Exploring Potential Epigenetic Biomarkers for Colorectal Cancer Metastasis
Abstract
:1. Introduction
2. DNA Methylation Biomarkers for CRC Metastasis
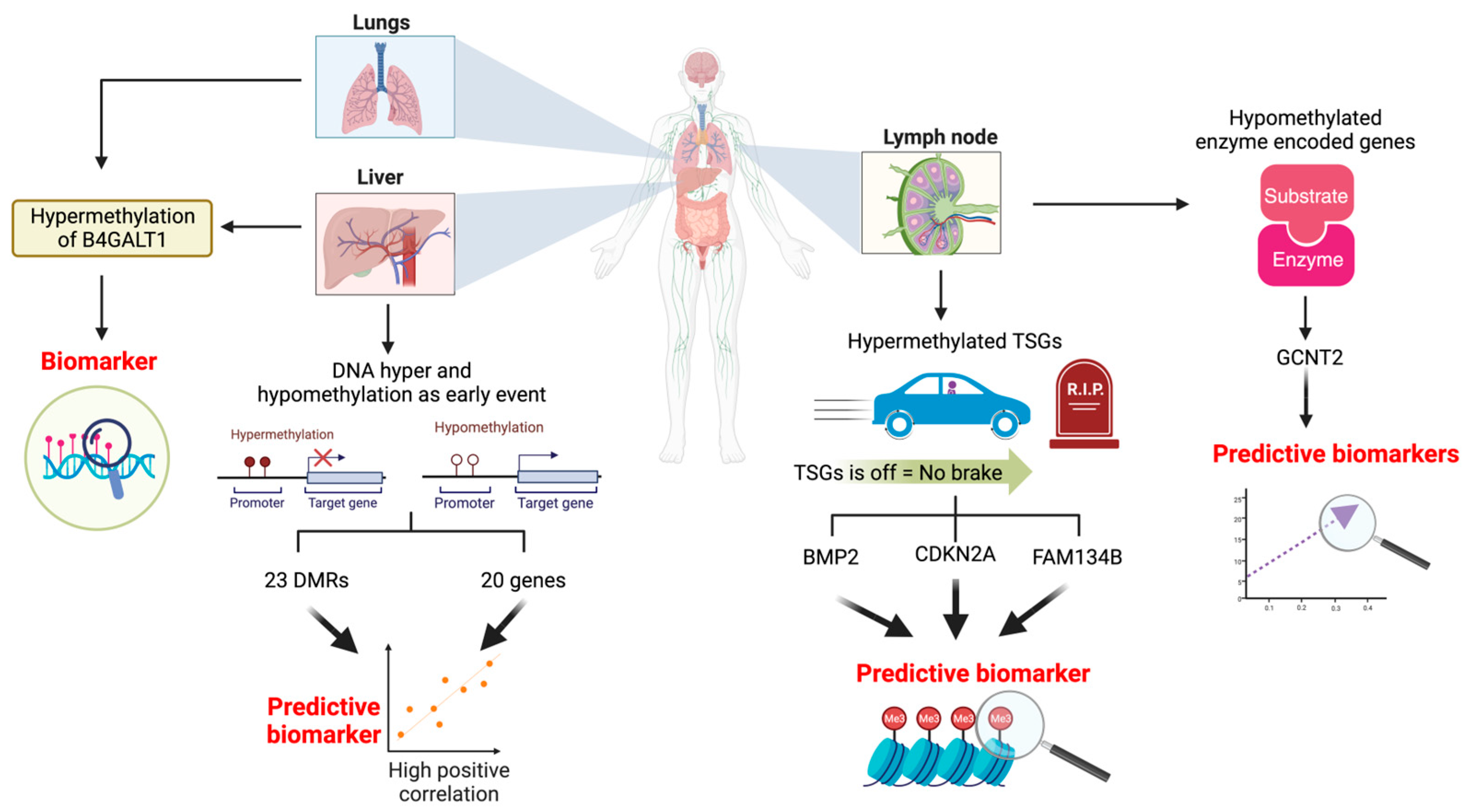
2.1. DNA Hypermethylation of Tumour Suppressor Genes as a Biomarker for Lymph Node Metastasis
2.2. Hypomethylation of the Enzyme-Encoding Gene as a Biomarker for Lymph Node Metastasis
2.3. DNA Hyper and Hypomethylation as an Early Event to Predict Liver Metastasis
2.4. The Role of DNA Hypermethylation in Distant Metastasis
3. Histone Modifications as Potential Biomarkers for CRC Metastasis
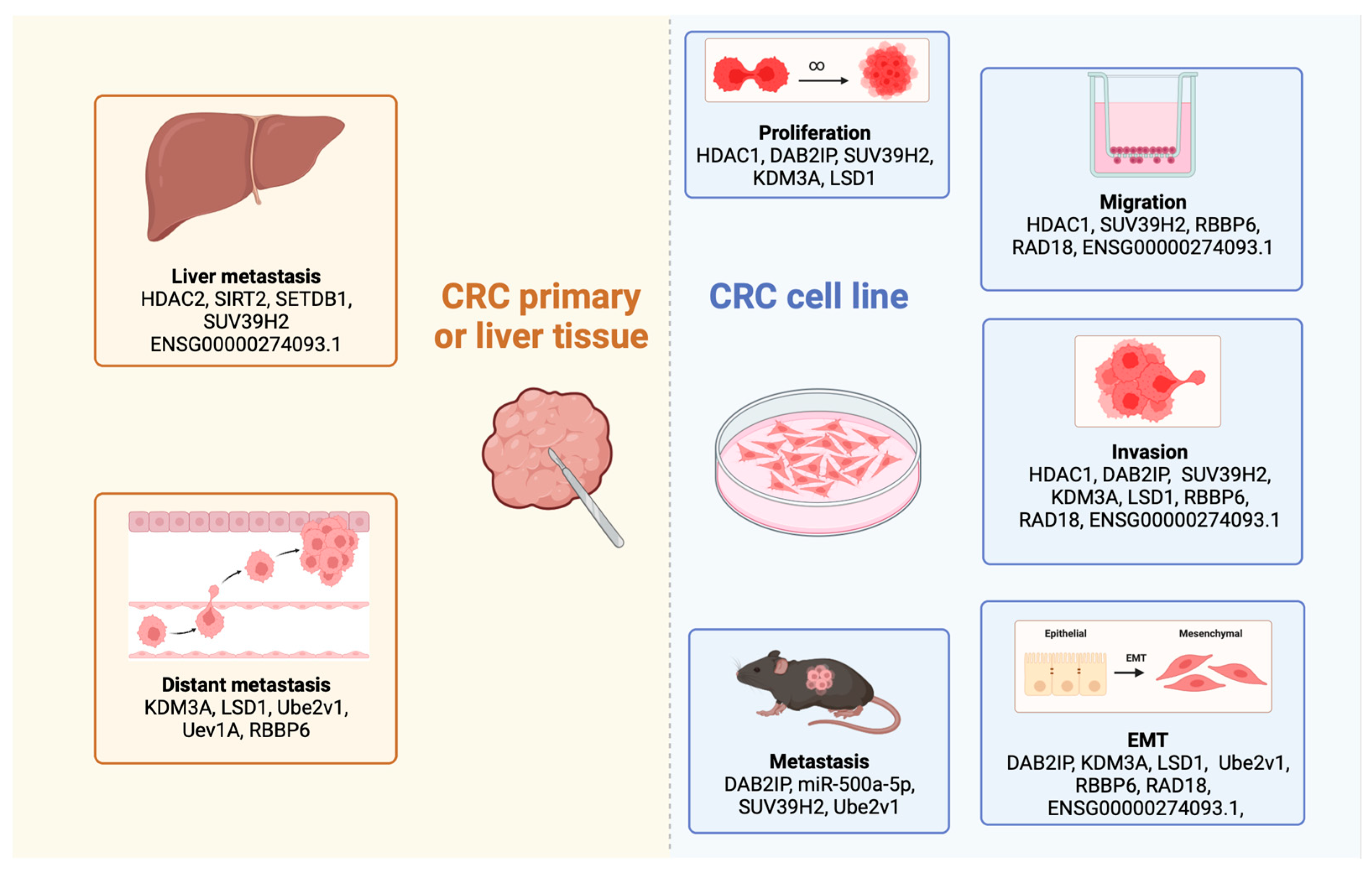
3.1. The Role of Histone Deacetylase Enzymes in CRC Metastasis
3.1.1. Histone Deacetylases Influence the Progression of Epithelial-to-Mesenchymal Transition
3.1.2. Involvement of Histone Deacetylases in CRC Liver Metastasis
3.2. Histone Methyltransferases as Potential Oncogenes in CRC Liver Metastasis
3.3. Lysine-Specific Histone Demethylases Promote CRC Metastasis
3.4. Understanding the Influence of Ubiquitination in CRC Metastasis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Moons, L.; Mariman, A.; Vermeir, P.; Colemont, L.; Clays, E.; Van Vlierberghe, H.; Vogelaers, D. Sociodemographic factors and strategies in colorectal cancer screening: A narrative review and practical recommendations. Acta Clin. Belg. 2020, 75, 33–41. [Google Scholar] [CrossRef]
- Keum, N.; Giovannucci, E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 713–732. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R. A comparative examination of colorectal cancer burden in European Union, 1990–2019: Estimates from Global Burden of Disease 2019 Study. Int. J. Clin. Oncol. 2022, 27, 1309–1320. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Borràs, J.M.; Castells, A.; Ciardiello, F.; Ducreux, M.; Haq, A.; Schmoll, H.-J.; Tabernero, J. Improving outcomes in colorectal cancer: Where do we go from here? Eur. J. Cancer 2013, 49, 2476–2485. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.L.F.; Barbosa, L.E.R.; Teixeira, J.P.M.d.A. Prognosis in colorectal cancer beyond TNM. J. Coloproctol. 2020, 40, 404–411. [Google Scholar] [CrossRef]
- Vardy, J.L.; Dhillon, H.M.; Pond, G.R.; Renton, C.; Clarke, S.J.; Tannock, I.F. Prognostic indices of inflammatory markers, cognitive function and fatigue for survival in patients with localised colorectal cancer. ESMO Open 2018, 3, e000302. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, Y.; Xu, G.; Baklaushev, V.P.; Chekhonin, V.P.; Peltzer, K.; Ma, W.; Wang, X.; Wang, G.; Zhang, C. Nomogram for predicting overall survival in colorectal cancer with distant metastasis. BMC Gastroenterol. 2021, 21, 103. [Google Scholar] [CrossRef]
- Amin, M.B.; Edge, S.B.; Greene, F.L.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C. AJCC Cancer Staging Manual; Springer: Berlin/Heidelberg, Germany, 2017; Volume 1024. [Google Scholar]
- Martinelli, E.; Ciardiello, D.; Martini, G.; Troiani, T.; Cardone, C.; Vitiello, P.P.; Normanno, N.; Rachiglio, A.M.; Maiello, E.; Latiano, T.; et al. Implementing anti-epidermal growth factor receptor (EGFR) therapy in metastatic colorectal cancer: Challenges and future perspectives. Ann. Oncol. 2020, 31, 30–40. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, C.; Ma, W.; Tian, F.; Xu, G.; Han, X.; Sun, P.; Baklaushev, V.P.; Bryukhovetskiy, A.S.; Wang, G.; et al. Patterns of bone metastases in newly diagnosed colorectal cancer: A real-world analysis in the SEER database. Int. J. Color. Dis. 2019, 34, 533–543. [Google Scholar] [CrossRef]
- Liu, X.; Xu, D.; Liu, Z.; Li, Y.; Zhang, C.; Gong, Y.; Jiang, Y.; Xing, B. THBS1 facilitates colorectal liver metastasis through enhancing epithelial–mesenchymal transition. Clin. Transl. Oncol. 2020, 22, 1730–1740. [Google Scholar] [CrossRef] [PubMed]
- Tie, J.; Wang, Y.; Cohen, J.; Li, L.; Hong, W.; Christie, M.; Wong, H.L.; Kosmider, S.; Wong, R.; Thomson, B.; et al. Circulating tumor DNA dynamics and recurrence risk in patients undergoing curative intent resection of colorectal cancer liver metastases: A prospective cohort study. PLoS Med. 2021, 18, e1003620. [Google Scholar] [CrossRef]
- Koo, T.; Kim, K.; Park, H.J.; Han, S.-W.; Kim, T.-Y.; Jeong, S.-Y.; Park, K.J.; Chie, E.K. Prognostic factors for survival in colorectal cancer patients with brain metastases undergoing whole brain radiotherapy: Multicenter retrospective study. Sci. Rep. 2020, 10, 4340. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Chun, Y.J.; Kim, H.S.; Kim, J.H.; Lee, C.-K.; Beom, S.-H.; Shin, S.J.; Ahn, J.B. Clinical features and KRAS mutation in colorectal cancer with bone metastasis. Sci. Rep. 2020, 10, 21180. [Google Scholar] [CrossRef] [PubMed]
- Quan, J.-C.; Guan, X.; Ma, C.-X.; Liu, Z.; Yang, M.; Zhao, Z.-X.; Sun, P.; Zhuang, M.; Wang, S.; Jiang, Z.; et al. Prognostic scoring system for synchronous brain metastasis at diagnosis of colorectal cancer: A population-based study. World J. Gastrointest. Oncol. 2020, 12, 195–204. [Google Scholar] [CrossRef]
