Omics Studies of Tumor Cells under Microgravity Conditions
Abstract
:1. Introduction
2. Microgravity Platforms

3. Results for Omics Studies in Tumor Cells Exposed to Microgravity
3.1. Brain Tumors
3.2. Hematological Malignancies
3.3. Sarcomas
3.4. Thyroid Cancer
3.5. Prostate Cancer
3.6. Breast Cancer
3.7. Gynecologic Cancer
3.8. Gastrointestinal Cancer
3.9. Lung Cancer
3.10. The Potential Biases or Limitations of This Review
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Cell Line | Omics | Microgravity | Devices/Platforms | Ref. |
|---|---|---|---|---|
| BRAIN TUMORS | ||||
| K562 | Transcriptomics: mRNA levels of STIM1, STIM2, ORAI1, and ORAI were accessed. S-µg downregulated ORAI1. Proteomics: Reduced levels of ORAI1 protein were observed following incubation under µg conditions. | s-µg | 2D clinostat | [84] |
| A-172 and HUVEC | Proteomics: Lower expression of s-µg YAP-1 in A-172 cells. Remodeling of Cadherin junction protein in HUVECs | s-µg | RPM | [85,86] |
| NSC | Genomics: r-µg alters division of NSC Proteomics: Reduced expression of Tubulin was observed in r-µg exposed NSCs. | r-µg | ISS | [87] |
| HEMATOLOGICAL DISORDERS | ||||
| K562 | Metabolomics: Increase production of ROS | s-µg | Clinostat | [100] |
| Raji cells | Genomics: Reduced DNA repair Proteomics: Reduction of ATM expression Metabolomics: Higher ROS | s-µg | RWV | [101] |
| U937 | Proteomics: No express of 5-Lox | s-µg | RPM | [102] |
| Proteomics: Increased expression of PKC, interleukin production significantly reduced | r-µg | Space shuttle flight | [103] | |
| Proteomics: Decrease in the expression of Actin. Changes in the distribution of vinculin and be-ta-tubulin Upregulation of Hsp70; decrease in proteasome activity | s-µg | RWV | [116] | |
| Proteomics: Reduction phosphorylation of tyrosine and activation of transcription factor C-Jun. Increase the phosphorylation of p53 protein | s-µg r-µg | 2D Clinostat PFC | [113] | |
| Genomics: Altered in the response of HIF-related genes Transcriptomics: HIF-1α, HIF-1 were transcripts differently regulated. Alteration in transcriptome. Proteomics: Increase the phosphorylation of MEK | r-µg | PFC | [115] | |
| Proteomics: Increase the expression of ICAM-1 | s-µg | 2D Clinostat | [112] | |
| Transcriptomics: Profound alteration in the transcriptome associated with DNA replication. Changes in mRNA processing | r-µg | PFC/TEXUS-49 | [117] | |
| Proteomics: Reduction in PKS translocation | r-µg | STS-81 Space Shuttle mission | [122] | |
| TK6 | Transcriptomics: Expression of several miRNAs was changed significantly in the simulated microgravity condition including miR-150, miR-34a, miR-423-5p, miR-22, miR-141, miR-618, and miR-222 | s-µg | HARV | [110] |
| Epigenomics: 3204 DMRs differentially methylated regions, 1286 DMRs associated with hypermethylation (gain of 5mc) and 1918 DMRs associated with hypomethylation (loss of 5mc). Transcriptions start sites of the genes WBSCR22, DOCK6, C3, DEFB119, FXYD6 were hyperhydroxymethylated whereas of the gene MTRNR2L2 were hypo-hydroxy methylated Transcriptomics: Transcription of genes TSPAN5, SPG20 associated with loss of 5mc is upregulated, whereas genes as PLIN2, MAP3K13, FBX01 were downregulated | s-µg | HARV | [109] | |
| Genomics: Increasing of HPRT mutants and broken chromosome fragment (micronucleus) | s-µg | RWV | [105] | |
| MOLT-4 | Genomics: 349 genes were upregulated, and 444 genes were downregulated Epigenomics: Dysregulation of post transcriptional gene silencing machinery Transcriptomics: Significant dysregulation of several microRNA host genes including MIR17HG, MIR21HG. MIR22HG are upregulated | s-µg | HARV | [111] |
| Jurkat cells | Proteomics: Enhance the phosphorylation of MAP kinase ERK-1/2, MEK and P38 Inhibition of NFKB | s-µg r-µg | 2D Clinostat PFC 8th DLR | [113] |
| Proteomics: Different expression of cytoskeletal proteins Vimentin and β-actin were in-creased ß-tubulin reduced and hardly detect-able release of IL-2, TNFα, GM-CSF Metabolomics: Decreased intra-cellular Ca2+ and ROS. Decreased glucose and lactate content | s-µg | RPM | [119] | |
| Genomics: Downregulated of CD69 gene Proteomics: Increase of Fas/Apo-1 protein Metabolomics: Significant increase in glucose consumption | r-µg | STS-80 STS-95 Space Shuttle missions | [124,133] | |
| Genomics: Upregulation of CAPN1 gene. Increase cytosolic DNA fragments Transcriptomics: Increased mRNA significantly (~2-fold) Proteomics: Upregulation µ-Calpain and INF-y significantly reduction of cytokines LIF, IL-4, IL-2 Metabolomics: Increase activity of µ-Calpain (~2-fold). The cytosolic content of cytochrome c increased and lost it in the mitochondria. | s-µg | RCCS | [126] | |
| Genomics: TSC2 and cell cycle-related genes were upregulated. 2% of 20,000 genes were upregulated. 10 cytoskeletal genes upregulated (Plectin; C-NAP1, Calponin, MLC-2, Ankyrin, and Dynactin) and gene encoding gelsolin downregulated Transcriptomics: mRNA for Plectin, C-NAP1, and Calponin were upregulated Proteomics: Upregulation of CDK6, Tuberin, actionlike protein and EST | r-µg | STS-95 Space Shuttle mission | [130] | |
| Proteomics: VEGFR-1, VEGFR-2, VEGFR-3 were downregulated | s-µg | RWV | [134] | |
| HL cells (L-540 and HDLM-2) | Genomics: Upregulation of NADPH oxidase family genes gp91, p22, p47, p67-Phox. And upregulation of ULK1, ATG14, BECN1, and LC3 Transcriptomics: mRNA expression downregulated of ATP1A1 and ATP5A1 Proteomics: Increase the phosphorylated ULK1, ATF4, Beclin-1, and LC3; Decrease the phosphorylation of BCl-2, MCl-1; Upregulation of LKB1, AMPK; Downregulation of Akt/mTOR/S6K Metabolomics: ROS levels were increased | s-µg | 3D clinostat | [118] |
| HL-60 | Proteomics: Reduced expression of CD116 antigen Metabolomics: Reduced ROIS respiratory impulses | s-µg | RWV | [253] |
| Proteomics: Increase of IL-6, IL-8 and MCP-1 Metabolomics: Upregulation of nitric oxide | s-µg | RCCS | [136] | |
| Genomics: Increase DNA damage Transcriptomics: Upregulation of transcript of ATM, ATR, CHK1, CHK2, and RAD51. Downregulation of transcript of XPC, MLH1, and PMS2 Proteomics: Reduced expression of PCNA, ERK1/2AKK; Increase the phosphorylation of ATM; Upregulation of H2A.x, KU70, KU80, DNA-PKCs, Rad 51 Protein, Caspase-3, PARP, and Bax; Downregulation of Bcl-2 Metabolomics: Enhanced ROS formation | s-µg | RCCS | [137] | |
| UT-7/EPO | Transcriptomics: Decrease of mRNA expression of EPOR Proteomics: Activation of caspases-3 and downregulation of Bcl-xL | s-µg | RCCS | [138] |
| SARCOMAS | ||||
| Hu09 | Proteomics: alkaline phosphatase activity and osteocalcin production significantly reduced; 1,25-dihydroxy-vtamin D3-induced secretion of bone γ-carboxyglutamic acid-containing protein (BGP) | s-µg, 7 d | Clinostat | [143] |
| A673 | Transcriptomics: CXCR4 and CD44: elevated in MCS; DKK2, and VEGFA; downregulated in AD and MCS; EWS/FLI1 significantly upregulated in AD and MCS Proteomics: EWS/FLI1 protein was elevated in AD | s-µg, 24 h | RPM | [144] |
| MG-63 | Transcriptomics: COL1A1 reduced Proteomics: osteocalcin, ALP, and vitamin receptor (VDR) protein levels: reduced | s-µg, 3 d, 8 rpm and 16 rpm; microcarrier beads | RCCS/STLVs | [145] |
| Transcriptomics: COL1A1, BGLAP, and ALPL reduced Proteomics: ALP activity following treatment at µg increased 1.8-fold, compared to 3.8-fold at 1g; no change in collagen type I synthesis µg vs. 1g | Foton 10 satellite, r-µg; 9 d | spaceflight | [146] | |
| Transcriptomics: VEGF121, VEGF165: increased and VEGF189: decreased in both SiScr anSiRhoA cells; SiCdc42 cells only showed VEGF165 upregulation Proteomics: In space fibrinogen was significantly reduced in all cell types except for SiRac1 cells | FOTON M3 satellite; r-µg, 69 h | spaceflight | [148] | |
| THYROID CANCER | ||||
| ML-1 | Proteomics: collagen I, III, fibronectin, laminin, chondroitin sulfate, vimentin, TSH receptor Fas/Apo-1, 85-kDa PARP fragment and p53 increase, thyroglobulin decrease. Secretomics: reduced fT3, fT4 secretion. | s-µg, 24 h | 3D Clinostat | [150] |
| Transcriptomics: 148 significantly regulated genes. | r-µg, 31 × 22 s | PF | [151] | |
| ML-1 WRO | Proteomics: β-actin, cytokine release and cytoskeletal protein expression. | s-µg, 3 + 7 d | RPM, 2D Clinostat | [152] |
| WRO | Transcriptomics: VEGFA, VEGFD, MSN, and MMP3 upregulation | s-µg, 24 h | RPM | [153] |
| FTC-133 | Proteomics: alpha-enolase, phosphoglycerate kinase 1, annexin 1 and 2 downregulation, glutathione S-transferase upregulation. | s-µg, 72 h | RPM | [154] |
| Transcriptomics: CTGF, TLN1, IL6, CXCL8, CD44, SPP1, PCDHB5, PCDH7, CX3CL1, RAPGEF3, and 13 ribosomal protein coding genes. Proteomics: RelA upregulation. | s-µg, 24 h | RPM | [155] | |
| Transcriptomics: EGF and CTGF. | r-µg, s-µg, 10 d | Shenzhou 8, RPM | [156] | |
| Transcriptomics: IL6, CXCL8, IL15, SSP1, VEGFA, VEGFD, FGF17, MMP2, MMP3, TIMP1, PRKAA, and PRKACA. | r-µg, s-µg, 10 d | Shenzhou 8, RPM | [42] | |
| Transcriptomics: CAV1 and CTGF. | s-µg, 72 h | RPM, 2D Clinostat | [157] | |
| Proteomics: 47 and 13 unique proteins in ground control and flown cells, respectively. | r-µg, 32 d | SpaceX CRS-3/ISS | [158] | |
| Proteomics: caveolin-1, plasminogen | r-µg, 32 d | SpaceX CRS-3/ISS | [159] | |
| Exosomes: differences in the distribution of subpopulations and alteration of their population regarding the tetraspanin surface expression. | r-µg, 32 d | SpaceX CRS-3/ISS | [160] | |
| Exosomes: altered relative quantification of 119 miRNAs. | r-µg, 32 d | SpaceX CRS-3/ISS | [161] | |
| Transcriptomics: VCL, PXN, ITGB1, RELA, ERK1, and ERK2 downregulation. Secretomics: angiopoetin-2. | r-µg, 5 + 10 d | SpaceX CRS-13/ISS | [162] | |
| Transcriptomics: NGAL, VEGFA, OPN, IL6, and IL17. Secretomics: VEGFA, IL-17, and IL-6 | s-µg, 14 d | RPM | [163] | |
| Transcriptomics: ACTB, TUBB1, VIM, LAMA, BAX, BCL2, and EGF downregulation. | r-µg, 6 min | TEXUS-53 | [164] | |
| Transcriptomics: COL1A1, VCL, CFL1, PTK2, IL6, CXCL8, and MMP14. | 18g, 1 min | MuSIC centrifuge | [165] | |
| Transcriptomics: reactions on dexamethasone treatment, NFκB components, genes of Wnt/β-catenin and TGF-β metabolic pathways. | s-µg, 4 h + 3 d | RPM | [166] | |
| FTC-133, WRO | Transcriptomics: Proteomics: reactions on dexamethasone treatment | s-µg, 3 d | RPM | [167] |
| PROSTATE CANCER | ||||
| DU-145 | Proteomics: cytokeratin 18, actin and vimentin increase | s-µg 11 d | HARV | [169] |
| Proteomics: ceramides, phospholipase, cAMP | s-µg, 2–14 d | HARV | [174] | |
| LNCaP | Proteomics: increase in PSA production under dihydrotesterone (DHT) treatment | s-µg, 10 d | low-turning lateral vessel (STLV) | [170] |
| LNCaP, DU-145, PC-3 | Proteomics: increased expression of E cadherin and CD44 in LNCaP | s-µg, 21 d | HARV | [171] |
| PC-3 | Transcriptomics: VEGF, SRC1, AKT, MTOR and COL1A1 (downregulation), ERK1/2, FN1, VCL1 (upregulation). Secretome: VEGF and NGAL (downregulation). | s-µg, 3–5 d | RPM | [178] |
