Cardiometabolic Changes in Sirtuin1-Heterozygous Mice on High-Fat Diet and Melatonin Supplementation
Abstract
:1. Introduction
2. Results
2.1. Sirtuin1 Content in Mouse Heart
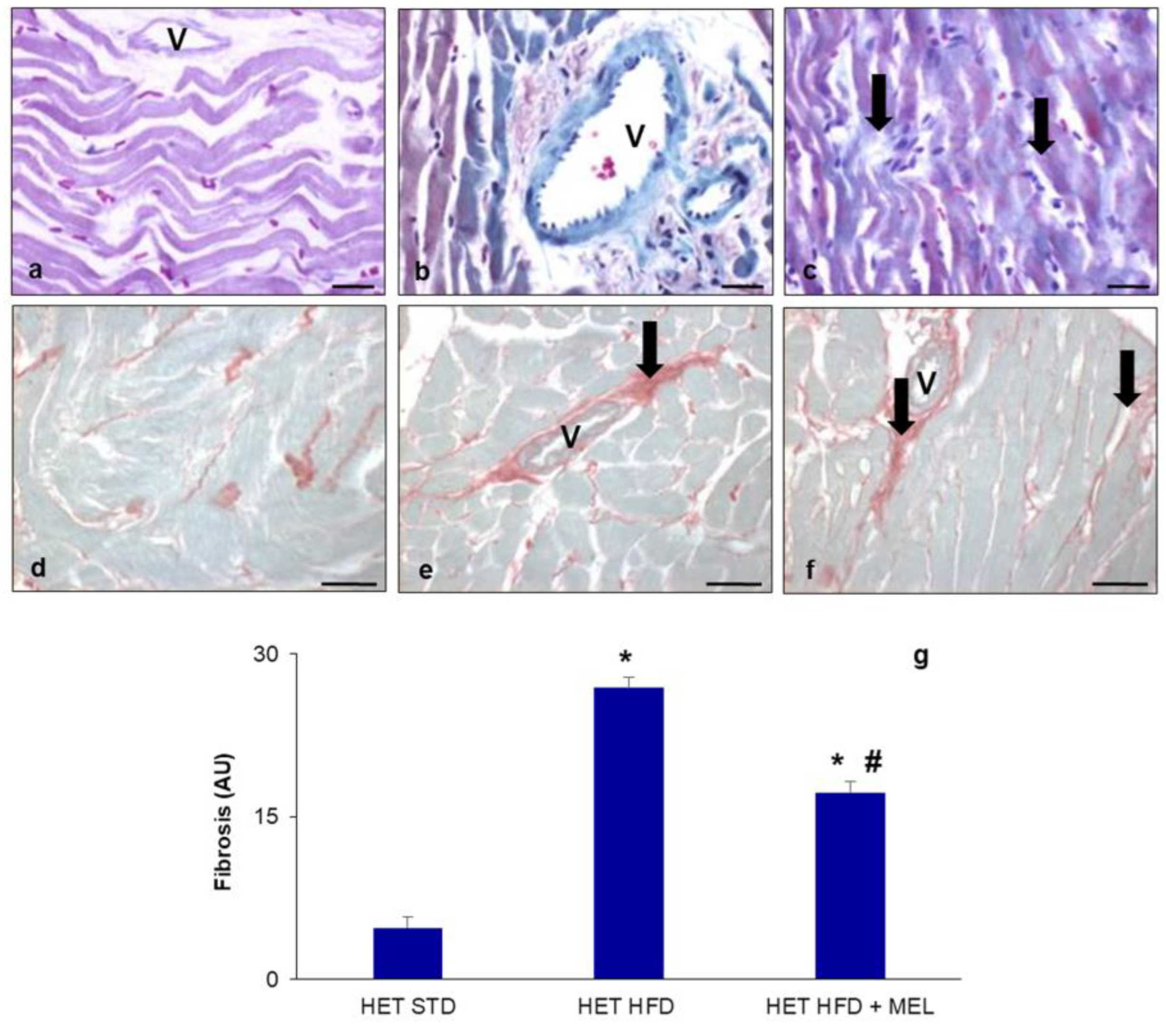
2.2. Metabolic Data and Cardiac Fibrosis
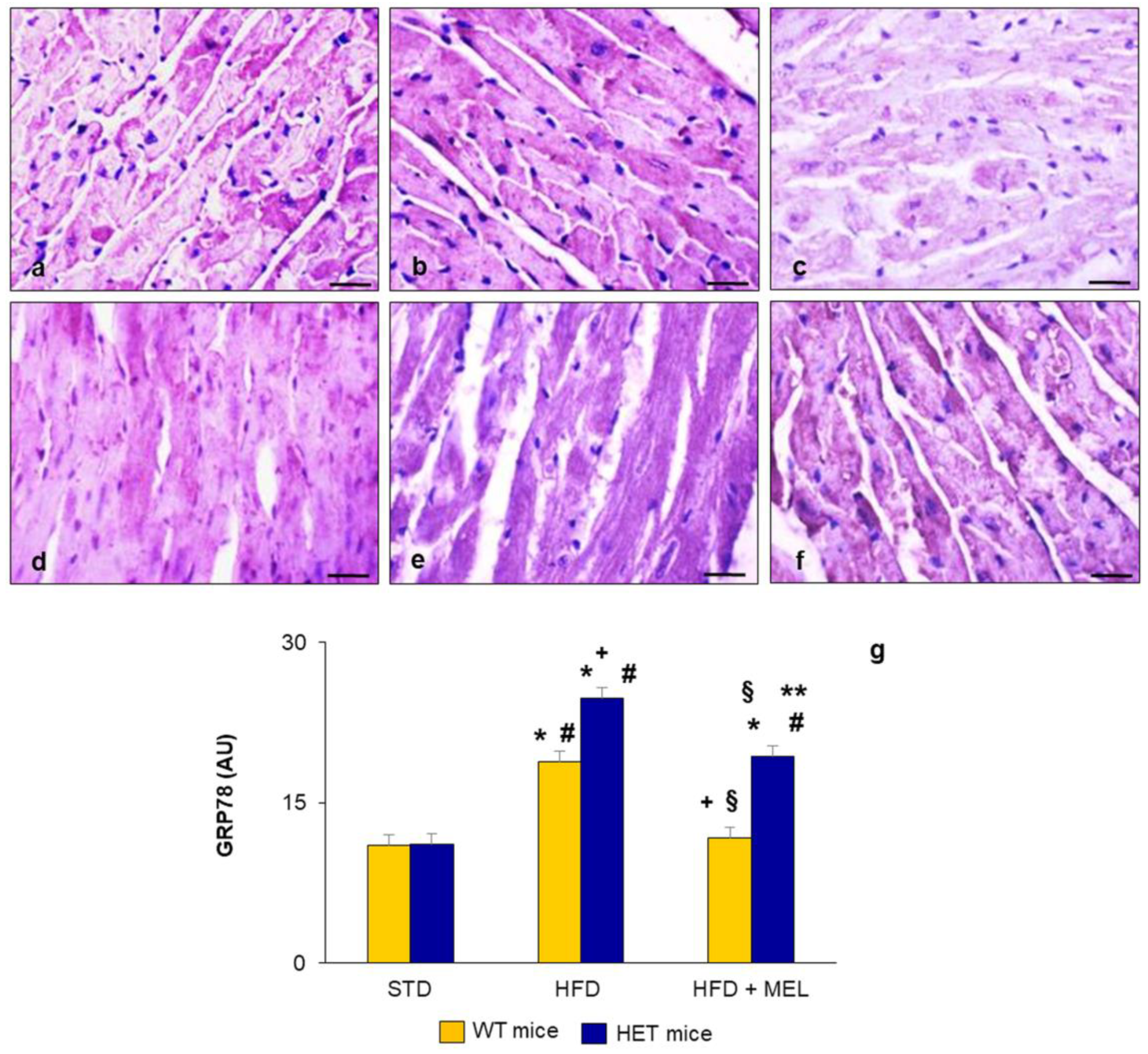
2.3. Heart Lipid Peroxidation and Endoplasmic Reticulum Stress
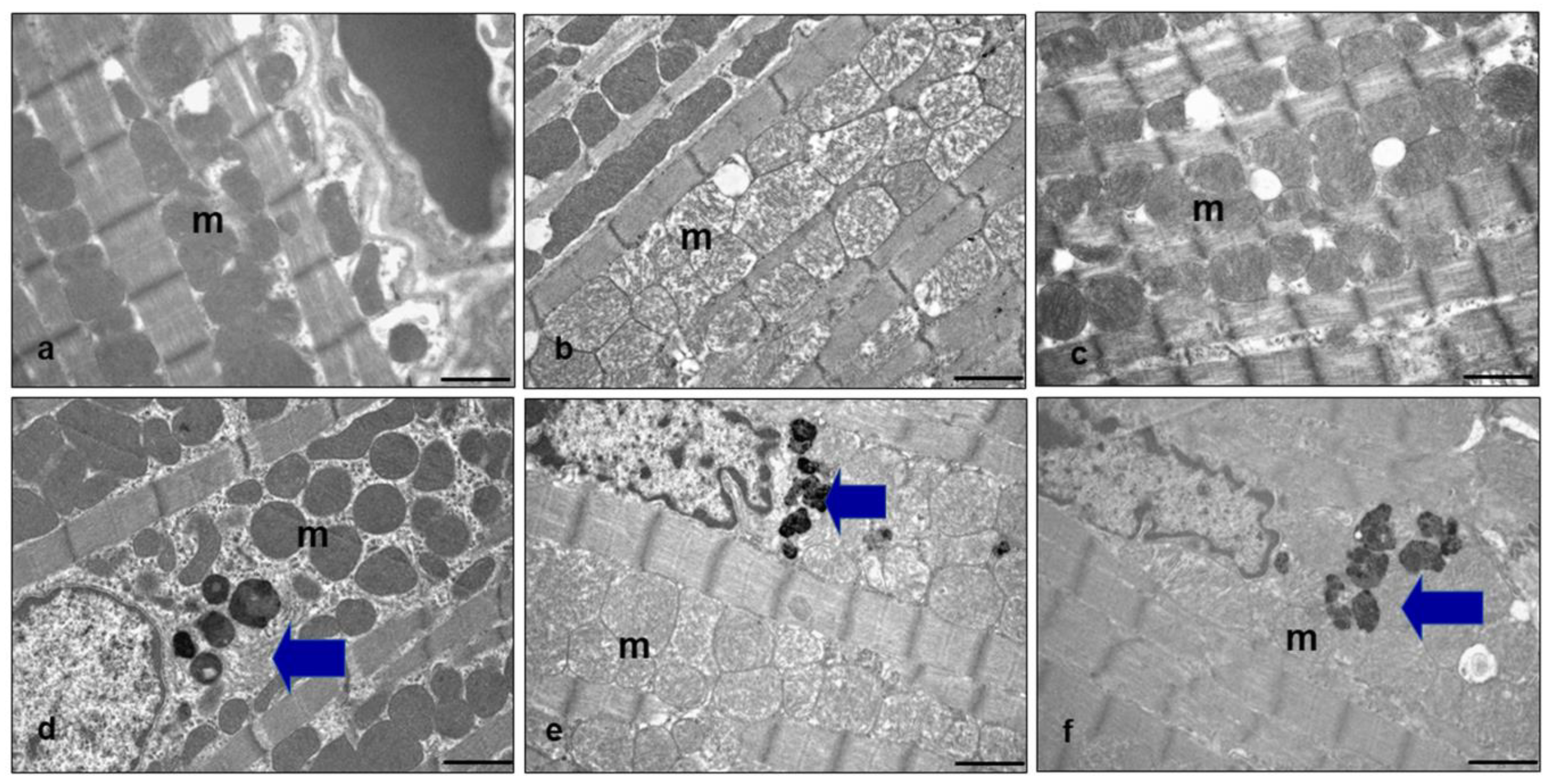
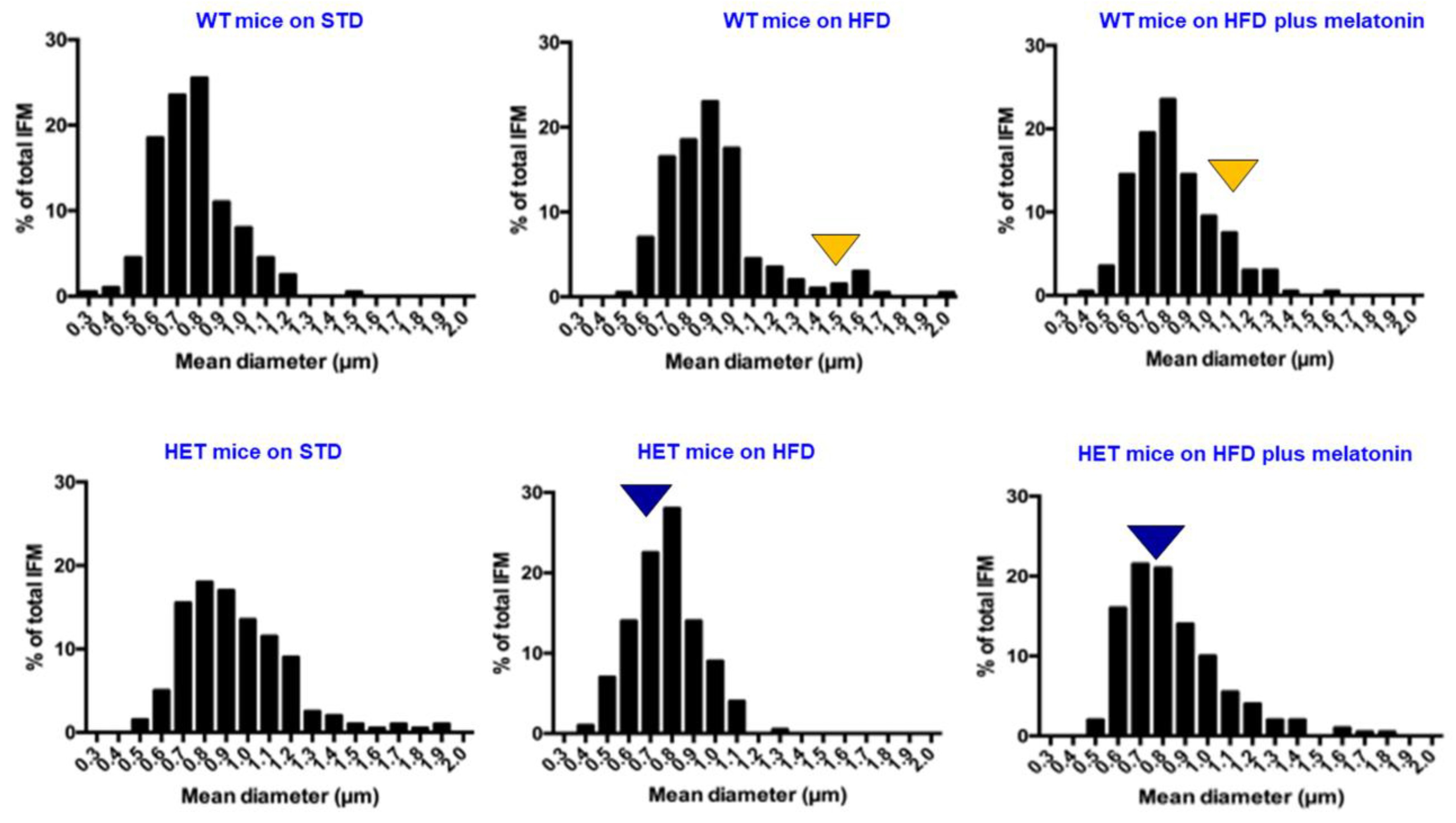
2.4. Ultrastructural Analysis of Heart Mitochondria
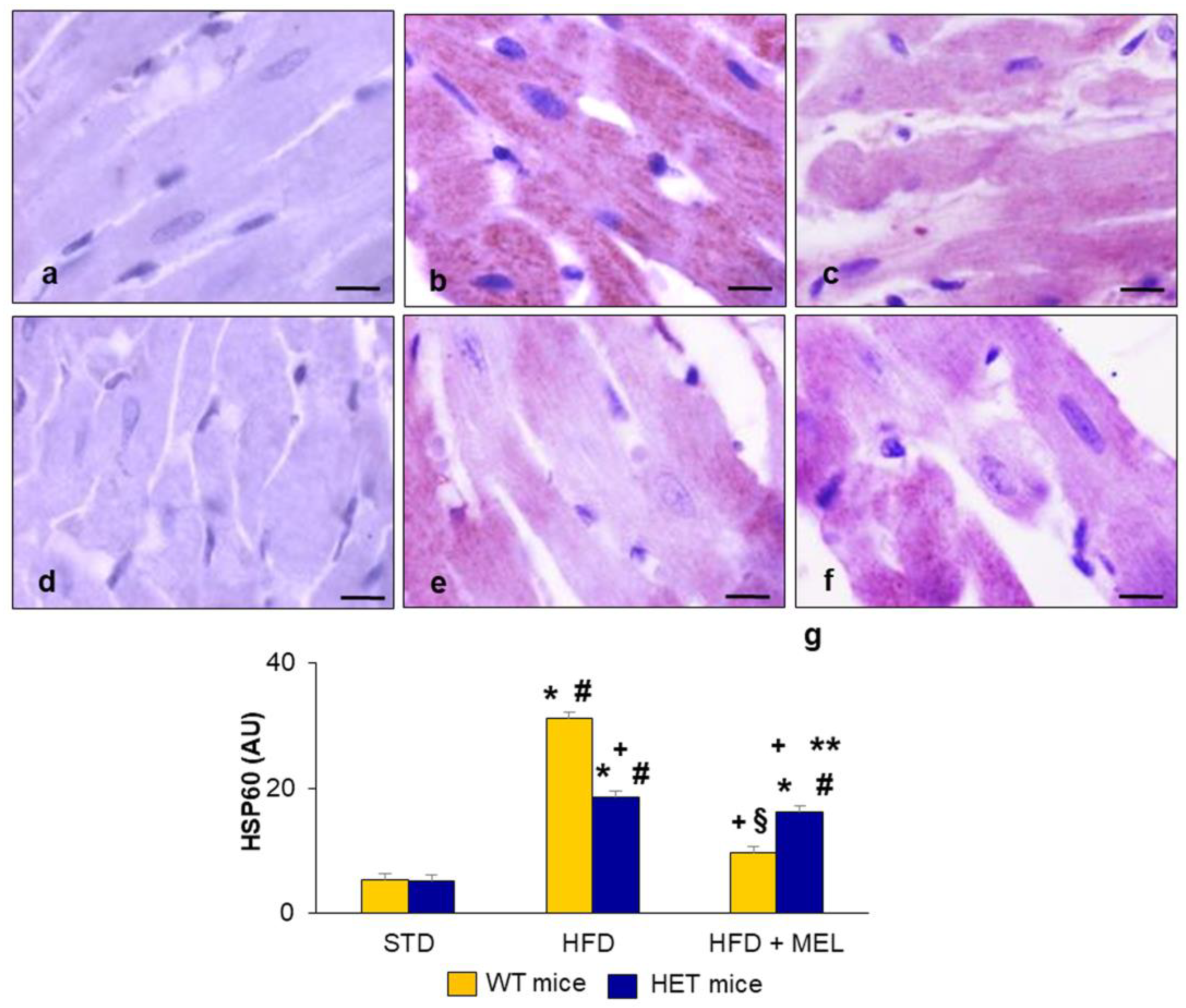
2.5. Heart Heat Shock Protein60 Expression
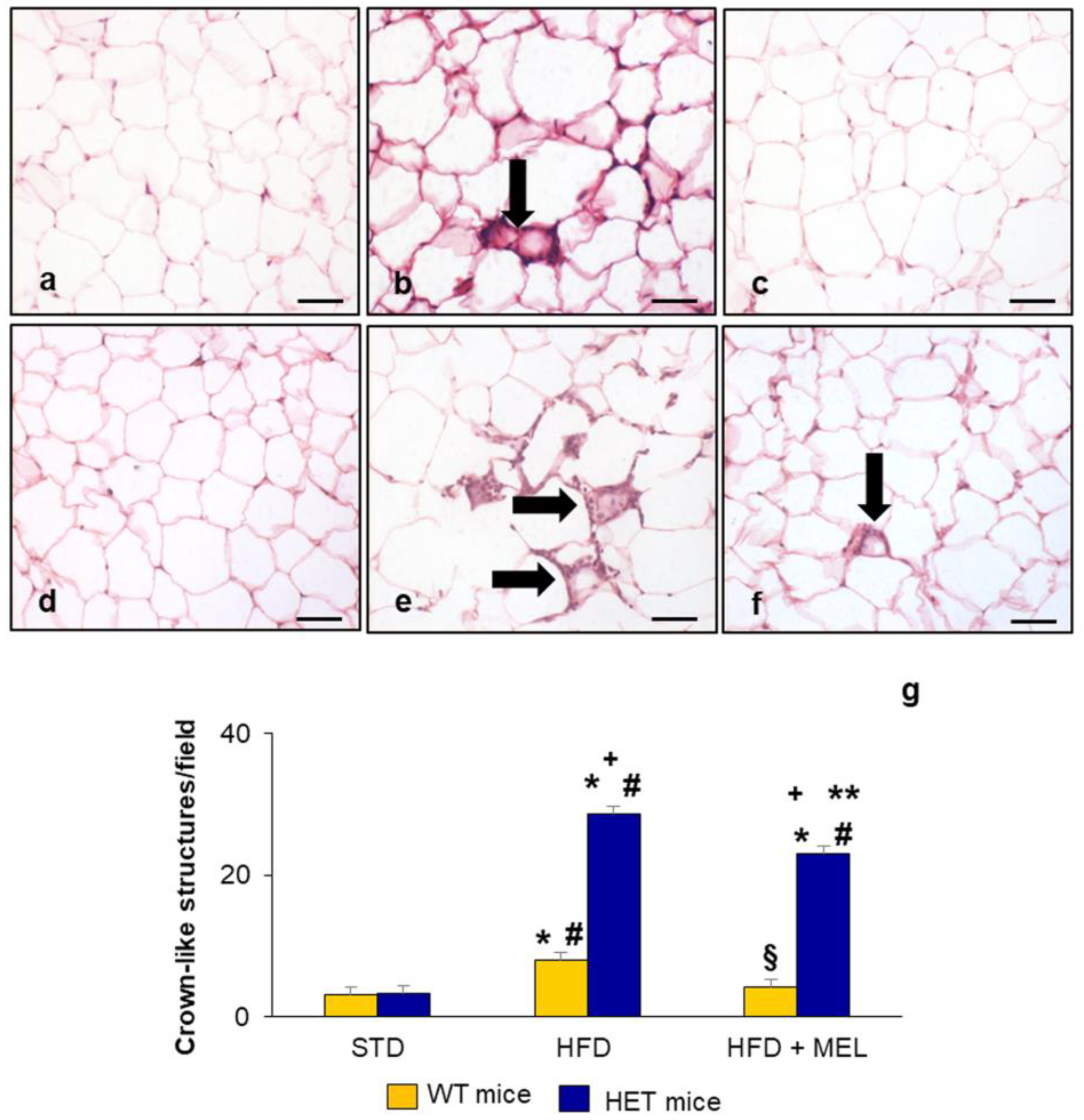
2.6. Epididymal Adipose Tissue and Brown Adipose Tissue Evaluation
3. Discussion
4. Materials and Methods
4.1. Animal Model and Dietary Treatments
4.2. Morphological and Morphometrical Analysis
4.3. Immunohistochemical Analysis
4.4. Transmission Electron Microscopy
4.5. Western Blotting
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lavie, C.; Ozemek, C.; Carbone, S.; Katzmaryk, P.; Blair, S. Sedentary behaviour, exercise, and cardiovascular health. Circ. Res. 2019, 124, 799–815. [Google Scholar] [CrossRef] [PubMed]
- Van Bakel, B.; Bakker, E.; de Vries, F.; Thijssen, D.; Eijsvogels, T. Changes in physical activity and sedentary behavior in cardiovascular disease patients during the COVID-19 lockdown. Int. J. Environ. Res. Public. Health 2021, 18, 11929. [Google Scholar] [CrossRef] [PubMed]
- Beydoun, H.; Beydoun, M.; Gautam, R.; Alemu, B.; Weiss, J.; Hossain, S.; Zonderman, A. COVID-19 pandemic impact on trajectories in cardiometabolic health, physical activity, and functioning among adults from 2006–2020 health and retirement study. J. Gerontol. Ser. A 2022, 77, 1371–1379. [Google Scholar] [CrossRef]
- Powell-Wiley, T.; Poirer, P.; Burke, L.; Despres, J.; Gordon-Larsen, P.; Lavie, C.; Lear, S.; Ndumele, C.; Neeland, I.; Sanders, P.; et al. Obesity and cardiovascular disease: A scientific statement from American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef] [PubMed]
- Tune, J.; Goodwill, A.; Sassoon, D.; Mather, K. Cardiovascular consequences of metabolic syndrome. Trasl. Res. 2017, 183, 57–70. [Google Scholar] [CrossRef]
- Veit, M.; van Asten, R.; Olie, A.; Prinz, P. The role of dietary sugars, overweight, and obesity in type 2 diabetes mellitus: A narrative review. Eur. J. Clin. Nutr. 2022, 76, 1497–1501. [Google Scholar] [CrossRef]
- Calligaris, S.; Lecanda, M.; Solis, F.; Ezquer, M.; Gutierrez, J.; Brandan, E.; Leiva, A.; Sobrevia, L.; Conget, P. Mice long-term high fat diet feeding recapitulates human cardiovascular alterations: An animal model to study the early phases of diabetic cardiomyopathy. PLoS ONE 2013, 8, e60931. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, L.; Tso, A.; Wang, S.; Fang, X.; Ouyang, K.; Han, Z. Mitochondrial chaperones and proteases in cardiomyocytes and heart failure. Front. Mol. Biosci. 2021, 8, 630332. [Google Scholar] [CrossRef]
- Khan, C.; Wang, G.; Lee, K. Altered adipose tissue and adipocyte function in the pathogenesis of metabolic syndrome. J. Clin. Investig. 2019, 129, 3990–4000. [Google Scholar] [CrossRef]