- Liu, L.L.; Sun, J.D.; Xiang, Z.L. Survival nomograms for colorectal carcinoma patients with lung metastasis and lung-only metastasis, based on the SEER database and a single-center external validation cohort. BMC Gastroenterol. 2022, 22, 446. [Google Scholar] [CrossRef] [PubMed]
- Paulatto, L.; Dioguardi Burgio, M.; Sartoris, R.; Beaufrère, A.; Cauchy, F.; Paradis, V.; Vilgrain, V.; Ronot, M. Colorectal liver metastases: Radiopathological correlation. Insights Into Imaging 2020, 11, 99. [Google Scholar] [CrossRef]
- Li, T.; Huang, H.; Zhang, S.; Zhang, Y.; Jing, H.; Sun, T.; Zhang, X.; Lu, L.; Zhang, M. Predictive models based on machine learning for bone metastasis in patients with diagnosed colorectal cancer. Front. Public Health 2022, 10, 984750. [Google Scholar] [CrossRef]
- Li, W.; Wang, T.; Zhu, Y.; Yu, H.; Ma, L.; Ding, Y.; Hong, G.; Lei, D. Brain metastasis from colorectal cancer: Treatment, survival, and prognosis. Medicine 2022, 101, e30273. [Google Scholar] [CrossRef]
- Biller, L.H.; Schrag, D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA 2021, 325, 669–685. [Google Scholar] [CrossRef]
- Tsili, A.C.; Alexiou, G.; Naka, C.; Argyropoulou, M.I. Imaging of colorectal cancer liver metastases using contrast-enhanced US, multidetector CT, MRI, and FDG PET/CT: A meta-analysis. Acta Radiol. 2020, 62, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Hancerliogullari, O.; Okuyucu, K.; Ince, S.; Peker, S.; Arslan, N. Prognostic parameters in recurrent colorectal cancer: A role of control or restaging by FDG-PET/CT. Vojnosanit. Pregl. 2020, 77. [Google Scholar] [CrossRef]
- Kudo, S.-E.; Ichimasa, K.; Villard, B.; Mori, Y.; Misawa, M.; Saito, S.; Hotta, K.; Saito, Y.; Matsuda, T.; Yamada, K.; et al. Artificial Intelligence System to Determine Risk of T1 Colorectal Cancer Metastasis to Lymph Node. Gastroenterology 2021, 160, 1075–1084.e2. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Song, B.-I.; Kim, B.W.; Kim, H.W.; Won, K.S.; Bae, S.U.; Jeong, W.K.; Baek, S.K. Predictive Value of [18F]FDG PET/CT for Lymph Node Metastasis in Rectal Cancer. Sci. Rep. 2019, 9, 4979. [Google Scholar] [CrossRef]
- Elzaki, A. Assessment of the Use of Preoperative CT Scan Image for Predicting Lymph Nodes for Resection of Colorectal Cancer: A Retrospective Study. Dubai Med. J. 2022, 5, 171–176. [Google Scholar] [CrossRef]
- Hunter, C.; Blake, H.; Jeyadevan, N.; Abulafi, M.; Swift, I.; Toomey, P.; Brown, G. Local staging and assessment of colon cancer with 1.5-T magnetic resonance imaging. Br. J. Radiol. 2016, 89, 20160257. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, S.H.; Lee, S.M.; Lee, J.S.; Han, J.K. CT volumetric measurement of colorectal cancer helps predict tumor staging and prognosis. PLoS ONE 2017, 12, e0178522. [Google Scholar] [CrossRef]
- Mou, A.; Li, H.; Chen, X.-L.; Fan, Y.-H.; Pu, H. Tumor size measured by multidetector CT in resectable colon cancer: Correlation with regional lymph node metastasis and N stage. World J. Surg. Oncol. 2021, 19, 179. [Google Scholar] [CrossRef]
- Chen, H.; Huang, S.; Zeng, Q.; Zhang, M.; Ni, Z.; Li, X.; Xu, X. A retrospective study analyzing missed diagnosis of lung metastases at their early stages on computed tomography. J. Thorac. Dis. 2019, 11, 3360–3368. [Google Scholar] [CrossRef]
- Daza, J.F.; Solis, N.M.; Parpia, S.; Gallinger, S.; Moulton, C.-A.; Belley-Cote, E.P.; Levine, M.N.; Serrano, P.E. A meta-analysis exploring the role of PET and PET-CT in the management of potentially resectable colorectal cancer liver metastases. Eur. J. Surg. Oncol. 2019, 45, 1341–1348. [Google Scholar] [CrossRef]
- Borello, A.; Russolillo, N.; Lo Tesoriere, R.; Langella, S.; Guerra, M.; Ferrero, A. Diagnostic performance of the FDG-PET/CT in patients with resected mucinous colorectal liver metastases. Surgeon 2021, 19, e140–e145. [Google Scholar] [CrossRef] [PubMed]
- Uzun, A.K.; Güveli, T.K.; Özülker, F.; Özülker, T. The Efficacy of 18F-FDG PET/CT in Detecting Colorectal Cancer Recurrences. Eur. Arch. Med. Res. 2021, 37, 236–243. [Google Scholar] [CrossRef]
- Seo, H.J.; Min, B.W.; Eo, J.S.; Lee, S.I.; Kang, S.H.; Jung, S.Y.; Oh, S.C.; Choe, J.G. Usefulness of 18F-FDG PET/CT to Detect Metastatic Mucinous Adenocarcinoma Within an Inguinal Hernia. Nucl. Med. Mol. Imaging 2016, 50, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.A.; Kim, Y.K.; Min, J.H.; Song, K.D.; Sohn, I.; Ahn, H.S. Non-contrast liver MRI as an alternative to gadoxetic acid-enhanced MRI for liver metastasis from colorectal cancer. Acta Radiol. 2019, 60, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Diane, M.; Mehdi, O.; David, F.; Philippe, M.; Johann, P.; Henri, D.; Jean-Marc, R.; Laetitia, D.; Igor, S.; Bernard, S. Patients with Brain Metastases from Colorectal Cancer Are Not Condemned. Anticancer Res. 2013, 33, 5645. [Google Scholar]