| Transcriptomics: ACTB, MSN, COL1A1, FN1, IL1A, IL6, CXCL8, LAMA3, TIMP1, FLT1, HIF1A and EGFR1 upregulation. Secretome: cytokines, TNF-α, collagen-1α1, MMP-2 and osteopontin (early) downregulation. | s-µg, 0.5–24 h | RPM | [177] | |
| Transcriptomics: 298 gravisensitive genes, chemokines enriched | r-µg, 31 × 22 s | PFC | [176] | |
| BREAST CANCER | ||||
| MDA-MB-231 | Proteomics: MCS: reduced cyclin-D1 protein content; Proapoptotic factors (BAX, PARP) increase in suspended RPM-cultured cells; prosurvival factors (Bcl-2, Survivin) are significantly decreased; p-AKT and p-ERK were significantly reduced in suspended cell aggregates | s-µg, 24 h, 72 h | RPM | [186] |
| Proteomics: Proteomic analysis of both EVs and cells further revealed a significant correlation with GTPases and proliferation | s-µg, 96 h | Gravite® | [189] | |
| Transcriptomics: upregulation of ICAM1, CD44 and ERK1 mRNAs after the first parabola (P1) and a delayed upregulation of NFKB1, NFKBIA, NFKBIB, and FAK1 after the last parabola (P31). Proteomics: ICAM-1, VCAM-1 and CD44 protein levels were elevated, whereas the NFκB subunit p-65, annexin-A2 protein, and osteopontin protein levels were reduced after the 31st parabola (P31) | r-µg; 31 parabolas | PFC | [192] | |
| MCF-7 | Transcriptomics: The ACTB, TUBB, EZR, RDX, FN1, VEGFA, FLK1 Casp9, Casp3, PRKCA mRNAs were downregulated in 5d-MCS-samples. ESR1 was upregulated in AD, and PGR1 in both phenotypes after 5 d. Proteomics: Increase in beta-actin, pan-cytokeratin protein in 5-day AD and MCS vs. 1g; Decrease in radixin in 5-day AD and MCS vs. 1g; Decrease in laminin and integrin-b1 in 5-day MCS vs. AD Elevation of fibronectin in MCS vs. AD; No change in VEFGA protein in 5-day study | s-µg, up to 5 d | RPM | [95] |
| Transcriptomics: Relative expression of BCAR1, MAPK8, and CDH1 is downregulated in MCS Proteomics: Proteins in the AD and MCS group with LFQs deviated at least twofold compared with 1g control cells. E-cadherin was reduced in MCS cells, elevated proteins of the E-cadherin auto-degradation pathways. Higher concentration of BCAR1 and MAPK8 (JNK1) in AD than in MCS cells. The protein changes correspond rather well to the LFQ values as well as to the corresponding mRNA expression in MCS cells | s-µg, 14 d | RPM | [187] | |
| Transcriptomics: early upregulation of KRT8, RDX, TIMP1, CXCL8 mRNAs, and a downregulation of VCL after the first parabola of a parabolic flight Proteomics: Increase in VEGFA protein during the r-µg phase (TEXUS), decrease in IL-6, IL-8 and MMP-9 proteins after the hyper-g phase (TEXUS); Significant downregulation of ITGB-1 protein and focal adhesion proteins after the first and 31st parabolas: E-cadherin, vinculin; Increase in VEGFA and Il-8 after the first and 31st parabola | r-µg | TEXUS sounding rocket flight, PFC | [191] | |
| MCF-7 MDA-MB-231 | Transcriptomics: MCS: ERK1, AKT1, MAPK14, EGFR, CTNNA1, CTNNB1, ITGB1, COL4A5, ACTB, and TUBB mRNAs differentially regulated | s-µg, 14 d | RPM | [196] |
| CRL2351 | Transcriptomics: AD, MCS: Upregulation of VIM, RHOA, MAPK1, and BRCA1; MCS: upregulation of ERBB2; VEGFA mRNA was unaltered Proteomics: Increase in vimentin and MAPK1 protein, no changes were detected for RHOA; and BRCA1 proteins in AD and MCS; reduced VEGF protein in AD and MCS | s-µg, 5 d | RPM | [194] |
| GYNELOGIC CANCER | ||||
| CaSki | Transcriptomics: SOX4, MALAT-1, COX4I1, C14orf45, ZNF12, HNRPH1, DENND1A, SMUG1, MELK, GPI, MMP7, STC1, JAGN1, STAU1, SUB1, ACTB, SHOC2, RBMS3, DIS3L2, SGEF, PDGFRL, NUCKS1, TP53BP1, AKR1B1, SMC1A, ARL6IP1, B2M, 28. PSME1, CAPZA1, H2AFV, TBC1D20, NDUFA4, FTH1, GAPDH | r-µg | Shenzhou-4 | [204] |
| OV-90, TOV-21G, and Caov-3 | Proteomics: Downregulation of caveolin-1 protein | r-µg | Clinostat | [205] |
| GASTROINTESTINAL TUMORS | ||||
| HCT116 | Proteomics: ATG5 ATG12, FOXO3, PTEN upregulated and strong YAP nuclear localization in s-µg | s-µg | RCCS | [209] |
| MIP-101 | Proteomics: lower expression of EGF-R, TGF-alpha, or TGF-beta in both r-µg and s-µg. greater CEA expression in r-µg | r-µg in low orbit, and s-µg | RWV | [210] |
| DLD1 | Genomics: ARRDC3, ATF3, CCPG1, CDKN2AIP, CDKN2D, CREBBP, CREBRF, CXCL3, DDIT3, EGR2, ETS1, ETV5, FGF7, GORAB, HDAC9, HINT3, HIVEP2, IRS2, JUN, MIR1304, NCOA7, NDFIP2, PIBF1, PLEKHF2, PTEN, RAB30, SKIL, SMAD7, TNFAIP3, XIAP, ZFAND2A, ZFY, ZMYM5 Genes have been upregulated. ASIC1, CD24, CDKN1C, DHFR, DNHD1, DUT, EEF1A1, EIF4A1, ENSA, FANCL, FAR1, FGFR3, GSPT1, GSTA4, HES4, HMGB1, HMGB3, HSPA4, IFI30, IFRD2, JPH1, NRBP2, MTPAP, NEURL1B, NFIA, PARP1, PHKA1, PLXNA1, PNPT1, POLR3H, RBBP4, TUBB Genes have been downregulated. Transcriptomics: mRNA levels of cell cycle genes CDK1, CDK2, CCNB1, and CCNE1 diminished in and Cell cycle inhibitors CDKN2B (p1INK4b) and CDKN2D (p16INK4d) were significantly higher in s-µg. Proteomics: The protein expression of p38 MAPK, STAT3, E-Cadherin, PTEN, and MnSOD upregulated. The protein levels of AKT, as well as phosphorylated forms pAKTs473 and pAKTt308, the phosphorylated form of GSK-3β, diminished in s-µg. | s-µg | HARV | [111,211] |
| LS180 | Genomics: The P-gp efflux pump gene expression in spheroids was increased after treatment with paclitaxel. Proteomics: intracellular ATP content increased, and AK decreased in s-µg. Metabolimics: Glucose consumption increased | s-µg | Clinostat | [212] |
| HGC-27 | Metabolomics: Phosphatidyl choline, phosphatidyl ethanolamine, arachidonic acid, and sphinganine upregulated. Phosphatidyl serine, sphingomyelin, phosphatidic acid, creatine, L-proline, pantothenic acid, adenosine triphosphate, oxidized glutathione, and adenosine triphosphate downregulated. | s-µg | RCCS | [213] |
| PaCa44 | Transcriptomics: PI3k/Akt and NFκB signaling upregulated and eIF2 signaling downregulated after 9 days in s-µg. Proteomics: Upregulation of stemness-related proteins, including EpCAM, ALDH1A3, ALDHA3A2, S100A4, ROHA, and ITGA3, glycolysis-related proteins logFC for ENO1, GAPDH, ALDOA, PGAM1, TPI1, and PKMI. Metabolomics: Glycolysis was upregulated and LDH downregulated after 7 and 9 days in s-µg. Total TG levels decreased at 1 and 7 days but increased at 9 days. Total lysophosphatidylcholines (LPCs), lysophosphatidylethanolamine (LPEs), and polyunsaturated fatty acids (FAs) increased at 1 and 7 days but decreased at 9 days in s-µg. | s-µg | RPM | [215] |
| Caco-2 | Proteomics: The proteins associated with ATP synthesis and mitochondrial functions MTFR2, MT-ATP8 were downregulated, and ubiquitin associated proteins UBAP1, UBR3 and cadherin 17 upregulated in s-µg. Heterogeneous nuclear ribonucleoprotein D-like (HNRNPDL), specifically its subunit DnaJ homolog subfamily C member 5 and CDK2 were downregulated | s-µg | 2D clinostat | [216] |
| HepG2 | Genomics: serine hydroxymethyltransferase 2, insulin receptor, apolipoprotein E, cyclophilin F, as KIAA0073 protein, EST (Hs.55153) upregulated. CYP1A1, AKR1C1, EPHX1, LTB4DH, LDLR, HMGCR Metabolic genes were upregulated. Metabolomics: Genes responsible for lipid transporter activities, such as APOA1, APOA2, and APOB, were significantly downregulated. | s-µg | RCCS, RWV | [217,218,219] |
| EPG85–257 RDB, EPG85–257 P | Genomics: ABCB1 gene in EPG85-257 RDB cells downregulated in presence and absence of DOX in s-µg. ABCB1 and ABCG2 genes downregulated in EPG85–257 P in presence of DOX in s-µg. | s-µg | RCCS | [214] |
| LUNG CANCER | ||||
| A549 | Transcriptomics: MMP2, MKI67 mRNAs reduced in s-µg (ns). | s-µg, 72 h | clinostat | [222] |
| Transcriptomics: differentially expressed miRNAs: cell cycle (via P53, CDKN, E2F), apoptosis (via BCL2, BIRC5), and stress response (via FOS, MAPK). | s-µg, 48 h | RPM | [225] | |
| Transcriptomics: two datasets from the Gene Gene Expression Omnibus (GEO) database (GSE78210 and GSE36931: gene expression profiles for A549 and Colo699) Candidate genes: AZGP1, CFB, NOX1, VTCN1, AGR3, GDA, TCN1, CST1, F5, CEACAM6, BPIFB1, FCGBP, and BPIFA1. Increase in CDH1, significant decreases in CDH2 and MMP2—induction of mesenchymal–epithelial transition | s-µg, 24 h, 48 h, 72 h | RWV | [226] | |
| A549 and H1703 | Transcriptomics: MMP2, MMP9, TIMP1, and TIMP2 revealed a significant downregulation of all genes in A549 cells under s-µg. In H1703 cells, MMP2, MMP9: significantly downregulated Proteomics: MMP-2, MMP-9, and TIMP-1: increase after 24 h; decrease after 36 h of s-µg | s-µg, 36 h | 3D clinostat | [223] |
| CRL-5889 | Transcriptomics: AD cells showed differentially increased expression of genes involved in cell death/apoptosis, such as TP53, CDKN2A, PTEN, and RB1. | s-µg; max. 96 h | RPM | [227] |
References
- White, R.J.; Averner, M. Humans in space. Nature 2001, 409, 1115–1118. [Google Scholar] [CrossRef]
- Monici, M.; van Loon, J.; Choukér, A.; Iorio, C.S. Editorial: Wound management and healing in space. Front. Bioeng. Biotechnol. 2022, 10, 1078986. [Google Scholar] [CrossRef] [PubMed]
- Penchev, R.; Scheuring, R.A.; Soto, A.T.; Miletich, D.M.; Kerstman, E.; Cohen, S.P. Back Pain in Outer Space. Anesthesiology 2021, 135, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Shibata, S.; Wakeham, D.J.; Thomas, J.D.; Abdullah, S.M.; Platts, S.; Bungo, M.W.; Levine, B.D. Cardiac Effects of Long-Duration Space Flight. J. Am. Coll. Cardiol. 2023, 82, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Hughson, R.L.; Helm, A.; Durante, M. Heart in space: Effect of the extraterrestrial environment on the cardiovascular system. Nat. Rev. Cardiol. 2018, 15, 167–180. [Google Scholar] [CrossRef]
- Sy, M.R.; Keefe, J.A.; Sutton, J.P.; Wehrens, X.H.T. Cardiac function, structural, and electrical remodeling by microgravity exposure. Am. J. Physiol. Heart Circ. Physiol. 2023, 324, H1–H13. [Google Scholar] [CrossRef] [PubMed]
- Bonanni, R.; Cariati, I.; Marini, M.; Tarantino, U.; Tancredi, V. Microgravity and Musculoskeletal Health: What Strategies Should Be Used for a Great Challenge? Life 2023, 13, 1423. [Google Scholar] [CrossRef]
- Cialdai, F.; Risaliti, C.; Monici, M. Role of fibroblasts in wound healing and tissue remodeling on Earth and in space. Front. Bioeng. Biotechnol. 2022, 10, 958381. [Google Scholar] [CrossRef]
- Belgrado, J.P.; Bonetti, G.; Maloizelle-Delaunay, J.; Stoichkova, V.; Tartaglia, G.M.; Chiurazzi, P.; Cecchin, S.; Bertelli, M. Lymphatic circulation in astronauts: Basic knowledge, challenges and perspectives. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 119–126. [Google Scholar] [CrossRef]
- Jacob, P.; Oertlin, C.; Baselet, B.; Westerberg, L.S.; Frippiat, J.P.; Baatout, S. Next generation of astronauts or ESA astronaut 2.0 concept and spotlight on immunity. NPJ Microgravity 2023, 9, 51. [Google Scholar] [CrossRef]
- Ax, T.; Ganse, B.; Fries, F.N.; Szentmary, N.; de Paiva, C.S.; March de Ribot, F.; Jensen, S.O.; Seitz, B.; Millar, T.J. Dry eye disease in astronauts: A narrative review. Front. Physiol. 2023, 14, 1281327. [Google Scholar] [CrossRef]