- Mulder, P.; Morrison, M.; Wielinga, P.; Van Duyvenvoorden, W.; Koostra, T.; Kleemann, R. Surgical removal of inflamed epididymal white adipose tissue attenuates the development of non-alcoholic steatohepatitis in obesity. Int. J. Obes. 2016, 40, 675–684. [Google Scholar] [CrossRef]
- Oh, D.; Morinaga, H.; Talukadar, S.; Bae, J.; Olefsky, J. Increased macrophages migration into adipose tissue in obese mice. Diabetes 2012, 61, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Valgas da Silva, C.; Shettigar, V.; Baer, L.; Abay, E.; Madaris, K.; Mehling, M.; Hernandez-Savedra, D.; Pinckard, K.; Seculov, N.; Ziolo, M.; et al. Brown adipose tissue prevents glucose intolerance and cardiac remodeling in high-fat-fed mice after a mild myocardial infarction. Int. J. Obes. 2022, 46, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Poret, J.; Souza-Smith, F.; Marcell, S.; Gaudet, D.; Tzeng, T.; Douglas Braymer, H.; Harrison-Bernard, L.; Primeaux, S. High fat diet consumption differentially affects adipose tissue inflammation and adipocyte size in obesity-prone and obesity-resistant rats. Int. J. Obes. 2018, 42, 535541. [Google Scholar] [CrossRef] [PubMed]
- Akoumianakis, I.; Antoniades, C. The interplay between adipose tissue and the cardiovascular system: Is. fat always bad? Cardiovasc. Res. 2017, 113, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Bertero, E.; Maack, C. Metabolic remodelling in heart failure. Nat. Rev. Cardiol. 2018, 15, 457–470. [Google Scholar] [CrossRef]
- Ha, E.; Bauer, R. Emerging roles for adipose tissue in cardiovascular disease. Arter. Thromb. Vasc. Biol. 2018, 38, e137–e144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Syed, W.; Liu, R.; Yu, J. Role of endoplasmic reticulum stress, autophagy, and inflammation in cardiovascular disease. Front. Cardiovasc. Med. 2017, 4, 29. [Google Scholar] [CrossRef]
- Ghemrawi, R.; Battaglia-Hsu, S.; Arnold, C. Endoplasmic reticulum stress in metabolic disorders. Cells 2018, 7, 63. [Google Scholar] [CrossRef]
- Hu, H.; Okada, K.; Liao, Y.; Tsukamoto, O.; Isomura, T.; Asai, M.; Sawada, T.; Okuda, K.; Asano, Y.; Sanada, S.; et al. Ablation of C/EBP homologous protein attenuates endoplasmic reticulum-mediated apoptosis and cardiac dysfunction induced by pressure overload. Circulation 2010, 122, 361–369. [Google Scholar] [CrossRef]
- Minamino, T.; Kitakaze, M. ER stress in cardiovascular disease. J. Mol. Cell Cardiol. 2010, 48, 1105–1110. [Google Scholar] [CrossRef]
- Fan, F.; Duan, Y.; Yang, F.; Trexler, C.; Wang, H.; Huang, L.; Li, Y.; Tang, H.; Wang, G.; Fang, X.; et al. Deletion of heat shock protein 60 in adult mouse cardiomyocytes perturbs mitochondrial protein homeostasis and causes heart failure. Cell Death Differ. 2020, 27, 587–600. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, D.; Brundel, B.; Wiersma, M. Imbalance of ER and mitochondria interactions: Prelude to cardiac ageing and disease? Cells 2019, 8, 1617. [Google Scholar] [CrossRef] [PubMed]
