Mesenchymal Stem Cells Cultured in a 3D Microgel Environment Containing Platelet-Rich Plasma Significantly Modify Their Chondrogenesis-Related miRNA Expression
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Gelatin Modification to Add Tyramine Groups
4.3. PRP Collection
4.4. Preparation of the Microspheres
4.5. Evaluation of Platelet Activation
4.6. Cell Isolation and Culture
4.7. Analysis of Relative Gene Expression of Chondrocyte-Related Genes
4.8. miRNAs Analysis
4.9. Data Presentation and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anitua, E.; Sánchez, M.; Orive, G.; Andia, I. Delivering growth factors for therapeutics. Trends Pharmacol. Sci. 2008, 29, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Alsousou, J.; Thompson, M.; Hulley, P.; Noble, A.; Willett, K. The biology of platelet-rich plasma and its application in trauma and orthopaedic surgery: A review of the literature. J. Bone Jt. Surg. Br. 2009, 91, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Weibrich, G.; Hansen, T.; Kleis, W.; Buch, R.; Hitzler, W.E. Effect of platelet concentration in platelet-rich plasma on peri-implant bone regeneration. Bone 2004, 34, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Marx, R.E. Platelet-rich plasma: Evidence to support its use. J. Oral Maxillofac. Surg. 2004, 62, 489–496. [Google Scholar] [CrossRef]
- Schmidt-Bleek, K.; Willie, B.M.; Schwabe, P.; Seemann, P.; Duda, G.N. BMPs in bone regeneration: Less is more effective, a paradigm-shift. Cytokine Growth Factor Rev. 2016, 27, 141–148. [Google Scholar] [CrossRef]
- Vukicevic, S.; Oppermann, H.; Verbanac, D.; Jankolija, M.; Popek, I.; Curak, J.; Brkljacic, J.; Pauk, M.; Erjavec, I.; Francetic, I.; et al. The clinical use of bone morphogenetic proteins revisited: A novel biocompatible carrier device OSTEOGROW for bone healing. Int. Orthop. 2014, 38, 635–647. [Google Scholar] [CrossRef]
- Jain, E.; Chinzei, N.; Blanco, A.; Case, N.; Sandell, L.J.; Sell, S.; Rai, M.F.; Zustiak, S.P. Platelet-rich plasma released from polyethylene glycol hydrogels exerts beneficial effects on human chondrocytes. J. Orthop. Res. 2019, 37, 2401–2410. [Google Scholar] [CrossRef]