- Hicks, J.; Olson, M.; Mitchell, C.; Juran, C.M.; Paul, A.M. The Impact of Microgravity on Immunological States. Immunohorizons 2023, 7, 670–682. [Google Scholar]
- Milner, D.C.; Subramanian, P.S. Insights into spaceflight-associated neuro-ocular syndrome with review of intraocular and orbital findings. Curr. Opin. Ophthalmol. 2023, 34, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Olde Engberink, R.H.G.; van Oosten, P.J.; Weber, T.; Tabury, K.; Baatout, S.; Siew, K.; Walsh, S.B.; Valenti, G.; Chouker, A.; Boutouyrie, P.; et al. The kidney, volume homeostasis and osmoregulation in space: Current perspective and knowledge gaps. NPJ Microgravity 2023, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Capri, M.; Conte, M.; Ciurca, E.; Pirazzini, C.; Garagnani, P.; Santoro, A.; Longo, F.; Salvioli, S.; Lau, P.; Moeller, R.; et al. Long-term human spaceflight and inflammaging: Does it promote aging? Ageing Res. Rev. 2023, 87, 101909. [Google Scholar] [CrossRef]
- Arshad, I.; Ferre, E.R. Cognition in zero gravity: Effects of non-terrestrial gravity on human behaviour. Q. J. Exp. Psychol. 2023, 76, 979–994. [Google Scholar] [CrossRef]
- Alon, D.M.; Mittelman, K.; Stibbe, E.; Countryman, S.; Stodieck, L.; Doraisingam, S.; Leal Martin, D.M.; Hamo, E.R.; Pines, G.; Burstein, D. CRISPR-based genetic diagnostics in microgravity. Biosens. Bioelectron. 2023, 237, 115479. [Google Scholar] [CrossRef]
- Rea, G.; Cristofaro, F.; Pani, G.; Pascucci, B.; Ghuge, S.A.; Corsetto, P.A.; Imbriani, M.; Visai, L.; Rizzo, A.M. Microgravity-driven remodeling of the proteome reveals insights into molecular mechanisms and signal networks involved in response to the space flight environment. J. Proteomics 2016, 137, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.A.; Suarez-Meade, P.; Brooks, M.; Bhargav, A.G.; Freeman, M.L.; Harvey, L.M.; Quinn, J.; Quinones-Hinojosa, A. Behavior of glioblastoma brain tumor stem cells following a suborbital rocket flight: Reaching the “edge” of outer space. NPJ Microgravity 2023, 9, 92. [Google Scholar] [CrossRef]
- Garbacki, N.; Willems, J.; Neutelings, T.; Lambert, C.; Deroanne, C.; Adrian, A.; Franz, M.; Maurer, M.; De Gieter, P.; Nusgens, B.; et al. Microgravity triggers ferroptosis and accelerates senescence in the MG-63 cell model of osteoblastic cells. NPJ Microgravity 2023, 9, 91. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Zhong, G.; Xu, Q.; Tan, Y.; Xing, W.; Cao, D.; Wang, Y.; Liu, C.; Li, J.; et al. Vascular smooth muscle cell-specific miRNA-214 deficiency alleviates simulated microgravity-induced vascular remodeling. FASEB J. 2024, 38, e23369. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.; Rampoldi, A.; Forghani, P.; Li, D.; Fite, J.; Boland, G.; Maher, K.; Xu, C. Space microgravity increases expression of genes associated with proliferation and differentiation in human cardiac spheres. NPJ Microgravity 2023, 9, 88. [Google Scholar] [CrossRef]
- Miranda, S.; Vermeesen, R.; Radstake, W.E.; Parisi, A.; Ivanova, A.; Baatout, S.; Tabury, K.; Baselet, B. Lost in Space? Unmasking the T Cell Reaction to Simulated Space Stressors. Int. J. Mol. Sci. 2023, 24, 16943. [Google Scholar] [CrossRef]
- Li, K.; Desai, R.; Scott, R.T.; Steele, J.R.; Machado, M.; Demharter, S.; Hoarfrost, A.; Braun, J.L.; Fajardo, V.A.; Sanders, L.M.; et al. Explainable machine learning identifies multi-omics signatures of muscle response to spaceflight in mice. NPJ Microgravity 2023, 9, 90. [Google Scholar] [CrossRef]
- Bizzarri, M.; Monici, M.; van Loon, J.J. How microgravity affects the biology of living systems. Biomed. Res. Int. 2015, 2015, 863075. [Google Scholar] [CrossRef]
- Demontis, G.C.; Germani, M.M.; Caiani, E.G.; Barravecchia, I.; Passino, C.; Angeloni, D. Human Pathophysiological Adaptations to the Space Environment. Front. Physiol. 2017, 8, 547. [Google Scholar] [CrossRef] [PubMed]
- Dai, K.; Wang, Y.; Yan, R.; Shi, Q.; Wang, Z.; Yuan, Y.; Cheng, H.; Li, S.; Fan, Y.; Zhuang, F. Effects of microgravity and hypergravity on platelet functions. Thromb Haemost. 2009, 101, 902–910. [Google Scholar] [PubMed]
- Le Bourg, E. A review of the effects of microgravity and of hypergravity on aging and longevity. Exp. Gerontol. 1999, 34, 319–336. [Google Scholar] [CrossRef]
- Maier, J.A.; Cialdai, F.; Monici, M.; Morbidelli, L. The impact of microgravity and hypergravity on endothelial cells. Biomed. Res. Int. 2015, 2015, 434803. [Google Scholar] [CrossRef]
- Wu, X.T.; Yang, X.; Tian, R.; Li, Y.H.; Wang, C.Y.; Fan, Y.B.; Sun, L.W. Cells respond to space microgravity through cytoskeleton reorganization. FASEB J. 2022, 36, e22114. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Guo, S.; Li, B.B.; Jiang, N.; Li, A.; Yan, H.F.; Yang, H.M.; Zhou, J.L.; Li, C.L.; Cui, Y. Effect of Weightlessness on the 3D Structure Formation and Physiologic Function of Human Cancer Cells. Biomed. Res. Int. 2019, 2019, 4894083. [Google Scholar] [CrossRef]
- Grimm, D.; Wehland, M.; Corydon, T.J.; Richter, P.; Prasad, B.; Bauer, J.; Egli, M.; Kopp, S.; Lebert, M.; Krüger, M. The effects of microgravity on differentiation and cell growth in stem cells and cancer stem cells. Stem Cells Transl. Med. 2020, 9, 882–894. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lv, W.; Peng, X.; Cheng, Y.; Tu, Y.; Song, G.; Luo, Q. Simulated microgravity attenuates skin wound healing by inhibiting dermal fibroblast migration via F-actin/YAP signaling pathway. J. Cell Physiol. 2023, 238, 2751–2764. [Google Scholar] [CrossRef] [PubMed]
- Handwerk, L.; Schreier, H.K.; Kraft, D.; Shreder, K.; Hemmersbach, R.; Hauslage, J.; Bonig, H.; Wiesmuller, L.; Fournier, C.; Rall-Scharpf, M. Simulating Space Conditions Evokes Different DNA Damage Responses in Immature and Mature Cells of the Human Hematopoietic System. Int. J. Mol. Sci. 2023, 24, 13761. [Google Scholar] [CrossRef] [PubMed]
- ElGindi, M.; Sapudom, J.; Garcia Sabate, A.; Chesney Quartey, B.; Alatoom, A.; Al-Sayegh, M.; Li, R.; Chen, W.; Teo, J. Effects of an aged tissue niche on the immune potency of dendritic cells using simulated microgravity. NPJ Aging 2023, 9, 14. [Google Scholar] [CrossRef]
- Winkelmaier, G.; Jabbari, K.; Chien, L.C.; Grabham, P.; Parvin, B.; Pluth, J. Influence of Simulated Microgravity on Mammary Epithelial Cells Grown as 2D and 3D Cultures. Int. J. Mol. Sci. 2023, 24, 7615. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, X.; Li, G.; Zhang, L.; Zhang, S.; Shi, F.; Hu, Z. Inhibition of SIRT1 by miR-138-5p provides a mechanism for inhibiting osteoblast proliferation and promoting apoptosis under simulated microgravity. Life Sci. Space Res. 2023, 36, 59–69. [Google Scholar] [CrossRef]
- Carlsson, S.I.; Bertilaccio, M.T.; Ballabio, E.; Maier, J.A. Endothelial stress by gravitational unloading: Effects on cell growth and cytoskeletal organization. Biochim. Biophys. Acta 2003, 1642, 173–179. [Google Scholar] [CrossRef]
- Cazzaniga, A.; Locatelli, L.; Castiglioni, S.; Maier, J.A.M. The dynamic adaptation of primary human endothelial cells to simulated microgravity. FASEB J. 2019, 33, 5957–5966. [Google Scholar] [CrossRef]
- Mariotti, M.; Maier, J.A. Gravitational unloading induces an anti-angiogenic phenotype in human microvascular endothelial cells. J. Cell Biochem. 2008, 104, 129–135. [Google Scholar] [CrossRef]
- Versari, S.; Villa, A.; Bradamante, S.; Maier, J.A. Alterations of the actin cytoskeleton and increased nitric oxide synthesis are common features in human primary endothelial cell response to changes in gravity. Biochim. Biophys. Acta 2007, 1773, 1645–1652. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Pietsch, J.; Wehland, M.; Schulz, H.; Saar, K.; Hübner, N.; Bauer, J.; Braun, M.; Schwarzwälder, A.; Segerer, J.; et al. Differential gene expression profile and altered cytokine secretion of thyroid cancer cells in space. FASEB J. 2014, 28, 813–835. [Google Scholar] [CrossRef]
- Berrios, D.C.; Galazka, J.; Grigorev, K.; Gebre, S.; Costes, S.V. NASA GeneLab: Interfaces for the exploration of space omics data. Nucleic Acids Res. 2021, 49, D1515–D1522. [Google Scholar] [CrossRef] [PubMed]
- Corydon, T.J.; Schulz, H.; Richter, P.; Strauch, S.M.; Böhmer, M.; Ricciardi, D.A.; Wehland, M.; Krüger, M.; Erzinger, G.S.; Lebert, M.; et al. Current Knowledge about the Impact of Microgravity on Gene Regulation. Cells 2023, 12, 1043. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Grimm, D.; Schulz, H.; Krüger, M.; Cortés-Sánchez, J.L.; Egli, M.; Kraus, A.; Sahana, J.; Corydon, T.J.; Hemmersbach, R.; Wise, P.M.; et al. The Fight against Cancer by Microgravity: The Multicellular Spheroid as a Metastasis Model. Int. J. Mol. Sci. 2022, 23, 3073. [Google Scholar] [CrossRef]
- Becker, J.L.; Souza, G.R. Using space-based investigations to inform cancer research on Earth. Nat. Rev. Cancer 2013, 13, 315–327. [Google Scholar] [CrossRef]
- Beysens, D.A.; Van Loon, J.J. Generation and Applications of Extra-Terrestrial Environments on Earth; Taylor & Francis: Abingdon, UK, 2015. [Google Scholar]
- Brungs, S.; Egli, M.; Wuest, S.L.; Christianen, P.C.M.; van Loon, J.J.W.A.; Ngo Anh, T.J.; Hemmersbach, R. Facilities for Simulation of Microgravity in the ESA Ground-Based Facility Programme. Microgravity Sci. Technol. 2016, 28, 191–203. [Google Scholar] [CrossRef]
- Herranz, R.; Anken, R.; Boonstra, J.; Braun, M.; Christianen, P.C.; de Geest, M.; Hauslage, J.; Hilbig, R.; Hill, R.J.; Lebert, M.; et al. Ground-based facilities for simulation of microgravity: Organism-specific recommendations for their use, and recommended terminology. Astrobiology 2013, 13, 1–17. [Google Scholar] [CrossRef]
- Pletser, V.; Rouquette, S.; Friedrich, U.; Clervoy, J.-F.; Gharib, T.; Gai, F.; Mora, C. The First European Parabolic Flight Campaign with the Airbus A310 ZERO-G. Microgravity Sci. Technol. 2016, 28, 587–601. [Google Scholar] [CrossRef]
- Karmali, F.; Shelhamer, M. The dynamics of parabolic flight: Flight characteristics and passenger percepts. Acta Astronaut. 2008, 63, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Pletser, V. European aircraft parabolic flights for microgravity research, applications and exploration: A review. REACH 2016, 1, 11–19. [Google Scholar] [CrossRef]
- Seibert, G. The History of Sounding Rockets and Their Contribution to European Space Research; Battrick, B., Ed.; ESA Publications Division: Noordwijk, The Netherlands, 2006. [Google Scholar]
- Duan, E.; Long, M. Life Science in Space: Experiments on Board the SJ-10 Recoverable Satellite; Springer: Singapore, 2019. [Google Scholar]
- Cosmos/Bion: The age of the biosatellites. In Animals in Space: From Research Rockets to the Space Shuttle; Springer: New York, NY, USA, 2007; pp. 277–305.
- Reference Guide to the International Space Station—Assembly Complete Edition; National Aeronautics and Space Administration (NASA): Washington, DC, USA, 2010.