- Ramaccini, D.; Montoya-Uribe, V.; Aan, F.; Modesti, L.; Potes, Y.; Wieckowski, M.; Krga, I.; Glibetic, M.; Pinton, P.; Giorgi, C.; et al. Mitochondrial function, and dysfunction in dilated cardiomyopathy. Front. Cell Dev. Biol. 2021, 8, 624216. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Ramirez, F.; Ramos-Mondragon, R.; Garcia-Rivas, G. Mitochondrial and sarcoplasmic reticulum interconnection in cardiac arrhythmia. Front. Cell Dev. Biol. 2020, 8, 623381. [Google Scholar] [CrossRef] [PubMed]
- Hollander, J.; Thapa, D.; Sheperd, D. Physiological and structural differences in spatially distinct subpopulations of cardiac mitochondria: Influence of cardiac pathologies. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H1–H14. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Abdullah, C.; Aishwarya, R.; Morshed, M.; Bhuiyan, S. Molecular perspectives of mitochondrial adaptations and their role in cardiac proteostasis. Front. Physiol. 2020, 11, 1054. [Google Scholar] [CrossRef]
- Qin, K.; Tang, H.; Ren, Y.; Yang, D.; Li, Y.; Huang, W.; Wu, Y.; Yin, Z. Melatonin promotes sirtuin 1 expression and inhibits IRE1α-XBP1S-CHOP to reduce endoplasmic reticulum stress-mediated apoptosis in chondrocytes. Front. Pharmacol. 2022, 13, 940629. [Google Scholar] [CrossRef]
- Archer, S. Mitochondrial dynamics-mitochondrial fission and fusion in human diseases. N. Engl. J. Med. 2013, 369, 2236–2251. [Google Scholar] [CrossRef]
- Chen, Q.; Samidurai, A.; Thompson, J.; Hu, Y.; Das, A.; Willard, B.; Lesnefsky, E. Endoplasmic reticulum stress-mediated mitochondrial dysfunction in aged hearts. Biochimica Biophys. Acta BBA Mol. Basis Dis. 2020, 1866, 165899. [Google Scholar] [CrossRef]
- Santin, Y.; Fazal, L.; Sainte-Marie, Y.; Sicard, P.; Maggiorani, D.; Tortosa, F.; Yucel, Y.; Teyssedre, L.; Rouquette, J.; Marcellin, M.; et al. Mitochondrial 4HNE derived from MAO-A promotes mitoCa2+ overload in chronic postischemic cardiac remodelling. Cell Death Diff. 2020, 27, 1907–1923. [Google Scholar] [CrossRef]
- Chang, H.; Guarente, L. SIRT1 and other sirtuins in metabolism. Trends Endocr. Metab. 2014, 25, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Bai, P.; Gen Jin, Z. Sirtuins in cardiovascular health and diseases. Trends Endocrinol. Metab. 2016, 27, 677–678. [Google Scholar] [CrossRef] [PubMed]
- Favero, G.; Stacchiotti, A.; Castrezzati, S.; Bonomini, F.; Albanese, M.; Rezzani, R.; Rodella, L.F. Melatonin reduces obesity and restores adipokine patterns and metabolism in obese (ob/ob) mice. Nutr. Res. 2015, 95, 891–900. [Google Scholar] [CrossRef]
- D’Onofrio, N.; Servillo, L.; Balestrieri, M. SIRT1 and SIRT6 signaling pathways in cardiovascular disease protection. Antiox Redox Signal 2018, 28, 711–732. [Google Scholar] [CrossRef] [PubMed]
- Paramesha, B.; Soheb Anwar, M.; Meghwani, H.; Maulik, S.; Arava, S.; Banerjee, S. Sirt1 and Sirt3 activation improved cardiac function of diabetic rats via modulation of mitochondrial function. Antioxidants 2021, 10, 338. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, S.; Sadoshima, J. The role of sirtuins in cardiac disease. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1375–H1389. [Google Scholar] [CrossRef]