- Ishida, K.; Kuroda, R.; Miwa, M.; Tabata, Y.; Hokugo, A.; Kawamoto, T.; Sasaki, K.; Doita, M.; Kurosaka, M. The regenerative effects of platelet-rich plasma on meniscal cells in vitro and its in vivo application with biodegradable gelatin hydrogel. Tissue Eng. 2007, 13, 1103–1112. [Google Scholar] [CrossRef]
- Saito, M.; Takahashi, K.A.; Arail, Y.; Inoue, A.; Sakao, K.; Tonomura, H.; Honjo, K.; Nakagawa, S.; Inoue, H.; Tabata, Y.; et al. Intraarticular administration of platelet-rich plasma with biodegradable gelatin hydrogel microspheres prevents osteoarthritis progression in the rabbit knee. Clin. Exp. Rheumatol. 2009, 27, 201–207. [Google Scholar]
- Nagae, M.; Ikeda, T.; Mikami, Y.; Hase, H.; Ozawa, H.; Matsuda, K.I.; Sakamoto, H.; Tabata, Y.; Kawata, M.; Kubo, T. Intervertebral disc regeneration using platelet-rich plasma and biodegradable gelatin hydrogel microspheres. Tissue Eng. 2007, 13, 147–158. [Google Scholar] [CrossRef]
- Xu, X.; Hu, J.; Lu, H. Histological observation of a gelatin sponge transplant loaded with bone marrow-derived mesenchymal stem cells combined with platelet-rich plasma in repairing an annulus defect. PLoS ONE 2017, 12, e0171500. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhen, J.; Zhang, X.; Yang, Z.; Zhang, L.; Hao, D.; Ren, B. Effect of autologous platelet-rich plasma and gelatin sponge for tendon-to-bone healing after rabbit anterior cruciate ligament reconstruction. Arthroscopy 2019, 35, 1486–1497. [Google Scholar] [CrossRef]
- Akeda, K.; An, H.S.; Okuma, M.; Attawia, M.; Miyamoto, K.; Thonar, E.J.M.A.; Lenz, M.; Sah, R.; Masuda, K. Platelet-rich plasma stimulates porcine articular chondrocyte proliferation and matrix biosynthesis. Osteoarthr. Cartil. 2006, 14, 1272–1280. [Google Scholar] [CrossRef] [PubMed]
- Jeyakumar, V.; Niculescu-Morzsa, E.; Bauer, C.; Lacza, Z.; Nehrer, S. Platelet-rich plasma supports proliferation and redifferentiation of chondrocytes during in vitro expansion. Front. Bioeng. Biotechnol. 2017, 5, 75. [Google Scholar] [CrossRef] [PubMed]