- Rai, A.; Robinson, J.A.; Tate-Brown, J.; Buckley, N.; Zell, M.; Tasaki, K.; Karabadzhak, G.; Sorokin, I.V.; Pignataro, S. Expanded benefits for humanity from the International Space Station. Acta Astronaut. 2016, 126, 463–474. [Google Scholar] [CrossRef]
- Wuest, S.L.; Richard, S.; Kopp, S.; Grimm, D.; Egli, M. Simulated Microgravity: Critical Review on the Use of Random Positioning Machines for Mammalian Cell Culture. BioMed Res. Int. 2015, 2015, 971474. [Google Scholar] [CrossRef]
- Hammond, T.; Allen, P. The Bonn Criteria: Minimal Experimental Parameter Reporting for Clinostat and Random Positioning Machine Experiments with Cells and Tissues. Microgravity Sci. Technol. 2011, 23, 271–275. [Google Scholar] [CrossRef]
- van Loon, J.J.W.A. Some history and use of the random positioning machine, RPM, in gravity related research. Adv. Space Res. 2007, 39, 1161–1165. [Google Scholar] [CrossRef]
- Dedolph, R.R.; Dipert, M.H. The physical basis of gravity stimulus nullification by clinostat rotation. Plant Physiol. 1971, 47, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Briegleb, W. Some qualitative and quantitative aspects of the fast-rotating clinostat as a research tool. ASGSB Bull. 1992, 5, 23. [Google Scholar]
- Hammond, T.G.; Hammond, J.M. Optimized suspension culture: The rotating-wall vessel. Am. J. Physiol. Renal. Physiol. 2001, 281, F12–F25. [Google Scholar] [CrossRef]
- Ayyaswamy, P.S.; Mukundakrishnan, K. Optimal conditions for simulating microgravity employing NASA designed rotating wall vessels. Acta Astronaut. 2007, 60, 397–405. [Google Scholar] [CrossRef]
- Klaus, D.M.; Todd, P.; Schatz, A. Functional weightlessness during clinorotation of cell suspensions. Adv. Space Res. 1998, 21, 1315–1318. [Google Scholar] [CrossRef]
- Wuest, S.L.; Stern, P.; Casartelli, E.; Egli, M. Fluid Dynamics Appearing during Simulated Microgravity Using Random Positioning Machines. PLoS ONE 2017, 12, e0170826. [Google Scholar] [CrossRef]
- Leguy, C.A.D.; Delfos, R.; Pourquie, M.J.B.M.; Poelma, C.; Westerweel, J.; van Loon, J.J.W.A. Fluid dynamics during Random Positioning Machine micro-gravity experiments. Adv. Space Res. 2017, 59, 3045–3057. [Google Scholar] [CrossRef]
- Hauslage, J.; Cevik, V.; Hemmersbach, R. Pyrocystis noctiluca represents an excellent bioassay for shear forces induced in ground-based microgravity simulators (clinostat and random positioning machine). NPJ Microgravity 2017, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Wuest, S.L.; Richard, S.; Walther, I.; Furrer, R.; Anderegg, R.; Sekler, J.; Egli, M. A Novel Microgravity Simulator Applicable for Three-Dimensional Cell Culturing. Microgravity Sci. Technol. 2014, 26, 77–88. [Google Scholar] [CrossRef]
- Beaugnon, E.; Tournier, R. Levitation of water and organic substances in high static magnetic fields. J. De Phys. III 1991, 1, 1423–1428. [Google Scholar] [CrossRef]
- Beaugnon, E.; Tournier, R. Levitation of organic materials. Nature 1991, 349, 470. [Google Scholar] [CrossRef]
- Berry, M.V.; Geim, A.K. Of flying frogs and levitrons. Eur. J. Phys. 1997, 18, 307. [Google Scholar] [CrossRef]
- Valles, J.M., Jr.; Lin, K.; Denegre, J.M.; Mowry, K.L. Stable magnetic field gradient levitation of Xenopus laevis: Toward low-gravity simulation. Biophys. J. 1997, 73, 1130–1133. [Google Scholar] [CrossRef]
- Hemmersbach, R.; Simon, A.; Wasser, K.; Hauslage, J.; Christianen, P.C.; Albers, P.W.; Lebert, M.; Richter, P.; Alt, W.; Anken, R. Impact of a high magnetic field on the orientation of gravitactic unicellular organisms—A critical consideration about the application of magnetic fields to mimic functional weightlessness. Astrobiology 2014, 14, 205–215. [Google Scholar] [CrossRef]
- Denegre, J.M.; Valles, J.M., Jr.; Lin, K.; Jordan, W.B.; Mowry, K.L. Cleavage planes in frog eggs are altered by strong magnetic fields. Proc. Natl. Acad. Sci. USA 1998, 95, 14729–14732. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.; Dinesan, M.; Ajayakumar, T. Survival and quality of life analysis in glioblastoma multiforme with adjuvant chemoradiotherapy: A retrospective study. Rep. Pract. Oncol. Radiother. 2022, 27, 1026–1036. [Google Scholar] [CrossRef]
- Fukazawa, T.; Tanimoto, K.; Shrestha, L.; Imura, T.; Takahashi, S.; Sueda, T.; Hirohashi, N.; Hiyama, E.; Yuge, L. Simulated microgravity enhances CDDP-induced apoptosis signal via p53-independent mechanisms in cancer cells. PLoS ONE 2019, 14, e0219363. [Google Scholar] [CrossRef] [PubMed]
- Marfia, G.; Navone, S.E.; Guarnaccia, L.; Campanella, R.; Locatelli, M.; Miozzo, M.; Perelli, P.; Della Morte, G.; Catamo, L.; Tondo, P.; et al. Space flight and central nervous system: Friends or enemies? Challenges and opportunities for neuroscience and neuro-oncology. J. Neurosci. Res. 2022, 100, 1649–1663. [Google Scholar] [CrossRef] [PubMed]
- Ohgaki, H.; Kleihues, P. Epidemiology and etiology of gliomas. Acta Neuropathol. 2005, 109, 93–108. [Google Scholar] [CrossRef]
- Takeda, M.; Magaki, T.; Okazaki, T.; Kawahara, Y.; Manabe, T.; Yuge, L.; Kurisu, K. Effects of simulated microgravity on proliferation and chemosensitivity in malignant glioma cells. Neurosci. Lett. 2009, 463, 54–59. [Google Scholar] [CrossRef]
- Uva, B.M.; Masini, M.A.; Sturla, M.; Bruzzone, F.; Giuliani, M.; Tagliafierro, G.; Strollo, F. Microgravity-induced apoptosis in cultured glial cells. Eur. J. Histochem. 2002, 46, 209–214. [Google Scholar] [CrossRef]
- Shi, Z.-x.; Rao, W.; Wang, H.; Wang, N.-d.; Si, J.-W.; Zhao, J.; Li, J.-c.; Wang, Z.-r. Modeled microgravity suppressed invasion and migration of human glioblastoma U87 cells through downregulating store-operated calcium entry. Biochem. Biophys. Res. Commun. 2015, 457, 378–384. [Google Scholar] [CrossRef]
- Silvani, G.; Basirun, C.; Wu, H.; Mehner, C.; Poole, K.; Bradbury, P.; Chou, J. A 3D-Bioprinted Vascularized Glioblastoma-on-a-Chip for Studying the Impact of Simulated Microgravity as a Novel Pre-Clinical Approach in Brain Tumor Therapy. Adv. Ther. 2021, 4, 2100106. [Google Scholar] [CrossRef]
- Silvani, G.; Bradbury, P.; Basirun, C.; Mehner, C.; Zalli, D.; Poole, K.; Chou, J. Testing 3D printed biological platform for advancing simulated microgravity and space mechanobiology research. NPJ Microgravity 2022, 8, 19. [Google Scholar] [CrossRef]
- Silvano, M.; Miele, E.; Valerio, M.; Casadei, L.; Begalli, F.; Campese, A.; Besharat, Z.M.; Alfano, V.; Abballe, L.; Catanzaro, G.; et al. Consequences of Simulated Microgravity in Neural Stem Cells: Biological Effects and Metabolic Response. J. Stem Cell Res. Ther. 2015, 5, 1000289. [Google Scholar] [CrossRef]
- Shaka, S.; Carpo, N.; Tran, V.; Cepeda, C.; Espinosa-Jeffrey, A. Space Microgravity Alters Neural Stem Cell Division: Implications for Brain Cancer Research on Earth and in Space. Int. J. Mol. Sci. 2022, 23, 14320. [Google Scholar] [CrossRef] [PubMed]
- Vardiman, J.W.; Thiele, J.; Arber, D.A.; Brunning, R.D.; Borowitz, M.J.; Porwit, A.; Harris, N.L.; Le Beau, M.M.; Hellström-Lindberg, E.; Tefferi, A.; et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: Rationale and important changes. Blood 2009, 114, 937–951. [Google Scholar] [CrossRef]
- The Leukemia & Lymphoma Society. Facts 2022–2023. Updated Data on Blood Cancers; The Leukemia & Lymphoma Society: Rye Brook, NY, USA, 2023. [Google Scholar]
- Mattiuzzi, C.; Lippi, G. Cancer statistics: A comparison between World Health Organization (WHO) and Global Burden of Disease (GBD). Eur. J. Public Health 2020, 30, 1026–1027. [Google Scholar] [CrossRef] [PubMed]
- Jun, C. Tumor Cells in Microgravity. In Into Space; Thais, R., Lucas, R., Eds.; IntechOpen: Rijeka, Croatia, 2018; Chapter 9. [Google Scholar]
- McKinley, S.; Taylor, A.; Peeples, C.; Jacob, M.; Khaparde, G.; Walter, Y.; Ekpenyong, A. Simulated Microgravity-Induced Changes to Drug Response in Cancer Cells Quantified Using Fluorescence Morphometry. Life 2023, 13, 1683. [Google Scholar] [CrossRef] [PubMed]
- Topal, U.; Zamur, C. Microgravity, Stem Cells, and Cancer: A New Hope for Cancer Treatment. Stem Cells Int. 2021, 2021, 5566872. [Google Scholar] [CrossRef]
- Kopp, S.; Slumstrup, L.; Corydon, T.J.; Sahana, J.; Aleshcheva, G.; Islam, T.; Magnusson, N.E.; Wehland, M.; Bauer, J.; Infanger, M.; et al. Identifications of novel mechanisms in breast cancer cells involving duct-like multicellular spheroid formation after exposure to the Random Positioning Machine. Sci. Rep. 2016, 6, 26887. [Google Scholar] [CrossRef]
- Medha, M.; Roy, A. Microgravity: New aspect for breast cancer treatment, a review. Acta Astronaut. 2022, 190, 62–73. [Google Scholar] [CrossRef]
- Hsieh, W.C.; Budiarto, B.R.; Wang, Y.F.; Lin, C.Y.; Gwo, M.C.; So, D.K.; Tzeng, Y.S.; Chen, S.Y. Spatial multi-omics analyses of the tumor immune microenvironment. J. Biomed. Sci. 2022, 29, 96. [Google Scholar] [CrossRef]
- Pan, D.; Jia, D. Application of Single-Cell Multi-Omics in Dissecting Cancer Cell Plasticity and Tumor Heterogeneity. Front. Mol. Biosci. 2021, 8, 757024. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Saha, R.; Palanisamy, A.; Ghosh, M.; Biswas, A.; Roy, S.; Pal, A.; Sarkar, K.; Bagh, S. A systems biology pipeline identifies new immune and disease related molecular signatures and networks in human cells during microgravity exposure. Sci. Rep. 2016, 6, 25975. [Google Scholar] [CrossRef] [PubMed]
- Prasanth, D.; Suresh, S.; Prathivadhi-Bhayankaram, S.; Mimlitz, M.; Zetocha, N.; Lee, B.; Ekpenyong, A. Microgravity Modulates Effects of Chemotherapeutic Drugs on Cancer Cell Migration. Life 2020, 10, 162. [Google Scholar] [CrossRef] [PubMed]
- Brinley, A.A.; Theriot, C.A.; Nelman-Gonzalez, M.; Crucian, B.; Stowe, R.P.; Barrett, A.D.; Pierson, D.L. Characterization of Epstein-Barr virus reactivation in a modeled spaceflight system. J. Cell Biochem. 2013, 114, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Maccarrone, M.; Battista, N.; Meloni, M.; Bari, M.; Galleri, G.; Pippia, P.; Cogoli, A.; Finazzi-Agrò, A. Creating conditions similar to those that occur during exposure of cells to microgravity induces apoptosis in human lymphocytes by 5-lipoxygenase-mediated mitochondrial uncoupling and cytochrome c release. J. Leukoc. Biol. 2003, 73, 472–481. [Google Scholar] [CrossRef]
- Schmitt, D.A.; Hatton, J.P.; Emond, C.; Chaput, D.; Paris, H.; Levade, T.; Cazenave, J.P.; Schaffar, L. The distribution of protein kinase C in human leukocytes is altered in microgravity. FASEB J. 1996, 10, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Hughes-Fulford, M.; Chang, T.T.; Martinez, E.M.; Li, C.F. Spaceflight alters expression of microRNA during T-cell activation. FASEB J. 2015, 29, 4893–4900. [Google Scholar] [CrossRef]
- Canova, S.; Fiorasi, F.; Mognato, M.; Grifalconi, M.; Reddi, E.; Russo, A.; Celotti, L. “Modeled microgravity” affects cell response to ionizing radiation and increases genomic damage. Radiat. Res. 2005, 163, 191–199. [Google Scholar] [CrossRef]