- Hoshino, N.; Murakami, K.; Hida, K.; Sakamoto, T.; Sakai, Y. Diagnostic accuracy of magnetic resonance imaging and computed tomography for lateral lymph node metastasis in rectal cancer: A systematic review and meta-analysis. Int. J. Clin. Oncol. 2019, 24, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.; Zhang, Y.; Wei, M.; Yang, X.; Wang, Z. Magnetic resonance imaging evaluation of the accuracy of various lymph node staging criteria in rectal cancer: A systematic review and meta-analysis. Front. Oncol. 2021, 11, 709070. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Bi, X.; Liu, F.; Huang, J.; Zhang, Z. Predictive Efficacy of Circulating Tumor Cells in First Drainage Vein Blood from Patients with Colorectal Cancer Liver Metastasis. Cancer Investig. 2022, 40, 767–776. [Google Scholar] [CrossRef]
- Vatandoost, N.; Ghanbari, J.; Mojaver, M.; Avan, A.; Ghayour-Mobarhan, M.; Nedaeinia, R.; Salehi, R. Early detection of colorectal cancer: From conventional methods to novel biomarkers. J. Cancer Res. Clin. Oncol. 2016, 142, 341–351. [Google Scholar] [CrossRef]
- Ruiz-Bañobre, J.; Kandimalla, R.; Goel, A. Predictive Biomarkers in Metastatic Colorectal Cancer: A Systematic Review. JCO Precis. Oncol. 2019, 3, 1–17. [Google Scholar] [CrossRef]
- Rodger, E.J.; Gimenez, G.; Ajithkumar, P.; Stockwell, P.A.; Almomani, S.; Bowden, S.A.; Leichter, A.L.; Ahn, A.; Pattison, S.; McCall, J.L.; et al. An epigenetic signature of advanced colorectal cancer metastasis. iScience 2023, 26, 106986. [Google Scholar] [CrossRef] [PubMed]
- Condelli, V.; Calice, G.; Cassano, A.; Basso, M.; Rodriquenz, M.G.; Zupa, A.; Maddalena, F.; Crispo, F.; Pietrafesa, M.; Aieta, M.; et al. Novel Epigenetic Eight-Gene Signature Predictive of Poor Prognosis and MSI-Like Phenotype in Human Metastatic Colorectal Carcinomas. Cancers 2021, 13, 158. [Google Scholar] [CrossRef]
- Afrăsânie, V.-A.; Marinca, M.-V.; Gafton, B.; Alexa-Stratulat, T.; Rusu, A.; Froicu, E.-M.; Sur, D.; Lungulescu, C.V.; Popovici, L.; Lefter, A.-V.; et al. Clinical, Pathological and Molecular Insights on KRAS, NRAS, BRAF, PIK3CA and TP53 Mutations in Metastatic Colorectal Cancer Patients from Northeastern Romania. Int. J. Mol. Sci. 2023, 24, 12679. [Google Scholar] [CrossRef] [PubMed]
- Postwala, H.; Shah, Y.; Parekh, P.S.; Chorawala, M.R. Unveiling the genetic and epigenetic landscape of colorectal cancer: New insights into pathogenic pathways. Med. Oncol. 2023, 40, 334. [Google Scholar] [CrossRef]
- Han, M.; Jia, L.; Lv, W.; Wang, L.; Cui, W. Epigenetic Enzyme Mutations: Role in Tumorigenesis and Molecular Inhibitors. Front. Oncol. 2019, 9, 194. [Google Scholar] [CrossRef]
- Chatterjee, A.; Rodger, E.J.; Eccles, M.R. Epigenetic drivers of tumourigenesis and cancer metastasis. Semin. Cancer Biol. 2018, 51, 149–159. [Google Scholar] [CrossRef]
- Banerjee, R.; Smith, J.; Eccles, M.R.; Weeks, R.J.; Chatterjee, A. Epigenetic basis and targeting of cancer metastasis. Trends Cancer 2022, 8, 226–241. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.F.; Yan, Q. The roles of epigenetics in cancer progression and metastasis. Biochem. J. 2021, 478, 3373–3393. [Google Scholar] [CrossRef]
- Shao, K.; Pu, W.; Zhang, J.; Guo, S.; Qian, F.; Glurich, I.; Jin, Q.; Ma, Y.; Ju, S.; Zhang, Z.; et al. DNA hypermethylation contributes to colorectal cancer metastasis by regulating the binding of CEBPB and TFCP2 to the CPEB1 promoter. Clin. Epigenet. 2021, 13, 89. [Google Scholar] [CrossRef]
- Chatterjee, A.; Stockwell, P.A.; Ahn, A.; Rodger, E.J.; Leichter, A.L.; Eccles, M.R. Genome-wide methylation sequencing of paired primary and metastatic cell lines identifies common DNA methylation changes and a role for EBF3 as a candidate epigenetic driver of melanoma metastasis. Oncotarget 2017, 8, 6085–6101. [Google Scholar] [CrossRef]
- Rodger, E.J.; Chatterjee, A.; Stockwell, P.A.; Eccles, M.R. Characterisation of DNA methylation changes in EBF3 and TBC1D16 associated with tumour progression and metastasis in multiple cancer types. Clin. Epigenet. 2019, 11, 114. [Google Scholar] [CrossRef] [PubMed]
- Bi, B.; Qiu, M.; Liu, P.; Wang, Q.; Wen, Y.; Li, Y.; Li, B.; Li, Y.; He, Y.; Zhao, J. Protein post-translational modifications: A key factor in colorectal cancer resistance mechanisms. Biochim. Biophys. Acta (BBA)-Gene Regul. Mech. 2023, 1866, 194977. [Google Scholar] [CrossRef]
- Chatterjee, A.; Rodger, E.J.; Morison, I.M.; Eccles, M.R.; Stockwell, P.A. Tools and Strategies for Analysis of Genome-Wide and Gene-Specific DNA Methylation Patterns. In Oral Biology: Molecular Techniques and Applications; Seymour, G.J., Cullinan, M.P., Heng, N.C.K., Eds.; Springer: New York, NY, USA, 2017; pp. 249–277. [Google Scholar]
- Miura, T.; Ishiguro, M.; Ishikawa, T.; Okazaki, S.; Baba, H.; Kikuchi, A.; Yamauchi, S.; Matsuyama, T.; Uetake, H.; Kinugasa, Y. Methylation of bone morphogenetic protein 2 is associated with poor prognosis in colorectal cancer. Oncol. Lett. 2020, 19, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Bihl, M.P.; Foerster, A.; Lugli, A.; Zlobec, I. Characterization of CDKN2A(p16) methylation and impact in colorectal cancer: Systematic analysis using pyrosequencing. J. Transl. Med. 2012, 10, 173. [Google Scholar] [CrossRef] [PubMed]