- Vetrano, M.; Ranieri, D.; Nanni, M.; Pavan, A.; Malisan, F.; Vulpiani, M.C.; Visco, V. Hyaluronic acid (HA), platelet-rich plasm and extracorporeal shock wave therapy (ESWT) promote human chondrocyte regeneration in vitro and ESWT-mediated increase of CD44 expression enhances their susceptibility to HA treatment. PLoS ONE 2019, 14, e0218740. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Pan, Z.; Xia, L.H.; Liu, X.N.; Guo, X.L.; He, Y.; Zhou, J.; Qu, Z.H.; Mei, G.; Jin, D.; et al. Bilayered poly(Lactide-co-glycolide) scaffold with platelet-rich plasma and mesenchymal stem cells improves restoration of osteochondral defects. J. Biomater. Tissue Eng. 2015, 5, 757–765. [Google Scholar] [CrossRef]
- Filardo, G.; Kon, E.; Buda, R.; Timoncini, A.; Di Martino, A.; Cenacchi, A.; Fornasari, P.M.; Giannini, S.; Marcacci, M. Platelet-rich plasma intra-articular knee injections for the treatment of degenerative cartilage lesions and osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 528–535. [Google Scholar] [CrossRef]
- Kon, E.; Buda, R.; Filardo, G.; Di Martino, A.; Timoncini, A.; Cenacchi, A.; Fornasari, P.M.; Giannini, S.; Marcacci, M. Platelet-rich plasma: Intra-articular knee injections produced favorable results on degenerative cartilage lesions. Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 472–479. [Google Scholar] [CrossRef]
- Battaglia, M.; Guaraldi, F.; Vannini, F.; Rossi, G.; Timoncini, A.; Buda, R.; Giannini, S. Efficacy of ultrasound-guided intra-articular injections of platelet-rich plasma versus hyaluronic acid for hip osteoarthritis. Orthopedics 2013, 36, e1501–e1509. [Google Scholar] [CrossRef]
- Li, N.; Long, B.; Han, W.; Yuan, S.; Wang, K. MicroRNAs: Important regulators of stem cells. Stem Cell Res. Ther. 2017, 8, 110. [Google Scholar] [CrossRef]
- Stelcer, E.; Kulcenty, K.; Rucinski, M.; Jopek, K.; Richter, M.; Trzeciak, T.; Suchorska, W.M. The role of microRNAs in early chondrogenesis of human induced Pluripotent Stem Cells (hiPSCs). Int. J. Mol. Sci. 2019, 20, 4371. [Google Scholar] [CrossRef] [PubMed]
- Paganopoulos, P.K.; Lambrou, G.I. The involvement of microRNAs in osteoarthritis and recent developments: A narrative review. Mediterr. J. Theumatol. 2018, 29, 67–79. [Google Scholar] [CrossRef]