- Mognato, M.; Celotti, L. Modeled microgravity affects cell survival and HPRT mutant frequency, but not the expression of DNA repair genes in human lymphocytes irradiated with ionising radiation. Mutat. Res. 2005, 578, 417–429. [Google Scholar] [CrossRef]
- Degan, P.; Sancandi, M.; Zunino, A.; Ottaggio, L.; Viaggi, S.; Cesarone, F.; Pippia, P.; Galleri, G.; Abbondandolo, A. Exposure of human lymphocytes and lymphoblastoid cells to simulated microgravity strongly affects energy metabolism and DNA repair. J. Cell Biochem. 2005, 94, 460–469. [Google Scholar] [CrossRef]
- Risso, A.; Tell, G.; Vascotto, C.; Costessi, A.; Arena, S.; Scaloni, A.; Cosulich, M.E. Activation of human T lymphocytes under conditions similar to those that occur during exposure to microgravity: A proteomics study. Proteomics 2005, 5, 1827–1837. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, B.; Seetharam, A.; Wang, Z.; Liu, Y.; Lossie, A.C.; Thimmapuram, J.; Irudayaraj, J. A Study of Alterations in DNA Epigenetic Modifications (5mC and 5hmC) and Gene Expression Influenced by Simulated Microgravity in Human Lymphoblastoid Cells. PLoS ONE 2016, 11, e0147514. [Google Scholar] [CrossRef]
- Mangala, L.S.; Zhang, Y.; He, Z.; Emami, K.; Ramesh, G.T.; Story, M.; Rohde, L.H.; Wu, H. Effects of simulated microgravity on expression profile of microRNA in human lymphoblastoid cells. J. Biol. Chem. 2011, 286, 32483–32490. [Google Scholar] [CrossRef]
- Vidyasekar, P.; Shyamsunder, P.; Arun, R.; Santhakumar, R.; Kapadia, N.K.; Kumar, R.; Verma, R.S. Genome Wide Expression Profiling of Cancer Cell Lines Cultured in Microgravity Reveals Significant Dysregulation of Cell Cycle and MicroRNA Gene Networks. PLoS ONE 2015, 10, e0135958. [Google Scholar] [CrossRef]
- Paulsen, K.; Tauber, S.; Dumrese, C.; Bradacs, G.; Simmet, D.M.; Gölz, N.; Hauschild, S.; Raig, C.; Engeli, S.; Gutewort, A.; et al. Regulation of ICAM-1 in cells of the monocyte/macrophage system in microgravity. Biomed. Res. Int. 2015, 2015, 538786. [Google Scholar] [CrossRef]
- Paulsen, K.; Thiel, C.; Timm, J.; Schmidt, P.M.; Huber, K.; Tauber, S.; Hemmersbach, R.; Seibt, D.; Kroll, H.; Grote, K.-H.; et al. Microgravity-induced alterations in signal transduction in cells of the immune system. Acta Astronaut. 2010, 67, 1116–1125. [Google Scholar] [CrossRef]
- Paulsen, K.; Tauber, S.; Goelz, N.; Simmet, D.M.; Engeli, S.; Birlem, M.; Dumrese, C.; Karer, A.; Hunziker, S.; Biskup, J.; et al. Severe disruption of the cytoskeleton and immunologically relevant surface molecules in a human macrophageal cell line in microgravity—Results of an in vitro experiment on board of the Shenzhou-8 space mission. Acta Astronaut. 2014, 94, 277–292. [Google Scholar] [CrossRef]
- Vogel, J.; Thiel, C.S.; Tauber, S.; Stockmann, C.; Gassmann, M.; Ullrich, O. Expression of Hypoxia-Inducible Factor 1α (HIF-1α) and Genes of Related Pathways in Altered Gravity. Int. J. Mol. Sci. 2019, 20, 436. [Google Scholar] [CrossRef]
- Maier, J.A. Impact of simulated microgravity on cell cycle control and cytokine release by U937 cells. Int. J. Immunopathol. Pharmacol. 2006, 19, 279–286. [Google Scholar] [CrossRef]
- Thiel, C.S.; Tauber, S.; Christoffel, S.; Huge, A.; Lauber, B.A.; Polzer, J.; Paulsen, K.; Lier, H.; Engelmann, F.; Schmitz, B.; et al. Rapid coupling between gravitational forces and the transcriptome in human myelomonocytic U937 cells. Sci. Rep. 2018, 8, 13267. [Google Scholar] [CrossRef]
- Jeong, A.J.; Kim, Y.J.; Lim, M.H.; Lee, H.; Noh, K.; Kim, B.H.; Chung, J.W.; Cho, C.H.; Kim, S.; Ye, S.K. Microgravity induces autophagy via mitochondrial dysfunction in human Hodgkin’s lymphoma cells. Sci. Rep. 2018, 8, 14646. [Google Scholar] [CrossRef] [PubMed]
- Morabito, C.; Lanuti, P.; Caprara, G.A.; Marchisio, M.; Bizzarri, M.; Guarnieri, S.; Mariggiò, M.A. Physiological Responses of Jurkat Lymphocytes to Simulated Microgravity Conditions. Int. J. Mol. Sci. 2019, 20, 1892. [Google Scholar] [CrossRef] [PubMed]
- Mylabathula, P.L.; Bigley, A.B.; Li, L.; Crucian, B.E.; Pierson, D.L.; Mehta, S.K.; Rezvani, K.; Simpson, R.J. Simulated microgravity ‘disarms’ human Natural Killer cells and suppresses cytotoxic activity against tumor target cells. Brain Behav. Immun. 2017, 66, e31. [Google Scholar] [CrossRef]
- Boonyaratanakornkit, J.B.; Cogoli, A.; Li, C.F.; Schopper, T.; Pippia, P.; Galleri, G.; Meloni, M.A.; Hughes-Fulford, M. Key gravity-sensitive signaling pathways drive T cell activation. FASEB J. 2005, 19, 2020–2022. [Google Scholar] [CrossRef]
- Hatton, J.P.; Gaubert, F.; Cazenave, J.P.; Schmitt, D. Microgravity modifies protein kinase C isoform translocation in the human monocytic cell line U937 and human peripheral blood T-cells. J. Cell Biochem. 2002, 87, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.T.; Walther, I.; Li, C.F.; Boonyaratanakornkit, J.; Galleri, G.; Meloni, M.A.; Pippia, P.; Cogoli, A.; Hughes-Fulford, M. The Rel/NF-κB pathway and transcription of immediate early genes in T cell activation are inhibited by microgravity. J. Leukoc. Biol. 2012, 92, 1133–1145. [Google Scholar] [CrossRef]
- Cubano, L.A.; Lewis, M.L. Fas/APO-1 protein is increased in spaceflown lymphocytes (Jurkat). Exp. Gerontol. 2000, 35, 389–400. [Google Scholar] [CrossRef]
- Kim, Y.J.; Jeong, A.J.; Kim, M.; Lee, C.; Ye, S.K.; Kim, S. Time-averaged simulated microgravity (taSMG) inhibits proliferation of lymphoma cells, L-540 and HDLM-2, using a 3D clinostat. Biomed. Eng. Online 2017, 16, 48. [Google Scholar] [CrossRef]
- Gasperi, V.; Rapino, C.; Battista, N.; Bari, M.; Mastrangelo, N.; Angeletti, S.; Dainese, E.; Maccarrone, M. A functional interplay between 5-lipoxygenase and μ-calpain affects survival and cytokine profile of human Jurkat T lymphocyte exposed to simulated microgravity. Biomed. Res. Int. 2014, 2014, 782390. [Google Scholar] [CrossRef]
- Maccarrone, M.; Battista, N.; Bari, M.; Finazzi-Agrò, A. Lipoxygenase activity in altered gravity. Adv. Space Biol. Med. 2002, 8, 1–17. [Google Scholar] [CrossRef]
- Maccarrone, M.; Putti, S.; Finazzi Agro, A. Altered gravity modulates 5-lipoxygenase in human erythroleukemia K562 cells. J. Gravit. Physiol. 1998, 5, 97–98. [Google Scholar]
- Maccarrone, M.; Bari, M.; Lorenzon, T.; Finazzi-Agrò, A. Altered gravity modulates prostaglandin H synthase in human K562 cells. J. Gravit. Physiol. 2000, 7, 61–62. [Google Scholar]
- Lewis, M.L.; Cubano, L.A.; Zhao, B.; Dinh, H.K.; Pabalan, J.G.; Piepmeier, E.H.; Bowman, P.D. cDNA microarray reveals altered cytoskeletal gene expression in space-flown leukemic T lymphocytes (Jurkat). FASEB J. 2001, 15, 1783–1785. [Google Scholar] [CrossRef]
- Shao, D.; Ye, L.; Zhu, B.; Li, Q.; Yang, H.; Shi, J.; Huang, Q.; Zhao, W. Mechanisms of the Effect of Simulated Microgravity on the Cytotoxicity of NK Cells Following the DNA Methylation of NKG2D and the Expression of DAP10. Microgravity Sci. Technol. 2021, 33, 6. [Google Scholar] [CrossRef]
- Cubano, L.A.; Lewis, M.L. Effect of vibrational stress and spaceflight on regulation of heat shock proteins hsp70 and hsp27 in human lymphocytes (Jurkat). J. Leukoc. Biol. 2001, 69, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.L.; Reynolds, J.L.; Cubano, L.A.; Hatton, J.P.; Lawless, B.D.; Piepmeier, E.H. Spaceflight alters microtubules and increases apoptosis in human lymphocytes (Jurkat). FASEB J. 1998, 12, 1007–1018. [Google Scholar] [CrossRef]
- Puca, A.; Russo, G.; Giordano, A. Properties of mechano-transduction via simulated microgravity and its effects on intracellular trafficking of VEGFR’s. Oncotarget 2012, 3, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Sciola, L.; Cogoli-Greuter, M.; Cogoli, A.; Spano, A.; Pippia, P. Influence of microgravity on mitogen binding and cytoskeleton in Jurkat cells. Adv. Space Res. 1999, 24, 801–805. [Google Scholar] [CrossRef]
- Wang, C.; Li, N.; Zhang, C.; Sun, S.; Gao, Y.; Long, M. Effects of Simulated Microgravity on Functions of Neutrophil-like HL-60 Cells. Microgravity Sci. Technol. 2015, 27, 515–527. [Google Scholar] [CrossRef]
- Singh, R.; Rajput, M.; Singh, R.P. Simulated microgravity triggers DNA damage and mitochondria-mediated apoptosis through ROS generation in human promyelocytic leukemic cells. Mitochondrion 2021, 61, 114–124. [Google Scholar] [CrossRef]
- Zou, L.-x.; Cui, S.-y.; Zhong, J.; Yi, Z.-c.; Sun, Y.; Fan, Y.-b.; Zhuang, F.-y. Simulated microgravity induce apoptosis and down-regulation of erythropoietin receptor of UT-7/EPO cells. Adv. Space Res. 2010, 46, 1237–1244. [Google Scholar] [CrossRef]
- Ferrari, A.; Dirksen, U.; Bielack, S. Sarcomas of Soft Tissue and Bone. Prog. Tumor Res. 2016, 43, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Hui, J.Y. Epidemiology and Etiology of Sarcomas. Surg. Clin. N. Am. 2016, 96, 901–914. [Google Scholar] [CrossRef] [PubMed]
- Burningham, Z.; Hashibe, M.; Spector, L.; Schiffman, J.D. The epidemiology of sarcoma. Clin. Sarcoma Res. 2012, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Lahat, G.; Lazar, A.; Lev, D. Sarcoma epidemiology and etiology: Potential environmental and genetic factors. Surg. Clin. N. Am. 2008, 88, 451–481. [Google Scholar] [CrossRef] [PubMed]
- Kunisada, T.; Kawai, A.; Inoue, H.; Namba, M. Effects of simulated microgravity on human osteoblast-like cells in culture. Acta Med. Okayama 1997, 51, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Romswinkel, A.; Infanger, M.; Dietz, C.; Strube, F.; Kraus, A. The Role of C-X-C Chemokine Receptor Type 4 (CXCR4) in Cell Adherence and Spheroid Formation of Human Ewing’s Sarcoma Cells under Simulated Microgravity. Int. J. Mol. Sci. 2019, 20, 6073. [Google Scholar] [CrossRef]
- Narayanan, R.; Smith, C.L.; Weigel, N.L. Vector-averaged gravity-induced changes in cell signaling and vitamin D receptor activity in MG-63 cells are reversed by a 1,25-(OH)2D3 analog, EB1089. Bone 2002, 31, 381–388. [Google Scholar] [CrossRef]
- Carmeliet, G.; Nys, G.; Stockmans, I.; Bouillon, R. Gene expression related to the differentiation of osteoblastic cells is altered by microgravity. Bone 1998, 22, 139s–143s. [Google Scholar] [CrossRef]
- Carmeliet, G.; Nys, G.; Bouillon, R. Microgravity reduces the differentiation of human osteoblastic MG-63 cells. J. Bone Miner. Res. 1997, 12, 786–794. [Google Scholar] [CrossRef]
- Guignandon, A.; Faure, C.; Neutelings, T.; Rattner, A.; Mineur, P.; Linossier, M.T.; Laroche, N.; Lambert, C.; Deroanne, C.; Nusgens, B.; et al. Rac1 GTPase silencing counteracts microgravity-induced effects on osteoblastic cells. FASEB J. 2014, 28, 4077–4087. [Google Scholar] [CrossRef]
- American Cancer Society. Thyroid Cancer. Available online: https://www.cancer.org/cancer/types/thyroid-cancer.html (accessed on 10 December 2023).