- Majeed, Y.; Halabi, N.; Madani, A.; Enelke, R.M.; Bhagwat, A.; Abdesselem, H.; Agha, M.; Vakayil, M.; Courjart, R.; Goswani, N.; et al. SIRT1 promotes lipid metabolism and mitochondrial biogenesis in adipocytes and coordinates adipogenesis by targeting key enzymatic pathways. Sci. Rep. 2021, 11, 8177. [Google Scholar] [CrossRef]
- Herranz, D.; Serrano, M. Sirt1: Recent lessons from mouse models. Nat. Rev. Cancer 2010, 10, 819–823. [Google Scholar] [CrossRef]
- Planavila, A.; Dominuez, E.; Navarro, M.; Vinciguerra, M.; Iglesias, R.; Giralt, M.; Lope-Piedrafita, S.; Ruberte, J.; Villarroya, F. Dilated cardiomyopathy and mitochondrial dysfunction in Sirt1-deficient mice: A role for Sirt1-Mef2 in adult heart. J. Mol. Cell Cardiol. 2012, 53, 521–531. [Google Scholar] [CrossRef]
- Hsu, Y.; Hsu, S.; Hsu, C.; Chen, Y.; Chang, Y.; Sadoshima, J.; Huang, S.; Tsai, C.; Lin, C. Sirtuin1 protects the aging heart from contractile dysfunction mediated through the inibittion of endoplasmic reticulum stress-mediated apoptosis in cardiac specific knockout mouse model. Int. J. Cardiol. 2017, 228, 543–552. [Google Scholar] [CrossRef]
- Prola, A.; Da Silva, J.; Guilbert, A.; Lecnu, L.; Piquereau, J.; Ribeiro, M.; Mateo, P.; Gressette, M.; Fortin, D.; Boursier, C.; et al. SIRT1 protects the heart from ER-stress-induced cell death through eIF2α deacetylation. Cell Death Diff. 2017, 24, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Meng, T.; Qin, W.; Liu, B. SIRT1 antagonizes oxidative stress in diabetic vascular complication. Front. Endocrinol. 2020, 11, 568861. [Google Scholar] [CrossRef] [PubMed]
- Alcendor, R.; Gao, S.; Zhai, P.; Zablocki, D.; Holle, E.; Yu, X.; Tian, B.; Wagner, T.; Vatner, S.; Sadoshima, J. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ. Res. 2007, 100, 1512–1521. [Google Scholar] [CrossRef] [PubMed]
- Hui, X.; Zhang, M.; Gu, P.; Li, K.; Gao, Y.; Wu, D.; Wang, Y.; Xu, A. Adipocyte SIRT1 controls systemic insulin sensitivity by modulating macrophages in adipose tissue. EMBO Rep. 2017, 18, 645–657. [Google Scholar] [CrossRef]
- Arendt, J. Melatonin: Counteracting chaotic time cues. Front. Endocrinol. 2019, 10, 391. [Google Scholar] [CrossRef]
- Guan, Q.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Mechanisms of melatonin in obesity: A review. Int. J. Mol. Sci. 2022, 23, 218. [Google Scholar] [CrossRef]
- Tan, D.; Manchester, L.; Qin, L.; Reiter, R. Melatonin: A mitochondrial targeting molecule involving mitochondrial protection and dynamics. Int. J. Mol. Sci. 2016, 17, 2124. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Feng, N.; Tang, D.; Feng, J.; Li, Z.; Jia, M.; Liu, Z.; Gu, X.; Wang, Y.; Fu, F.; et al. Melatonin prevents Drp1-mediated mitochondrial fission in diabetic hearts through SIRT1-PGC1α pathway. J. Pineal Res. 2018, 65, e12491. [Google Scholar] [CrossRef]
- Stacchiotti, A.; Favero, G.; Giugno, L.; Golic, I.; Korac, A.; Rezzani, R. Melatonin efficacy in obese leptin-deficient mice heart. Nutrients 2017, 9, 1323. [Google Scholar] [CrossRef]
- Xu, L.; Li, D.; Li, H.; Zhang, O.; Huang, Y.; Shao, H.; Wang, Y.; Cai, S.; Zhu, Y.; Jin, S.; et al. Suppression of obesity by melatonin through increasing energy expenditure and accelerating lipolysis in mice fed a high-fat diet. Nutr. Diabetes 2022, 12, 42. [Google Scholar] [CrossRef]