- Zurriaga Carda, J.; Lastra, M.L.; Antolinos-Turpin, C.M.; Morales-Román, R.M.; Sancho-Tello, M.; Perea-Ruiz, S.; Milián, L.; Fernández, J.M.; Cortizo, A.M.; Carda, C.; et al. A cell-free approach with a supporting biomaterial in the form of dispersed microspheres induces hyaline cartilage formation in a rabbit knee model. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 1428–1438. [Google Scholar] [CrossRef] [PubMed]
- Bello, A.B.; Kim, D.; Kim, D.; Park, H.; Lee, S.H. Engineering and functionalization of gelatin biomaterials: From cell culture to medical applications. Tissue Eng. Part B Rev. 2020, 26, 164–180. [Google Scholar] [CrossRef]
- Sulaiman, S.B.; Idrus, R.B.H.; Hwei, N.M. Gelatin microsphere for cartilage tissue engineering: Current and future strategies. Polymers 2020, 12, 2404. [Google Scholar] [CrossRef] [PubMed]
- Poveda-Reyes, S.; Moulisova, V.; Sanmartín-Masiá, E.; Quintanilla-Sierra, L.; Salmerón-Sánchez, M.; Ferrer, G.G. Gelatin-hyaluronic acid hydrogels with tuned stiffness to counterbalance cellular forces and promote cell differentiation. Macromol. Biosci. 2016, 16, 1311–1324. [Google Scholar] [CrossRef]
- Oliver-Ferrándiz, M.; Milián, L.; Sancho-Tello, M.; de Llano, J.J.M.; Roca, F.G.; Martínez-Ramos, C.; Carda, C.; Mata, M. Alginate-agarose hydrogels improve the in vitro differentiation of human dental pulp stem cells in chondrocytes. A histological study. Biomedicines 2021, 9, 834. [Google Scholar] [CrossRef]
- Infante, A.; Rubio-Azpeitia, E.; Sánchez, P.; Alberdi, R.; Rodriguez, C.I.; Andia, I. Platelet rich plasma and culture configuration affect the matrix forming phenotype of bone marrow stromal cells. Tissue Eng. Regen. Med. 2017, 14, 567–577. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Zhang, B.; Zhao, Q.; Liu, Y.; Qi, W. Effect of activated platelet-rich plasma on chondrogenic differentiation of rabbit bone marrow-derived mesenchymal stem cells. Stem Cells Int. 2021, 2021, 9947187. [Google Scholar] [CrossRef]
- Colnot, C.I.; Helms, J.A. A molecular analysis of matrix remodeling and angiogenesis during long bone development. Mech. Dev. 2001, 100, 245–250. [Google Scholar] [CrossRef]
