB2 | 26% | 90% | 32% |
| AptaB3 | 10% | 90% | 18% |
| AptaB4 | 44% | 60% | 46% |
| AptaB5 | 52% | 70% | 54% |
| AptaB4 + AptaB5 | 77% | 40% | 73% |
| AptaB2 + AptaB4 + AptaB5 | 87% | 30% | 81% |
| AptaB2 + AptaB3 + AptaB4 + AptaB5 | 89% | 30% | 83% |
| AptaB1 + AptaB2 + AptaB3 + AptaB4 + AptaB5 | 96% | 30% | 89% |
| Protein–AptaB1 | Haddock Score | |
|---|---|---|
| A | CSKP–AptaB1 | −34.9 +/− 7.5 |
| B | TM9S3–AptaB1 | −76.7 +/− 33.4 |
| C | TMEM205–AptaB1 | −48.1 +/− 8.0 |
| D | CD151–AptaB1 | 2.8 +/− 9.4 |
| Protein–AptaB2 | Haddock Score | |
| A | CSKP–AptaB2 | −8.3 +/− 8.9 |
| B | TM9S3–AptaB2 | −11.1 +/− 18.0 |
| C | TMEM205–AptaB2 | −46.7 +/− 6.1 |
| D | CD151–AptaB2 | −53.1 +/− 9.2 |
| Protein–AptaB3 | Haddock Score | |
| A | CSKP–AptaB3 | −8.7 +/− 21.7 |
| B | TM9S3–AptaB3 | −31.2 +/− 3.9 |
| C | TMEM205–AptaB3 | −34.2 +/− 3.5 |
| D | CD151–AptaB3 | 17.3 +/− 9.9 |
| Protein–AptaB4 | Haddock Score | |
| A | CSK–AptaB4 | −34.7 +/− 11.1 |
| B | TM9S3–AptaB4 | −22.3 +/− 4.0 |
| C | TMEM205–AptaB4 | −23.9 +/− 6.5 |
| D | CD151–AptaB4 | −8.4 +/− 25.8 |
| Protein–AptaB5.1 | Haddock Score | |
| A | CSKP–AptaB5.1 | 20.5 +/− 27.0 |
| B | TM9S3–AptaB5.1 | −41.2 +/− 16.2 |
| C | TMEM205–AptaB5.1 | 12.3 +/− 11.5 |
| D | CD151–AptaB5.1 | 18.0 +/− 13.2 |
| Protein–AptaB5.2 | Haddock Score | |
| A | CSKP–AptaB5.2 | −4.7 +/− 22.0 |
| B | TM9S3–AptaB5.2 | −81.2 +/− 4.4 |
| C | TMEM205–AptaB5.2 | −3.7 +/− 13.9 |
| D | CD151–AptaB5.2 | 6.5 +/− 6.5 |
| System | ΔEvdw | Δele | Δegb | ΔGesurf | ΔGbind |
|---|---|---|---|---|---|
| TM9S3AptaB1 | −212.04 | 4821.73 | −4654.34 | −23.45 | −68.1 ± 2.7 |
| CD151AptaB2 | −31.49 | 983.92 | −952.11 | −3.99 | −03.6 ± 3.7 |
| TM205AptaB3 | −52.77 | 874.05 | −842.43 | −13.90 | −35.0 ± 3.3 |
| CSKPAptaB4 | −246.40 | 5174.56 | −5015.07 | −30.90 | −117.8 ± 2.2 |
| TMS9AptaB 5.1 | −209.31 | 4883.27 | −4703.32 | −24.24 | −53.6 ± 2.1 |
| TMS9AptaB 5.2 | −210.58 | 4925.62 | −4745.78 | −28.66 | −59.4 ± 2.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Araújo, N.S.; Moreira, A.d.S.; Abreu, R.d.S.; Junior, V.V.; Antunes, D.; Mendonça, J.B.; Sassaro, T.F.; Jurberg, A.D.; Ferreira-Reis, R.; Bastos, N.C.; et al. Aptamer-Based Recognition of Breast Tumor Cells: A New Era for Breast Cancer Diagnosis. Int. J. Mol. Sci. 2024, 25, 840. https://doi.org/10.3390/ijms25020840
de Araújo NS, Moreira AdS, Abreu RdS, Junior VV, Antunes D, Mendonça JB, Sassaro TF, Jurberg AD, Ferreira-Reis R, Bastos NC, et al. Aptamer-Based Recognition of Breast Tumor Cells: A New Era for Breast Cancer Diagnosis. International Journal of Molecular Sciences. 2024; 25(2):840. https://doi.org/10.3390/ijms25020840
Chicago/Turabian Stylede Araújo, Natassia Silva, Aline dos Santos Moreira, Rayane da Silva Abreu, Valdemir Vargas Junior, Deborah Antunes, Julia Badaró Mendonça, Tayanne Felippe Sassaro, Arnon Dias Jurberg, Rafaella Ferreira-Reis, Nina Carrossini Bastos, and et al. 2024. "Aptamer-Based Recognition of Breast Tumor Cells: A New Era for Breast Cancer Diagnosis" International Journal of Molecular Sciences 25, no. 2: 840. https://doi.org/10.3390/ijms25020840
APA Stylede Araújo, N. S., Moreira, A. d. S., Abreu, R. d. S., Junior, V. V., Antunes, D., Mendonça, J. B., Sassaro, T. F., Jurberg, A. D., Ferreira-Reis, R., Bastos, N. C., Fernandes, P. V., Guimarães, A. C. R., Degrave, W. M. S., Tilli, T. M., & Waghabi, M. C. (2024). Aptamer-Based Recognition of Breast Tumor Cells: A New Era for Breast Cancer Diagnosis. International Journal of Molecular Sciences, 25(2), 840. https://doi.org/10.3390/ijms25020840