- Aouichat, S.; Raya, E.; Molina-Carballo, A.; Munoz-Hoyos, A.; Aloweidi, A.; Elmahallawy, E.; Agil, A. Dose-dependent effect of melatonin on BAT thermogenesis in Zucker diabetic fatty rat: Future clinical implications for obesity. Antioxidants 2022, 11, 1646. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Gonzalez, J.L.; Sanchez-Quintero, D.; Proaño-Bernal, L.; Santana-Apreza, R.; Jimenez-Chavarria, M.A.; Luna-Alvarez-Amezquita, J.A.; Straface, J.I.; Perez-Partida, A.M.; Berarducci, J.; Armenta-Moreno, J.I.; et al. Role of the antioxidant activity of melatonin in myocardial ischemia-reperfusion injury. Antioxidants 2022, 11, 627. [Google Scholar] [CrossRef] [PubMed]
- Nduhirabandi, F.; Maarman, G. Melatonin in heart failure: A promising therapeutic strategy? Molecules 2018, 23, 1819. [Google Scholar] [CrossRef] [PubMed]
- Stacchiotti, A.; Grossi, I.; Garcia-Gomez, R.; Patel, G.; Salvi, A.; De Petro, G.; Monsalve, M.; Rezzani, R. Melatonin effects on non-alcoholic fatty liver disease are related to microRNA-34a-5p/Sirt1 axis and autophagy. Cells 2019, 8, 1053. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Zheng, X.; Lin, B.; Liang, H.; Cai, M.; Cao, H.; Ye, J.; Weng, J. Diet-induced obesity and insulin resistance are associated with brown fat degeneration in SIRT1-deficient mice. Obesity 2016, 24, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Nepal, P.; Odelade, A.; Freely, F.D.; Belton, D.M.; Graves, J.L., Jr.; Maldonado-Devincci, A.M. High-fat diet-induced weight gain, behavioral deficits, and dopamine changes in young C57BL/6J mice. Front. Nutr. 2021, 7, 591161. [Google Scholar] [CrossRef]
- Gianazza, E.; Brioschi, M.; Martinez Fernandez, A.; Casalnuovo, F.; Altomare, A.; Aldini, G.; Banfi, C. Lipid Peroxidation in atherosclerotic cardiovscular diseases. Antioxid. Redox Signal. 2020, 34, 49–98. [Google Scholar] [CrossRef]
- Chaanine, A.H.; LeJemtel, T.H.; Delafontaine, P. Mitochondrial Pathobiology and Metabolic Remodeling in Progression to Overt Systolic Heart Failure. J. Clin. Med. 2020, 9, 3582. [Google Scholar] [CrossRef]
- Smyrnias, I.; Gray, S.P.; Okonko, D.O.; Sawyer, G.; Zoccarato, A.; Catibog, N.; López, B.; González, A.; Ravassa, S.; Díez, J.; et al. Cardioprotective effect of the mitochondrial unfolded protein response during chronic pressure overload. J. Am. Coll. Cardiol. 2019, 73, 1795–1806. [Google Scholar] [CrossRef]
- Altintas, M.M.; Rossetti, M.A.; Nayer, B.; Puig, A.; Zagallo, P.; Ortega, L.M.; Johnson, K.B.; McNamara, G.; Reiser, J.; Mendez, A.J.; et al. Apoptosis, mastocytosis, and diminished adipocytokine gene expression accompany reduced epididymal fat mass in long-standing diet-induced obese mice. Lipids Health Dis. 2011, 10, 198. [Google Scholar] [CrossRef]