- Lefebvre, V.; Behringer, R.R.; De Crombrugghe, B. L-Sox5, Sox6 and SOx9 control essential steps of the chondrocyte differentiation pathway. Osteoarthr. Cartil. 2001, 9, S69–S75. [Google Scholar] [CrossRef] [PubMed]
- Suomi, S.; Taipaleenmäki, H.; Seppänen, A.; Ripatti, T.; Väänänen, K.; Hentunen, T.; Säämänen, A.-M.; Laitala-Leinonen, T. MicroRNAs regulate osteogenesis and chondrogenesis of mouse bone marrow stromal cells. Gene Regul. Syst. Biol. 2008, 2, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.J.; Liu, J.; Qin, J. MiR-138 suppressed the progression of osteoarthritis mainly through targeting p65. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2177–2184. [Google Scholar]
- Vonk, L.A.; Kragten, A.H.M.; Dhert, W.J.A.; Saris, D.B.F.; Creemers, L.B. Overexpression of hsa-miR-148a promotes cartilage production and inhibits cartilage degradation by osteoarthritic chondrocytes. Osteoarthr. Cartil. 2014, 22, 145–153. [Google Scholar] [CrossRef]
- Tian, Y.; Guo, R.; Shi, B.; Chen, L.; Yang, L.; Fu, Q. MicroRNA-30a promotes chondrogenic differentiation of mesenchymal stem cells through inhibiting Delta-like 4 expression. Life Sci. 2016, 148, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Melnik, S.; Hofmann, N.; Gabler, J.; Hecht, N.; Richter, W. MiR-181a targets RSPO2 and regulates bone morphogenetic protein—WNT signaling crosstalk during chondrogenic differentiation of mesenchymal stromal cells. Front. Cell. Dev. Biol. 2021, 9, 747057. [Google Scholar] [CrossRef]
- Xu, R.; Wei, Y.; Yin, X.; Shi, B.; Li, J. miR-20a suppresses chondrogenic differentiation of ATDC5 cells by regulating Atg7. Sci. Rep. 2019, 9, 9243. [Google Scholar] [CrossRef]
- Yan, C.; Wang, Y.; Shen, X.Y.; Yang, G.; Jian, J.; Wang, H.S.; Chen, G.Q.; Wu, Q. MicroRNA regulation associated chondrogenesis of mouse MSCs grown on polyhydroxyalkanoates. Biomaterials 2011, 32, 6435–6444. [Google Scholar] [CrossRef]
- Karlsen, T.A.; Jakobsen, R.B.; Mikkelsen, T.S.; Brinchmann, J.E. microRNA-140 targets RALA and regulates chondrogenic differentiation of human mesenchymal stem cells by translational enhancement of SOX9 and ACAN. Stem Cells Dev. 2014, 23, 290–304. [Google Scholar] [CrossRef]