- Islam, F.; Gopalan, V.; Pillai, S.; Lu, C.T.; Kasem, K.; Lam, A.K.Y. Promoter hypermethylation inactivate tumor suppressor FAM134B and is associated with poor prognosis in colorectal cancer. Genes Chromosomes Cancer 2018, 57, 240–251. [Google Scholar] [CrossRef]
- Xing, X.; Cai, W.; Shi, H.; Wang, Y.; Li, M.; Jiao, J.; Chen, M. The prognostic value of CDKN2A hypermethylation in colorectal cancer: A meta-analysis. Br. J. Cancer 2013, 108, 2542–2548. [Google Scholar] [CrossRef]
- Vuong, L.D.; Nguyen, H.V.; Truong, V.-L.; Nguyen, Q.N. Aberrant methylation of CDKN2A, RASSF1A and WIF1 in sporadic adenocarcinomatous colorectal cancer: Associations with clinicopathological features. J. Adv. Biotechnol. Exp. Ther. 2021, 4, 305–310. [Google Scholar] [CrossRef]
- Nakamura, K.; Yamashita, K.; Sawaki, H.; Waraya, M.; Katoh, H.; Nakayama, N.; Kawamata, H.; Nishimiya, H.; Ema, A.; Narimatsu, H. Aberrant methylation of GCNT2 is tightly related to lymph node metastasis of primary CRC. Anticancer Res. 2015, 35, 1411–1421. [Google Scholar]
- Konishi, K.; Watanabe, Y.; Shen, L.; Guo, Y.; Castoro, R.J.; Kondo, K.; Chung, W.; Ahmed, S.; Jelinek, J.; Boumber, Y.A. DNA methylation profiles of primary colorectal carcinoma and matched liver metastasis. PLoS ONE 2011, 6, e27889. [Google Scholar] [CrossRef]
- Ajithkumar, P.; Gimenez, G.; Stockwell, P.A.; Almomani, S.; Bowden, S.A.; Leichter, A.L.; Ahn, A.; Pattison, S.; Schmeier, S.; Frizelle, F.A.; et al. DNA Methylome and Transcriptome Maps of Primary Colorectal Cancer and Matched Liver Metastasis. Data 2024, 9, 8. [Google Scholar] [CrossRef]
- Ramalho-Carvalho, J.; Henrique, R.; Jerónimo, C. Methylation-Specific PCR. In DNA Methylation Protocols; Tost, J., Ed.; Springer: New York, NY, USA, 2018; pp. 447–472. [Google Scholar]
- Noguera-Castells, A.; García-Prieto, C.A.; Álvarez-Errico, D.; Esteller, M. Validation of the new EPIC DNA methylation microarray (900K EPIC v2) for high-throughput profiling of the human DNA methylome. Epigenetics 2023, 18, 2185742. [Google Scholar] [CrossRef] [PubMed]
- De Chiara, L.; Leiro-Fernandez, V.; Rodríguez-Girondo, M.; Valverde, D.; Botana-Rial, M.I.; Fernández-Villar, A. Comparison of Bisulfite Pyrosequencing and Methylation-Specific qPCR for Methylation Assessment. Int. J. Mol. Sci. 2020, 21, 9242. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Rodger, E.J.; Stockwell, P.A.; Weeks, R.J.; Morison, I.M. Technical Considerations for Reduced Representation Bisulfite Sequencing with Multiplexed Libraries. J. Biomed. Biotechnol. 2012, 2012, 741542. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Raman, A.T.; Wang, X.; Gaiti, F.; Chaligne, R.; Mohammad, A.W.; Arczewska, A.; Smith, Z.D.; Landau, D.A.; Aryee, M.J.; et al. Smart-RRBS for single-cell methylome and transcriptome analysis. Nat. Protoc. 2021, 16, 4004–4030. [Google Scholar] [CrossRef] [PubMed]
- Rodger, E.J.; Stockwell, P.A.; Almomani, S.; Eccles, M.R.; Chatterjee, A. Protocol for generating high-quality genome-scale DNA methylation sequencing data from human cancer biospecimens. STAR Protoc. 2023, 4, 102714. [Google Scholar] [CrossRef] [PubMed]
- Weichert, W.; Röske, A.; Niesporek, S.; Noske, A.; Buckendahl, A.-C.; Dietel, M.; Gekeler, V.; Boehm, M.; Beckers, T.; Denkert, C. Class I histone deacetylase expression has independent prognostic impact in human colorectal cancer: Specific role of class I histone deacetylases in vitro and in vivo. Clin. Cancer Res. 2008, 14, 1669. [Google Scholar] [CrossRef] [PubMed]
- Higashijima, J.; Kurita, N.; Miyatani, T.; Yoshikawa, K.; Morimoto, S.; Nishioka, M.; Iwata, T.; Shimada, M. Expression of histone deacetylase 1 and metastasis-associated protein 1 as prognostic factors in colon cancer. Oncol. Rep. 2011, 26, 343–348. [Google Scholar]
- Chen, C.; Wei, M.; Wang, C.; Sun, D.; Liu, P.; Zhong, X.; He, Q.; Yu, W. The histone deacetylase HDAC1 activates HIF1α/VEGFA signal pathway in colorectal cancer. Gene 2020, 754, 144851. [Google Scholar] [CrossRef]
- Qi, Z.-P.; Yalikong, A.; Zhang, J.-W.; Cai, S.-L.; Li, B.; Di, S.; Lv, Z.T.; Xu, E.-P.; Zhong, Y.-S.; Zhou, P.-H. HDAC2 promotes the EMT of colorectal cancer cells and via the modular scaffold function of ENSG00000274093.1. J. Cell. Mol. Med. 2021, 25, 1190–1197. [Google Scholar] [CrossRef]
- Hu, Y.; Dai, M.; Zheng, Y.; Wu, J.; Yu, B.; Zhang, H.; Kong, W.; Wu, H.; Yu, X. Epigenetic suppression of E-cadherin expression by Snail2 during the metastasis of colorectal cancer. Clin. Epigenet. 2018, 10, 154. [Google Scholar] [CrossRef]
- García-Domínguez, D.J.; Hontecillas-Prieto, L.; Kaliszczak, M.; He, M.; Burguillos, M.A.; Bekay, R.; Abdul-Salam, V.B.; Khozoie, C.; Shah, K.; O’Neill, K.; et al. Novel nuclear role of HDAC6 in prognosis and therapeutic target for colorectal cancer. bioRxiv 2020. [Google Scholar] [CrossRef]