- De Rosa, M.; Gambardella, J.; Shu, J.; Santulli, G. Dietary fats a key determinant in balancing mitochondrial dynamics in heart failure: A novel mechanism underlying the obesity paradox. Cardiovasc. Res. 2018, 114, 925–927. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Lavie, C.; Borer, J.; Vallakati, A.; Goel, S.; Lopez-Jimenez, F.; Arbab-Zadeh, A.; Mukherejee, D.; Lazar, J. Meta-analysis of the relation of body mass index to all-cause and cardiovascular mortality and hospitalization in patients with chronic heart failure. Am. J. Cardiol. 2015, 115, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Potes, Y.; de Luxan-Delgado, B.; Rublo-Gonzalez, A.; Reiter, R.; Coto-Montes, A. Dose-dependent beneficial effect of melatonin on obesity: Interaction of melatonin and leptin. Melatonin Res. 2019, 2, 1–8. [Google Scholar] [CrossRef]
- Dominguez-Rodriguez, A.; Abreu-Gonzales, P.; Reiter, R. The potential usefulness of serum melatonin levelto predict heart failure in patients with hypertensive cardiomyopathy. Int. J. Cardiol. 2014, 174, 415–417. [Google Scholar] [CrossRef] [PubMed]
- Misaka, T.; Yoshihiisa, A.; Yokokawa, T.; Sato, T.; Oikawa, M.; Kobayashi, A.; Yamaki, T.; Sugimoto, K.; Kunii, H.; Nakazato, K.; et al. Plasma levels of melatonin in dilated cardiomyopathy. J. Pineal Res. 2019, 66, e12564. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Young, M.; Cui, L.; Lopaschuk, C.; Liao, R.; Tian, R. Increased glucose uptake and oxidation in mouse hearts prevents high fatty acid oxidation but cause cardiac dysfunction in diet-induced obesity. Circulation 2009, 119, 2818–2828. [Google Scholar] [CrossRef]
- Purushotham, A.; Xu, Q.; Li, X. Systematic SIRT1 insufficiency results in disruption in energy homeostasis and steroid hormone metabolism upon high-fat-diet feeding. FASEB J. 2012, 26, 656–667. [Google Scholar] [CrossRef]
- Villarroya, J.; Redondo-Angulo, I.; Iglesias, R.; Giralt, M.; Villarroya, F.; Planavila, A. Sirt1 mediates the effects of a short-term high-fat diet on the heart. J. Nutr. Biochem. 2015, 26, 1328–1337. [Google Scholar] [CrossRef]
- Yamamoto, T.; Endo, J.; Kataoka, M.; Matsuhashi, T.; Katsumata, Y.; Shirakawa, K.; Yoshida, N.; Isobe, S.; Moriyama, H.; Goto, S.; et al. Sirt1 counteracts decrease in membrane phospholipid unsaturation and diastolic dysfunction during saturated fatty acid overload. J. Mol. Cell Cardiol. 2019, 133, 1–11. [Google Scholar] [CrossRef]
- Sanz, M.; Grimbert, L.; Moulin, M.; Gressette, M.; Rucker-Martin, C.; Lemaire, C.; Mericsaky, M.; Veksler, V.; Ventura-Clapier, R.; Garnier, A.; et al. Inducible cardiac-specific deletion of Sirt1 in male mice reveals progressive cardiac dysfunction and sensitization of the heart to pressure overload. Int. J. Mol. Sci. 2019, 20, 5005. [Google Scholar] [CrossRef]
- Kruszewska, J.; Cudnoch-Jedrzejewska, A.; Czarzasta, I. Remodelling and fibrosis of the cardiac muscle in the course of obesity-Pathogenesis and involvement of the extracellular matrix. Int. J. Mol. Sci. 2022, 23, 4195. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Weihrauch, D.; Kersten, J.R.; Toth, J.M.; Passerini, A.G.; Rajamani, A.; Schrepfer, S.; LaDisa, J.F., Jr. Alagebrium inhibits neointimal hyperplasia and restores distributions of wall shear stress by reducing downstream vascular resistance in obese and diabetic rats. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1130–H1140. [Google Scholar] [CrossRef] [PubMed]