- Melnik, S.; Gabler, J.; Dreher, S.I.; Hecht, N.; Hofmann, N.; Großner, T.; Richter, W. MiR-218 affects hypertrophic differentiation of human mesenchymal stromal cells during chondrogenesis via targeting RUNX2, MEF2C, and COL10A1. Stem Cell Res. Ther. 2020, 11, 532. [Google Scholar] [CrossRef]
- Yang, B.; Guo, H.; Zhang, Y.; Dong, S.; Ying, D. The microRNA expression profiles of mouse mesenchymal stem cell during chondrogenic differentiation. BMB Rep. 2011, 44, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Jin, E.H.; Kim, D.; Kim, K.Y.; Chun, C.H.; Jin, E.J. MicroRNA-222 regulates MMP-13 via targeting HDAC-4 during osteoarthritis pathogenesis. BBA Clin. 2015, 3, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Huang, R.; Ma, H.; Zhao, X.; Wang, G. MiRNA-411 regulates chondrocyte autophagy in osteoarthritis by targeting hypoxia-inducible factor 1 alpha (HIF-1α). Med. Sci. Monit. 2020, 26, e921155. [Google Scholar] [CrossRef] [PubMed]
- Moulisová, V.; Poveda-Reyes, S.; Sanmartín-Masiá, E.; Quintanilla-Sierra, L.; Salmerón-Sánchez, M.; Gallego Ferrer, G. Hybrid Protein-Glycosaminoglycan Hydrogels Promote Chondrogenic Stem Cell Differentiation. ACS Omega 2017, 2, 7609–7620. [Google Scholar] [CrossRef]
- Milian, L.; Mata, M.; Alcacer, J.; Oliver, M.; Sancho-Tello, M.; de Llano, J.J.M.; Camps, C.; Galbis, J.; Carretero, J.; Carda, C. Cannabinoid receptor expression in non-small cell lung cancer. Effectiveness of tetrahydrocannabinol and cannabidiol inhibiting cell proliferation and epithelial-mesenchymal transition in vitro. PLoS ONE 2020, 15, e0228909. [Google Scholar] [CrossRef]
- Sarrion, I.; Milian, L.; Juan, G.; Ramon, M.; Furest, I.; Carda, C.; Gimeno, J.C.; Roig, M.M. Role of circulating miRNAs as biomarkers in idiopathic pulmonary arterial hypertension: Possible relevance of miR-23a. Oxid. Med. Cell. Longev. 2015, 2015, 792846. [Google Scholar] [CrossRef]
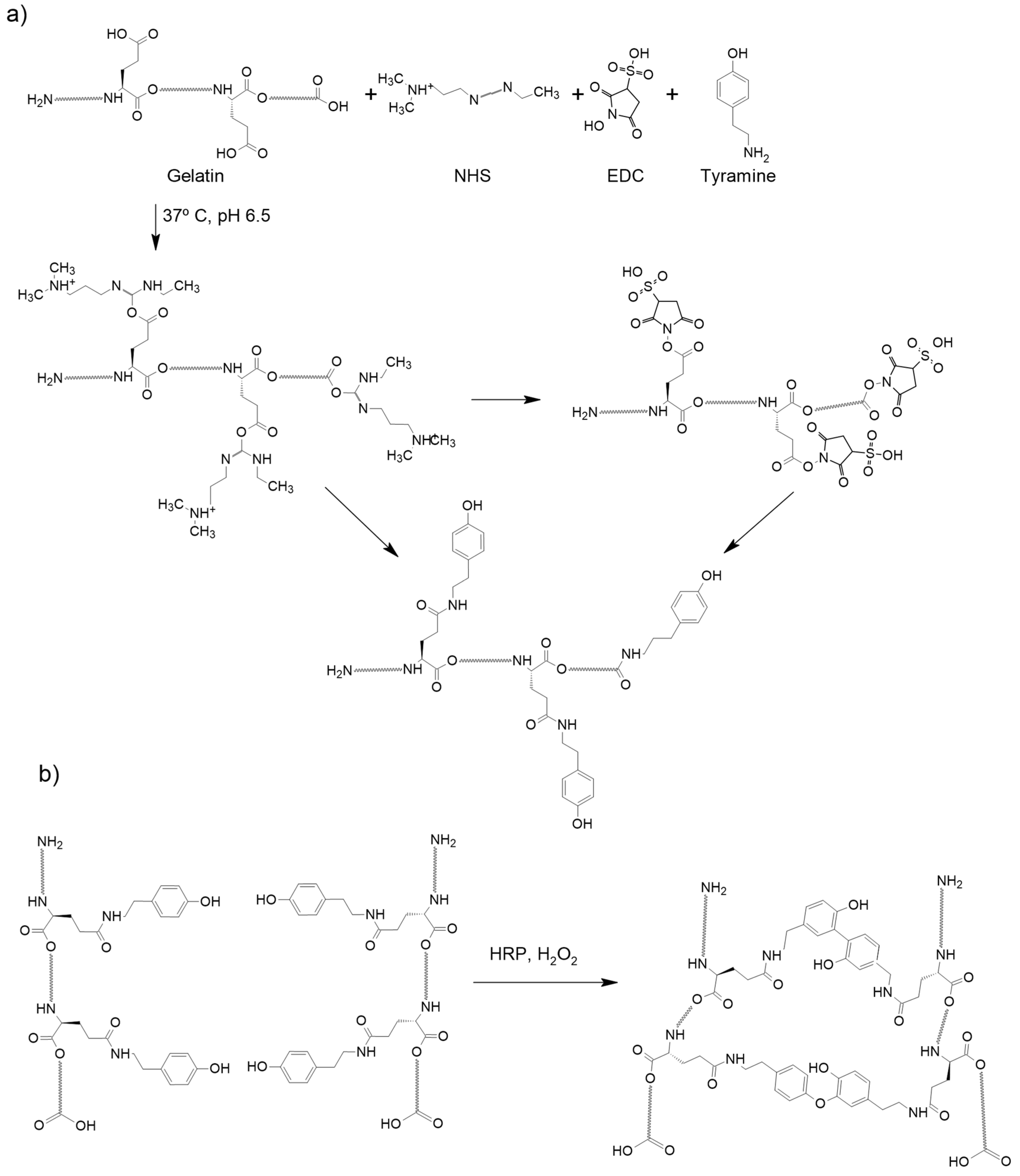
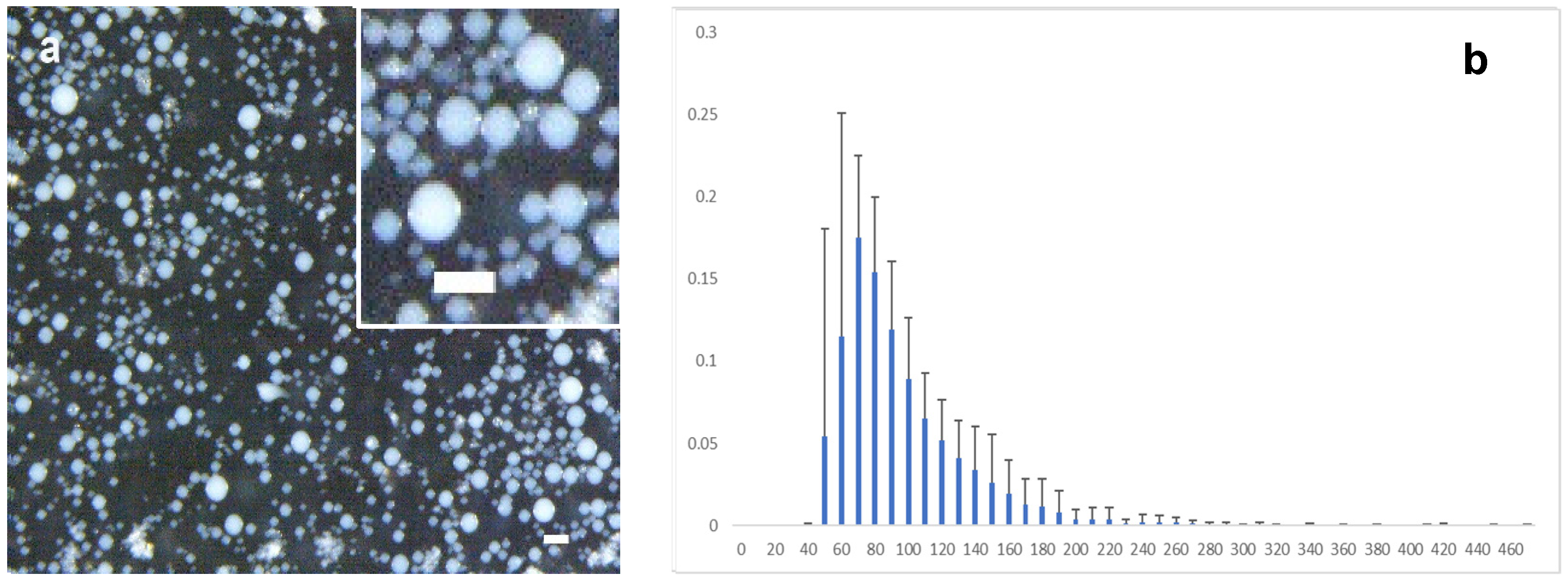

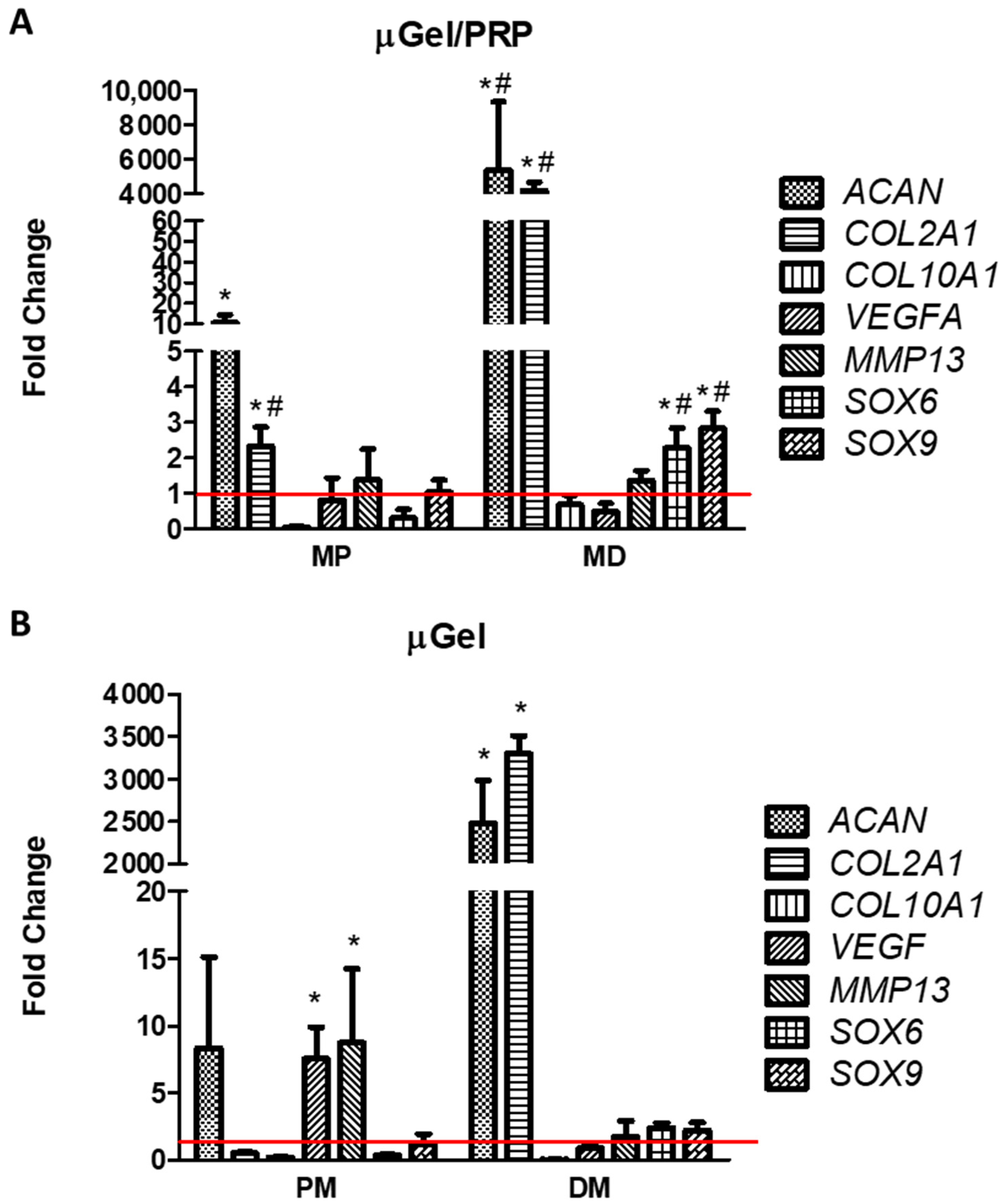