- Zhang, S.-L.; Du, X.; Tan, L.-N.; Deng, F.-H.; Zhou, B.-Y.; Zhou, H.-J.; Zhu, H.-Y.; Chu, Y.; Liu, D.-L.; Tan, Y.-Y. SET7 interacts with HDAC6 and suppresses the development of colon cancer through inactivation of HDAC6. Am. J. Transl. Res. 2020, 12, 602–611. [Google Scholar] [PubMed]
- Zhang, L.-L.; Zhan, L.; Jin, Y.-D.; Min, Z.-L.; Wei, C.; Wang, Q.; Chen, Y.-J.; Wu, Q.-M.; Hu, X.-M.; Yuan, Q. SIRT2 mediated antitumor effects of shikonin on metastatic colorectal cancer. Eur. J. Pharmacol. 2017, 797, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Ye, Y.; Yang, X.; Liu, B.; Wang, Z.; Chen, S.; Jiang, K.; Zhang, W.; Jiang, H.; Mustonen, H.; et al. SIRT2-dependent IDH1 deacetylation inhibits colorectal cancer and liver metastases. EMBO Rep. 2020, 21, e48183. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, X.; Hu, J.; He, G.; Li, X.; Wu, P.; Ren, X.; Wang, F.; Liao, W.; Liang, L.; et al. The positive feedback between Snail and DAB2IP regulates EMT, invasion and metastasis in colorectal cancer. Oncotarget 2015, 6, 27427–27439. [Google Scholar] [CrossRef] [PubMed]
- Shuai, W.; Wu, J.; Chen, S.; Liu, R.; Ye, Z.; Kuang, C.; Fu, X.; Wang, G.; Li, Y.; Peng, Q.; et al. SUV39H2 promotes colorectal cancer proliferation and metastasis via tri-methylation of the SLIT1 promoter. Cancer Lett. 2018, 422, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Sun, L.; Xu, F.; Hu, F.; Lan, J.; Song, D.; Feng, Y.; Wang, J.; Luo, X.; Hu, J.; et al. Blocking histone methyltransferase SETDB1 inhibits tumorigenesis and enhances cetuximab sensitivity in colorectal cancer. Cancer Lett. 2020, 487, 63–73. [Google Scholar] [CrossRef]
- Liu, J.; Liang, T.; Zhangsun, W. KDM3A is associated with tumor metastasis and modulates colorectal cancer cell migration and invasion. Int. J. Biol. Macromol. 2019, 126, 318–325. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, Z.M.; Xia, Y.; Liao, G.Q.; Pan, Y.; Liu, S.; Zhang, Y.; Yan, Z.S. LSD1-mediated epigenetic modification contributes to proliferation and metastasis of colon cancer. Br. J. Cancer 2013, 109, 994–1003. [Google Scholar] [CrossRef]
- Jie, D.; Zhongmin, Z.; Guoqing, L.; Sheng, L.; Yi, Z.; Jing, W.; Liang, Z. Positive Expression of LSD1 and Negative Expression of E-cadherin Correlate with Metastasis and Poor Prognosis of Colon Cancer. Dig. Dis. Sci. 2013, 58, 1581–1589. [Google Scholar] [CrossRef]
- Shen, T.; Cai, L.-D.; Liu, Y.-H.; Li, S.; Gan, W.-J.; Li, X.-M.; Wang, J.-R.; Guo, P.-D.; Zhou, Q.; Lu, X.-X.; et al. Ube2v1-mediated ubiquitination and degradation of Sirt1 promotes metastasis of colorectal cancer by epigenetically suppressing autophagy. J. Hematol. Oncol. 2018, 11, 95. [Google Scholar] [CrossRef]
- Wu, Z.; Neufeld, H.; Torlakovic, E.; Xiao, W. Uev1A-Ubc13 promotes colorectal cancer metastasis through regulating CXCL1 expression via NF-кB activation. Oncotarget 2018, 9, 15952–15967. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, Y.; Hu, C.; Jing, H.; Cao, Y.; Liu, X. Association of clinicopathological features with UbcH10 expression in colorectal cancer. J. Cancer Res. Clin. Oncol. 2010, 136, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tang, H.; Wu, Z.; Zhou, C.; Jiang, T.; Xue, Y.; Huang, G.; Yan, D.; Peng, Z. Overexpression of RBBP6, Alone or Combined with Mutant TP53, Is Predictive of Poor Prognosis in Colon Cancer. PLoS ONE 2013, 8, e66524. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Wu, G.; Zhou, Z.; Zhang, X.; Wang, Y.; Song, G.; Ding, E.; Sun, X.; Zhong, L.; Li, S.; et al. RBBP6, a RING finger-domain E3 ubiquitin ligase, induces epithelial–mesenchymal transition and promotes metastasis of colorectal cancer. Cell Death Dis. 2019, 10, 833. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; He, C.; Gao, A.; Yan, X.; Xia, X.; Zhou, J.; Wu, J. RAD18 promotes colorectal cancer metastasis by activating the epithelial-mesenchymal transition pathway. Oncol. Rep. 2020, 44, 213–223. [Google Scholar] [CrossRef]
- Nakazawa, T.; Kondo, T.; Ma, D.; Niu, D.; Mochizuki, K.; Kawasaki, T.; Yamane, T.; Iino, H.; Fujii, H.; Katoh, R. Global histone modification of histone H3 in colorectal cancer and its precursor lesions. Hum. Pathol. 2012, 43, 834–842. [Google Scholar] [CrossRef]
- Chanda, A.; Chan, A.; Deng, L.; Kornaga, E.N.; Enwere, E.K.; Morris, D.G.; Bonni, S. Identification of the SUMO E3 ligase PIAS1 as a potential survival biomarker in breast cancer. PLoS ONE 2017, 12, e0177639. [Google Scholar] [CrossRef]
- Noberini, R.; Robusti, G.; Bonaldi, T. Mass spectrometry-based characterization of histones in clinical samples: Applications, progress, and challenges. FEBS J. 2022, 289, 1191–1213. [Google Scholar] [CrossRef]
- Begum, H.; Murugesan, P.; Tangutur, A.D. Western blotting: A powerful staple in scientific and biomedical research. BioTechniques 2022, 73, 58–69. [Google Scholar] [CrossRef]