- Brainard, R.; Watson, L.; DeMartino, A.; Brittian, K.; Readnower, R.; Boakye, A.; Zhang, D.; Hoetker, J.; Bhatagar, A.; Baba, S.; et al. High fat feeding in mice is insufficient to induce cardiac dysfunction and does not exacerbate heart failure. PLoS ONE 2013, 8, e83174. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.; Jung, T.; Grune, T.; Siems, W. 4-hydroxynonenal (HNE) modified proteins in metabolic diseases. Free Rad. Biol. Med. 2017, 111, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.; Macario, A.; Conway de Macario, E.; Gouni-Berthold, I.; Berthold, H.; Rini, G.; Zummo, G.; Cappello, F. Heat shock protein 60 and risk for cardiovascular disease. Curr. Pharm. Des. 2011, 17, 3662–3668. [Google Scholar] [CrossRef]
- Duan, Y.; Tang, H.; Mitchell-Silbaugh, K.; Fang, X.; Han, Z.; Ouyang, K. Heat shock protein 60 in cardiovascular physiology and diseases. Front. Mol. Biosci. 2020, 7, 73. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shao, M.; Yao, J.; Yang, S.; Cheng, W.; Ma, L.; Li, W.; Cao, J.; Zhang, Y.; Hu, Y.; et al. Neocryptotanshinone protects against myocardial ischemia-reperfusion injury by promoting autolysosome degradation of protein aggregates via the ERK1/2-Nrf2-LAMP2 pathway. Phytomedicine 2023, 110, 154625. [Google Scholar] [CrossRef]
- Li, W.; Wang, H.; Tan, Y.; Wang, Y.; Yu, S.; Li, Z. Reducing lipofuscin accumulation and cardiomyocytic senescence of aging heart by enhancing autophagy. Exp. Cell Res. 2021, 403, 112585. [Google Scholar] [CrossRef]
- Hohn, A.; Grune, T. Lipofuscin: Formation, effects and role of macroautophagy. Redox Biol. 2013, 1, 140–144. [Google Scholar] [CrossRef]
- Song, M.; Franco, A.; Fleischer, J.; Zhang, L.; Dorn, G. Abrogating mitochondrial dynamics in mouse hearts accelerates mitochondrial senescence. Cell Metab. 2017, 26, 872–883. [Google Scholar] [CrossRef]
- Zhang, J.; He, Z.; Fedorova, J.; Logan, C.; Bates, L.; Davitt, K.; Le, V.; Murphy, J.; Li, M.; Wang, M.; et al. Alterations in mitochondrial dynamics with age-related sirtuin1/sirtuin3 deficiency impair cardiomyocyte contractility. Aging Cell 2021, 20, e13419. [Google Scholar] [CrossRef]
- Nawaz, A.; Mehmood, A.; Kanatani, Y.; Kado, T.; Igarashi, Y.; Takikawa, A.; Yamamoto, S.; Okabe, K.; Nakagawa, T.; Yagi, K.; et al. Sirt1 activator induces proangiogenic genes in preadipocytes to rescue insulin resistance in diet-induced obese mice. Sci. Rep. 2018, 8, 11370. [Google Scholar] [CrossRef] [PubMed]
- Braud, L.; Pini, M.; Stec, D.; Manin, S.; Derumeaux, G.; Stec, D.; Foresti, R.; Motterlini, R. Increased Sirt1 secreted from visceral white adipose tissue is associated with improved glucose tolerance in obese Nrf2-deficient mice. Redox Biol. 2021, 38, 101805. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.; Ronconi Sousa, K.; Morra, A.; Lima Val, P.; Porto Leite, M.; Vassallo, D.; Figueiredo, S.; Stefanon, I. Sex differences in the regulation of spatially distinct cardiac mitochondrial subpopulations. Mol. Cell Biochem. 2016, 419, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.; Kraus, G.; Kim, I.; Spurlock, M.; Bailey, T.; Zhang, Q.; Beitz, D. A mitochondria-targeted vitamin E derivative decreases hepatic oxidative stress and inhibits fat deposition in mice. J. Nutr. 2010, 140, 1425–1431. [Google Scholar] [CrossRef]