- Grimm, D.; Bauer, J.; Kossmehl, P.; Shakibaei, M.; Schöberger, J.; Pickenhahn, H.; Schulze-Tanzil, G.; Vetter, R.; Eilles, C.; Paul, M.; et al. Simulated microgravity alters differentiation and increases apoptosis in human follicular thyroid carcinoma cells. FASEB J. 2002, 16, 604–606. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, C.; Pietsch, J.; Grosse, J.; Wehland, M.; Schulz, H.; Saar, K.; Hübner, N.; Hauslage, J.; Hemmersbach, R.; Braun, M.; et al. Differential gene regulation under altered gravity conditions in follicular thyroid cancer cells: Relationship between the extracellular matrix and the cytoskeleton. Cell Physiol. Biochem. 2011, 28, 185–198. [Google Scholar] [CrossRef]
- Svejgaard, B.; Wehland, M.; Ma, X.; Kopp, S.; Sahana, J.; Warnke, E.; Aleshcheva, G.; Hemmersbach, R.; Hauslage, J.; Grosse, J.; et al. Common Effects on Cancer Cells Exerted by a Random Positioning Machine and a 2D Clinostat. PLoS ONE 2015, 10, e0135157. [Google Scholar] [CrossRef] [PubMed]
- Riwaldt, S.; Bauer, J.; Wehland, M.; Slumstrup, L.; Kopp, S.; Warnke, E.; Dittrich, A.; Magnusson, N.E.; Pietsch, J.; Corydon, T.J.; et al. Pathways Regulating Spheroid Formation of Human Follicular Thyroid Cancer Cells under Simulated Microgravity Conditions: A Genetic Approach. Int. J. Mol. Sci. 2016, 17, 528. [Google Scholar] [CrossRef]
- Pietsch, J.; Kussian, R.; Sickmann, A.; Bauer, J.; Weber, G.; Nissum, M.; Westphal, K.; Egli, M.; Grosse, J.; Schönberger, J.; et al. Application of free-flow IEF to identify protein candidates changing under microgravity conditions. Proteomics 2010, 10, 904–913. [Google Scholar] [CrossRef] [PubMed]
- Grosse, J.; Wehland, M.; Pietsch, J.; Schulz, H.; Saar, K.; Hübner, N.; Eilles, C.; Bauer, J.; Abou-El-Ardat, K.; Baatout, S.; et al. Gravity-sensitive signaling drives 3-dimensional formation of multicellular thyroid cancer spheroids. FASEB J. 2012, 26, 5124–5140. [Google Scholar] [CrossRef] [PubMed]
- Pietsch, J.; Ma, X.; Wehland, M.; Aleshcheva, G.; Schwarzwälder, A.; Segerer, J.; Birlem, M.; Horn, A.; Bauer, J.; Infanger, M.; et al. Spheroid formation of human thyroid cancer cells in an automated culturing system during the Shenzhou-8 Space mission. Biomaterials 2013, 34, 7694–7705. [Google Scholar] [CrossRef]
- Warnke, E.; Pietsch, J.; Wehland, M.; Bauer, J.; Infanger, M.; Görög, M.; Hemmersbach, R.; Braun, M.; Ma, X.; Sahana, J.; et al. Spheroid formation of human thyroid cancer cells under simulated microgravity: A possible role of CTGF and CAV1. Cell Commun. Signal. 2014, 12, 32. [Google Scholar] [CrossRef]
- Riwaldt, S.; Pietsch, J.; Sickmann, A.; Bauer, J.; Braun, M.; Segerer, J.; Schwarzwälder, A.; Aleshcheva, G.; Corydon, T.J.; Infanger, M.; et al. Identification of proteins involved in inhibition of spheroid formation under microgravity. Proteomics 2015, 15, 2945–2952. [Google Scholar] [CrossRef]
- Riwaldt, S.; Bauer, J.; Pietsch, J.; Braun, M.; Segerer, J.; Schwarzwälder, A.; Corydon, T.J.; Infanger, M.; Grimm, D. The Importance of Caveolin-1 as Key-Regulator of Three-Dimensional Growth in Thyroid Cancer Cells Cultured under Real and Simulated Microgravity Conditions. Int. J. Mol. Sci. 2015, 16, 28296–28310. [Google Scholar] [CrossRef] [PubMed]
- Wise, P.M.; Neviani, P.; Riwaldt, S.; Corydon, T.J.; Wehland, M.; Braun, M.; Krüger, M.; Infanger, M.; Grimm, D. Changes in Exosome Release in Thyroid Cancer Cells after Prolonged Exposure to Real Microgravity in Space. Int. J. Mol. Sci. 2021, 22, 2132. [Google Scholar] [CrossRef] [PubMed]
- Wise, P.M.; Neviani, P.; Riwaldt, S.; Corydon, T.J.; Wehland, M.; Braun, M.; Krüger, M.; Infanger, M.; Grimm, D. Changes in Exosomal miRNA Composition in Thyroid Cancer Cells after Prolonged Exposure to Real Microgravity in Space. Int. J. Mol. Sci. 2021, 22, 12841. [Google Scholar] [CrossRef]
- Melnik, D.; Krüger, M.; Schulz, H.; Kopp, S.; Wehland, M.; Bauer, J.; Baselet, B.; Vermeesen, R.; Baatout, S.; Corydon, T.J.; et al. The CellBox-2 Mission to the International Space Station: Thyroid Cancer Cells in Space. Int. J. Mol. Sci. 2021, 22, 8777. [Google Scholar] [CrossRef] [PubMed]
- Kopp, S.; Warnke, E.; Wehland, M.; Aleshcheva, G.; Magnusson, N.E.; Hemmersbach, R.; Corydon, T.J.; Bauer, J.; Infanger, M.; Grimm, D. Mechanisms of three-dimensional growth of thyroid cells during long-term simulated microgravity. Sci. Rep. 2015, 5, 16691. [Google Scholar] [CrossRef] [PubMed]
- Kopp, S.; Krüger, M.; Feldmann, S.; Oltmann, H.; Schütte, A.; Schmitz, B.; Bauer, J.; Schulz, H.; Saar, K.; Huebner, N.; et al. Thyroid cancer cells in space during the TEXUS-53 sounding rocket mission—The THYROID Project. Sci. Rep. 2018, 8, 10355. [Google Scholar] [CrossRef]
- Kopp, S.; Krüger, M.; Bauer, J.; Wehland, M.; Corydon, T.J.; Sahana, J.; Nassef, M.Z.; Melnik, D.; Bauer, T.J.; Schulz, H.; et al. Microgravity Affects Thyroid Cancer Cells during the TEXUS-53 Mission Stronger than Hypergravity. Int. J. Mol. Sci. 2018, 19, 4001. [Google Scholar] [CrossRef] [PubMed]
- Melnik, D.; Sahana, J.; Corydon, T.J.; Kopp, S.; Nassef, M.Z.; Wehland, M.; Infanger, M.; Grimm, D.; Krüger, M. Dexamethasone Inhibits Spheroid Formation of Thyroid Cancer Cells Exposed to Simulated Microgravity. Cells 2020, 9, 367. [Google Scholar] [CrossRef]
- Melnik, D.; Cortés-Sánchez, J.L.; Sandt, V.; Kahlert, S.; Kopp, S.; Grimm, D.; Krüger, M. Dexamethasone Selectively Inhibits Detachment of Metastatic Thyroid Cancer Cells during Random Positioning. Cancers 2023, 15, 1641. [Google Scholar] [CrossRef]
- American Cancer Society. Prostate Cancer. Available online: https://www.cancer.org/cancer/types/prostate-cancer.html (accessed on 10 December 2023).
- Clejan, S.; O’Connor, K.C.; Cowger, N.L.; Cheles, M.K.; Haque, S.; Primavera, A.C. Effects of simulated microgravity on DU 145 human prostate carcinoma cells. Biotechnol. Bioeng. 1996, 50, 587–597. [Google Scholar] [CrossRef]
- Zhau, H.E.; Goodwin, T.J.; Chang, S.M.; Baker, T.L.; Chung, L.W. Establishment of a three-dimensional human prostate organoid coculture under microgravity-simulated conditions: Evaluation of androgen-induced growth and PSA expression. In Vitro Cell Dev. Biol. Anim. 1997, 33, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Ingram, M.; Techy, G.B.; Saroufeem, R.; Yazan, O.; Narayan, K.S.; Goodwin, T.J.; Spaulding, G.F. Three-dimensional growth patterns of various human tumor cell lines in simulated microgravity of a NASA bioreactor. In Vitro Cell Dev. Biol. Anim. 1997, 33, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Margolis, L.; Hatfill, S.; Chuaqui, R.; Vocke, C.; Emmert-Buck, M.; Linehan, W.M.; Duray, P.H. Long term organ culture of human prostate tissue in a NASA-designed rotating wall bioreactor. J. Urol. 1999, 161, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Markin, A.A.; Zhuravleva, O.A.; Morukov, B.V.; Zabolotskaia, I.V.; Vostrikova, L.V.; Kuzichkin, D.S. Metabolic effects of physical countermeasures against deficient weight-bearing in an experiment with 7-day immersion. Aviakosm. Ekolog. Med. 2011, 45, 28–34. [Google Scholar] [CrossRef]
- Clejan, S.; O’Connor, K.; Rosensweig, N. Tri-dimensional prostate cell cultures in simulated microgravity and induced changes in lipid second messengers and signal transduction. J. Cell. Mol. Med. 2001, 5, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Twombly, R. Prostate modeling experiment success becomes part of legacy of shuttle astronauts. J. Natl. Cancer Inst. 2003, 95, 505–507. [Google Scholar] [CrossRef]
- Schulz, H.; Dietrichs, D.; Wehland, M.; Corydon, T.J.; Hemmersbach, R.; Liemersdorf, C.; Melnik, D.; Hübner, N.; Saar, K.; Infanger, M.; et al. In Prostate Cancer Cells Cytokines Are Early Responders to Gravitational Changes Occurring in Parabolic Flights. Int. J. Mol. Sci. 2022, 23, 7876. [Google Scholar] [CrossRef]
- Dietrichs, D.; Grimm, D.; Sahana, J.; Melnik, D.; Corydon, T.J.; Wehland, M.; Krüger, M.; Vermeesen, R.; Baselet, B.; Baatout, S.; et al. Three-Dimensional Growth of Prostate Cancer Cells Exposed to Simulated Microgravity. Front. Cell. Dev. Biol. 2022, 10, 841017. [Google Scholar] [CrossRef]
- Hybel, T.E.; Dietrichs, D.; Sahana, J.; Corydon, T.J.; Nassef, M.Z.; Wehland, M.; Krüger, M.; Magnusson, N.E.; Bauer, J.; Utpatel, K.; et al. Simulated Microgravity Influences VEGF, MAPK, and PAM Signaling in Prostate Cancer Cells. Int. J. Mol. Sci. 2020, 21, 1263. [Google Scholar] [CrossRef]
- de Jesús, T.J.; Ramakrishnan, P. NF-κB c-Rel Dictates the Inflammatory Threshold by Acting as a Transcriptional Repressor. iScience 2020, 23, 100876. [Google Scholar] [CrossRef]
- Breastcancer.org. Breast Cancer Facts and Statistics. Available online: https://www.breastcancer.org/facts-statistics (accessed on 3 November 2023).
- Benitez Fuentes, J.D.; Morgan, E.; de Luna Aguilar, A.; Mafra, A.; Shah, R.; Giusti, F.; Vignat, J.; Znaor, A.; Musetti, C.; Yip, C.H.; et al. Global Stage Distribution of Breast Cancer at Diagnosis: A Systematic Review and Meta-Analysis. JAMA Oncol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Vassy, J.; Portet, S.; Beil, M.; Millot, G.; Fauvel-Lafève, F.; Gasset, G.; Schoevaert, D. Weightlessness acts on human breast cancer cell line MCF-7. Adv. Space Res. 2003, 32, 1595–1603. [Google Scholar] [CrossRef] [PubMed]
- Vassy, J.; Portet, S.; Beil, M.; Millot, G.; Fauvel-Lafève, F.; Karniguian, A.; Gasset, G.; Irinopoulou, T.; Calvo, F.; Rigaut, J.P.; et al. The effect of weightlessness on cytoskeleton architecture and proliferation of human breast cancer cell line MCF-7. FASEB J. 2001, 15, 1104–1106. [Google Scholar] [CrossRef] [PubMed]
- Coinu, R.; Galleri, G.; Pippia, P.; Tilocca, M.G.; Meloni, M.; Covelli, B.; Chiaviello, A.; Palumbo, G. Microgravity alters basal and insulin-mediated metabolic activity of normal and neoplastic cells. J. Gravit. Physiol. 2004, 11, 185–186. [Google Scholar]