- Rumbaugh, G.; Miller, C.A. Epigenetic changes in the brain: Measuring global histone modifications. Methods Mol. Biol. 2011, 670, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Yörüker, E.E.; Holdenrieder, S.; Gezer, U. Potential of circulating nucleosome-associated histone modifications in cancer. Transl. Cancer Res. 2017, 7, S185–S191. [Google Scholar] [CrossRef]
- Liu, J.; Li, H.; Sun, L.; Shen, S.; Zhou, Q.; Yuan, Y.; Xing, C. Epigenetic alternations of MicroRNAs and DNA methylation contribute to liver metastasis of colorectal cancer. Dig. Dis. Sci. 2019, 64, 1523–1534. [Google Scholar] [CrossRef] [PubMed]
- Visone, R.; Bacalini, M.G.; Franco, S.D.; Ferracin, M.; Colorito, M.L.; Pagotto, S.; Laprovitera, N.; Licastro, D.; Marco, M.D.; Scavo, E.; et al. DNA methylation of shelf, shore and open sea CpG positions distinguish high microsatellite instability from low or stable microsatellite status colon cancer stem cells. Epigenomics 2019, 11, 587–604. [Google Scholar] [CrossRef]
- Bach, S.; Paulis, I.; Sluiter, N.R.; Tibbesma, M.; Martin, I.; van de Wiel, M.A.; Tuynman, J.B.; Bahce, I.; Kazemier, G.; Steenbergen, R.D.M. Detection of colorectal cancer in urine using DNA methylation analysis. Sci. Rep. 2021, 11, 2363. [Google Scholar] [CrossRef]
- Kopfnagel, V.; Klopp, N.; Bernemann, I.; Nizhegorodtseva, N.; Wilson, R.; Gronauer, R.; Seifert, M.; Illig, T. Effects of Repeated Freeze and Thaw Cycles on the Genome-Wide DNA Methylation Profile of Isolated Genomic DNA. Biopreserv. Biobanking 2023. ahead of print. [Google Scholar] [CrossRef]
- Ili, C.; Buchegger, K.; Demond, H.; Castillo-Fernandez, J.; Kelsey, G.; Zanella, L.; Abanto, M.; Riquelme, I.; López, J.; Viscarra, T.; et al. Landscape of Genome-Wide DNA Methylation of Colorectal Cancer Metastasis. Cancers 2020, 12, 2710. [Google Scholar] [CrossRef]
- Gregory, G.L.; Copple, I.M. Modulating the expression of tumor suppressor genes using activating oligonucleotide technologies as a therapeutic approach in cancer. Mol. Ther. Nucleic Acids 2023, 31, 211–223. [Google Scholar] [CrossRef]
- Tahoon, A.; El-Khateeb, D.; Mosbeh, A.; Tantawy El Sayed, I.; Khalil, A. Significance of promoter methylation of multiple tumor suppressor genes in hepatocellular carcinoma. Egypt. J. Med. Hum. Genet. 2022, 23, 22. [Google Scholar] [CrossRef]
- Das, J.; Chandra, L.; Gandhi, G.; Amle, D.B.; Patnayak, R.L.; Khurana, N.; Saxena, A. Evaluation of promoter hypermethylation of tumor suppressor gene BRCA1 in epithelial ovarian cancer. J. Cancer Res. Ther. 2022, 18, 1578–1582. [Google Scholar] [CrossRef]
- Chen, Z.; Fan, Y.; Liu, X.; Shang, X.; Qi, K.; Zhang, S. Clinicopathological significance of DAPK gene promoter hypermethylation in non-small cell lung cancer: A meta-analysis. Int. J. Biol. Markers 2022, 37, 47–57. [Google Scholar] [CrossRef]
- Su, Y.; Huang, Q.; Lu, L.; Qu, H.; Wang, D.; Qiu, J.; Li, W.; Lin, M.; Liu, H.; Wang, Z.; et al. Promoter Methylation-Mediated NPTX2 Silencing Promotes Tumor Growth in Human Prostate Cancer. J. Cancer 2022, 13, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Vishnubalaji, R.; Yue, S.; Alfayez, M.; Kassem, M.; Liu, F.-F.; Aldahmash, A.; Alajez, N.M. Bone morphogenetic protein 2 (BMP2) induces growth suppression and enhances chemosensitivity of human colon cancer cells. Cancer Cell Int. 2016, 16, 77. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.W.; Yang, S.X.; Chen, R.P.; Zhou, Y.H.; Ye, M.S.; Miao, L.; Xue, Z.X.; Lu, G.R. Prognostic Value of Lymphovascular Invasion in Patients with Stage III Colorectal Cancer: A Retrospective Study. Med. Sci. Monit. 2019, 25, 6043–6050. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.-B.; Yu, C.S.; Jang, S.J.; Kim, T.W.; Kim, J.H.; Kim, J.C. Prognostic Significance of Lymphovascular Invasion in Sporadic Colorectal Cancer. Dis. Colon Rectum 2010, 53, 377–384. [Google Scholar] [CrossRef]
- Hewitt, G.; Korolchuk, V.I. Repair, Reuse, Recycle: The Expanding Role of Autophagy in Genome Maintenance. Trends Cell Biol. 2017, 27, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Zhang, W.; Xie, J.; Mao, J. CDKN2A inhibits cell proliferation and invasion in cervical cancer through LDHA-mediated AKT/mTOR pathway. Clin. Transl. Oncol. 2021, 23, 222–228. [Google Scholar] [CrossRef]
- Nothling, M.D.; Xiao, Z.; Bhaskaran, A.; Blyth, M.T.; Bennett, C.W.; Coote, M.L.; Connal, L.A. Synthetic Catalysts Inspired by Hydrolytic Enzymes. ACS Catal. 2019, 9, 168–187. [Google Scholar] [CrossRef]
- Ganatra, S.; Kekunnaya, R.; Sachdeva, V. Bilateral congenital membranous cataracts due to Glucosaminyl (N-Acetyl) Transferase 2 (GCNT2) mutation: Life-saving genetic analysis. Indian J. Ophthalmol. 2022, 70, 2622–2623. [Google Scholar] [CrossRef]
- Xu, X.; Wu, Y.; Jia, G.; Zhu, Q.; Li, D.; Xie, K. A signature based on glycosyltransferase genes provides a promising tool for the prediction of prognosis and immunotherapy responsiveness in ovarian cancer. J. Ovarian Res. 2023, 16, 5. [Google Scholar] [CrossRef]