| μGel/PRP | Gelatin/PRP Microspheres |
| μGel/PRP-A | Gelatin/PRP microspheres activated with CaCl2 |
| bGel/PRP | Bulk Gelatin/PRP |
| bGel/PRP-A | Bulk Gelatin/PRP activated with CaCl2 |
| PRP | Pure PRP |
| PRP-A | Pure PRP activated with CaCl2 |
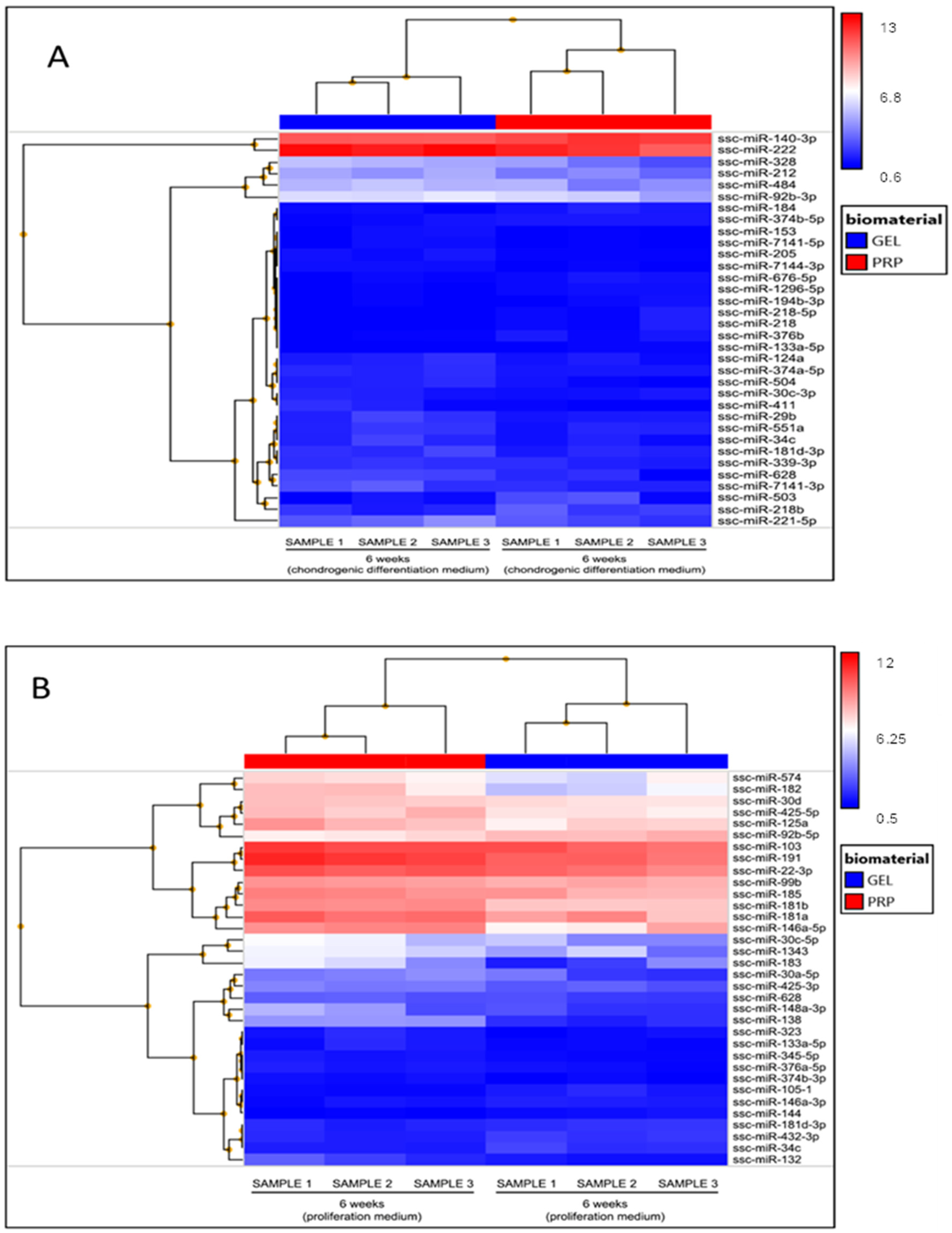
| Proliferation Medium | Chondrogenic Differentiation Medium | ||||
|---|---|---|---|---|---|
| miRNA | Fold Change | Adjusted p-Value | miRNA | Fold Change | Adjusted p-Value |
| ssc-miR-374b-3p | 1.75 | 0.0706 | ssc-miR-503 | 3.36 | 0.1456 |
| ssc-miR-132 | 1.98 | 0.0542 | ssc-miR-140-3p | 2.06 | 0.0137 |
| ssc-miR-181b | 2.55 | 0.00000402 | ssc-miR-218b | 1.99 | 0.1005 |
| ssc-miR-323 | 1.78 | 0.0994 | ssc-miR-376b | 1.87 | 0.0612 |
| ssc-miR-125a | 2.08 | 0.0653 | ssc-miR-133a-5p | 1.82 | 0.0226 |
| ssc-miR-144 | −1.94 | 0.0167 | ssc-miR-194b-3p | 1.78 | 0.174 |
| ssc-miR-105-1 | −1.96 | 0.0236 | ssc-miR-1296-5p | 1.78 | 0.0269 |
| ssc-miR-432-3p | −2.13 | 0.0161 | ssc-miR-676-5p | 1.74 | 0.0882 |
| ssc-miR-138 | 4.63 | 0.0000379 | ssc-miR-184 | 1.73 | 0.0856 |
| ssc-miR-148a-3p | 4.51 | 0.0889 | ssc-miR-218 | 1.73 | 0.083 |
| ssc-miR-181a | 2.42 | 0.041 | ssc-miR-218-5p | 1.73 | 0.083 |
| ssc-miR-30d | 2.07 | 0.0098 | ssc-miR-374b-5p | 1.71 | 0.0364 |
| ssc-miR-103 | 1.89 | 0.0903 | ssc-miR-124a | −1.71 | 0.1324 |
| ssc-miR-628 | 1.73 | 0.0598 | ssc-miR-7141-5p | −1.71 | 0.1605 |
| ssc-miR-146a-3p | −1.91 | 0.0282 | ssc-miR-205 | −1.72 | 0.0238 |
| ssc-miR-92b-5p | −2.62 | 0.0076 | ssc-miR-7144-3p | −1.72 | 0.008 |
| ssc-miR-22-3p | 2.1 | 0.0644 | ssc-miR-339-3p | −1.74 | 0.0988 |
| ssc-miR-30c-5p | 5.88 | 0.0861 | ssc-miR-374a-5p | −1.76 | 0.0084 |
| ssc-miR-99b | 1.9 | 0.028 | ssc-miR-92b-3p | −1.76 | 0.1694 |
| ssc-miR-1343 | 3.73 | 0.0838 | ssc-miR-181d-3p | −1.78 | 0.1152 |
| ssc-miR-185 | 2.08 | 0.058 | ssc-miR-30c-3p | −1.78 | 0.1384 |
| ssc-miR-133a-5p | 1.76 | 0.0941 | ssc-miR-153 | −1.79 | 0.1415 |
| ssc-miR-30a-5p | 3.17 | 0.0763 | ssc-miR-34c | −1.81 | 0.1241 |
| ssc-miR-425-3p | 2.15 | 0.0073 | ssc-miR-551a | −1.83 | 0.1258 |
| ssc-miR-183 | 9.24 | 0.0538 | ssc-miR-29b | −1.91 | 0.0807 |