- Coinu, R.; Chiaviello, A.; Galleri, G.; Franconi, F.; Crescenzi, E.; Palumbo, G. Exposure to modeled microgravity induces metabolic idleness in malignant human MCF-7 and normal murine VSMC cells. FEBS Lett. 2006, 580, 2465–2470. [Google Scholar] [CrossRef] [PubMed]
- Masiello, M.G.; Cucina, A.; Proietti, S.; Palombo, A.; Coluccia, P.; D’Anselmi, F.; Dinicola, S.; Pasqualato, A.; Morini, V.; Bizzarri, M. Phenotypic switch induced by simulated microgravity on MDA-MB-231 breast cancer cells. Biomed. Res. Int. 2014, 2014, 652434. [Google Scholar] [CrossRef]
- Sahana, J.; Nassef, M.Z.; Wehland, M.; Kopp, S.; Krüger, M.; Corydon, T.J.; Infanger, M.; Bauer, J.; Grimm, D. Decreased E-Cadherin in MCF7 Human Breast Cancer Cells Forming Multicellular Spheroids Exposed to Simulated Microgravity. Proteomics 2018, 18, e1800015. [Google Scholar] [CrossRef]
- Bauer, J.; Wehland, M.; Infanger, M.; Grimm, D.; Gombocz, E. Semantic Analysis of Posttranslational Modification of Proteins Accumulated in Thyroid Cancer Cells Exposed to Simulated Microgravity. Int. J. Mol. Sci. 2018, 19, 2257. [Google Scholar] [CrossRef]
- Chen, Y.; Xue, F.; Russo, A.; Wan, Y. Proteomic Analysis of Extracellular Vesicles Derived from MDA-MB-231 Cells in Microgravity. Protein J. 2021, 40, 108–118. [Google Scholar] [CrossRef]
- Wise, P.M.; Sahana, J.; Neviani, P.; Corydon, T.J.; Schulz, H.; Wehland, M.; Infanger, M.; Grimm, D. Prolonged Exposure to Simulated Microgravity Changes Release of Small Extracellular Vesicle in Breast Cancer Cells. Int. J. Mol. Sci. 2022, 23, 16095. [Google Scholar] [CrossRef]
- Nassef, M.Z.; Kopp, S.; Wehland, M.; Melnik, D.; Sahana, J.; Krüger, M.; Corydon, T.J.; Oltmann, H.; Schmitz, B.; Schütte, A.; et al. Real Microgravity Influences the Cytoskeleton and Focal Adhesions in Human Breast Cancer Cells. Int. J. Mol. Sci. 2019, 20, 3156. [Google Scholar] [CrossRef] [PubMed]
- Nassef, M.Z.; Kopp, S.; Melnik, D.; Corydon, T.J.; Sahana, J.; Krüger, M.; Wehland, M.; Bauer, T.J.; Liemersdorf, C.; Hemmersbach, R.; et al. Short-Term Microgravity Influences Cell Adhesion in Human Breast Cancer Cells. Int. J. Mol. Sci. 2019, 20, 5730. [Google Scholar] [CrossRef] [PubMed]
- Strube, F.; Infanger, M.; Wehland, M.; Delvinioti, X.; Romswinkel, A.; Dietz, C.; Kraus, A. Alteration of Cytoskeleton Morphology and Gene Expression in Human Breast Cancer Cells under Simulated Microgravity. Cell J. 2020, 22, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Strube, F.; Infanger, M.; Dietz, C.; Romswinkel, A.; Kraus, A. Short-term effects of simulated microgravity on morphology and gene expression in human breast cancer cells. Physiol. Int. 2019, 106, 311–322. [Google Scholar] [CrossRef]
- Monti, N.; Masiello, M.G.; Proietti, S.; Catizone, A.; Ricci, G.; Harrath, A.H.; Alwasel, S.H.; Cucina, A.; Bizzarri, M. Survival Pathways Are Differently Affected by Microgravity in Normal and Cancerous Breast Cells. Int. J. Mol. Sci. 2021, 22, 862. [Google Scholar] [CrossRef] [PubMed]
- Sahana, J.; Cortés-Sánchez, J.L.; Sandt, V.; Melnik, D.; Corydon, T.J.; Schulz, H.; Cai, Z.; Evert, K.; Grimm, D.; Wehland, M. Long-Term Simulation of Microgravity Induces Changes in Gene Expression in Breast Cancer Cells. Int. J. Mol. Sci. 2023, 24, 1181. [Google Scholar] [CrossRef] [PubMed]
- Calvaruso, M.; Militello, C.; Minafra, L.; La Regina, V.; Torrisi, F.; Pucci, G.; Cammarata, F.P.; Bravatà, V.; Forte, G.I.; Russo, G. Biological and Mechanical Characterization of the Random Positioning Machine (RPM) for Microgravity Simulations. Life 2021, 11, 1190. [Google Scholar] [CrossRef]
- Sahana, J.; Corydon, T.J.; Wehland, M.; Krüger, M.; Kopp, S.; Melnik, D.; Kahlert, S.; Relja, B.; Infanger, M.; Grimm, D. Alterations of Growth and Focal Adhesion Molecules in Human Breast Cancer Cells Exposed to the Random Positioning Machine. Front. Cell. Dev. Biol. 2021, 9, 672098. [Google Scholar] [CrossRef]
- Becker, J.L. Women’s health issues and space-based medical technologies. Earth Space Rev. 1994, 3, 15–19. [Google Scholar]
- Cheng, K.; Feng, X.a.; Yang, C.; Ma, C.; Niu, S.; Jia, L.; Yang, X.; Liang, J.; Bo, Y.; Geng, K.; et al. Simulated microgravity reduces quality of ovarian follicles and oocytes by disrupting communications of follicle cells. NPJ Microgravity 2023, 9, 7. [Google Scholar] [CrossRef]
- Cho, H.J.; Baek, M.O.; Khaliq, S.A.; Chon, S.J.; Son, K.H.; Lee, S.H.; Yoon, M.S. Microgravity inhibits decidualization via decreasing Akt activity and FOXO3a expression in human endometrial stromal cells. Sci. Rep. 2019, 9, 12094. [Google Scholar] [CrossRef] [PubMed]
- Chopra, V.; Dinh, T.V.; Hannigan, E.V. Three-dimensional endothelial-tumor epithelial cell interactions in human cervical cancers. In Vitro Cell. Dev. Biol. Anim. 1997, 33, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.; Maarsingh, J.D.; Herbst-Kralovetz, M.M.; Van Doorslaer, K. 3D Oral and Cervical Tissue Models for Studying Papillomavirus Host-Pathogen Interactions. Curr. Protoc. Microbiol. 2020, 59, e129. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Li, Y.; Liu, Y.; Huang, J.; Zhang, Z.; Wang, J.; Li, Y.; Hu, J.; Li, G. Identification of genes associated with tumor development in CaSki cells in the cosmic space. Mol. Biol. Rep. 2012, 39, 6923–6931. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Nguyen, Q.T.T.; Lee, S.; Choi, K.M.; Lee, E.J.; Park, J.Y. Customized small-sized clinostat using 3D printing and gas-permeable polydimethylsiloxane culture dish. NPJ Microgravity 2023, 9, 63. [Google Scholar] [CrossRef]
- Przystupski, D.; Górska, A.; Szewczyk, A.; Drąg-Zalesińska, M.; Kulbacka, J. 3D Clinorotation Affects Drug Sensitivity of Human Ovarian Cancer Cells. Microgravity Sci. Technol. 2021, 33, 42. [Google Scholar] [CrossRef]
- Drago-Ferrante, R.; Di Fiore, R.; Karouia, F.; Subbannayya, Y.; Das, S.; Aydogan Mathyk, B.; Arif, S.; Guevara-Cerdán, A.P.; Seylani, A.; Galsinh, A.S.; et al. Extraterrestrial Gynecology: Could Spaceflight Increase the Risk of Developing Cancer in Female Astronauts? An Updated Review. Int. J. Mol. Sci. 2022, 23, 7465. [Google Scholar] [CrossRef]
- Reynolds, R.; Little, M.P.; Day, S.; Charvat, J.; Blattnig, S.; Huff, J.; Patel, Z.S. Cancer incidence and mortality in the USA Astronaut Corps, 1959-2017. Occup. Environ. Med. 2021, 78, 869–875. [Google Scholar] [CrossRef]
- Arun, R.P.; Sivanesan, D.; Patra, B.; Varadaraj, S.; Verma, R.S. Simulated microgravity increases polyploid giant cancer cells and nuclear localization of YAP. Sci. Rep. 2019, 9, 10684. [Google Scholar] [CrossRef]
- Jessup, J.M.; Frantz, M.; Sonmez-Alpan, E.; Locker, J.; Skena, K.; Waller, H.; Battle, P.; Nachman, A.; Weber, M.E.; Thomas, D.A.; et al. Microgravity culture reduces apoptosis and increases the differentiation of a human colorectal carcinoma cell line. In Vitro Cell. Dev. Biol. Anim. 2000, 36, 367–373. [Google Scholar] [CrossRef]
- Arun, R.P.; Sivanesan, D.; Vidyasekar, P.; Verma, R.S. PTEN/FOXO3/AKT pathway regulates cell death and mediates morphogenetic differentiation of Colorectal Cancer Cells under Simulated Microgravity. Sci. Rep. 2017, 7, 5952. [Google Scholar] [CrossRef]
- Smit, T.; Calitz, C.; Willers, C.; Svitina, H.; Hamman, J.; Fey, S.J.; Gouws, C.; Wrzesinski, K. Characterization of an Alginate Encapsulated LS180 Spheroid Model for Anti-colorectal Cancer Compound Screening. ACS Med. Chem. Lett. 2020, 11, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Y.; Jiang, N.; Guo, S.; Li, B.B.; Yang, J.Q.; Chai, S.B.; Yan, H.F.; Sun, P.M.; Zhang, T.; Sun, H.W.; et al. Effect of simulated microgravity on metabolism of HGC-27 gastric cancer cells. Oncol. Lett. 2020, 19, 3439–3450. [Google Scholar] [CrossRef]
- Rembiałkowska, N.; Baczyńska, D.; Dubińska-Magiera, M.; Choromańska, A.; Bieżuńska-Kusiak, K.; Gajewska-Naryniecka, A.; Novickij, V.; Saczko, J.; Przystupski, D.; Kulbacka, J. RCCS Bioreactor-Based Modeled Microgravity Affects Gastric Cancer Cells and Improves the Chemotherapeutic Effect. Membranes 2022, 12, 448. [Google Scholar] [CrossRef] [PubMed]
- Masini, M.A.; Bonetto, V.; Manfredi, M.; Pastò, A.; Barberis, E.; Timo, S.; Vanella, V.V.; Robotti, E.; Masetto, F.; Andreoli, F.; et al. Prolonged exposure to simulated microgravity promotes stemness impairing morphological, metabolic and migratory profile of pancreatic cancer cells: A comprehensive proteomic, lipidomic and transcriptomic analysis. Cell. Mol. Life Sci. 2022, 79, 226. [Google Scholar] [CrossRef] [PubMed]
- La Barbera, G.; Capriotti, A.L.; Michelini, E.; Piovesana, S.; Calabretta, M.M.; Zenezini Chiozzi, R.; Roda, A.; Laganà, A. Proteomic analysis and bioluminescent reporter gene assays to investigate effects of simulated microgravity on Caco-2 cells. Proteomics 2017, 17, 1700081. [Google Scholar] [CrossRef] [PubMed]
- Khaoustov, V.I.; Risin, D.; Pellis, N.R.; Yoffe, B. Microarray analysis of genes differentially expressed in HepG2 cells cultured in simulated microgravity: Preliminary report. In Vitro Cell. Dev. Biol. Anim. 2001, 37, 84–88. [Google Scholar] [CrossRef]
- Chang, T.T.; Hughes-Fulford, M. Monolayer and spheroid culture of human liver hepatocellular carcinoma cell line cells demonstrate distinct global gene expression patterns and functional phenotypes. Tissue Eng. Part A 2009, 15, 559–567. [Google Scholar] [CrossRef]