- Dimitroff, C.J. I-branched carbohydrates as emerging effectors of malignant progression. Proc. Natl. Acad. Sci. USA 2019, 116, 13729–13737. [Google Scholar] [CrossRef] [PubMed]
- Svetlana, B.; James, R.; Andrew, W.; Rasheed, S.; Paris, T.; Diana, T.; David, C.; Brown, G. Diagnostic accuracy of high-resolution MRI as a method to predict potentially safe endoscopic and surgical planes in patients with early rectal cancer. BMJ Open Gastroenterol. 2017, 4, e000151. [Google Scholar] [CrossRef]
- Furukawa, K.; Haruki, K.; Taniai, T.; Hamura, R.; Shirai, Y.; Yasuda, J.; Shiozaki, H.; Onda, S.; Gocho, T.; Ikegami, T. Osteosarcopenia is a potential predictor for the prognosis of patients who underwent hepatic resection for colorectal liver metastases. Ann. Gastroenterol. Surg. 2021, 5, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Guo, L.; Tang, W.; Ma, Y.; Wang, X.; Shao, Y.; Zhao, H.; Ying, J. Identification of DNA methylation biomarkers for risk of liver metastasis in early-stage colorectal cancer. Clin. Epigenet. 2021, 13, 126. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.-P.; Song, Q.-Z.; Liu, S.; Xie, J.-Y.; Lv, G.-Q. Predicting lymph node metastasis in colorectal cancer: An analysis of influencing factors to develop a risk model. World J. Gastrointest. Surg. 2023, 15, 2234–2246. [Google Scholar] [CrossRef] [PubMed]
- Picardo, F.; Romanelli, A.; Muinelo-Romay, L.; Mazza, T.; Fusilli, C.; Parrella, P.; Barbazán, J.; Lopez-López, R.; Barbano, R.; De Robertis, M. Diagnostic and prognostic value of B4GALT1 hypermethylation and its clinical significance as a novel circulating cell-free DNA biomarker in colorectal cancer. Cancers 2019, 11, 1598. [Google Scholar] [CrossRef]
- Sun, J.; Wang, C.; Zhang, Y.; Xu, L.; Fang, W.; Zhu, Y.; Zheng, Y.; Chen, X.; Xie, X.; Hu, X.; et al. Genomic signatures reveal DNA damage response deficiency in colorectal cancer brain metastases. Nat. Commun. 2019, 10, 3190. [Google Scholar] [CrossRef]
- Jonas, F.; Vidavski, M.; Benuck, E.; Barkai, N.; Yaakov, G. Nucleosome retention by histone chaperones and remodelers occludes pervasive DNA–protein binding. Nucleic Acids Res. 2023, 51, 8496–8513. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, M.; Wang, Y. The roles of histone modifications in tumorigenesis and associated inhibitors in cancer therapy. J. Natl. Cancer Cent. 2022, 2, 277–290. [Google Scholar] [CrossRef]
- Ebrahimi, V.; Soleimanian, A.; Ebrahimi, T.; Azargun, R.; Yazdani, P.; Eyvazi, S.; Tarhriz, V. Epigenetic modifications in gastric cancer: Focus on DNA methylation. Gene 2020, 742, 144577. [Google Scholar] [CrossRef]
- Teng, L.; Li, Z.; Shi, Y.; Gao, Z.; Yang, Y.; Wang, Y.; Bi, L. Development and validation of a microenvironment-related prognostic model for hepatocellular carcinoma patients based on histone deacetylase family. Transl. Oncol. 2022, 26, 101547. [Google Scholar] [CrossRef] [PubMed]
- Ropero, S.; Esteller, M. The role of histone deacetylases (HDACs) in human cancer. Mol. Oncol. 2007, 1, 19–25. [Google Scholar] [CrossRef]
- Zhang, K.; Yao, Y.; Tu, Z.; Liao, C.; Wang, Z.; Qiu, Y.; Chen, D.; Hamilton, D.J.; Li, Z.; Jiang, S. Discovery of class I histone deacetylase inhibitors based on romidpesin with promising selectivity for cancer cells. Future Med. Chem. 2020, 12, 311–323. [Google Scholar] [CrossRef]
- van Zijl, F.; Krupitza, G.; Mikulits, W. Initial steps of metastasis: Cell invasion and endothelial transmigration. Mutat. Res./Rev. Mutat. Res. 2011, 728, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Hua, W.; ten Dijke, P.; Kostidis, S.; Giera, M.; Hornsveld, M. TGFβ-induced metabolic reprogramming during epithelial-to-mesenchymal transition in cancer. Cell. Mol. Life Sci. 2020, 77, 2103–2123. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Ong, S.L.; Tran, L.M.; Jing, Z.; Liu, B.; Park, S.J.; Huang, Z.L.; Walser, T.C.; Heinrich, E.L.; Lee, G.; et al. Chronic IL-1β-induced inflammation regulates epithelial-to-mesenchymal transition memory phenotypes via epigenetic modifications in non-small cell lung cancer. Sci. Rep. 2020, 10, 377. [Google Scholar] [CrossRef] [PubMed]
- Emmanuel, N.K.; Antonios, K.; Athanasios, S.; Dimitrios, S.; Aikaterini, M.; Nikolaos, G.; Michail, D.; Kyveli, A.; Georgios, T.; Athanasios, P.; et al. Role of Oncogenes and Tumor-suppressor Genes in Carcinogenesis: A Review. Anticancer Res. 2020, 40, 6009. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, D.; Hu, T.; Zhao, H.; Zhao, X.; Lou, Z.; He, Y.; Qin, W.; Xia, J.; Zhang, X.; et al. KMT2A histone methyltransferase contributes to colorectal cancer development by promoting cathepsin Z transcriptional activation. Cancer Med. 2019, 8, 3544–3552. [Google Scholar] [CrossRef]
- Berlin, C.; Cottard, F.; Willmann, D.; Urban, S.; Tirier, S.M.; Marx, L.; Rippe, K.; Schmitt, M.; Petrocelli, V.; Greten, F.R.; et al. KMT9 Controls Stemness and Growth of Colorectal Cancer. Cancer Res. 2022, 82, 210–220. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, J.; Ma, Y.; Wu, C.; Cui, W.; Wang, L. Histone methyltransferase and drug resistance in cancers. J. Exp. Clin. Cancer Res. 2020, 39, 173. [Google Scholar] [CrossRef]