| ssc-miR-376a-5p | 1.71 | 0.0444 | ssc-miR-504 | −1.98 | 0.03 |
| ssc-miR-425-5p | 1.8 | 0.0188 | ssc-miR-628 | −1.99 | 0.0747 |
| ssc-miR-345-5p | 1.72 | 0.0043 | ssc-miR-411 | −2.07 | 0.0789 |
| ssc-miR-34c | −1.97 | 0.0341 | ssc-miR-484 | -2.22 | 0.1198 |
| ssc-miR-181d-3p | −1.94 | 0.0561 | ssc-miR-221-5p | −2.27 | 0.1237 |
| ssc-miR-574 | 2.59 | 0.0699 | ssc-miR-7141-3p | −2.34 | 0.0945 |
| ssc-miR-182 | 6.46 | 0.0163 | ssc-miR-222 | −2.51 | 0.083 |
| ssc-miR-146a-5p | 5.3 | 0.0401 | ssc-miR-212 | −2.6 | 0.0398 |
| ssc-miR-191 | 1.85 | 0.0115 | ssc-miR-328 | −3.38 | 0.0559 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mata, M.; Salvador-Clavell, R.; Ródenas-Rochina, J.; Sancho-Tello, M.; Gallego Ferrer, G.; Gómez Ribelles, J.L. Mesenchymal Stem Cells Cultured in a 3D Microgel Environment Containing Platelet-Rich Plasma Significantly Modify Their Chondrogenesis-Related miRNA Expression. Int. J. Mol. Sci. 2024, 25, 937. https://doi.org/10.3390/ijms25020937
Mata M, Salvador-Clavell R, Ródenas-Rochina J, Sancho-Tello M, Gallego Ferrer G, Gómez Ribelles JL. Mesenchymal Stem Cells Cultured in a 3D Microgel Environment Containing Platelet-Rich Plasma Significantly Modify Their Chondrogenesis-Related miRNA Expression. International Journal of Molecular Sciences. 2024; 25(2):937. https://doi.org/10.3390/ijms25020937
Chicago/Turabian StyleMata, Manuel, Rubén Salvador-Clavell, Joaquín Ródenas-Rochina, María Sancho-Tello, Gloria Gallego Ferrer, and José Luis Gómez Ribelles. 2024. "Mesenchymal Stem Cells Cultured in a 3D Microgel Environment Containing Platelet-Rich Plasma Significantly Modify Their Chondrogenesis-Related miRNA Expression" International Journal of Molecular Sciences 25, no. 2: 937. https://doi.org/10.3390/ijms25020937
APA StyleMata, M., Salvador-Clavell, R., Ródenas-Rochina, J., Sancho-Tello, M., Gallego Ferrer, G., & Gómez Ribelles, J. L. (2024). Mesenchymal Stem Cells Cultured in a 3D Microgel Environment Containing Platelet-Rich Plasma Significantly Modify Their Chondrogenesis-Related miRNA Expression. International Journal of Molecular Sciences, 25(2), 937. https://doi.org/10.3390/ijms25020937