- Clement, J.Q.; Lacy, S.M.; Wilson, B.L. Genome-wide gene expression profiling of microgravity effect on human liver cells. J. Gravit. Physiol. 2007, 14, 121–122. [Google Scholar]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Bade, B.C.; Dela Cruz, C.S. Lung Cancer 2020: Epidemiology, Etiology, and Prevention. Clin. Chest Med. 2020, 41, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.; Xu, H.; Guo, Y.; Jiang, X.; Liu, Y.; Li, K.; Pan, C.; Yuan, M.; Wang, J.; Li, T.; et al. Simulated microgravity alters the metastatic potential of a human lung adenocarcinoma cell line. In Vitro Cell. Dev. Biol. Anim. 2013, 49, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.H.; Ahn, C.B.; Son, K.H.; Yi, E.; Son, H.S.; Kim, H.S.; Lee, S.H. Simulated Microgravity Effects on Nonsmall Cell Lung Cancer Cell Proliferation and Migration. Aerosp. Med. Hum. Perform. 2017, 88, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.B.; Lee, J.H.; Han, D.G.; Kang, H.W.; Lee, S.H.; Lee, J.I.; Son, K.H.; Lee, J.W. Simulated microgravity with floating environment promotes migration of non-small cell lung cancers. Sci. Rep. 2019, 9, 14553. [Google Scholar] [CrossRef] [PubMed]
- Degan, P.; Cortese, K.; Pulliero, A.; Bruno, S.; Gagliani, M.C.; Congiu, M.; Izzotti, A. Simulated Microgravity Effects on Human Adenocarcinoma Alveolar Epithelial Cells: Characterization of Morphological, Functional, and Epigenetic Parameters. Int. J. Mol. Sci. 2021, 22, 6951. [Google Scholar] [CrossRef] [PubMed]
- Baghoum, H.; Alahmed, H.; Hachim, M.; Senok, A.; Jalaleddine, N.; Al Heialy, S. Simulated Microgravity Influences Immunity-Related Biomarkers in Lung Cancer. Int. J. Mol. Sci. 2022, 24, 10155. [Google Scholar] [CrossRef] [PubMed]
- Dietz, C.; Infanger, M.; Romswinkel, A.; Strube, F.; Kraus, A. Apoptosis Induction and Alteration of Cell Adherence in Human Lung Cancer Cells under Simulated Microgravity. Int. J. Mol. Sci. 2019, 20, 3601. [Google Scholar] [CrossRef]
- Lv, W.; Peng, X.; Tu, Y.; Shi, Y.; Song, G.; Luo, Q. YAP Inhibition Alleviates Simulated Microgravity-Induced Mesenchymal Stem Cell Senescence via Targeting Mitochondrial Dysfunction. Antioxidants 2023, 12, 990. [Google Scholar] [CrossRef]
- Roggan, M.D.; Kronenberg, J.; Wollert, E.; Hoffmann, S.; Nisar, H.; Konda, B.; Diegeler, S.; Liemersdorf, C.; Hellweg, C.E. Unraveling astrocyte behavior in the space brain: Radiation response of primary astrocytes. Front. Public Health 2023, 11, 1063250. [Google Scholar] [CrossRef]
- Tyrina, E.A.; Andreeva, E.R.; Buravkova, L.B. Simulated microgravity affects stroma-dependent ex vivo myelopoiesis. Tissue Cell 2023, 80, 101987. [Google Scholar] [CrossRef]
- Vergnes, L.; Foucaud, B.; Cepeda, C.; Espinosa-Jeffrey, A. Metabolomics Profile of the Secretome of Space-Flown Oligodendrocytes. Cells 2023, 12, 2249. [Google Scholar] [CrossRef] [PubMed]
- Parafati, M.; Giza, S.; Shenoy, T.S.; Mojica-Santiago, J.A.; Hopf, M.; Malany, L.K.; Platt, D.; Moore, I.; Jacobs, Z.A.; Kuehl, P.; et al. Human skeletal muscle tissue chip autonomous payload reveals changes in fiber type and metabolic gene expression due to spaceflight. NPJ Microgravity 2023, 9, 77. [Google Scholar] [CrossRef]
- Gesualdi, L.; Berardini, M.; Scicchitano, B.M.; Castaldo, C.; Bizzarri, M.; Filippini, A.; Riccioli, A.; Schiraldi, C.; Ferranti, F.; Liguoro, D.; et al. ERK Signaling Pathway Is Constitutively Active in NT2D1 Non-Seminoma Cells and Its Inhibition Impairs Basal and HGF-Activated Cell Proliferation. Biomedicines 2023, 11, 1894. [Google Scholar] [CrossRef]
- Mao, X.; Stanbouly, S.; Holley, J.; Pecaut, M.; Crapo, J. Evidence of Spaceflight-Induced Adverse Effects on Photoreceptors and Retinal Function in the Mouse Eye. Int. J. Mol. Sci. 2023, 24, 7362. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.; Carpo, N.; Cepeda, C.; Espinosa-Jeffrey, A. Oligodendrocyte Progenitors Display Enhanced Proliferation and Autophagy after Space Flight. Biomolecules 2023, 13, 201. [Google Scholar] [CrossRef]
- Ogneva, I.V.; Golubkova, M.A.; Biryukov, N.S.; Kotov, O.V. Drosophila melanogaster Oocytes after Space Flight: The Early Period of Adaptation to the Force of Gravity. Cells 2022, 11, 3871. [Google Scholar] [CrossRef] [PubMed]
- Veliz, A.L.; Mamoun, L.; Hughes, L.; Vega, R.; Holmes, B.; Monteon, A.; Bray, J.; Pecaut, M.J.; Kearns-Jonker, M. Transcriptomic Effects on the Mouse Heart Following 30 Days on the International Space Station. Biomolecules 2023, 13, 371. [Google Scholar] [CrossRef]
- Albi, E.; Ambesi-Impiombato, F.S.; Peverini, M.; Damaskopoulou, E.; Fontanini, E.; Lazzarini, R.; Curcio, F.; Perrella, G. Thyrotropin receptor and membrane interactions in FRTL-5 thyroid cell strain in microgravity. Astrobiology 2011, 11, 57–64. [Google Scholar] [CrossRef]
- Aissiou, A.K.; Jha, S.; Dhunnoo, K.; Ma, Z.; Li, D.X.; Ravin, R.; Kunze, M.; Wong, K.; Adesida, A.B. Transcriptomic response of bioengineered human cartilage to parabolic flight microgravity is sex-dependent. NPJ Microgravity 2023, 9, 5. [Google Scholar] [CrossRef]
- Mao, X.W.; Sandberg, L.B.; Gridley, D.S.; Herrmann, E.C.; Zhang, G.; Raghavan, R.; Zubarev, R.A.; Zhang, B.; Stodieck, L.S.; Ferguson, V.L.; et al. Proteomic Analysis of Mouse Brain Subjected to Spaceflight. Int. J. Mol. Sci. 2018, 20, 7. [Google Scholar] [CrossRef]
- Proshchina, A.; Gulimova, V.; Kharlamova, A.; Krivova, Y.; Barabanov, V.; Saveliev, S. Cytoskeleton Markers in the Spinal Cord and Mechanoreceptors of Thick-Toed Geckos after Prolonged Space Flights. Life 2022, 12, 100. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Sun, S.; Zhang, F.; Luo, C.; Zheng, L.; Wu, Y.; Li, N.; Zhang, C.; Wang, C.; Chen, Q.; et al. Microgravity-induced hepatogenic differentiation of rBMSCs on board the SJ-10 satellite. FASEB J. 2019, 33, 4273–4286. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, D.; Zhao, L.; Zhang, M.; Sun, Y. Effects of microgravity on DNA damage response in Caenorhabditis elegans during Shenzhou-8 spaceflight. Int. J. Radiat. Biol. 2015, 91, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Freed, L.E.; Langer, R.; Martin, I.; Pellis, N.R.; Vunjak-Novakovic, G. Tissue engineering of cartilage in space. Proc. Natl. Acad. Sci. USA 1997, 94, 13885–13890. [Google Scholar] [CrossRef] [PubMed]
- Stamenkovic, V.; Keller, G.; Nesic, D.; Cogoli, A.; Grogan, S.P. Neocartilage formation in 1g, simulated, and microgravity environments: Implications for tissue engineering. Tissue Eng. Part A 2010, 16, 1729–1736. [Google Scholar] [CrossRef]
- Carpo, N.; Tran, V.; Biancotti, J.C.; Cepeda, C.; Espinosa-Jeffrey, A. Space Flight Enhances Stress Pathways in Human Neural Stem Cells. Biomolecules 2024, 14, 65. [Google Scholar] [CrossRef]
- Camberos, V.; Baio, J.; Mandujano, A.; Martinez, A.F.; Bailey, L.; Hasaniya, N.; Kearns-Jonker, M. The Impact of Spaceflight and Microgravity on the Human Islet-1+ Cardiovascular Progenitor Cell Transcriptome. Int. J. Mol. Sci. 2021, 22, 3577. [Google Scholar] [CrossRef]
- Li, N.; Wang, C.; Sun, S.; Zhang, C.; Lu, D.; Chen, Q.; Long, M. Microgravity-Induced Alterations of Inflammation-Related Mechanotransduction in Endothelial Cells on Board SJ-10 Satellite. Front. Physiol. 2018, 9, 1025. [Google Scholar] [CrossRef]
- Rampoldi, A.; Forghani, P.; Li, D.; Hwang, H.; Armand, L.C.; Fite, J.; Boland, G.; Maxwell, J.; Maher, K.; Xu, C. Space microgravity improves proliferation of human iPSC-derived cardiomyocytes. Stem Cell Rep. 2022, 17, 2272–2285. [Google Scholar] [CrossRef]
- Cialdai, F.; Bolognini, D.; Vignali, L.; Iannotti, N.; Cacchione, S.; Magi, A.; Balsamo, M.; Vukich, M.; Neri, G.; Donati, A.; et al. Effect of space flight on the behavior of human retinal pigment epithelial ARPE-19 cells and evaluation of coenzyme Q10 treatment. Cell. Mol. Life Sci. 2021, 78, 7795–7812. [Google Scholar] [CrossRef]
- Versari, S.; Longinotti, G.; Barenghi, L.; Maier, J.A.; Bradamante, S. The challenging environment on board the International Space Station affects endothelial cell function by triggering oxidative stress through thioredoxin interacting protein overexpression: The ESA-SPHINX experiment. FASEB J. 2013, 27, 4466–4475. [Google Scholar] [CrossRef] [PubMed]
- Barravecchia, I.; De Cesari, C.; Forcato, M.; Scebba, F.; Pyankova, O.V.; Bridger, J.M.; Foster, H.A.; Signore, G.; Borghini, A.; Andreassi, M.; et al. Microgravity and space radiation inhibit autophagy in human capillary endothelial cells, through either opposite or synergistic effects on specific molecular pathways. Cell. Mol. Life Sci. 2021, 79, 28. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.H.; Long, J.P. Simulated microgravity impairs respiratory burst activity in human promyelocytic cells. In Vitro Cell. Dev. Biol. Anim. 2001, 37, 209–215. [Google Scholar] [CrossRef] [PubMed]


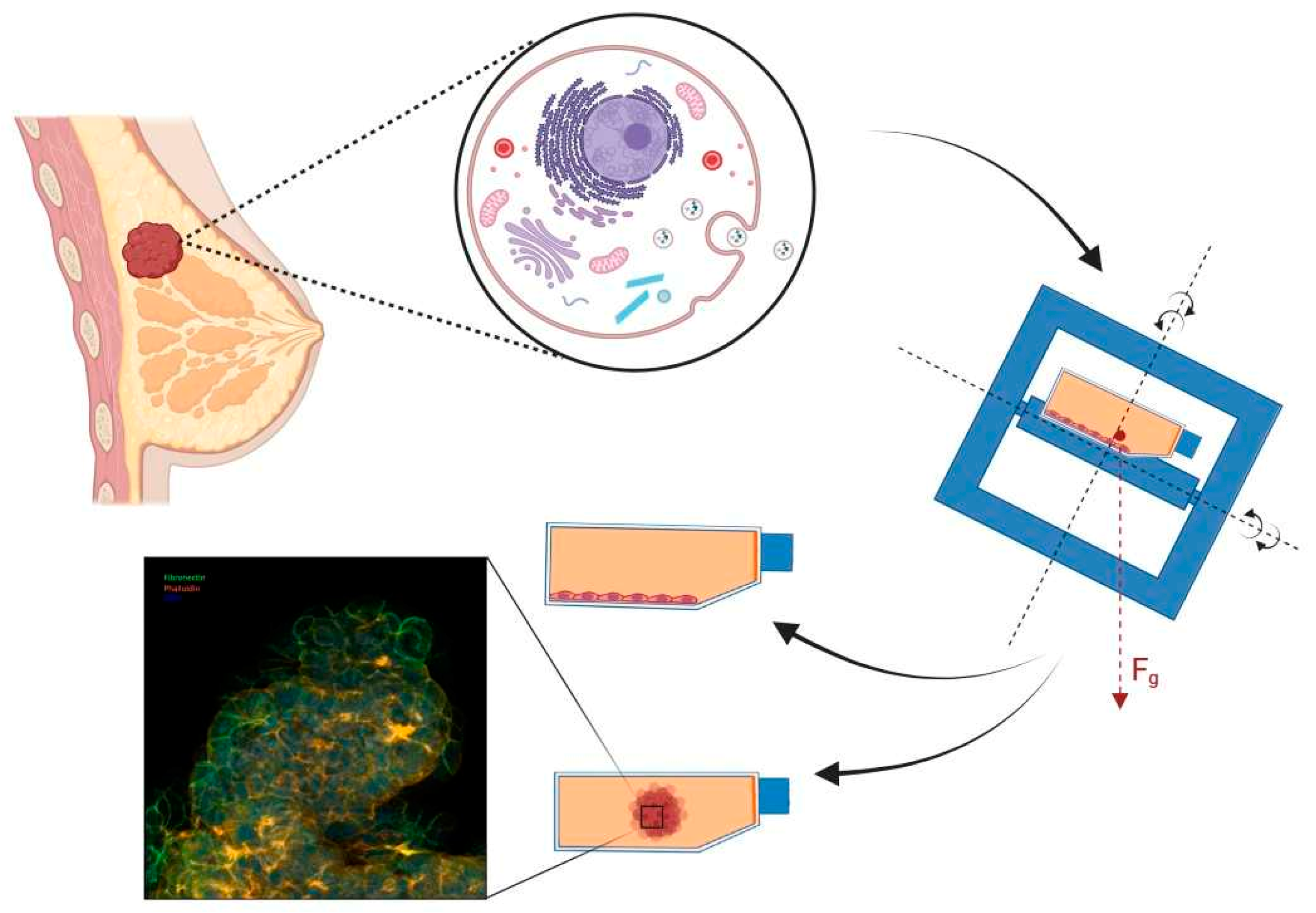
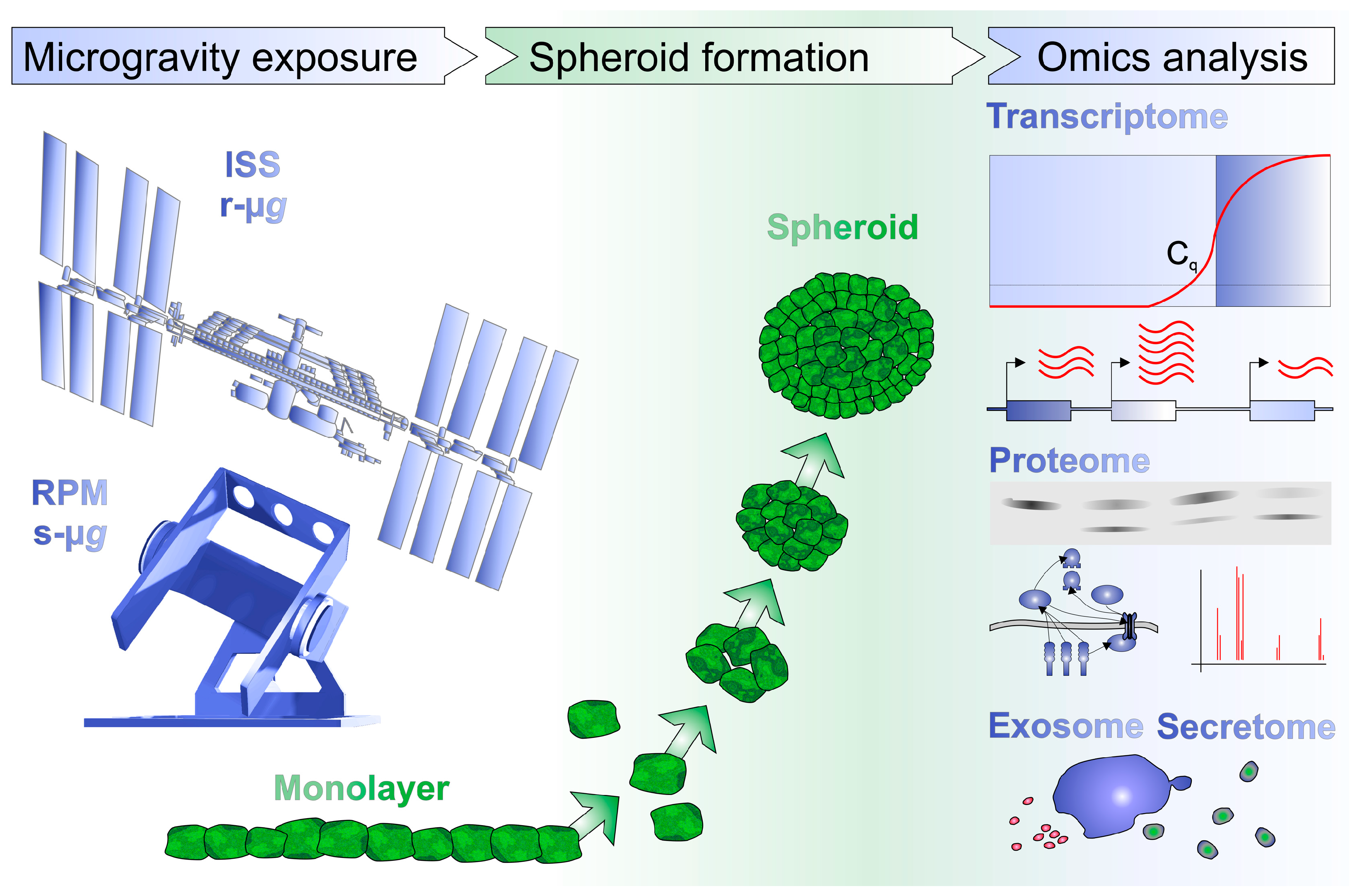
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graf, J.; Schulz, H.; Wehland, M.; Corydon, T.J.; Sahana, J.; Abdelfattah, F.; Wuest, S.L.; Egli, M.; Krüger, M.; Kraus, A.; et al. Omics Studies of Tumor Cells under Microgravity Conditions. Int. J. Mol. Sci. 2024, 25, 926. https://doi.org/10.3390/ijms25020926
Graf J, Schulz H, Wehland M, Corydon TJ, Sahana J, Abdelfattah F, Wuest SL, Egli M, Krüger M, Kraus A, et al. Omics Studies of Tumor Cells under Microgravity Conditions. International Journal of Molecular Sciences. 2024; 25(2):926. https://doi.org/10.3390/ijms25020926
Chicago/Turabian StyleGraf, Jenny, Herbert Schulz, Markus Wehland, Thomas J. Corydon, Jayashree Sahana, Fatima Abdelfattah, Simon L. Wuest, Marcel Egli, Marcus Krüger, Armin Kraus, and et al. 2024. "Omics Studies of Tumor Cells under Microgravity Conditions" International Journal of Molecular Sciences 25, no. 2: 926. https://doi.org/10.3390/ijms25020926
APA StyleGraf, J., Schulz, H., Wehland, M., Corydon, T. J., Sahana, J., Abdelfattah, F., Wuest, S. L., Egli, M., Krüger, M., Kraus, A., Wise, P. M., Infanger, M., & Grimm, D. (2024). Omics Studies of Tumor Cells under Microgravity Conditions. International Journal of Molecular Sciences, 25(2), 926. https://doi.org/10.3390/ijms25020926