- He, D.N.; Wang, N.; Wen, X.L.; Li, X.H.; Guo, Y.; Fu, S.H.; Xiong, F.F.; Wu, Z.Y.; Zhu, X.; Gao, X.L.; et al. Multi-omics analysis reveals a molecular landscape of the early recurrence and early metastasis in pan-cancer. Front. Genet. 2023, 14, 1061364. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.H.P.; Sarkar, S.; Yang, S.Y.; Seifalian, A.M.; Winslet, M.C. In vivo models for early development of colorectal liver metastasis. Int. J. Exp. Pathol. 2008, 89, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jing, L.; Li, M.; He, L.; Guo, Z. Regulation of histone arginine methylation/demethylation by methylase and demethylase (Review). Mol. Med. Rep. 2019, 19, 3963–3971. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Yin, T.; Zhao, Y.; Lv, W.; Liu, Z.; Chen, C.; Liu, K.; Shan, S.; Zhou, R.; Li, X.; et al. Histone demethylases in the regulation of immunity and inflammation. Cell Death Discov. 2023, 9, 188. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, R.; Wu, L.; Chen, Y.; Liu, S.; Zhao, H.; Wang, Y.; Wang, L.; Shao, Z. Histone methyltransferase KMT2D cooperates with MEF2A to promote the stem-like properties of oral squamous cell carcinoma. Cell Biosci. 2022, 12, 49. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, M. Histone Demethylation Profiles in Nonalcoholic Fatty Liver Disease and Prognostic Values in Hepatocellular Carcinoma: A Bioinformatic Analysis. Curr. Issues Mol. Biol. 2023, 45, 3640–3657. [Google Scholar] [CrossRef]
- Oser, M.G.; Sabet, A.H.; Gao, W.; Chakraborty, A.A.; Schinzel, A.C.; Jennings, R.B.; Fonseca, R.; Bonal, D.M.; Booker, M.A.; Flaifel, A. The KDM5A/RBP2 histone demethylase represses NOTCH signaling to sustain neuroendocrine differentiation and promote small cell lung cancer tumorigenesis. Genes Dev. 2019, 33, 1718–1738. [Google Scholar] [CrossRef]
- Liu, Q.; Pang, J.; Wang, L.-a.; Huang, Z.; Xu, J.; Yang, X.; Xie, Q.; Huang, Y.; Tang, T.; Tong, D.; et al. Histone demethylase PHF8 drives neuroendocrine prostate cancer progression by epigenetically upregulating FOXA2. J. Pathol. 2021, 253, 106–118. [Google Scholar] [CrossRef]
- Verigos, J.; Karakaidos, P.; Kordias, D.; Papoudou-Bai, A.; Evangelou, Z.; Harissis, H.V.; Klinakis, A.; Magklara, A. The Histone Demethylase LSD1/ΚDM1A Mediates Chemoresistance in Breast Cancer via Regulation of a Stem Cell Program. Cancers 2019, 11, 1585. [Google Scholar] [CrossRef]
- Liu, R.; Wu, J.; Guo, H.; Yao, W.; Li, S.; Lu, Y.; Jia, Y.; Liang, X.; Tang, J.; Zhang, H. Post-translational modifications of histones: Mechanisms, biological functions, and therapeutic targets. MedComm 2023, 4, e292. [Google Scholar] [CrossRef]
- Nishizawa, Y.; Nishida, N.; Konno, M.; Kawamoto, K.; Asai, A.; Koseki, J.; Takahashi, H.; Haraguchi, N.; Nishimura, J.; Hata, T.; et al. Clinical Significance of Histone Demethylase NO66 in Invasive Colorectal Cancer. Ann. Surg. Oncol. 2017, 24, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Kang, Y. E-Cadherin: Context-Dependent Functions of a Quintessential Epithelial Marker in Metastasis. Cancer Res. 2021, 81, 5800–5802. [Google Scholar] [CrossRef] [PubMed]
- Cockram, P.E.; Kist, M.; Prakash, S.; Chen, S.-H.; Wertz, I.E.; Vucic, D. Ubiquitination in the regulation of inflammatory cell death and cancer. Cell Death Differ. 2021, 28, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Fhu, C.W.; Ali, A. Dysregulation of the Ubiquitin Proteasome System in Human Malignancies: A Window for Therapeutic Intervention. Cancers 2021, 13, 1513. [Google Scholar] [CrossRef]
- Zhou, R.; Chen, J.; Xu, Y.; Ye, Y.; Zhong, G.; Chen, T.; Qiu, L. PRPF19 facilitates colorectal cancer liver metastasis through activation of the Src-YAP1 pathway via K63-linked ubiquitination of MYL9. Cell Death Dis. 2023, 14, 258. [Google Scholar] [CrossRef]
- Yassi, M.; Chatterjee, A.; Parry, M. Application of deep learning in cancer epigenetics through DNA methylation analysis. Brief. Bioinform. 2023, 24, bbad411. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ajithkumar, P.; Vasantharajan, S.S.; Pattison, S.; McCall, J.L.; Rodger, E.J.; Chatterjee, A. Exploring Potential Epigenetic Biomarkers for Colorectal Cancer Metastasis. Int. J. Mol. Sci. 2024, 25, 874. https://doi.org/10.3390/ijms25020874
Ajithkumar P, Vasantharajan SS, Pattison S, McCall JL, Rodger EJ, Chatterjee A. Exploring Potential Epigenetic Biomarkers for Colorectal Cancer Metastasis. International Journal of Molecular Sciences. 2024; 25(2):874. https://doi.org/10.3390/ijms25020874
Chicago/Turabian StyleAjithkumar, Priyadarshana, Sai Shyam Vasantharajan, Sharon Pattison, John L. McCall, Euan J. Rodger, and Aniruddha Chatterjee. 2024. "Exploring Potential Epigenetic Biomarkers for Colorectal Cancer Metastasis" International Journal of Molecular Sciences 25, no. 2: 874. https://doi.org/10.3390/ijms25020874