- Rangel-Azevedo, C.; Santana-Oliveira Araujo, D.; Santos Miranda, C.; Ferreira Martins, F.; Mandarim de Lacerda, C.; Souza-Mello, V. Progressive brown adipocyte dysfunction: Whitening and impaired nonshivering thermogenesis as long-term obesity complication. J. Nutr. Biochem. 2022, 105, 109002. [Google Scholar] [CrossRef]
- Nardo, L.; Rezzani, R.; Facchetti, L.; Favero, G.; Franco, C.; Abdelhafez, Y.G.; Badawi, R.D.; Guindani, M.; Seo, Y.; Pampaloni, M. Beneficial effects of melatonin on apolipoprotein-E knockout mice by morphological and 18F-FDG PET/CT assessments. Int. J. Mol. Sci. 2020, 21, 2920. [Google Scholar] [CrossRef]
- Borsani, E.; Ballini, A.; Buffoli, B.; Muzio, L.L.; Di Domenico, M.; Boccellino, M.; Scacco, S.; Nocini, R.; Dibello, V.; Rezzani, R.; et al. Peripheral purinergic modulation in pediatric orofacial inflammatory pain affects brainstem nitroxidergic system: A translational research. Biomed. Res. Int. 2022, 2022, 1326885. [Google Scholar] [CrossRef]



| STD (n = 6) | HFD (n = 10) | HFD + MEL (n = 10) | |
|---|---|---|---|
| WT body weight—T0 (g) | 26.35 ± 0.8 | 27.58 ± 0.7 | 26.49 ± 0.9 |
| WT body weight—T1 (g) | 29.09 ± 0.9 | 42.35 ± 1.1 A,C | 34.05 ± 0.7 B |
| WT body weight gain (g) | 2.74 | 14.77 | 7.56 |
| HET body weight—T0 (g) | 27.03 ± 0.8 | 27.34 ± 0.9 | 27.65 ± 0.8 |
| HET body weight—T1 (g) | 30.06 ± 0.7 | 40.42 ± 1.0 A | 38.10 ± 0.6 A |
| HET body weight gain (g) | 3.03 | 13.08 | 10.45 |
| WT eWAT weight—T0 (g) | 0.82 ± 0.04 | 1.12 ± 0.02 A,C | 0.93 ± 0.03 A,B |
| HET eWAT weight—T1 (g) | 0.78 ± 0.02 | 0.70± 0.02 A | 0.68 ± 0.04 A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Favero, G.; Golic, I.; Arnaboldi, F.; Cappella, A.; Korac, A.; Monsalve, M.; Stacchiotti, A.; Rezzani, R. Cardiometabolic Changes in Sirtuin1-Heterozygous Mice on High-Fat Diet and Melatonin Supplementation. Int. J. Mol. Sci. 2024, 25, 860. https://doi.org/10.3390/ijms25020860
Favero G, Golic I, Arnaboldi F, Cappella A, Korac A, Monsalve M, Stacchiotti A, Rezzani R. Cardiometabolic Changes in Sirtuin1-Heterozygous Mice on High-Fat Diet and Melatonin Supplementation. International Journal of Molecular Sciences. 2024; 25(2):860. https://doi.org/10.3390/ijms25020860
Chicago/Turabian StyleFavero, Gaia, Igor Golic, Francesca Arnaboldi, Annalisa Cappella, Aleksandra Korac, Maria Monsalve, Alessandra Stacchiotti, and Rita Rezzani. 2024. "Cardiometabolic Changes in Sirtuin1-Heterozygous Mice on High-Fat Diet and Melatonin Supplementation" International Journal of Molecular Sciences 25, no. 2: 860. https://doi.org/10.3390/ijms25020860
APA StyleFavero, G., Golic, I., Arnaboldi, F., Cappella, A., Korac, A., Monsalve, M., Stacchiotti, A., & Rezzani, R. (2024). Cardiometabolic Changes in Sirtuin1-Heterozygous Mice on High-Fat Diet and Melatonin Supplementation. International Journal of Molecular Sciences, 25(2), 860. https://doi.org/10.3390/ijms25020860












